Abstract
Study Design
Retrospective Cohort.
Objective
The aim of this study was to develop a clinical tool to pre-operatively risk-stratify patients undergoing spine surgery based on their likelihood to have high postoperative analgesic requirements.
Methods
A total of 1199 consecutive patients undergoing elective spine surgery over a 2-year period at a single center were included. Patients not requiring inpatient admission, those who received epidural analgesia, those who had two surgeries at separate sites under one anesthesia event, and those with a length of stay greater than 10 days were excluded. The remaining 860 patients were divided into a derivation and validation cohort. Pre-operative factors were collected by review of the electronic medical record. Total postoperative inpatient opioid intake requirements were converted into morphine milligram equivalents to standardize postoperative analgesic requirements.
Results
The postoperative analgesic intake needs (PAIN) score was developed after the following predictor variables were identified: age, race, history of depression/anxiety, smoking status, active pre-operative benzodiazepine use and pre-operative opioid use, and surgical type. Patients were risk-stratified based on their score with the high-risk group being more likely to have high opioid consumption postoperatively compared to the moderate and low-risk groups in both the derivation and validation cohorts.
Conclusion
The PAIN Score is a pre-operative clinical tool for patients undergoing spine surgery to risk stratify them based on their likelihood for high analgesic requirements. The information can be used to individualize a multi-modal analgesic regimen rather than utilizing a “one-size fits all” approach.
Keywords: postoperative pain, opioid intake, multi-modal analgesia, spine surgery
Introduction
The management of postoperative pain is a primary focus of both the surgeon and the patient following surgical intervention. Poorly controlled post-operative pain has been shown to be associated with increased length of stay, delayed postoperative mobilization, higher rates of perioperative complications such as postoperative delirium and thromboembolic events, and decreased overall patient satisfaction.1,2 On the other hand, over-treatment with analgesics may in itself lead to complications including but not limited to delirium, ileus, urinary retention, respiratory depression, and drug dependence. 3 Traditionally, opioids have been the powerhouse for the treatment of postoperative pain. However, a better understanding of the complications associated with opioids in light of the current opioid epidemic has led to a shift toward a focus on multi-modal analgesia.1,4 Despite this shift, opioids remain a mainstay in the treatment of postoperative pain, particularly in the United States and in surgical fields associated with painful procedures. 5
Spine surgery certainly falls into this category due to the extent of muscle dissection, bony work, spinal realignment, and placement of instrumentation that is often involved. Multi-modal analgesia in spine surgery frequently involves a combination of acetaminophen, muscle relaxants, neuromodulating agents, and opioids. 1 The use of NSAIDs, although effective, is unfortunately often limited given the associated risk of bleeding and risk of pseudoarthrosis in patients undergoing a bony fusion. Therefore, more invasive and often more expensive adjuncts are alternatively used, including epidural analgesia, ketamine, intravenous (IV) lidocaine, liposomal bupivacaine, and interfascial blocks.6-8 Determining which patients may benefit from these interventions while avoiding them in those where the benefits are minimal and do not necessarily justify the risks and costs is often a difficult decision to make. As such, the aim of this study was to develop a clinically useful tool to help identify patients who may experience a greater degree of postoperative pain and therefore require a higher degree of postoperative analgesia, with the hope of providing clinicians with information that would be useful in personalizing a multi-modal pain regimen following surgical intervention. We hypothesized that the PAIN score will allow us to risk-stratify patients according to their predicted need for postoperative narcotic medication, in the context of spine surgery.
Methods
Patient Selection
After obtaining Institutional Review Board approval (IRB #STU-2019-0519), patient data was collected retrospectively by reviewing the electronic medical records of all adult patients undergoing elective spine surgery at a single institution between August 2015 and August 2017. Patients who did not require inpatient admission, those who undergo a catheter placement for epidural analgesia, those who had two surgeries at separate sites under one anesthesia event, and those who had a length of stay greater than 10 days were excluded for analysis. We excluded patients who had a protracted length of stay after surgery as this was due to pending insurance approval for rehabilitation purposes, or because of an unforeseen complication that created a deviation from the standard postoperative course and would falsely skew our predictive model.
