Abstract
Osteoarthritis (OA) is a common debilitating degenerative disease of the elderly. We aimed to study the therapeutic effects of combining curcumin and swimming in monosodium iodoacetate (MIA)-induced OA in a rat model. The rats were divided into 5 groups (n = 9). Group 1 received saline and served as a control group. Groups 2–5 were injected intra-articularly in the right knee with 100 μL MIA. One week later, groups 3 and 5 were started on daily swimming sessions that gradually increased to 20-mins per session, and for groups 4 and 5, oral curcumin was administered at a dose of 200 mg/kg for 4 weeks. The combination therapy (curcumin + swimming) showed the most effective results in alleviating pain and joint stiffness as well as improving histological and radiological osteoarthritis manifestations in the knee joints. The combination modality also reduced serum C-reactive protein and tissue cartilage oligomeric matrix protein levels. Mechanistically, rats received dual treatment exhibited restoration of miR-130a and HDAC3 expression. The dual treatment also upregulated PPAR-γ alongside downregulation of NF-κB and its inflammatory cytokine targets TNF-α and IL-1β. Additionally, there was downregulation of MMP1 and MMP13 in the treated rats. In conclusion, our data showed that there is a therapeutic potential for combining curcumin with swimming in OA, which is attributed, at least in part, to the modulation of miR-130a/HDAC3/PPAR-γ signaling axis.
Keywords: Osteoarthritis, MIA, Curcumin, Swimming, MiR-130a, HDAC3, PPAR-γ
1. Introduction
Osteoarthritis (OA) is one of the most widespread diseases and is among the prevalent incapacitating illnesses, affecting more than one-third of individuals over 65 [28]. Although OA has not yet been linked to a single identifiable cause, a growing body of evidence points to the interplay of molecular mechanisms in the affected joints as the likely culprit [10]. The underlying causes of OA include dysregulation of several regulatory pathways. For instance, in articular chondrocytes isolated from OA cartilage, altered gene expressions of matrix-degrading proteases (MMPs), inflammatory mediators, and extracellular matrix (ECM)-related genes including collagens and proteoglycans have been previously reported [23,33]. The regulation of the expression of these genes in OA cartilage is still not completely elucidated. One of the erroneous epigenetic alterations is the regulation of gene expression by histone modifications [32] as lysine acetylation, which is controlled by the histone acetyltransferases (HATs) and histone deacetylases (HDACs) enzymes [16]. Histone acetylation levels are often directly correlated with the transcriptional activity of genes. The equilibrium of the opposing activities of HATs and HDACs leads to steady-state levels of acetylation of the histones (and other non-histone proteins). When the balance between HATs and HDACs is thrown off, diseases like cancer and chronic inflammation may occur [39].
Altered HDAC activity is implicated in the development of OA where chondrocyte protection and cartilage preservation are obtained via the use of HDAC inhibitors (HDACi) [32]. HDAC3 plays a significant role in maintaining the cartilage and chondrocytes healthy [8]. Deregulation of HDAC3 has been linked to inflammatory conditions such as carditis [68], spinal cord injury [12], and macrophage inflammatory gene expression [13]. In the cartilage of OA patients, an elevated level of HDAC3 has been found [47]. Furthermore, the association between HDACs and microRNAs (miRNAs) in the pathophysiology of OA has been shown in several studies [45,47]. MicroRNAs are small non-coding RNA molecules that play crucial roles in gene regulation. They are highly conserved across different species, indicating their importance in biological processes. One such miRNA, miR-130a, has been shown to be evolutionarily conserved in both humans and mice. The miR-130a belongs to a miRNA family that involves five members: miR-130a, miR-130b, miR-454, miR-301a, and miR-301b. These miRNA genes share the same seed sequence (6–8 nt long). Therefore, they potentially can silence the expression of the same target genes or gene family [6, 42]. Recently, a bioinformatics comparative analysis found that the sequence of human miR-130a was conserved in all primates and rodents [69]. This conservation suggests that miR-130a plays a fundamental role in various physiological and pathological processes in these organisms. The primary function of miRNAs is to regulate cell division and proliferation [41]. Although specifically miR-130a was found to be involved in a variety of human diseases [40], little is known about its role in controlling chondrogenesis during osteoarthritis progression.
Despite the recent development in OA treatments, current medical management only concentrates on symptom relief rather than slowing or stopping disease progression. There is no single medicine that can cure this disease with a complicated genesis. Therapies are aimed at articular cartilage, synovial components, or subchondral bone [26,56]. Therefore, developing cutting-edge therapeutic approaches is essential to significantly improve the outcome of OA. Curcumin, turmeric’s polyphenolic ingredient, possesses potent anti-inflammatory and antioxidant properties as well as enhanced ability to influence epigenetic processes [54].
Complementary to pharmacological approach, Osteoarthritis Research Society International (ORSI) recommends non-pharmacologic treatments for optimum OA cure. As an alternative to surgery, physical exercise has drawn a lot of attention as a potential strategy to successfully alleviate OA [46]. Exercise reduces pain and strengthens the muscles and ligaments [24]. In particular, underwater exercise is used to build muscles while simultaneously reduces direct joint loads [35]. Moderate intensity exercise increases glycosaminoglycan content in the cartilage and prevents its damage through modulating extracellular matrix loss, chondrocyte apoptosis, and inflammation [29]. Furthermore, Sleiman et al., [55] discovered that mechanic-epigenetics, commonly known as mechanical intervention or physical exercise, can control HDACs expression [55]. However, the mechanism by which exercise reduces HDAC3 has not yet been unraveled.
In this study, we aimed to investigate the possible curative effects of curcumin combined with swimming in attenuating inflammation in the articular cartilage via modulating the expression of miR-130a/HDAC3/PPAR-γ axis in monosodium iodoacetate (MIA)-induced OA rat model.
2. Materials and methods
2.1. Animals
The study was approved by Institutional Animal Care and Use Committee of Cairo University (Permit number: PT3045), and adheres to the Guide for Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 2011). Male Wistar rats weighing 200–250 g (age: 10–12 weeks) were obtained from the animal facility of the Faculty of Veterinary Medicine, Cairo University. They were housed in plastic cages under controlled environmental conditions (temperature: 20 ± 5 °C, 12 h light/dark cycle, and humidity: 55 ± 15%). Rats were allowed free access to standard diet and water.
Forty-five rats were randomly divided into 5 groups (total number of rats per group = 9): Group (1) served as the control group which received an intra-articular injection of saline in the right knee together with oral administration of saline. Rats in groups (2, 3, 4, 5) were intraarticularly injected with 100 μL MIA in the right knee on day 0. One week later, OA rats (group 2) were given oral saline for a total of 4 weeks. Meanwhile, the 20-minutes swimming sessions were introduced to rats of groups 3 and 5 and curcumin was orally administered to the 4th and 5th groups in a dose of 200 mg/kg [25] till the end of the experimental period (35 days).
The animals’ weights and rotarod-falling latency were recorded weekly and then after 24 h of the last intervention, x-ray imaging was performed, the degree of knee swelling was estimated, and the animals were subjected to von Frey and accelerated rotarod tests. Rats were anaesthetized using thiopental (20 mg/kg, i.p.) and blood samples were collected from the retro-orbital sinus. After which, rats were euthanized by cervical dislocation. Three right knee joints from each group were preserved in 10% (v/v) formalin for histopathological investigation. The right knee joint was separated from the rest of the animals to be stored at − 80 °C for biochemical measurements.
2.2. Drugs and chemicals
Monosodium iodoacetate (MIA) and curcumin were purchased from Sigma-Aldrich (Merck, Darmstadt, Germany), and normal saline was used as a solvent for both drugs. Rats were administered curcumin solution at a volume of 5 mL/kg. All other chemicals were of standard analytical grade.
2.3. Osteoarthritis (OA) induction
Excluding the control group, an intra-articular injection of 100 μL (2 mg) MIA was used to induce osteoarthritis in the right knee of the rats in all other groups [52]. Control rats received an intra-articular injection of saline instead of MIA. Injections were administered after rats were anesthetized using thiopental sodium (20 mg/kg, i.p.) [34] and the knees were shaved.
2.4. Swimming protocol
To assess the therapeutic effects of swimming on osteoarthritis, one week after MIA injection, rats were introduced into a rounded water tank (30 °C) of 100 cm-depth in which they were left to swim for 5 mins and gradually increasing the time every day to a maximum of 20 mins “full session”. The protocol was implied 5 times per week for a total of 4 weeks with the elimination of the escape behavior [43]. A trial of increasing the swimming time to 30 mins was performed but the rats drowned.
2.5. Knee swelling assessment
The changes in knee diameter were obtained as the difference between left (untreated) and right (injected) knee joints using a digital caliper (HC Kenshin, HuiChuang Technology) [1].
2.6. Radiologic evaluation
Rats were anaesthetized using ketamine (50 mg/kg)/xylazine (2 mg/kg) mixture and positioned with hind limbs straightened by an adhesive tape. Imaging of the lateromedial and craniocaudal views of the right stifle were obtained using a Fisher® X-ray device (Fisher R183, Emerald tube 125) [25].
2.7. Von Frey test for mechanical pain sensitivity
To allow easy application of the von Frey filaments (Cat. # 37450–275, Ugo Basile, Italy) against the plantar surface of the right paw, animals were placed on a perforated metal platform and left to acclimatize for 30 mins. Then, the filaments were perpendicularly pressed against the middle of the right hind paw until the filament bends then, held for 1–2 s. A paw withdrawal reflex was considered a positive response and the result was expressed as paw withdrawal threshold (g) following the up-down paradigm [9].
2.8. Accelerated rotarod to evaluate articular motor function
Rats’ locomotor function was assessed weekly using accelerating rotarod apparatus (Cat. # 7750, Ugo Basile, Italy) as per Vonsy et al. [57] with modifications.
Before the experiment, animals were trained on the rotarod by placing them in separate lanes at 5 revolutions per minute (rpm) such that rats walk forward to keep balance and the procedure is repeated three times separated by 10 mins intertrial intervals.
At the time of the experiment, the apparatus was started at 10 rpm and accelerated to 45 rpm where the falling latency was recorded and expressed in seconds with a 120 s cut-off. Trial starts when acceleration begins and ends when animal falls off the rod and the procedure is repeated three times separated by 15 mins intertrial intervals.
