Abstract
Protamine, a polycationic peptide (mol. wt 4000–4500), was evaluated as a potential penetration enhancer for phosphodiester antisense oligonucleotides (ODNs). Unique complexes in the form of nanoparticles were spontaneously formed, which we call ‘proticles’. The stability of the particles and the ODNs bound into the proticles was examined in foetal calf serum and cell culture medium. FITC-labelled ODNs bound to protamine showed an increased cellular uptake into human histiocytic lymphoma U 937 cells compared to free ODNs. Proticles significantly decreased cellular growth in a cell proliferation assay using ODNs against the c-myc proto-oncogene.
INTRODUCTION
The c-myc proto-oncogene is an evolutionarily conserved gene expressed in numerous cell types including human promonocytic leukaemia U 937 cells (1). It has often been associated with transformation, differentiation and cell cycle progression (2,3). The c-Myc protein has two distinct properties: (i) Myc specifically binds to nucleotide sequences in DNA and regulates gene transcription in the form of heterodimers with other proteins; (ii) Myc specifically binds to retinoblastoma protein, which in turn regulates cell cycle events (4). Without a second survival signal, such as that provided by growth factor receptor interaction, c-Myc induces apoptosis. If the second signal is present, the cells are allowed to progress through the cell cycle (5).
The c-myc gene has a higher level of expression in proliferating cells than in differentiated cells, which usually have lower proliferation rates, depending on the level of differentiation. Gene transfer experiments have demonstrated that constitutive expression of c-myc inhibits induced differentiation of cell lines, supporting the fact that there is an inverse association between c-myc expression and differentiation. However, exceptions to these events have been reported (6,7). Under certain conditions aberrant expression of c-myc may drive the cell through the cell cycle leading to malignant transformation. c-Myc is overexpressed in various tumours, including breast cancer and promyelocytic leukaemia. Investigators working with both tumours have found that cancer cell growth is inhibited when exposed to antisense oligonucleotides (ODNs) directed against the human c-myc AUG translation initiation codon site mRNA sequence (8–10). Analysis of the DNA content of cells incubated with 4 µM antisense oligomer showed a generalised prolongation of the cell cycle, but no significant accumulation of cells in any specific phase (6). Furthermore, phosphorothioate and phosphodiester antisense oligonucleotides corresponding to c-myc blocked activation-induced cell death (4). In most studies modified ODNs, such as antisense c-myc phosphorothioates or oligonucleotide N3′→P5′ phosphoramidates (11), were used to increase stability against exo- and endonucleases. But this may involve sequence-independent effects like non-specific growth inhibition as a result of exposure of the cells to phosphorothioates, possibly leading to cell surface receptor binding (8,12,13). Other investigators have documented sequence-independent actions of phosphorothioate oligodeoxynucleotides that may involve other cellular targets in addition to specific mRNA transcripts as well as both selective and non-selective protein binding and non-selective translation inhibition (8).
Another way to circumvent stability problems of phosphodiester oligonucleotides is the use of carrier systems, such as cationic liposomes (14,15) and nanoparticles (16,17). In the present study a new carrier for antisense oligonucleotides, the cationic peptide protamine, is introduced. A new method for preparing solid particles from oligonucleotides together with this protein in the form of a nanosuspension was tested. Protamine complexes oligonucleotides in the form of particles in the size range 100–200 nm, which we call ‘proticles’. Such an aggregation into compact structures with short segments of single-stranded DNA has only been reported with the polycation poly(l-lysine) (18). Proticles exhibited a high protection against enzymatic digestion, especially for phosphodiester oligonucleotides. Consequently, a high stability in cell culture medium occurred. The cellular uptake of FITC-labelled phosphodiester c-myc ODNs into human promonocytic leukaemia U 937 cells as well as the antisense effect using an in vitro cell proliferation assay were determined.
