Introduction
Stress is an experience that produces both protective and harmful outcomes (McEwen, 1998). When stress surpasses an intrinsic threshold, the sympathetic nervous system is activated, setting off a chain-reaction of neurochemical events that trigger negative behavioral and psychological consequences (see review: (Klein and Corwin, 2002)). Across species, adolescents demonstrate heightened vulnerability to stress compared to adults. In humans, adolescents exhibit elevated activity in areas associated with emotional reactivity, such as the amygdala, following exposure to threat (fearful faces) (Hare et al., 2008). Adolescent rats (Postnatal Days [P]28–50) demonstrate 1) elevated corticosterone (CORT) response to stressors and 2) a prolonged return to baseline CORT levels compared to adult counterparts (Brown and Spencer, 2013). This enhanced activity within the hypothalamus–pituitary–adrenal (HPA) axis has been similarly observed in adolescent primate and avian models, and appears to wane by adulthood (Andersen and Teicher, 2008). This age-specific activity is hypothesized to involve extra-hypothalamic regions which continue to develop in adolescence, although the exact mechanisms are still under investigation.
Sex has also been shown to dictate behavioral and neural responses to stress, primarily in adults. For instance, sex can determine whether a stressor enhances or diminishes cognitive performance in fear-learning assessments (Brown and Spencer, 2013). However, the direction and degree of stress-induced response in females is still widely contended. We and others have shown that compared to males, females demonstrate sex-specific resilience to the negative effects of stress in both hamsters (Faruzzi et al., 2005) and rats (Bowman et al., 2003; Varlinskaya et al., 2020), an effect which was blocked in rats with administration of an estrogen antagonist (Wood and Shors, 1998). However, several independent studies have also reported that females exhibit greater stress-induced HPA activity compared to males (Kudielka and Kirschbaum, 2005; Walker and McCormick, 2009; Young et al., 2007). Notably, sex-differences in HPA and autonomic responses appear to be most prominent when females were assessed within a pubertal-menopausal window (Kajantie and Phillips, 2006). This suggests that age as a biological factor contributes to stress reactivity.
The central amygdala (CeA) is a region well-characterized for its role in regulating stress and anxiety-like responses. This striatal-like structure is composed primarily of GABAergic local interneurons and projection neurons (Babaev et al., 2018b; Ehrlich et al., 2009). Inhibitory tone within the medial subnucleus of the CeA (CeM) has been associated with reduced response to stress-inducing stimuli (Gilpin et al., 2015), possibly through direct efferents to brain regions responsible for the expression of anxious behaviors (Babaev et al., 2018b). This region is also rich in concentrations of the stress peptide corticotropin-releasing factor (CRF). CRF is a 41 amino-acid peptide found in many mammalian species, and is identical across human and rat species (Dautzenberg and Hauger, 2002). Throughout the brain, high concentrations of CRF and activation of one of its primary receptors, CRFR1, have been recognized for their anxiogenic influence in adult populations (Kehne, 2007; Magalhaes et al., 2010).
Within the CeM of adult male rodents, CRF potentiates γ-aminobutyric acid (GABA) release through presynaptic CRFR1 (Roberto et al., 2010) and selective antagonism of CeM-CRFR1 blunts anxiety-like behavior (Skorzewska et al., 2017). However, response to CRFR1 activation in adolescents is largely unexplored. One investigation of presumed-adolescent male Sprague Dawley rats (125–150g) demonstrated that CRF superfusion into the CeA results in neuronal hyperpolarization (Rainnie et al., 1992), however, this study did not directly examine synaptic transmission. A more recent study in the lateral habenula showed a CRFR1-induced reduction in GABA transmission in post-weanling rats (Authement et al., 2018), opposite of what has been established in the adults within the CeA (Bajo et al., 2008; Nie et al., 2004; Roberto et al., 2010). These findings suggest a potential developmental shift in CRFR1-regulation of GABA transmission, however, age and sex as biological factors have not been systematically investigated.
Thus, the objective of the present study was to determine if the biological factors of age and sex influence CRF system function within the CeM. Using whole cell patch-clamp electrophysiology, we determined that these factors significantly influence basal GABA transmission, as well as the instrinsic excitability of CeM neurons. Furthermore, CRFR1-regulated GABAergic transmission within this region was age and sex-specific in both the sensitivity of presynaptic CRFR1 and the direction of receptor-regulated GABA release. Finally, we determined that adult males, and not adolescent males or adolesent/adult females, demonstrate tonic CRFR1 activity.
Materials and Methods
Animals.
To avoid introducing shipping stress during development, all experimental subjects were bred in-house as previously described (Rouzer et al., 2017) Adult male and female Sprague Dawley breeders were obtained from Envigo/Harlan (Indianapolis, IN) and permitted to acclimate at least one week prior to breeding. Upon detection of sperm in vaginal smears, pregnant dams were isolated and housed with a plastic hut and crinkle paper as nesting material. After parturition, litters were culled to 5:5 males/females on P2 and housing conditions remained the same with a plastic hut and crinkle paper. Pups were weaned from their mother on P21 and housed with same-sex littermates until experimentation in mid-adolescence (P40–48) or adulthood (P70–95). This specific period of adolescence has been repeatedly used by our lab to investigate impairments engendered by developmental exposures, including alcohol, and the results of this study will be used to inform further research on the effects of early-life insults on the CRF system. All animals were group-housed (2–3 animals per cage) in a temperature-controlled (22°C) vivarium and maintained on a 12:12 h light:dark cycle (lights on at 7:00 h). Subjects were provided ad libitum access to food (5L0D PicoLab Laboratory Rodent diet) and water throughout the duration of experimentation. All animal procedures were approved by the Binghamton University Institutional Animal Care and Use Committee.
Whole-cell Electrophysiology.
Drugs and Chemicals.
All chemicals and kynurenic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA). APV, tetrodotoxin (TTX), Stressin-1, CGP 55845 and NBI 35965 were purchased from Tocris/R&D systems (Bristol, UK).
Slice Preparation.
Adolescent (P40–48) and adult (P70–95) male and female rats were sedated with a 250 mg/kg dose of ketamine and quickly decapitated, as previously performed by our lab (Przybysz et al., 2017). Brains were rapidly removed and immersed in ice-cold oxygenated (95% O2-5% CO2) sucrose artificial cerebrospinal fluid (ACSF) containing (in mM): sucrose (220), KCl (2), NaH2PO4 (1.25), NaHCO3 (26), glucose (10), MgSO4 (12), CaCl2 (0.2), and ketamine (0.43). Coronal slices (300 μM) containing the CeM were collected with a Vibratome (Leica Microsystems. Bannocknurn, IL, USA). Slices were incubated in 34°C normal ACSF (in mM): NaCl (125), KCl (2), NaH2PO4 (1.3), NaHCO3 (26), glucose (10), CaCl2 (0.1), MgSO4 (0.1), ascorbic acid (0.04), and continuously bubbled at 95% O2-5% CO2 for at least 40 minutes before recording. All experiments were performed within 6 hours of slice preparation.
Whole-cell patch-clamp recordings.
Following incubation, slices were transferred to a recording chamber, where oxygenated ACSF was warmed to 32°C and continuously superfused over the submerged slice at 3.3 ml/min. Recording electrodes of 3–5 MΩ tip resistance were pulled from borosilicate glass capillary tubing (Sutter Instruments) using a Flaming-Brown puller (Sutter Instruments). Recordings were collected from the CeM with patch pipettes filled with experiment-specific internal solutions (see below). Membrane properties (averaged across entire recordings) were provided by the membrane test in pClamp 10 (Molecular Devices) and electrophysiology data were acquired with a MultiClamp 700B (Molecular Devices, Sunnyvale, CA) at 10 kHz, filtered at 1 kHz, and stored for later analysis using pClamp 10 software (Molecular Devices).
Voltage-clamp recordings:
For voltage-clamp experiments, a KCl-based internal solution was used for detecting GABAA receptor-mediated currents, containing (in mM): KCl (135), HEPES (10), MgCl2 (2), EGTA (0.5), Mg-ATP (5), Na-GTP (1), and QX314-Cl (1); 300 mOsm; 7.3 pH with KOH. For recordings of GABAA receptor-mediated spontaneous inhibitory postsynaptic currents (sIPSCs), AMPA and NMDA glutamate receptors were pharmacologically blocked using 1 mM kynurenic acid and 50 μM APV, respectively, and GABAB receptors were blocked with CGP 36645 (1 μM). For data collection of miniature IPSCs (mIPSCs), recordings were made in the presence of the Na+ channel blocker, TTX (1 μM). Baseline recordings of sIPSCs and mIPSCs were allowed to equilibrate for at least 5 min before recording began. A 3-min baseline period was recorded prior to drug application.
For electrophysiological assessment of CRFR1 function, the CRFR1 selective-agonist, Stressin-1 (10 nM, 100 nM and 1 μM) or the selective antagonist, NBI 35965 (1 μM), was applied for 15 min. As CeM neurons within the CeM exhibited generally stable access resistance, this length of time ensured that changes in transmission could be visualized for stability. To determine the timing of a stable drug effect, a time course of drug exposure was constructed during the pilot phase of experimentation and stable activity was observed 5 min following drug application. Therefore, the first 5 min of drug application was removed from final analyses. Additionally, for all experiments, only recordings with an access resistance change of <20% were included in final analyses.
Current-clamp recordings.