2.9. Quantitative real-time polymerase chain reaction (qRT-PCR)
Using the Direct-zol RNA Miniprep Plus kit (Cat. # R2072, Zymo Research, Irvine, CA, USA), total RNA was extracted from the tissue lysate, and the quantity of purified RNA was calculated spectrophotometrically. Then, synthesis of cDNA was performed using SuperScript IV One-Step RT-PCR kit (Cat. # 12594100, Thermo Fisher Scientific, Waltham, MA, USA). This kit enables both cDNA synthesis and PCR amplification in a single reaction using gene-specific primers: HDAC3 (Forward 5′-TGGCTTCTGCTATGTCAACG-3′ and Reverse 5′-GCACGTGGGTTGGTAGAAGT-3′), miR-130a (Forward 5′-TTCCGAATGTGTTTTGTCCT-3′ and Reverse 5′-CTGCGGTCCTGAGAGGG-3′). GADPH (Forward 5′- TGGATTTGGACGCATTGGTC-3′ and Reverse 5′- TTTGCACTGGTACGTGTTGAT-3′) was used as a housekeeping gene to normalize the resultant miR-130a and HDAC3 relative expression. Gene expression values were represented using the 2-ΔΔCt method.
2.10. ELISA
Serum C-reactive protein (CRP) was determined at one-week after MIA injection and at the end of experimental period following CusaBio kit’s instruction (Cat. # CSB-E07922r, Houston, USA). In addition, cartilage oligomeric matrix protein (COMP) and peroxisome proliferator-activated receptor γ (PPAR-γ) were measured in the knee joint homogenate according to manufacturer’s instructions (Cat. # CSB-E13833r, CSB-E08624r, respectively; CusaBio). Moreover, the inflammatory markers NF-κB (Cat. # SEB824Ra), tumor necrosis factor-α (TNF-α) (Cat. # SEA133Ra) and interleukin-1β (IL-1β) (Cat. # SEA563Ra) were measured in the knee joint homogenate using rat specific Cloud Clone kits (Wuhan, China).
2.11. Western blotting
Tissue samples were homogenized in radio-immunoprecipitation (RIPA) buffer and protein concentration in each sample was assessed using Bradford protein assay kit (Cat. # SK3041, Bio Basic, Ontario, Canada). Proteins (20 μg) were separated on sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE), then transferred to nitrocellulose membrane. Tris-buffered saline with Tween 20 (TBST) and 3% bovine serum albumin (BSA) was used to block the membrane. After which, the membrane was incubated with primary antibodies against MMP1 (Cat. # sc-21731; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and MMP13 (Cat. # sc-515284; Santa Cruz Biotechnology) and β-actin (Cat. # sc-47778; Santa Cruz Biotechnology) was used for normalization. After washing the membranes, incubation with horseradish peroxidase (HRP)-conjugated secondary antibody was done, then the chemiluminescent substrate (Cat. #170–5060; ClarityTM Western ECL substrate, Bio-Rad, Hercules, CA, USA) was applied to the blot according to the manufacturer’s recommendations. The yielded blots were captured, and intensities of the bands were measured using image analysis software (ChemiDoc MP Imaging System, Bio-Rad).
2.12. Histological examination
After the rats were sacrificed by cervical dislocation under light anesthesia, the right knee joints were fixed in 10% formalin for 48 h after which 20% EDTA was used to decalcify the joints. The joints were then removed and embedded in paraffin. Sections of 4–6 μm were stained with hematoxylin and eosin (H&E) and examined under a light microscope. Semiquantitative scoring of the histopathological derangements were graded as follows: 0, normal; 1, minimal synovitis without cartilage/bone erosion; 2, synovitis with some marginal erosion but with joint architecture maintained; 3, moderate synovitis and erosion with a change in joint architecture, and 4, severe synovitis and erosion with loss of normal joint architecture [18].
2.13. Immunohistochemical analysis
Knee Sections (4–5 μm) were sliced and deparaffinized and underwent retrieval steps. Then, the slides were incubated overnight at the refrigerator (4 °C) with the primary antibody against NF-κB (Cat. # sc-8008; Santa Cruz biotechnology) and TNF-α (Cat. # sc-52746; Santa Cruz biotechnology) at a dilution of 1:200. The HRP-labelled anti-mouse secondary antibody (Abcam, Cambridge, UK) was added to the slides and kept for two hours, in addition to DAB (3,3’Diaminobenzidine) Substrate kit (Thermo Scientific) for detection. Positive staining was determined by calculating the area % of positive cells in averaged 5 random non-overlapping fields from each animal using CellSens dimensions (Olympus software) [19].
2.14. Statistical analysis
GraphPad Prism software version 9 (GraphPad Software, Inc., San Diego, CA, USA) was used in the current study. Data were presented as means ± standard deviation (SD) and statistically analyzed by using one-way analysis of variance (ANOVA) except for weekly rotarod falling latency was done by two-way ANOVA. For groups’ comparison, Tukey’s post-hoc multiple comparison test was performed. The significance level was presented as: ns; non-significant, *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001.
3. Results
3.1. Concomitant curcumin and swimming reduced osteoarthritis-related swelling and pain and improved articular motor function independent of body weight change
Weight load can affect cartilage development; for that reason, body weight was measured weekly throughout the experiment. All groups did not demonstrate any significant differences in the average rats’ weights at any point (Fig. 1 (A)). We therefore concluded that weight changes did not affect our results.
Fig. 1.
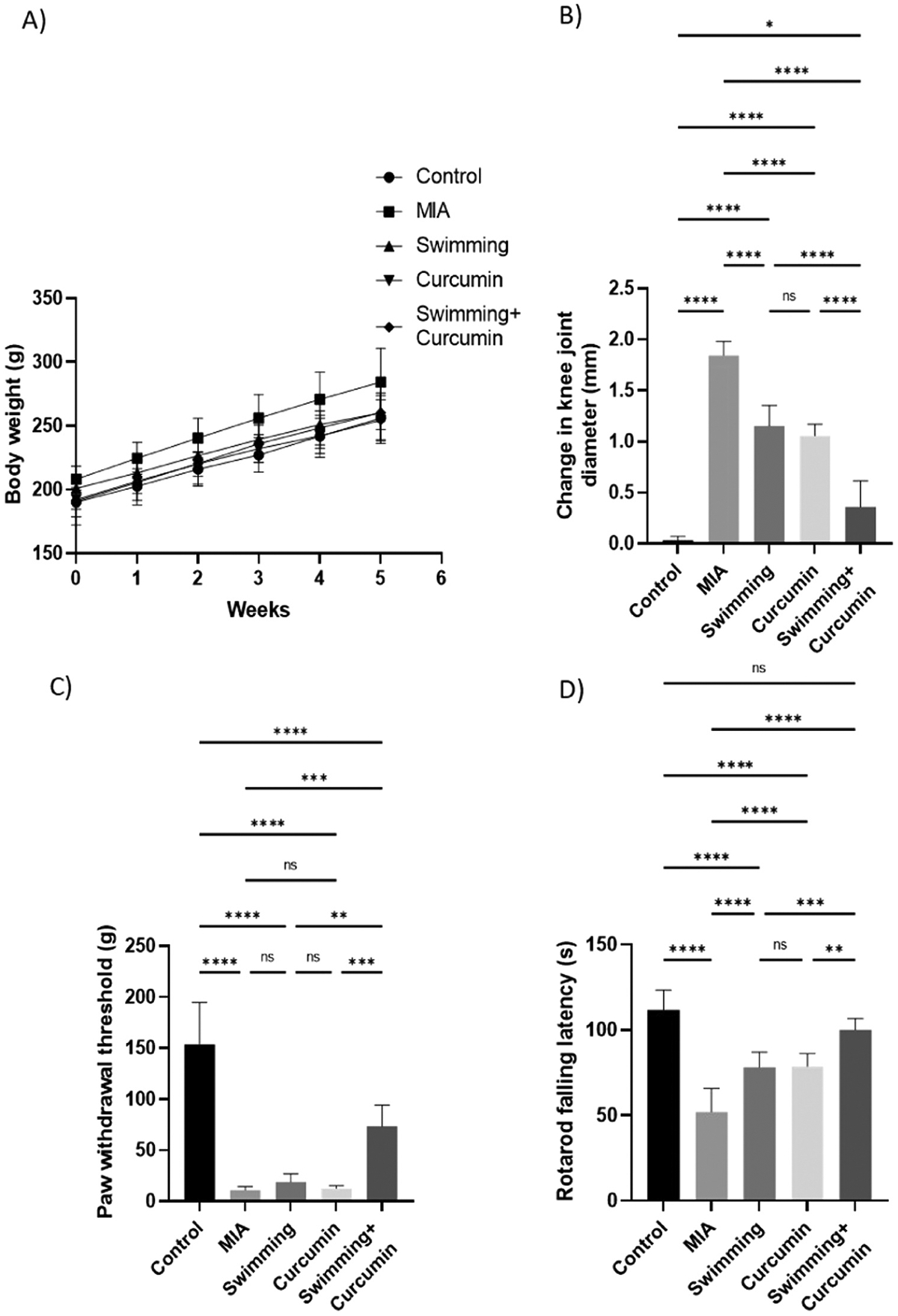
Analysis of A) body weight, B) change in knee joint diameter, C) Von Frey paw withdrawal threshold, and D) rotarod falling latency in rats subjected to 4 weeks of swimming, curcumin or swimming combined with curcumin. One-way ANOVA was used as a statistical test followed by Tukey post-hoc test for significance ns; non-significant, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, n = 6–8/group.
MIA-induced knee swelling was assessed by comparing the left and right joint diameters. As illustrated in Fig. 1 (B), there was a significant increase in the right joint diameters in rats of the MIA group that reached 52-fold increase compared to the control group, while the left joints remained unaffected. After swimming and curcumin treatment, knee swelling was significantly reduced by 20- and 22-fold, respectively, compared to the untreated group. Combining swimming and curcumin treatments resulted in the greatest inhibition of the knee swelling by 40-fold compared to the MIA group as shown in Fig. 1 (B).
MIA-treated rats also exhibited reduction in the threshold of response to mechanical stimuli compared to the control group (Fig. 1 (C)). Rats treated with curcumin and swimming exhibited reduced tactile hypersensitivity with a threshold 40% higher than the MIA group. Individual swimming and curcumin treatments resulted in a non-significant change in the paw withdrawal threshold compared to the untreated group.
As illustrated in Fig. 1 (D), concomitant swimming and curcumin likewise improved the articular motor function of the rats detected through an increase in the time spent on the rotarod by 39% compared to the MIA group. Swimming or curcumin monotherapy resulted in a significant increase in the rotarod time by only 22%. Table 1 also shows the weekly rotarod falling latency time. Combined swimming and curcumin resulted in a significant increase in the time spent on the rotarod compared to the monotherapies starting from the 3rd week of treatment. Fig. 2.
Table 1.