MATERIALS AND METHODS
Materials
A phosphodiester oligonucleotide (5′-ACG TTC CTC CTG CGG GAA G-3′, anti HSV-I; 19) was used for preliminary stability studies and was purchased from MWG-Biotech GmbH (Ebersberg, Germany). The c-myc 20mer phosphodiester oligonucleotides, with an antisense sequence (AS, 5′-AAG CTA ACG TTG AGG GGC AT-3′), sense sequence (S, 5′-ATG CCC CTC AAC GTT AGC TT-3′) or scrambled sequence (SC, 5′-TTG CAT TCG AAG TGG GGC TA-3′) (10), which were used for the cellular uptake and the antisense effect studies were obtained from ARK Scientific (Darmstadt, Germany). All ODNs were delivered as HPLC grade. Protamine free base, ethyleneglycol-bis(β-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and RPMI 1640 medium were obtained from Sigma (Deisenhofen, Germany), tris-(hydroxymethyl)aminomethane, 1,4-diazabicyclo(2.2.2)octane (DABCO) and potassium dihydrogen phosphate from Fluka (Buchs, Switzerland), 0.1 and 1 N sodium hydroxide, paraformaldehyde, potassium chloride, sodium chloride and disodium hydrogen phosphate from Merck (Darmstadt, Germany) and glycerol from Wasserfuhr GmbH (Bonn, Germany). Mowiol 4-88 was a gift from Clariant GmbH (Frankfurt, Germany). Alexa–concanavalin A was purchased from Molecular Probes (MoBiTec, Göttingen, Germany), Earle’s minimal essential medium (MEM), foetal calf serum (FCS), newborn calf serum (CS), a stabilised solution of l-glutamine from Seromed (Biochrom KG, Berlin, Germany), penicillin/streptomycin from Gibco (Eggenstein, Germany) and sodium lauryl sulphate from Henkel (Düsseldorf, Germany). All chemicals were of analytic grade and were used as delivered. The 150 mM phosphate buffer solution, pH 7.4, (PBS) was prepared to result in 136.90 mM sodium chloride, 2.68 mM potassium chloride, 1.47 mM potassium dihydrogen phosphate and 8.10 mM disodium hydrogen phosphate.
Sample preparation
Phosphodiester oligonucleotide (ODNs) were bound with protamine free base in mass ratios (protamine/ODNs) ranging from 0.5:1 to 5:1. Aliquots of 5–50 µg protamine were dissolved in 500 µl of double-distilled water and 10 µg ODNs, also dissolved in 500 µl of double-distilled water, were added, followed by intensive vortexing. Proticle formation is indicated by an instant turbidity of the samples.
Size determination of the particles
The hydrodynamic diameters of the resulting proticles were first measured in double-distilled water and additionally at physiological ionic strengths in FCS and cell culture medium. The influence on particle size of adsorption of serum compounds to the particle surface was measured with proticles prepared in double-distilled water followed by a 10-fold dilution with FCS or RPMI 1640 medium supplemented with 10% FCS and 1% penicillin/streptomycin. After an incubation time of 30 min at 37°C and 700 r.p.m. using an Eppendorf Thermomixer 5437 (Eppendorf, Germany), the particle sizes were measured by photon correlation spectroscopy (PCS) using a BI-200 SM Goniometer version 2.0. (Brookhaven Instruments Corp., Holtsville, NY) with a 30 mW He/Ne laser (Melles Griot, Cincinnati, CA) and a BI-2030-AT Digital Correlator (Brookhaven). The measurements were carried out at a scattering angle of 90° at room temperature. Additionally, scanning electron microscopy (SEM) (S-4500 Hitachi field emission electron microscope, 15 kV, upper detector) of proticles was performed for further confirmation of the results obtained by PCS measurements. The samples were filtered through a membrane filter with an exclusion size of 30 000 Da. Then the supernatants were dried at 40°C and sputtered with platine (15.2 mA, 15 s, 5.0 × 10–2 mbar) (Hochvakuum-Kleinbeschichtungsanlage MED 020; Bal-Tec, Balzers, Liechtenstein).
Surface charge
The zeta potential of the proticles was determined in double-distilled water and after incubation in FCS by measuring the electrophoretic mobility using a Model 501 Lazer Zee Meter™ (PenKem, Bedford Hills, NY). The final oligonucleotide concentration was 5 µg/ml. The measured zeta potential was corrected for a reference temperature of 20°C.
Oligonucleotide loading and release
The oligonucleotide content of the protamine/ODN complexes was determined by a strong anion exchange HPLC assay, using a Dionex Nucleopac™ PA 100 4 × 250 column (Dionex, Idstein, Germany) and a Merck-Hitachi HPLC system (Merck, Darmstadt, Germany). The HPLC system was equipped with an AS-2000 A autosampler, a L-6220 gradient pump, a D-6000 A interface, an L-4500 diode array detector (DAD) and a model D-6500 DAD system manager. A two-eluent system with eluent A consisting of 50 mM sodium chloride in 25 mM sodium hydroxide solution and eluent B containing 1 M sodium chloride in 25 mM sodium hydroxide was used as described previously (16). A linear eluent gradient of from 50 to 1000 mM sodium chloride at a flow rate of 1 ml/min was employed for 20 min, except in the case of stability testing in cell culture medium, when elution for 28 min was used. The samples were centrifugated at 100 000 g for 2 h (Ultracentrifuge Optima L-80; Beckmann, Germany). From each supernatant 20 µl were injected. The ODN concentration was determined by measuring the absorbance at 260 nm. Bound ODNs were calculated as the difference between the amount of ODN added to the complex preparation and the ODN content in the supernatant.