Study Variables
Pre-operative factors including patient demographics (age, gender, and race), social history (alcohol use, smoking status, and recreational drug use), psychiatric history (depression, anxiety, obsessive compulsive disorder, and post-traumatic stress disorder), and pre-operative opioid and benzodiazepine use were collected. Age was divided into three groups: 18–44, 45–64, and 65 and older. Race was divided into White/Caucasian, Black/African American, Hispanic, and Other. Current smoking status, alcohol use, and recreational drug use were each divided into two groups: yes or no. Pre-operative opioid use was divided into three categories: Controlled Substance Act (CSA) Schedule II (hydrocodone, oxycodone, morphine, hydromorphone, fentanyl), CSA Schedule III or IV (Tylenol-codeine, tramadol), and none. The division of pre-operative opioid users into separate groups was done to better characterize patients by the potency and addictive potential of the medication they were on at the time of surgery. If a patient was on more than one opioid medication pre-operatively, the patient was placed into the category of the more addictive medication. Pre-operative benzodiazepine use was determined by an active benzodiazepine prescription at the time of surgery (yes or no). Regarding surgical type, patients were initially divided into five surgical categories: Anterior Cervical, Posterior Cervical, Pure Thoracic, Thoraco-Lumbar: Short segment, and Thoraco-Lumbar: Long segment. A surgery involving three segments or less was considered short segment, while four segments or more was considered long segment. Surgical categories were then further subdivided into three groups: a low-risk group consisting of anterior cervical, a moderate-risk group consisting of posterior cervical, thoracic, and short segment thoraco-lumbar surgery, and a high-risk group consisting of long segment thoraco-lumbar surgery. The primary outcome of interest was postoperative inpatient opioid consumption. Total opioid use while inpatient was collected from oral (PO), intravenous (IV), and pain-controlled analgesia (PCA) routes, and then converted into morphine milligram equivalents (MME) to standardize the opioid consumption as previously reported in the literature.9,10 Opioid intake pre-operatively, intra-operatively, or while the patient was in the post-anesthesia care unit was not included. The total MME was then divided by length of stay (LOS) to calculate an average opioid consumption per hospital day.
Statistical Analysis
SPSS software was used to divide the total sample size into two halves (v26 SPSS Inc, Chicago, IL). Stepwise regression analysis using SAS version 9.4 was then used to determine the variables in the derivation sample predictive of average MME use per day. Entry and stay categories were set at .10. Possible predictor variables included: age categories, gender, indicator variables for White/Caucasian, Black/African American, and Hispanic, alcohol use, current smoker, recreational drug user, diagnosis of a psychiatric disorder, active opioid use at the time of surgery (none, schedule III/IV or schedule II), active benzodiazepine use at the time of surgery, and each surgical risk category. The results of the stepwise regression analysis were then utilized in a multiple linear regression to include levels of the predictor variables in order to develop the overall risk score. Where there were three levels for a predictor variable, the two highest were included in the regression analysis and the lowest was assigned a score of 0. The resulting regression coefficients were divided by a common factor based on the lowest value in order to arrive at the score for all levels of the predictor variables. Patients were then assigned an overall PAIN score according to the model, and further stratified into low-risk, moderate-risk, and high-risk groups. The distribution of scores into 4 MME categories (0 to 49.9, 50.0 to 99.9, 100.0 to 149.9 and 150 and greater) was assessed using chi-square. The MME quantity was stratified in a way that would maximize the predictive power of our model, while at the same time keeping the MME intervals simple. In addition, odds ratios (OR) for a high-risk score for the MME category were determined for two groups: MME less than 100 vs MME of 100 or more for the derivation and for the validation sample. 95% confidence intervals were determined and the OR’s were compared using Cochran–Mantel–Haenszel technique.
Results
A total of 1199 patients were reviewed, and 860 patients were included for analysis after removing patients who met the exclusion criteria. Using the remaining 860 patients, the total sample was divided into a derivation group and a validation group as previously described in the methods section, with 432 patients and 428 patients in each group, respectively. The patient characteristics and outcomes for the total sample, derivation group, and validation group are shown in Table 1. There were no statistically significant differences between the baseline characteristics of the validation and derivation groups.
Table 1.
Summary of Demographic, Operative Characteristics, and Postoperative Outcomes in the Derivation and Validation Cohorts.
