Abstract
The most common type of cancer in the world is lung cancer. Traditional treatments have an important role in cancer therapy. In the present review, the most recent findings on the effects of medicinal plants and their constituents or natural products (NP) in treating lung cancer are discussed. Empirical studies until the end of March 2022 were searched using the appropriate keywords through the databases PubMed, Science Direct and Scopus. The extracts and essential oils tested were all shown to effect lung cancer by several mechanisms including decreased tumour weight and volume, cell viability and modulation of cytokine. Some plant constituents increased expression of apoptotic proteins, the proportion of cells in the G2/M phase and subG0/G1 phase, and Cyt c levels. Also, natural products (NP) activate apoptotic pathways in lung cancer cell including p‐JNK, Akt/mTOR, PI3/ AKT\ and Bax, Bcl2, but suppressed AXL phosphorylation. Plant‐derived substances altered the cell morphology, reduced cell migration and metastasis, oxidative marker production, p‐eIF2α and GRP78, IgG, IgM levels and reduced leukocyte counts, LDH, GGT, 5′NT and carcinoembryonic antigen (CEA). Therefore, medicinal plant extracts and their constituents could have promising therapeutic value for lung cancer, especially if used in combination with ordinary anti‐cancer drugs.
Keywords: apoptosis, in vitro, in vivo, lung cancer, medical plants, metastasis
1. INTRODUCTION
It is estimated that 70% of cancer cases in the world are occurring in low‐ and middle‐income countries and, as with many cancers, early diagnosis increases the survival rate by up to 20%. 1 , 2 Tobacco smoke accounts for more than 80% of lung cancer cases, making it the most preventable cancer of this century. 3 , 4 , 5 However, familial history, genetics and biological gender also pose effective risk factors. It was reported that lung cancer is more prevalent in women that smoke or who are exposed to cigarette or tobacco smoke, and a lifetime of smoking increases the risk of developing lung cancer by 20‐ to 30‐fold. 6 Non‐invasive screening approaches such as chest imaging, 7 monitoring of biomarkers: proteins, 8 , 9 , 10 auto‐antibodies, early CDT‐Lung test, 11 gene expression profiles 12 and microRNAs 13 in the blood or airway epithelium, evaluating mutations or methylation in circulating tumour DNA (ctDNA), 14 , 15 , 16 , 17 , 18 as well as learning the history of chronic exposure to smoke and autoimmune diseases 13 are helpful for early diagnosis of lung cancer. The two main types of lung cancer are small cell lung cancer (SCLC) and non‐small cell lung cancer (NSCLC). SCLC occurs mainly in the central airways including bronchioloalveolar cells, whereas NSCLC types originate in the neuroendocrine system. 4 Radiotherapy, chemotherapy and surgery are common treatments, depending on the stage of the cancer. For example, in the early stages of cancer surgery is best, and in advanced stages, a combination of radiotherapy and surgery are appropriate treatment methods. Cisplatin or carboplatin‐based combination therapies are standard treatments of metastatic lung cancer. 19
Alternative treatments in complement with traditional methods have been shown to be helpful in the treatment of various types of cancer, and more recently, medicinal plants 20 , 21 , 22 are reported as effective alternative treatments of lung cancer. Their anti‐cancer properties include cytotoxicity (being toxic to cancer cells), inducing cancer cell apoptosis, inhibiting tumour angiogenesis and inhibiting cancer cell proliferation and growth. These impacts on cancer cells by medicinal plants and their derivatives occur through a variety of cellulare pathways and molecular processes, including: altering reactive oxygen species (ROS) generation, modulating transcriptional activators, causing mitochondrial membrane potential loss by P53 (promoter of apoptosis), inhibiting the proliferation of the transformed cell by CD95, Cyt.c and Smac release, down‐regulating Nuclear factor kappa B (NFk‐B), activator protein 1 (AP‐1), early growth response protein 1 (EGR‐1), cyclooxygenase 2 (COX‐2), lipoxygenase (LOX), matrix metallopeptidase 9 (MMP‐9), tumour necrosis factor (TNF), chemokine, epidermal growth factor receptor (EGFR), human epidermal growth factor receptor 2 (HER2) and inhibiting c‐Jun N‐terminal kinases (JNK) and serine/threonine kinase (STK4) pathway. Furthermore, medicinal plant extracts have been found to inhibit metastasis in various tumour cancers (breast, hepatic, colon and stomach cancer) by reducing MMP‐2 release of cytochrome c (Cyt c), activating Cas‐3 and ‐9, down‐regulating anti‐apoptotic proteins Bcl‐XL and integrin associated proteins and altering the ratio of Bcl2 family proteins. 23
In the present article, we discuss the 11 medicinal plants and their components or natural products (NP) that have proven most effective against lung cancer. We collate findings from in vitro and in vivo studies and clinical trials and provide the proposed molecular and cellular mechanisms of action. The biochemical properties of each plant and its constituents are described before exploring their potential as an alternative and complementary treatment of lung cancer.
2. METHODS
In vivo and in vitro reports regarding the effect of NP on lung cancer were explored using the databases PubMed, Science Direct and Scopus until the end of March 2022. Accordingly, keywords including lung cancer, medicinal plants, plant constituents, NP, in vivo, in vitro and clinical studies were used.
Several criteria were used to select eligible studies for this review: (1) in vitro, in vivo (animal) and human studies, (2) NP supplementation and their dosage, (3) the effect of NP on lung cancer, (5) English language articles published in well‐known international journals. The reference classification is depicted in Figure 1.
FIGURE 1.

Flowchart of the selecting studies in the current review.
3. RESULTS
3.1. The effect of various medicinal herbs and their constituents on lung cancer
3.1.1. Zataria multiflora
Zataria multiflora (Z. multiflora) is a traditional herb belonging to the Lamiaceae family and grows in the southern and central regions of Iran. Application of Z. multiflora extract, and its constituent carvacrol, led to various pharmacological effects including anti‐inflammation, immunomodulation and antioxidation. The plant extract and carvacrol also reduced paraquat (herbicide)‐induced lung injury via activation of PPAR‐γ. 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 Anti‐cancer effects of Z. multiflora and carvacrol have been reported in several studies that we discuss below.
Regarding the safety and efficacy of Z. multiflora, the results of two animal studies indicated that, due to phenol components including carvacrol and thymol, reduction of ROS levels in tissue but in doses more than 0.4 mL/kg for 14 days, Z. multiflora reduced the safety but increased toxicity. 34 , 35 However, in healthy human carvacrol at doses of 1 and 2 mg/kg/day for 1 month did not show any side effects. 36 The effects of Z. multiflora extract and carvacrol on lung cancer are summarized in Table 1.
TABLE 1.
The effects of Zataria multiflora and Nigella sativa and their derivatives, carvacrol and thymoquinone, on lung cancer.
| Study type | Type of lung cancer | Preparations | Dose | Effects | Ref. |
|---|---|---|---|---|---|
| A549 cell | CN | 0–150 μg/mL | Decreased MAPK p38, ERK activation, COX‐2 and VEGF expression | 39 | |
| NSCLC | Carvacrol | 0, 30, 100 and 300 μM | Decreased AXL protein expiration, inhibited AXL phosphorylation | 44 | |
| NSCLC | Carvacrol nanoemulsion | 25, 50 and 100 μg/mL | Decreased ROS, regulated p‐JNK, Bax, Bcl2, increased Cyt c, caspase, apoptosis, down‐regulated CHOP, p‐eIF2α, GRP78 expression | 43 | |
| NSCLC | Carvacrol | 100–1000 μM | Decreased morphology changes, cell growth | 42 | |
| Doxorubicin resistant‐A549 cell line | Carvacrol nanoemulsion | 5, 25 and 50 μg/mL after 24 h | Elevated Bax, Cyt c, cleaved caspase 3 and 9, p21 protein expression, reduced CDK2, CDK4, CDK6, Cyclin E, cyclin D1 | 43 | |
| NCI‐H460 cells | TQ | 1.25, 0.5, 5 μM, for 24, 48 and 72 hrs | Reduced cell proliferation, cytokines expression, ENA‐78, GRO‐alpha, increased apoptosis level, cell viability | 47 | |
| NSCLC | TQ | 10, 20, 40 μmol/L | Inhibited cell proliferation, migration PCNA, cyclin D1, MMP2, MMP9 mRNA, activation ERK1/2 pathway, cell cycle | 55 | |
| A549 non‐small cell | TQ | 2, 5, 10 μM | Up‐regulated Bax, p21, receptor 1 and 2 expressions, Bax/Bcl2 ratio, down‐regulated Bcl2 proteins, inflammatory markers, ROS, cyclin D, NF‐kappa B and IKK1 expression | 56 | |
| In vivo | Tumour‐bearing BALB/c mice | Z. multiflora Essential oil | 500 mg/kg | Increased TNF‐α, IFN‐γ, IL‐2, decreased IL‐4 level | 38 |
| Lung cancer induced‐B(a)P | Carvacrol | 25 and 50 mg/kg, 7 days | Enhanced antioxidants, iNOS, NF‐κB and COX‐2 expressions, decreased positive stained cells | 40 | |
| Lung cancer model (athymic nude mice) | Carvacrol | 50 and 100 mg/kg | Reduced tumour growth and weight, increased p‐JNK, Bax, Bcl2, Cyt C, caspase‐3, caspase‐9 and β‐ Actin expression | 43 | |
| Rat multi‐organ carcinogenesis | N. sativa volatile oil | 1000 or 4000 ppm, 30 weeks | Reduced carcinogenesis incidences, multiplicities | 54 | |
| Lung cancer model of mice | TQ | 5, 20 mg/kg | Decreased tumour volume and weight, NF‐KB | 47 | |
| CD1‐nude mice | TQ | 5, 10, 10 mg/kg | Reduced p‐AKT, p‐mTOR, caspase‐3, p‐53, NF‐κB expression | 58 | |
| B(a)P‐induced rat's lung cancer | TQ | 50 mg/kg b.w. | Decreased NFk‐B expression, ROS, MDA, NO, increased apoptosis, CAT and SOD activities | 62 |
Abbreviations: B(a)P, benzo(a)pyrene; CN, carvacrol nanoemulsion; IFN‐γ, interferon‐gamma, IL‐2, interleukin‐2; NF‐κB, nuclear factor kappa B; NSCLC, non‐small cell lung cancer; NSE, seed extract; Ref, references; ROS, reactive oxygen species; SPBP, soy‐phospholipid‐based phytosomes; TNF alpha, tumour necrosis factor‐alpha; TQ, thymoquinone; VEGF, vascular endothelial growth factor.
Extract preparation
For the preparation of methanolic extract of Z. multiflora its dried powder was mixed with 85% methanol for 48 h and filtered and the ethanol was removed by rotary at 40C°. 37 Also, Z. multiflora essential oil was prepared from the air‐dried plants through hydrodistillation for 3 h using an all‐glass Clevenger‐type apparatus. The essential oil was dehydrated over anhydrous sodium sulphate and stored at 4°C. 38
Growth‐inhibitory activity in cell culture
Treatment with Z. multiflora methanolic extract down‐regulated c‐MYC levels and up‐regulated p53 in the U266 multiple myeloma cell line. 37 Pre‐treatment A549 (human lung carcinoma), HCT‐116 (human colorectal carcinoma), HepG2 (human hepatocellular carcinoma) and B16F10 (mouse melanoma) cells with carvacrol (25 and 50 mg/kg for 7 days, orally) before inducing benzo(a)pyrene [B(a)P] enhanced antioxidant enzyme activity. 39 Furthermore, carvacrol pre‐treatment down‐regulated protein expression of inducible nitric oxide synthase (iNOS), NF‐κB and COX‐2 causing a decrease in the number of cancer marker cells and restoring histopathological changes made to the lung tissues. 40 Administration of carvacrol nanoemulsion (CANE, 0–150 μg/mL) decreased MAPK p38 and ERK activation as well as COX‐2 and vascular endothelial growth factor (VEGF) expression. 39 CN can also bind to COX‐2 and VEGF and activate allosteric sites of CD31 with low binding energy in A549 (adenocarcinoma cell line) cells. 39 Another study found that carvacrol suppressed the viability of NCI‐H1299 cells by inducing apoptosis by inhibiting protein and mRNA expression levels of Cas‐9 and elevating the expression of MMP‐9 and TIMP‐1. 41 Treatment with carvacrol (100, 250, 500 and 1000 μM) for 24 h caused morphological changes in NSCLC (A549) cells by improving cell rounding, causing cytoplasmic blebbing and irregularity in shape, leading to fewer cells and inhibiting rates of cell growth. 42 Carvacrol nanoemulsion (25, 50 and 100 μg/mL) also decreased ROS and regulated apoptosis via p‐JNK, Bax and Bcl2 and increased Cyt c and activated Cas cascades in human lung adenocarcinoma A549 cells. Following administration of carvacrol, an MTT assay revealed increased A549 cell viability and further testing found increases in ROS production, a decrease in Ca2+ release, down‐regulation of CHOP, p‐eIF2α, GRP78 and apoptotic markers. 43 Western blots and reverse transcription polymerase chain reaction (RT‐PCR) tests found that 24 h administration of carvacrol inhibited the expression of AXL proteinase and suppressed AXL phosphorylation upon stimulation of neuronal NSCLC cells with ligands. 44 In addition, carvacrol treatment led to inhibited cell proliferation and migration of the NSCLC cells. 44 Finally, CN treatment was found to reduce the number of cell proliferation enzymes: cyclin‐dependent kinase 2 (CDK2), CDK4, CDK6, Cyclin E, Cyclin D1 and enhanced expression of p21 protein in the doxorubicin resistant‐A549 cell line. 45
Anti‐tumour activity in vivo
Z. multiflora essential oil (500 mg/kg) reduced tumour weight and balanced T helper 1 levels by increasing the secretion of TNF‐α, Interferon‐gamma (IFN‐γ) and IL‐2 and decreasing IL‐4 levels. However, no significant effects on aminotransferase and alanine aminotransferase activity were found. 38
Two doses of carvacrol nanoemulsion (CANE, and 100 mg/Kg) over 4 weeks in an athymic nude mouse model significantly reduced tumour growth and tumour weight by 34.2% and 62.1%, respectively, and increased the expression of p‐JNK, Bax, Bcl2, Cyt C, Cas‐3, Cas‐9 and β‐actin in lung tissue. 43 In vivo indicated that CN treatment (50,100 mg/kg) decreased cell growth and levels of MMP, decreased the activation of MAPK p38 and ERK, decreased the expression of VEGF and CD31 and decreased anti‐angiogenesis effects. 39
Overall, treatment with Z. multiflora extract, and its main constituent carvacrol, reduced lung cancer virality by decreasing tumour weight and volume, increasing the expression of apoptotic proteins, modulating cytokine levels and cancer cell viability, as well as regulating specific pathways and cancer tumour cell numbers. Therefore, Z. multiflora extract and carvacrol are worth testing as a part of contemporary treatment strategies for lung cancer.
3.1.2. Nigella sativa
A variety of diseases have been treated with Nigella sativa (N. sativa) seeds for thousands of years across the African and Asian continents. For example, in the south‐west of Asia, this plant has been used both as a food additive and herbal medicine. 46 A powerful anti‐cancer compound extracted from N. sativa seeds is thymoquinone (TQ). 47 Several studies have found both N. sativa and TQ to have anti‐inflammatory, anti‐oxidative and immunomodulatory properties, and are also effective treatments against allergic disorders, lung cancer and tumour morphology. 48 , 49 , 50 , 51
Regarding the safety and toxicity of black seeds and TQ, dependent on the type of animal model and administration manner, the results demonstrated that the toxicity effects of oral administration were lower than intraperitoneal injection and administration of more than 2 and 3 g/kg in the mice increased the risk of the organ toxicity of N. sativa extract and TQ. 52 The results of a phase I clinical trial to evaluate the safety of thymoquinone‐rich black cumin oil (BlaQmax®) on healthy subjects consuming black cumin oil formulation containing 5% at a dose of 200 mg/adult/day for 90 days showed a well‐safe profile of the plant. 53 As shown in Table 1, N. sativa and TQ are both potential treatments for lung cancer.
Extract preparation
Cleaned and dried seeds of N. sativa were extracted with 95% aqueous methanol for 24 h. The extract was filtered by a Buchner funnel and re‐extracted with 0% methanol for an additional 2 h and methanol was removed with a rotatory evaporator at 40°C. 49 N. sativa volatile oil used in Salim's study was purchased from Kahira Pharm. and Chem. Co., Cairo, Egypt. 54
Growth‐inhibitory activity in cell culture
The administration of essential oil N. sativa gold nanoparticles (NsEO‐AuNPs) decreased the hydrophobicity index causing significant suppression of S. aureus (78%) and V. harveyi (46%) biofilm formation, which are common infections in patients with lung cancer. 54 A study using N. sativa plant seed extract (NSE) treatment found it caused a significant down‐regulation of cancer cell viability and altered their morphology. Similarly, NSE treatment (0.01, 0.025, 0.05, 0.1, 0.25, 0.50, 1 mg/mL for 24 h) on human lung cancer cells significantly reduced their viability. 49 TQ treatment (1.25, 2.5 and 5 μM, for 24, 48 and 72 h) of NCI‐H460 cells (NSCLC cell line) reduced cell proliferation, expression of cytokines, epithelial‐neutrophil activating peptide (ENA‐78) and GRO‐alpha and increased apoptosis occurrence and reduced cell viability. 47 Administration of TQ (10, 20, 40 μmol/L) on a SCLC cell line also inhibited cell proliferation, migration, proliferating cell nuclear antigen (PCNA), cyclin D1, MMP2 and MMP9 mRNA levels, activated the extracellular signal‐regulated kinase 1/2) ERK1/2 (pathway and inhibited cell cycle via P16 expression and the gelatinase activities of MMP2 and MMP9. 55 Treatment with TQ (2, 5, 10 μM) on NCLC cells exposed to B(a)P up‐regulated Bax and p21 levels, increased receptor 1 and 2 expression and the Bax/Bcl2 ratio, whilst down‐regulating Bcl2 proteins, inflammatory markers, ROS, cyclin D, NF‐kappa B and IKK1 expression. 56 Treatment with TQ‐loaded soy‐phospholipid‐based phytosomes (0.5, 1.0, 2.0, 4.0, 6.0, 8.0 and 12.0 h) was found to activate Cas‐3 and reduce oxidative stress markers in A549 cells using the annexin V staining technique. 57 Combined application of indirubin‐3‐monoxime and TQ on A549 cancer cells did increase apoptosis markers and also reduced the Bcl‐2/Bax ratio, inhibiting migration and metastasis of the cancer cells. 58 Administration of TQ also increased natural killer (NK) cell tumoricidal activity and enhanced IFN‐γ secretion via NK cells and NK cell‐mediated killing of NSCLC cells. 59 In addition, inhibition of GTPase KRas, Sir‐2, ALK5 and β‐catenin were shown when TQ was applied to lung cancer cells. 60
Anti‐tumour activity in vivo
The use of honey and N. sativa (0.2 g N. sativa or 5 g honey/day) as supplements in Sprague Dawley rats showed protection against oxidative carcinogenesis induced by methyl nitrosourea in lung, skin and colon tissues. 61 Oral administration of N. sativa volatile oil (1000 or 4000 ppm) for 30 weeks to male Wistar rats reduced the size of some tumours, including in the lungs. 54 Similarly, administration of TQ (5 and 20 mg/kg) decreased the size and weight of tumours and NF‐KB in an animal lung cancer xenograft model. 47 Treatment of a mouse lung cancer model with indirubin‐3‐monoxime and TQ (5, 10, 10 mg/kg) significantly reduced the expression of Phospho‐Akt (p‐AKT), Phospho‐Mammalian target of rapamycin (p‐mTOR), Cas‐3, p‐53 and NF‐κB. 58 Finally, administration of TQ (50 mg/kg) decreased NFk‐B expression, ROS, MDA and NO levels and increased apoptosis and CAT and SOD activity in B(a)P‐induced lung cancer in rats. 62
The above in vitro and in vivo results indicate that N. sativa and its constituent TQ could be promising candidates for treating lung cancer. Their therapeutic actions involve decreasing cancer cell viability and altering their morphology and levels of Bcl2 proteins, inflammatory markers, oxidative stress parameters and NF‐kappa whilst increasing Bax/Bcl2 ratio and levels of apoptosis.