To determine changes in oligonucleotide content after exposure of proticles to physiologically buffered solutions, particles were formed in the mass ratios 5:1, 2.5:1, 1:1 and 0.5:1 in double-distilled water and then diluted 1:1 with 2× PBS. After incubation times of 0.5, 1, 2, 6 and 24 h at 37°C and 700 r.p.m. (Eppendorf Thermomixer 5437; Eppendorf, Germany) the samples were centrifuged and analysed as already described.
Stability in cell culture medium
The stability of ODNs (HSV I sequence) in MEM cell culture medium containing FCS was determined as follows. ODNs were bound to protamine in mass ratios ranging from 0.5:1 to 5:1 in double-distilled water. Aliquots of 30 µl of this dispersion and of an ODN control were incubated with 120 µl of MEM containing FCS for 0, 2, 4, 8 and 24 h at 37°C and 700 r.p.m. using an Eppendorf Thermomixer 5437 (Eppendorf, Germany). Degradation of the ODNs by the medium was terminated by addition of 50 µl of 1 M EGTA solution into a 150 µl sample. The complexes were then dissolved by further addition of 100 µl of 4 M sodium chloride solution overnight. The oligonucleotides and their digested fragments were analysed by HPLC as described.
Cell cultures
U 937 (human histiocytic lymphoma) cells were grown in RPMI 1640 medium supplemented with 10% FCS and 1% penicillin/streptomycin in a humidified incubator containing 5% CO2 at 37°C.
Laser scanning confocal microscopic study (CLSM)
Cellular uptake of protamine/ODN particles was investigated with U 937 cells in vitro. 5′-FITC-labelled c-myc antisense oligonucleotides (FODNs) were mixed in different mass ratios with protamine solutions in double-distilled water to prepare fluorescent proticles. Then all preparations (a medium control, an oligonucleotide control and the 2.5:1 and 1:1 particles) were diluted 10-fold with RPMI 1640 medium supplemented with 10% FCS and 1% penicillin/streptomycin. Approximately 100 000 cells were incubated with the samples in a 24-well suspension cell culture plate (Greiner GmbH, Frickenhausen, Germany) at 37°C for 4 h. After incubation, the cells were washed three times with cold PBS by centrifugating each time in an Eppendorf Centrifuge 5417 (Eppendorf, Germany). The cells were left to adhere for 5 min on the microscope slide (a gift from Paul Marienfeld GmbH, Bad Mergentheim, Germany). After a further washing step, cell membranes were stained for 2 min with a 5 × 10–3% (w/v) solution of Alexa-labelled concanavalin A in PBS, then the cells were fixed with a 5% (w/w) solution of paraformaldehyde in PBS for 7 min. Finally, the cells were embedded in a gel containing 22.73% (w/w) glycerine, 9.1% (w/w) Mowiol 4-88, 22.73% (w/w) double-distilled water, 45.45% (w/w) 0.2 M Tris buffer, pH 8.5, and 2.5% (w/w) DABCO as an antifading agent. After 12 h, confocal microscopy was performed with a Leitz DM IRB microscope (Leitz, Wetzlar, Germany) equipped with an argon/krypton laser. Two channel image recording at 488 and 568 nm laser excitation was used. Optical filters were chosen for the FITC and TRITC range. All optical sections were recorded with the same laser and detector settings using Scanware TCS v.5.1 software. Further image processing was performed with Imaris software (Bitplane, Zürich, Switzerland) on a Silicon Graphics computer system. Confocal stacks of green and red fluorescence were visualised in section view mode. Current sections are indicated as small triangles on the border.
Cell proliferation assay
U 937 cells were cultured in 24-well plates (Greiner GmbH, Frickenhausen, Germany) with an initial concentration of 1 × 105 cells/well in RPMI 1640 medium supplemented with 10% FCS and 1% penicillin/streptomycin. Pure c-myc antisense, sense and scrambled oligonucleotides (10 µg/ml), their corresponding proticle preparations at mass ratios of 2.5:1 and 1:1 and a protamine control (25 µg/ml) were added three times, at 0, 24 and 48 h. Proticles were formed ~30 min before addition in double-distilled water. After a 4 day incubation, the number of cells was determined by MTT assay (20). The cells were incubated for 2 h with a 0.25% (w/v) solution of MTT in PBS. After centrifugation the cells were dissolved in a 25% (w/w) solution of sodium lauryl sulphate in 0.1 N NaOH. The number of living cells was calculated by measuring the absorption at 550 nm (reference 630 nm) (Dynatech MR 5000 ELISA Reader; Dynatech, Denkendorf, Germany). In a further experiment, c-myc antisense oligonucleotides (10 µg/ml) and their preparations with protamine (mass ratios 2.5:1 and 1:1) were added three times to the cells as already described. For 4 days the number of cells was determined every 24 h with a haemocytometer according to Neubauer (Blaubrand, Brand, Germany).
