Abstract
Background:
Inflammatory injury in the donor lung remains a persistent challenge in lung transplantation that limits donor organ usage and post-transplant outcomes. Inducing immunomodulatory capacity in donor organs could address this unsolved clinical problem. We sought to apply CRISPR-Cas technologies to the donor lung to fine-tune immunomodulatory gene expression, exploring for the first time the therapeutic use of CRISPR-mediated transcriptional activation in the whole donor lung.
Methods:
We explored the feasibility of CRISPR-mediated transcriptional upregulation of IL-10, a key immunomodulatory cytokine, in vitro and in vivo. We first evaluated the potency, titratability, and multiplexibility of the gene activation in rat and human cell lines. Next, in vivo CRISPR-mediated IL-10 activation was characterized in rat lungs. Finally, the IL-10-activated donor lungs were transplanted into recipient rats to assess the feasibility in a transplant setting.
Results:
The targeted transcriptional activation induced robust and titrable IL-10 upregulation in vitro. The combination of guide RNAs also facilitated multiplex gene modulation, i.e. simultaneous activation of IL-10 and IL1 receptor antagonist. In vivo profiling demonstrated that adenoviral delivery of Cas9-based activators to the lung was feasible with the use of immunosuppression, which is routinely applied to organ transplant recipients. The transcriptionally modulated donor lungs retained IL-10 upregulation in isogeneic and allogeneic recipients.
Conclusions:
Our findings highlight the potential of CRISPR epigenome editing to improve lung transplant outcomes by creating a more favorable immunomodulatory environment in the donor organ, a paradigm that may be extendable to other organ transplants.
Keywords: CRISPR, gene activation, dSaCas9-VPR, IL-10, lung transplant
Introduction
Inflammatory donor lung injury remains a significant challenge in lung transplantation (LTx). Clinically, inflammatory injury contributes to primary graft dysfunction (PGD) and chronic lung allograft dysfunction (CLAD), which limit both short- and long-term outcomes. Furthermore, concerns about donor lung injury substantially lower the donor lung utilization rate to approximately 20%.(1, 2)
Pre-implantation transcriptome modulation of the donor lung holds promise for expanding the donor pool and improving outcomes in LTx. Preclinical studies using in vivo gene therapy have shown that delivery of an immunomodulatory gene to the donor lung effectively ameliorates graft injury(3-7) and rejection.(8) Furthermore, the development of ex vivo lung perfusion (EVLP) system(9-13) has enabled ex vivo donor lung modulation.(14-16) Indeed, pre-implantation donor organ optimization is realistically becoming a practical treatment.
CRISPR-Cas genome editing technologies have opened a new era of genetic engineering and continue to expand the potential of genome-targeting therapies.(17, 18) Beyond genome editing, the simple and versatile platform has enabled flexible modulation of a broad range of genes.(19-23) We hypothesized that a CRISPR-Cas-based therapy could fine-tune immunomodulatory gene expressions, thus advancing donor lung engineering. However, the feasibility of this approach remains uncertain.
In this study, we explored the feasibility of deploying CRISPR-Cas-mediated transcriptional activators in the donor lung using rat models as the first step toward a translational path.
Materials and Methods
Vector design and construction
Plasmids expressing dSaCas9-VPR were a gift from Dr. George Church at Harvard University (Addgene plasmid # 68495). gRNAs were designed to bind the region between 1- and 500-nt upstream of the rat IL-10 (Supplementary Methods). The gRNA expression cassettes were synthesized as gBlock (Integrated DNA Technologies, Coralville, IA). Single vectors were generated using standard cloning methods. Golden Gate Assembly was also used when appropriate. The plasmids were purified using the EndoFree Plasmid Maxi Kit (Qiagen, Hilden, Germany) to transfect cells.
Adenoviral vector
The high-titer recombinant adenoviruses, which E1/E3 deleted replication-incompetent human adenovirus serotype 5, were generated by SIRION Biotech (Martinsried, Germany). The functional titer of the viruses was measured upon arrival to our lab using a QuickTiter™ Adenovirus Titer Immunoassay Kit (CellBiolab, San Diego, CA). The titer determined at our site was used for the experiments.
Animals
Male inbred Lewis rats (LEW/SsNHsd, 250–350 g) and Brown Norway rats (BN/RijHsd, 250–350 g) were purchased from Envigo (Huntingdon, United Kingdom). and maintained in a pathogen-free environment. Experiments were performed following Animal Usage Protocol # 6057 approved by the Committee of Animal Resources Centre at University Health Network. Data were collected from five to ten rats for each group. The number of rats in each experiment is described in the figure legends.
Viral administration in vivo
Rats were anesthetized using isoflurane, intubated with a 16G catheter (BD382258, BD, Franklin Lakes, NJ), and ventilated (Harvard Apparatus, Holliston, MA) at a tidal volume of 10 ml/kg, positive end-expiratory pressure (PEEP) 2 cmH2O, fraction of inspired oxygen (FiO2) 1.0, and a respiratory rate (RR) of 80 breaths per minute in the supine position. The intubation tube was then advanced into the left bronchus that was confirmed by observing the movement of the thoracic cavity. The rat was changed to the left-side down position and 500 μl of diluent (HEPES 100 mM, MgCl2 20 mM) with or without viral vectors was administered to the left lung through the intubation tube. After pulling back the intubation tube into the trachea, the rats were ventilated in the left-side down position until awakening.
In single lung transplant experiments, viral vectors were delivered to the left lung of a donor rat in vivo 24 hours prior to the donor lung retrieval.
Immunosuppression regimen
For transient immunosuppression, cyclosporine (15 mg/kg/day), azathioprine (6 mg/kg/day), and methylprednisolone (30 mg/kg) were intraperitoneally injected 2h before viral vector administration on days 0, 1, and 2. At 2h post-delivery, 30 mg/kg methylprednisolone alone was intraperitoneally injected. In the continued immunosuppression group, triple immunosuppressants (cyclosporine 15 mg/kg/day, azathioprine 6 mg/kg/day, and methylprednisolone 2.5 mg/kg/day) were subcutaneously injected daily following the transient immunosuppression protocol.
Rat single lung transplant surgery
Orthotopic left lung transplantation was performed as previously described.(3-5) Briefly, donor rats were anesthetized with isoflurane, intubated with a 14G catheter, and ventilated at a tidal volume of 10 ml/kg, PEEP of 2 cmH2O, FiO2 of 0.5, and 80 breaths per minute in the supine position. After laparotomy, 1000 USP units/kg of heparin and 30 mg/kg of methylprednisolone were injected into inferior vena cava (IVC). Blood was drawn from the abdominal aorta to measure the donor P/F ratio. The thoracic cavity was opened by median sternotomy. The lungs were flushed with 20 ml of low potassium dextran solution (LPD)(24) through main pulmonary artery (PA) using gravity flow. Three cuffs were placed on the grafts for anastomoses. The retrieved donor lung was kept on an ice-cold plate during cuff attachment. Upon completion of cuff placement, the graft was transferred and stored in the 4°C fridge until the time of anastomosis.