3.1.3. Crocus sativus
Crocus sativus L (C. sativus) is mainly cultivated in Iran and is a member of the Iridaceae family. Chemical analyses of C. sativus extract indicate that the most critical constituents are carotenoids, crocin, crocetin and the monoterpene aldehydes crocin and safranal. 63 Studies have reported that C. sativus and its critical constituents decrease white blood cell (WBC) counts, reduce oxidative stress markers, impact airway responsiveness and also have immunomodulatory effects. 64 , 65 , 66
Regarding the safety of C. sativus, its oral administration of 200 and 400 mg/day for 7 days, indicated the changes in some haematological and biochemical parameters but, these changes were in normal ranges which were not important clinically. 67 The effect of C. sativus and its constituents on lung cancer and their potential mechanism was also reported in numerous studies (Table 2).
TABLE 2.
The effects of Crocus sativus, Ocimum basilicum, Ferula persica and Ferula szowitsiana and their constituents on lung cancer.
| Study type | Type of lung cancer | Preparations | Dose | Effects | Ref. |
|---|---|---|---|---|---|
| In vitro | L929 cell line | Saffron ethanolic extract | 500–2000 μg/mL, 24 and 48 h | Reduced cell number and viability | 68 |
| GCL‐induced A549 cells | Crocin | 500 μM, 48 h | Reduced GSHand ROS, enhanced GCL expression, activated Nrf2 | 72 | |
| A549 cell | Linalool | 0–2.0 mM | Inhibited cell proliferation, migration, increased G0/G1 cell cycle arrest, anti‐metastatic | 81 | |
| A549 cell | F. persica methanolic extract | 400 μg/mL, 48 and 72 h | Up‐regulated P53, Bax and caspase‐9, activated multiple apoptotic pathways | 86 | |
| A549 cell | F. persica methanolic extract | 100 μg/mL | Decreased cytotoxicity and cell proliferation | 85 | |
| In vivo | Mice lung cancer model | 100 mg/kg, for 28 days | Decreased xenograft tumour size and reduced Cas‐3, −8 and − 9 expression | 70 |
Abbreviations: Bax, B‐cell lymphoma 2‐associated X protein; Bcl‐2, B‐cell lymphoma 2; Ref, references; and ROS, reactive oxygen species.
Extract preparation
For the preparation of ethanolic extract, 1 g of the dried saffron stigma was extracted with 10 mL ethanol (96%) for 2 h in an ultrasonic bath. Then the extract was filtered and concentrated in a vacuum evaporator. 68
Growth‐inhibitory activity in cell culture
Treatment with ethanolic saffron extract (500, 1000, 1500 and 2000 μg/mL for 24 and 48 h) reduced cell number and viability of L929 lung cancer cells. 68 The increased apoptotic level was indicated using annexin V‐fluorescein isothiocyanate in A549 cancer cells that had been treated with C. sativus extract. 69 Furthermore, administration of saffron extract (100 mg/kg for 28 days) decreased xenograft tumour size and reduced Cas‐3, −8 and −9 expression. 70
Treatment of lung cancer cells with crocin (1, 2, 4, 8 and 16 mg/mL) markedly enhanced the mRNA levels of p53 and Bax and significantly down‐regulated Bcl‐2 mRNA expression. 71 Another study using crocin treatment (500 μM for 48 h) on glutamate‐cysteine ligase (GCL)‐stimulated A549 cancer cells found it reduced GSH levels by enhancing GCL expression via activation of Nrf2, but inhibited the release of ROS in. 72
Anti‐tumour activity in vivo
Albino mice with B(a)P‐induced lung cancer were treated with crocetin (50 mg/kg) and cancer cell proliferation was reduced by 68% and 45% in weeks 8 and 18 respectively, and glycoproteins and polyamines were significantly altered. 71
The above results suggest that C. sativus, crocin and crocetin down‐regulate mRNA levels of major proteins of apoptosis in lung cancer cells. Therefore, C. sativus, crocin and crocetin could be considered as treatment strategies for lung cancer.
3.1.4. Ocimum basilicum
Ocimum basilicum L. (O. basilicum), or basil, is the main part of the oil crop used in traditional medicine that belongs to the Lamiaceae family. It is widely found in regions of central and southeast Asia, including Iran and Pakistan. 48 , 73 Previous studies indicated that linalool is the medicinal constituent of O. basilicum. 74 The anti‐cancer effects of O. basilicum and its constituents have been demonstrated by Alkhateeb et al. 75 Experimental and clinical studies found O. basilicum and its derivatives reduced inflammation and oxidative stress and improved immune function in patients with asthma, COPD, lung cancer and allergy diseases. 76 , 77 , 78
Safety results demonstrated that acute effects of O. basilicum were shown in the LD50 > 5 mg/kg such as haematocrit, platelets and RBC decreasing and oral toxicity in patients. 79 It was shown that O. basilicum consumption in food and drug preparations is safe. However, a concentration limit for alkylbenzenes should be considered, and the plant chemotypes with equal or lower levels of these alkylbenzenes should be used. 80 The effects of O. basilicum on lung tumours are summarized in Table 2.
Growth‐inhibitory activity in cell culture
Treatment of A549 cancer cells with linalool (0–2.0 mM) inhibited cell proliferation and migration but increased the G0/G1 cell cycle arrest. Linalool treatment also sensitized cancer cells and anti‐metastatic markers were recorded in the treated A549 cells. 81 Finally, linalool significantly decreased cancer cell viability even if only administered for 4 h. 81 The above in vitro studies support the hypothesis that O. basilicum and its constituent linalool are a natural therapeutic against lung cancer cells and support further research to test the hypothesis.
3.1.5. Ferula assa‐foetida and F. Szowitsiana
The genus Ferula are perennial flowering plants belonging to the Apiaceae (Umbelliferae) family. Ferula growth spans the eastern Mediterranean regions all the way to central Asia. 82 This genus consists of about 170 species of which 30 are found in Iran. The anti‐tumour and cytotoxic effects against multifarious cancer cell lines, anti‐inflammatory, antioxidant, immunomodulatory and anti‐bacterial activities, reduced lipid peroxidation and down‐regulation of matrix metalloprotease expression caused by F. szowitsiana and its constituents have been reported in several studies (75–82). As well as F. szowitsiana, studies have found F. persica and its derivative coumarin may possess therapeutic properties.
Administration F. persica known as food additive until 1.5–2 mg/kg incresed the fibroblast cells in male patients and improved libido but did not report serious or acute side effects. 83 , 84 The effects of F. persica and coumarin on lung cancer are summarized in Table 2.
Extract preparation
Total plant extracts were obtained by extraction of dried and milled aerial parts of the plants with 80% methanol (1:10) using the maceration method for 4 days. After every 24 h, the mixture was filtered and a new solvent was added to the plant powder. The combined extracts were concentrated to dryness under vacuum pressure. 85 In the other study, the extract of the air‐dried roots of F. szowitsiana (100 g) was prepared using the Soxhlet‐extracted method with dichloromethane and methanol (1 L of each) and then was dried by evaporator. 86
Growth‐inhibitory activity in cell culture
Treatment of A549 cells with F. szowitsiana extract (400 μg/mL for 48 and 72 h) altered anti‐apoptosis, pro‐apoptosis and tumour suppressor gene expression, up‐regulated P53, Bax and Cas‐9 and also activated multiple apoptotic pathways and antiproliferation activity. 86 Treatment with F. persica extract (100 μg/mL) decreased cell viability and cell proliferation in MDBK, A549, HT29, HepG2 and MCF7 cell lines. 85
Cell proliferation, invasion, metastasis and autophagy were reduced in lung cancer cells treated with terpenoids. The inhibitory effects of terpenoids on cyclins and CDK were shown. 81 Triterpenoids (25 mg/kg) inhibited the ROS accumulation via cytotoxicity of cisplatin by blocking autophagic flux. 87 Human lung cancer cells treated with F. szowitsiana extract showed decreased cytotoxicity via SK‐LU‐1, SPC‐A‐1 and 95D tests and were also less viable. 88 Inhibition of cell growth via increasing G1 cell cycle arrest, morphological cells and annexin were reported in NSCLC cell lines treated with coumarin (10–160 μg/m). 89 The level of ROS and glutathione (GSH) in human alveolar epithelial A549 cancer cells was decreased and thiobarbituric acid reactive substance (TBARS) was markedly increased when they were treated with coumarin. 90 Coumarin derivatives (12 μM) significantly decreased the migration of IL‐1β‐stimulated cells and IL‐1β levels and inhibited F‐actin reorganization in A549 cell lines. 91 An earlier study found that administration of coumarin‐based benzopyranone derivatives (20 μM) up‐regulated the apoptotic pathway via Bax protein expression but down‐regulated Bcl‐2 protein expression in human lung (A549) cancer cells. 92
Based on the above findings, further investigation in F. persica and its main constituent coumarin as a possible treatment for lung cancer via the inhibition of cyclins and CDK, their reducing effects on cancer cell migration and metastasis mediated by cytokines such as IL‐1β, oxidative marker production, cell viability and cytotoxicity warrant further investigation.
3.1.6. Curcuma longa
Turmeric, also known as Curcuma longa (C. longa), is a part of the Zingiberaceae family found in some regions of Asia, being particularly abundant in India. In addition to its multiple health benefits, C. longa is an important ingredient in recipes. 93 , 94 Previous studies demonstrate various pharmacological properties of C. longa and curcumin (CUR) including: antioxidant (7), anti‐inflammatory, 95 anti‐tumour, 96 anti‐tussive, 97 anti‐diabetic, 98 anti‐convulsant 99 and hepatoprotective effects. 100 The anti‐inflammatory, antioxidant and immunomodulatory effects of CUR have also been reported in experimental lung disorders. 101 Some studies have looked at the potential anti‐tumour mechanisms that C. longa and CUR adopt but further investigation is still needed to ascertain how they work.
Clinical results indicated that administration of C. longa and curcumin for 3 months, up to 8000 mg/d did not show side effects in patients, thus, it was recommended that using 2500 mg of C. longa was safe in humans. 102 Table 3 summarizes the effects of C. longa and CUR on lung cancer.
TABLE 3.
The effects of Curcuma longa and Achillea millefoliumand their constituents on lung cancer.
| Study type | Type of lung cancer | Preparations | Dose | Effects | Ref. |
|---|---|---|---|---|---|
| In vitro | A549 and H460 cells | CURoid extract | 0.2%–2%, 8 days | Increased caspase‐3, caspase‐8 and caspase‐9 activities and Cyt c, CDK1 and cyclin B expression | 104 |
| Lung cancer in vitro model | N‐hexane extract of C. longa | IC50, 0.23–0.28 mg/mL, 24 | Inhibited telomerase‐ effect on cell line A549 | 103 | |
| Lung cancer stem cells | CUR | 5–40 μM, 7 days | Inhibited Wnt/β‐catenin, Sonic Hedgehog pathways | 106 | |
| A549 cells | CUR | 25–100 μM, 6–48 h | Down‐regulated UCA1, inhibited LIN‐28A through the activation of miRNA‐98 | 108 | |
| A549 cell | Nanoencapsulated CUR‐Fe3O4 | 0–120 μM, 24, 48 and 72 h | Reduced proliferation and hTERT gene expression | 109 | |
| Lung cancer cells | CUR | 160 μM, 12–72 h | Decreased p53, BCL‐2, BCL‐XL, Bak and Caspase genes expression | 111 | |
| Lung cancer cells | CUR | 10 μM | Regulated axon guidance, glioma, ErbB tyrosine kinase receptor signalling pathways | 112 | |
| Human NCI‐H292 LSCC cell line | CUR | 40 μM, 4 h | Inhibited human NCI‐H292 cells growth, increased FOXA2 expression | 113 | |
| H460 cells | CUR | 20 and 40 μM | Inhibited JAK2 activity, reduced tumour spheres by inhibiting JAK2/STAT3 pathway | 115 | |
| NSCLC patients | CUR | 1 g/kg, once daily | Enhanced efficacy of EGFR‐TKIs and overcome the EGFR‐TKI resistance | 116 | |
| A549 cells | CUR | 6 μM, 48 h | Suppressed EZH2 but reduced NOTCH1 expression via the EZH2 suppression | 124 | |
| Lung cancer cells | CUR | 15, 30, 45 and 60 μmol/L | Suppressed GLUT1, MT1‐MMP, MMP2 expressions, attenuated GLUT1/MT1‐MMP/MMP2 pathway | 125 | |
| NSCLC cells | CUR | 10, 20 and 30 μM, 2 h | Decreased MTP, ROS, activated DNA‐ DRP, mitochondrial apoptosis | 127 | |
| A549 cells | CUR derivative MHMM‐41 | 8 μM and 16 μM for 12 h | Induced ROS‐mediated apoptosis, blocked migration | 128 | |
| NCI‐H460 cells | CUR | 30 μmol/L | Inhibited cell migration, tube formation, transfected pMXs‐Stat3C | 129 | |
| A549 cells | Kaempferol | 17.5, 35, 52.5, 70 μM | Inhibited Akt‐1 phosphorylation, MEK1/2, activated MAPK, induced cell apoptosis | 142 | |
| A549 cells | Kaempferol | 10, 25, 50 μM | Down‐regulated Smad3PCPC formation with Smad4NT under TGF‐β1 stimulation | 143 | |
| A549 cells | Kaempferol | 25 μM | Decreased GST expression, NQO1, HO1, triggered Nrf2 by tBHQ | 146 | |
| NSCLC cells | Kaempferol | 20, 40, 60, 80, 100, 120, 140 μM | Reduced migration, metastasis, induced cell mortality, apoptosis, modulated EMT protein expression | 147 | |
| In vivo | BALB/c mice injected A549 SP cells | CUR | 100 mg/kg, every other day | Reduced tumour weight, size, down‐regulated Notch HIF‐1 mRNA, VEGF, NF‐κB expression | 105 |
Abbreviations: DNA‐DRP, DNA damage/repair pathway; MAPK, mitogen‐activated protein kinase; MTP, mitochondrial transmembrane potential; NCI‐H292, human lung cancer cell; NF‐κB, nuclear factor kappa B; Ref, references; Smad3PCPC, Smad3 phosphorylation, C‐terminus phosphorylation, complex; Smad4NT, Smad4, nuclear translocation; tBHQ, tert‐butylhydroquinone; VEGF, vascular endothelial growth factor.
Extract preparation
C. longa rhizome powder (100 g) was dissolved in 200 mL n‐hexane and the solution was shaken for 4 h at 45°C. Then, the supernatant was transferred to a tube. This step was performed three times. Then, the residue of n‐hexane extraction was dissolved in 200 mL dichloromethane instead of n‐hexane and the same steps were repeated. The debris of extraction with dichloromethane was dissolved in 200 mL methanol and in the same way, supernatant was collected. Finally, the solvents of all three phases were dried by rotatory‐evaporator and the remaining powders were stored at—20°C until used. 103
Growth‐inhibitory activity in cell culture
N‐hexane extract of C. longa at concentrations of 0.114, 0.142, 0.171 and 0.199 mg/mL inhibited telomerase in A549 lung cancer cells dose‐dependently. 103 Administration of CUR (1%), in diet before and during weekly NTHi exposure in mice, significantly decreased lung tumours number in the absence of NTHi exposure and in the presence of NTHi exposures (85% and 53% respectively). In addition, direct anti‐tumoral effects of CUR was reported in in vitro of murine K‐ras‐induced lung adenocarcinoma cell lines (LKR‐10 and LKR‐13) through reduction of cell viability, colony formation and apoptosis induction. 104 In addition, tumour size and weight, Notch and HIF1 mRNA and tumour VEGF and NFB expression were all decreased by CUR (100 mg/kg). VEGF signalling was also inhibited by CUR, consequently inhibiting cancer cell growth. 105
CUR (0, 5,10, 20 and 40 μM) applied to lung cancer stem cells for 7 days acted as an interventional agent by inhibiting both the Wnt/ß‐catenin and the Sonic Hedgehog pathways. 106 CUR inhibits Wnt and mTOR pathways by down‐regulating urothelial cancer‐associated 1 (UCA1). 107 CUR (25–100 μM, for 6–48 h) also inhibited lung cancer cell growth by down‐regulating UCA1, and by enhancing miRNA‐98 activity. 108
Both pure CUR and nanoencapsulated CUR (CUR‐Fe3O4) (0–120 mM, 24 and 48 h) reduced proliferation and hTERT gene expression in lung cancer cells but CUR‐Fe3O4 was most effective. 109 Additionally, CUR‐Fe3O4 decreased migration of human lung cancer cells and ROS‐mediated apoptosis. 110 Lung cancer cells in 160 mM CUR for 12 to 72 h showed increased p53, BCL‐2 (promoter of apoptosis), BCL‐XL (promoter of apoptosis), Bak (promoter of apoptosis) and Cas gene expression. 111 CUR (10 M) has also been found to regulate axon guidance, glioma, ErbB tyrosine kinase receptor signalling pathways and reduce metastasis in lung cancer cells. 112
As a result of increasing FOXA2 expression and modulating STAT3 signalling pathways, CUR treatment (40 μ M, 4 h) inhibited human NCI‐H292 cell growth. 113 In NCI‐H460 cells, CUR causes DNA repair and DNA damage. 114 CUR (20 and 40 mM) reduced tumorspheres formed by H460 lung cancer cells and also inhibited proliferation and colony formation of the cancer cells. The inhibitory action of CUR on the JAK2/STAT3 pathway is implicated in the reduction of tumour spores and the suppression of tumour growth in mice bearing lung cancer xenografts. 115
Gefitinib is a medication for cancer that inhibits EGFR in target cells. In primary NSCLC cell lines that are gefitinib‐resistant (H157 and H1294), CUR reinstated the anti‐cancer effects of gefitinib. 116 CUR also inhibited SCLC cells, up‐regulated the cyclin‐dependent kinase inhibitors: p27 and p21 and down‐regulated cyclin D1. 117 Additionally, CUR activated the ERK1/2 signalling pathway to increase the expression of forkhead box protein O1 (FOXO1). 117 PI3K/Akt/mTOR was also inhibited by CUR, consequently inhibiting the growth of NSCLC cells by inducing autophagy and apoptosis 118 , 119 , 120 , 121 and modulating calcium signalling. 122 CUR also inhibits adiponectin receptor 1, preventing cancer cells from migrating and invading their host.