For assessments of intrinsic excitability and resting membrane potential (RMP), recordings were collected from the CeM with patch pipettes filled with a K-gluconate-based internal solution containing (in mM): K-Gluconate (120), KCl (15), EGTA (0.1), HEPES (10), MgCl2 (4.0), MgATP (4.0), Na3GTP (0.3) and Phosphocreatine (7.0); 280–290 mOsm; 7.25 pH with KOH. Cells were opened in a voltage-clamp configuration (holding potential = −70 mV) and switched to current-clamp settings for at least 7 minutes to allow neurons to dialyze prior to applying a series of depolarizing current steps (increments of 15 pA, 500 ms duration). Subsequent firing activity was recorded for future analysis. Rheobase, time to first action potential, and action potential threshold, peak and half-width were determined with the first action potential fired with the lowest stimulation current as assessed by pClamp 10 software (Molecular Devices). A liquid junction potential of −19.4 mV was also calculated using this software, and all reported membrane potentials have been adjusted to account for this disparity.
Experimental Design and Statistical Analysis.
Based on preliminary data, a power analysis (G*Power 3.1.9.2) for a two-way (sex x age) analysis of variance (ANOVA) and an alpha of 0.05 suggested a sample size of 8 units per group. The unit of analyses for all experiments was cell, with no more than 2 cells per animal used for any given experiment. Furthermore, no more than 2 subjects per litter were used for any given experiment to reduce litter effects in our sample population. To avoid experimenter bias, all data analyses were conducted by an individual blind to the conditions of the subject. All statistical analyses were performed using GraphPad 8 Software (Prism). Measures of membrane properties, RMP, intrinsic excitability, and basal s/mIPSCs were analyzed with 2 (age: adolescents, adults) x 2 (sex: male, female) between-subjects ANOVA. Upon discovering a significant sex x age interaction in basal sIPSC frequency in the CeM, all CRFR1-targetted analyses were subsequently analyzed independently in each sex. Concentration-response activity following CRFR1 activation were reported as % change from baseline mIPSC activity and analyzed independently in each sex using 2 (age) x 3 (concentration: 10 nM, 100 nM, 1 μM) between-subjects ANOVAs. CRFR1 activity was measured in each age/sex group by performing a one-sample t-test, statistically comparing the average change in mIPSC baseline activity to a null mIPSC change (0). In within-cell analyses between baseline mIPSCs and drug application, effect of drug is reported as significant only when a statistical comparison between baseline activity and drug application differs by p < 0.05. In all assessments, significance was defined as p ≤ 0.05 unless otherwise noted. In the event of significant main effects or interactions, post hoc Sidak’s multiple comparison tests were performed to determine specific group differences. All data were assessed for outliers using the ROUT method of regression (GraphPad 8 Software, Prism), and all identified outliers were removed from statistical analyses. All data are presented as mean ± standard error of the mean (SEM) unless otherwise specified.
Results
Membrane properties of CeM neurons do not differ across age and sex.
The average access resistance of electrophysiological recordings of CeM neurons was 15.96 (± 0.717), with no difference in access resistance between experimental groups. Assessment of membrane properties across age (adolescent and adult) and sex (male and female) revealed no significant group differences in membrane capacitance [age: F(1,60) = 0.758, p = 0.388; sex: F(1,60) = 2.085, p = 0.154; age x sex interaction: F(1,60) = 0.028, p = 0.868, n = 12–20 cells per group] or membrane resistance [age: F(1,60) = 0.163, p = 0.689; sex: F(1,60) = 2.455, p = 0.122; age x sex interaction: F(1,60) = 0.003, p = 0.955, n = 12–20 cells per group] (Table 1).
Table 1.
Cell properties of CeM interneurons across age and sex, reported as Mean (SEM). Experimental groups do not differ in native membrane properties or resting membrane potential. However, adults demonstrate generally lower action potential thresholds than adolescents, an effect most pronounced in males. Independent of sex, age influences time to action potential peak following current injection, with adults demonstrating the quickest times. Action potential amplitudes are higher in males than females, independent of age, while neither age nor sex appear to influence action potential half-widths.
| Adolescent Males (n=14) | Adolescent Females (n=18) | Adult Males (n=16) | Adult Females (n=18) | |
|---|---|---|---|---|
| Membrane Resistance (MΩ) | 424.21 (70.00) | 529.05 (82.78) | 392.32 (41.70) | 504.98 (56.00) |
| Membrane Capacitance (pF) | 41.66 (4.56) | 46.69 (4.37) | 37.57 (2.43) | 43.92 (3.38) |
| Resting Membrane Potential (mV) | −51.59 (2.80) | −48.99 (1.86) | −51.63 (1.77) | −49.76 (2.10) |
| AP Threshold (mV) * # | −45.55 (2.41) | −43.40 (1.31) | −50.07 (1.70) | −45.54 (1.12) |
| Rheobase (pA) | 52.50 (7.16) | 55.00 (8.47) | 50.00 (6.12) | 56.36 (9.37) |
| Time to 1st AP (ms) * | 123.75 (15.36) | 101.58 (22.28) | 73.18 (18.78) | 67.96 (12.94) |
| AP Amplitude (mv) # | 96.96 (5.17) | 90.214 (3.21) | 102.04 (1.69) | 93.52 (0.95) |
| AP Half-Width (ms) | 2.04 (0.25) | 2.27 (0.10) | 2.26 (0.15) | 1.99 (0.13) |
indicates significant main effect of age (p < 0.05)
indicates significant main effect of sex (p < 0.05).
Both sex and age influence the excitability of CeM neurons.
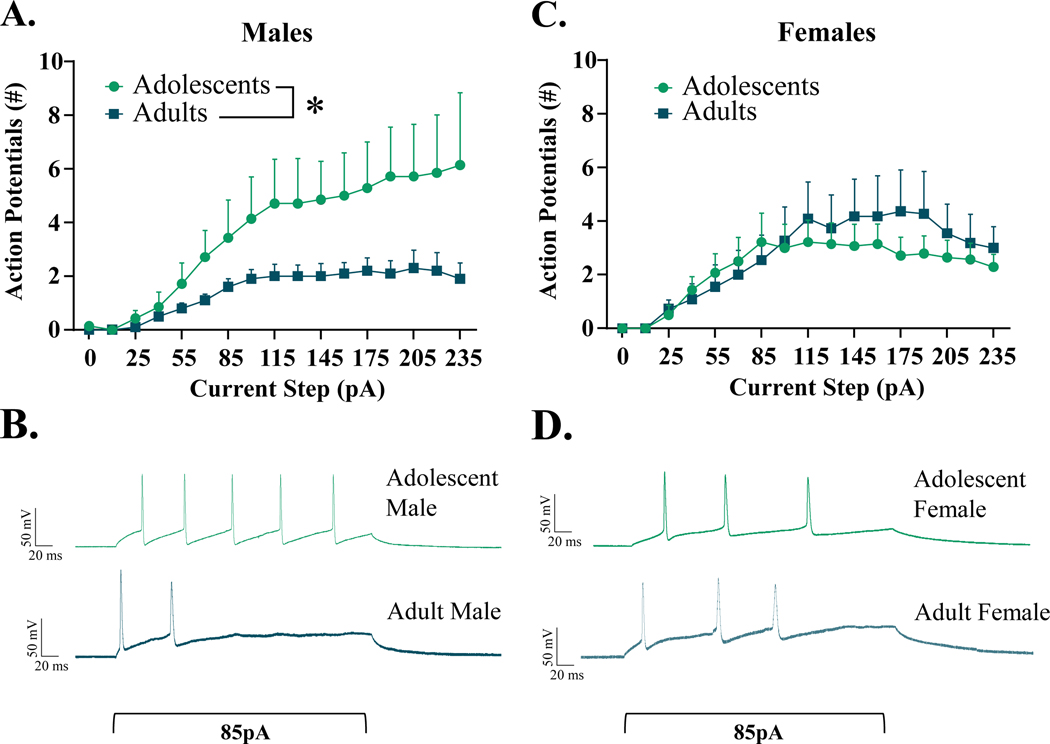
In a current-neutral configuration, cells were assessed for resting membrane potential. Neither age nor sex influenced resting membrane potential [age: F(1,62) = 0.037, p = 0.849; sex: F(1,62) = 1.104, p = 0.297; age x sex interaction: F(1,62) = 0.029, p = 0.865, n = 14–18 cells per group] (Table 1). Cells were then held at −70 mV to assess and normalize differences in excitability across groups. Notably, the proportion of cells that demonstrated firing activity following current injection were considerably lower in adolescent males compared to adolescent females (Males: 6/14, Females: 14/18) but comparable between sexes in adulthood (Males: 10/16, Females: 11/18).
From cells that were responsive to current injection, we found no differences in rheobase across ages and sexes [age: F(1,36) = 2.109, p = 0.155; sex: F(1,36) = 0.041, p = 0.840; age x sex interaction: F(1,36) = 0.077, p = 0.784] (Table 1). However, age did significantly influence the membrane potential at first AP [F(1,36) = 4.356, p = 0.044], with adults demonstrating lower thresholds than adolescents, an effect driven by adult males (Table 1). Furthermore, females demonstrated significantly higher thresholds than males [F(1,36) = 4.403, p = 0.043]. There was no significant interaction between these two variables [F(1,36) = 0.555, p = 0.461]. Quantification of firing activity revealed a significant age x sex interaction [F(1,703) = 19.48, p < 0.001], whereupon adolescent males exhibited significantly greater activity than adult males [t(1,23) = 3.300, p = 0.0031] (Fig. 1A). This effect of age was absent in females [t(1,32) = 0.962, p = 0.343] (Fig. 1C). We also found a significant main effect of age when examining time to first AP, with adults demonstrating quicker onset of the first action potential than adolescents [F(1,37) = 4.141, p = 0.049] (Table 1 and depicted in exemplar traces in Fig. 1B&D). This activity was not influenced by sex, either as a main effect [F(1,37) = 0.438, p = 0.512] or as an interacting variable [F(1,37) = 0.168, p = 0.684]. Action potential amplitude statistically differed between sexes [F(1,37) = 6.140, p = 0.018], with males demonstrating slightly higher amplitudes than females, with no effect of age [F(1,37) = 1.851, p = 0.182] or an interaction of age and sex [F(1,37) = 0.083, p = 0.775]. Finally, action potential half-widths did not differ by age [F(1,37) = 0.025, p = 0.876], sex [F(1,37) = 0.038, p = 0.847] or an interaction of these variables [F(1,37) = 2.787, p = 0.103] in recorded cells.