Recipient rats were anesthetized and intraperitoneally injected with the triple immunosuppressive agents described above. After establishing ventilation at FiO2 of 1.0, recipient rats were placed in the lateral position. Following left thoracotomy, the three anastomoses (left PA, PV, and bronchus), were completed by securing the inserted cuff. Warm ischemic time was set at 15 min. The cold ischemic time, which is defined as the time from flushing to transferring the lung from the fridge to the surgical field, was median (interquartile range) 69 (61) min. There were no statistically significant differences in CIT between the diluent and two gRNAs groups in both iso- and allo-transplantation in the analysis using Mann Whitney test. After 5 min of reperfusion, blood was taken from the left PV for blood gas analysis, which represented graft oxygenation on POD0. Fluid supplementation and analgesia were applied until POD2.
Lewis donor to Lewis recipient transplantation was performed for iso-transplant, while Brown Norway donor to Lewis recipient transplantation was used for allo-transplant.
Sample collection from rats
Rats were anesthetized, intubated, and ventilated. Arterial blood was collected from the abdominal aorta for blood gas analysis. The left lung was divided into four portions for formalin fixation, storage in RNAlater (Thermo Fisher Scientific), snap-frozen, and wet-to-dry weight ratio measurement in the sequence from the apical to basal part to be used for histological analysis, RNA assay, and protein assay.
Statistics
Data were analyzed using the Prism software (GraphPad Software, San Diego, CA). A two-tailed unpaired Student’s t-test was used in in vitro experiments. For in vivo experiments, the Mann-Whitney test or Kruskal-Wallis test followed by Dunn’s correction was used to compare two and multiple groups, respectively. Two-way ANOVA followed by Bonferroni’s correction was used to analyze the experimental groups at different time points. Statistical significance is set at p < 0.05.
Other methods used in this study are described in the supplementary methods.
Results
Activation of endogenous IL-10 via CRISPR-Cas system in vitro
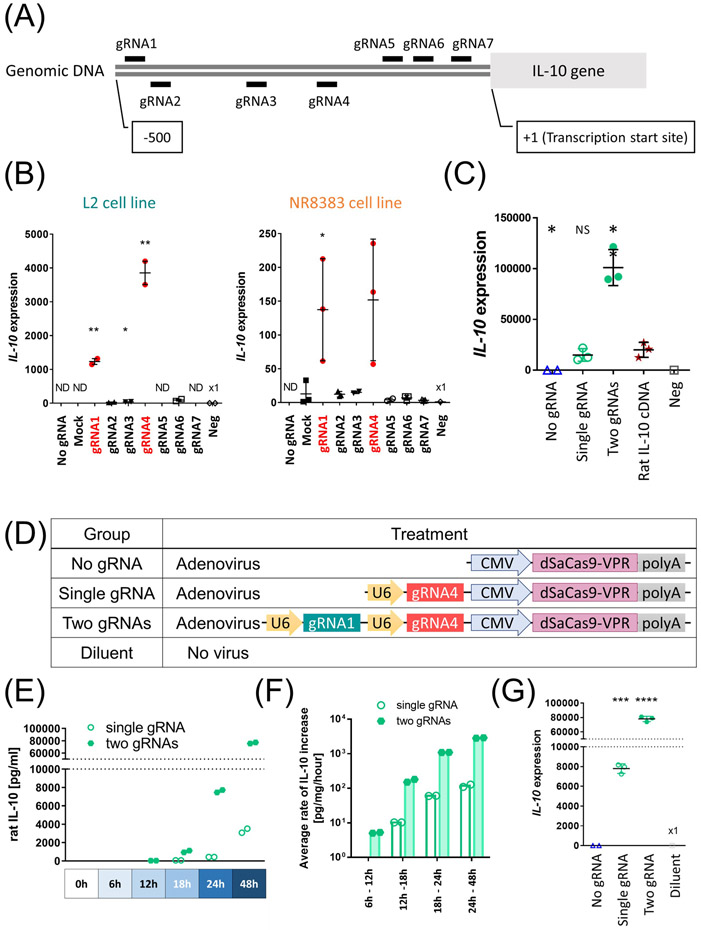
We employed the dSaCas9-VPR,(25) which is a single-component Cas9-based activator comprised of a nuclease-deficient Staphylococcus aureus Cas9 fused with a tripartite transcriptional activation effector.(25) We selected interleukin 10 (IL-10), which encodes a key anti-inflammatory and immunomodulatory cytokine, as a primary target to explore the potential for immunomodulation.
In vitro screening of gRNAs (Fig. 1A) identified two gRNAs that strongly activated IL-10 in a rat lung epithelial cell line (Fig. 1B, left panel). The dSaCas9-VPR construct also enhanced IL-10 expression in a rat lung macrophage cell line, which represents a primary source of IL-10 (Fig. 1B, right panel). As in previous reports on other genes,(19, 26) combination of the two distinct gRNAs (two-gRNA group) synergistically enhanced IL-10 activation. The IL-10 expression in the two-gRNA group surpassed that of rat IL-10 cDNA delivery driven by cytomegalovirus (CMV) promoter (Fig. 1C).
Figure 1.
Transcriptional activation of the endogenous IL-10 using dSaCas9-VPR in rat lung cell lines. (A) The design of gRNAs. The short lines indicate the genomic loci targeted by each gRNA (gRNA1 to gRNA7). (B) Each gRNA was expressed along with dSaCas9 by co-transfection of two plasmids in either L2 cells (n=2, independent transfections) or NR8383 cells (n=3, independent transfections). IL-10 expression was measured after 48 hours by qPCR. Mean (SD) are shown. Negative control (Neg) received transfection reagent without any plasmids. Ct values were normalized to a housekeeping gene peptidylprolyl isomerase A (PPIA). Values detected after 40 cycles were shown as fold expression to Neg. Mock represents effects of non-targeting gRNA. Student’s t-test was applied to compare each group with negative control (L2 cells) or Mock group (NR8383 cells). (C) L2 cells were transfected with a single plasmid or co-transfected with two plasmids (equimolar quantity) and assessed for IL-10 gene expression after 48h (n=3 independent transfections). Each group was compared with the rat IL-10 cDNA group (Rat IL-10 ORF) using Student’s t-test. (D) Experimental groups used for in vitro assessment. (E, F) L2 cells were transduced with adenoviral vector at multiplicity of infection (MOI) of 250 (n=2, independent transductions). (E) IL-10 protein concentration of the cell culture supernatants was measured by ELISA at different time points. The undetected values were set as 0, which was a back-calculated concentration of the sample with the lowest OD. (F) Calculated average rates of IL-10 protein increase. Values above detection limits were used for calculation. (G) IL-10 transcript levels 48h after adenoviral transduction measured by qPCR (n=3, independent transductions). Student’s t-test was used to compare with no gRNA group. ND; not detectable, NS; not significant, *: p ≤ 0.05, **: p ≤ 0.01, ***: p ≤ 0.001, ****: p ≤ 0.0001.
We also confirmed that the same approach upregulated the endogenous human IL-10 in human cell line (Fig. S1) with the synergistic activation capability (Fig. S1).
These findings suggest that targeted transcriptional activation facilitates robust and titrable IL-10 activation by selection and combination of gRNAs.
In vitro kinetics of IL-10 activation after adenoviral delivery
For delivery to the lung, we generated recombinant adenoviruses expressing dSaCas9-VPR and either one or two functional gRNAs (Fig. 1D). In vitro validation demonstrated a time-dependent and accelerated increase in secreted IL-10 protein in both the single- and two-gRNA groups (Fig. 1E and 1F). After 48 h, IL-10 transcript levels were higher in the single- and two-gRNA groups than in the diluent group (Fig. 1G).