| Overall | Derivation sample | Validation sample | P Value | |
|---|---|---|---|---|
| N | 860 | 432 | 428 | |
| Age | 61.2 (SD = 13.9) | 61.8 (SD = 14.0) | 60.6 (SD = 13.8) | .232 |
| Age <46 | 119 | 57 (13.2%) | 62 (14.5%) | .583 |
| Age 46 to 64 | 333 | 164 (38.0%) | 169 (39.5%) | .647 |
| Age 65+ | 408 | 211 (48.8%) | 197 (46.0%) | .409 |
| Male gender | 432 (50.2%) | 219 (50.7%) | 213 (49.8%) | .786 |
| Race—African American | 72 (8.4%) | 36 (8.3%) | 36 (8.4%) | .967 |
| Hispanic | 50 (5.8%) | 23 (5.3%) | 27 (6.3%) | .537 |
| White | 680 (79.1%) | 347 (80.3%) | 333 (77.8%) | .364 |
| Other | 18 (2.1%) | 7 (1.6%) | 11 (2.6%) | .306 |
| Unknown | 40 (4.7%) | 19 (4.4%) | 21 (4.9%) | .728 |
| Active alcohol use | 438 (50.9%) | 228 (52.8%) | 210 (49.1%) | .276 |
| Current smoker | 64 (7.4%) | 32 (7.4%) | 32 (7.5%) | .937 |
| Recreational drug use | 14 (1.6%) | 7 (1.6%) | 7 (1.6%) | .986 |
| Affective disorder | 299 (34.8%) | 147 (34.0%) | 152 (35.5%) | .647 |
| Anxiety | 171 (19.9%) | 87 (20.1%) | 84 (19.6%) | .851 |
| Depression | 236 (27.4%) | 112 (25.9%) | 124 (29.0%) | .317 |
| PreOp narcotics—None | 462 (53.7%) | 230 (53.2%) | 232 (54.2%) | .427 |
| CSA sched 3 and 4 | 136 (15.8%) | 63 (14.6%) | 73 (17.1%) | .316 |
| CSA sched 2 | 262 (30.5%) | 139 (32.2%) | 123 (28.7%) | |
| PreOp benzodiazepine | 159 (18.5%) | 77 (17.8%) | 82 (19.2%) | .614 |
| Surgery—Anterior cervical | 175 (20.4%) | 85 (19.7%) | 90 (21.0%) | .636 |
| Lumbar short | 516 (60.0%) | 257 (59.9%) | 259 (60.5%) | .857 |
| Posterior cervical | 97 (11.3%) | 53 (12.3%) | 44 (10.3%) | .356 |
| Thoracic | 25 (2.9%) | 14 (3.2%) | 11 (2.6%) | .6 |
| Lumbar long | 47 (5.5%) | 23 (5.3%) | 24 (5.6%) | .846 |
| Surgery risk—low | 175 (20.4%) | 85 (19.7%) | 90 (21.0%) | .860 |
| Moderate | 638 (74.2%) | 324 (75.0%) | 314 (73.4%) | .592 |
| High | 47 (5.5%) | 23 (5.3%) | 24 (5.6%) | .846 |
| Renew narc—3 mos | 251 (29.2%) | 113 (26.2%) | 138 (32.3%) | .047 |
| Renew narc—12 mos | 89 (10.4%) | 44 (10.2%) | 45 (10.5%) | .865 |
| ED visits—1 or more | 37 (4.3%) | 18 (4.2%) | 19 (4.4%) | .844 |
| Readmissions | 20 (2.3%) | 10 (2.3%) | 10 (2.3%) | .983 |
| Length of stay (days) | 2.61 (SD = 2.2) | 2.53 (SD = 2.1) | 2.70 (SD = 2.2) | .225 |
| Morphine per day—MME | 73.2 (SD = 56.3) | 72.1 (SD = 55.6) | 74.3 (SD = 57.1) | .567 |
| 0–49.99 | 336 (39.1%) | 173 (40.1%) | 163 (38.1%) | .548 |
| 50–99.99 | 324 (37.7%) | 162 (37.5%) | 162 (37.9%) | .904 |
| 100–149.99 | 117 (13.6%) | 55 (12.7%) | 62 (14.5%) | .441 |
| 150 or greater | 83 (9.7%) | 42 (9.7%) | 41 (9.6%) | .96 |
The stepwise regression identified the following predictor variables: age categories, Caucasian/White, current smoker, diagnosis of a psychiatric disorder, pre-operative benzodiazepine use, pre-operative narcotic use, and surgical risk categories. The results of additional regression analyses as described in the methods, summarized in Table 2, were then utilized to develop the model shown in Table 3. A PAIN score of 0 to 5 was considered low risk, 6 to 10 as moderate risk, and 11 or more as high risk. Of the 432 patients in the derivation sample, 152 were in the low-risk group (35.2%), 206 were in the moderate-risk group (47.7%), and 74 were in the high-risk group (17.1%). Of the 428 patients in the validation sample, 160 fell into the low-risk group (37.4%), 190 were in the moderate-risk group (44.4%), and 78 were in the high-risk group (18.2%).
Table 2.