In vitro studies have shown that CUR (10 μ M for 4 days) induces the maturation of lung cancer patient‐isolated regulatory cells (Treg) to T helper (Th)‐1 cells by inhibiting DNA transcription of forkhead protein‐3 (Foxp3) and increasing expression of IFN‐γ. Treatment with CUR in lung cancer patients led to a significant increase in peripheral Tregs compared to healthy subjects. 123 Treatment of A549 cells with CUR (6 M, for 48 h) increased the expression of the enzyme EZH2 through microRNAs (miR)‐7c and miR‐101 and reduced the expression of NOTCH1 via EZH2 activation. 124 CUR (0, 15, 30, 45 and 60 μ mol/L) significantly inhibited lung cancer invasion and metastasis by inhibiting GLUT1/MT1‐MMP/MMP2 pathways and was also found to significantly inhibit GLUT1/MT1‐MMP/MMP2 expression and invasion in mice. 125 Wang et al (123) demonstrated that CUR‐induced reduction in NSCLC (A549) viability is related to oxidative stress and its cytotoxicity by causing ROS accumulation. 126 In the same study, CUR prevented NSCLC proliferation by modulating the Wnt/ß‐catenin pathway (123). Furthermore, by reducing mitochondrial transmembrane potential, CUR (0, 10, 20 and 30 μM, for 2 h) activated the mitochondrial apoptosis and DNA damage repair pathways. 127 MHMM‐41 (8 μM and 16 μM for 12 h) exerted even better antiproliferative effects on A549 cancer cells and also induced ROS‐mediated apoptosis and migration of A549 cells. 128 CUR (30 μM) inhibited migration and tube formation in NCI‐H460 cells but this could be reversed by infecting the cells with the dominant‐active variant STAT3C. This result suggests that CUR's inactivation of STAT3 is responsible for its effect on tumour angiogenesis. 129 LCLC 801D cells were effectively inhibited from migrating and invasively spreading by low‐toxic levels of CUR that disrupted Rac1/PAK1 signalling pathways and MMP‐2 and MMP‐9 expression. 130
A study was performed using CUR dry powders and liposomal (LCDs) against human lung cancer A549 cells and normal human bronchial epithelial BEAS‐2B cells. CUR and LCDs reduced TNF‐α levels in bronchoalveolar lavage fluid) BALF, (malondialdehyde (MDA) and Cas‐3 levels in lung tissue and expression of VEGF and B‐cell lymphoma 2 (BCL‐2). However, CUR powder showed a much stronger anti‐cancer effect than LCDs. 131 Treatment with CUR also reduced activation of HLJ1 via activation of the JNK/JunD pathway and also led to increased expression of E‐cadherin in lung cancer cells. 132
Anti‐tumour activity in vivo
In an animal model of lung cancer, the anti‐carcinogenic effect of CUR (100 mg/kg, 0.2 mL, once every other day) was shown by reduced tumour volume and weight, down‐regulated Notch and HIF1 mRNA expression and inhibited VEGF and NFB. 105 In another study conducted on mice, CUR administration reduced the number of visible lung tumours and inhibited NTH's ability to increase neutrophil chemokine levels. 133 In a rat model of lung cancer, administration of CUR and resveratrol decreased Cyt c activity and oxidative enzymes, increased lipid peroxidation, SOD and CAT activities and reduced LPO. 134 CUR liposomes increased apoptosis in cells and adiponectin expression and tumour growth in vivo were both inhibited by CUR treatment. CUR inhibited adiponectin expression by inhibiting NF‐ĸ B/MMP pathways, resulting in decreased migration and invasiveness of the A549 cancer cells. 135
In both in vitro and in vivo studies, C. longa and CUR have been found to suppress cancer cell proliferation and induce apoptosis of lung cancer cells. Additionally, CUR altered the expression of EGFR, microRNAs and autophagy in cancer stem cells. It is believed that CUR influences the expression of different genes in the cancer cells themselves, such as NF‐kB, STAT‐3 and AP‐1, as well as protein kinases, including MAPK and enzymes such as COX and LOX. Thus, CUR makes for a promising therapeutic target in treating lung cancer.
3.1.7. Achillea millefolium
Achillea millefolium (A. millefolium), also known as yarrow, is a member of the Asteraceae family and found across Europe, Asia, North Africa and North America. 136 , 137
The anti‐inflammation and antioxidant and improvement effects of A. millefoliumi on pain and estrogenical wounds were reported and chronic administration (ED50 = 32 mg/kg، p.o) increased above the affects in rats for 90 days but it did not show any serious side effect or toxicity. 138 In review articles, it was stated that A. millefolium extracts are safe in the concentrations used in cosmetics. 139 Several in vitro studies have explored the anti‐tumour potential of A. millefolium and its constituents, and the findings of A. millefolium effects on lung cancer are summarized in Table 3.
Extract preparation
The lyophilized powder (1 g) of the plant was extracted with 40 mL of methanol by stirring (25°C, at 10,000 x g) for 1 h and filtered through filter paper. The extract was evaporated at 40°C in rotary to dryness. The ethanolic extract was prepared using the dry fruiting bodies (1 g) with 30 mL of 90% ethanol by stirring for 48 h at 70°C. The extract was filtered and centrifuged to get a clear liquid and evaporated at 40°C. 140
Growth‐inhibitory activity in cell culture
Administration of two doses of A. millefolium extract on human lung cancer cells (NCI‐H292) increased the level of apoptosis, up‐regulated p53 protein and Bax, activated Cas‐3 protein expression and increased activity of the ER stress pathway, GRP78, Cas‐12 and Cas‐8. 141 Similarly, A. millefolium extract (75 and 100 μg/mL) altered the cycle profiles, increased apoptosis and increased p53 and p21 expression in HCT‐15 and NCI‐H460 cells. 140 Administration of kaempferol (17.5, 35, 52.5, 70 μM) inhibited Akt‐1 phosphorylation and MEK1/2, activated mitogen‐activated protein kinase (MAPK), induced cell apoptosis via cleavage of Cas‐7 and induced poly ADP‐ribose polymerase (PARP) activity in A549 cells. 142 In addition, kaempferol (10, 25, 50 μM) treatment of A549 lung cancer cells, followed by TGFß‐1 stimulation, resulted in phosphorylation of Smad3 (although phosphorylation at residue Thr179 was down‐regulated) and the formation and nuclear translocation of Smad4 complexes. 143 Kaempferol treatment combined with radiation therapy to kill tumours increased the amount of tumour death, both in vitro and in vivo, by suppressing the AKT/PI3K and ERK pathways and activating the mitochondria apoptosis pathway. 144 In the same study, kaempferol was also found to induce G2/M cell cycle arrest, inhibit clonogenic survival and elevate tumour cell apoptosis. Administration of kaempferol‐3‐O‐rutinoside significantly stimulated cancer cell cytoskeleton collapse, mitochondrial dysfunction and apoptosis via the calcium signalling pathway. 145 Kaempferol treatment (25 μM) for 48 h also decreased the expression of GST, NQO1 and HO1, triggered Nrf2 by tert‐butylhydroquinone (tBHQ), reduced ROS levels and induced apoptosis in treated cells. 146 Finally, Hang et al 147 found various doses of kaempferol (20, 40, 60, 80, 100, 120 and 140 μM) reduced migration and metastasis of cells, induced cell mortality and apoptosis and modulated EMT protein expression in human NSCLC cells. These results suggest that the anti‐cancer mechanisms of A. millefolium and its constituent kaempferol are broad and lead to cancer cell immobility and death. Compared to the other plant species discussed, A. millefolium has also been reported to disrupt the cytoskeleton of lung cancer cells.
3.1.8. Portulaca oleraceae
Portulaca oleracea (P. oleracea) belongs to the Portulacaceae family and grows in Iran, India, Japan, China and southern Europe. 148 Genistein and luteolin 149 are natural flavonoid constituents of P. oleracea with potential anti‐cancer, antioxidant and anti‐inflammatory properties. 150 Previous studies showed that P. oleracea and genistein treatment reduced inflammation in lung tissue, reduced oxidative stress and improved the Th1/Th2 balance, among other immunological indices. 151 , 152 , 153
Results were reported that administration P. oleracea did not show cytotoxic effects in in vitro studies and the IC50 value of more than 10 μg/mL but moderate toxic effects were shown in LD50 value of 1853 mg/kg. 154 It was also reported that human consumption of P. oleracea poses minimal dangers in humans. 155 Several studies have reported a therapeutic effect of P. oleracea, genistein and luteolin on lung cancer cells, which are discussed below and summarized in Table 4.
TABLE 4.
The effects of Portulaca oleracea, Allium cepa, Brassica juncea and Aloe Vera their constituents, on lung cancer.
| Study type | Typ of lung cancer | Preparations | Dose | Effects | Ref. |
|---|---|---|---|---|---|
| In vitro | NCIH‐522 cell line | Portulaca oleracea whole plant | 10–1000 g/μg for 24 h | Reduction in cell viability and changes in cell morphology were recorded | 156 |
| NCI‐H209 | Quercetin | 5 μM | Decreased cell viability, MMP, Cyt c, increased cell cycle, G2/M and subG0/G1 phase cells, cyclin B, Cdc25c‐ser‐216‐p expressions, G2/M arrest, up‐regulated Bax, down‐regulated caspase‐3, poly(ADP‐ribose) polymerase activations cleavage | 179 | |
| A549 cell | Quercetin | 14.5, 29, 43.5, 58 μM | Reduced cell viability, DNA synthesis and Bcl‐2, increased Bax, Bad and Bcl‐x(L) | 180 | |
| A549 cell line | Genistein | 25, 50, 75 and 100 μM | Inhibited cell proliferation, ERK1/2 phosphorylation, PI3K/Akt pathways, down‐regulated MMP‐2 expression | 159 | |
| H460 non‐small‐cell | Genistein | 5, 10, 20, 30 and 50 μM | Increased Bax expression, induction of p21 protein, induced apoptosis via the p53‐independent pathway | 160 | |
| A549 human lung cancer | Genistein | 5–200 μM | Reduced miR‐27a, MET protein expression | 161 | |
| A549 cells | Sinapic acid | 5–10,000 μg/mL | Decreased necroptotic marker proteins cyclophilin A HMGB1 | 195 | |
| H460 cells | Aloe‐emodin | 20 μM | Increased apoptosis or necrosis, cell death, MAP kinase members activation MPTP, changed Cyt c protein expression | 200 | |
| A549 and NCI–H1299 cells | Aloe‐emodin | 10, 20, 40 μM | Stimulated autophagy through activation of MAPK, inhibited Akt/mTOR pathway, reduced ROS, mediated autophagy | 201 | |
| CH27 | Aloe‐emodin | 40 μM | Increased Cyt c, Bcl‐2 family protein expression such as Bcl‐XL, Bag‐1 and Bak | 203 | |
| H460 cell | Aloe‐emodin | 20 μM, 2 h | Increased PKCδ expression, cell death, decreased CRP expression, including sarcoma RAS, RHO, p38, HSP27, FAK, α‐actinin, tubulin | 204 | |
| H460 cell | Aloe‐emodin | 50 μM, 16 h | Increased cAMP‐dependent protein kinase, protein kinase C, Bcl‐2, caspase‐3, p38 | 206 | |
| In vivo | Nicotine treated rats | A. cepa oil | 100 mg/kg, 21 days | Increased CAT and SOD activity | 182 |
| B(a)P‐induced LC in mice | Sinapic acid | 30 mg/kg, orally | Declined IgG, IgM levels, leukocyte counts, lipid peroxidation, pro‐inflammatory cytokines, AHH, LDH, GGT, 5′NT, CEA | 194 |
Abbreviations: A549 cell line, human non‐small cell lung cancer cells; B(a)P, benzo(a)pyrene CAT, catalase; Bax, B‐cell lymphoma 2‐associated X protein; Bcl‐2, B‐cell lymphoma 2; CRP, cytoskeleton‐related protein; FAK, focal adhesion kinase; HMGB1, high mobility group box 1; HSP27, heat shock protein 27; LC, lung cancer; miR‐27a, microRNA‐27a; MMP, mitochondrial membrane potential; MPTP, mitochondrial permeability transition pore; PKCδ, protein kinase Cδ; Ref, references; RHO, ras homologue gene family member.
Extract preparation
The oil from dried and grounded P. oleracea seeds was extracted by continuous extraction in Soxhlet apparatus for 12 h using petroleum ether (60–80°C boiling range) as a solvent. Then the solvent was evaporated and kept at −4°C. 156 , 157
Growth‐inhibitory activity in cell culture
Following treatment of HepG2 and A‐549 cells with P. oleracea treatment (10–1000 ng/mL m for 24 h), a dose‐dependent reduction in cell viability and changes in cell morphology were recorded. In addition, the lung adenocarcinoma cell line (A549) showed decreased proliferation and a higher apoptotic rate compared to controls. 156
Considering mRNA and protein levels, glutein therapy increased Bax but decreased Bcl‐2 (anti‐apoptotic factor) in A549 cells. 158 Similarly, genistein may enhance the production of Bax and down‐regulate Bcl‐2, as well as inhibit lung cancer cell proliferation and induce their apoptosis.
The treatment resulted in inhibited tumour growth by activating miR‐128 in the tumour cells. P. oleracea cytotoxic effects on human lung (K562 and A549) and breast cancer (MCF‐7 and MDA‐MB‐435) cells were examined using four alkaloids isolated from air‐dried aerial parts. The alkaloids isolated from P. oleracea showed moderate cytotoxic activity against A549 cells and only weak cytotoxic activity against K562 cells. 157 The alkaloids did not appear to have any cytotoxic effects on MCF‐7 and MDA‐MB‐435 (breast cancer) cells.
Genestein (25, 50, 75 and 100 μM) also suppressed cell proliferation and reduced MMP‐2 expression in human NSCLC cells (A549 cell line) in a dose‐dependent manner. 159 As a result, ERK1/2 and PI3K/Akt phosphorylation was inhibited, leading to these protective effects against lung cancer. Furthermore, genistein (5, 10, 20, 30 and 50 μM) increased Bax expression and p21 protein levels in H460 NSCLC and H322 cells, independent of the p53 pathway. 160 Moreover, genistein (5, 10, 25, 50, 100 and 200 μM) exerted anti‐cancer effects on the A549 human lung cancer cell by regulating Cas‐3/9 activity, miRNA27a activity and the expression of the MET protein. 161 A549 cells, expressing TRAIL‐resistant human adenocarcinoma genes, were treated with genistein (0, 10, 20 and 40 μM) which led to enhancing p62, activating Cas‐3 and Cas‐8 and increasing TRAIL‐induced tumour cell death by activated autophagy. 162 Apoptosis and autophagy of NSCLC cells were also increased by genistein in vitro by inhibiting Bcl‐xL distribution in the cytoplasm, lowering cytoplasmic Bcl‐xL levels and increasing Bcl‐xL and Beclin‐1 dissociation. 163 Bcl‐xL distribution and levels in the cytoplasm and Bcl‐xL and Beclin‐1 dissociation are closely associated with NSCLC radiosensitivity by facilitating DNA damage‐induced apoptosis and autophagy. Therefore, genestein could be considered as a therapeutic agent for enhancing the sensitivity of radiation therapy in NSCLC patients.
3.1.9. Allium cepa
Allium cepa L. (A. cepa), or onion, belongs to the Liliaceae family and includes over 250 genera and 3700 species. 164 , 165 , 166 Also, Garlic (Allium sativum) is a member of the onion (Amaryllidaceae) family and is classified in the same genus to which onion, leek, chive and shallot belong. 167 The plant grows all over the world but is most common in zones with moderate climates and originates from central Asia. 168 , 169 Anti‐fungal, 170 anti‐cancer, anti‐inflammatory, 171 antioxidant, antispasmodic, 168 , 172 antimicrobial, anti‐mutagenic, 173 antidiabetic, 174 , 175 antiplatelet 176 and anti‐asthmatic properties 177 of its main constituent quercetin (QT) have been reported.
To our knowledge, there is no published data regarding the safety of A. cepa in humans. Several studies have found A. cepa and CUR works against lung cancer cells and the potential mechanisms are explored below and summarized in Table 4.