Simultaneous activation of multiple anti-inflammatory genes
We explored the potential of multiplexed anti-inflammatory gene modulation. We selected interleukin 1 receptor antagonist (IL-1RN) as the second target gene. IL-1RN is a naturally occurring protein that blocks the inflammatory signaling of both IL-1α and IL-1β, thus could provide additional therapeutic benefits. Additional expression of two distinct gRNAs targeting IL-1RN induced simultaneous activation of IL-10 and IL-1RN expression as designed (Supplementary Fig. 2A). Assessment using single adenoviral vectors demonstrated increased secretion of both cytokines (Supplementary Fig. 2B). These findings highlight the feasibility of manipulating multiple immunomodulatory gene expressions using CRISPR-Cas system.
Enriched dSaCas9-VPR binding to the target loci
Chromatin immunoprecipitation (ChIP)-qPCR using rat primary lung fibroblasts transduced with adenoviral vectors detected enriched dSaCas9-VPR binding to both on-target genomic loci in the two-gRNAs group compared to the no gRNA and diluent buffer groups (Supplementary Fig. 3A). These results suggest that IL-10 upregulation is attributed to dSaCas9-VPR recruitment to the target genomic regions.
Immune response after delivery of Ad-dSaCas9-VPR to the lung
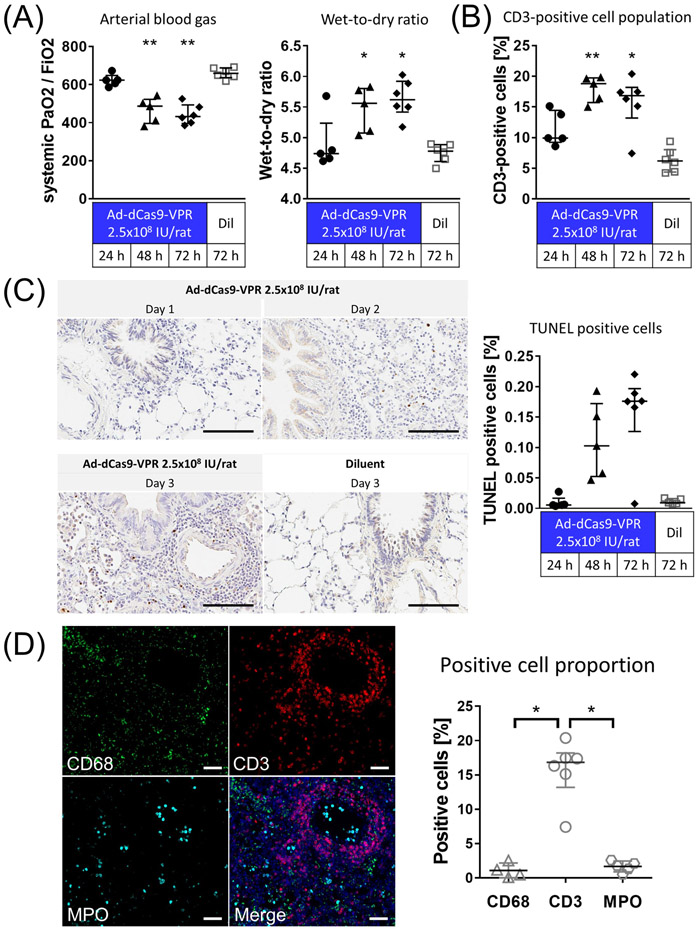
For in vivo profiling, we first evaluated the immune response after trans-airway delivery of adenovirus expressing dSaCas9-VPR (Ad-dSaCas9-VPR). Delivery of Ad-dCas9-VPR at high doses evoked a strong immune response in the rat lungs, as shown in progressive worsening of systemic oxygenation (Fig. 2A), increased wet-to-dry lung weight ratio (Fig. 2A), and exacerbated infiltration of CD3-positive lymphocytes in the lung tissue (Fig. 2B) and an increase in TUNEL-positive apoptotic cells (Fig. 2C). Immunofluorescence (IF) demonstrated the perivascular infiltration of lymphocytes as shown by significantly higher percentage of CD3-positive cells compared to those of CD68-positive macrophages and myeloperoxidase (MPO)-positive neutrophils (Fig. 2D). These inflammatory responses initiated by the adenoviral vectors are similar to what we (5, 15, 27) and others(28) have previously described.
Figure 2.
Assessment of inflammation evoked by trans-airway delivery of higher-dose Ad-dSaCas9-VPR in the lung. (A) Ad-dSaCas9-VPR was administered to the left lung via the airways at a dose of 2.5x108 infectious unit (IU)/rat or diluent buffer alone (Ad-dCas9-VPR at 24h and 48h, n=5; others, n=6). PaO2 was analyzed using arterial blood drawn from the abdominal aorta after 5 min ventilation at FiO2 of 1.0. Wet-to-Dry ratio of the lung was calculated by weighing the lung tissue before and after incubation in the oven at 85 °C. Median (interquartile range) were presented. Symbols indicate *: p ≤ 0.05 and **: p ≤ 0.01 (Kruskal-Wallis followed by Dunn’s correction compared to the diluent group). (B) CD3-positive cell population in immunohistochemistry were measured using the Cytonuclear IHC algorithm in HALO image analysis platform. Median (interquartile range) were presented. Symbols indicate *: p ≤ 0.05 and **: p ≤ 0.01 (Kruskal-Wallis followed by Dunn’s correction compared to the diluent group). (C) TUNEL staining of lung tissues fixed at 1-3 days after adenoviral vector delivery. Scale bars, 100 μm. The percentage of cells that are strongly positive for TUNEL staining was quantified using the Cytonuclear IHC algorithm in HALO image analysis platform (n=6 for Ad-dCas9-VPR on day 3, n=5 for other groups). Median (interquartile range) are presented. (C) Immunofluorescence of lung tissue fixed 3 days after Ad-dSaCas9-VPR delivery. MPO; myeloperoxidase. Scale bars, 50 μm. The proportion of cells positive for each marker was analyzed using the Cytonuclear IHC algorithm in HALO image analysis platform. Median (interquartile range) were presented. Symbols indicate *: p ≤ 0.05 (Kruskal-Wallis followed by Dunn’s correction).
