Abstract
Introduction:
Asthma is a common pediatric disease. Identification of exacerbating factors is important to gain better asthma control. One potential exacerbation trigger is NSAID-hypersensitivity (NSAID-H). Studies regarding pediatric NSAID-H have varied demographics, methodologies, and conclusions. However, most studies find NSAID-H more prevalent in asthmatic patients.
Methods:
The objective was to determine the prevalence, symptoms, and factors associated with NSAID-H in a pediatric severe asthma population. One hundred children aged 6 to 18 years old from the Severe Asthma Clinic at Riley Hospital for Children in Indianapolis, IN, between 11/2020 and 5/2022 completed a survey about asthma triggers, allergies, co-morbid diagnoses, sinus symptoms, and NSAID reaction history.
Results:
Nineteen percent of participants reported a reaction to at least one NSAID. Ibuprofen (16%), aspirin (9%), and acetaminophen (9%) were the most implicated NSAIDs. Most common symptoms were dyspnea, wheezing, coughing, lightheadedness, and abdominal pain appearing within 30 minutes. Associated factors included history of a medication other than an NSAID triggering asthma (p = 0.02), nasal polyps (p = 0.01), ageusia (p = 0.01), cold-induced asthma (p = 0.02), and chronic sinusitis in immediate family member (p = 0.04).
Conclusions:
Prevalence of NSAID-H in a large children’s hospital pediatric severe asthma clinic was 19%. The most common drug was ibuprofen and the most common symptoms were respiratory and gastrointestinal. Associated factors included medication and cold triggered asthma, nasal polyps, ageusia, and family history of chronic sinusitis. This highlights the importance of a thorough history in severe asthma patients who may be at higher risk for NSAID-H. Future studies should focus on looking at the rate of NSAID-H in a larger severe asthma population and if social determinants of health play a role in the increased incidence of reacting.
To the editor:
Asthma is a common pediatric disease characterized by bronchial smooth muscle constriction and inflammation of the airway mucosa. Identification of exacerbating factors is important to gain better asthma control and reduce exacerbations. One potential exacerbation trigger is NSAID-hypersensitivity (NSAID-H).
Studies regarding NSAIDs as a cause of drug hypersensitivity in children have historically variedly greatly. NSAID-H prevalence varies with geography, ethnicity, age, and co-morbidities with international estimates ranging from 0.9–19.6%.1,2 This highlights the variability in NSAID-H prevalence among studies and is further complicated by variances in classification and diagnostic methods (oral provocation test [OPT] versus clinical diagnosis).3,4 One consistency among studies is that rates of NSAID-H are higher in asthmatics compared to general pediatric populations.1,2,3,5 However, no studies have specifically looked at children with severe asthma and if the prevalence and presenting symptoms of NSAID-H were more severe than children with intermittent or mild persistent asthma.
In this study, the aims were to determine the prevalence of NSAID-H, the most common presenting symptoms, and factors associated with NSAID-H within a pediatric severe asthma population. It was hypothesized that children with severe asthma have an increased prevalence of NSAID-H than previously reported.
This single-center, cross-sectional, survey-based project was performed in the pediatric severe asthma clinic at Riley Hospital for Children at Indiana University Health from November 2020 to May 2022 after receiving Institutional Review Board approval. Participants were 6–18 years of age and had to have severe asthma based on National Heart, Lung, and Blood Institute (NHLBI) guidelines (Expert Panel Report 4) as a requisite for inclusion. Analyses were performed to determine if there were differences in demographic factors and outcome measures between the participants reporting a reaction to NSAIDs (reactor) and those who did not report a reaction to NSAIDs (non-reactor). Descriptive statistics were generated for demographics, showing mean (standard deviation) for continuous variables and frequencies (percentages) for categorical variables. Student’s t-tests and Chi-Square tests were used for continuous and categorical variables, respectively. Fisher’s Exact tests were used to verify Chi-Square results when expected cell counts were small. Multivariable logistic regression analyses were also performed, to determine which significant variables from the bivariate analyses remained independently associated with NSAID use. Due to the sample size, we started only with those variables that had p<0.20 in the bivariate analyses, as well as including the patient characteristic variables of age, sex, and race. We then ran a backward selection model, including and retaining any variable which had a p-value of 0.20 or smaller. All analytic assumptions were verified, and all statistical analyses were performed using SAS v9.4 (SAS Institute, Cary, NC). A p-value of <0.05 was considered statistically significant.
Fifty-one percent of participants were male and mean age was 12.4 years. Twenty-nine percent self-identified as White, 63% as Black, and 6% as more than one race. Six percent self-identified as Hispanic. There was no statistical difference between the non-reactor and reactor groups in terms of gender, age, race, or ethnicity (Table 1).