Weekly rotarod falling latency in rats subjected to MIA injection followed by 4 weeks of swimming, curcumin or swimming combined with curcumin.
| Groups Week | Control | MIA | Swimming | Curcumin | Swimming + Curcumin |
|---|---|---|---|---|---|
| 0 | 101.10 ± 6.10 | 96.13 ± 5.27 | 99.25 ± 7.04 | 101.8 ± 5.06 | 103.9 ± 5.98 |
| 1 | 103.50 ± 5.68 | 77.63 ± 5.57a | 82.63 ± 10.39a | 85.88 ± 6.03a | 90.13 ± 6.64ab |
| 2 | 100.40 ± 9.66 | 72.00 ± 10.09a | 79.88 ± 8.95a | 75.63 ± 4.80a | 83.00 ± 4.78a |
| 3 | 108.10 ± 11.46 | 65.50 ± 13.27a | 78.88 ± 5.64a | 75.50 ± 8.01a | 84.38 ± 4.74a |
| 4 | 110.40 ± 11.58 | 62.75 ± 13.49a | 75.00 ± 6.99a | 78.75 ± 8.41a | 95.63 ± 5.42bcd |
| 5 | 111.80 ± 11.54 | 52.00 ± 13.74a | 78.13 ± 8.91ab | 78.75 ± 7.44ab | 100.00 ± 6.71bcd |
Two-way ANOVA was used as a statistical test followed by Tukey post-hoc test for significance.
significantly different from control,
significantly different from MIA,
significantly different from swimming,
significantly different from curcumin.
Fig. 2.
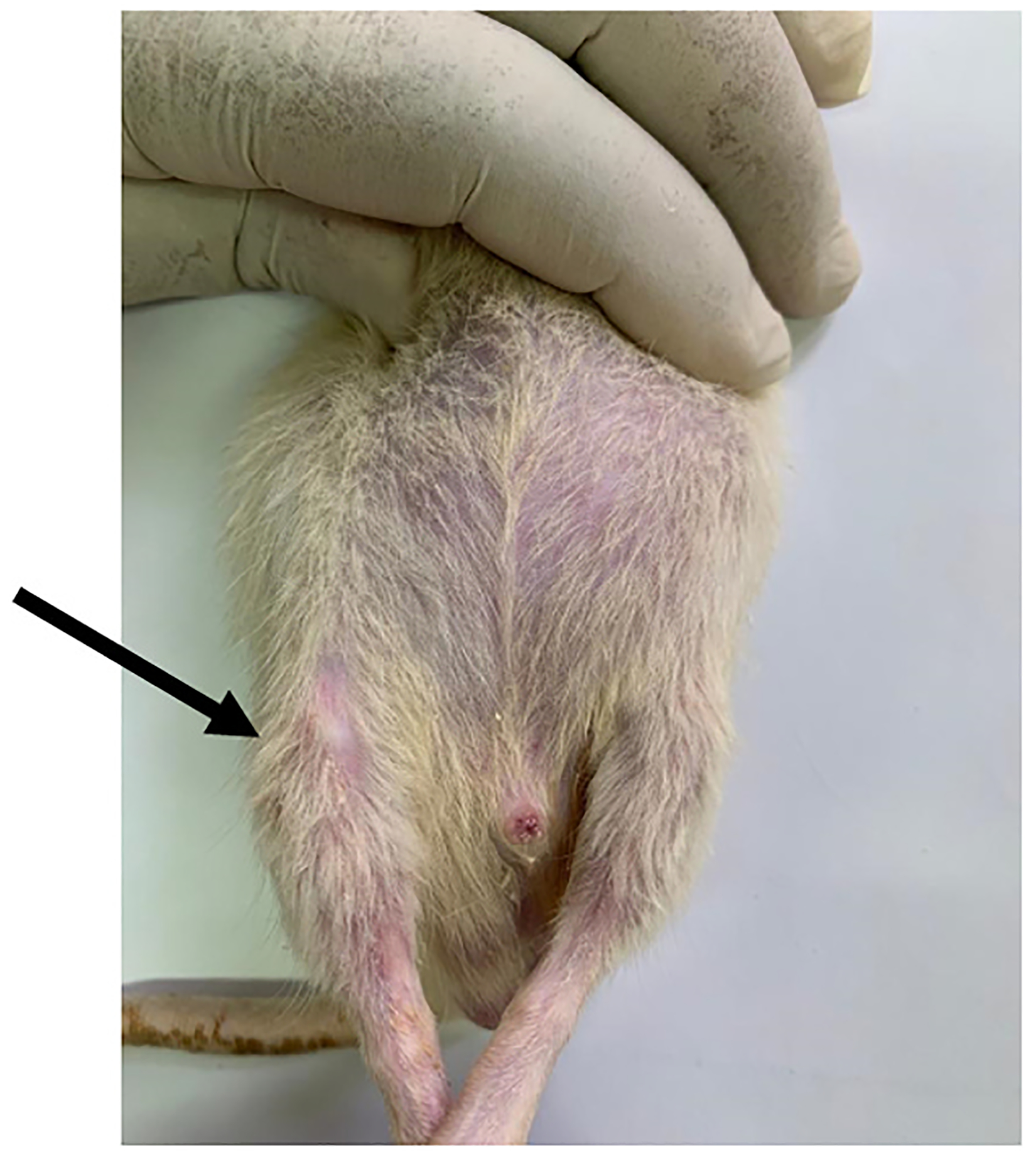
Photo of a rat knee injected with MIA (black arrow) showing edema compared to the left non-injected knee.
3.2. Curcumin combined with swimming significantly attenuated the knee joint damage
Fig. 3 shows the serum CRP and knee COMP levels in the different experimental groups. Rats injected with MIA showed increased CRP and COMP levels by 6.3- and 3-fold, respectively, when compared to the control group. After subjecting the rats to swimming, the CRP and COMP levels decreased by 0.5- and 0.7-fold, respectively, while treatment with curcumin resulted in a 1.9-fold decrease in CRP and 1.2-fold decrease in COMP levels compared to the MIA group. When curcumin was administered with swimming, a decrease in CRP and COMP levels by 3.6- and 1.7-fold, respectively, was observed compared to the untreated MIA group. Thus, the curcumin + swimming group was the group that showed the greatest improvement in CRP and COMP levels.
Fig. 3.
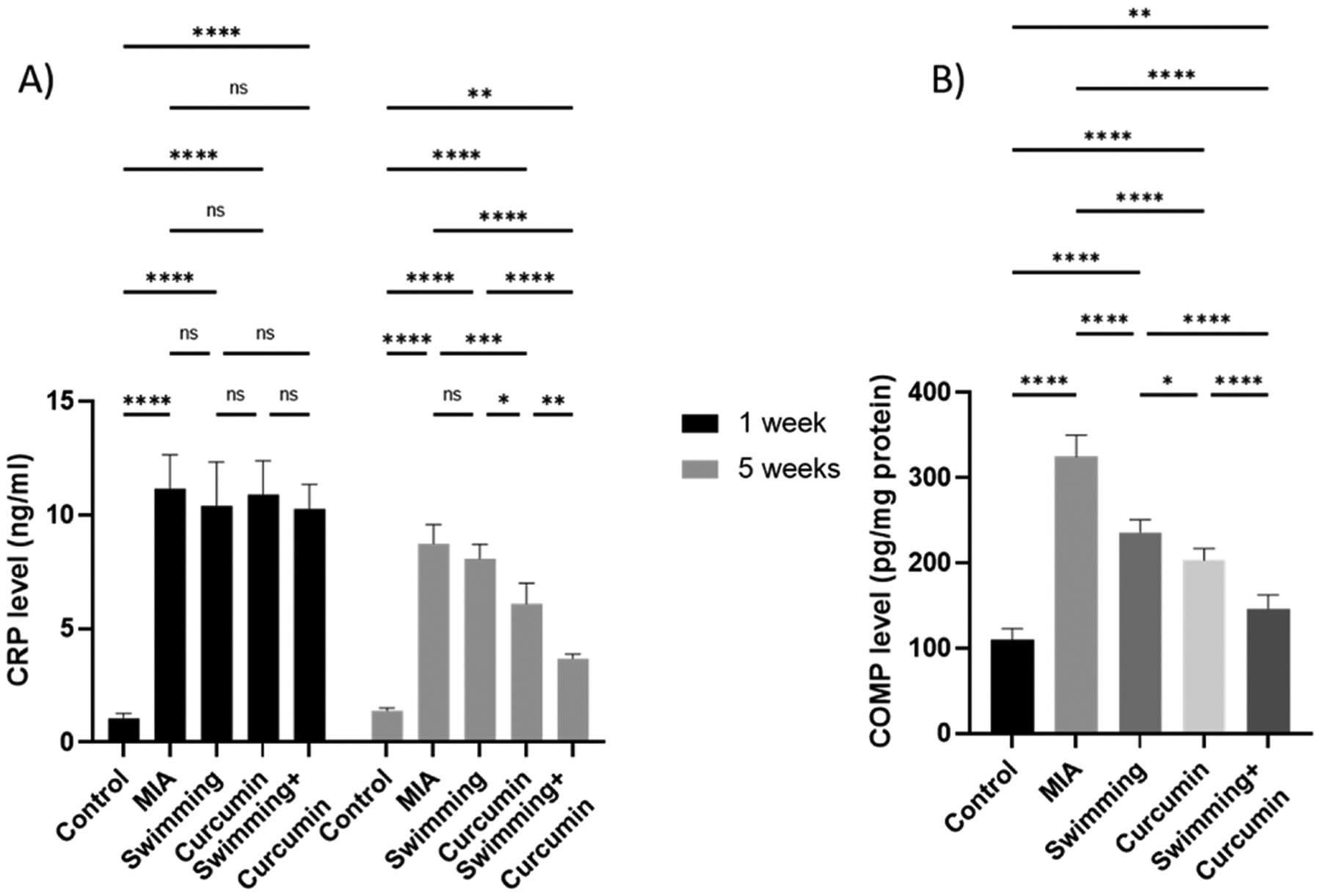
Levels of A) serum CRP at two different time points and B) tissue COMP from the rats subjected to 4 weeks of swimming, curcumin or swimming combined with curcumin. One-way ANOVA was used as a statistical test followed by Tukey post-hoc test for significance ns: non-significant, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, n = 6/group.
3.3. Combined treatment with curcumin and swimming decreases the alteration in miR-130a and HDAC3
As illustrated in Fig. 4, a 77% pronounced drop in miR-130a accompanied by a 7-fold elevation in the HDAC3 expression was observed in the untreated MIA group.
Fig. 4.