Regression Analysis.
| Derivation Group only Coefficients of P ossible P redictors of MME I ntake per day with S moking | ||||||
| The REG procedure | ||||||
| Model: MODEL1 | ||||||
| Dependent variable: mor_LOS morphine equivalents per day | ||||||
| Number of observations read | 432 | |||||
| Number of observations used | 432 | |||||
| Analysis of variance | ||||||
| Source | DF | Sum of squares | Mean square | F value | Pr > F | |
| Model | 10 | 289 463 | 28 946 | 11.69 | <.0001 | |
| Error | 421 | 1 042 515 | 2476.28173 | |||
| Corrected total | 431 | 1 331 977 | ||||
| Root MSE | 49.76225 | R-square | .2173 | |||
| Dependent mean | 72.11400 | Adj R-Sq | .1987 | |||
| Coeff var | 69.00498 | |||||
| Parameter estimates | ||||||
| Variable | Label | DF | Parameter estimate | Standard error | t Value | Pr > |t| |
| Intercept | Intercept | 1 | 15.64459 | 8.77626 | 1.78 | .0754 |
| young | 1 | 64.00477 | 7.66357 | 8.35 | <.0001 | |
| mature | 1 | 23.87111 | 5.47339 | 4.36 | <.0001 | |
| Cau | 1 | 17.37471 | 6.22593 | 2.79 | .0055 | |
| smoke | Current smoker | 1 | 21.86550 | 9.20184 | 2.38 | .0179 |
| affect | Presence of an affective disorder | 1 | 6.39418 | 5.24709 | 1.22 | .2237 |
| schedIII | 1 | 16.14714 | 7.12032 | 2.27 | .0239 | |
| schedII | 1 | 28.98421 | 5.42069 | 5.35 | <.0001 | |
| prebenzo | Pre-op active benzodiazepine use | 1 | 8.71031 | 6.45741 | 1.35 | .1781 |
| modrisk | 1 | 8.72460 | 6.34483 | 1.38 | .1698 | |
| highrisk | 1 | 26.93258 | 11.93640 | 2.26 | .0246 | |
Table 3.
Table Showcasing PAIN Score Categories and Risk Stratification.
| PAIN score | Risk Stratification Low risk = 0–5 Moderate risk = 6–10 High risk = 11+ |
|
| Age Less than or equal to 44 45 to 64 Greater than or equal to 65 |
8 3 0 |
|
| Race White/Caucasian Other |
2 0 |
|
| History of depression and/or anxiety Yes No |
1 0 |
|
| Active pre-operative benzodiazepine use Yes No |
1 0 |
|
| Active pre-operative opioid use CSA schedule II narcotic CSA schedule III/IV narcotic None |
4 2 0 |
|
| Smoking history Current Former or never |
3 0 |
|
| Surgery category Lumbar—long Segment Posterior cervical, thoracic, or lumbar—short Segment Anterior cervical |
3 1 0 |
|
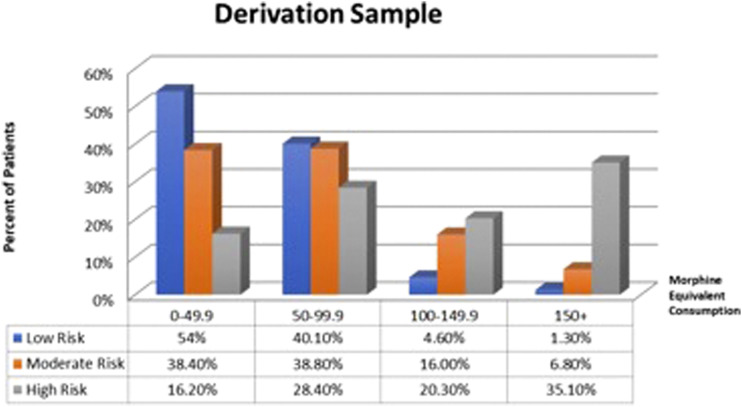
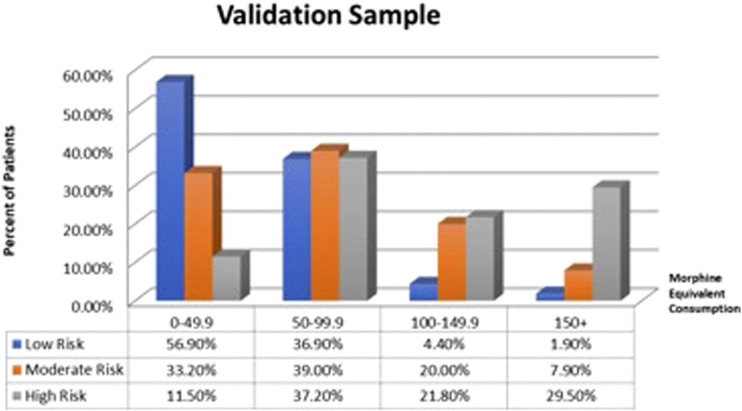
The distribution of scores in MME categories (0 to 49.9, 50 to 99.9, 100 to 149.9, and 150 and over) was assessed using Chi-square for each of the risk stratification groups, with the results represented Figures 1 and 2. In the derivation and validation samples, respectively, 35.1% and 29.5% of patients in the high-risk group fell into the highest intake category (150+ MME/day), compared to only 1.3% and 1.9% of patients in the low-risk group, while 54.0% and 56.9% of patients in the low-risk group fell into the lowest intake category (0-49.9 MME/day) compared to 16.2% and 11.5% of the high-risk group.