Statistical analyses were performed using Microsoft Excel 97. Significance was determined using Student’s t-test, with P < 0.05 being significant.
RESULTS
Particle size determination
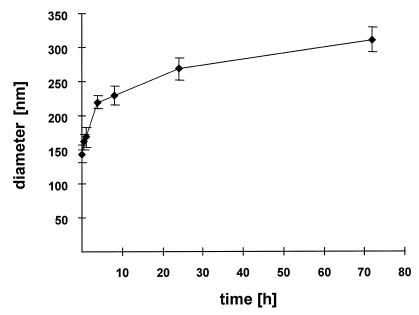
ODNs were bound to protamine in a mass ratio of 5:1 by combining aliquots of 10 µg/ml oligonucleotide with solutions of protamine in double-distilled water at concentrations of 50 µg/ml. Immediately after preparation the hydrodynamic diameter was measured for 72 h by PCS (Fig. 1). Protamine and the oligonucleotides spontaneously aggregated in the form of particles, which we call ‘proticles’, with mean diameters of 150–170 nm within 30 min. Over the measured time the mean proticle sizes increased and reached diameters of ~300–330 nm after 3 days.
Figure 1.
Hydrodynamic diameters of protamine/ODN particles versus time.
The dependency on protamine content of particle formation was evaluated by complexing ODNs with protamine in different mass ratios (5:1, 2.5:1, 1:1 and 0.5:1). The hydrodynamic diameters were measured 5 min after mixing. Proticles with mass ratios of 5:1 and 2.5:1 had initial diameters of ~110 nm. The 1:1 and 0.5:1 particles were smaller, i.e. 95 (1:1) and 90 nm (0.5:1), respectively. In addition, SEM of proticles of different mass ratios of protamine/ODN was performed to verify the formation of solid particles (Fig. 2). The diameters of the 5:1 and 2.5:1 proticles ranged between 46 and 138 nm. The 1:1 particle diameter was between 81 and 219 nm and the diameter of the 0.5:1 aggregates between 67 and 100 nm. Because of the time dependency of particular sizes the following antisense experiments were performed with proticles of 150–170 nm mean diameter, which resulted with incubation times of ~30 min.
Figure 2.

Scanning electron micrograph of 1:1 proticles.
These measurements were carried out at low ionic strengths. Additionally, particle sizes were measured in FCS and cell culture medium. After 30 min incubation at 37°C, the hydrodynamic diameters were measured by PCS (Table 1). Depending on the proticle composition, different mean diameters were obtained after FCS exposure compared to water. Particles with a 5:1 mass ratio slightly increased in diameter. The mean difference between the particle size in double-distilled water (mean diameter 203.8 nm) and FCS (mean diameter 222.8 nm) was ~20 nm. This may result from adsorption of serum compounds, for example opsonins. In contrast, 2.5:1 particles showed a decrease in the mean hydrodynamic diameter, which might be caused by different hydration under physiological ionic strength. Proticles of 1:1 mass ratio resulted in a similar mean hydrodynamic diameter compared to water. In cell culture medium 5:1 and 1:1 proticles became much smaller (150.8 and 82.1 nm, respectively), a consequence of a lower protein content than in FCS. The reduction in the mean size of 2.5:1 particles was similar to that in FCS (144.2 nm).
Table 1. Particle sizes of proticles with different protamine/ODNs ratios after 30 min incubation in double-distilled water, FCS and cell culture medium.
| Mass ratio | Mean diameter (nm) |
||
|---|---|---|---|
| protamine/ODN | in double-distilled water | in FCS | in cell culture medium |
| 5:1 | 203.8 | 222.8 | 150.8 |
| 2.5:1 | 241.7 | 152.2 | 144.2 |
| 1:1 | 143.0 | 145.9 | 82.1 |
Surface charge
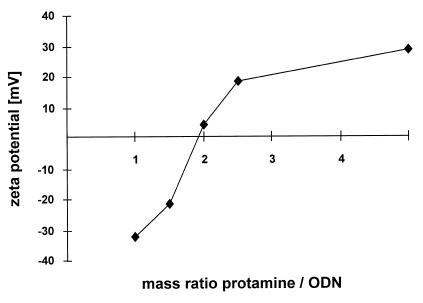
In addition, the zeta potentials of the complexes consisting of protamine and 19mer phosphodiester ODNs were determined in double-distilled water and in FCS. In water, particles with protamine/ODNs mass ratios below 2:1 were negatively charged. Above this mass ratio positive zeta potentials were obtained (Fig. 4). In contrast, after incubation in FCS all proticles were negatively charged. The zeta potentials of the 5:1 and 2.5:1 particles were –16.75 and –17.10 mV in FCS and +28.80 and +18.58 mV in double-distilled water, respectively. This is further evidence for a possible adsorption of serum proteins on the positively charged proticles in cell culture medium.