Figure 1.
Excitability of interneurons within the CeM, Males (A&B) and Females (C&D). Increasing current steps (pA) produce significantly more firing activity in adolescent males than adult males, with no difference in females between adolescent and adult age groups. * indicates significant difference between groups (p < 0.05)
Basal synaptic transmission in the CeM is sex- and age-specific.
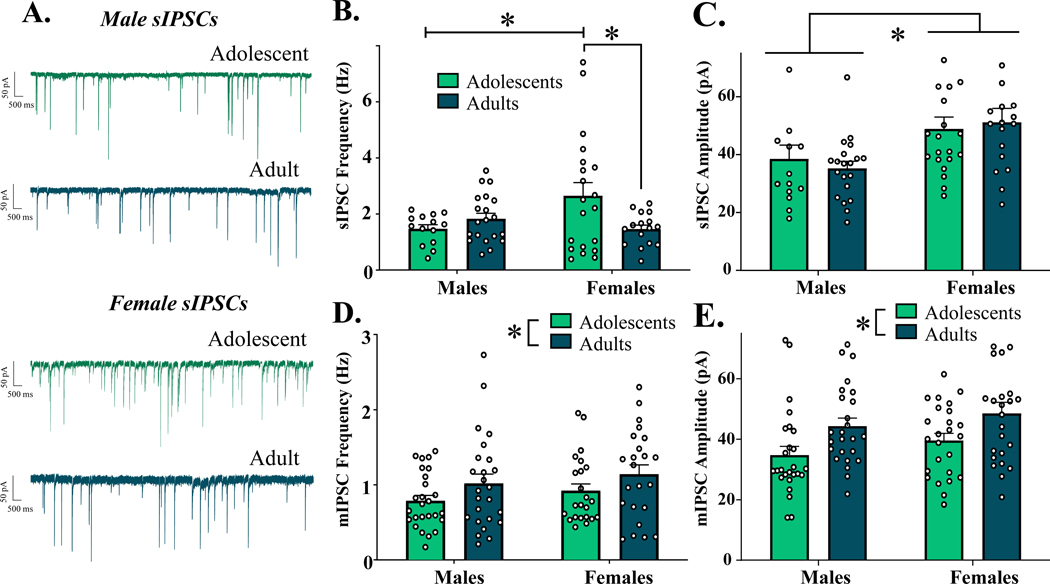
To determine if factors of age and sex influenced basal inhibitory synaptic activity in the CeM, both sIPSCs and mIPSCs were recorded. As represented in Fig. 2A, analyses of basal sIPSC frequency revealed a significant interaction of age x sex [F(1,66) = 6.296, p = 0.014] (Fig. 2B), with no main effects of age [F(1,66) = 1.830, p = 0.181] or sex [F(1,66) = 1.720, p = 0.194]. Post-hoc analyses revealed significantly higher sIPSC frequency in adolescent females compared to both age-matched males (n = 14–20, p = 0.048), and adult females (n = 16–20, p = 0.035). Notably, males did not differ in sIPSC frequency between age groups (n = 14–20, p = 0.853). Assessment of sIPSC amplitude revealed a significant main effect of sex [F(1,67) = 10.590, p = 0.002], with females consistently exhibiting greater sIPSC amplitudes than males (Fig. 2C), while age did not influence sIPSC amplitude (age: [F(1,67) = 0.016, p = 0.900]; age x sex interaction: [F(1,67) = 0.4737, p = 0.494]).
Figure 2.
Native IPSCs in the CeM across age and sex. (A) Representative sIPSC activity of neurons in the CeM. B) Spontaneous IPSC frequency across experimental groups. Adolescent females exhibit significantly greater sIPSC frequency than both adolescent males and adult females. C) Spontaneous IPSC amplitude across experimental groups. Females exhibit significantly higher sIPSC amplitude than males, independent of age. D) Miniature IPSC frequency across experimental groups. Adults demonstrate greater mIPSC frequency than adolescents, independent of sex. E) Miniature IPSC amplitude across experimental groups. Adults demonstrate higher mIPSC amplitude than adolescents, independent of sex. * indicates significant difference between groups (p < 0.05)
In contrast, action potential-independent mIPSC frequency significantly differed only between age groups [F(1,93) = 4.559, p = 0.035], with adults exhibiting higher mIPSC frequency than adolescents (Fig. 2D). This effect was not significantly different between sexes (main effect of sex: [F(1,93) = 1.461, p = 0.223]; age x sex interaction: [F(1,93) = 0.002, p = 0.964]). A similar pattern emerged in assessment of mIPSC amplitude, with adults demonstrating greater amplitude than adolescents [F(1,93) = 10.49, p = 0.002] (Fig. 2E), independent of sex (main effect of sex: [F(1,93) = 2.462, p = 0.120; age x sex interaction: [F(1,93) = 0.010, p = 0.924]). In further analyses of postsynaptic receptor kinetics, summarized in Table 2, we observed a significant effect of age on mIPSC area [F(1,93) = 16.54, p < 0.001], with adults exhibiting greater area than adolescents. There was a non-significant effect of sex on area [F(1,93) = 3.150, p = 0.079], and no interaction of age x sex on this measure [F(1,93) = 1.021, p = 0.315]. Interestingly, there was no age-specific effect in rise time [F(1,93) = 0.002, p = 0.9969] or decay [F(1,93) = 0.051, p = 0.821]. A statistically significant effect of sex was observed in rise time [F(1,93) = 4.576, p = 0.035], with females exhibiting faster rise times than males. Sex had no effect on decay [F(1,93) = 0.459, p = 0.499], nor did interactions of age x sex influence rise time [F(1,93) = 0.213, p = 0.646] or decay [F(1,93) = 0.391, p = 0.534].
Table 2.
mIPSC postsynaptic kinetic properties of CeM neurons across age and sex, reported as Mean (SEM). mIPSC area is significantly greater in adults than adolescents, while sex did not produce a statistically significant difference in either measure. Sex did influence rise time, with females demonstrating faster rise times than males in both age groups. Decay (τ) was not influenced by either age or sex.
| Area (pA*ms) ** | Rise time (ms) # | τ (ms) | |
|---|---|---|---|
| Adolescent Males (n=26) | 672.24 (39.34) | 1.33 (0.08) | 39.62 (2.26) |
| Adolescent Females (n=25) | 833.10 (54.28) | 1.21 (0.07) | 39.46 (3.26) |
| Adult Males (n=25) | 965.47 (50.73) | 1.37 (0.09) | 42.30 (3.54) |
| Adult Females (n=24) | 1009.60 (80.76) | 1.18 (0.06) | 38.20 (3.26) |
indicates significant main effect of age (p < 0.01)
indicates significant main effect of sex (p < 0.05).
CRFR1 activation bi-directionally regulates GABAergic transmission in adolescent and adult males.
Given the age x sex interactions found in basal sIPSC activity, CRFR1-regulated activity was subsequently analyzed independently in each sex. Previous research in adult, male Sprague Dawley rats has shown that CRFR1 activation within the CeA increases mIPSC frequency without changing mIPSC amplitude (Herman et al., 2013a; Kang-Park et al., 2015; Roberto et al., 2010). To determine if the function of CRFR1 on GABA transmission is age-dependent, we assessed the effect of the CRFR1-selective agonist, Stressin-1 (10 nM, 100 nM and 1 μM) on mIPSCs within the CeM of adolescent and adult males. Analyses of drug effects were analyzed by % change in activity from baseline, as detailed below. Changes in raw frequency values were also statistically assessed; significant raw value comparisons mirrored significant % changes from baseline, and have been summarized in Supplementary Table 2.