Table 1 –
Comparison of Reactor and Non-Reactor Groups. N = number of participants.
| Factor | Reactor Group (n = 19) | Non-Reactor Group (n = 81) | P-value |
|---|---|---|---|
|
| |||
| Demographics | |||
|
| |||
| Age (years) | 13.7 (4.0) | 12.0 (4.1) | .10 |
|
| |||
| Age category | |||
| <=10 years | 5 (26.3) | 21 (27.6) | .91 |
| >10 years | 14 (73.7) | 55 (72.4) | |
|
| |||
| Gender | |||
| Male | 10 (52.6) | 41 (51.3) | .91 |
| Female | 9 (47.4) | 49 (48.8) | |
|
| |||
| Race | |||
| White | 7 (38.9) | 22 (27.5) | .63 |
| Black | 10 (55.6) | 53 (66.3) | |
| Multiracial | 1 (5.6) | 5 (6.3) | |
|
| |||
| Hispanic | 1 (5.3) | 5 (6.3) | .87 |
|
| |||
| Have allergies | 16 (84.2) | 69 (85.2) | .91 |
|
| |||
| Allergy Triggers | |||
|
| |||
| Dust mites | 8 (42.1) | 42 (51.9) | .44 |
|
| |||
| Pollen | 11 (58.9) | 44 (54.3) | .78 |
|
| |||
| Animal Dander | 7 (36.8) | 40 (49.4) | .32 |
|
| |||
| Mold | 7 (36.8) | 38 (46.9) | .43 |
|
| |||
| Food | 6 (31.6) | 30 (37.0) | .66 |
|
| |||
| Medications | 5 (26.3) | 6 (7.4) | .02 |
|
| |||
| Other | 1 (5.3) | 6 (7.4) | .74 |
|
| |||
| Co-Morbid Conditions | |||
|
| |||
| Rhinitis | 3 (15.8) | 12 (14.8) | .91 |
|
| |||
| Eczema | 10 (53.6) | 39 (48.2) | .73 |
|
| |||
| Hives | 1 (5.3) | 3 (3.7) | .75 |
|
| |||
| Autoimmune | 0 (0) | 1 (1.2) | .63 |
|
| |||
| Symptoms | |||
|
| |||
| Sinus symptoms | 13 (68.4) | 39 (48.2) | .11 |
|
| |||
| Nasal polyps | 6 (31.6) | 8 (9.9) | .01 |
|
| |||
| Lost sense of taste | 5 (26.3) | 5 (6.2) | .01 |
|
| |||
| Lost sense of smell | 4 (21.1) | 6 (7.4) | .07 |
|
| |||
| Sinus surgery | 4 (21.1) | 6 (7.4) | .07 |
|
| |||
| Asthma Triggers | |||
|
| |||
| Trigger exercise | 11 (57.9) | 55 (67.9) | .41 |
|
| |||
| Trigger laughter | 5 (26.3) | 13 (16.1) | .29 |
|
| |||
| Trigger crying | 2 (10.5) | 11 (13.6) | .72 |
|
| |||
| Trigger cold temperature | 15 (79.0) | 40 (49.4) | .02 |
|
| |||
| Trigger heat | 9 (47.4) | 32 (39.5) | .53 |
|
| |||
| Trigger virus | 11 (57.9) | 54 (66.7) | .47 |
|
| |||
| Trigger seasons | 13 (68.4) | 64 (79.0) | .32 |
|
| |||
| Trigger pollen | 12 (63.2) | 41 (50.6) | .32 |
|
| |||
| Trigger animal | 8 (42.1) | 36 (44.4) | .85 |
|
| |||
| Trigger dust mites | 9 (47.4) | 42 (51.9) | .74 |
|
| |||
| Trigger mold | 11 (57.9) | 30 (37.0) | .10 |
|
| |||
| Trigger strong smells | 13 (68.4) | 45 (55.6) | .31 |
|
| |||
| Trigger other | 1 (5.3) | 1 (1.2) | .26 |
|
| |||
| Immediate Family History | |||
|
| |||
| Family w/ asthma | 11 (57.9) | 50 (62.5) | .71 |
|
| |||
| Family w/ allergies | 10 (52.6) | 46 (57.5) | .70 |
|
| |||
| Family w/ drug allergy | 4 (21.1) | 15 (18.8) | .82 |
|
| |||
| Family w/ sinusitis | 7 (36.8) | 13 (16.3) | .04 |
|
| |||
| Family w/ eczema | 4 (21.1) | 36 (45.0) | .06 |
Out of all the participants, 19% (n=19) reported a reaction to at least one NSAID and 79% (n=15) of reactors reported a reaction to more than one NSAID. Among reactors, the median number of NSAIDs they reported a reaction to was two. The most implicated drug was ibuprofen (84% of reactors, 16% of participants), followed by aspirin (47% of reactors, 9% of participants), acetaminophen (47% of reactors, 9% of participants), and naproxen (42% of reactors, 8% of participants).