Extract preparation
Onion bulbs (100 g) was peeled, washed, cut into pieces and crushed and percolated in 500 mL of distilled water for 3 days with intermittent shaking. Using Whattman no.1 filter paper the extract was filtered to get fine. 178
Growth‐inhibitory activity in cell culture
NCI‐H209 lung cancer cells treated with QT (5 μM) glucuronides are less likely to survive but they exhibit a higher percentage of cells in the G2/M phase, subG0/G1 phase and in the G2/M phase over time. 179 Raising cyclin B expression and glucuronic acid, Cdc25c‐ser‐216‐pand Wee1 levels were also reduced, indicating G2/M arrest. Several features of apoptosis were also observed after QT treatment, including the release of Cyt c, the induction of Bax and Bcl‐2, the activation of Cas‐3 and the cleavage of poly (ADP‐ ribose) polymerase. 179 Increases in Bax, Bad and Bcl‐x (L) levels occurred dose‐dependently following treatment with QT (14.5, 29, 43.5, 58 μM). 180 In the same study, QT was also found to inhibit Akt‐1 and p‐Akt‐1, phosphorylated ERK and MEK1/2 in a dose‐dependent manner. Several studies have indicated that QT is capable of causing apoptosis via activation of caspase‐3 in A549 lung carcinoma cells. 180 Researchers found that consumption of QT‐rich foods negatively correlated with lung cancer risk in non‐tumour lung tissue from 38 adenocarcinoma patients. This correlation was not different between genotypes of P450s or GSTs, gender or subtypes of lung cancer, and the correlation was strongest in smoker subjects (who smoked >20 cigarettes per day). 179 Furthermore, high consumption of QT‐rich foods led to higher expression of GSTM1, GSTM2, GSTT2 and GSTP1 relative to the low consumption group, but there was a lower expression of some P450 genes. According to these findings, QT intake is able to reduce smoking‐induced lung cancer risk. 179
A QT‐containing diet is also associated with poorer miRNA expression profiles of the tumour suppressor let‐7 family in lung cancer tissue samples from 264 cases (144 adenocarcinomas and 120 squamous cell carcinomas). 181 QT‐rich diet also significantly differentiates miR‐146, miR‐26 and miR‐17, which are cancer‐related miRNAs. It was also found that 33 miRs were differentially expressed between high and low QT‐rich food consumers in former and current smokers with adenocarcinoma. QT‐rich foods in adenocarcinoma demonstrate differential expression of biologically functional miRs in this study, indicating that QT may have a therapeutic effect on lung cancer. Crocin (1, 2, 4, 8 and 16 mg/mL for 24 and 48 h) reduced mRNA expressions of B‐cell lymphoma 2 and Bax, but increased expressions of p53. Two lung cancer cell lines showed that QT also inhibited proliferation. 71
Anti‐tumour activity in vivo
A. cepa extract (10 g/L) showed an antiproliferative effect and mitotic indices appeared to be reduced in association with concentrations of the extracts. 178 Moreover, the extract showed no anti‐mutagenic or genotoxic properties. It is possible that the effects of onion extracts are caused by phenolic compounds. An antioxidant effect of A. cepa oil (10 mg/kg for 21 days) was shown in rats that had been exposed to nicotine, with higher levels of catalase (CAT) and superoxide dismutase (SOD) noted. 182 Administration of crocetin (50 mg/kg for 8 and q8 weeks) reduced cell proliferation, glycoprotein and polyamine synthesis in B(a)P‐induced lung cancer in mice. 183
The anti‐cancer effects of A. cepa and its main constituent QT reported in the above studies indicate that A. cepa and QT could inhibit adenocarcinomas parameters by modulating the expression of B‐cell lymphoma, Bax mRNA, Bcl‐2 mRNA, cell viability and DNA synthesis in lung cancer cells.
3.1.10. Brassica juncea
Brassica juncea (B. juncea), or mustard leaves, is a cruciferous vegetable consumed in different Asian and African countries. 184 , 185 , 186 Different biological activities of mustard and its constituents, such as antioxidant, 185 , 187 , 188 anti‐inflammatory 189 and anti‐depressant activities 190 have been reported. B. juncea has also demonstrated cytotoxic effects against cancer cells from different organs, including the colon, stomach, lungs and breast. 191
Adminstration of F. suspensa extract did not show hepatotoxicity, hemolytic activity or other side effects indicating its safety when used at the studies doses. 192 The effects of mustard and its constituents on lung cancer are summarized in Table 4.
Extract preparation
Dried B. juncea powder (20 g) was dissolved in 100 mL of 100% methanol and kept in a rectangular double‐walled water bath at 50°C for 4 h. Then the excerpt was filtered with filter paper (diameter of 125 mm) and the filtrate was kept at 50°C until it turned into a semisolid form. 193
Growth‐inhibitory activity in cell culture
DPPH assay analysis found that a combination of A. deliciosa and B. juncea extracts increased antioxidant and antiproliferation activity markers and apoptosis in human lung carcinoma cells. 193 Treatment of lung cancer cells with sinapic acid decreased potential cytotoxic and apoptosis activity, reduced ROS production levels and elevated Cas activity (Cas‐3 and Cas‐9). 194 Similarly, administration of sinapic acid (500, 1000, 2000, 5000 and 10,000 μg/mL) to A549 cells was found to increase Cas‐3 activity, as well as lactate dehydrogenase (LDH) activity and decrease necroptotic marker proteins, cyclophilin A and high mobility group box 1 (HMGB1). 195
Anti‐tumour activity in vivo
In vivo results showed that treatment with sinapic acid (30 mg/kg in corn oil, orally) improved lung and body weight, reduced levels of IgG, IgM and leukocyte counts, reduced the function of neutrophils, lipid peroxidation, pro‐inflammatory cytokines, AHH, LDH, GGT, 5′NT and CEA and elevated the phagocytic index, activity index and activity of antioxidant enzymes. 194
The above in vitro and in vivo results indicate that mustard and its constituents could be effective in lung cancer treatment via increasing antioxidant activities and antiproliferation activity markers, apoptosis and improving levels of immunoglobulins, Cas, HMGB1, LDH and pro‐inflammatory cytokines in lung cancer models.
3.1.11. Aloe vera
Aloe Vera (A. vera) is used to treat wounds in traditional Chinese medicine but is also widely used as a food, a remedy for constipation, wound healing and anti‐cancer purposes in Egypt, Greece and China. These medicinal properties have also been tested in pharmacological studies. 196 , 197 , 198
Regarding the safety of the plant, administration of tree doses (832.5, 1665 and 3330 mg/kg/bw by gavage), did not show any toxicity and mortality. 199 Several studies have indicated an effect of A. vera and its derivatives on lung cancer and the findings are discussed below and summarized in Table 4.
Growth‐inhibitory activity in cell culture
Administration of Aloe emodin (A. emodin, 20 μM) to lung cancer (H460) cells increased apoptosis, necrosis and cell death, increased MAP kinase membrane activation of a mitochondrial permeability transition pore, changed Cyt c protein expression and mediated localization of F‐actin. 200 In addition, treatment of A549 and NCI–H1299 cells with A. emodin (10, 20 and 40 μM) stimulated autophagy through activation of MAPK signalling, inhibition of the Akt/mTOR pathways, reduced ROS levels and mediated autophagy. 201 A study looking at the impact of A. emodin on CH27 and H460 cells found apoptosis was induced via nuclear morphological changes and DNA fragmentation, cystolic Cyt c fractions were enhanced, Cas‐3 was activated and protein kinase C (PKC) expression was increased. 202 Similarly, Lee et al 203 found A. emodin (40 μM) treatment of CH27 cells also triggered apoptosis by DNA fragmentation and enhanced expression of Bcl‐2 family proteins such as Bcl‐XL, Bag‐1 and Bak which increased Cyt c in the cytosolic fraction. A. emodin administration (20 μM for 2 h) to H460 cells also stimulated protein kinase Cδ (PKCδ) expression and cell death and decreased expression of cytoskeleton‐related proteins, including sarcoma (RAS), RAS homologue gene family member A (RHO), p38, heat shock protein 27 (HSP27), focal adhesion kinase (FAK), α‐actinin and tubulin. 204 Lee et al 205 found the treatment of carcinoma H460 cells with A. emodin (40 μM) increased the amount of proform and fragments of nucleophosmin, inducing apoptosis. In a more recent study, apoptosis‐inducing proteins such as cAMP‐dependent protein kinase, protein kinase C, Bcl‐2, Cas‐3 and p38 were also measured in carcinoma H460 cells following treatment with A. emodin nanoparticles (50 μM) for 16 h. 206 These A. emodin nanoparticles cleaved Cas‐3, Poly (ADP‐ribose) polymerase (PARP), Cas‐8 and Cas‐9 and simultaneously activated MAPKs and inhibited PI3K/AKT, leading to marked inhibition of cancer cell proliferation, induced cell cycle arrest and apoptosis. 206
Based on the above studies, A. vera and A. emodin showed anti‐carcinoma effects on lung cancer cells. Therefore, A. vera and A. emodin could be effective in lung cancer therapy by inhibiting cell proliferation, cytoskeleton activation, stimulated cell apoptosis as well as Cas‐3 and PKC activation, MAPK signalling, inhibition of ROS production, Akt/mTOR and PI3K/AKT pathways. 206
3.2. Different mechanisms of natural products against lung cancer
In the present article, the effects of the most effective 11 medicinal plants and their constituents against lung cancer were reviewed. The anti‐cancer effects of the above medicinal plants and their derivatives on lung cancer were mediated through different mechanisms of action including inhibition or abnormal cell growth and proliferation, cancer cell apoptosis and death, oxidative stress and inhibition of migration and or metastasis. 37 , 207 , 208 , 209 , 210 Below we summarize the mode of action and molecular strategies of medicinal plants and their derivatives against lung cancer, as evidenced by empirical study.
3.2.1. Cell proliferation, cell viability and tumour growth
In lung cancer, increasing cell proliferation and viability of cancer cells demonstrates the advancement of the disease. Therefore, the effect of natural products on lung cancer cell proliferation and viability is an indicator of their therapeutic effects. The in vitro and in vivo studies demonstrated the inhibitory effects of A. vera, C. longa, C. sativus, B. juncea, A. cepa, P. oleracea, Z. multiflora, N. sativa and their constituents on lung cancer cell proliferation. 43 , 45 , 68 , 81 , 115 , 127 , 156 , 159 , 180 In addition to cell proliferation and viability, tumour growth rate is used to diagnose the stage and severity of cancer. A. vera, C.longa, F. szowitsiana, P. oleracea and Z. multiflora all demonstrated an ability to block or disrupt tumour growth in lung cancer models and cell lines. 39 , 90 , 131 , 135 , 211 Figure 2 illustrates the effects of these natural products on cell proliferation and tumour growth in lung cancer.
FIGURE 2.
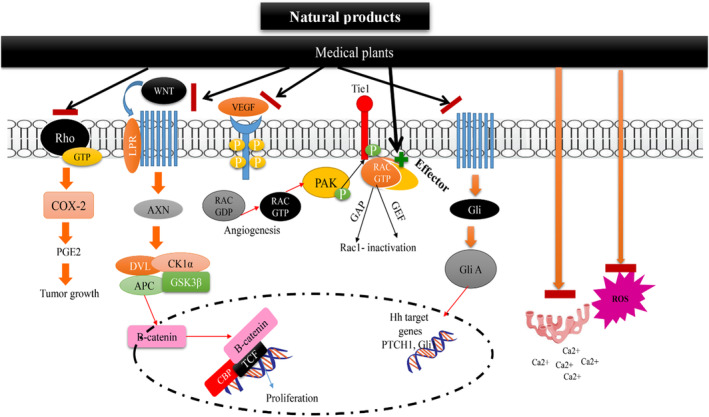
Anti‐apoptosis, cell death and anti‐cell migration mechanisms of natural products on lung cancer.
3.2.2. Apoptosis
Increased apoptosis reduced rates of lung cancer cell proliferation, growth and prevented metastasis, making it a powerful effect against lung cancer. Several studies reported that the natural products: O. basilicum, P. oleracea, A. vera, C. longa, A. cepa, C. sativus, N. sativa, Z. multiflora and their constituents increased the level of apoptosis in lung cancer models or cell lines. 58 , 86 , 128 , 142 , 146 , 161 , 180 , 206 How these natural products induce apoptosis in lung cancer is shown in Figure 2.
3.2.3. Growth of lung cancer
The extracellular matrix (ECM) is a crucial component of the tumour microenvironment (TME) and is present in both interstitial and epithelial vessels. It plays a dual role in cancer progression, as it facilitates interactions between cancer cells and stromal cells, promoting carcinogenesis, while also acting as a barrier against tumour metastasis. 212 Degradation of the ECM allows cancer cells to traverse it and enter blood vessels. Subsequently, with the assistance of certain cytokines, cancer cells can pass through vessel walls and extravasate to secondary sites, where they continue to proliferate and form metastatic lesions. 213 Matrix metalloproteinases (MMPs), a family of zinc‐dependent endopeptidases produced by various cell types, including fibroblasts, epithelial cells and immune cells, are the primary proteases responsible for ECM degradation. The urokinase‐type plasminogen activator (u‐PA) is a key enzyme involved in ECM degradation and the activation of proMMPs, including MMP‐2. Abnormal expression of membrane type 1‐matrix metalloproteinase (MT1‐MMP) in tumours is associated with the regulation of MMP‐2 activity. 214 MMP‐9, another member of the MMP family, has been identified as a biomarker for various cancers. 215 Therefore, inhibiting the activity or expression of MMPs may be a potential strategy to suppress tumour invasion and metastasis. In lung cancer and other cancers, overexpression of GLUT1 (glucose transporter 1) is associated with poor prognosis. CUR, a natural compound with diverse activities, has been studied for its potential therapeutic effects. 216 Liao et al. demonstrated that CUR treatment reduced the expression of GLUT1, MT1‐MMP and MMP2 in A549 lung cancer cells. Conversely, in GLUT1‐overexpressing A549 cells, CUR's anti‐migration and anti‐invasion effects were impaired, and MT1‐MMP and MMP2 expression were up‐regulated. Consistent with these findings, in vivo experiments using nude mice showed that CUR treatment significantly reduced metastatic rates in A549 cells, but this effect was hindered in GLUT1‐overexpressing cells. The study concluded that CUR suppresses migration and invasion by modulating the GLUT1/MT1‐MMP/MMP2 pathway in A549 cells. 125 Another compound, honokiol, derived from Magnolia officinalis, was found to inhibit migration and invasion in H1299 lung cancer cells by disrupting the expression of MMP‐9 and the Hsp90/MMP‐9 interactions mediated by HDAC6. Honokiol promoted the degradation of MMP‐9 through the ubiquitin‐proteasome pathway, rather than inhibiting its transcription. HDAC6, a deacetylase, regulates the stability of Hsp90, which is involved in the activation of MMP‐2/9. Honokiol inhibited the expression of acetyl‐α‐tubulin, a specific substrate of HDAC6, and further experiments confirmed the regulation of MMP‐9 by HDAC6. 217 Other natural compounds, such as theaflavin and theaflavin digallate from black tea, were found to exert anti‐metastasis effects by inhibiting type IV collagenase in mouse LLC cells. 218 (‐)‐Epigallocatechin‐3‐gallate (EGCG), a polyphenol found in green tea, has also been extensively studied. Deng et al. reported that EGCG inhibits invasion in CL1‐5 lung cancer cells by suppressing the mRNA and protein levels of MMP‐2 through the JNK pathway. EGCG was also found to enhance the anti‐cancer effects of docetaxel and reduce MMP‐2 expression. Additionally, EGCG was shown to suppress migration and invasion by inhibiting epithelial‐mesenchymal transition (EMT) and angiogenesis induced by nicotine. 219 , 220 , 221 Another important target of cancer treatments is decreasing the amount of tumour cell migration and metastasis. There are several in vitro and even in vivo studies that found A. millefolium, C. longa, Ferula spp., N. sativa and their constituents reduced lung cancer cell migration 44 , 58 , 81 , 91 , 130 , 143 and lung cancer metastasis. 58 , 81 , 112 , 125 , 147 The effects of these natural products on migration and metastasis of lung cancer are shown in Figure 2.
3.2.4. Signalling pathways underlying the growth‐suppressive activity of medicinal plants and their constituents
Studies investigating various types of cancer have revealed some of the molecular events behind cancer cell proliferation, viability, migration and metastasis. For example, enhanced tumour growth correlated with increased AMPK and fatty acid oxidation in ovarian cancer cells, linked to dysregulation of mTORC1 expression in renal cell carcinoma and has been connected to the hypoxia and oncogenic HRAS or KRAS pathways in glioblastoma and bladder cancer. 222
Hence, p53‐deficient KRasG12D NSCLC had dysfunctional mitochondria, lipid accumulation and defects in fatty acid oxidation, resulting in reduced tumour growth. 223 In addition, mutations in MYC, TP53, Ros‐related oncogenes, the AMPK and PI3K signalling pathways of the NSCLCs enhanced expression of GLUT1, negatively impacting cell proliferation and growth. 223
In microglial, bladder cancer and HepG2 cells, cell proliferation, migration and survival could be enhanced by up‐regulation of the Gadd45 family member Gadd45β/Myd118, which associates with MKK7/JNKK2, p38 expression, transforming growth factor‐β and TGF‐β via the JAK/STAT pathway, ultimately activating toll‐like receptors and interferon‐γin. 224 , 225 , 226
Increasing accumulation and expression of angiogenesis factors such as VPF and VGEF enhanced metastasis and cell growth in tumour cells. Apoxemia and realizing ROS also triggered angiogenesis factors in human glioma cells. 227 , 228 Breast cancer cells tend to proliferate when ß‐actin, FOXM1, FBXW7, Fascin, eNOS, MMP‐2 and HER2‐receptor are expressed or stimulated. 229 Expressions of Wnt1, β‐catenin and cyclin D1 at the protein level in NSCLC cells increased the risk of mutagenesis, increased cancer cell viability and decreased apoptosis. 230 Activation of Signal Transducer And Activator Of Transcription 3 (STAT3) signalling induced EMT and increased miR‐193a‐3p, miR‐210‐3p and miR‐5100 levels, prompting lung cancer metastasis. 231 It was reported that proliferation was suppressed via inhibiting the PHLPP1/AKT pathway. 232
The cancer suppression effects of N. sativa, A. emodin, F. Szowitsiana and A. millefolium extracts as well as some derivatives of medicinal plants such as CUR, TQ, carvacrol, genistein, crocin work by increasing apoptosis and authophagy, reducing cell viability or proliferation via RTK and reducing cytotoxicity via inhibition of ROS release and expression. 60 , 116 , 204 The suppression effects of the above plants on P38, 39 , 116 , 206 P53, 37 , 71 , 233 Akt 58 , 62 , 159 and activation of mitochondrial protein expression such as Bcl, Bcl2, 43 , 56 , 58 Cyto C, 43 , 104 Cas‐3 and ‐8 down‐regulation of Ca2+ release mediated NF‐κB/MMP 43 and inhibiting the PI3K/Akt/mTOR, JAK2/STAT3 pathways 135 , 206 , 231 were also shown. C. longa, A. millefolium, C. sativus, szowitsiana and CURwere also found to Block the Wnt/β‐catenin and Sonic Hedgehog pathways, reduce metastasis and migration of lung cancer cells via inhibition of NF‐κB and AP‐1, MMp2, JAK/STAT3 pathways, increase FOXA2 expression via regulation of STAT3 signalling pathways, enhance GCL expression via activation of Nrf2, LC3‐II, p62 expression rand decrease cancer cell cytotoxicity via inhibition of ROS and inflammatory markers. 101 , 113 , 130 Therefore natural plant products, including medicinal plants and their derivatives, are promising agents for the treatment of lung cancer by impacting apoptosis, autophagy, cell viability or proliferation, metastasis and migration. The molecular pathways of these anti‐cancer effects are summarized in Figure 3.