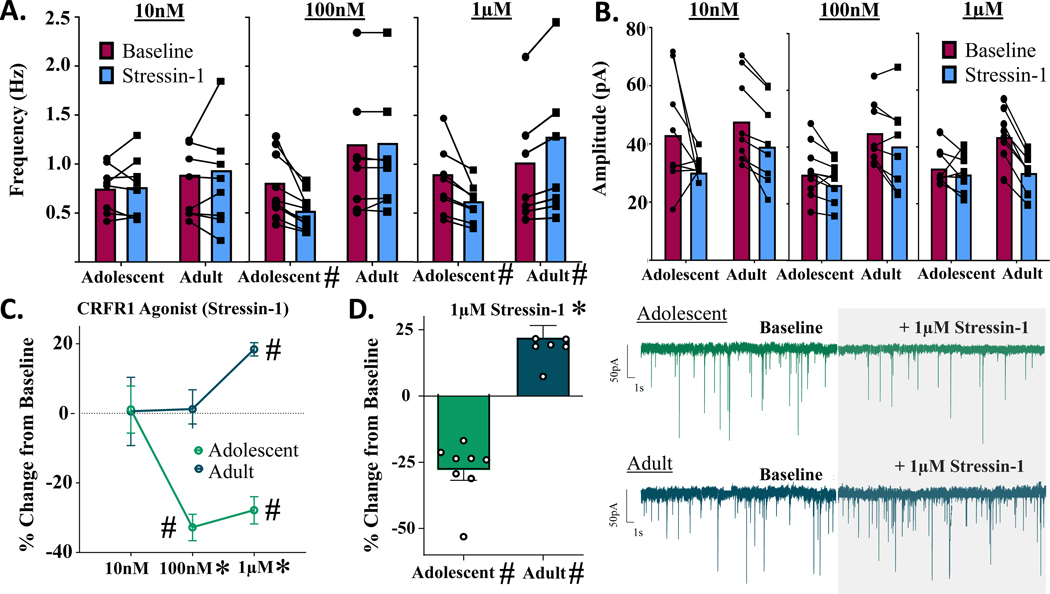
Analysis of Stressin-1-induced changes in mIPSC frequency revealed significant main effects of both age [F(1,43) = 30.700, p < 0.001] and concentration of drug [F(2,43) = 4.364, p = 0.019] in males, and a significant age x concentration interaction [F(2,43) = 8.320, p < 0.001], with a leftward shift in concentration in adolescent males (Fig. 3A,C). Post-hoc analyses revealed no significant difference between adolescents and adults at the 10 nM concentration [t(1,43) = 0.066, p > 0.999], whereupon 10 nM Stressin-1 did not produce a significant change in mIPSC frequency in either adolescents [t(1,7) = 0.164, p = 0.875, n = 8 cells] or adults [t(1,7) = 0.056, p = 0.957, n = 8 cells]. At 100 nM, drug effects were significantly different between age groups [t(1,43) = 4.294, p < 0.001]; this concentration produced no significant change in mIPSC frequency in adults [t(1,7) = 0.218, p = 0.884, n = 8 cells], however 100 nM Stressin-1 significantly reduced mIPSC frequency in adolescents [t(1,9) = 8.642, p < 0.001, n = 10 cells]. Finally, at 1 μM, a significant difference between age groups was also observed [t(1,43) = 5.356, p < 0.001]. At this concentration, Stressin-1 continued to significantly reduce mIPSC frequency in adolescents [t(1,7) = 7.089, p < 0.001, n = 8 cells], yet produced an opposite and significant increase in mIPSC frequency in adults [t(1,7) = 9.576, p < 0.001, n = 8 cells] (Fig. 3D).
Figure 3.
Males: change in mIPSCs following bath application of selective CRFR1 agonist, Stressin-1. A) mIPSC frequency activity across three doses of CRFR1 agonist Stressin-1. CRFR1 activation produces significant dose-dependent changes in both adolescents and adults. In adolescents, 100nM and 1μM Stressin-1 significantly decrease mIPSC frequency, while 1μM Stressin-1 produces a significant increase in mIPSC frequency in adults. B) mIPSC amplitude activity across three doses of CRFR1 agonist Stressin-1. CRFR1 activation does not produce significant changes in postsynaptic GABA-A amplitudes as a function of age or CRFR1 agonist dose. C) mIPSC frequency activity across three doses of CRFR1 agonist, reported as % change in baseline activity. D) Bar graph and representative traces of % change in mIPSC frequency from adolescent and adult males following bath application of 1μM Stressin-1. * indicates significant effect of age (p < 0.05) # signifies significant effect of drug (p < 0.05)
Analysis of drug-induced % change in mIPSC amplitude in these same cells revealed no significant main effects of age [F(1,43) = 2.172, p = 0.148] or dose of drug [F(2,43) = 0.255, p = 0.776] in males, nor a significant age x drug interaction [F(2,43) = 0.884, p = 0.421] (Fig. 3B; Supplementary Fig. 1). Analysis of raw value changes in mIPSC amplitude revealed similar null results: the dose of CRFR1 agonist did not change mIPSC amplitudes in either adolescent [F(2,23) = 2.023, p = 0.155] or adult males [F(2,23) = 0.524, p = 0.600].
CRFR1 activation inhibits GABAergic transmission in females.
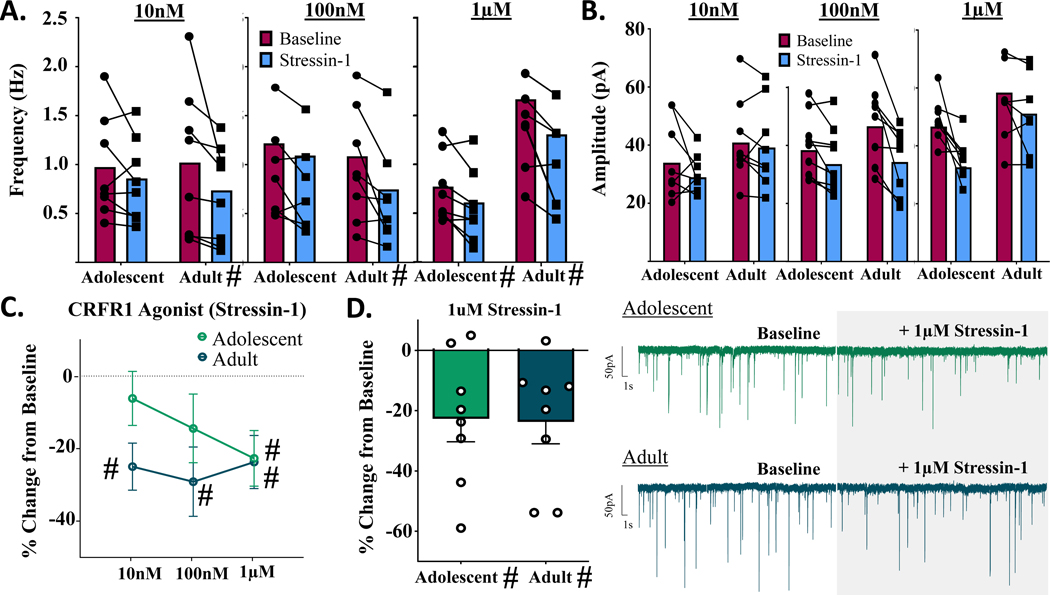
Analysis of Stressin-1-induced changes in mIPSC frequency revealed no significant main effect of concentration of drug in females [F(1,42) = 0.505, p = 0.607], nor a significant age x concentration interaction [F(2,42) = 0.668, p = 0.518] (Fig. 4A). Although the main effect of age did not reach statistical significance [F(2,42) = 3.049, p = 0.081], age-specific patterns of response were observable (Fig. 4C) and indicated CRFR1 activation produced changes in mIPSC frequency in females; specifically, in adolescents the magnitude of decrease in mIPSC frequency became larger with increasing agonist concentrations, while adults demonstrated consistent decreases in mIPSC frequency across concentrations. Consistent with these observations, in adolescent females, only the highest Stressin-1 concentration (1 μM) significantly reduced mIPSC frequency from baseline levels [10 nM: t(1,7) = 1.102, p = 0.307, n = 8 cells; 100 nM: t(1,7) = 1.527, p = 0. 171, n = 8 cells; 1 μM: t(1,7) = 2.958, p = 0.021, n = 8 cells] (Fig. 4A,D). In contrast, all three concentrations of Stressin-1 produced a statistically significant reduction in mIPSC frequency in adult females [10 nM: t(1,7) = 3.857, p = 0.006, n = 8 cells; 100 nM: t(1,7) = 3.050, p = 0. 019, n = 8 cells; 1 μM: t(1,7) = 3.235, p = 0.014, n = 8 cells].
Figure 4.
Females: change in mIPSCs following bath application of selective CRFR1 agonist, Stressin-1. A) mIPSC frequency activity across three doses of a CRFR1 agonist. In adults, CRFR1 activation produces significant changes in mIPSC frequency at all agonist doses, while producing no significant changes in mIPSC frequency in adolescents. B) mIPSC amplitude activity across three doses of CRFR1 agonist Stressin-1. CRFR1 activation does not produce significant changes in postsynaptic GABA-A amplitudes as a function of age or CRFR1 agonist dose. C) mIPSC frequency activity across three doses of CRFR1 agonist, reported as % change in baseline activity. In adolescents, 1μM Stressin-1 significantly decreases mIPSC frequency, while all three doses of Stressin-1 produce a significant decrease in mIPSC frequency in adults. D) Bar graph and representative traces of % change in mIPSC frequency from adolescent and adult females following bath application of 1μM Stressin-1. * indicates significant effect of age (p < 0.05) # signifies significant difference from 0.
Analysis of mIPSC amplitude in these same cells revealed no significant main effects of age [F(1,43) = 0.142, p = 0.708] or concentration of drug [F(1,43) = 2.699, p = 0.079] in females, nor a significant age x drug interaction [F(2,43) = 2.366, p = 0.106] (Fig. 4B; Supplementary Fig. 1). Analysis of raw value changes in mIPSC amplitude revealed similar null results: the concentrations of CRFR1 agonist did not change mIPSC amplitudes in either adolescent [F(2,22) = 1.858, p = 0.180] or adult females [F(2,23) = 2.660, p = 0.093].
Tonic CRFR1 regulates GABAergic transmission in the CeM in an age- and sex-specific manner.