Figure 4.
Zeta potentials of proticles at different mass ratios protamine/ODNs.
Oligonucleotide loading and release
The protamine/oligonucleotide ratio required to adsorb all ODNs was investigated by mixing the oligonucleotides with the peptide in different mass ratios (0.5:1, 1:1, 2.5:1 and 5:1), followed by centrifugation. The supernatants were analysed by HPLC. Bound ODNs were calculated as the difference between the amount of ODNs added and the oligonucleotide content in the supernatants. In the 0.5:1 samples, 82% unbound ODNs were found. At a mass ratio of 1:1, 40% ODNs remained in solution. The 2.5:1 and 5:1 proticles quantitatively incorporated ODNs in the form of particles.
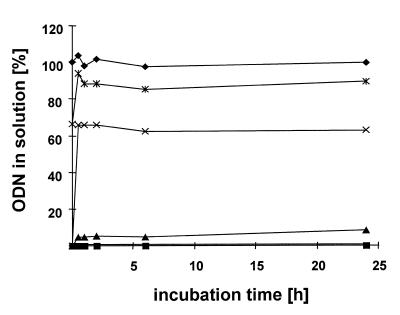
The stability of the proticles and the release of ODNs were investigated in PBS at 37°C (Fig. 3). After 24 h, no ODN release occurred in the 5:1 proticle samples. The 2.5:1 particles showed a minor release of ODNs; 8.0% unbound ODNs were found. In the 1:1 and 0.5:1 samples, 63.0 (1:1) and 90.0% (0.5:1) ODNs were found in solution.
Figure 3.
Release of oligonucleotides from proticles in PBS. Free ODN (diamonds), proticles with mass ratios of 5:1 (squares), 2.5:1 (triangles), 1:1 (crosses) and 0.5:1 (double crosses).
Stability in cell culture medium
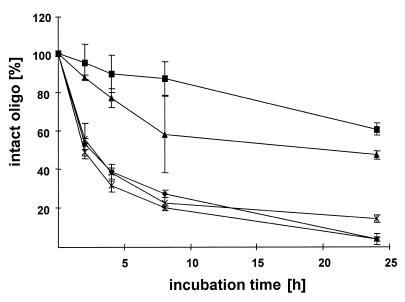
The protective effect against nucleases of adsorption of ODNs to protamine in MEM cell culture medium containing non-heat-inactivated FCS was evaluated with 5:1, 2.5:1, 1:1 and 0.5:1 proticles as well as with unprotected phosphodiester ODNs. The stability of the proticles and the content of intact ODNs was measured for 24 h (Fig. 5). The oligonucleotides and their digested fragments were determined by HPLC. Unbound oligonucleotides were nearly completely digested within a 1 day incubation. After 24 h only 3.5% intact ODNs were detected, whereas in a control experiment with unbound ODN, in which enzymatic digestion was immediately stopped, no degradation occurred. The 0.5:1 proticles showed similar degradation rates; only 3.2% undigested ODNs were found. Significant stabilisation of ODNs started with the 1:1 proticle preparation (13.9% undigested ODNs). Protamine/ODN particles with 2.5:1 and 5:1 mass ratios were degraded much slower than the control. After 24 h, 47.2% (2.5:1) and 60.7% (5:1) ODNs remained intact.
Figure 5.
Stability of free and complexed ODNs in cell culture medium versus incubation time. Free ODN (diamonds), proticles with mass ratios of 5:1 (squares), 2.5:1 (trianges), 1:1 (crosses) and 0.5:1 (double crosses).