FIGURE 3.
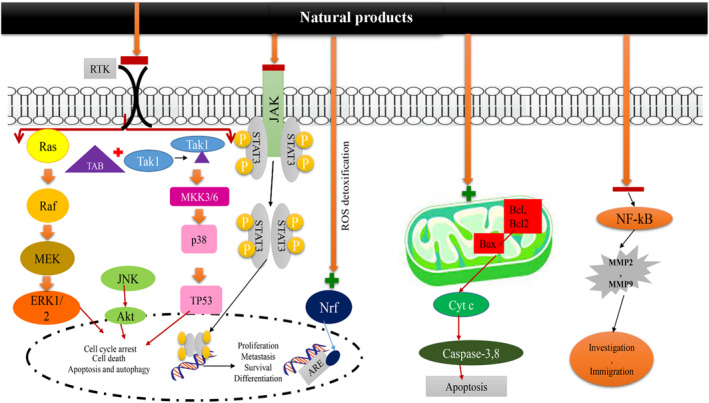
Anti‐cell proliferation, anti‐oxidative and anti‐tumour growth mechanisms of natural products on lung cancer.
3.2.5. Chemosensitization activity
Despite the availability of numerous conventional anti‐cancer drugs with diverse mechanisms of action, the majority of them ultimately induce cell death through necrosis, apoptosis or autophagy by stimulating genotoxic stress. 234 However, these chemotherapeutic agents primarily target rapidly dividing cells, which often results in adverse effects on normal dividing cells, leading to a multitude of side effects. 235 While some of these side effects are short‐term, such as nausea, myelosuppression or alopecia, others, including infertility, weight gain, cardiac dysfunction or secondary leukaemia, are long‐term and serious. Additionally, the development of chemoresistance, particularly multidrug resistance (MDR), is another significant obstacle to the success of chemotherapy. 235 To overcome this challenge, researchers have conducted extensive research on molecules that may enhance the therapeutic index of anti‐tumoral drugs by making tumour cells more sensitive to chemotherapeutic agents, known as chemosensitizers. However, many chemosensitizers have shown toxicity or low efficacy in clinical trials. 236 , 237 , 238
Given these limitations, natural compounds have emerged as a promising source of new molecules for chemosensitization due to their unique structures and mechanisms of action. Many plant‐derived molecules have been found to target multiple pathways involved in cancer and are consumed as food or used as traditional remedies. Natural products have played a significant role in the development of small molecules approved for cancer treatment. For instance, between the 1940s and the end of 2014, 75% of the small molecules approved for cancer treatment were other than synthetic, with 49% being natural products or directly derived from it. Therefore, scientists are currently exploring new molecules, such as natural compounds, to address the important problem of MDR in cancer. 239 , 240 , 241
Natural compounds have emerged as a promising source of new molecules for chemosensitization in cancer therapy due to their unique structures and mechanisms of action. They can be used as chemotherapeutic agents, chemopreventive agents and chemosensitizing agents to improve the effectiveness of conventional chemotherapy. 242 , 243 Natural products, especially edible phytochemicals‐nutraceuticals, are attractive partners to be used in combination with chemotherapy due to their high biodiversity, good oral bioavailability and relatively low intrinsic toxicity. 244 When associated with conventional chemotherapeutics, plant‐based chemosensitizers enhance the cytotoxic effect of the anti‐cancer drugs, promoting a synergistic effect even in cells with acquired resistance. Nutraceuticals represent a specific segment of natural compounds that are consumed as part of a normal diet and are considered pharmacologically safe. However, challenges regarding the limited bioavailability of these compounds have been discussed, and novel formulations and analogues have been developed to increase their efficacy. 52 , 245 , 246 Over the past few decades, numerous studies have indicated that cancer cells can develop resistance to drugs, which can be overcome by combining multiple drugs that act on redundant signalling nodes. EGFRi can initiate various side effects, which can be alleviated by incorporating natural products into the therapeutic regimen. 247 For instance, Honeysuckle, a natural product obtained from Lonicera japonica Thunb, was shown to reduce acneiform rash incidences and severities induced by EGFRi in lung cancer patients. Additionally, natural bioactive components can help reverse EGFRi resistance in cancer cells. Bruceine H, a derivative of Brucea javanica (L.), was found to overcome resistance to receptor tyrosine kinase (RTK)‐EGFRi in non‐small cell lung cancer (NSCLC) models by suppressing Notch3, EGFR activation and β‐catenin expression., thereby increasing gefitinib response. This combination also induced Foxo3a expression, which correlates with better response to EGFR inhibitors and overall survival in NSLCC patients. Similarly, cryptotanshinone (CTS), a bioactive component of Salvia miltiorrhiza, was shown to sensitize gefitinib‐resistant EGFR‐mutant lung cancer cells by targeting catalase (CAT), heme oxygenase 1 (HMOX1) and stearoyl‐CoA desaturase (SCD). These proteins were identified through proteomic analysis and suggested to be potential therapeutic targets in lung cancers. 248
4. CONCLUSION
This review article presented the potential therapeutic effects of medicinal plants and their constituents on lung cancer based on in vivo and in vitro findings. Medicinal plants and their constituents increase the anti‐cancer effects of many chemotherapeutic agents and radiotherapy, as well as reverse the effects of chemotherapeutic drug resistance, which is noteworthy since the emergence of chemotherapeutic drug resistance will eventually compromise the treatment of cancer.
The reviewed papers showed that the extract of Z. multiflora extract and its main constituent carvacrol, reduce lung cancer virality, increases the expression of apoptotic proteins, modulates cytokine levels, cancer cell viability and regulate specific pathways. The effect of C. sativus, crocin and crocetin on down‐regulation of mRNA levels of major proteins of apoptosis in lung cancer cells was also reported. F. persica and its main constituent coumarin are shown as a possible treatment for lung cancer via the inhibition of cyclins and CDK, their reducing effects on cancer cell migration and metastasis mediated by cytokines such as IL‐1β, oxidative marker production, cell viability and cytotoxicity.
C. longa and CUR suppress cancer cell proliferation and induce apoptosis of lung cancer cells. CUR changed the expression of EGFR, microRNAs and autophagy in cancer stem cells and influences the expression of different genes in the cancer cells, such as NF‐kB, STAT‐3 and AP‐1, as well as protein kinases, including MAPK and enzymes such as COX and LOX. The anti‐cancer mechanisms of A. millefolium and its constituent, kaempferol by change in cancer cell immobility and death as well as disruption the cytoskeleton of lung cancer cells were shown. P. oleracea and its constituent such genestein reduce cell viability, cell proliferation, Bcl‐2 level and MMP‐2 expression but increase apoptotic, cytotoxicity, Bax expression, miR‐128 activation and p21 protein level, inhibit NSCLC cells ERK1/2 and PI3K/Akt phosphorylation, regulate Cas‐3/9 activity, miRNA27a activity and the expression of the MET protein enhancing p62, activating Cas‐3 and Cas‐8 and increasing TRAIL‐induced tumour cell death by activated autophagy.
The anti‐cancer effects of A. cepa and its main constituent QT are shown by adenocarcinomas parameters inhibition through modulating the expression of B‐cell lymphoma, Bax mRNA, Bcl‐2 mRNA, cell viability and DNA synthesis in lung cancer cells. The effects of B. juncea or mustard and its constituents on lung cancer treatment are shown via increasing antioxidant activities and antiproliferation activity markers, apoptosis and improving levels of immunoglobulins, Cas, HMGB1, LDH and pro‐inflammatory cytokines. Based on the above studies, A. vera and A. emodin showed anti‐carcinoma effects on lung cancer cells. It was shown that A. vera and A. emodin are effective in lung cancer therapy by inhibiting cell proliferation, cytoskeleton activation, stimulated cell apoptosis as well as Cas‐3 and PKC activation, MAPK signalling, inhibition of ROS production, Akt/mTOR and PI3K/AKT pathways.
Therefore, promising effects of medicinal plants and their constituents on lung cancer based on many experimental findings were indicated. However, it is necessary to pay attention to the pharmacodynamic and pharmacokinetic limitations of each medicinal plant. In addition, further clinical studies of the plants and their constituents on lung cancer are needed to justify their use in clinical practice for the treatment of these diseases.
AUTHOR CONTRIBUTIONS
Arghavan Memarzia: Writing – original draft (equal). Saeideh Saadat: Writing – original draft (equal); writing – review and editing (equal). Fereshteh Asgharzadeh: Writing – original draft (equal); writing – review and editing (equal). Sepide Behrouz: Writing – original draft (equal). Gert Folkerts: Writing – review and editing (equal). Mohammad Hossein Boskabady: Formal analysis (equal); methodology (equal); supervision (equal); writing – review and editing (equal).
FUNDING INFORMATION
Not applicable.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
CONSENT TO PARTICIPATE
Not applicable.
CONSENT TO PUBLISH
Not applicable.
ACKNOWLEDGEMENTS
Not applicable.
Memarzia A, Saadat S, Asgharzadeh F, Behrouz S, Folkerts G, Boskabady MH. Therapeutic effects of medicinal plants and their constituents on lung cancer, in vitro, in vivo and clinical evidence. J Cell Mol Med. 2023;27:2841‐2863. doi: 10.1111/jcmm.17936
DATA AVAILABILITY STATEMENT
Not applicable (This is a review article).
REFERENCES
- 1. De Angelis R, Sant M, Coleman MP, et al. Cancer survival in Europe 1999–2007 by country and age: results of EUROCARE‐5—a population‐based study. Lancet Oncol. 2014;15:23‐34. [DOI] [PubMed] [Google Scholar]
- 2. de Groot PM, Wu CC, Carter BW, Munden RF. The epidemiology of lung cancer. Transl Lung Cancer Res. 2018;7:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dadashi A, Mohammadi A, MohammadEbrahimi S, et al. Spatial analysis of the 10 most prevalent cancers in North‐Eastern Iran, 2017–2018. J Spat Sci. 2023;269:281‐301. [Google Scholar]
- 4. Minna JD, Roth JA, Gazdar AF. Focus on lung cancer. Cancer Cell. 2002;1:49‐52. [DOI] [PubMed] [Google Scholar]
- 5. Rahimi F, Heidari M. Time trend analysis of stomach cancer incidence in the west of Iran. J Health Dev. 2012;1:100‐111. [Google Scholar]
- 6. Lippman SM, Spitz MR. Lung cancer chemoprevention: an integrated approach. J Clin Oncol. 2001;19:74S‐82S. [PubMed] [Google Scholar]
- 7. Mazzone PJ, Sears CR, Arenberg DA, et al. Evaluating molecular biomarkers for the early detection of lung cancer: when is a biomarker ready for clinical use? An official American Thoracic Society policy statement. Am J Respir Crit Care Med. 2017;196:e15‐e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chaturvedi AK, Caporaso NE, Katki HA, et al. C‐reactive protein and risk of lung cancer. J Clin Oncol. 2010;28:2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guida F, Sun N, Bantis LE, et al. Assessment of lung cancer risk on the basis of a biomarker panel of circulating proteins. JAMA Oncol. 2018;4:e182078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tang H, Bai Y, Shen W, et al. Clinical significance of combined detection of interleukin‐6 and tumour markers in lung cancer. Autoimmunity. 2018;51:191‐198. [DOI] [PubMed] [Google Scholar]
- 11. Tang Z‐M, Ling Z‐G, Wang C‐M, Wu Y‐B, Kong J‐L. Serum tumor‐associated autoantibodies as diagnostic biomarkers for lung cancer: a systematic review and meta‐analysis. PLoS One. 2017;12:e0182117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Silvestri GA, Vachani A, Whitney D, et al. A bronchial genomic classifier for the diagnostic evaluation of lung cancer. N Engl J Med. 2015;373:243‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Seijo LM, Peled N, Ajona D, et al. Biomarkers in lung cancer screening: achievements, promises, and challenges. J Thorac Oncol. 2019;14:343‐357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abbosh C, Birkbak NJ, Wilson GA, et al. Phylogenetic ctDNA analysis depicts early‐stage lung cancer evolution. Nature. 2017;545:446‐451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lennon AM, Buchanan AH, Kinde I, et al. Feasibility of blood testing combined with PET‐CT to screen for cancer and guide intervention. Science. 2020;369:eabb9601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu M, Oxnard G, Klein E, et al. Sensitive and specific multi‐cancer detection and localization using methylation signatures in cell‐free DNA. Ann Oncol. 2020;31:745‐759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Phallen J, Sausen M, Adleff V, et al. Direct detection of early‐stage cancers using circulating tumor DNA. Sci Transl Med. 2017;9:eaan2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shen SY, Singhania R, Fehringer G, et al. Sensitive tumour detection and classification using plasma cell‐free DNA methylomes. Nature. 2018;563:579‐583. [DOI] [PubMed] [Google Scholar]
- 19. Lemjabbar‐Alaoui H, Hassan OU, Yang Y‐W, Buchanan P. Lung cancer: biology and treatment options. Biochim Biophys Acta. 2015;1856:189‐210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ağagündüz D, Şahin TÖ, Yılmaz B, Ekenci KD, Duyar Özer Ş, Capasso R. Cruciferous vegetables and their bioactive metabolites: from prevention to novel therapies of colorectal cancer. Evid Based Complement Alternat Med. 2022;2022:1534083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ferrarini EG, Paes RS, Baldasso GM, et al. Broad‐spectrum cannabis oil ameliorates reserpine‐induced fibromyalgia model in mice. Biomed Pharmacother. 2022;154:113552. [DOI] [PubMed] [Google Scholar]
- 22. Wang P, Ma XM, Geng K, Jiang ZZ, Yan PY, Xu Y. Effects of Camellia tea and herbal tea on cardiometabolic risk in patients with type 2 diabetes mellitus: a systematic review and meta‐analysis of randomized controlled trials. Phytother Res. 2022;36:4051‐4062. [DOI] [PubMed] [Google Scholar]
- 23. Desai AG, Qazi GN, Ganju RK, et al. Medicinal plants and cancer chemoprevention. Curr Drug Metab. 2008;9:581‐591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Amin F, Memarzia A, Kazerani HR, Boskabady MH. Carvacrol and Zataria multiflora influenced the PPARγ agonist effects on systemic inflammation and oxidative stress induced by inhaled paraquat in rat. Iran J Basic Med Sci. 2020;23:930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boskabady MH, Gholami ML. Effect of the Zataria multiflora on systemic inflammation of experimental animals model of COPD. Biomed Res Int. 2014;2014:802189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khazdair MR, Ghorani V, Alavinezhad A, Boskabady MH. Effect of Zataria multiflora on serum cytokine levels and pulmonary function tests in sulfur mustard‐induced lung disorders: a randomized double‐blind clinical trial. J Ethnopharmacol. 2020;248:112325. [DOI] [PubMed] [Google Scholar]
- 27. Boskabady MH, Mehrjardi SS, Rezaee A, Rafatpanah H, Jalali S. The impact of Zataria multiflora Boiss extract on in vitro and in vivo Th1/Th2 cytokine (IFN‐γ/IL4) balance. J Ethnopharmacol. 2013;150:1024‐1031. [DOI] [PubMed] [Google Scholar]
- 28. Boskabady MH, Gholami ML. Lung inflammation changes and oxidative stress induced by cigarette smoke exposure in Guinea pigs affected by Zataria multiflora and its constituent, carvacrol. BMC Complement Altern Med. 2015;15:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Amin F, Roohbakhsh A, Memarzia A, Kazerani HR, Boskabady MH. Paraquat‐induced systemic inflammation and increased oxidative markers in rats improved by Zataria multiflora extract and carvacrol. Avicenna J Phytomed. 2020;10:513‐522. [PMC free article] [PubMed] [Google Scholar]
- 30. Khazdair MR, Ghorani V, Alavinezhad A, Boskabady MH. Pharmacological effects of Zataria multiflora Boiss L. and its constituents focus on their anti‐inflammatory, antioxidant, and immunomodulatory effects. Fundam Clin Pharmacol. 2018;32:26‐50. [DOI] [PubMed] [Google Scholar]