Figure 1.
Graph showing the distribution of patients within the low, moderate, and high-risk categories in each morphine equivalent groups (0–49.9; 50–99.9; 100–149.9; 150+) in the derivation sample.
Figure 2.
Graph showing the distribution of patients within the low, moderate, and high-risk categories in each morphine equivalent groups (0–49.9; 50–99.9; 100–149.9; 150+) in the validation sample.
In addition, a dichotomized comparison (<100 MME/day vs > 100 MME/day) between the groups was performed. In the derivation cohort, 55.4% of patients in the high-risk group had an opioid intake of greater than 100 MME/day, compared to 22.8% in the moderate group and only 5.9% of the low-risk group. Similar results were seen in the validation cohort, with 51.3%, 27.9%, and 6.3% of patients taking greater than 100 MME/day in the high risk, moderate risk, and low-risk groups, respectively. When determining the likelihood of being in the >100 MME/day category, the OR for the high-risk group in the derivation sample was 6.70 (95% confidence interval of 3.91 to 11.50) and 4.80 (95% confidence interval of 2.85 to 8.07) in the validation sample. The common OR was 5.62 for the entire high-risk cohort with no significant difference between the two ORs (P = .382), signifying similar results between the two groups.
Discussion
Overview
The PAIN score was developed as a potential clinical tool to help identify patients who may be at risk for high narcotic analgesic requirements postoperatively. The results in our population suggest the model was effective at identifying patients both at low risk and high risk for high narcotic requirements based on pre-operative factors, with comparable results in both the derivation and validation samples. By design, the PAIN score is constructed exclusively by factors that are readily available pre-operatively and easy to measure in order to risk-stratify patients prior to surgery. The information garnered can help determine which patients may benefit from a more extensive postoperative analgesic regimen in order to minimize opioid intake and opioid-related complications, while still adequately controlling postoperative pain.
In addition to a standard multi-modal pain regimen including acetaminophen, muscle relaxants, neuropathic medications, and non-steroidal anti-inflammatory drugs (NSAIDs) when clinically appropriate, 1 studies have demonstrated decreased opioid requirements and improved postoperative pain with patient controlled epidural analgesia (PCEA),6,11 IV lidocaine, 12 IV ketamine, 13 and thoraco-lumbar interfascial blocks6,7 to name a few. While these analgesic adjuncts are often highly effective at improving pain and minimizing opioid requirements, they carry with them varying degrees of invasiveness, as well their own adverse effects and associated costs. With this in mind, patients deemed low risk might be treated more conservatively by avoiding certain non-opioid analgesics while still achieving adequate pain control with minimal opioid intake. As we move more toward an age of personalized medicine, the PAIN score may provide additional information to the surgical team to design a patient-specific postoperative analgesic regimen rather than utilizing a “one-size fits all” approach.
Modifiable Score Components
The PAIN score is devised using both non-modifiable and potentially modifiable components. The modifiable components include pre-operative opioid use, pre-operative benzodiazepine use, and active smoking status. While further study is needed to determine if modifying these risk factors pre-operatively leads to a decrease in postoperative analgesic needs, they do provide a potential source for pre-operative intervention in attempts to optimize patients for surgery.
Pre-operative opioid use is one of the most consistent predictors of postoperative opioid use listed in the literature, in addition to overall complications, readmissions, and persistent postoperative pain.14-19 In our model, we aimed to further stratify pre-operative opioid users by the potency and potential for abuse of the narcotic medication involved in accordance with the CSA schedule listing. As expected, patients taking CSA Schedule II narcotics had greater MME intake per day compared to those taking CSA Schedule III/IV narcotics, with both groups showing greater intake than opioid-naïve patients. While it may be difficulty to wean patients from opioids completely pre-operatively, taking a patient from a CSA Schedule II narcotic to a CSA Schedule III or IV narcotic may still provide some clinical benefit in the perioperative period. Further study is needed to investigate this potential application of our score.