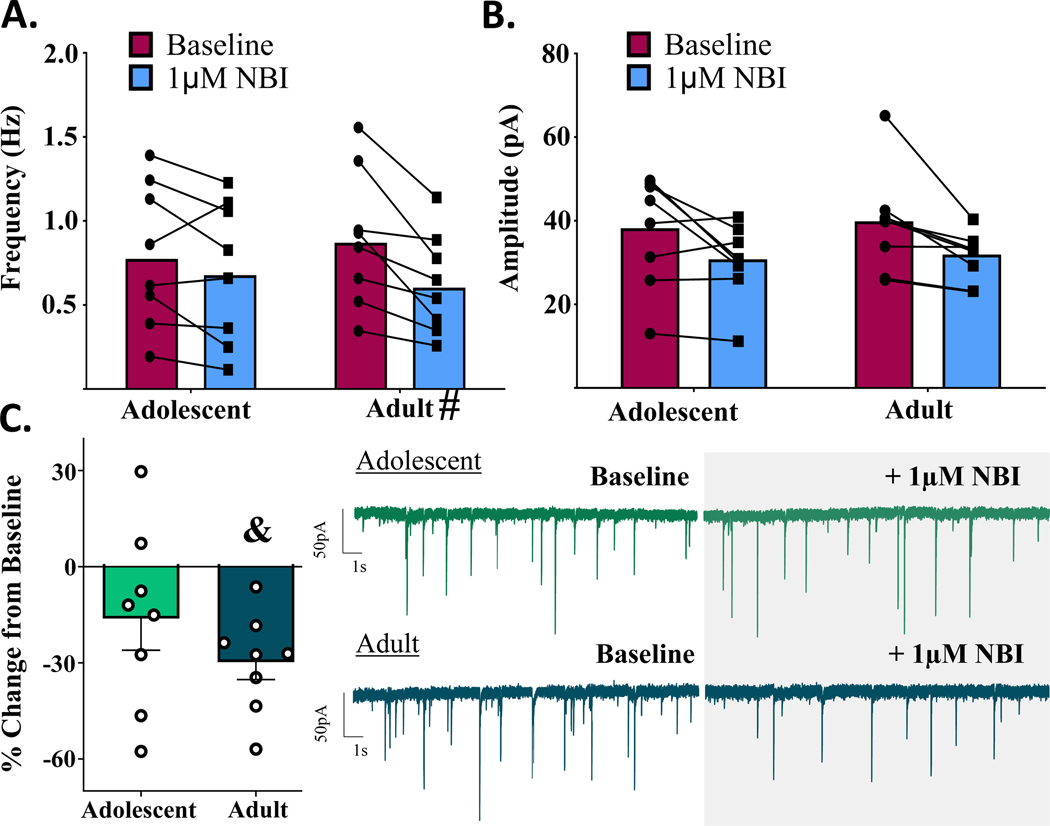
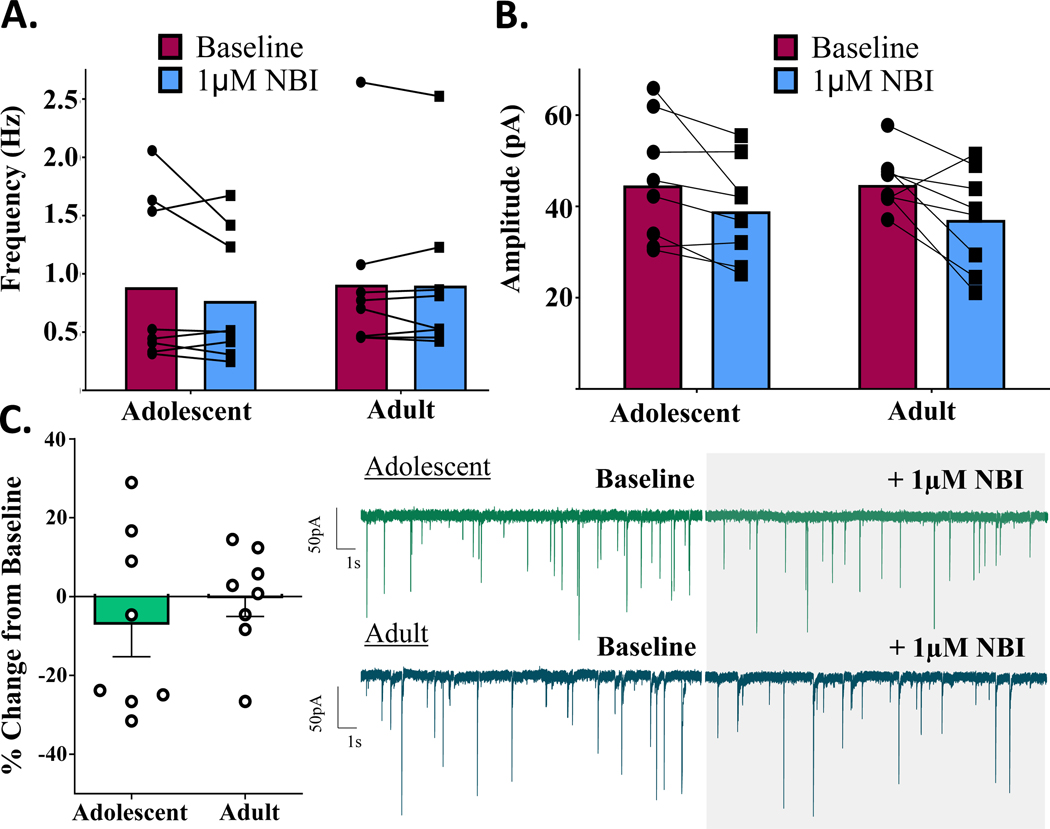
To assess tonic activation of CRFR1 in the CeM, the CRFR1-selective antagonist, NBI 35965 (1 μM) was bath applied following baseline recordings of mIPSCs. There were no main effects of age within males [t(1,14) = 1.200, p = 0.250, n = 8 cells] or females [t(1,14) = 0.717, p = 0.485, n = 8 cells] in this experiment. As depicted in Figs. 5–6, within-group responses to the CRFR1 antagonist were highly variable, particularly within adolescents, and drug application did not produce overall significant changes in mIPSC frequency in either adolescent males [t(1,7) = 1.629, p = 0.147, n = 8 cells] or adolescent females [t(1,7) = 0.876, p = 0.410, n = 8 cells] (Figs. 5C,6C). In adults, however, CRFR1 blockade significantly reduced mIPSC frequency in males [t(1,7) = 5.436, p = 0.001, n = 8 cells] while producing no significant change in mIPSC frequency in females [t(1,7) = 0.090, p = 0.931, n = 8 cells].
Figure 5.
Males: change in mIPSCs following bath application of selective CRFR1-receptor antagonist, NBI (1μM). A) mIPSC frequency activity before and after CRFR1 blockade. 1μM NBI significantly attenuates mIPSC frequency in adult males, while producing no significant change in adolescent males. B) mIPSC amplitude activity before and after CRFR1 blockade. Age did not significantly influence 1μM NBI–induced changes in postsynaptic GABA-A amplitudes. C) mIPSC frequency activity CRFR1 blockade, reported as % change in baseline activity. Only adult males demonstrate significant tonic activation of CRFR1. # signifies significant difference from 0.
Figure 6.
Females: change in mIPSCs following bath application of selective CRFR1-receptor antagonist, NBI (1μM). A) mIPSC frequency activity before and after CRFR1 blockade. 1μM NBI produces no significant change in mIPSC frequency of adolescent or adult females. B) mIPSC amplitude activity before and after CRFR1 blockade. Age did not significantly influence 1μM NBI–induced changes in postsynaptic GABA-A amplitudes. C) mIPSC frequency activity CRFR1 blockade, reported as % change in baseline activity. Females of neither age group demonstrate significant tonic activation of CRFR1 in the CeM. # signifies significant difference from 0.
To determine if membrane properties and basal mIPSC frequency contributed to the notable variability in tonic CRF activity, we performed within-cell Pearson correlations for each sex x age group between 1) % change in mIPSC frequency following NBI bath application, and 2) measures of mIPSC frequency, Rm and Cm. The results, summarized in Supplementary Table 1, revealed no significant correlations across the four groups, indicating that these cell properties are not sufficient to explain the variability in tonic CRF-regulated mIPSC frequency.
Analysis of % change in mIPSC amplitude in these same cells revealed no significant main effects of age in males [t(1,14) = 0.172, p = 0.866, n = 8 cells] or females [t(1,14) = 0.648, p = 0.528, n = 8 cells] in response to the CRFR1 antagonist (Figs 5B, 6B; Supplementary Fig. 1). Similarly, changes in raw mIPSC amplitude values did not differ by age in males [F(1,14) = 0.084, p = 0.776] or females [F(1,14) = 0.038, p = 0.847].
In a subset of adult males (n = 4 cells/3 animals), following completion of NBI experiments, 1 μM Stressin-1 was added to the NBI bath and recorded for an additional 10 min to verify the selectivity of the agonist. This concentration was selected because it previously produced significant potentiation of mIPSC frequency in adult males (Fig. 3C). In the presence of NBI, however, there were no significant changes in mIPSC frequency with the addition of the agonist [t(1,3) = 0.348, p = 0.751; Δ difference: 0.036 Hz ± 0.1035 Hz], and no significant changes in mIPSC amplitude [t(1,3) = 0.477, p = 0.666; Δ difference: 1.12 pA ± 2.348 pA].
Length of experimental procedure impacts post-synaptic, but not presynaptic, function.
Although the results of this research indicate that age and sex did not significantly influence CRFR1-selective drug effects on mIPSC amplitude, we did observe a consistent reduction in mIPSC amplitude across the four drug groups (three concentrations of Stressin-1 and one concentration of NBI) (Figs. 3–6, Supplementary Fig 1). Importantly, these effects did not differ by drug/concentration. We therefore hypothesized that observed declines in amplitude reflected a natural reduction in GABA-A receptor functioning during slice recordings under our experimental conditions (averaging ~ 25 min length). To test this possibility, a sample of CeM neurons in adult males (n = 5 cells/3 animals) were patched and recorded for 30 min in ACSF only. Recordings were then assessed for run-down effects of both sIPSC frequency and amplitude.