- 31. Boskabady MH, Tabatabaee A, Jalali S. Potential effect of the extract of Zataria multiflora and its constituent, carvacrol, on lung pathology, total and differential WBC, IgE and eosinophil peroxidase levels in sensitized Guinea pigs. J Funct Foods. 2014;11:49‐61. [Google Scholar]
- 32. Amin F, Memarzia A, Roohbakhsh A, Shakeri F, Boskabady MH. Zataria multiflora and pioglitazone affect systemic inflammation and oxidative stress induced by inhaled paraquat in rats. Mediat Inflamm. 2021;2021:5575059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Alavinezhad A, Ghorani V, Rajabi O, Boskabady MH. Zataria multiflora extract influenced asthmatic patients by improving respiratory symptoms, pulmonary function tests and lung inflammation. J Ethnopharmacol. 2022;285:114888. [DOI] [PubMed] [Google Scholar]
- 34. Mahboubi M. Potential effect and mechanism of action of Zataria multiflora in liver disorders. J Pre‐Clin Clin Res. 2021;15:192‐198. [Google Scholar]
- 35. Mahmoudvand H, Kareshk AT, Moradi MN, et al. Efficacy and safety of Zataria multiflora Boiss essential oil against acute toxoplasmosis in mice. Iran J Parasitol. 2020;15:22. [PMC free article] [PubMed] [Google Scholar]
- 36. Ghorani V, Alavinezhad A, Rajabi O, Mohammadpour AH, Boskabady MH. Safety and tolerability of carvacrol in healthy subjects: a phase I clinical study. Drug Chem Toxicol. 2021;44:177‐189. [DOI] [PubMed] [Google Scholar]
- 37. Anani H, Baluchi I, Farsinejad A, Fatemi A, Khalilabadi RM. Zataria multiflora methanolic extract has antitumor properties on U266 multiple myeloma cell line. Gene Reports. 2020;20:100655. [Google Scholar]
- 38. Azadi M, Jamali T, Kianmehr Z, Kavoosi G, Ardestani SK. In‐vitro (2D and 3D cultures) and in‐vivo cytotoxic properties of Zataria multiflora essential oil (ZEO) emulsion in breast and cervical cancer cells along with the investigation of immunomodulatory potential. J Ethnopharmacol. 2020;257:112865. [DOI] [PubMed] [Google Scholar]
- 39. Khan I, Bhardwaj M, Shukla S, et al. Carvacrol encapsulated nanocarrier/nanoemulsion abrogates angiogenesis by downregulating COX‐2, VEGF and CD31 in vitro and in vivo in a lung adenocarcinoma model. Colloids Surf B Biointerfaces. 2019;181:612‐622. [DOI] [PubMed] [Google Scholar]
- 40. Barnwal P, Vafa A, Afzal S, et al. Benzo (a) pyrene induces lung toxicity and inflammation in mice: prevention by carvacrol. Hum Exp Toxicol. 2018;37:752‐761. [DOI] [PubMed] [Google Scholar]
- 41. Luo L, Wang Y, Li S. Study on the carvacrol induced apoptosis in human non‐small cell lung cancer cell line H1299. Tianjin Med J. 2016;25:1418‐1422. [Google Scholar]
- 42. Koparal AT, Zeytinoğlu M. Effects of carvacrol on a human non‐small cell lung cancer (NSCLC) cell line, A549. Cytotechnol. 2003;43:149‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Khan I, Bahuguna A, Kumar P, Bajpai VK, Kang SC. In vitro and in vivo antitumor potential of carvacrol nanoemulsion against human lung adenocarcinoma A549 cells via mitochondrial mediated apoptosis. Sci Rep. 2018;8:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jung CY, Kim S‐Y, Lee C. Carvacrol targets AXL to inhibit cell proliferation and migration in non‐small cell lung cancer cells. Anticancer Res. 2018;38:279‐286. [DOI] [PubMed] [Google Scholar]
- 45. Khan I, Bahuguna A, Bhardwaj M, Pal Khaket T, Kang SC. Carvacrol nanoemulsion evokes cell cycle arrest, apoptosis induction and autophagy inhibition in doxorubicin resistant‐A549 cell line. Artif Cells Nanomed Biotechnol. 2018;46:664‐675. [DOI] [PubMed] [Google Scholar]
- 46. Tembhurne S, Feroz S, More B, Sakarkar D. A review on therapeutic potential of Nigella sativa (kalonji) seeds. J Med Plant Res. 2014;8:167‐177. [Google Scholar]
- 47. Jafri SH, Glass J, Shi R, Zhang S, Prince M, Kleiner‐Hancock H. Thymoquinone and cisplatin as a therapeutic combination in lung cancer: in vitro and in vivo. J Exp Clin Cancer Res. 2010;29:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gholamnezhad Z, Shakeri F, Saadat S, Ghorani V, Boskabady MH. Clinical and experimental effects of Nigella sativa and its constituents on respiratory and allergic disorders. Avicenna J Phytomed. 2019;9:195. [PMC free article] [PubMed] [Google Scholar]
- 49. Al‐Sheddi ES, Farshori NN, Al‐Oqail MM, Musarrat J, Al‐Khedhairy AA, Siddiqui MA. Cytotoxicity of Nigella sativa seed oil and extract against human lung cancer cell line. Asian Pac J Cancer Prev. 2014;15:983‐987. [DOI] [PubMed] [Google Scholar]
- 50. Boskabady MH, Vahedi N, Amery S, Khakzad MR. The effect of Nigella sativa alone, and in combination with dexamethasone, on tracheal muscle responsiveness and lung inflammation in sulfur mustard exposed Guinea pigs. J Ethnopharmacol. 2011;137:1028‐1034. [DOI] [PubMed] [Google Scholar]
- 51. Mokhtari‐Zaer A, Norouzi F, Askari VR, et al. The protective effect of Nigella sativa extract on lung inflammation and oxidative stress induced by lipopolysaccharide in rats. J Ethnopharmacol. 2020;253:112653. [DOI] [PubMed] [Google Scholar]
- 52. Hannan M, Rahman M, Sohag A, et al. Black cumin (Nigella sativa L.): a comprehensive review on phytochemistry, health benefits, molecular pharmacology, and safety. Nutrients. 2021;13(6):1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Thomas JV, Mohan M, Prabhakaran P, Maliakel B, Krishnakumar I. A phase I clinical trial to evaluate the safety of thymoquinone‐rich black cumin oil (BlaQmax®) on healthy subjects: randomized, double‐blinded, placebo‐controlled prospective study. Toxicol Rep. 2022;9:999‐1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Salim EI. Cancer chemopreventive potential of volatile oil from black cumin seeds, Nigella sativa L., in a rat multi‐organ carcinogenesis bioassay. Oncol Lett. 2010;1:913‐924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yang J, Kuang X‐R, Lv P‐T, Yan X‐X. Thymoquinone inhibits proliferation and invasion of human nonsmall‐cell lung cancer cells via ERK pathway. Tumor Biol. 2015;36:259‐269. [DOI] [PubMed] [Google Scholar]
- 56. Ulasli SS, Celik S, Gunay E, et al. Anticancer effects of thymoquinone, caffeic acid phenethyl ester and resveratrol on A549 non‐small cell lung cancer cells exposed to benzo (a) pyrene. Asian Pac J Cancer Prev. 2013;14:6159‐6164. [DOI] [PubMed] [Google Scholar]
- 57. Alhakamy NA, Badr‐Eldin SM, A Fahmy U, et al. Thymoquinone‐loaded soy‐phospholipid‐based phytosomes exhibit anticancer potential against human lung cancer cells. Pharmaceutics. 2020;12:761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dera AA, Rajagopalan P, Al Fayi M, Ahmed I, Chandramoorthy HC. Indirubin‐3‐monoxime and thymoquinone exhibit synergistic efficacy as therapeutic combination in in‐vitro and in‐vivo models of lung cancer. Arch Pharm Res. 2020;43:655‐665. [DOI] [PubMed] [Google Scholar]
- 59. Singh SK, Mishra MK, Lillard JW, Singh R. Thymoquinone enhanced the tumoricidal activity of nk cells against lung cancer. Am Assoc Immnol. 2018;200:124‐125. [Google Scholar]
- 60. Durga B, Julius A. In‐silico docking studies of thymoquinone as potential anti‐cancer drug target on lung cancer cells. Eur J Mol Clin Med. 2020;7:1706‐1716. [Google Scholar]
- 61. Mabrouk G, Moselhy S, Zohny S, et al. Inhibition of methylnitrosourea (MNU) induced oxidative stress and carcinogenesis by orally administered bee honey and nigella grains in Sprague Dawely rats. J Exp Clin Cancer Res. 2002;21:341‐346. [PubMed] [Google Scholar]
- 62. Alzohairy MA, Khan AA, Alsahli MA, Almatroodi SA, Rahmani AH. Protective effects of thymoquinone, an active compound of Nigella sativa, on rats with benzo (a) pyrene‐induced lung injury through regulation of oxidative stress and inflammation. Molecules. 2021;26:3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Champalal K, Nilakshi N, Vijay G, Abhyankar M. Detailed profile of Crocus sativus . Int J Pharm Bio Sci. 2011;2:530‐540. [Google Scholar]
- 64. Boskabady MH, Gholamnezhad Z, Ghorani V, Saadat S. The effect of Crocus sativous (saffron) on the respiratory system: traditional and experimental evidence. Science. 2019;1:28‐52. [Google Scholar]
- 65. Khazdair MR, Gholamnezhad Z, Rezaee R, Boskabady MH. A qualitative and quantitative comparison of Crocus sativus and Nigella sativa immunomodulatory effects. Biomed Pharmacother. 2021;140:111774. [DOI] [PubMed] [Google Scholar]
- 66. Mokhtari‐Zaer A, Saadat S, Ghorani V, Memarzia A, Boskabady MH. The effects of saffron (Crocus Sativus) and its constituents on immune system: experimental and clinical evidence. Elsevier; 2020:193‐217. [Google Scholar]
- 67. Modaghegh M‐H, Shahabian M, Esmaeili H‐A, Rajbai O, Hosseinzadeh H. Safety evaluation of saffron (Crocus sativus) tablets in healthy volunteers. Phytomedicine. 2008;15:1032‐1037. [DOI] [PubMed] [Google Scholar]
- 68. Samarghandian S, Boskabady MH, Davoodi S. Use of in vitro assays to assess the potential antiproliferative and cytotoxic effects of saffron (Crocus sativus L.) in human lung cancer cell line. Pharmacogn Mag. 2010;6:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Samarghandian S, Tavakkol Afshari J, Davoodi S. Suppression of pulmonary tumor promotion and induction of apoptosis by Crocus sativus L. extraction. Appl Biochem Biotechnol. 2011;164:238‐247. [DOI] [PubMed] [Google Scholar]
- 70. Liu D‐D, Ye Y‐L, Zhang J, Xu J‐N, Qian X‐D, Zhang Q. Distinct pro‐apoptotic properties of Zhejiang saffron against human lung cancer via a caspase‐8‐9‐3 cascade. Asian Pac J Cancer Prev. 2014;15:6075‐6080. [DOI] [PubMed] [Google Scholar]
- 71. Chen S, Zhao S, Wang X, et al. Crocin inhibits cell proliferation and enhances cisplatin and pemetrexed chemosensitivity in lung cancer cells. Transl Lung Cancer Res. 2015;4:775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Radan M, Dianat M, Badavi M, Mard SA, Bayati V, Ahmadizadeh M. The association of cigarette smoke exposure with lung cellular toxicity and oxidative stress: the protective role of crocin. Inflammation. 2020;43:135‐145. [DOI] [PubMed] [Google Scholar]
- 73. Sajjadi SE. Analysis of the essential oils of two cultivated basil (Ocimum basilicum L.) from Iran. DARU J Pharm Sci. 2006;14:128‐130. [Google Scholar]
- 74. Rezzoug M, Bakchiche B, Gherib A, et al. Chemical composition and bioactivity of essential oils and ethanolic extracts of Ocimum basilicum L. and Thymus algeriensis Boiss. & Reut. From the Algerian Saharan atlas. BMC Complement Altern Med. 2019;19:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Alkhateeb MA, Al‐Otaibi WR, AlGabbani Q, et al. Low‐temperature extracts of purple blossoms of basil (Ocimum basilicum L.) intervened mitochondrial translocation contributes prompted apoptosis in human breast cancer cells. Biol Res. 2021;54:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Aminian AR, Mohebbati R, Boskabady MH. The effect of Ocimum basilicum L. and its main ingredients on respiratory disorders: an experimental, Preclinical, and Clinical Review. Front Pharmacol. 2021;12:805391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Eftekhar N, Moghimi A, Boskabady MH. The effects of Ocimum basilicum extract and its constituent, rosmarinic acid on total and differential blood WBC, serum levels of NO, MDA, thiol, SOD, and CAT in ovalbumin sensitized rats. Iran J Pharm Res. 2018;17:1371. [PMC free article] [PubMed] [Google Scholar]
- 78. Eftekhar N, Moghimi A, Hossein Boskabady M, Kaveh M, Shakeri F. Ocimum basilicum affects tracheal responsiveness, lung inflammatory cells and oxidant–antioxidant biomarkers in sensitized rats. Drug Chem Toxicol. 2019;42:286‐294. [DOI] [PubMed] [Google Scholar]
- 79. Rasekh HR, Hosseinzadeh L, Mehri S, Kamli‐Nejad M, Aslani M, Tanbakoosazan F. Safety assessment of Ocimum basilicum hydroalcoholic extract in wistar rats: acute and subchronic toxicity studies. Iran J Basic Med Sci. 2012;15:645. [PMC free article] [PubMed] [Google Scholar]
- 80. Sestili P, Ismail T, Calcabrini C, et al. The potential effects of Ocimum basilicum on health: a review of pharmacological and toxicological studies. Expert Opin Drug Metab Toxicol. 2018;14:679‐692. [DOI] [PubMed] [Google Scholar]
- 81. Rodenak‐Kladniew B, Castro MA, Crespo R, Galle M, de Bravo MG. Anti‐cancer mechanisms of linalool and 1, 8‐cineole in non‐small cell lung cancer A549 cells. Heliyon. 2020;6:e05639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Iranshahi M, Rezaee R, Najafi MN, Haghbin A, Kasaian J. Cytotoxic activity of the genus Ferula (Apiaceae) and its bioactive constituents. Avicenna J Phytomed. 2018;8:296. [PMC free article] [PubMed] [Google Scholar]
- 83. EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) , Bampidis V, Azimonti G, et al. Safety and efficacy of a feed additive consisting of an essential oil from the gum resin of Ferula Assa‐foetida L.(asafoetida oil) for use in dogs and cats (FEFANA asbl). EFSA J. 2022;20:e07688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kassis E, Fulder S, Khalil K, et al. Efficacy and safety assessments of Ferula Assa‐foetida L., traditionally used in Greco‐Arab herbal medicine for enhancing male fertility, libido and erectile function. Open Complement Med J. 2009;1:1. [Google Scholar]
- 85. Esmaeili S, Hajimehdipoor H, Ramezani A, Mosaddegh M. The cytotoxic effects of Ferula persica var. persica and Ferula hezarlalehzarica against HepG2, A549, HT29, MCF7 and MDBK cell lines. Iran J Pharm Res. 2012;8:115‐119. [Google Scholar]
- 86. Soltanzad F. Evaluation of Cytotoxicity and Anti‐Tumor Effect of Chrozophora Tinctoria and Ferula Szowitsiana's Extracts on Lung Cancer A549 Cell‐Line. Tabriz University of Medical Sciences, Faculty of Pharmacy; 2012. [Google Scholar]
- 87. Wang K, Liu X, Liu Q, et al. Hederagenin potentiated cisplatin‐and paclitaxel‐mediated cytotoxicity by impairing autophagy in lung cancer cells. Cell Death Dis. 2020;11:1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Weng K‐G, Yuan Y‐L. Synthesis and evaluation of coumarin derivatives against human lung cancer cell lines. Braz J Med Biol Res. 2017;50:e6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lopez‐Gonzalez JS, Prado‐Garcia H, Aguilar‐Cazares D, Molina‐Guarneros JA, Morales‐Fuentes J, Mandoki JJ. Apoptosis and cell cycle disturbances induced by coumarin and 7‐hydroxycoumarin on human lung carcinoma cell lines. Lung Cancer. 2004;43:275‐283. [DOI] [PubMed] [Google Scholar]
- 90. Usman M, Zaki M, Khan RA, Alsalme A, Ahmad M, Tabassum S. Coumarin centered copper (II) complex with appended‐imidazole as cancer chemotherapeutic agents against lung cancer: molecular insight via DFT‐based vibrational analysis. RSC Adv. 2017;7:36056‐36071. [Google Scholar]
- 91. de Araújo RSA, Carmo JOS, de Omena Silva SL, et al. Coumarin derivatives exert anti‐lung cancer activity by inhibition of epithelial–mesenchymal transition and migration in A549 cells. Pharmaceuticals. 2022;15:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Musa MA, Badisa VL, Latinwo LM, Patterson TA, Owens MA. Coumarin‐based benzopyranone derivatives induced apoptosis in human lung (A549) cancer cells. Anticancer Res. 2012;32:4271‐4276. [PubMed] [Google Scholar]
- 93. Gupta SC, Sung B, Kim JH, Prasad S, Li S, Aggarwal BB. Multitargeting by turmeric, the golden spice: from kitchen to clinic. Mol Nutr Food Res. 2013;57:1510‐1528. [DOI] [PubMed] [Google Scholar]
- 94. Kocher A, Schiborr C, Behnam D, Frank J. The oral bioavailability of curcuminoids in healthy humans is markedly enhanced by micellar solubilisation but not further improved by simultaneous ingestion of sesamin, ferulic acid, naringenin and xanthohumol. J Funct Foods. 2015;14:183‐191. [Google Scholar]