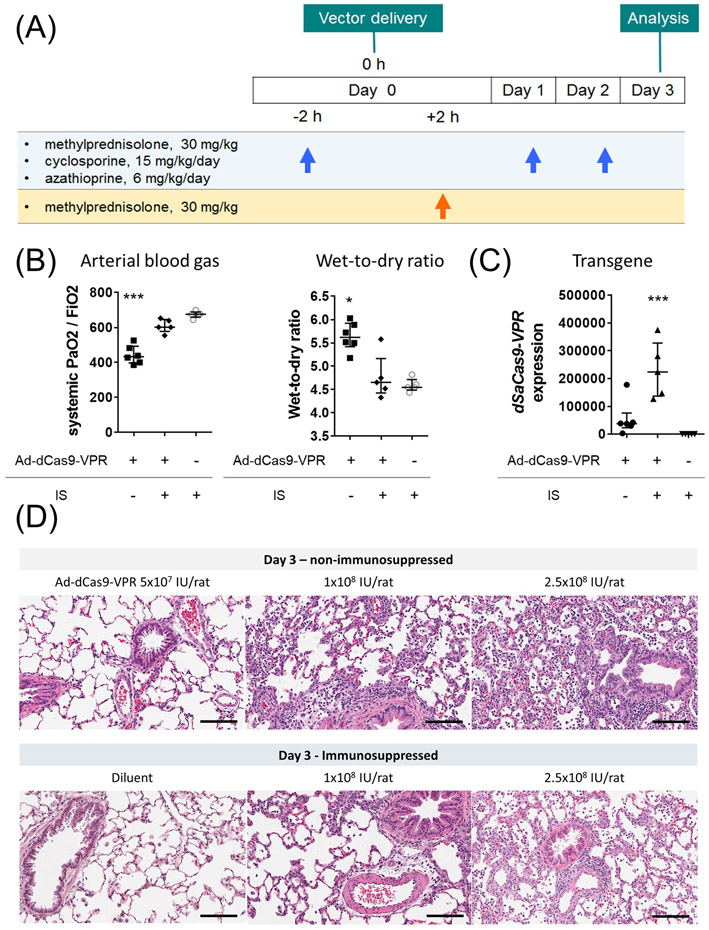
Immunosuppression for efficient delivery of Ad-dSaCas9-VPR
We tested whether transplant immunosuppression could mitigate the inflammation associated with Ad-dSaCas9-VPR delivery. Triple immunosuppression, consisting of methylprednisolone, cyclosporine, and azathioprine, was administered before and after the delivery of high-dose Ad-dSaCas9-VPR (Fig. 3A). We optimized the regimen of prior work(27) by supplementing high-dose methylprednisolone 2h after viral vector delivery to maintain its effects. Indeed, this immunosuppressive regimen effectively circumvented inflammation as shown in improved oxygenation, decreased lung edema (Fig. 3B), enhanced dSaCas9-VPR expression (Fig. 3C), and less inflammatory cell infiltration (Fig. 3D).
Figure 3.
Impact of triple immunosuppression on ameliorating inflammation after dSaCas9-VPR delivery to the lung. (A) Experimental design and immunosuppression protocol. Immunosuppressants were intraperitoneally injected. (B, C) The left lungs were treated with Ad-dSaCas9-VPR at a dose of 2.5x108 IU/rat and analyzed on day 3. Systemic PaO2 was measured using arterial blood taken from abdominal aorta. Wet-to-dry ratio and dSaCas9 expression were measured using lung tissues collected (Ad-dCas9-VPR without IS, n=6; others, n=5). Median (interquartile range) are presented. A Kruskal-Wallis test followed by Dunn’s correction was used for comparison. *: p ≤ 0.05, ***: p ≤ 0.001. (D) Representative images of hematoxylin and eosin staining of the lung tissues treated with different doses of Ad-dCas9-VPR with or without immunosuppression. Scale bars, 100 μm. IS: immunosuppression.
These results illustrate that immunosuppressive medications used in clinical organ transplantation facilitates efficient delivery of CRISPR epigenome editors using adenoviral vectors in the lung. This triple immunosuppression protocol was therefore used in subsequent experiments.
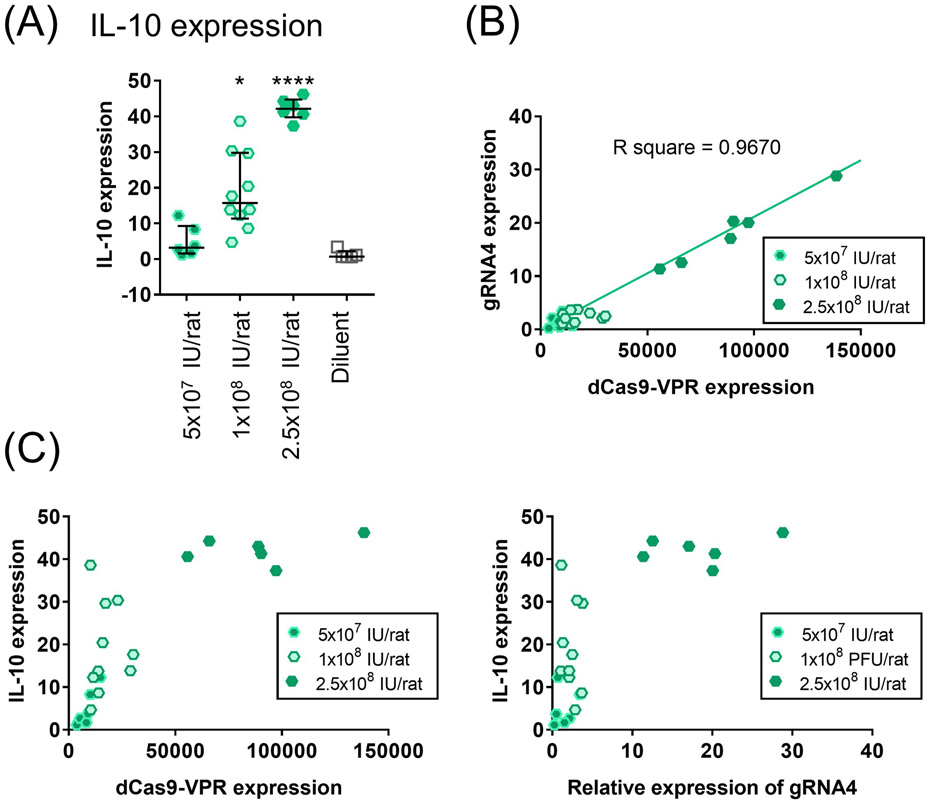
In vivo IL-10 activation in the lung
The delivery of two-gRNA adenoviral vector upregulated IL-10 expression in the lung in a dose-dependent (Fig. 4A). While the dSaCas9-VPR and gRNA expression exhibited a linear relationship (Fig. 4B), IL-10 expression reached a plateau in the high-dose cohort (Fig. 4C), suggesting an upper limit of IL-10 expression.
Figure 4.
Dose-effect relationship of adenoviral vector-mediated expression of dSaCas9-VPR and two gRNAs in the lung. (A) IL-10 expression in the lungs treated with two gRNAs virus after 3 days. Lower-, medium- and higher-dose represent 5x107, 1x108 and 2.5x108 IU/rat of adenoviral vector (lower- and higher-, n=6; medium-, n=10; diluent, n=5). Median (interquartile range) are shown. A Kruskal-Wallis test followed by Dunn’s correction was used for comparison. Symbols indicate *: p ≤ 0.05, and ****: p ≤ 0.0001. (B, C) Scatter plots present the relationships between gRNA4, dSaCas9-VPR and IL-10 expression at day 3. The same cases as in A were presented. The best-fit line and R square value from a simple linear regression were computed using GraphPad software and are shown on the graph.