Compared to an initial 5 min baseline recording, sIPSC frequency did not decrease after 25 min [t(1,5) = 1.392, p = 0.228] (Supplementary Fig 1). Importantly, however, sIPSC amplitude demonstrated a significant and notable decline after 25 min [t(1,5) = 3.876, p = 0.018; −13.88% ± 3.581% from baseline], independent of any manipulation.
Discussion
CRF is well-established as a regulator of the physiological stress response, stimulating hormone secretion within the hypothalamus and modulating neurotransmission within extra-hypothalamic regions, including the CeM. Notably, studies incorporating diverse subject samples (i.e. females, distinct age groups) are minimal within established CRF literature, despite clinical accounts of age- and sex-specific stress responsivity in humans (Andersen, 2003; Verma et al., 2011). A recently-published review comprehensively addressed the importance of these subject factors in understanding the role of CRF system function in alcohol use disorders (Agoglia et al., 2020a), and importantly, emphasized the need for future research investigating the intersections of these variables. To our knowledge, the present study is the first to directly assess how factors of age and sex interact to influence CRF’s neuromodulatory function in drug-naïve subjects. Overall, our data provide compelling evidence of a developmental shift in native and CRFR1-regulated GABAergic activity within the CeM, with distinct characteristics between males and females.
Although unanticipated, our data uncovered native differences in GABAergic activity within the CeM between age groups and sexes. Adolescent females demonstrated higher sIPSC frequency than both adult females and age-matched males, an interaction which was not observed in action potential-independent mIPSCs. Together, these findings suggest that sex-specific differential input onto CeM neurons may be driving sIPSC differences. In addition to local phasic inhibition, GABAergic inputs from multiple brain regions are known to innervate the CeM, including the medial paracapsular cells/intercalated cell mass (Marowsky et al., 2005) and the lateral sub-nucleus of the CeA (Pitkanen et al., 1997), it is yet unclear whether activation of these projections are age and sex-distinct.
Furthermore, independent of age, females exhibited greater post-synaptic GABA-A receptor amplitudes than males. This sex-specific receptor function could be attributed to differences in post-synaptic receptor expression, with our findings suggesting higher quantities in females than males. Alternatively, differences in GABA-A receptor subunit composition could influence receptor functioning, a hypothesis supported by our findings of sex differences in postsynaptic receptor rise time. Within the central amygdala, the predominant GABA-A receptor α-subunits are α2 and α4 (Esmaeili et al., 2009), with moderate α1 expression present within the medial sub-region (Babaev et al., 2018a). Differences in rise time have been reported between GABA-A receptors expressing α1 and α3/α4 subunits (Barberis et al., 2007; Lagrange et al., 2007). Specifically within the central amygdala, a moderate difference in rise time has been distinguished between α1 and α2-containing receptors (Marowsky et al., 2004). We believe these studies, together with our own findings, support future investigations of sex-specific expression of GABA-A receptor composition within the CeM, and the functional impact on GABAergic inhibition.
In action-potential independent mIPSCs, adults exhibited greater frequencies and amplitudes than adolescents, regardless of sex. The increase in pre- and post-synaptic GABAergic activity in adults may be indicative of developmental maturation of this system, which we have captured at an immature state in our adolescent groups. Future research incorporating younger adolescents would be beneficial for testing this theory. Additionally, as sexually-dimorphic basal GABAergic activity was present in the CeM, we believe future investigations of this region should account for the influence of estrous cycle at the time of experimentation.
In our assessment of CRFR1-regulated GABA transmission in the CeM, we uncovered both age and sex-specific regulatory function. As previously established in adult males (Herman et al., 2013b; Roberto et al., 2010), we found that CRFR1 activation significantly increased mIPSC frequency at the 1 μM concentration of Stressin-1. However, in adolescent males, CRFR1 activation reduced mIPSC frequency at this concentration, opposite of what we observed in adults. Surprisingly, this significant attenuation of mIPSCs was also observed at the 100 nM concentration, indicative of increased CRFR1-sensitivity in adolescent males compared to adults. Together, these findings may highlight an immature, hyper-sensitive function of presynaptic CRFR1 in adolescent males, and may implicate a developmental shift in this stress system. Importantly, females did not exhibit the developmental switch in CRF1R function, as both ages showed CRFR1-dependent inhibition of GABA release, with a potential increase in sensitivity in adult females. These observations in females may implicate the existence of a developmental shift in either CRFR1 function or expression, and requires future investigation.
It is possible that intracellular signaling pathways of CRFR1 are contributing to these observed age/sex effects. Although it is well-established that CRFR1 primarily couples with G-proteins and uses cyclic (c) AMP as a secondary messenger in signaling cascades, multiple intracellular signal transduction pathways have been associated with this receptor within a single brain region (Dautzenberg and Hauger, 2002). This includes Gs-coupling to stimulate adenylyl cyclase and activate PKA pathways, and Gq -coupling to activate intracellular PKC pathways, both of which are associated with unique gene transcription and protein phosphorylation. Furthermore, a G-protein independent pathway has been linked with β-arrestin binding and subsequent CRFR1 downregulation (Valentino et al., 2013). Importantly, coupling of CRFR1 to specific G-proteins is sex-biased under stressful conditions, with males demonstrating a stress-induced preference toward CRFR1- β-arrestin association and consequential receptor internalization, which is absent in females (Bangasser et al., 2010). Established literature hints at the importance of intracellular signaling pathways for dynamic regulation of synaptic activity; for instance, previous research in the anterior pituitary has demonstrated that sustained stress exposure can desensitize CRF-stimulated cAMP and downregulate CRFR1 (Hauger and Dautzenberg, 2000). Furthermore, evidence of CRFR1 coupling to Gi-proteins has been reported in human and rodent cells (Blank et al., 2003; Brar et al., 2004; Grammatopoulos et al., 1999; Grammatopoulos et al., 2000; Wietfeld et al., 2004), which may explain the attenuation of mIPSCs observed in our adolescent groups/adult females. Thus, shifts in proportional reliance on signaling pathways may elucidate on the responses to CRFR1 activation observed in this study, and further electrophysiological investigations are required to investigate sex and age-specific reliance on distinct intracellular signaling pathways.
In addition to activity-dependent intracellular signaling pathways and GABA-A receptor subunit composition, other neurotransmitter systems may contribute to our observed mIPSC frequency attenuation in adolescents. A recent investigation of neuronal activity in the lateral habenula found that CRF reduced GABAergic neurotransmission in early-adolescent subjects (P21–28), as we observed in mid-late adolescent subjects (P40–48), and this effect was mediated by endocannabinoid signaling (Authement et al., 2018). We have therefore considered that CRF’s modulation of presynaptic GABA release is not only the result of direct CRF-CRFR1 association, but also an interaction of the CRF system with presynaptic cannabinoid receptors.
It is worth noting that CRFR1 activation attenuated synaptic GABA release in adult females at concentrations insufficient to change mIPSC frequency in adult males. This sexually-dimorphic sensitivity has been similarly observed in the locus coeruleus, whereupon females respond to significantly lower doses of synthetic CRF than males, further supporting our conclusion that adult females demonstrate hypersensitive CRFR1 activation. Together with the opposing direction of effect observed between adult males and females, we have provided neurophysiological evidence supporting clinical reports that females and males differ in their response to stress (Kudielka and Kirschbaum, 2005; Walker and McCormick, 2009; Young et al., 2007). Of course, to determine whether these age and sex-specific physiological findings correspond to differences in the expression of anxiety-like behavior and stress response, additional experiments are required assessing and manipulating the CeM CRF system in vivo.
It is important to acknowledge that the data collected from our CRFR1 experiments contained more variability within females than males. Influences of sex hormones such as estrogen could potentially account for this variability in activity (Marrocco and McEwen, 2016); however, in the few instances in which a female subject contributed two data points to the same experiment under random assignment, responses from that same female were also highly variable. This would suggest that distinct sub-populations of cells (with diverse CRFR1 function/responsivity) contribute to the variability observed in these data, more so than hormonal influences. Importantly, this variability was also similar between adolescent and adult females, reducing the likelihood of pubertal influence. However, it should be acknowledged that this adolescent age range (P40–48) represents a different stage of puberty between males and females (Bell, 2018), with an earlier onset (~P35) and conclusion (~P42) of puberty in female rats. Theoretically, as our experimental females were tested within a more mature pubertal stage than males, this could explain why distinct age differences were observed in the data collected from males but not females. To empirically test this theory, future research can investigate the CRF system within the CeM of pre-pubertal animals (~P30–33). As alluded to earlier, investigations of younger subjects would also pinpoint when shifts in CRFR1 function can be first detected, which is important for early intervention of CRFR1-targetted drug treatments for stress disorders.
In general, our experimental recordings revealed moderate attenuation of mIPSC amplitude, independent of drug application. In rodent brain tissue, GABA-A receptors have previously demonstrated this “rundown effect”, which refers to a progressive loss of receptor response in the absence of an agonist (Mathers, 1991). Independent of this rundown effect, prior literature has demonstrated that 200 nM CRF is not sufficient to produce changes in non-evoked postsynaptic amplitudes, either sIPSC (Agoglia et al., 2020b) or mIPSC (Herman et al., 2013b). In congruence, our experiments did not find main effects of drug on mIPSC amplitude in any age or sex following bath application of CRFR1-targetting Stressin-1. However, we must be cautious when drawing comparisons between experiments including CRF vs CRFR1-selective agonists, as they may not impose identical effects on postsynaptic receptor amplitude. Specifically, CRF may affect both CRFR1 and CRFR2 receptors as a non-selective agonist, producing changes in amplitude that may differ from the selective CRFR1 activation used in this study. This further emphasizes importance of using non-selective CRF in future experiments assessing CRF activity in the CeM.