Benzodiazepines and nicotine are two additional highly addictive agents that have been associated with increased postoperative pain.17,19-21 In our cohort, both were shown to have a moderate impact on postoperative opioid consumption. Animal models have demonstrated the development of peripheral and central sensitization in cases of prolonged nicotine exposure, providing a possible mechanism to explain increased pain in smokers.19,22-24 Smoking and tobacco use have been identified as a risk factor for a number of surgical complications, including impaired wound healing, pseudoarthrosis, and cardiovascular injury, in addition to poorly controlled postoperative pain.25,26 For these reasons, patients are often counseled extensively on the importance of smoking cessation prior to surgery, and this impact on postoperative pain serves as an additional point of discussion. Ensuring that patients have the tools and recourses necessary to quit should be a critical step in the pre-operative evaluation phase.
Non-modifiable Score Components
In addition to the modifiable components, the PAIN score consists of several non-modifiable factors that were shown to influence postoperative opioid consumption. Younger age (age <45) has been shown to be a consistent predictor of postoperative pain in the literature5,15,19 and was the greatest risk factor for high opioid consumption postoperatively in our cohort. Studies evaluating age-related changes in pain perception have revealed evidence of increases in pain threshold with age, suggesting that aging reduces pain sensitivity, particularly for lower pain intensities. 26 A higher percentage of muscle mass in the younger population may result in worsening postoperative pain, especially in cases involving extensive muscle dissection. Other explanations could include better awareness of opioid-related side-effects by the elderly population as well as more judicious opioid prescribing habits for these patients, both of which may have influenced our findings.
Race contributed a modest amount to the model, with White/Caucasian patients demonstrating a slightly higher degree of postoperative opioid consumption compared to other races in our cohort. In addition, the presence of a psychiatric disorder was shown to contribute to postoperative opioid consumption. Psychological factors have been long demonstrated to affect both the perception of pain and the ability to cope with pain, with numerous studies demonstrating increased postoperative pain in patients suffering from anxiety, depression, and various other mood disorders.19,27,28
Finally, the surgical site was an additional component that was shown to have a strong influence of the amount of postoperative opioid intake in our patient population. Anterior cervical operations typically involve small incisions with minimal muscle disruption. In contrast, long segment thoraco-lumbar, and lumbar procedures involve large incisions with extensive muscle disruption and often realignment of the spine in cases of spinal deformity. Minimally invasive approaches, when applicable, could help compensate for that effect.
Limitations
The PAIN score is a predictive model that was developed from a retrospective review of a population at a single institution over a 2-year period. As such, different postoperative management strategies may affect the performance of the model at different institutions or at different time points. However, the primary goal is not to predict a certain amount of opioid consumption, but rather to pre-operatively risk-stratify patients according to anticipated postoperative analgesic requirements in a given population. External validation may improve the generalizability of our model. Furthermore, while the score is constructed in part by potentially modifiable risk factors, it has not been established that modifying these factors pre-operatively may decrease a patient’s risk for high opioid consumption post-operatively.
In addition, there are many other factors that may influence an individual’s postoperative analgesic requirements that were not included in the score. While the list is long, these may include patient factors such as comorbidities affecting the ability to metabolize medications (e.g., chronic kidney disease, liver disease) and intra-operative factors including operative time, choice of anesthetic, and analgesics administered intra-operatively. Also, while pre-operative narcotic users were separated based upon the CSA class of the agent, we were unable to further separate them according to the duration of use of these agents due to the retrospective nature of the study and lack of availability of this data. Furthermore, out of an attempt to keep the score simple and easy to use, a more detailed breakdown of surgical classification was not attempted. However, we recognize this classification of surgical risk categories does not fully take into consideration all surgical factors (e.g., minimally invasive vs open instrumentation) that may influence postoperative analgesic requirements.
Finally, our model was designed for use in patients undergoing surgery of the spine. Given that surgical risk category is included in the model, its application to other surgical subspecialties may be limited. However, if surgical risk categories in other surgical subspecialties can be established, the model could potentially be utilized in surgical specialties outside of spine surgery as well.