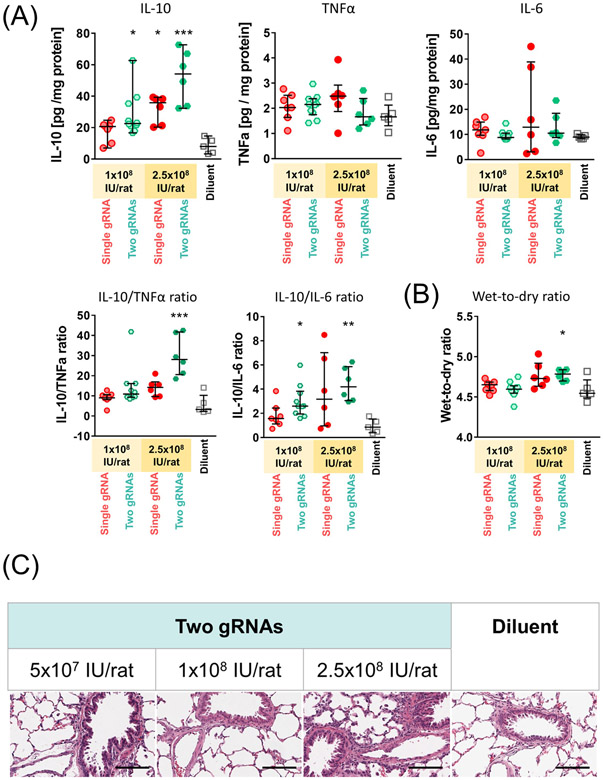
Treatment with the two-gRNA-vector at medium and high doses significantly elevated IL-10 protein levels in lung tissue relative to the diluent group (Fig. 5A). Both IL-10 protein levels (median [IQR], 28.4 [14.0] pg/mg and 52.3 [16.9] pg/mg, respectively; Fig. 5A) fell within the target therapeutic window known to inhibit post-transplant lung injury.(4, 5, 29)
Figure 5.
Analysis of protein levels and histology of the lungs treated with adenoviral-vector mediated IL-10 activation. (A) Concentrations of each target protein in the lung tissue were measured by enzyme-linked immunosorbent assay (ELISA) using lung tissues collected 3 days after the treatment (1x108 IU/rat single gRNA, n=7; 1x108 IU/rat two gRNAs, n=10; 2.5x108 IU/rat single gRNA and two gRNAs, n=6; and Diluent, n=5). IL-10/TNF-α and IL-10/IL-6 ratio were calculated using the concentrations of each protein obtained by ELISA. (B) Wet-to-dry weight ratios of the lung tissues after 3 days of the vector administration. Median (interquartile range) in the same cases as in A are shown. A Kruskal-Wallis test followed by Dunn’s correction was used for comparison. Symbols indicate: *: p ≤ 0.05, **: p ≤ 0.01, ***: p ≤ 0.001. (C) Representative images of hematoxylin and eosin staining of the lung tissues fixed at day 3. Scale bars, 100 μm.
Compared with the diluent group, there were no significant increases in the levels of IL-6 and TNF-α proteins, which represent pro-inflammatory cytokines, in the treatment group (Fig. 5A). The levels of IFNγ protein were below the lowest detection limit in both groups. Based on the histology and W/D weight ratio (Figs. 5B and 5C), the medium dose was determined to be optimal and was used for subsequent experiments.
Distribution of transduced cells
In situ hybridization (ISH) revealed that most dSaCas9-VPR-expressing cells were localized in the alveolar region, and a few in the airway region (Supplementary Fig. 4A) and showed IL-10 mRNA staining. A certain population of transduced cells in the alveolar space was positive for podoplanin, a marker of alveolar type 1 cells (Supplementary Fig. 4B).
Continued immunosuppression prolonged IL-10 activation
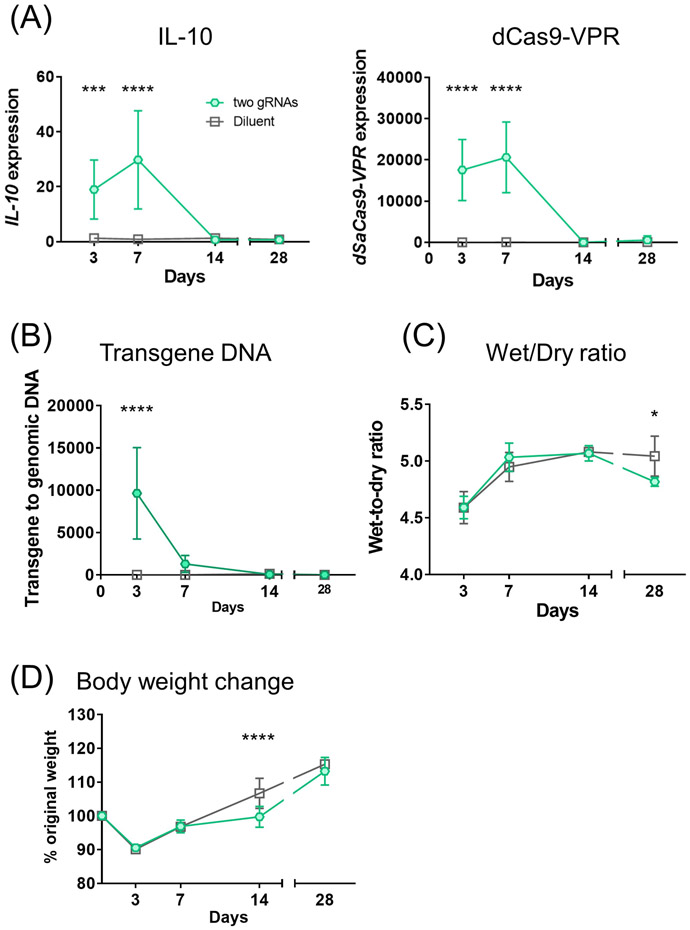
We observed IL-10 upregulation and dSaCas9-VPR expression were sustained for 7 days and then declined by day 14 with transient immunosuppression (Fig. 6A) along with a rapid drop in transgene DNA levels by day 7 (Fig. 6B). Compared with the diluent group, treated lungs presented comparable or lower W/D ratios (Fig. 6C). Body weight gain was slightly slower in the treatment cohort on day 14 but fully recovered by day 28 (Fig. 6D).
Figure 6.
Time course of parameters related to targeted IL-10 activation and general conditions of rats under transient immunosuppression. (A, B) Gene expression and transgene DNA measured by qPCR. (C) Wet-to-dry ratios of the lungs collected at each timepoint. (D) Body weight change was calculated as % of original weight. Two gRNAs on day 3, n=10; others, n=5 at each time point. Mean (SD) are shown. Two-way ANOVA followed by Bonferroni’s correction was used. *: p ≤ 0.05, ***: p ≤ 0.001, ****: p ≤ 0.0001.
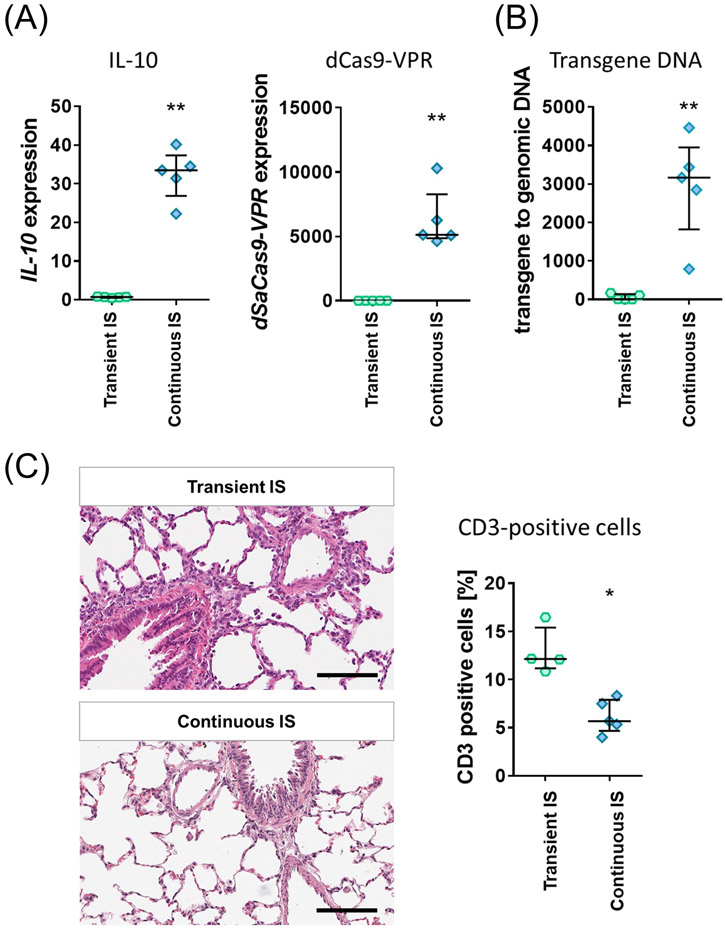
We asked if continued immunosuppression in transplant recipients could prolong the targeted IL-10 activation. Under continued immunosuppression, IL-10 expression was sustained for 14 days (Fig. 7A), as were the dSaCas9-VPR expression and transgene DNA (Figs. 7A and 7B). The daily immunosuppression improved CD3-positive lymphocyte cell infiltration on day 14 as well (Fig. 7C).