Assessment of tonic CRF-regulated GABA transmission further identified age-specific differences in males, with CRFR1 blockade significantly reducing mIPSC frequency in adult males and producing no consistent change in adolescents of the same sex. In contrast, age did not influence tonic CRF-regulated GABA transmission in females. These results suggest that adult males may exhibit significantly greater CRF-CRFR1 regulated GABA release, possibly attributable to higher tonic release of CRF in this group compared to younger males and females. This is consistent with previous reports of reduced CRF mRNA in the CeA of early adolescent (P30) males and females and adult (P60) females relative to adult (P60) males (Viau et al., 2005). It is important to note that CRF peptide is both locally-generated and recruited from afferent CRF+ fibers originating from various areas, including the BNST (Schreiber and Gilpin, 2018), and the quantification of CRF concentrations within the CeM requires further targeted investigation.
By isolating mIPSCs in CRFR1-targetting experiments, we were able to assess drug-induced changes occurring specifically via presynaptic action potential-independent GABA release, which has been shown to be one of the primary sites of action of CRFR1. However, we acknowledge that assessments of CRFR1 effects on both sIPSC and mIPSC function would be highly informative, particularly following a recent investigation of CRF effects on adult CeA GABA transmission which found CRF-induced increases in sIPSC frequency in both males and females (Agoglia et al., 2020b). Ideally, such research would analyze IPSC effects across age and sex using a consistent drug – CRF, for nonspecific CRF receptor activation, or Stressin-1, for targeted CRFR1 activation – to determine whether CRF differentially regulates sIPSC and mIPSC activity in this region.
When assessing cellular excitability, we determined that CeM neuronal membrane properties and resting membrane potential did not differ between age groups and sexes. However, adults demonstrated lower AP thresholds than adolescents, and males demonstrated lower AP thresholds than females, although this effect appears to be predominantly driven by adult male subjects. Furthermore, adults of both sexes demonstrated quicker response to current injection than adolescents without a difference in the amount of current injection required to produce an AP (rheobase) in either age. Effects of sex were found among certain characteristics of firing activity: for instance, males on average exhibited slightly higher AP amplitudes than females regardless of age. Additionally, when AP threshold was met, adolescent males exhibited robustly more activity than adult males, an age difference that was absent in females. However, adolescent males also demonstrated notably fewer tonically active cells than adult counterparts, a disparity that was more modest in females.
Together, these findings may point to age- and sex-specific differences in voltage-gated ion channel function. Alternatively, the amount of synaptic input could explain these group differences in neuronal excitability. As adolescent and adult males did not differ in sIPSC frequency, local phasic inhibition is not likely responsible for this effect. However, tonic GABA inhibition and glutamatergic input is natively abundant within the CeA and also a target of CRF (Herman et al., 2013a; Liu et al., 2004; Silberman and Winder, 2013). Since CRF acts on both inhibitory and excitatory systems, it would be worthwhile to investigate CRF1R effects on tonic GABA inhibition and the glutamatergic system within the CeM across sex and ontogeny. It would further be beneficial to assess how CRFR1 activation influences intrinsic excitability of CeM neurons, given our findings of sex and age-specific CRFR1-regulated inhibition.
Our novel and compelling findings regarding the role of the CRF system in a primary stress- and anxiety-associated brain area may underlie age and sex-specific differences in response to stressful stimuli. Established literature has positively associated CRFR1 activation with anxiogenic behavior (see review: (Schreiber and Gilpin, 2018), in part via CRFR1- mediated increased GABA release in the CeM of adult male subjects (Herman et al., 2013b; Roberto et al., 2010), an effect we replicated. Importantly, our data suggest that CRFR1 activation decreases GABA release in adolescent males and females of both ages, suggesting these groups may respond to CRFR1 activation with reduced anxiety-like behavior. When considering age-specific stress response in humans, it is important to acknowledge that adolescents are not only phenotypically characterized with increased sensitivity to stressful situations, but also with inappropriate responses to anxiety-inducing events. This includes high rates of risk-taking and impaired decision-making while undergoing stress (Andersen and Teicher, 2008; Lee et al., 2003; Romeo and McEwen, 2006), behavior which has been attributed to incomplete maturation of brain regions sensitive to stress, including the amygdala (Giedd and Rapoport, 2010; Tottenham, 2017; Tottenham and Sheridan, 2009). Thus, we hypothesize that this inappropriate stress response may be attributable, in part, to age-specific CRF function within the CeM. Our lab is currently investigating the influence of this system in vivo in offspring prenatally exposed to alcohol, to elucidate on the causal relationship between CeM physiology and stress-related behaviors.
Across species, females exhibit greater susceptibility and sensitivity to stress than males, particularly within the peri-pubertal and post-pubertal window of development (see review: (Bale and Epperson, 2015)). Although our CRFR1 assessments support this characteristic hypersensitivity, the opposing direction of CRFR1-regulated GABA transmission between sexes would implicate distinct behavioral responses to stress between males and females as well. It has been previously suggested that estrogen contributes to a stress-resilient phenotype in females (Kajantie and Phillips, 2006), an effect which corresponds to altered neurotransmitter levels within the hippocampus, frontal cortex and amygdala, although this has yet to be causally investigated. However, several additional studies have reported exactly the opposite: greater stress-induced HPA activity in females compared to males (Kudielka and Kirschbaum, 2005; Walker and McCormick, 2009; Young et al., 2007). From these studies, in combination with our own findings, we hypothesize that the stress response in females is dynamic across brain regions, and perhaps exemplary of a “stress-protective” response in extra-hypothalamic regions which seeks to compensate or correct for increased HPA axis sensitivity during development. However, targeted behavioral assessments will be essential for determining the sex-specific contributions of different brain regions to stress response in naïve animals.
In conclusion, this study highlights how factors of age and sex influence neurophysiological activity, and we recommend these factors be considered and reported in future investigations of the CRF system and/or CeA physiology. Furthermore, as both age and sex influence neurophysiological response to CRFR1 activation, these factors may also challenge the efficacy of CRFR1-targetted drug treatments for stress disorders in humans, and should therefore be considered for their influence in future clinical trials.
Supplementary Material
Acknowledgments:
The experiments included in this manuscript were supported by NIAAA grants P50 AA0178230, T32 AA025606 and F31 AA028166.
Footnotes
Conflict of interest statement: The authors declare no competing financial interests.
References
- Agoglia AE, Crofton EJ, Herman MA, 2020a. Biological intersection of sex, age, and environment in the corticotropin releasing factor (CRF) system and alcohol. Neuropharmacology 170, 108045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agoglia AE, Tella J, Herman MA, 2020b. Sex differences in corticotropin releasing factor peptide regulation of inhibitory control and excitability in central amygdala corticotropin releasing factor receptor 1-neurons. Neuropharmacology 180, 108296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, 2003. Trajectories of brain development: point of vulnerability or window of opportunity? Neuroscience & Biobehavioral Reviews 27, 3–18. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH, 2008. Stress, sensitive periods and maturational events in adolescent depression. Trends in neurosciences 31, 183–191. [DOI] [PubMed] [Google Scholar]
- Authement ME, Langlois LD, Shepard RD, Browne CA, Lucki I, Kassis H, Nugent FS, 2018. A role for corticotropin-releasing factor signaling in the lateral habenula and its modulation by early-life stress. Sci Signal 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babaev O, Chatain CP, Krueger-Burg D, 2018a. Inhibition in the amygdala anxiety circuitry. Experimental & molecular medicine 50, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babaev O, Chatain CP, Krueger-Burg D, 2018b. Inhibition in the amygdala anxiety circuitry. Experimental & molecular medicine 50, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo M, Cruz MT, Siggins GR, Messing R, Roberto M, 2008. Protein kinase C epsilon mediation of CRF- and ethanol-induced GABA release in central amygdala. Proc Natl Acad Sci U S A 105, 8410–8415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Epperson CN, 2015. Sex differences and stress across the lifespan. Nature Neuroscience 18, 1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H, Van Bockstaele EJ, Valentino RJ, 2010. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry 15, 877, 896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberis A, Mozrzymas JW, Ortinski PI, Vicini S, 2007. Desensitization and binding properties determine distinct α1β2γ2 and α3β2γ2 GABAA receptor‐channel kinetic behavior. European Journal of Neuroscience 25, 2726–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MR, 2018. Comparing Postnatal Development of Gonadal Hormones and Associated Social Behaviors in Rats, Mice, and Humans. Endocrinology 159, 2596–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank T, Nijholt I, Grammatopoulos DK, Randeva HS, Hillhouse EW, Spiess J, 2003. Corticotropin-releasing factor receptors couple to multiple G-proteins to activate diverse intracellular signaling pathways in mouse hippocampus: role in neuronal excitability and associative learning. J Neurosci 23, 700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman RE, Beck KD, Luine VN, 2003. Chronic stress effects on memory: sex differences in performance and monoaminergic activity. Hormones and behavior 43, 48–59. [DOI] [PubMed] [Google Scholar]