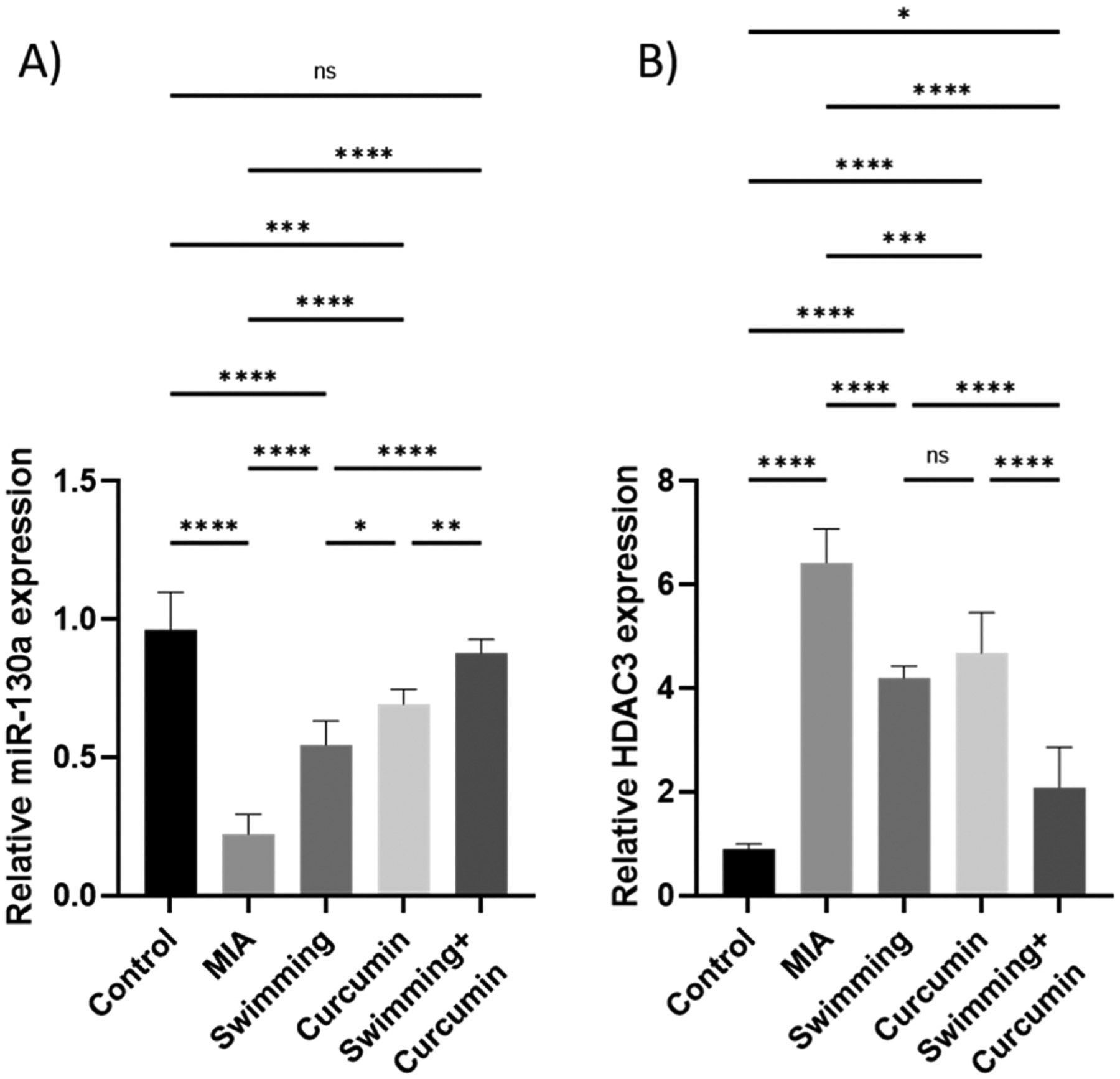
Relative expression of A) miR-130a and B) HDAC3 in the knee joint homogenate of the rats subjected to 4 weeks of swimming, curcumin or swimming combined with curcumin. One-way ANOVA was used as a statistical test followed by Tukey post-hoc test for significance. ns: non-significant, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, n = 6/group.
Treatment by swimming or curcumin alone resulted in 34% and 49% increase in miR-130a level and a 2.4- and 1.9-fold decrease in HDAC expression, respectively, in comparison to the MIA group. The combination of curcumin and swimming resulted in the greatest attenuation of the MIA effect with a 68% increase in miR-130a and a 4.8-fold decrease in HDAC3 expression.
3.4. Suppression of MMP1 and MMP13 expression after curcumin and swimming treatment
After osteoarthritis induction by MIA, the levels of MMP1 and MMP13 significantly increased to 3.6- and 3.9-fold, respectively, in contrast to the control group (Fig. 5). Subjecting the rats to swimming and curcumin individual treatments for 4 weeks attenuated the increase resulting from MIA by a 0.9- and 1.5-fold in MMP1 and a 0.5- and 1.2-fold in MMP13, respectively. Combining both modalities resulted in approximately 2.2-fold decrease in MMP1 and MMP13 compared to the MIA group.
Fig. 5.
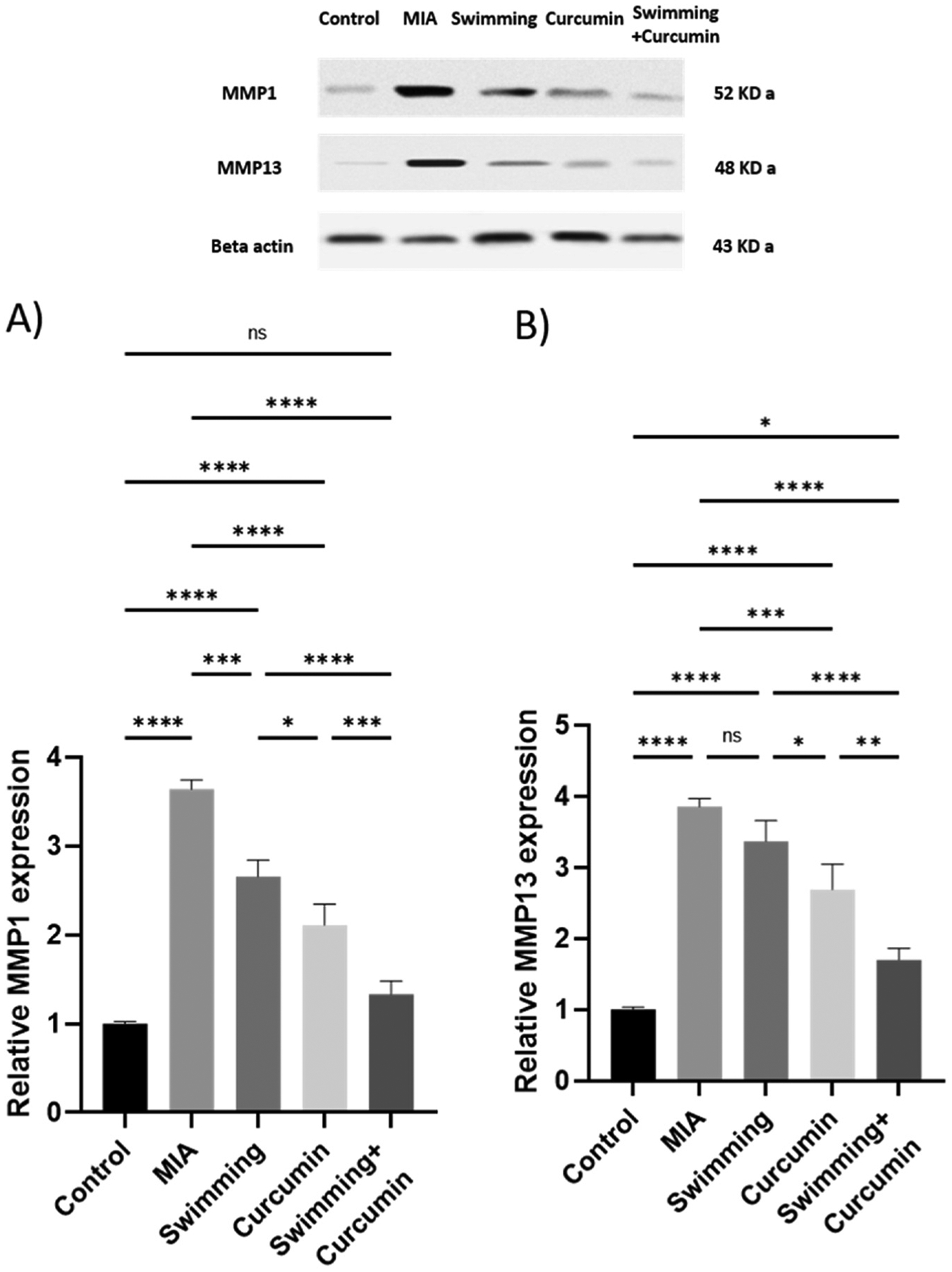
Expression of MMP1 and MMP13 in the knee joint homogenate of the rats subjected to 4 weeks of swimming, curcumin or swimming combined with curcumin shown in the upper pannel. Beta-actin confirms equal loading of the protein. Western blot was performed in triplicates with similar results. One representative blot is shown. One-way ANOVA was used as a statistical test followed by Tukey post-hoc test for significance ns; non-significant, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, n = 6/group.
3.5. Suppression of inflammation after combined swimming and curcumin treatment
In comparison to the control group, PPAR-γ decreased in the MIA group by 74%. After swimming, curcumin, and combined swimming and curcumin treatments, PPAR-γ increased by 22%, 33%, and 49%, respectively, compared to the MIA group (Fig. 6). NF-κB, IL-1β, and TNF-α showed a significant elevation in the MIA group to about 7-, 5.6-, and 4.3-fold the control group. Swimming and curcumin treatment attenuated the inflammation which was reflected in the levels of the three measured parameters. Swimming resulted in a 3-, 1-, and 1.8-fold lower NF-κB, IL-1β, and TNF-α levels, while curcumin showed a 4-, 1.3-, and 2.2-fold decrease when compared to the MIA group. Swimming and curcumin co-treatment resulted in a 5-, 3.5-, and 3-fold attenuation in NF-κB, IL-1β, and TNF-α levels compared to the MIA group. These results were confirmed by the immunohistochemical staining of the sections. As shown in Fig. 7, MIA increased NF-κB by 9 folds. The different treatment groups of swimming, curcumin, and both together showed an attenuation of this decrease by 5.3-, 3.7-, and 3.4-fold respectively. Fig. 8 also shows TNF-α which increased by 14.2-fold after osteoarthritis induction by MIA. Swimming, curcumin and their combination resulted in a decrease of 6.6-, 5.8-, and 12-fold compared to the MIA group.
Fig. 6.