- 95. Anna KT, Suhana M, Das S, Faizah O, Hamzaini A. Anti‐inflammatory effect of Curcuma longa (turmeric) on collagen‐induced arthritis: an anatomico‐radiological study. Clin Ter. 2011;162:201‐207. [PubMed] [Google Scholar]
- 96. Kuttan R, Bhanumathy P, Nirmala K, George M. Potential anticancer activity of turmeric (Curcuma longa). Cancer Lett. 1985;29:197‐202. [DOI] [PubMed] [Google Scholar]
- 97. Gupta Y, Katyal J, Kumar G, et al. Evaluation of antitussive activity of formulations with herbal extracts in sulphur dioxide (SO2) induced cough model in mice. Indian J Physiol Pharmacol. 2009;53:61‐66. [PubMed] [Google Scholar]
- 98. Sukandar E, Permana H, Adnyana I, et al. Clinical study of turmeric (Curcuma longa L.) and garlic (Allium sativum L.) extracts as antihyperglycemic and antihyperlipidemic agent in type‐2 diabetes‐dyslipidemia patients. Int J Pharmacol. 2010;6:456‐463. [Google Scholar]
- 99. Orellana‐Paucar AM, Serruys A‐SK, Afrikanova T, et al. Anticonvulsant activity of bisabolene sesquiterpenoids of Curcuma longa in Zebrafish and mouse seizure models. Epilepsy Behav. 2012;24:14‐22. [DOI] [PubMed] [Google Scholar]
- 100. Song E‐K, Cho H, Kim J‐S, et al. Diarylheptanoids with free radical scavenging and hepatoprotective activity in vitro from Curcuma longa . Planta Med. 2001;67:876‐877. [DOI] [PubMed] [Google Scholar]
- 101. Memarzia A, Saadat S, Behrouz S, Boskabady MH. Curcuma longa and curcumin affect respiratory and allergic disorders, experimental and clinical evidence: a comprehensive and updated review. Biofactors. 2022;48:521‐551. [DOI] [PubMed] [Google Scholar]
- 102. Chainani‐Wu N. Safety and anti‐inflammatory activity of curcumin: a component of tumeric (Curcuma longa). J Altern Complement Med. 2003;9:161‐168. [DOI] [PubMed] [Google Scholar]
- 103. Mohammad P, Nosratollah Z, Mohammad R, Abbas A, Javad R. The inhibitory effect of Curcuma longa extract on telomerase activity in A549 lung cancer cell line. Afr J Biotechnol. 2010;9:912‐919. [Google Scholar]
- 104. Moghaddam S, Barta P, Mirabolfathinejad S, et al. Curcumin inhibits COPD‐like airway inflammation and lung cancer progression in mice. Carcinogenesis. 2009;30:1949‐1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Li X, Ma S, Yang P, et al. Anticancer effects of curcumin on nude mice bearing lung cancer A549 cell subsets SP and NSP cells. Oncol Lett. 2018;16:6756‐6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Zhu JY, Yang X, Chen Y, et al. Curcumin suppresses lung cancer stem cells via inhibiting Wnt/β‐catenin and sonic hedgehog pathways. Phytother Res. 2017;31:680‐688. [DOI] [PubMed] [Google Scholar]
- 107. Wang W‐H, Chen J, Zhang B‐R, et al. Curcumin inhibits proliferation and enhances apoptosis in A549 cells by downregulating lncRNA UCA1. Pharmazie. 2018;73:402‐407. [DOI] [PubMed] [Google Scholar]
- 108. Liu W‐L, Chang J‐M, Chong I‐W, et al. Curcumin inhibits LIN‐28A through the activation of miRNA‐98 in the lung cancer cell line A549. Molecules. 2017;22:929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Sadeghzadeh H, Pilehvar‐Soltanahmadi Y, Akbarzadeh A, Dariushnejad H, Sanjarian F, Zarghami N. The effects of nanoencapsulated curcumin‐Fe3O4 on proliferation and hTERT gene expression in lung cancer cells. Anticancer Agents Med Chem. 2017;17:1363‐1373. [DOI] [PubMed] [Google Scholar]
- 110. Dong Y, Yang Y, Wei Y, et al. Facile synthetic nano‐curcumin encapsulated bio‐fabricated nanoparticles induces ROS‐mediated apoptosis and migration blocking of human lung cancer cells. Process Biochem. 2020;95:91‐98. [Google Scholar]
- 111. Pillai GR, Srivastava AS, Hassanein TI, Chauhan DP, Carrier E. Induction of apoptosis in human lung cancer cells by curcumin. Cancer Lett. 2004;208:163‐170. [DOI] [PubMed] [Google Scholar]
- 112. Jw Z, Dm J, Wang Y, et al. Integrated micro RNA and gene expression profiling reveals the crucial mi RNA s in curcumin anti‐lung cancer cell invasion. Thoracic Cancer. 2017;8:461‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Tang L, Liu J, Zhu L, et al. Curcumin inhibits growth of human NCI‐H292 lung squamous cell carcinoma cells by increasing FOXA2 expression. Front Pharmacol. 2018;9:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ting C‐Y, Wang H‐E, Yu C‐C, Liu H‐C, Liu Y‐C, Chiang I‐T. Curcumin triggers DNA damage and inhibits expression of DNA repair proteins in human lung cancer cells. Anticancer Res. 2015;35:3867‐3873. [PubMed] [Google Scholar]
- 115. Wu L, Guo L, Liang Y, Liu X, Jiang L, Wang L. Curcumin suppresses stem‐like traits of lung cancer cells via inhibiting the JAK2/STAT3 signaling pathway. Oncol Rep. 2015;34:3311‐3317. [DOI] [PubMed] [Google Scholar]
- 116. Chen P, Huang H‐P, Wang Y, et al. Curcumin overcome primary gefitinib resistance in non‐small‐cell lung cancer cells through inducing autophagy‐related cell death. J Exp Clin Cancer Res. 2019;38:1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Chong L, Zhang W, Nie Y, et al. Protective effect of curcumin on acute airway inflammation of allergic asthma in mice through Notch1–GATA3 signaling pathway. Inflammation. 2014;37:1476‐1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Liu F, Gao S, Yang Y, et al. Antitumor activity of curcumin by modulation of apoptosis and autophagy in human lung cancer A549 cells through inhibiting PI3K/Akt/mTOR pathway. Oncol Rep. 2018;39:1523‐1531. [DOI] [PubMed] [Google Scholar]
- 119. Zhang Q, Qiao H, Wu D, et al. Curcumin potentiates the galbanic acid‐induced anti‐tumor effect in non‐small cell lung cancer cells through inhibiting Akt/mTOR signaling pathway. Life Sci. 2019;239:117044. [DOI] [PubMed] [Google Scholar]
- 120. Jin H, Qiao F, Wang Y, Xu Y, Shang Y. Curcumin inhibits cell proliferation and induces apoptosis of human non‐small cell lung cancer cells through the upregulation of miR‐192‐5p and suppression of PI3K/Akt signaling pathway. Oncol Rep. 2015;34:2782‐2789. [DOI] [PubMed] [Google Scholar]
- 121. Wang A, Wang J, Zhang S, Zhang H, Xu Z, Li X. Curcumin inhibits the development of non‐small cell lung cancer by inhibiting autophagy and apoptosis. Exp Ther Med. 2017;14:5075‐5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Xu X, Chen D, Ye B, Zhong F, Chen G. Curcumin induces the apoptosis of non‐small cell lung cancer cells through a calcium signaling pathway. Int J Mol Med. 2015;35:1610‐1616. [DOI] [PubMed] [Google Scholar]
- 123. Zou JY, Su CH, Luo HH, et al. Curcumin converts Foxp3+ regulatory T cells to T helper 1 cells in patients with lung cancer. J Cell Biochem. 2018;119:1420‐1428. [DOI] [PubMed] [Google Scholar]
- 124. Wu G‐Q, Chai K‐Q, Zhu X‐M, et al. Anti‐cancer effects of curcumin on lung cancer through the inhibition of EZH2 and NOTCH1. Oncotarget. 2016;7:26535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Liao H, Wang Z, Deng Z, Ren H, Li X. Curcumin inhibits lung cancer invasion and metastasis by attenuating GLUT1/MT1‐MMP/MMP2 pathway. Int J Clin Exp Med. 2015;8:8948. [PMC free article] [PubMed] [Google Scholar]
- 126. Wang J, Wang X, Wang X, et al. Curcumin inhibits the growth via Wnt/beta‐catenin pathway in non‐small‐cell lung cancer cells. Eur Rev Med Pharmacol Sci. 2018;22:7492‐7499. [DOI] [PubMed] [Google Scholar]
- 127. Wang C, Song X, Shang M, et al. Curcumin exerts cytotoxicity dependent on reactive oxygen species accumulation in non‐small‐cell lung cancer cells. Future Oncol. 2019;15:1243‐1253. [DOI] [PubMed] [Google Scholar]
- 128. Zhou G‐Z, Li A‐F, Sun Y‐H, Sun G‐C. A novel synthetic curcumin derivative MHMM‐41 induces ROS‐mediated apoptosis and migration blocking of human lung cancer cells A549. Biomed Pharmacother. 2018;103:391‐398. [DOI] [PubMed] [Google Scholar]
- 129. Xu X, Zhu Y. Curcumin inhibits human non‐small cell lung cancer xenografts by targeting STAT3 pathway. Am J Transl Res. 2017;9:3633. [PMC free article] [PubMed] [Google Scholar]
- 130. Chen Q‐y, Zheng Y, Jiao D‐m, et al. Curcumin inhibits lung cancer cell migration and invasion through Rac1‐dependent signaling pathway. J Nutr Biochem. 2014;25:177‐185. [DOI] [PubMed] [Google Scholar]
- 131. Zhang T, Chen Y, Ge Y, Hu Y, Li M, Jin Y. Inhalation treatment of primary lung cancer using liposomal curcumin dry powder inhalers. Acta Pharm Sin B. 2018;8:440‐448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Chen H‐W, Lee J‐Y, Huang J‐Y, et al. Curcumin inhibits lung cancer cell invasion and metastasis through the tumor suppressor HLJ1. Cancer Res. 2008;68:7428‐7438. [DOI] [PubMed] [Google Scholar]
- 133. Chang H‐B, Chen B‐H. Inhibition of lung cancer cells A549 and H460 by curcuminoid extracts and nanoemulsions prepared from Curcuma longa Linnaeus. Int J Nanomedicine. 2015;10:5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Liu D, He B, Lin L, Malhotra A, Yuan N. Potential of curcumin and resveratrol as biochemical and biophysical modulators during lung cancer in rats. Drug Chem Toxicol. 2019;42:328‐334. [DOI] [PubMed] [Google Scholar]
- 135. Tsai J‐R, Liu P‐L, Chen Y‐H, et al. Curcumin inhibits non‐small cell lung cancer cells metastasis through the adiponectin/NF‐κb/MMPs signaling pathway. PLoS One. 2015;10:e0144462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Csupor‐Löffler B, Hajdú Z, Zupkó I, et al. Antiproliferative effect of flavonoids and sesquiterpenoids from Achillea millefolium sl on cultured human tumour cell lines. Phytother Res. 2009;23:672‐676. [DOI] [PubMed] [Google Scholar]
- 137. Dias MI, Barros L, Dueñas M, et al. Chemical composition of wild and commercial Achillea millefolium L. and bioactivity of the methanolic extract, infusion and decoction. Food Chem. 2013;141:4152‐4160. [DOI] [PubMed] [Google Scholar]
- 138. Cavalcanti AM, Baggio CH, Freitas CS, et al. Safety and antiulcer efficacy studies of Achillea millefolium L. after chronic treatment in Wistar rats. J Ethnopharmacol. 2006;107:277‐284. [DOI] [PubMed] [Google Scholar]
- 139. Becker LC, Bergfeld WF, Belsito DV, et al. Safety assessment of Achillea millefolium as used in cosmetics. Int J Toxicol. 2016;35:5S‐15S. [DOI] [PubMed] [Google Scholar]
- 140. Pereira JM, Peixoto V, Teixeira A, et al. Achillea millefolium L. hydroethanolic extract inhibits growth of human tumor cell lines by interfering with cell cycle and inducing apoptosis. Food Chem Toxicol. 2018;118:635‐644. [DOI] [PubMed] [Google Scholar]
- 141. Wang S, Wang Y, Wu Y, Dong M, Ni Z. Mechanism of Inhibitory Effect of Terpenoid Compound from Achillea Millefolium on Human Lung Cancer Cell Proliferation. Wolters Kluwer Health; 2021. [Google Scholar]
- 142. Nguyen T, Tran E, Ong C, et al. Kaempferol‐induced growth inhibition and apoptosis in A549 lung cancer cells is mediated by activation of MEK‐MAPK. J Cell Physiol. 2003;197:110‐121. [DOI] [PubMed] [Google Scholar]
- 143. Jo E, Park SJ, Choi YS, Jeon W‐K, Kim B‐C. Kaempferol suppresses transforming growth factor‐β1–induced epithelial‐to‐mesenchymal transition and migration of A549 lung cancer cells by inhibiting Akt1‐mediated phosphorylation of Smad3 at threonine‐179. Neoplasia. 2015;17:525‐537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Kuo W‐T, Tsai Y‐C, Wu H‐C, et al. Radiosensitization of non‐small cell lung cancer by kaempferol. Oncol Rep. 2015;34:2351‐2356. [DOI] [PubMed] [Google Scholar]
- 145. Li Y, Yu X, Wang Y, Zheng X, Chu Q. Kaempferol‐3‐O‐rutinoside, a flavone derived from Tetrastigma hemsleyanum, suppresses lung adenocarcinoma via the calcium signaling pathway. Food Funct. 2021;12:8351‐8365. [DOI] [PubMed] [Google Scholar]
- 146. Fouzder C, Mukhuty A, Kundu R. Kaempferol inhibits Nrf2 signalling pathway via downregulation of Nrf2 mRNA and induces apoptosis in NSCLC cells. Arch Biochem Biophys. 2021;697:108700. [DOI] [PubMed] [Google Scholar]
- 147. Hang M, Zhao F, Chen S‐B, Sun Q, Zhang C‐X. Kaempferol modulates the metastasis of human non‐small cell lung cancer cells by inhibiting epithelial‐mesenchymal transition. Bangladesh J Pharmacol. 2015;10:267‐270. [Google Scholar]
- 148. Zargari A. Medicinal plants. Vol 1050400844. Tehran University Publications; 1995. [Google Scholar]
- 149. Fernández J, Silván B, Entrialgo‐Cadierno R, et al. Antiproliferative and palliative activity of flavonoids in colorectal cancer. Biomed Pharmacother. 2021;143:112241. [DOI] [PubMed] [Google Scholar]
- 150. Lee J‐p, Li Y‐c, Chen H‐y, et al. Protective effects of luteolin against lipopolysaccharide‐induced acute lung injury involves inhibition of MEK/ERK and PI3K/Akt pathways in neutrophils. Acta Pharmacol Sin. 2010;31:831‐838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Askari VR, Rezaee SA, Abnous K, Iranshahi M, Boskabady MH. The influence of hydro‐ethanolic extract of Portulaca oleracea L. on Th1/Th2 balance in isolated human lymphocytes. J Ethnopharmacol. 2016;194:1112‐1121. [DOI] [PubMed] [Google Scholar]
- 152. Kaveh M, Eidi A, Nemati A, Boskabady MH. Modulation of lung inflammation and immune markers in asthmatic rats treated by Portulaca oleracea . Avicenna J Phytomed. 2017;7:409. [PMC free article] [PubMed] [Google Scholar]
- 153. Khazdair MR, Saadat S, Aslani MR, Shakeri F, Boskabady MH. Experimental and clinical studies on the effects of Portulaca oleracea L. and its constituents on respiratory, allergic, and immunologic disorders, a review. Phytother Res. 2021;35:6813‐6842. [DOI] [PubMed] [Google Scholar]
- 154. Iranshahy M, Javadi B, Iranshahi M, et al. A review of traditional uses, phytochemistry and pharmacology of Portulaca oleracea L. J Ethnopharmacol. 2017;205:158‐172. [DOI] [PubMed] [Google Scholar]
- 155. Heidari A, Jafarpour Chek Ab Z, Farzam M, Rouhani A. Human health risk assessment from consumption of (Portulaca oleracea) cultivated in nickel contaminated soil and modified with iron nanoparticles. J Res Environ Health. 2023;8:392‐405. [Google Scholar]
- 156. Al‐Sheddi ES, Farshori NN, Al‐Oqail MM, Musarrat J, Al‐Khedhairy AA, Siddiqui MA. Portulaca oleracea seed oil exerts cytotoxic effects on human liver cancer (HepG2) and human lung cancer (A‐549) cell lines. Asian Pac J Cancer Prev. 2015;16:3383‐3387. [DOI] [PubMed] [Google Scholar]
- 157. Tian J‐L, Liang X, Gao P‐Y, et al. Two new alkaloids from Portulaca oleracea and their cytotoxic activities. J Asian Nat Prod Res. 2014;16:259‐264. [DOI] [PubMed] [Google Scholar]
- 158. Zhang L, Ma X, Dong Y. Effect of genistein on apoptosis of lung adenocarcinoma a549 cells and expression of apoptosis factors. JBU off J Balk Union Oncol. 2018;23:641‐646. [PubMed] [Google Scholar]
- 159. Noori DM, Saffari M, Saydi DO, et al. Study of Antimetastatic Effect of Genistein through Inhibition of Expression of Matrix Metalloproteinase in A‐549 Cell Line. 2012.
- 160. Lian F, Li Y, Bhuiyan M, Sarkar FH. p53‐independent apoptosis induced by genistein in lung cancer cells. Nutr Cancer. 1999;33:125‐131. [DOI] [PubMed] [Google Scholar]
- 161. Yang Y, Zang A, Jia Y, et al. Genistein inhibits A549 human lung cancer cell proliferation via miR‐27a and MET signaling. Oncol Lett. 2016;12:2189‐2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Nazim UM, Park S‐Y. Genistein enhances TRAIL‐induced cancer cell death via inactivation of autophagic flux. Oncol Rep. 2015;34:2692‐2698. [DOI] [PubMed] [Google Scholar]
- 163. Zhang Z, Jin F, Lian X, et al. Genistein promotes ionizing radiation‐induced cell death by reducing cytoplasmic Bcl‐xL levels in non‐small cell lung cancer. Sci Rep. 2018;8:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Akash MSH, Rehman K, Chen S. Spice plant Allium cepa: dietary supplement for treatment of type 2 diabetes mellitus. Nutrition. 2014;30:1128‐1137. [DOI] [PubMed] [Google Scholar]
- 165. Ebhomielen J, Azeke M. e effects of sprouting on the antioxidant potentials of onions (Allium cepa L.). 2020.