The most common symptom reported with NSAID ingestion was dyspnea (43%) (Figure 1). Wheezing, coughing, lightheadedness, and abdominal pain were also reported frequently (36%). Less common symptoms were rhinorrhea (29%), congestion (21%), and tachypnea (21%). Interestingly, cutaneous symptoms (rash and swelling) were only reported in 7% of reactors. Time to symptom onset was typically within 30 minutes of ingestion (n = 11, 61.1% of reactors). The range of time to reaction was immediate to 24+ hours later.
Figure 1.
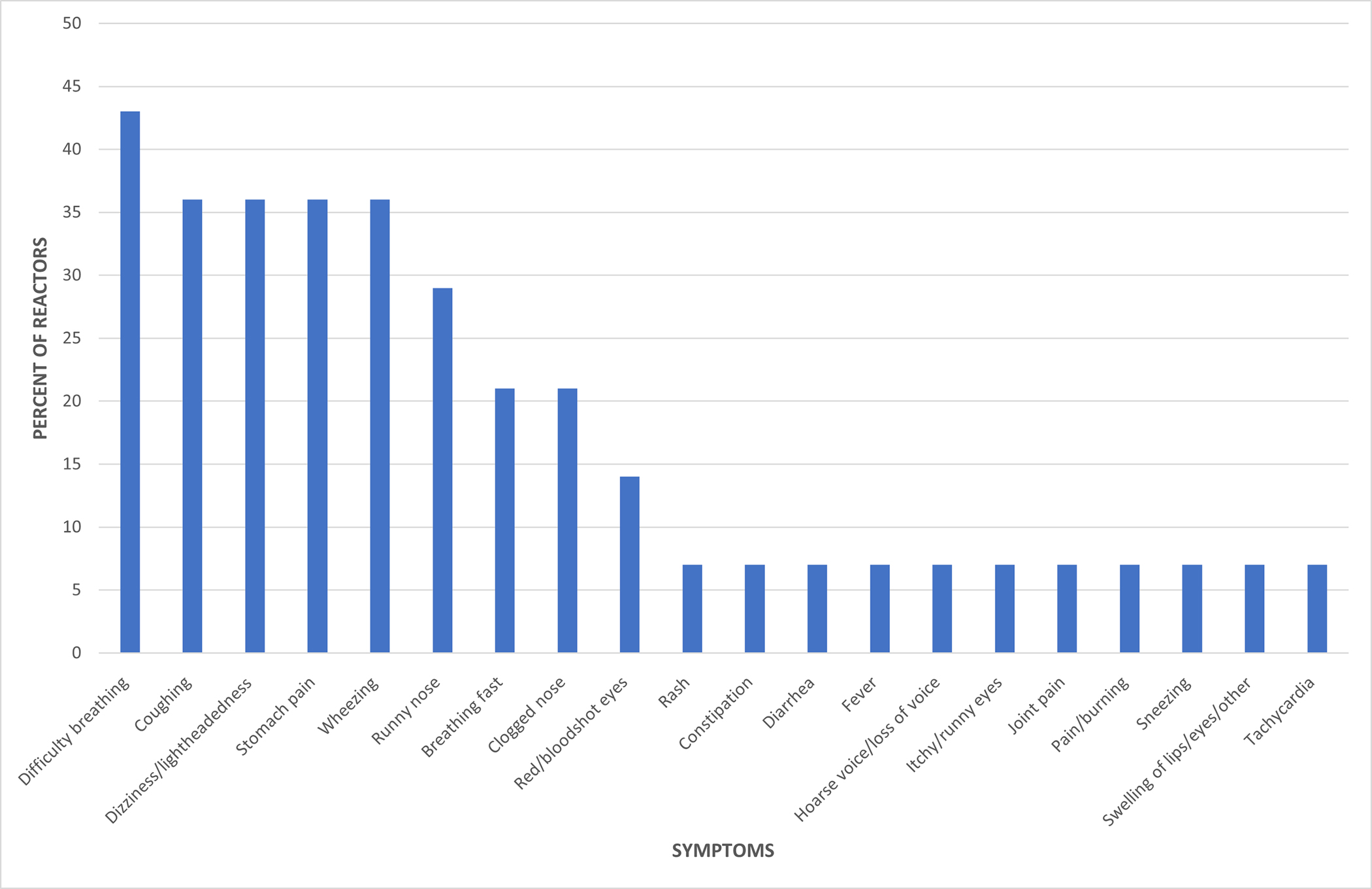
Percentage of reactors reporting specific symptoms after NSAID exposure.