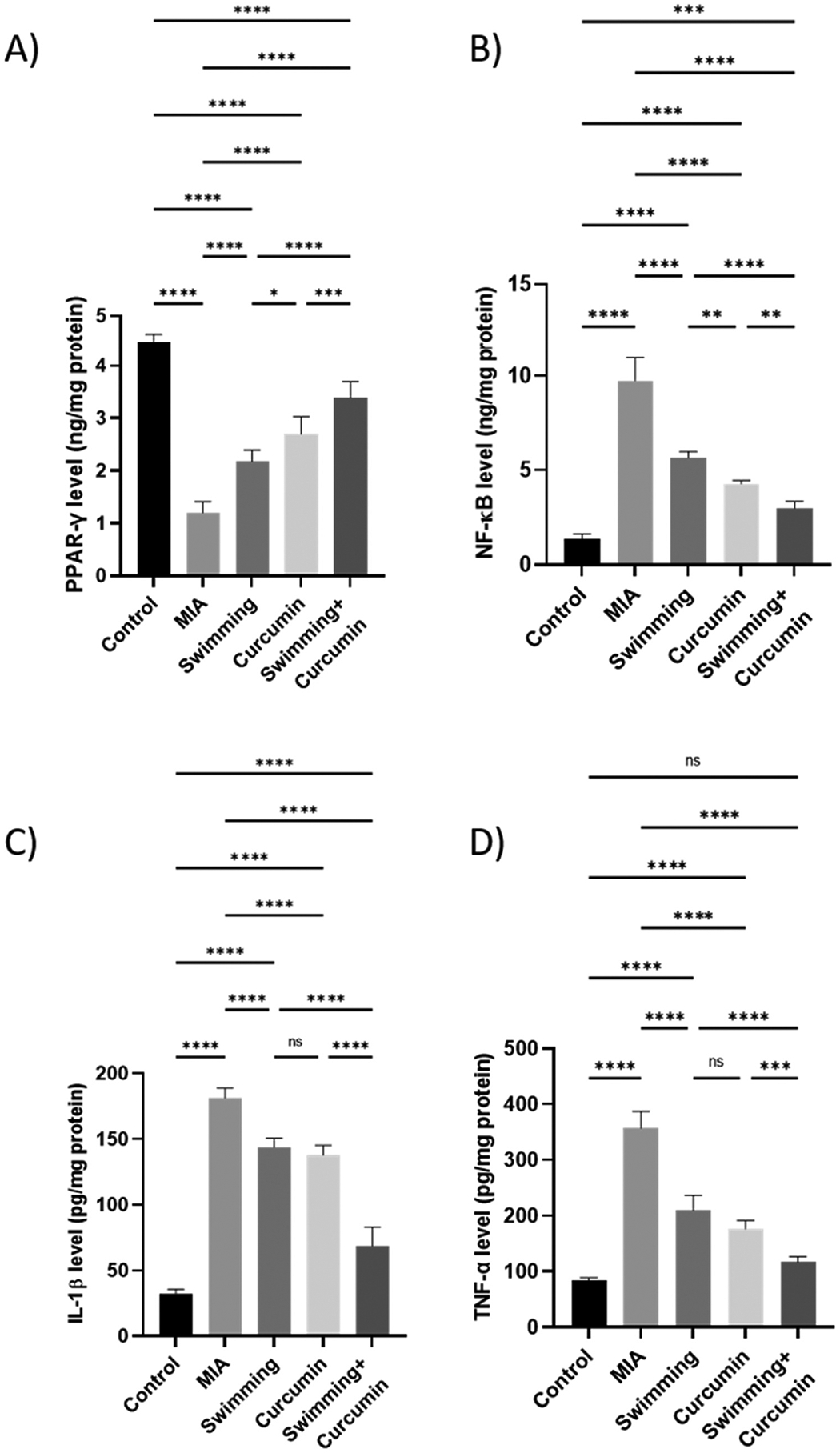
Levels of A) PPAR-γ, B) NF-κB, C) IL-1β, and D) TNF-α in the knee joint homogenate of the rats subjected to 4 weeks of swimming, curcumin or swimming combined with curcumin. One-way ANOVA was used as a statistical test followed by Tukey post-hoc test for significance ns; non-significant, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 n = 6/group.
Fig. 7.
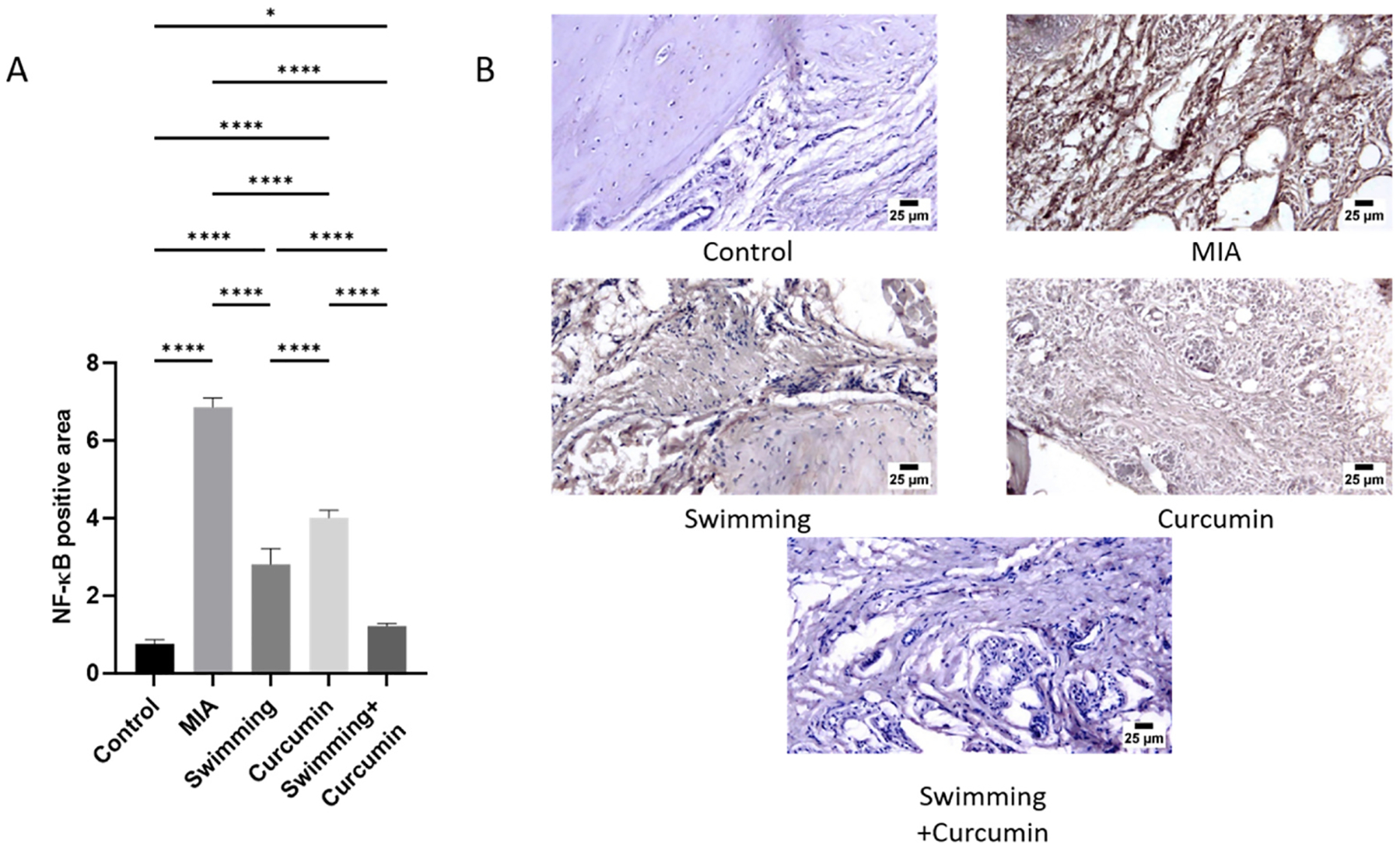
Immunohistochemical quantification (A) and photomicrographs (B) of NF-κB positive and negative areas. One-way ANOVA was used as a statistical test followed by Tukey post-hoc test for significance. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, n = 5/group.
Fig. 8.
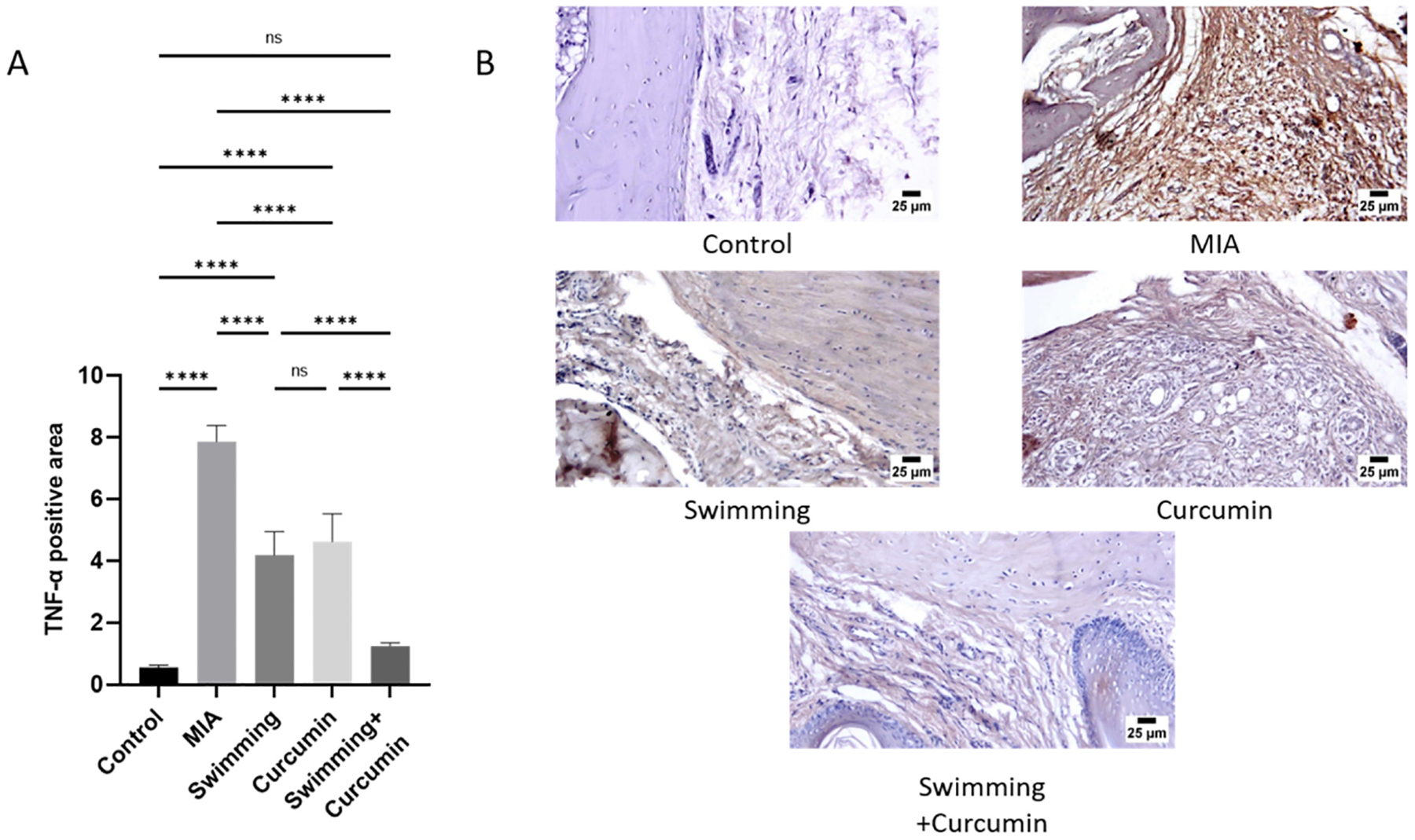
Immunohistochemical quantification (A) and photomicrographs (B) of TNF-α positive and negative areas. One-way ANOVA was used as a statistical test followed by Tukey post-hoc test for significance ns; non-significant, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, n = 5/group.