- 166. Gharirvand Eskandari E, Setorki M, Doudi M. Medicinal plants with antileishmanial properties: a review study. Pharma Biomed Res. 2020;6:1‐16. [Google Scholar]
- 167. Mitra S, Das R, Emran T, et al. (2022) Diallyl Disulfide: A Bioactive Garlic Compound with Anticancer Potential. Biological Aspects of Targeted Drug Discovery: Development of Novel Targets and/or Chemotherapies, and Drug Repurposing. 2023;16648714:128. [Google Scholar]
- 168. Albishi T, John JA, Al‐Khalifa AS, Shahidi F. Antioxidant, anti‐inflammatory and DNA scission inhibitory activities of phenolic compounds in selected onion and potato varieties. J Funct Foods. 2013;5:930‐939. [Google Scholar]
- 169. Benkeblia N. Antimicrobial activity of essential oil extracts of various onions (Allium cepa) and garlic (Allium sativum). LWT‐Food Sci Technol. 2004;37:263‐268. [Google Scholar]
- 170. Lanzotti V. The analysis of onion and garlic. J Chromatogr A. 2006;1112:3‐22. [DOI] [PubMed] [Google Scholar]
- 171. Elberry AA, Mufti S, Al‐Maghrabi J, et al. Immunomodulatory effect of red onion (Allium cepa Linn) scale extract on experimentally induced atypical prostatic hyperplasia in Wistar rats. Mediat Inflamm. 2014;2014:640746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Benmalek Y, Yahia OA, Belkebir A, Fardeau M‐L. Anti‐microbial and anti‐oxidant activities of illicium verum, crataegus oxyacantha ssp monogyna and Allium cepa red and white varieties. Bioengineered. 2013;4:244‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Shri R, Bora KS. Neuroprotective effect of methanolic extracts of Allium cepa on ischemia and reperfusion‐induced cerebral injury. Fitoterapia. 2008;79:86‐96. [DOI] [PubMed] [Google Scholar]
- 174. Nasri S, Anoush M, Khatami N. Evaluation of analgesic and anti‐inflammatory effects of fresh onion juice in experimental animals. Afr J Pharm Pharmacol. 2012;6:1679‐1684. [Google Scholar]
- 175. El‐Aasr M, Fujiwara Y, Takeya M, et al. Onionin a from Allium cepa inhibits macrophage activation. J Nat Prod. 2010;73:1306‐1308. [DOI] [PubMed] [Google Scholar]
- 176. Galmarini CR, Goldman IL, Havey MJ. Genetic analyses of correlated solids, flavor, and health‐enhancing traits in onion (Allium cepa L.). Mol Gen Genomics. 2001;265:543‐551. [DOI] [PubMed] [Google Scholar]
- 177. Takahashi M, Shibamoto T. Chemical compositions and antioxidant/anti‐inflammatory activities of steam distillate from freeze‐dried onion (Allium cepa L.) sprout. J Agric Food Chem. 2008;56:10462‐10467. [DOI] [PubMed] [Google Scholar]
- 178. Amrevuawho MO, Akinyemi AA, Oyewusi A, Bankole O, Ezeri GNO. Effects of onion (allium cepa) and chloramphenicol on haematological parameters, histopathology and survival of catfish clarias gariepinus (Burchell, 1822) sub‐adult infected with pseudomonas aeruginosa. Vom J Vet Sci. 2016;11:1‐12. [Google Scholar]
- 179. Yang J‐H, Hsia T‐C, Kuo H‐M, et al. Inhibition of lung cancer cell growth by quercetin glucuronides via G2/M arrest and induction of apoptosis. Drug Metab Dispos. 2006;34:296‐304. [DOI] [PubMed] [Google Scholar]
- 180. Nguyen T, Tran E, Nguyen T, Do P, Huynh T, Huynh H. The role of activated MEK‐ERK pathway in quercetin‐induced growth inhibition and apoptosis in A549 lung cancer cells. Carcinogenesis. 2004;25:647‐659. [DOI] [PubMed] [Google Scholar]
- 181. Rotunno M, Hu N, Su H, et al. A gene expression signature from peripheral whole blood for stage I lung adenocarcinoma. Cancer Prev Res. 2011;4:1599‐1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Helen A, Krishnakumar K, Vijayammal P, Augusti K. Antioxidant effect of onion oil (Allium cepa. Linn) on the damages induced by nicotine in rats as compared to alpha‐tocopherol. Toxicol Lett. 2000;116:61‐68. [DOI] [PubMed] [Google Scholar]
- 183. Magesh V, DurgaBhavani K, Senthilnathan P, Rajendran P, Sakthisekaran D. In vivo protective effect of crocetin on benzo (a) pyrene‐induced lung cancer in Swiss albino mice. Phytother Res. 2009;23:533‐539. [DOI] [PubMed] [Google Scholar]
- 184. Lee M‐A, Choi J‐H, Choi Y‐S, et al. The antioxidative properties of mustard leaf (Brassica juncea) kimchi extracts on refrigerated raw ground pork meat against lipid oxidation. Meat Sci. 2010;84:498‐504. [DOI] [PubMed] [Google Scholar]
- 185. Tiku AB, Abraham SK, Kale RK. Protective effect of the cruciferous vegetable mustard leaf (Brassica campestris) against in vivo chromosomal damage and oxidative stress induced by γ‐radiation and genotoxic chemicals. Environ Mol Mutagen. 2008;49:335‐342. [DOI] [PubMed] [Google Scholar]
- 186. Yokozawa T, Kim HY, Cho EJ, Yamabe N, Choi JS. Protective effects of mustard leaf (Brassica juncea) against diabetic oxidative stress. J Nutr Sci Vitaminol. 2003;49:87‐93. [DOI] [PubMed] [Google Scholar]
- 187. Yokozawa T, Kim HY, Cho EJ, Choi JS, Chung HY. Antioxidant effects of isorhamnetin 3, 7‐di‐O‐β‐D‐glucopyranoside isolated from mustard leaf (Brassica juncea) in rats with streptozotocin‐induced diabetes. J Agric Food Chem. 2002;50:5490‐5495. [DOI] [PubMed] [Google Scholar]
- 188. Young Kim H, Yokozawa T, Ju Cho E, Sik Cheigh H, Sue Choi J, Young CH. In vitro and in vivo antioxidant effects of mustard leaf (Brassica juncea). Phytother Res. 2003;17:465‐471. [DOI] [PubMed] [Google Scholar]
- 189. Kim HY, Yokozawa T, Cho EJ. Mustard leaf suppresses nitric oxide synthesis by mouse macrophages. J Nutr Sci Vitaminol. 2005;51:200‐203. [DOI] [PubMed] [Google Scholar]
- 190. Thakur AK, Chatterjee SS, Kumar V. Antidepressant‐like effects of Brassica juncea L. leaves in diabetic rodents. Indian J Exp Biol. 2014;52(6):613‐622. [PubMed] [Google Scholar]
- 191. Kim H‐K, Bang C‐S, Choi Y‐M, Lee J‐S. Antioxidant and antiproliferative activities of methanol extracts from leafy vegetables consumed in Korea. Food Sci Biotechnol. 2007;16:802‐806. [Google Scholar]
- 192. Bae W‐Y, Kim H‐Y, Choi K‐S, et al. Investigation of Brassica juncea, Forsythia suspensa, and Inula britannica: phytochemical properties, antiviral effects, and safety. BMC Complement Altern Med. 2019;19:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Manivasagan V, Subratha S, Daricca V, Saranya K, Babu NR. In‐Vitro Evaluation of Anti‐Cancer Activity of Actinidia Deliciosa And Brassica Juncea on Human Lung Carcinoma Cells (A549).
- 194. Hu X, Geetha RV, Surapaneni KM, et al. Lung cancer induced by benzo (a) pyrene: ChemoProtective effect of sinapic acid in swiss albino mice. Saudi J Biol Sci. 2021;28:7125‐7133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Lin C‐Y, Tam Ly M, Yang SH, et al. Tanshinone IIA shows higher antiproliferative activities than Sinapic acid in 4 cancer cell lines and simultaneously induces apoptosis and necroptosis in human lung cancer A549 cells. Nat Prod Commun. 2021;16:1934578X211050521. [Google Scholar]
- 196. Hekmatpou D, Mehrabi F, Rahzani K, Aminiyan A. The effect of aloe vera clinical trials on prevention and healing of skin wound: a systematic review. Iranian J Med Sci. 2019;44:1. [PMC free article] [PubMed] [Google Scholar]
- 197. Radha MH, Laxmipriya NP. Evaluation of biological properties and clinical effectiveness of aloe vera: a systematic review. J Tradit Complement Med. 2015;5:21‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Zagórska‐Dziok M, Furman‐Toczek D, Dudra‐Jastrzębska M, Zygo K, Stanisławek A, Kapka‐Skrzypczak L. Evaluation of clinical effectiveness of aloe vera–a review. J Pre‐Clin Clin Res. 2017;11:86. [Google Scholar]
- 199. Wu J, Zhang Y, Lv Z, Yu P, Shi W. Safety evaluation of aloe vera soft capsule in acute, subacute toxicity and genotoxicity study. PLoS One. 2021;16:e0249356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Lee H‐Z, Yang W‐H, Hour M‐J, et al. Photodynamic activity of aloe‐emodin induces resensitization of lung cancer cells to anoikis. Eur J Pharmacol. 2010;648:50‐58. [DOI] [PubMed] [Google Scholar]
- 201. Shen F, Ge C, Yuan P. Aloe‐emodin induces autophagy and apoptotic cell death in non‐small cell lung cancer cells via Akt/mTOR and MAPK signaling. Eur J Pharmacol. 2020;886:173550. [DOI] [PubMed] [Google Scholar]
- 202. Lee HZ. Protein kinase C involvement in aloe‐emodin‐and emodin‐induced apoptosis in lung carcinoma cell. Br J Pharmacol. 2001;134:1093‐1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Lee H‐Z, Hsu S‐L, Liu M‐C, Wu C‐H. Effects and mechanisms of aloe‐emodin on cell death in human lung squamous cell carcinoma. Eur J Pharmacol. 2001;431:287‐295. [DOI] [PubMed] [Google Scholar]
- 204. Chang W‐T, You B‐J, Yang W‐H, Wu C‐Y, Bau D‐T, Lee H‐Z. Protein kinase C delta‐mediated cytoskeleton remodeling is involved in aloe‐emodin‐induced photokilling of human lung cancer cells. Anticancer Res. 2012;32:3707‐3713. [PubMed] [Google Scholar]
- 205. Lee HZ, Wu CH, Chang SP. Release of nucleophosmin from the nucleus: involvement in aloe‐emodin–induced human lung non small carcinoma cell apoptosis. Int J Cancer. 2005;113:971‐976. [DOI] [PubMed] [Google Scholar]
- 206. Wu Y‐Y, Zhang J‐H, Gao J‐H, Li Y‐S. Aloe‐emodin (AE) nanoparticles suppresses proliferation and induces apoptosis in human lung squamous carcinoma via ROS generation in vitro and in vivo. Biochem Biophys Res Commun. 2017;490:601‐607. [DOI] [PubMed] [Google Scholar]
- 207. Patel S, Sarwat M, Khan TH. Mechanism behind the anti‐tumour potential of saffron (Crocus sativus L.): the molecular perspective. Crit Rev Oncol Hematol. 2017;115:27‐35. [DOI] [PubMed] [Google Scholar]
- 208. Shehzad A, Lee YS. Molecular mechanisms of curcumin action: signal transduction. Biofactors. 2013;39:27‐36. [DOI] [PubMed] [Google Scholar]
- 209. Teplicki E, Ma Q, Castillo DE, et al. The effects of aloe vera on wound healing in cell proliferation, migration, and viability. Wounds. 2018;30:263‐268. [PubMed] [Google Scholar]
- 210. Votto AP, Domingues BS, de Souza MM, et al. Toxicity mechanisms of onion (Allium cepa) extracts and compounds in multidrug resistant erythroleukemic cell line. Biol Res. 2010;43:429‐437. [PubMed] [Google Scholar]
- 211. Kwon T, Chandimali N, Huynh DL, et al. BRM270 inhibits cancer stem cell maintenance via microRNA regulation in chemoresistant A549 lung adenocarcinoma cells. Cell Death Dis. 2018;9:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196:395‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Kai F, Drain AP, Weaver VM. The extracellular matrix modulates the metastatic journey. Dev Cell. 2019;49:332‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Lee H, Chang K‐W, Yang H‐Y, Lin P‐W, Chen S‐U, Huang Y‐L. MT1‐MMP regulates MMP‐2 expression and angiogenesis‐related functions in human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2013;437:232‐238. [DOI] [PubMed] [Google Scholar]
- 215. Huang H. Matrix metalloproteinase‐9 (MMP‐9) as a cancer biomarker and MMP‐9 biosensors: recent advances. Sensors. 2018;18:3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Hewlings SJ, Kalman DS. Curcumin: a review of its effects on human health. Foods. 2017;6:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Pai JT, Hsu CY, Hsieh YS, Tsai TY, Hua KT, Weng MS. Suppressing migration and invasion of H1299 lung cancer cells by honokiol through disrupting expression of an HDAC6‐mediated matrix metalloproteinase 9. Food Sci Nutr. 2020;8:1534‐1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Sazuka M, Imazawa H, Shoji Y, Mita T, Hara Y, Isemura M. Inhibition of collagenases from mouse lung carcinoma cells by green tea catechins and black tea theaflavins. Biosci Biotechnol Biochem. 1997;61:1504‐1506. [DOI] [PubMed] [Google Scholar]
- 219. Chakrawarti L, Agrawal R, Dang S, Gupta S, Gabrani R. Therapeutic effects of EGCG: a patent review. Expert Opin Ther Pat. 2016;26:907‐916. [DOI] [PubMed] [Google Scholar]
- 220. Deng Y‐T, Lin J‐K. EGCG inhibits the invasion of highly invasive CL1‐5 lung cancer cells through suppressing MMP‐2 expression via JNK signaling and induces G2/M arrest. J Agric Food Chem. 2011;59:13318‐13327. [DOI] [PubMed] [Google Scholar]
- 221. Shi J, Liu F, Zhang W, Liu X, Lin B, Tang X. Epigallocatechin‐3‐gallate inhibits nicotine‐induced migration and invasion by the suppression of angiogenesis and epithelial‐mesenchymal transition in non‐small cell lung cancer cells. Oncol Rep. 2015;33:2972‐2980. [DOI] [PubMed] [Google Scholar]
- 222. Nieman KM, Kenny HA, Penicka CV, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498‐1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Boroughs LK, DeBerardinis RJ. Metabolic pathways promoting cancer cell survival and growth. Nat Cell Biol. 2015;17:351‐359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Ren K, Mo Z‐C, Liu X, et al. TGF‐β Down‐regulates apolipoprotein M expression through the TAK‐1‐JNK‐c‐Jun pathway in HepG2 cells. Lipids. 2017;52:109‐117. [DOI] [PubMed] [Google Scholar]
- 225. Ruan J, Qi Z, Shen L, et al. Crosstalk between JNK and NF‐κB signaling pathways via HSP27 phosphorylation in HepG2 cells. Biochem Biophys Res Commun. 2015;456:122‐128. [DOI] [PubMed] [Google Scholar]
- 226. Yin H, Yang X, Gu W, et al. HMGB1‐mediated autophagy attenuates gemcitabine‐induced apoptosis in bladder cancer cells involving JNK and ERK activation. Oncotarget. 2017;8:71642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Dvorak HF, Sioussat TM, Brown LF, et al. Distribution of vascular permeability factor (vascular endothelial growth factor) in tumors: concentration in tumor blood vessels. J Exp Med. 1991;174:1275‐1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992;359:845‐848. [DOI] [PubMed] [Google Scholar]
- 229. Hassan N, Rutsch N, Győrffy B, Espinoza‐Sánchez NA, Götte M. SETD3 acts as a prognostic marker in breast cancer patients and modulates the viability and invasion of breast cancer cells. Sci Rep. 2020;10:1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Gu B, Wang J, Song Y, Wang Q, Wu Q. microRNA‐383 regulates cell viability and apoptosis by mediating Wnt/β‐catenin signaling pathway in non–small cell lung cancer. J Cell Biochem. 2019;120:7918‐7926. [DOI] [PubMed] [Google Scholar]
- 231. Zhang X, Sai B, Wang F, et al. Hypoxic BMSC‐derived exosomal miRNAs promote metastasis of lung cancer cells via STAT3‐induced EMT. Mol Cancer. 2019;18:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Wang W, Chen M, Xu H, Lv D, Zhou S, Yang H. USP46 inhibits cell proliferation in lung cancer through PHLPP1/AKT pathway. Biomed Res Int. 2020;2020:2509529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Jabir AA‐H, Kadhim HS, Alzubaidy AA. Effects of fenugreek on lung cancer/in vitro study. Chemotherapy. 2015;3:4. [Google Scholar]
- 234. Gerl R, Vaux DL. Apoptosis in the development and treatment of cancer. Carcinogenesis. 2005;26:263‐270. [DOI] [PubMed] [Google Scholar]
- 235. Vinod BS, Maliekal TT, Anto RJ. Phytochemicals as chemosensitizers: from molecular mechanism to clinical significance. Antioxid Redox Signal. 2013;18:1307‐1348. [DOI] [PubMed] [Google Scholar]
- 236. Kathawala RJ, Gupta P, Ashby CR Jr, Chen Z‐S. The modulation of ABC transporter‐mediated multidrug resistance in cancer: a review of the past decade. Drug Resist Updat. 2015;18:1‐17. [DOI] [PubMed] [Google Scholar]
- 237. Li W, Zhang H, Assaraf YG, et al. Overcoming ABC transporter‐mediated multidrug resistance: molecular mechanisms and novel therapeutic drug strategies. Drug Resist Updat. 2016;27:14‐29. [DOI] [PubMed] [Google Scholar]
- 238. Varma MV, Ashokraj Y, Dey CS, Panchagnula R. P‐glycoprotein inhibitors and their screening: a perspective from bioavailability enhancement. Pharmacol Res. 2003;48:347‐359. [DOI] [PubMed] [Google Scholar]
- 239. Bharti AC, Vishnoi K, Singh SM, Aggarwal BB. Pathways linked to cancer chemoresistance and their targeting by nutraceuticals. Role of Nutraceuticals in Cancer. Elsevier; 2018:1‐30. [Google Scholar]
- 240. Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod. 2016;79:629‐661. [DOI] [PubMed] [Google Scholar]
- 241. Wang H, Oo Khor T, Shu L, et al. Plants vs. cancer: a review on natural phytochemicals in preventing and treating cancers and their druggability. Anticancer Agents Med Chem. 2012;12:1281‐1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Khan T, Ali M, Khan A, et al. Anticancer plants: a review of the active phytochemicals, applications in animal models, and regulatory aspects. Biomol Ther. 2019;10:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Shukla S, Mehta A. Anticancer potential of medicinal plants and their phytochemicals: a review. Rev Bras Bot. 2015;38:199‐210. [Google Scholar]
- 244. Hamed AR, Abdel‐Azim NS, Shams KA, Hammouda FM. Targeting multidrug resistance in cancer by natural chemosensitizers. Bullet Nat Res Centre. 2019;43:1‐14. [Google Scholar]
- 245. Gupta SC, Kannappan R, Reuter S, Kim JH, Aggarwal BB. Chemosensitization of tumors by resveratrol. Ann N Y Acad Sci. 2011;1215:150‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Jacquemin G, Shirley S, Micheau O. Combining naturally occurring polyphenols with TNF‐related apoptosis‐inducing ligand: a promising approach to kill resistant cancer cells? Cell Mol Life Sci. 2010;67:3115‐3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Wu J, He X, Xiong Z, et al. Bruceine H mediates EGFR‐TKI drug persistence in NSCLC by Notch3‐dependent β‐catenin activating FOXO3a signaling. Front Oncol. 2022;12:855603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Cai P, Sheng G, Jiang S, et al. Comparative proteomics analysis reveals the reversal effect of cryptotanshinone on gefitinib‐resistant cells in epidermal growth factor receptor‐mutant lung cancer. Front Pharmacol. 2022;13:837055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable (This is a review article).