Reactors were more likely to report an allergy to a medication that was not an NSAID (reactors 26.3%, non-reactors 7.4%, p = 0.02), nasal polyps (reactors 31.6%, non-reactors 9.9%, p = 0.01), ageusia (reactors 26.3%, non-reactors 6.2%, p = 0.01), viral-induced asthma (reactors 79%, non-reactors 49.4%, p = 0.02), and an immediate family member with chronic sinusitis (reactors 36.8%, non-reactors 16.3%, p = 0.04). Other factors that were more common in the reactors but did not reach clinical significance include anosmia with sinus symptoms (reactors 21.1%, non-reactors 7.4%, p = 0.07) and history of sinus surgery (reactors 21.1%, non-reactors 7.4%, p = 0.07). Of note, cutaneous symptoms were noted only in 7% (n = 1) of reactors. Results from the multivariable logistic regression model indicate that nasal polyps remain independently associated with being more likely to be in the Reactor group (Odds ratio (95% CI): 4.42 (1.06, 18.52), p=0.04). Medication trigger, cold trigger, and family history of sinusitis were attenuated. The symptoms of losing taste and smell were not included in the multivariable model due to their high collinearity with nasal polyps.
Nineteen percent of participants reported a reaction to at least one NSAID, a higher rate than previously estimated in most studies. 1,2,3,5 It is important to note that only Guvenir et al. (2018) highlighted the significance of asthma severity and its relationship to NSAID-H. They found that 78% of participants with confirmed NSAID-H had partially or uncontrolled asthma. Based on our literature review, we could not find other studies that specifically looked at the relationship of NSAID-H prevalence and asthma severity. The prevalence of NSAID-H in this study is potentially due to the severity of participant’s asthma; however, the pathophysiology behind this finding is yet unclear.
Previous general pediatric studies found that the most common symptoms were isolated skin involvement (urticaria, angioedema).6 However, this study found that children with severe asthma were more likely to report respiratory symptoms with NSAID ingestion and cutaneous symptoms were relatively uncommon. The reason behind this difference is unknown but could be from underlying airway inflammation in those with severe asthma. It also suggests that some children with severe asthma may be developing NERD (NSAID exacerbated respiratory disease) during childhood.
Ibuprofen was the most common instigating drug (84% of reactions), and most reactors were cross-intolerant to two or more NSAIDs.5 After ibuprofen, the most common cross-reactive drugs were aspirin and acetaminophen. One theory on why certain patients are cross-intolerant to multiple NSAIDs is that their pathology is not a true IgE-mediated allergy but rather a disruption in one or more inflammatory pathways - such as the arachidonic pathway -due to COX-1 inhibition.2,7 A related disease, aspirin-exacerbated respiratory disease (AERD), has NSAID-H as part of its diagnostic criteria and has been associated with genetic variations in the genes for the arachidonic acid pathway and ALOX5.7 As of yet, no studies have looked at NSAID-H and genetic variations. Additionally, acetaminophen, typically thought of as a COX-2 selective medication, was one of the more common drugs reports to cause a hypersensitivity reaction. The reason for this could be that compared to other COX-2 selective medications, acetaminophen displays higher levels of COX-1 inhibition and therefore could cause symptoms in patients sensitive to the consequences of COX-1 inhibition.8
Previous studies reported that participants with NSAID-H (diagnosed by history or OPT) frequently had reactions to more than one NSAID, had a history of atopy, and reported rhinoconjunctivitis symptoms with ingestion. 6 Interestingly, this study identified several additional, novel associations with NSAID-H including allergy to medications that are not NSAIDs, nasal polyps, ageusia, asthma triggered by viral infection, and an immediate family member with sinusitis. A multivariate analysis found that nasal polyps are independently associated with NSAID use, further suggesting that a subset of children with severe asthma are developing NERD. Finally, while NSAID-H is more common in adult females, this study found similar rates of NSAID-H in male and female children.1,2
One limitation of this study was its focus on children with severe asthma in a large-Midwestern city which may limit its generalizability. Previous NSAID-H studies have shown significant variability in prevalence depending on race/ethnicity and geographic location. Although this study had strong representation from self-identified Black and non-Hispanic white participants, prevalence studies require replication on a larger scale, in multiple geographic locations. Additionally, the strong representation of Black participants may be secondary to their increased risk of having severe asthma due to lower socioeconomic status and their effects on social determinants of health. Larger studies should examine the importance of race on NSAID-H prevalence. The final limitation is recall bias during survey completion. Despite these limitations, this study is one of the few to specifically examine prevalence of NSAID-H in a diverse pediatric severe asthma population and has strong implications for practice and future research.