Conclusion
The PAIN Score is a simple clinical model constructed of readily available pre-operative patient factors that can be used to identify individuals at high risk for increased postoperative narcotic analgesic requirements following spine surgery. The information garnered can help select patients who may benefit from more invasive or expensive non-opioid analgesic adjuncts to minimize postoperative opioid use and associated opioid-related complications. It can also potentially minimize the use of these adjuncts in low-risk patients to decrease associated cost and complications. The score may help optimize high-risk patients pre-operatively by treating modifiable factors pre-emptively.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was partially funded by the UTSW department of Neurosurgery Dr. Bagley receives royalties from K2M/Stryker.
Ethical approval: IRB compliance statement and ethical adherence: This study was written in compliance with our institutional ethical review board IRB approval was obtained. IRB #STU-2019-0519.
ORCID iDs
Zachary D. Johnson, MD https://orcid.org/0000-0001-9413-6256
Mark N. Pernik, BA https://orcid.org/0000-0002-7568-7308
Umaru Barrie, BS https://orcid.org/0000-0002-0365-7070
James P. Caruso, MD https://orcid.org/0000-0002-5562-6436
Salah G. Aoun, MD https://orcid.org/0000-0003-3499-7569
References
- 1.Kurd MF, Kreitz T, Schroeder G, Vaccaro AR. The role of multimodal analgesia in spine surgery. J Am Acad Orthop Surg. 2017;25:260-268. doi: 10.5435/JAAOS-D-16-00049. [DOI] [PubMed] [Google Scholar]
- 2.van Boekel RLM, Warlé MC, Nielen RGC, et al. Relationship between postoperative pain and overall 30-day complications in a broad surgical population. Ann Surg. 2019;269:856-865. doi: 10.1097/SLA.0000000000002583. [DOI] [PubMed] [Google Scholar]
- 3.de Boer HD, Detriche O, Forget P. Opioid-related side effects: postoperative ileus, urinary retention, nausea and vomiting, and shivering. A review of the literature. Best Pract Res Clin Anaesthesiol. 2017;31:499-504. doi: 10.1016/j.bpa.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Stoicea N, Costa A, Periel L, Uribe A, Weaver T, Bergese SD. Current perspectives on the opioid crisis in the US healthcare system. Medicine. 2019;98:e15425. doi: 10.1097/MD.0000000000015425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerbershagen HJ, Pogatzki-Zahn E, Aduckathil S, et al. Procedure-specific risk factor analysis for the development of severe postoperative pain. Anesthesiology. 2014;120:1237-1245. doi: 10.1097/ALN.0000000000000108. [DOI] [PubMed] [Google Scholar]
- 6.Cata JP, Noguera EM, Parke E, et al. Patient-controlled epidural analgesia (PCEA) for postoperative pain control after lumbar spine surgery. J Neurosurg Anesthesiol. 2008;20:256-260. doi: 10.1097/ANA.0b013e31817ffe90. [DOI] [PubMed] [Google Scholar]
- 7.Chen K, Wang L, Ning M, Dou L, Li W, Li Y. Evaluation of ultrasound-guided lateral thoracolumbar interfascial plane block for postoperative analgesia in lumbar spine fusion surgery: a prospective, randomized, and controlled clinical trial. PeerJ. 2019;7:e7967. doi: 10.7717/peerj.7967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh S, Choudhary NK, Lalin D, Verma VK. Bilateral ultrasound-guided erector spinae plane block for postoperative analgesia in lumbar spine surgery: a randomized control trial. J Neurosurg Anesthesiol. 2020;32:330-334. doi: 10.1097/ANA.0000000000000603. [DOI] [PubMed] [Google Scholar]
- 9.Adeyemo EA, Aoun SG, Barrie U, et al. Comparison of the effect of epidural versus intravenous patient controlled analgesia on inpatient and outpatient functional outcomes after adult degenerative scoliosis surgery: a comparative study. Spine J. 2021;21:765-771. doi: 10.1016/j.spinee.2020.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Adeyemo EA, Aoun SG, Barrie U, et al. Enhanced recovery after surgery reduces postoperative opioid use and 90-day readmission rates after open thoracolumbar fusion for adult degenerative deformity. Neurosurgery. 2020;88:295-300. doi: 10.1093/neuros/nyaa399. [DOI] [PubMed] [Google Scholar]
- 11.Fisher CG, Belanger L, Gofton EG, et al. Prospective randomized clinical trial comparing patient-controlled intravenous analgesia with patient-controlled epidural analgesia after lumbar spinal fusion. Spine. 1976 2003;28:739-743. [PubMed] [Google Scholar]