3.6. Radiographic evaluation
The control group showed no radiographic changes either in joint space or the articular surfaces. However, MIA injection demonstrated radiographic findings of OA manifested by joint capsule effusion with soft tissue swelling, joint space narrowing, endplate sclerosis and osteophytosis. Rats subject to swimming or curcumin showed distention in the joint capsule with effusion, clear articular surfaces, yet a rise in the radiodensity of the joint space with conserved identity appeared in the curcumin group. Joint space remained preserved with minimal detectable radiographic changes in the group with combination therapy (Fig. 9).
Fig. 9.
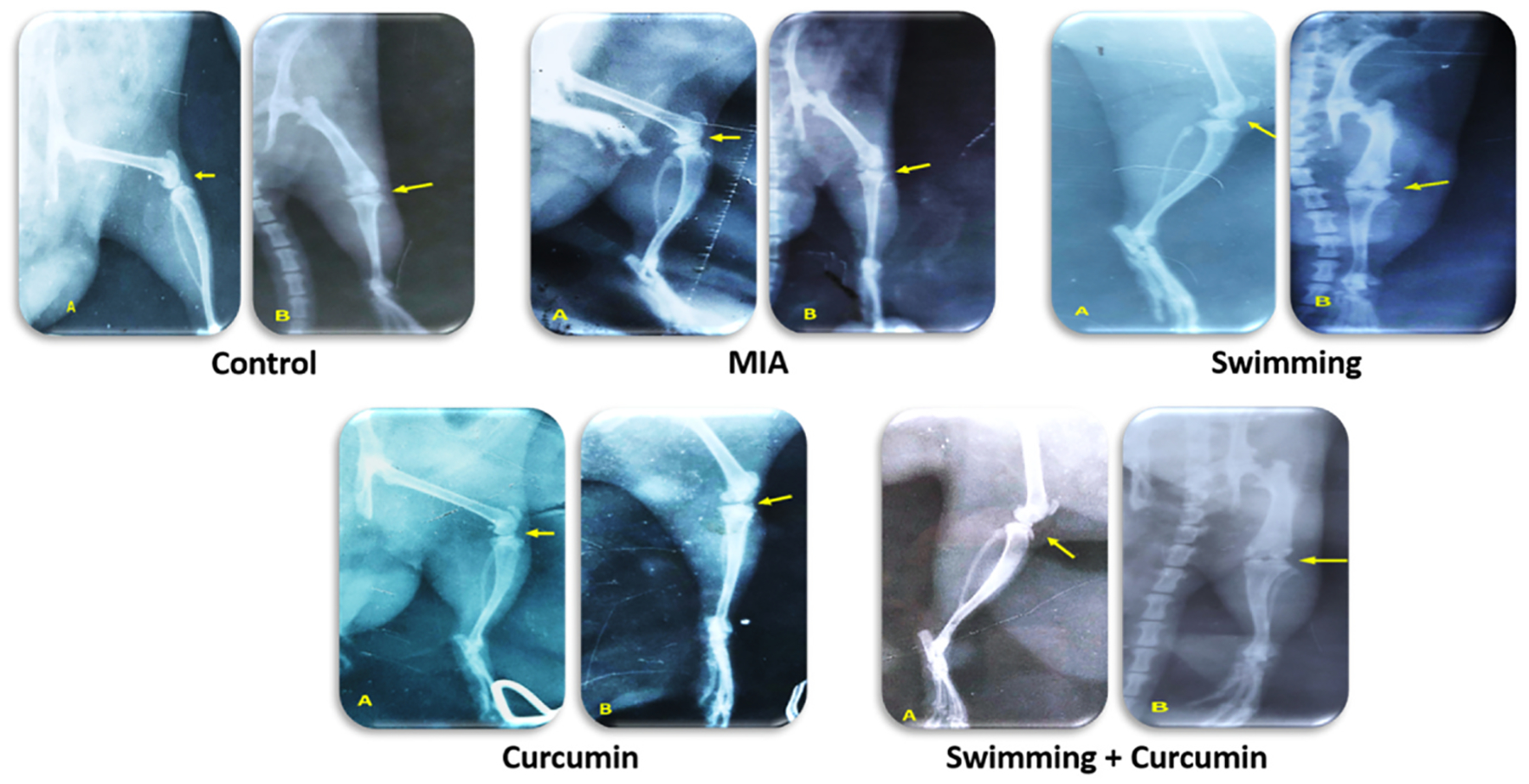
The radiography of the right knee joint of the five different groups: (A) Lateromedial and (B) Craniocaudal radiographic views of the right stifle (yellow arrow), n = 3/group.
3.7. Histopathological findings
Microscopic examination of joint and periarticular tissue from the control group revealed normal structure of all examined regions as shown in Fig. 10. On the contrary, the MIA group exhibited several histopathological alterations including that the periarticular tissue showed severe inflammation in the form of extensive infiltration by inflammatory cells and marked edema. The adjacent muscle bundles suffered from degeneration and necrosis with numerous inflammatory cell infiltration. The inflammatory reaction was extending deep into the joint capsule causing enhanced thickness of the capsule that also displayed pannus formation with numerous dilated blood vessels. Moderate improvements were detected in the swimming group where several rats’ joints showed apparently unremarkable articular surface, joint capsule, and surrounding tissues. However, some of the examined sections showed mild to moderate inflammatory reactions in the periarticular surface associated with perivascular mononuclear inflammatory cell infiltration. Mild improvements were observed in the curcumin group, which were characterized by apparently normal articular surface in several examined sections, however the joint capsules were moderately thickened with infiltration by inflammatory cells in the adjacent periarticular surface. As for the combination group, the most pronounced therapeutic effects were observed. Most of the sections showed normal histological features with the exception of one joint that revealed mild inflammation in the periarticular tissue.
Fig. 10.
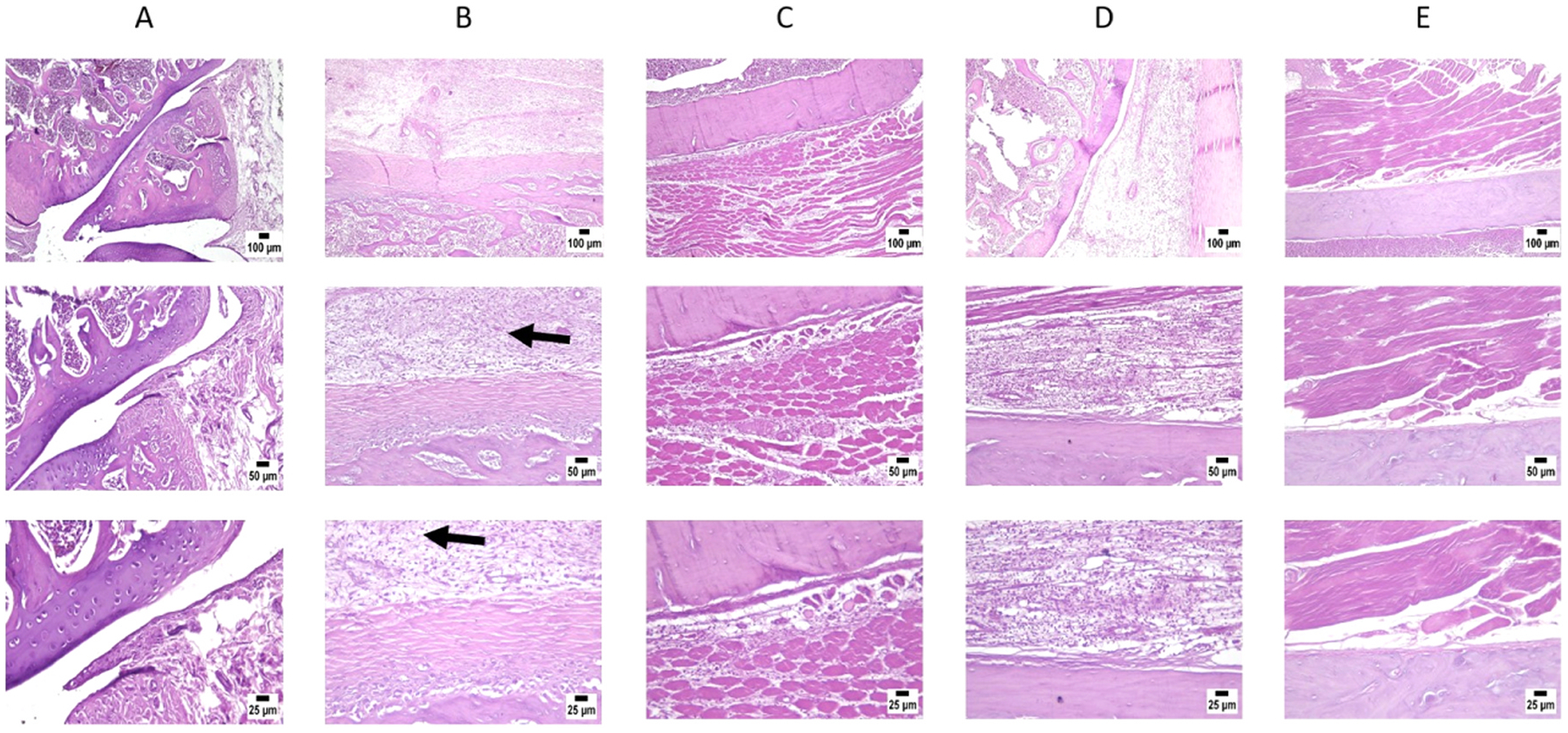
Photomicrographs showing; A) Control group, B) MIA group showing degeneration and destruction of the knee joint (arrow), C) Swimming group, D) Curcumin and E) Swimming + curcumin group. H&E stain (original magnifications x25, x50 and x100), n = 3/group.
Statistical analysis of histopathological lesion score showed a significant increase in group 2 that developed OA and didn’t receive treatment, when compared to all other groups. Meanwhile, absence of significant difference was noted between group 3 and group 4, which only underwent swimming protocol or only received curcumin, respectively. In contrast, a significant decrease was detected in group 5 (received combination therapy of curcumin + swimming) in comparison with the other treated groups 3 and 4 (Fig. 10).