Figure 7.
Impact of continuous immunosuppression on targeted IL-10 activation utilizing Ad-dSaCas9-VPR in the lung. (A, B) Rats were treated with two-gRNA adenoviruses under transient or continuous triple immunosuppression. Treated lungs were analyzed at day 14 for gene expression and transgene DNA in the lung tissue by qPCR (n=5 for each group). Median (interquartile range) are shown. (**: p ≤ 0.01; Mann-Whitney test). IS; immunosuppression. (C) Representative images of hematoxylin and eosin staining of the lungs 14 days after adenoviral vector administration. Scale bars, 100 μm. CD3-positive cell population in immunohistochemistry was measured using the Cytonuclear IHC algorithm in HALO image analysis platform. Median (interquartile range) were presented. Symbols indicate *: p ≤ 0.05 (Mann-Whitney test).
Feasibility of transplanting transcriptionally modulated donor lungs
Finally, we evaluated the feasibility of transplanting IL-10-activated donor lungs using a rat single lung transplant model.
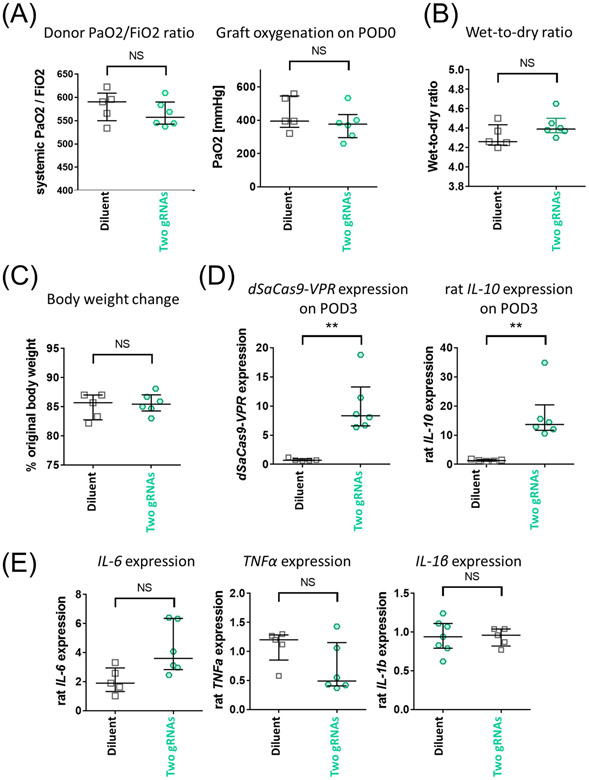
We delivered two-gRNA adenoviral vectors to the donor lung 24h before retrieval and transplanted into an isogeneic recipient with minimal cold ischemic time. The 24-hour post-viral delivery was selected as a timing when the IL-10 protein increase became evident in in vitro evaluation. The donor lung in the treatment group demonstrated comparable donor PaO2/FiO2 ratio and graft oxygenation at the time of reperfusion (Fig. 8A) to the diluent control. The treatment group showed significant transgene expression (Fig. 8B) and IL-10 upregulation (Fig. 8B) on postoperative day 3 (POD3). The persistent IL-10 activation was supported by the presence of cells positive for Cas9 and IL-10 (Fig. 8C). There were no significant differences in W/D ratio (Fig. 8D), the inflammatory cytokine gene expressions (Fig. 8E), and body weight changes (Fig. 8F).
Figure 8.
Assessment of IL-10-activated donor lungs in syngeneic single lung transplant non-injury survival model. Two-gRNA adenoviral vector or buffer only (diluent group) was delivered to left lung of donor Lewis rats in vivo using the same immunosuppression protocol illustrated in Fig. 3A. After 24h of viral delivery, the left lung grafts were transplanted into recipient Lewis rats. Grafts were assessed on postoperative day 3 (POD3). Recipients received triple immunosuppression until analysis (n=5 and n=6 for diluent and two gRNA group, respectively). (A) Donor PaO2/FiO2 ratio calculated from PaO2 of systemic arterial blood taken at FiO2 of 0.5 (left). Graft oxygenation on POD0 was assessed by measuring PaO2 of blood taken from the left pulmonary vein after reperfusion (right). (B) Transgene and rat IL-10 expression in the left lung graft on POD3 measured by qPCR. Values from the left lungs of untreated Lewis rats (n=2) were set as 1. (C) Lung tissue of grafts collected on POD3 were stained for SaCas9 (green) and rat IL-10 mRNA (red) using fluorescence in situ hybridization. Nuclei were counterstained with DAPI. The scale bar indicates 20 μm. (D) Wet-to-dry ratio of left lung graft collected on POD3. (E) Representative inflammatory gene expression was measured by qPCR. Values from the left lungs of untreated Lewis rats (n=2) were set as 1. (F) Recipient body weight on POD3 shown as a percentage of those on POD0. Median(interquartile range) is shown. The Mann-Whitney test was used for statistical comparisons. NS: not significant, **: p ≤ 0.01.
Consistent findings were obtained in an allogeneic transplantation model, where donor lung from Brown Norway rats were transplanted into Lewis recipient rats under triple immunosuppression. Donor lungs with minimal cold ischemia time (to avoid IR injury), expressed significantly higher levels of IL-10 without significant inflammation 3 days after transplantation (Supplementary Fig. 5).
Collectively, our findings demonstrated that transcriptionally modulated donor lungs can be transplanted and retain the targeted gene activation in recipients.
Discussion
In the present study, we activated the endogenous IL-10 gene in the rat lung using a CRISPR epigenome editor and demonstrated transplantation of transcriptionally modulated donor lungs in rat models. Our work shows the first proof-of-principle that transcriptional modulation of donor organs for transplantation is possible, opening the door to new organ engineering approaches.
This study highlights the potential of all-in-one adenoviral vectors in delivering a Cas-based effectors. The immunogenicity of Cas proteins has led to the use of less immunostimulatory vectors to deliver Cas enzymes for in vivo applications. Ongoing clinical trials on in vivo CRISPR-Cas genome editing have used adeno-associated virus (AAV) in subretinal injection(30) and lipid nanoparticles in systemic administration(31). However, the packaging capacity of AAV vectors necessitates a dual-vector system,(32) while non-viral vectors have limited transduction efficacy in the lung.(33) The rapid transgene expression and the scalable production of adenoviral vectors also hold advantages in pre-implantation donor lung modulation.(34, 35) Thus, single-package adenoviral vectors have favorable attributes for pulmonary application of CRISPR-Cas-based approaches when vector-related inflammation is managed.