Cellular uptake studies
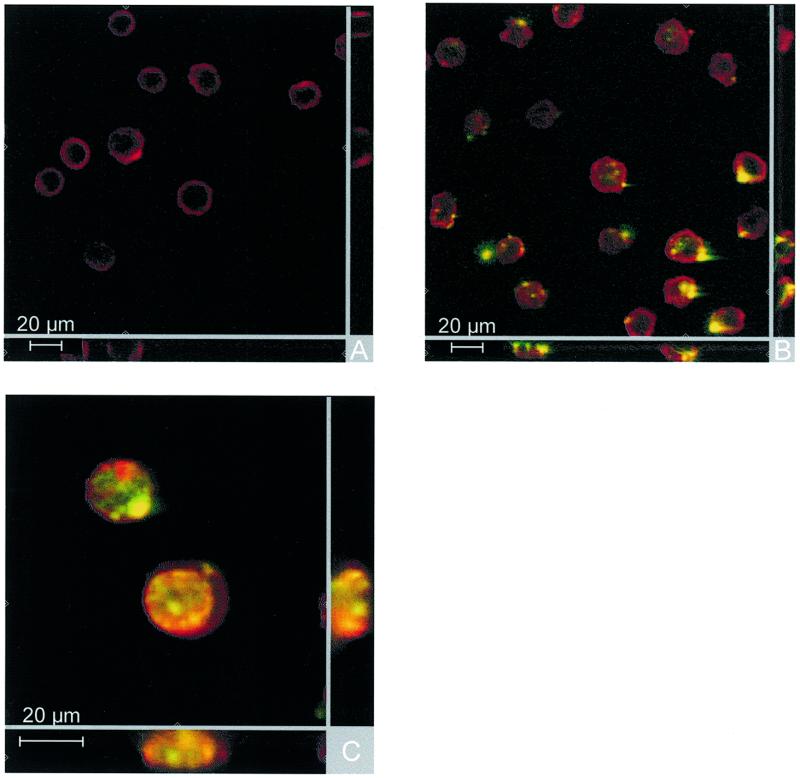
Cellular uptake of the protamine/FODN particles was investigated by CLSM in vitro using U 937 cells. 5′-FITC-labelled FODNs were incubated for 4 h at 37°C. The 2.5:1 and 1:1 proticles were employed in the uptake experiment. The adsorption of FODNs to protamine resulted in a shift of the green fluorescence of the FODNs to longer wavelengths. This shift was reversible; desorption of the oligonucleotides resulted in a shift back to green fluorescence (data not shown). This ability to change the colour of fluorescence was used to visualise unbound and complexed FODNs. Cells treated with unbound FODNs internalised no or only a minor portion of ODNs. There was no green fluorescence inside cells of which the membranes had been counterstained with Alexa–concanavalin A (Fig. 6A). In contrast, all proticles showed an increase in ODN uptake. At a mass ratio of 1:1 the green fluorescence of ODNs was visualised in the form of a spotted distribution inside the cells (Fig. 6B). The 2.5:1 proticles showed a higher internalisation than 1:1 proticles. Unbound FODNs as well as FODNs as proticles were observed in the cells (Fig. 6C). While the red fluorescence of complexed FODNs appeared to be distributed in a spotted area, the green fluorescence of free FODNs showed a spotted as well as a diffuse distribution. Furthermore, the FODNs accumulated in the nucleus.
Figure 6.
Laser scanning confocal microscopy of oligonucleotide uptake. U 937 cells were incubated for 4 h at 37°C with (A) free 5′-FITC-labelled phosphodiester oligonucleotides, (B) 1:1 protamine/FODN particles and (C) 2.5:1 protamine/FODN particles. These CLSM pictures are section views showing horizontal and vertical sections of 40 pictures.
Cell proliferation assay
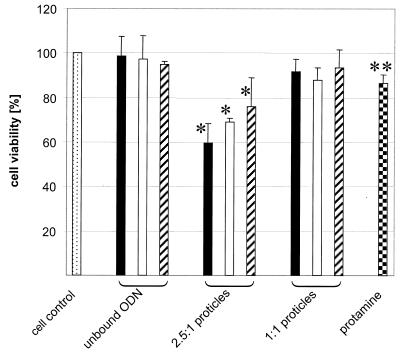
U 937 cells were cultured in 24-well plates at an initial concentration of 105 cells/well. The reduction in cell proliferation was determined in an experiment using an MTT assay for cell quantification (20). The influence of the peptide and different ODN sequences was measured with the protamine control and c-myc antisense, sense and scrambled oligonucleotides and with their protamine preparations at mass ratios of 2.5:1 and 1:1. After 4 days incubation, the cell number and viability of cells were determined by MTT assay (Fig. 7). Free phosphodiester oligonucleotides had no significant effect on cell proliferation. Antisense c-myc ODNs resulted in 98.6% (SD = 8.8%, n = 4), sense ODNs in 97.4% (SD = 10.5%, n = 3) and the scrambled sequence in 94.8% (SD = 1.3%, n = 4) cell viability. The 1:1 proticles with antisense, sense and scrambled sequences reduced cell proliferation slightly but not significantly. After 4 days incubation, 91.9% (SD = 5.5%, n = 6), 88.0% (SD = 5.5%, n = 3) and 93.5% (SD = 8.2%, n = 6) cell viability were found for the antisense ODNs, sense ODNs and scrambled ODNs, respectively, compared to an untreated control. In contrast, the 2.5:1 antisense ODN proticles reduced cell viability significantly to 59.6% (SD = 8.9%, n = 6). The sense and scrambled ODN proticles reduced cell proliferation to 69.2% (SD = 1.6%, n = 3) and 76.2% (SD = 12.8%, n = 6), respectively. This non-specific effect of the sense and scrambled 2.5:1 proticles was significantly lower than the reduction of cell number with the antisense proticles, although the scrambled ODN sequence contained four contiguous guanosine residues like the antisense sequence. This four guanosine motif might act as a potential proliferation inhibitor. For that reason it was preserved in the scrambled control to investigate this non-specific effect. Protamine as a control preparation without any oligonucleotide resulted in a similar non-specific effect. The reduction in cell count was 15.4% (SD = 8.1%, n = 8). No significant differences were found between oligonucleotides in solution and the protamine control in solution (Fig. 7).