- 12.Farag E, Ghobrial M, Sessler DI, et al. Effect of perioperative intravenous lidocaine administration on pain, opioid consumption, and quality of life after complex spine surgery. Anesthesiology. 2013;119:932-940. doi: 10.1097/ALN.0b013e318297d4a5. [DOI] [PubMed] [Google Scholar]
- 13.Pendi A, Field R, Farhan S-D, Eichler M, Bederman SS. Perioperative ketamine for analgesia in spine surgery. Spine. 1976 2018;43:E299-E307. doi: 10.1097/BRS.0000000000002318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armaghani SJ, Lee DS, Bible JE, et al. Preoperative opioid use and its association with perioperative opioid demand and postoperative opioid independence in patients undergoing spine surgery. Spine. 1976 2014;39:E1524-E1530. doi: 10.1097/BRS.0000000000000622. [DOI] [PubMed] [Google Scholar]
- 15.Behman R, Cleary S, McHardy P, et al. Predictors of post-operative pain and opioid consumption in patients undergoing liver surgery. World J Surg. 2019;43:2579-2586. doi: 10.1007/s00268-019-05050-7. [DOI] [PubMed] [Google Scholar]
- 16.Cozowicz C, Olson A, Poeran J, et al. Opioid prescription levels and postoperative outcomes in orthopedic surgery. Pain. 2017;158:2422-2430. doi: 10.1097/j.pain.0000000000001047. [DOI] [PubMed] [Google Scholar]
- 17.Cryar KA, Hereford T, Edwards PK, Siegel E, Barnes CL, Mears SC. Preoperative smoking and narcotic, benzodiazepine, and tramadol use are risk factors for narcotic use after hip and knee arthroplasty. J Arthroplasty. 2018;33:2774-2779. doi: 10.1016/j.arth.2018.03.066. [DOI] [PubMed] [Google Scholar]
- 18.Jain N, Phillips FM, Weaver T, Khan SN. Preoperative chronic opioid therapy. Spine. 1976 2018;43:1331-1338. doi: 10.1097/BRS.0000000000002609. [DOI] [PubMed] [Google Scholar]
- 19.Yang MMH, Hartley RL, Leung AA, et al. Preoperative predictors of poor acute postoperative pain control: a systematic review and meta-analysis. BMJ Open. 2019;9:e025091. doi: 10.1136/bmjopen-2018-025091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaulton TG, Wunsch H, Gaskins LJ, et al. Preoperative sedative-hypnotic medication use and adverse postoperative outcomes. Ann Surg. 2019;274:e108-e114. doi: 10.1097/SLA.0000000000003556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hozack BA, Rivlin M, Lutsky KF, et al. Preoperative exposure to benzodiazepines or sedative/hypnotics increases the risk of greater filled opioid prescriptions after surgery. Clin Orthop Relat Res. 2019;477:1482-1488. doi: 10.1097/CORR.0000000000000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hawkins JL, Denson JE, Miley DR, Durham PL. Nicotine stimulates expression of proteins implicated in peripheral and central sensitization. Neuroscience. 2015;290:115-125. doi: 10.1016/j.neuroscience.2015.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holtman JR, Jr., Crooks PA, Johnson-Hardy JK, Wala EP. The analgesic and toxic effects of nornicotine enantiomers alone and in interaction with morphine in rodent models of acute and persistent pain. Pharmacol Biochem Behav. 2010;94:352-362. doi: 10.1016/j.pbb.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jinks SL, Carstens E. Activation of spinal wide dynamic range neurons by intracutaneous microinjection of nicotine. J Neurophysiol. 1999;82:3046-3055. doi: 10.1152/jn.1999.82.6.3046. [DOI] [PubMed] [Google Scholar]
- 25.Jackson KL, 2nd, Devine JG. The effects of smoking and smoking cessation on spine surgery: a systematic review of the literature. Global Spine J. 2016;6:695-701. doi: 10.1055/s-0036-1571285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lautenbacher S, Peters JH, Heesen M, Scheel J, Kunz M. Age changes in pain perception: A systematic-review and meta-analysis of age effects on pain and tolerance thresholds. Neurosci Biobehav Rev. 2017;75:104-113. doi: 10.1016/j.neubiorev.2017.01.039. [DOI] [PubMed] [Google Scholar]
- 27.Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US Adults. JAMA Surgery. 2017;152:e170504. doi: 10.1001/jamasurg.2017.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Connell C, Azad TD, Mittal V, et al. Preoperative depression, lumbar fusion, and opioid use: an assessment of postoperative prescription, quality, and economic outcomes. Neurosurg Focus. 2018;44:E5. doi: 10.3171/2017.10.FOCUS17563. [DOI] [PubMed] [Google Scholar]