The immune response associated with Ad-dSaCas9-VPR is a complex reaction that involves both innate and adaptive immunity. The potential immunogens include viral capsids or genes, Cas genes, and Cas proteins that represent viral elements, transgenes, and transgene products, respectively.(36) Thus, inhibition of a broad range of immune responses is warranted to abrogate inflammation. The standard multidrug immunosuppression in solid organ transplantation can fulfill this demand and, importantly, is applicable in the clinical setting. It is also noteworthy that triple immunosuppression permits repeated dosing,(37, 38) indeed potentially facilitating recurrent administration of CRISPR-Cas therapeutics. Our results demonstrate that the standard triple immunosuppressive regimen permitted efficient delivery of Ad-dSaCas9-VPR to the lung with minimal post-delivery inflammation.
We demonstrated the feasibility of simultaneous activation of multiple immunomodulatory cytokines, using a single adenoviral vector. Given the complexity of the immune response in lung transplantation, multiplexed gene modulation is an attractive approach to immunomodulation. In this study, we combined IL1RN and IL-10 activation aiming to reinforce immunomodulation. This versatile gene manipulation platform would provide an exceptional opportunity to transcriptome modulation in donor organs.
The selection of a CRISPR platform from the expanding toolbox depends on the goal and the target gene of the application. In upregulating IL-10, we empirically concluded that the transcriptional activation was much more efficient than histone modification.(39) Besides, there is no evident CpG island near the IL-10 gene that can be targeted by demethylation approach.(40) While this technique has strengths of robustness and flexibility, a limitation is the need for continued expression of Cas9-based activator. A genome editing approach, which alters the sequence of the genomic DNA, could allow for persistent effects with a transient expression of the editing enzyme. In lung transplant setting, lentiviral vector mediated IL-10 gene delivery has been proposed and attempted as a therapeutic modality for treatment of CLAD.(8, 41, 42) The CRISPR-Cas 9 mediated gene editing may have benefits in both PGD and CLAD.
The major limitations of this study are as follows: (1) As a proof-of-concept study, we used in vivo delivery 24 hours prior to donor lung retrieval, to determine transcriptionally modulated donor lungs can produce more endogenous IL-10 post-transplantation. With positive results, the methods of organ modification during ex vivo lung perfusion will be further optimized for clinical practice. (2) Further studies using injury and rejection models are needed to evaluate the therapeutic benefits of this approach. (3) Pre-clinical studies, such as evaluation in a large animal model and human lung studies,(14) will be demanded for clinical translation.
In conclusion, our work demonstrated the feasibility of CRISPR-Cas-based therapy in pre-implantation donor lung modulation. This work lays the foundation for a broad range of transcriptome manipulation in donor lungs to improve donor organ quality and quantity for transplantation, and reduce the incidence of primary graft dysfunction, acute and chronic rejection post-transplantation.
Supplementary Material
Acknowledgments
This work was supported by the University Health Network Foundation, University of Toronto’s Medicine by Design (MbDPEFR1-2021-01), New Frontiers in Research Funding (NFRFT-2020-00787), and the Canadian Institutes of Health Research (CIHR PJT-173392). We thank Spatio-temporal Targeting and Amplification of Radiation Response (STTARR) at UHN and Advanced Optical Microscopy Facility (AOMF) at UHN for histology and imaging service. K.M. was supported by UHN Transplant Competition 2020, University of Toronto’s Institute of Medical Science Open Fellowship 2020–2023, and Canadian Society of Transplantation (CST) Research Training Award 2022. B.P.K. acknowledges support from National Institutes of Health (NIH) P01 HL142494.
List of non-standard abbreviations
- LTx
lung transplantation
- PGD
primary graft dysfunction
- CLAD
chronic lung allograft dysfunction
- CRISPR
clustered regularly interspaced short palindromic repeats
- Cas
CRISPR-associated
- gRNA
guide RNA
- VPR
VP64-p65-Rta
- dSaCas9
catalytically inactive Cas9 from Staphylococcus aureus
- EVLP
ex vivo lung perfusion
- IS
immunosuppression
- W/D ratio
wet-to-dry weight ratio
- P/F ratio
PaO2/FiO2 ratio
Footnotes
Competing interests
M.C. and S.K. are co-founders of Perfusix Canada and Scientific Advisors to Lung Bioengineering. S.K. is CMO of Traferox Technologies. University Health Network has a patent filed and pending for technologies related to donor organ modification, on which K.M., S.J., M.C., M.L., and S.K. are inventors. B.P.K is an inventor on patents and patent applications filed by Mass General Brigham that describe genome engineering technologies. B.P.K. is a consultant for EcoR1 capital and is an advisor to Acrigen Biosciences, Life Edit Therapeutics, and Prime Medicine.
Reference
- 1.Israni AK, Zaun D, Hadley N, et al. : OPTN/SRTR 2018 Annual Data Report: Deceased Organ Donation. Am J Transplant 2020;20 Suppl s1:509–41. [DOI] [PubMed] [Google Scholar]
- 2.Yeung JC, Keshavjee S: Overview of clinical lung transplantation. Cold Spring Harb Perspect Med 2014;4:a015628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fischer S, De Perrot M, Liu M, et al. : Interleukin 10 gene transfection of donor lungs ameliorates posttransplant cell death by a switch from cellular necrosis to apoptosis. J Thorac Cardiovasc Surg 2003;126:1174–80. [DOI] [PubMed] [Google Scholar]
- 4.Fischer S, Liu M, Maclean AA, et al. : In vivo donor adenoviral-mediated transtracheal transfection of human IL-10 (HIL-10) gene ameliorates ischemia-reperfusion (IR) injury and enhances transplanted lung function. J Heart Lung Transplant 2001;20:152–3. [DOI] [PubMed] [Google Scholar]
- 5.de Perrot M, Fischer S, Liu M, et al. : Impact of human interleukin-10 on vector-induced inflammation and early graft function in rat lung transplantation. Am J Respir Cell Mol Biol 2003;28:616–25. [DOI] [PubMed] [Google Scholar]
- 6.Itano H, Zhang W, Ritter JH, McCarthy TJ, Mohanakumar T, Patterson GA: Adenovirus-mediated gene transfer of human interleukin 10 ameliorates reperfusion injury of rat lung isografts. J Thorac Cardiovasc Surg 2000;120:947–56. [DOI] [PubMed] [Google Scholar]
- 7.Martins S, de Perrot M, Imai Y, et al. : Transbronchial administration of adenoviral-mediated interleukin-10 gene to the donor improves function in a pig lung transplant model. Gene Ther 2004;11:1786–96. [DOI] [PubMed] [Google Scholar]
- 8.Hirayama S, Sato M, Loisel-Meyer S, et al. : Lentivirus IL-10 gene therapy down-regulates IL-17 and attenuates mouse orthotopic lung allograft rejection. Am J Transplant 2013;13:1586–93. [DOI] [PubMed] [Google Scholar]
- 9.Cypel M, Yeung JC, Hirayama S, et al. : Technique for prolonged normothermic ex vivo lung perfusion. J Heart Lung Transplant 2008;27:1319–25. [DOI] [PubMed] [Google Scholar]