Figure 7.
Cellular viability after 5 days incubation in a medium control (dot pattern), with unbound and complexed antisense (filled bars), sense (empty bars) and scrambled c-myc ODNs (diagonal lines) or protamine control solution (chessboard pattern). Statistically significant differences (Student’s t-test) between unbound ODNs and proticle preparations (n = 3–6, P < 0.05), as well as between antisense proticles and scrambled proticles (n = 6, P < 0,05) (stars) no significant differences between unbound ODNs and protamine control solution (two stars).
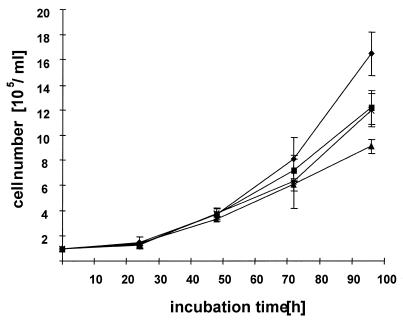
Additionally, the cell number during the 5 day experiment was determined every 24 h in the medium control and antisense samples. The addition of a daily dose of 10 µg/ml free c-myc antisense oligonucleotides for 3 days decreased the cell number to ~28% (SD = 10.9%, n = 3) in a 5 day period (Fig. 8). The degree of growth inhibition increased with time. Similar results were observed with the 1:1 proticles. At day 5 the number of cells was 70.7% (SD = 10.8%, n = 3) compared to untreated cells. However, the 2.5:1 proticle preparations inhibited cell proliferation to a higher extent. After the 5 day period cell proliferation was inhibited to ~50% (SD = 6.0%, n = 3). This study indicates that the biological activity of antisense c-myc phosphodiester oligonucleotides can be significantly enhanced with our protamine oligonucleotide particles.
Figure 8.
Cellular growth of U 937 cells incubated with medium (diamonds), free antisense c-myc phosphodiester ODNs (squares) and 1:1 (crosses) and 2.5:1 proticles (triangles).
DISCUSSION
Protamine is a relatively small polycationic peptide with a molecular weight of ~4000 Da. In the sperm of fish, especially salmon, it condenses DNA and delivers it to the nucleus of the egg after fertilisation. It is composed of two-thirds arginine residues and it possesses several amino acid sequences resembling a nuclear localisation signal (21). The natural function of the peptide has been exploited to condense plasmid DNA into compact particles which showed an increased stability against nuclease activity, increased cellular uptake and transfection efficiency (21,22).
Pharmaceutical preparations have used protamine as the major excipient for slow release insulin application for more than 30 years. Additionally, protamine is also used as a drug itself, the sulphate or hydrochloride salt being applied as a heparin antagonist. In our study we propose protamine as a pharmaceutical excipient to form nanoparticles with single-strand antisense oligonucleotides, which we call ‘proticles’. These nanoparticles were formed spontaneously in a size range depending on the mass ratio of protamine/ODN and the ionic strength of the buffer solution. Other cationic peptides, like poly(l-lysine) and poly(l-arginine), were unable to quantitatively form solid particles with ODNs in this way (data not shown). Degols et al. (23), for example, used ODNs which were covalently linked to poly(l-lysine) and administered in ternary complexes formed with heparin. Previous studies reported condensation and particle preparation with protamine only of larger double-stranded DNA. It was reported that at low DNA concentrations and at low ionic strengths DNA condensation resulted in monomolecular collapse in the form of particles with minimal hydrodynamic diameters of 50–100 nm (24,25). For that reason, in our study proticles were always prepared in double-distilled water. The protamine/ODN particles initially had the same range of diameters as those formed with plasmid DNA (25) or poly(l-lysine)-asialoorosomucoid with phosphorothioate oligonucleotides (18), but phosphodiester oligonucleotide particles showed a time-dependent increase in mean size which might be due to aggregation. This is why proticles were always incubated for ~30 min before applying them to the cells in the antisense experiments. The complexation of oligonucleotides with protamine resulted in an equilibrated product. Depending on the mass ratio, oligonucleotide or protamine was adsorbed to the surface of the particles and, therefore, different surface charges were found on zeta potential measurement. The 0.5:1 and 1:1 particles were negatively charged and proticles with mass ratios above 2:1 were positively charged. On the surfaces of the 0.5:1 and 1:1 proticles an excess of oligonucleotides seemed to be bound by electrostatic interactions, whereas the 2.5:1 and 5:1 preparations were free of unbound ODNs and an excess of non-neutralised protamine created a positively charged particle surface.