- Brar BK, Chen A, Perrin MH, Vale W, 2004. Specificity and regulation of extracellularly regulated kinase1/2 phosphorylation through corticotropin-releasing factor (CRF) receptors 1 and 2beta by the CRF/urocortin family of peptides. Endocrinology 145, 1718–1729. [DOI] [PubMed] [Google Scholar]
- Brown GR, Spencer KA, 2013. Steroid hormones, stress and the adolescent brain: a comparative perspective. Neuroscience 249, 115–128. [DOI] [PubMed] [Google Scholar]
- Dautzenberg FM, Hauger RL, 2002. The CRF peptide family and their receptors: yet more partners discovered. Trends in pharmacological sciences 23, 71–77. [DOI] [PubMed] [Google Scholar]
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Lüthi A, 2009. Amygdala inhibitory circuits and the control of fear memory. Neuron 62, 757–771. [DOI] [PubMed] [Google Scholar]
- Esmaeili A, Lynch JW, Sah P, 2009. GABAA receptors containing gamma1 subunits contribute to inhibitory transmission in the central amygdala. Journal of neurophysiology 101, 341–349. [DOI] [PubMed] [Google Scholar]
- Faruzzi AN, Solomon MB, Demas GE, Huhman KL, 2005. Gonadal hormones modulate the display of submissive behavior in socially defeated female Syrian hamsters. Hormones and behavior 47, 569–575. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Rapoport JL, 2010. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron 67, 728–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Herman MA, Roberto M, 2015. The central amygdala as an integrative hub for anxiety and alcohol use disorders. Biol Psychiatry 77, 859–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammatopoulos DK, Dai Y, Randeva HS, Levine MA, Karteris E, Easton AJ, Hillhouse EW, 1999. A novel spliced variant of the type 1 corticotropin-releasing hormone receptor with a deletion in the seventh transmembrane domain present in the human pregnant term myometrium and fetal membranes. Mol Endocrinol 13, 2189–2202. [DOI] [PubMed] [Google Scholar]
- Grammatopoulos DK, Randeva HS, Levine MA, Katsanou ES, Hillhouse EW, 2000. Urocortin, but not corticotropin-releasing hormone (CRH), activates the mitogen-activated protein kinase signal transduction pathway in human pregnant myometrium: an effect mediated via R1alpha and R2beta CRH receptor subtypes and stimulation of Gq-proteins. Mol Endocrinol 14, 2076–2091. [DOI] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey B, 2008. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological psychiatry 63, 927–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauger RL, Dautzenberg FM, 2000. Regulation of the stress response by corticotropin-releasing factor receptors. Neuroendocrinology in physiology and medicine. Springer, pp. 261–286. [Google Scholar]
- Herman MA, Contet C, Justice NJ, Vale W, Roberto M, 2013a. Novel subunit-specific tonic GABA currents and differential effects of ethanol in the central amygdala of CRF receptor-1 reporter mice. J Neurosci 33, 3284–3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MA, Kallupi M, Luu G, Oleata CS, Heilig M, Koob GF, Ciccocioppo R, Roberto M, 2013b. Enhanced GABAergic transmission in the central nucleus of the amygdala of genetically selected Marchigian Sardinian rats: alcohol and CRF effects. Neuropharmacology 67, 337–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajantie E, Phillips DI, 2006. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology 31, 151–178. [DOI] [PubMed] [Google Scholar]
- Kang-Park M, Kieffer BL, Roberts AJ, Siggins GR, Moore SD, 2015. Interaction of CRF and kappa opioid systems on GABAergic neurotransmission in the mouse central amygdala. J Pharmacol Exp Ther 355, 206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehne JH, 2007. The CRF1 receptor, a novel target for the treatment of depression, anxiety, and stress-related disorders. CNS Neurol Disord Drug Targets 6, 163–182. [DOI] [PubMed] [Google Scholar]
- Klein LC, Corwin EJ, 2002. Seeing the Unexpected: How Sex Differences in Stress Responses May Provide a New Perspective on the Manifestation of Psychiatric Disorders. Current Psychiatry Reports 4, 441–448. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C, 2005. Sex differences in HPA axis responses to stress: a review. Biological psychology 69, 113–132. [DOI] [PubMed] [Google Scholar]
- Lagrange AH, Botzolakis EJ, Macdonald RL, 2007. Enhanced macroscopic desensitization shapes the response of α4 subtype‐containing GABAA receptors to synaptic and extrasynaptic GABA. The Journal of physiology 578, 655–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PR, Brady D, Koenig JI, 2003. Corticosterone alters N-methyl-D-aspartate receptor subunit mRNA expression before puberty. Brain Res Mol Brain Res 115, 55–62. [DOI] [PubMed] [Google Scholar]
- Liu J, Yu B, Neugebauer V, Grigoriadis DE, Rivier J, Vale WW, Shinnick-Gallagher P, Gallagher JP, 2004. Corticotropin-Releasing Factor and Urocortin I Modulate Excitatory Glutamatergic Synaptic Transmission. The Journal of Neuroscience 24, 4020–4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhaes AC, Holmes KD, Dale LB, Comps-Agrar L, Lee D, Yadav PN, Drysdale L, Poulter MO, Roth BL, Pin JP, Anisman H, Ferguson SS, 2010. CRF receptor 1 regulates anxiety behavior via sensitization of 5-HT2 receptor signaling. Nat Neurosci 13, 622–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marowsky A, Fritschy JM, Vogt KE, 2004. Functional mapping of GABAA receptor subtypes in the amygdala. European Journal of Neuroscience 20, 1281–1289. [DOI] [PubMed] [Google Scholar]
- Marowsky A, Yanagawa Y, Obata K, Vogt KE, 2005. A specialized subclass of interneurons mediates dopaminergic facilitation of amygdala function. Neuron 48, 1025–1037. [DOI] [PubMed] [Google Scholar]
- Marrocco J, McEwen BS, 2016. Sex in the brain: hormones and sex differences. Dialogues Clin Neurosci 18, 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers DA, 1991. Activation and inactivation of the GABAA receptor: insights from comparison of native and recombinant subunit assemblies. Can J Physiol Pharmacol 69, 1057–1063. [DOI] [PubMed] [Google Scholar]
- McEwen BS, 1998. Protective and damaging effects of stress mediators. N Engl J Med 338, 171–179. [DOI] [PubMed] [Google Scholar]
- Nie Z, Schweitzer P, Roberts AJ, Madamba SG, Moore SD, Siggins GR, 2004. Ethanol augments GABAergic transmission in the central amygdala via CRF1 receptors. Science 303, 1512–1514. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Savander V, LeDoux JE, 1997. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci 20, 517–523. [DOI] [PubMed] [Google Scholar]
- Przybysz KR, Werner DF, Diaz MR, 2017. Age-dependent regulation of GABA transmission by kappa opioid receptors in the basolateral amygdala of Sprague-Dawley rats. Neuropharmacology 117, 124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainnie DG, Fernhout B, Shinnick-Gallagher P, 1992. Differential actions of corticotropin releasing factor on basolateral and central amygdaloid neurones, in vitro. Journal of Pharmacology and Experimental Therapeutics 263, 846–858. [PubMed] [Google Scholar]
- Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, Cottone P, Madamba SG, Stouffer DG, Zorrilla EP, 2010. Corticotropin releasing factor–induced amygdala gamma-aminobutyric acid release plays a key role in alcohol dependence. Biological psychiatry 67, 831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo RD, McEwen BS, 2006. Stress and the adolescent brain. Ann N Y Acad Sci 1094, 202–214. [DOI] [PubMed] [Google Scholar]
- Rouzer SK, Cole J, Herman JM, Varlinskaya EI, Diaz MR, 2017. Moderate maternal alcohol exposure on gestational day 12 impacts anxiety-like behavior in offspring. Frontiers in Behavioral Neuroscience 11, 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber AL, Gilpin NW, 2018. Corticotropin-Releasing Factor (CRF) Neurocircuitry and Neuropharmacology in Alcohol Drinking. Handb Exp Pharmacol 248, 435–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman Y, Winder DG, 2013. Corticotropin releasing factor and catecholamines enhance glutamatergic neurotransmission in the lateral subdivision of the central amygdala. Neuropharmacology 70, 316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorzewska A, Wislowska-Stanek A, Lehner M, Turzynska D, Sobolewska A, Krzascik P, Plaznik A, 2017. Corticotropin releasing factor receptor 1 antagonist differentially inhibits freezing behavior and changes gamma-aminobutyric acidergic activity in the amygdala in low- and high-anxiety rats. J Physiol Pharmacol 68, 35–46. [PubMed] [Google Scholar]
- Tottenham N, 2017. The Brain’s Emotional Development. Cerebrum 2017. [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Sheridan MA, 2009. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Front Hum Neurosci 3, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Bangasser D, Van Bockstaele EJ, 2013. Sex-biased stress signaling: the corticotropin-releasing factor receptor as a model. Mol Pharmacol 83, 737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Johnson JM, Przybysz KR, Deak T, Diaz MR, 2020. Adolescent forced swim stress increases social anxiety-like behaviors and alters kappa opioid receptor function in the basolateral amygdala of male rats. Prog Neuropsychopharmacol Biol Psychiatry 98, 109812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma R, Balhara YP, Gupta CS, 2011. Gender differences in stress response: Role of developmental and biological determinants. Ind Psychiatry J 20, 4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viau V, Bingham B, Davis J, Lee P, Wong M, 2005. Gender and puberty interact on the stress-induced activation of parvocellular neurosecretory neurons and corticotropin-releasing hormone messenger ribonucleic acid expression in the rat. Endocrinology 146, 137–146. [DOI] [PubMed] [Google Scholar]
- Walker C-D, McCormick C, 2009. Development of the stress axis: maternal and environmental influences. [Google Scholar]
- Wietfeld D, Heinrich N, Furkert J, Fechner K, Beyermann M, Bienert M, Berger H, 2004. Regulation of the coupling to different G proteins of rat corticotropin-releasing factor receptor type 1 in human embryonic kidney 293 cells. J Biol Chem 279, 38386–38394. [DOI] [PubMed] [Google Scholar]
- Wood GE, Shors TJ, 1998. Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proceedings of the National Academy of Sciences 95, 4066–4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EA, Korszun A, Figueiredo HF, Banks-Solomon M, Herman JP, 2007. Sex Differences in HPA axis regulation. Sex differences in the brain: From genes to behavior, 95–105. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.