4. Discussion
Patients with osteoarthritis frequently experience pain and discomfort, which can result in a variety of physical limitations [14]. The objectives of this study were to evaluate the therapeutic effects of curcumin combined with swimming in a rat model of OA. Our results showed that the oral supplementation of curcumin alongside swimming for 4 weeks is significantly more effective on reducing OA-related joint damage and inflammation than using any of these two modalities alone. This improvement was associated with reduced tactile hypersensitivity and improved articular motor function of the treated rats.
Previous studies have suggested that curcumin reduces pain and enhances physical function in patients with OA of their knees [51]. In OA patients, previous studies revealed a pronounced effect of curcumin on relieving pain and improving physical performance in OA patients [5, 60]. Of important note, curcumin induces its effects in OA with more tolerable gastrointestinal effects than that of the non-steroidal anti-inflammatory drugs (NSAIDs).
Regular physical exercise together with medication was previously highlighted as being more effective in alleviating osteoarthritis symptoms [59,65]. Among the physical exercise modalities, specifically swimming was found to decrease joint pain, stiffness, and functional restriction in OA patients [3]. Our current study demonstrated that swimming, accompanied by curcumin administration led to a better outcome in osteoarthritis patients.
Increased levels of pro-inflammatory cytokines is crucial for OA development [62]. In the present study, we demonstrated, for the first time, that the administration of curcumin combined with regular swimming for 4 weeks significantly decreases pro-inflammatory cytokines levels including CRP, IL-1β, and TNF-α. Notably, the effects of curcumin combined with swimming on decreasing the levels of pro-inflammatory cytokines were more pronounced than the effects of curcumin or swimming alone. Curcumin possesses anti-inflammatory characteristics that are comparable to those of NSAIDs, yet it doesn’t cause gastrointestinal untoward effects that frequently occur with NSAIDs administration. It has been shown that curcumin influences NF-κB activity to modify the signaling of pro-inflammatory cytokines [36]. NF-κB transcription factors are essential for the development of a wide range of cytokines, chemokines, and adhesion molecules that are involved in inflammatory and immunological responses as well as in cell survival [11]. A previous study showed that acetylation of regulatory proteins like NF-κB plays a critical role in controlling the strength, duration, and specificity of inflammatory reactions [38]. Our study demonstrated that curcumin decreased the levels of NF-κB, which could explain the decrease in IL-1β and TNF-α, which are known targets of NF-κB signaling. A study using a rat model of OA supported this finding by demonstrating a significant reduction in the expression of NF-κB, TNF-α IL-1β, and IL-6 in the synovial fluid after curcumin administration [67].
Among the biomarkers for OA monitoring are the cartilage oligomeric matrix proteins (COMP) and matrix metalloproteinases (MMPs) [30]. In the current study, an increase in COMP, MMP1, and MMP13 in the knees of rats with OA was observed, and was reversed by combining curcumin with swimming for 4 weeks. The fact that inflammation results in the activation of the MMPs such as MMP2, MMP3, MMP9, and MMP13, which induce degradation of the articular extracellular matrix (ECM) [7] elucidates the reason for their elevation after induction of OA by MIA. These enzymes play important roles in shaping the non-collagen matrix elements, proteoglycan aggrecan, and collagen. Curcumin has been demonstrated to reduce MMP1 and MMP3 levels in chondrocytes [49]. In an experimental rat model of OA, Yabas et al., [62] studied the anti-osteoarthritic effect of curcumin and found improved serum COMP, CRP, and MMP3 levels. Exercise also has an anti-inflammatory impact, which helps to slow the progression of OA’s degenerative alterations by preventing the ECM’s crucial degeneration [37].
In the present study, histological and radiological observations demonstrated normal periarticular tissue with almost complete disappearance of the inflammatory signs in the group receiving curcumin along with swimming exercise as compared to untreated group. This finding supports the anti-inflammatory role of combined curcumin and swimming in OA-induced joint inflammation and cartilage degradation. This was in accordance with previous studies that reported a decrease in the IL-1β expression levels after subjecting rats to an exercise program [4,48].
Several studies reported different pathways implicated in osteoarthritis as ERK/c-fos/NFATc1 pathway [21] and another involving HIF-1α [63]. Previous studies also documented the relationship between HDACs and miRNA in OA pathogenesis [64]. In agreement with our study, Wu et al., [61] observed significant difference in miRNAs expression profiles between OA and healthy controls. In the study of Zhang et al., [66], osteoarthritic patients showed conserved serum level of miR-130a which was related to apoptosis of chondrocytes, decreased cell proliferation and induction of inflammatory mediators such as NF-κB, TNF-α and IL-1β. The association between HDACs and its target miRNA-130a has been documented in other pathological conditions. For instance, according to Ma et al., [44], HDAC3 was shown to play a significant role in spinal cord injuries by regulating the expression of miR-130a. The down-regulation of miR-130a was associated with elevated expression of HDAC3 which, in turn, stimulated the inflammatory process as evidenced by enhanced NF-κB and TNF-α expression. NF-κB can be activated or deactivated by being (de)acetylated at different lysine residues [38]. However, the associations between HDAC3 and NF-κB in inflammatory signaling remain poorly understood. Jiang and Wang [31] also suggested that miR-130a through HDAC3 plays a role in ankylosing spondylitis. MiR-130a has been linked to the increase in NF-κB-mediated inflammation and transforming growth factor-1-mediated fibrosis in a rat model of acute myocardial infarction by blocking the protective action of the target gene PPAR-γ [15]. PPAR-γ is a transcription factor that regulates over 100 genes involved in inflammation, proliferation, and differentiation [2]. Numerous studies have demonstrated that HDAC3 nuclear translocation is necessary for the reduction of PPAR-γ transcriptional activity, whereas the inhibition of HDAC3 activity leads to the acetylation of proteins, which activates PPAR-γ [17,20,22]. PPAR-γ expression has been reported to be suppressed in OA [50]. A recent review on the implication of PPARs in OA [27] documented the role of PPAR-γ in halting the activation of NF-κB where a study done by Wang et al., [58] has shown that protection against lipopolysaccharide-induced chondrocytes injury in OA could be mediated through the crosstalk between PPARγ and NF-κB. Furthermore, in skeletal muscle cells, stimulation of PPAR-γ negatively impacted the transcriptional activity of NF-κB and reduced the expression of TNF-α and IL-1β [53]. Taken together, our combination appears to hamper OA through the modulation of miR-130a/HDAC3/PPAR-γ signaling pathway, which may have an impact on the expression of NF-κB and its downstream targets. However, to further confirm this idea, future studies are required.
5. Conclusions
This study highlights for the first time the therapeutic potential of the combined regimen of curcumin and swimming in OA, which successfully alleviated the OA pain, inflammation, and joint stiffness. The therapeutic efficacy of this strategy on alleviating OA is further supported by histological and radiological findings. Mechanistically, such strategy suppresses the miR-130a/HDAC3/PPAR-γ pathway, which leads to downregulation of NF-κB and its downstream targets of inflammatory cytokines IL-1β and TNF-α.
Acknowledgments
The authors acknowledge the efforts of the Pharmacology and Toxicology Department at Cairo University to facilitate the completion of this study.
Funding
HMA is supported by an R01 CA260872 grant from the National Institutes of Health.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Adães S, et al. , Intra-articular injection of collagenase in the knee of rats as an alternative model to study nociception associated with osteoarthritis, Arthritis Res. Ther 16 (2014) 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Al-Ghadban S, et al. , Increase in Leptin and PPAR-γ gene expression in lipedema adipocytes differentiated in vitro from adipose-derived stem cells, Cells 9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Alkatan Mohammed, et al. , Improved function and reduced pain after swimming and cycling training in patients with osteoarthritis, J. Rheumatol 43 (2016) 666–672. [DOI] [PubMed] [Google Scholar]
- [4].Assis L, et al. , Aerobic exercise training and low-level laser therapy modulate inflammatory response and degenerative process in an experimental model of knee osteoarthritis in rats, Osteoarthr. Cartil 24 (2016) 169–177. [DOI] [PubMed] [Google Scholar]
- [5].Bannuru RR, et al. , Efficacy of curcumin and Boswellia for knee osteoarthritis: systematic review and meta-analysis, Semin. Arthritis Rheum 48 (2018) 416–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Boldin MP, Baltimore D, MicroRNAs, new effectors and regulators of NF-κB, Immunol. Rev 246 (2012) 205–220. [DOI] [PubMed] [Google Scholar]
- [7].Cabral-Pacheco GA, et al. , The roles of matrix metalloproteinases and their inhibitors in human diseases, Int. J. Mol. Sci 21 (2020) 1–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Carpio LR et al. , 2017. Histone deacetylase 3 supports endochondral bone formation by controlling cytokine signaling and matrix remodeling HHS Public Access. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chaplan SR, et al. , Quantitative assessment of tactile allodynia in the rat paw, J. Neurosci. Methods 53 (1994) 55–63. [DOI] [PubMed] [Google Scholar]
- [10].Chen C, et al. , Biomechanical properties and mechanobiology of the articular chondrocyte, Am. J. Physiol. - Cell Physiol 305 (2013) 1202–1208. [DOI] [PubMed] [Google Scholar]
- [11].Chen LF, Greene WC, Shaping the nuclear action of NF-kappaB, Nat. Rev. Mol. Cell Biol 5 (2004) 392–401. [DOI] [PubMed] [Google Scholar]
- [12].Chen S, et al. , Valproic acid attenuates traumatic spinal cord injury-induced inflammation via STAT1 and NF-TB pathway dependent of HDAC3, J. Neuroinflamm 15 (2018) 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chen X, et al. , Requirement for the histone deacetylase Hdac3 for the inflammatory gene expression program in macrophages, Proc. Natl. Acad. Sci. U. S. A 109 (2012), E2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cheragh-Birjandi S, et al. , Impact of resistance exercises and nano-curcumin on synovial levels of collagenase and nitric oxide in women with knee osteoarthritis, Transl. Med. Commun 2020 51 5 (2020) 1–6. [Google Scholar]
- [15].Chu X, et al. , miR-130 aggravates acute myocardial infarction-induced myocardial injury by targeting PPAR-γ, J. Cell. Biochem 119 (2018) 7235–7244. [DOI] [PubMed] [Google Scholar]