This study highlights the importance of a thorough history, especially in children with severe asthma in which exacerbations can be frequent and severe and who may be at higher risk for NSAID-H compared to those with mild and controlled asthma. NSAID-H has traditionally been considered less frequent in children than in adults. However, this study showed 1 in 5 children with severe asthma report symptoms of NSAID-H. Inquiring about NSAID exposure and reactions during clinic is a quick and inexpensive part of history taking that may help reduce exacerbations in high-risk children. Based on history, clinicians can consider drug challenges given that only 31% to 68% of children with NSAID-H per history have a positive drug challenge.7 A negative drug challenge is helpful to reassure patients and families of the safety of NSAID administration while a positive drug challenge solidifies the diagnosis and could justify alternatives such as COX-2 inhibitors for NSAID needs. Future research in this subpopulation is needed to achieve disease control, improve quality of life, and reduce financial and healthcare resource burden from repeat exacerbations. Additional studies could also investigate the prevalence of AERD in the pediatric severe asthma population. Reactors were over three times more likely to report nasal polyps than non-reactors which could indicate a higher prevalence of AERD in this population as well.
Funding Source:
KMK: National Institute for Allergy, Infections Disease (NIAID) K23 Career development Award 1 K23 AI135094–01; National Institute for Health Loan Repayment Program L40 AI096442
Footnotes
Conflict of Interest: All authors have nothing to disclose.
References:
- 1.Guvenir H, Dibek Misirlioglu E, Capanoglu M, Buyuktiryaki B, Onay ZR, Ginis T, et al. The frequency of nonsteroidal anti-inflammatory drug hypersensitivity in children with asthma. Int Arch Allergy Immunol. 2018;176(1):26–32. [DOI] [PubMed] [Google Scholar]
- 2.Mori F, Atanaskovic-Markovic M, Blanca-Lopez N, Gomes E, Gaeta F, Sarti L, et al. A multicenter retrospective study on hypersensitivity reactions to nonsteroidal anti-inflammatory drugs (NSAIDs) in children: A report from the European network on drug allergy (ENDA) group. J Allergy Clin Immunol Pract. 2020;8(3):1022–1031.e1. [DOI] [PubMed] [Google Scholar]
- 3.Simsek I, Cogurlu MT, Aydogan M. Two approaches for diagnosis of nonsteroidal anti-inflammatory drug hypersensitivity in children. Ann Allergy Asthma Immunol. 2019;123(4):389–393. [DOI] [PubMed] [Google Scholar]
- 4.Arikoglu T, Aslan G, Yildirim DD, Batmaz SB, Kuyucu S. Discrepancies in the diagnosis and classification of nonsteroidal anti-inflammatory drug hypersensitivity reactions in children.Allergol Int. 2017;66(3):418–424. [DOI] [PubMed] [Google Scholar]
- 5.Kidon M, Blanca-Lopez N, Gomes E, Terreehorst I, Tanno L, Ponvert C, et al. EAACI/ENDA position paper: Diagnosis and management of hypersensitivity reactions to non-steroidal antiinflammatory drugs (NSAIDs) in children and adolescents. Pediatr Allergy Immunol.2018;29(5):469–480. [DOI] [PubMed] [Google Scholar]
- 6.Yuenyongviwat A, Chantaravisarut N, Phattarapongdilok W, Koosakulchai V, Jessadapakorn W,Sangsupawanich P. Characteristics and contributing factors related to nonsteroidal antiinflammatory drugs hypersensitivity. Int Arch Allergy Immunol. 2021;182(2):139–145.7. [DOI] [PubMed] [Google Scholar]
- 7.Khan D, Banerji A, Blumenthal K, et al. Drug allergy: A 2022 practice parameter update. J All and Clin Imm. 2022;150(6):1333–1393. [DOI] [PubMed] [Google Scholar]
- 8.Esh C, Chrismas B, Mauger A, Taylor L. Pharmacological hypotheses: Is acetaminophen selective in its cyclooxygenase inhibition? Pharmacol Res Perspect. 2021;9(4):e00835. [DOI] [PMC free article] [PubMed] [Google Scholar]