- 10.Cypel M, Yeung JC, Liu M, et al. : Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med 2011;364:1431–40. [DOI] [PubMed] [Google Scholar]
- 11.Warnecke G, Van Raemdonck D, Smith MA, et al. : Normothermic ex-vivo preservation with the portable Organ Care System Lung device for bilateral lung transplantation (INSPIRE): a randomised, open-label, non-inferiority, phase 3 study. The Lancet Respiratory Medicine 2018;6:357–67. [DOI] [PubMed] [Google Scholar]
- 12.Loor G, Warnecke G, Villavicencio MA, et al. : Portable normothermic ex-vivo lung perfusion, ventilation, and functional assessment with the Organ Care System on donor lung use for transplantation from extended-criteria donors (EXPAND): a single-arm, pivotal trial. The Lancet Respiratory medicine 2019;7:975–84. [DOI] [PubMed] [Google Scholar]
- 13.Nilsson T, Wallinder A, Henriksen I, et al. : Lung transplantation after ex vivo lung perfusion in two Scandinavian centres. Eur J Cardiothorac Surg 2019;55:766–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cypel M, Liu M, Rubacha M, et al. : Functional repair of human donor lungs by IL-10 gene therapy. Sci Transl Med 2009;1:4ra9. [DOI] [PubMed] [Google Scholar]
- 15.Yeung JC, Wagnetz D, Cypel M, et al. : Ex vivo adenoviral vector gene delivery results in decreased vector-associated inflammation pre- and post-lung transplantation in the pig. Mol Ther 2012;20:1204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Machuca TN, Cypel M, Bonato R, et al. : Safety and Efficacy of Ex Vivo Donor Lung Adenoviral IL-10 Gene Therapy in a Large Animal Lung Transplant Survival Model. Hum Gene Ther 2017;28:757–65. [DOI] [PubMed] [Google Scholar]
- 17.Doudna JA: The promise and challenge of therapeutic genome editing. Nature 2020;578:229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E: A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012;337:816–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casas-Mollano JA, Zinselmeier MH, Erickson SE, Smanski MJ: CRISPR-Cas Activators for Engineering Gene Expression in Higher Eukaryotes. Crispr j 2020;3:350–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tak YE, Kleinstiver BP, Nunez JK, et al. : Inducible and multiplex gene regulation using CRISPR-Cpf1-based transcription factors. Nat Methods 2017;14:1163–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakamura M, Srinivasan P, Chavez M, et al. : Anti-CRISPR-mediated control of gene editing and synthetic circuits in eukaryotic cells. Nat Commun 2019;10:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao Y, Xiong X, Wong S, Charles EJ, Lim WA, Qi LS: Complex transcriptional modulation with orthogonal and inducible dCas9 regulators. Nat Methods 2016;13:1043–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCarty NS, Graham AE, Studená L, Ledesma-Amaro R: Multiplexed CRISPR technologies for gene editing and transcriptional regulation. Nat Commun 2020;11:1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keshavjee SH, Yamazaki F, Cardoso PF, McRitchie DI, Patterson GA, Cooper JD: A method for safe twelve-hour pulmonary preservation. J Thorac Cardiovasc Surg 1989;98:529–34. [PubMed] [Google Scholar]
- 25.Chavez A, Scheiman J, Vora S, et al. : Highly efficient Cas9-mediated transcriptional programming. Nat Methods 2015;12:326–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perez-Pinera P, Kocak DD, Vockley CM, et al. : RNA-guided gene activation by CRISPR-Cas9-based transcription factors. Nat Methods 2013;10:973–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cassivi SD, Liu M, Boehler A, et al. : Transplant immunosuppression increases and prolongs transgene expression following adenoviral-mediated transfection of rat lungs. J Heart Lung Transplant 2000;19:984–94. [DOI] [PubMed] [Google Scholar]
- 28.Wilmott RW, Amin RS, Perez CR, et al. : Safety of adenovirus-mediated transfer of the human cystic fibrosis transmembrane conductance regulator cDNA to the lungs of nonhuman primates. Hum Gene Ther 1996;7:301–18. [DOI] [PubMed] [Google Scholar]
- 29.Tagawa T, Suda T, Daddi N, et al. : Low-dose endobronchial gene transfer to ameliorate lung graft ischemia-reperfusion injury. J Thorac Cardiovasc Surg 2002;123:795–802. [DOI] [PubMed] [Google Scholar]
- 30.Maeder ML, Stefanidakis M, Wilson CJ, et al. : Development of a gene-editing approach to restore vision loss in Leber congenital amaurosis type 10. Nat Med 2019;25:229–33. [DOI] [PubMed] [Google Scholar]
- 31.Gillmore JD, Gane E, Taubel J, et al. : CRISPR-Cas9 In Vivo Gene Editing for Transthyretin Amyloidosis. N Engl J Med 2021. [DOI] [PubMed] [Google Scholar]
- 32.Böhm S, Splith V, Riedmayr LM, et al. : A gene therapy for inherited blindness using dCas9-VPR-mediated transcriptional activation. Sci Adv 2020;6:eaba5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sondhi D, Stiles KM, De BP, Crystal RG: Genetic Modification of the Lung Directed Toward Treatment of Human Disease. Hum Gene Ther 2017;28:3–84. [DOI] [PubMed] [Google Scholar]
- 34.Lee CS, Bishop ES, Zhang R, et al. : Adenovirus-Mediated Gene Delivery: Potential Applications for Gene and Cell-Based Therapies in the New Era of Personalized Medicine. Genes Dis 2017;4:43–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bulcha JT, Wang Y, Ma H, Tai PWL, Gao G: Viral vector platforms within the gene therapy landscape. Signal Transduct Target Ther 2021;6:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shirley JL, de Jong YP, Terhorst C, Herzog RW: Immune Responses to Viral Gene Therapy Vectors. Mol Ther 2020;28:709–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suga M, Gladdy R, Xing Z, Keshavjee SH, Liu M: Transplant immunosuppression enhances efficiency of adenoviral-mediated gene retransfection: inhibition of interferon-gamma and immunoglobin G. Ann Thorac Surg 2002;73:1092–7. [DOI] [PubMed] [Google Scholar]
- 38.Cassivi SD, Liu M, Boehler A, et al. : Transgene expression after adenovirus-mediated retransfection of rat lungs is increased and prolonged by transplant immunosuppression. J Thorac Cardiovasc Surg 1999;117:1–7. [DOI] [PubMed] [Google Scholar]
- 39.Hilton IB, D'Ippolito AM, Vockley CM, et al. : Epigenome editing by a CRISPR-Cas9-based acetyltransferase activates genes from promoters and enhancers. Nat Biotechnol 2015;33:510–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu XS, Wu H, Krzisch M, et al. : Rescue of Fragile X Syndrome Neurons by DNA Methylation Editing of the FMR1 Gene. Cell 2018;172:979–92.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hirayama S, Sato M, Liu M, et al. : Local long-term expression of lentivirally delivered IL-10 in the lung attenuates obliteration of intrapulmonary allograft airways. Hum Gene Ther 2011;22:1453–60. [DOI] [PubMed] [Google Scholar]
- 42.Oishi H, Juvet SC, Martinu T, et al. : A novel combined ex vivo and in vivo lentiviral interleukin-10 gene delivery strategy at the time of transplantation decreases chronic lung allograft rejection in mice. J Thorac Cardiovasc Surg 2018;156:1305–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.