- [16].Dekker FJ, et al. , Small molecule inhibitors of histone acetyltransferases and deacetylases are potential drugs for inflammatory diseases, Drug Discov. Today 19 (2014) 654–660. [DOI] [PubMed] [Google Scholar]
- [17].Ding L, et al. , PPAR-γ is critical for HDAC3-mediated control of oligodendrocyte progenitor cell proliferation and differentiation after focal demyelination, Mol. Neurobiol 57 (2020) 4810–4824. [DOI] [PubMed] [Google Scholar]
- [18].El-Shiekh RA, et al. , Anti-inflammatory activity of Jasminum grandiflorum L. subsp. floribundum (Oleaceae) in inflammatory bowel disease and arthritis models, Biomed. Pharmacother 140 (2021), 111770. [DOI] [PubMed] [Google Scholar]
- [19].Elbaz EM, et al. , Donepezil halts acetic acid-induced experimental colitis in rats and its associated cognitive impairment through regulating inflammatory/oxidative/apoptotic cascades: an add-on to its anti-dementia activity, Int. Immunopharmacol (2023) 116. [DOI] [PubMed] [Google Scholar]
- [20].Fajas L, et al. , The retinoblastoma-histone deacetylase 3 complex inhibits PPARgamma and adipocyte differentiation, Dev. Cell 3 (2002) 903–910. [DOI] [PubMed] [Google Scholar]
- [21].Fang C, et al. , Diterbutyl phthalate attenuates osteoarthritis in ACLT mice via suppressing ERK/c-fos/NFATc1 pathway, and subsequently inhibiting subchondral osteoclast fusion, Acta Pharmacol. Sin 43 (2022) 1299–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gao Z, et al. , Regulation of nuclear translocation of HDAC3 by IkappaBalpha is required for tumor necrosis factor inhibition of peroxisome proliferator-activated receptor gamma function, J. Biol. Chem 281 (2006) 4540–4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Goldring SR, Goldring MB, Changes in the osteochondral unit during osteoarthritis: structure, function and cartilage–bone crosstalk, Nat. Rev. Rheumatol 12 (2016) 632–644. 2016 1211. [DOI] [PubMed] [Google Scholar]
- [24].Gordon R, Bloxham S, A systematic review of the effects of exercise and physical activity on non-specific chronic low back pain, Healthcare 4 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hamdalla HM, et al. , Ameliorative effect of curcumin nanoparticles against monosodium iodoacetate-induced knee osteoarthritis in rats, Mediat. Inflamm 2022 (2022) 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hu Y, et al. , Subchondral bone microenvironment in osteoarthritis and pain, Bone Res (2021) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Huang G, et al. , Role of peroxisome proliferator-activated receptors in osteoarthritis (Review), Mol. Med. Rep 23 (2021) 4–6. [DOI] [PubMed] [Google Scholar]
- [28].Hunter DJ, Bierma-Zeinstra S, Osteoarthritis, Lancet 393 (2019) 1745–1759. [DOI] [PubMed] [Google Scholar]
- [29].Iijima H, et al. , Effects of short-term gentle treadmill walking on subchondral bone in a rat model of instability-induced osteoarthritis, Osteoarthr. Cartil 23 (2015) 1563–1574. [DOI] [PubMed] [Google Scholar]
- [30].Jeong JW, et al. , Mori Folium water extract alleviates articular cartilage damages and inflammatory responses in monosodium iodoacetate-induced osteoarthritis rats, Mol. Med. Rep 16 (2017) 3841–3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jiang Y, Wang L, Role of histone deacetylase 3 in ankylosing spondylitis via negative feedback loop with microRNA-130a and enhancement of tumor necrosis factor-1α expression in peripheral blood mononuclear cells, Mol. Med. Rep 13 (2016) 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Khan NM, et al. , Epigenetics in osteoarthritis: potential of HDAC inhibitors as therapeutics Graphical Abstract HHS Public Access, Pharm. Res 128 (2018) 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Khan NM, et al. , Wogonin, a plant derived small molecule, exerts potent anti-inflammatory and chondroprotective effects through the activation of ROS/ERK/Nrf2 signaling pathways in human Osteoarthritis chondrocytes, Free Radic. Biol. Med 106 (2017) 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Khodir AE, et al. , Targeting Nrf2/HO-1 signaling by crocin: Role in attenuation of AA-induced ulcerative colitis in rats, Biomed. Pharmacother 110 (2018) 389–399. [DOI] [PubMed] [Google Scholar]
- [35].Kim S-H, et al. , Effects of swimming exercise and joint mobilization on HSP 70 levels in osteoarthritic rats, J. Korean Phys. Ther 26 (2014) 418–424. [Google Scholar]
- [36].Kocaadam B, Şanlier N, Curcumin, an active component of turmeric (Curcuma longa), and its effects on health, Crit. Rev. Food Sci. Nutr 57 (2017) 2889–2895. [DOI] [PubMed] [Google Scholar]
- [37].Kong H, et al. , Exercise for osteoarthritis: a literature review of pathology and mechanism, Front. Aging Neurosci 14 (2022) 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Leus NGJ, et al. , Histone deacetylase 3 (HDAC 3) as emerging drug target in NF-κB-mediated inflammation, Curr. Opin. Chem. Biol 33 (2016) 160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Li Y, Seto E, HDACs and HDAC inhibitors in cancer development and therapy, Cold Spring Harb. Perspect. Med (2016) 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Li ZC, et al. , Decreased expression of microRNA-130a correlates with TNF-α in the development of osteoarthritis, Int. J. Clin. Exp. Pathol 8 (2015) 2555. [PMC free article] [PubMed] [Google Scholar]
- [41].Lin Y, et al. , miR-638 regulates differentiation and proliferation in leukemic cells by targeting cyclin-dependent Kinase 2, J. Biol. Chem 290 (2015) 1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Liu F, et al. , MiRNA-130a promotes inflammation to accelerate atherosclerosis via the regulation of proliferator-activated receptor γ (PPARγ) expression, Anatol. J. Cardiol 25 (2021) 630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lunz W, et al. , Long-term aerobic swimming training by rats reduces the number of aberrant crypt foci in 1,2-dimethylhydrazine-induced colon cancer, Braz. J. Med. Biol. Res 41 (2008) 1000–1004. [DOI] [PubMed] [Google Scholar]
- [44].Ma YD, et al. , Increased HDAC3 and decreased miRNA-130a expression in PBMCs through recruitment HDAC3 in patients with spinal cord injuries, Int. J. Clin. Exp. Pathol 8 (2015) 1682. [PMC free article] [PubMed] [Google Scholar]
- [45].Mao G, et al. , Exosomal miR-95–5p regulates chondrogenesis and cartilage degradation via histone deacetylase 2/8, J. Cell. Mol. Med 22 (2018) 5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].McAlindon TE, et al. , OARSI guidelines for the non-surgical management of knee osteoarthritis, Osteoarthr. Cartil 22 (2014) 363–388. [DOI] [PubMed] [Google Scholar]
- [47].Meng F, et al. , MicroRNA-193b-3p regulates chondrogenesis and chondrocyte metabolism by targeting HDAC3, Theranostics 8 (2018) 2862–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Milares LP, et al. , Effectiveness of an aquatic exercise program and low-level laser therapy on articular cartilage in an experimental model of osteoarthritis in rats, Connect. Tissue Res 57 (2016) 398–407. [DOI] [PubMed] [Google Scholar]
- [49].Mobasheri A, et al. , Scientific evidence and rationale for the development of curcumin and resveratrol as nutraceutricals for joint health, Int. J. Mol. Sci 13 (2012) 4202–4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Núñez-Carro C, et al. , Epigenetics as a therapeutic target in osteoarthritis, Pharmaceuticals 16 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Paultre K, et al. , Therapeutic effects of turmeric or curcumin extract on pain and function for individuals with knee osteoarthritis: a systematic review, BMJ Open Sport Exerc. Med 7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ragab GH, et al. , Platelet-rich plasma ameliorates monosodium iodoacetateinduced ankle osteoarthritis in the rat model via suppression of inflammation and oxidative stress, Evid. -Based Complement. Altern. Med 2021 (2021) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Remels AHV, et al. , PPARγ inhibits NF-κB-dependent transcriptional activation in skeletal muscle, Am. J. Physiol. - Endocrinol. Metab 297 (2009) 174–183. [DOI] [PubMed] [Google Scholar]
- [54].Sharifi-Rad J, et al. , Turmeric and its major compound curcumin on health: bioactive effects and safety profiles for food, pharmaceutical, biotechnological and medicinal applications, Front. Pharmacol 11 (2020) 1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Sleiman SF, et al. , Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate, Elife 5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Tamaddon M, et al. , Osteochondral scaffolds for early treatment of cartilage defects in osteoarthritic joints: from bench to clinic, Biomater. Transl 1 (2020) 3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Vonsy JL, et al. , Differential analgesic effects of morphine and gabapentin on behavioural measures of pain and disability in a model of osteoarthritis pain in rats, Eur. J. Pain 13 (2009) 786–793. [DOI] [PubMed] [Google Scholar]
- [58].sheng Wang J, et al. , Galectin-3 deficiency protects lipopolysaccharide-induced chondrocytes injury via regulation of TLR4 and PPAR-γ-mediated NF-κB signaling pathway, J. Cell. Biochem 120 (2019) 10195–10204. [DOI] [PubMed] [Google Scholar]
- [59].Wang W, et al. , Physical therapy as a promising treatment for osteoarthritis: a narrative review, Front. Physiol 13 (2022) 2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Wang Z, et al. , Efficacy and safety of turmeric extracts for the treatment of knee osteoarthritis: a systematic review and meta-analysis of randomised controlled trials, Curr. Rheumatol. Rep 23 (2021). [DOI] [PubMed] [Google Scholar]
- [61].Wu W, et al. , Comparison of microRNA expression profiles of Kashin-Beck disease, osteoarthritis and rheumatoid arthritis, Sci. Rep 7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Yabas M, et al. , A next generation formulation of curcumin ameliorates experimentally induced osteoarthritis in rats via regulation of inflammatory mediators, Front. Immunol 12 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Zhang H, et al. , Maintaining hypoxia environment of subchondral bone alleviates osteoarthritis progression, Sci. Adv 9 (2023) eabo7868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Zhang H, et al. , The role of HDACs and HDACi in cartilage and osteoarthritis, Front. Cell Dev. Biol 8 (2020) 966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Zhang W, et al. , OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines, Osteoarthr. Cartil 16 (2008) 137–162. [DOI] [PubMed] [Google Scholar]
- [66].Zhang Y, et al. , MicroRNA-130a regulates chondrocyte proliferation and alleviates osteoarthritis through PTEN/PI3K/Akt signaling pathway, Int. J. Mol. Med 41 (2018) 3699–3708. [DOI] [PubMed] [Google Scholar]
- [67].Zhang Y, Zeng Y, Curcumin reduces inflammation in knee osteoarthritis rats through blocking TLR4/MyD88/NF-κB signal pathway, Drug Dev. Res 80 (2019) 353–359. [DOI] [PubMed] [Google Scholar]
- [68].Zhu H, et al. , Histone Deacetylase-3 activation promotes tumor necrosis Factor-α (TNF-α) expression in cardiomyocytes during lipopolysaccharide stimulation, J. Biol. Chem 285 (2010) 9429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Zhu Liang, et al. , Suppression of miR-130a-3p attenuates oxygen–glucose deprivation/reoxygenation-induced dendritic spine loss by promoting APP, Front. Neurosci 15 (2021), 601850. [DOI] [PMC free article] [PubMed] [Google Scholar]