As a major requirement for the application of proticles in vitro or in vivo the particles must be stable under physiological conditions. For this reason proticles consisting of different mass ratios of protamine and ODNs were incubated with FCS and cell culture medium. Depending on the medium applied, the 5:1 proticles slightly increased in mean diameter in FCS, which may have resulted from adsorption of serum compounds to the particle surface. Consequently, the particles became much smaller in cell culture medium, in which the protein content was lower than in FCS. Smaller particle diameters were also found with the 2.5:1 and 1:1 proticles. The zeta potential measurements confirmed this theory. After incubation with FCS all proticles had a negative surface charge, but this effect did not change the oligonucleotide content of the particles. Further, a major reason for a decreased hydrodynamic diameter in cell culture medium could be the smaller hydration layer under physiological buffer salt conditions.
Major functions of antisense oligonucleotide carrier systems include an increase in the stability of oligonucleotides against nucleases and the promotion of their cellular uptake. In MEM cell culture medium containing non-heat-inactivated FCS, unbound oligonucleotides were nearly completely digested within a 1 day incubation. Whereas proticles with 5:1 and 2.5:1 mass ratios stabilised ODNs to a very high level, up to 60% intact ODNs were found after 24 h.
The cellular uptake of 2.5:1 and 1:1 protamine/FODN particles was investigated by incubating U 937 cells with unbound and complexed 5′-FITC-labelled FODNs for 4 h at 37°C. Proticles with both mass ratios enhanced uptake due to an active process like endocytosis. Control experiments at 4°C supported this theory. No cellular uptake was observed at low temperature (data not shown). This effect was also reported for other types of nanoparticles in earlier studies (16).
Alexa–concanavalin A was introduced in this study to visualise the cell membranes. However, two different fluorochromes with the same emission properties, the alexa stain as well as the FODNs bound to proticles, are present, because the FITC excitation is shifted to a longer wavelength as long as the fluorescein-labelled oligonucleotide is in close contact with protamine. Therefore, differentiation between free and proticle-bound FODNs in the presence of the red alexa counterstain is only possible for inner cell structures, which are not stained by alexa–concanavalin A. In our study this was achieved by short-term staining (1–3 min) immediately followed by fixation of the specimen to prevent vesicular uptake of the concanavalin A. Control experiments with unstained specimens showed no interaction between membrane staining and intracellular FODN localisation (data not shown). However, these data can only be used for localisation of a fluorochrome. Predictions about intracellular stability of phosphodiester oligonucleotides cannot be drawn.
In U 937 cells the 2.5:1 proticles yielded an almost 50% reduction in cell proliferation compared to untreated cells. Sense and scrambled protamine/ODN particles showed a non-specific effect, but the effect with the antisense sequence was significantly higher. This non-specific effect might have resulted from protamine or a higher content of ODNs, and therefore higher contents of ODN degradation products, within the cells. The different ODNs would yield different levels of mononucleotides. However, compared to literature data reporting DNA toxicity, the ODN concentrations applied in our study have no pronounced cytotoxic or cytostatic effects on cells (26). Protamine itself has a slight but not significant effect on cell proliferation. But we suppose that the dissolved peptide might have other properties than the condensed form in proticles. For this reason, proticles with scrambled ODNs seemed to be more suitable controls in the antisense experiment. The scrambled ODN sequence used in this study also contains four contiguous guanosine residues like the antisense ODN sequence. Therefore, they could both form higher order structures, such as tetraplexes, which could mimic antisense effects in the cells. The fact that there is a significantly higher cell proliferation inhibition with the antisense than with the scrambled ODN 2.5:1 proticles indicates that the specific biological activity of antisense c-myc phosphodiester oligonucleotides can be enhanced with our protamine oligonucleotide particles.
c-myc antisense effects have already been confirmed at the protein level by western blot analysis by several research groups. In this context, Bergan et al. (27) demonstrated a sequence-dependant antisense effect by measuring c-Myc protein levels with western blot analysis in U 937 cells and Holt et al. (6) found that the addition of either a single or daily dose of 4 µM of the same antisense oligomer to HL-60 cells resulted in a 50% decrease in growth rate over a 5 day period. However, the previous studies used a heat-inactivated medium in which no enzymatic degradation should be expected. Additionally, Kanamaru et al. (10) found no significant effect on cell proliferation of unmodified phosphodiester and partially phosphorothioate ODNs with an antisense sequence. Phosphorothioate oligonucleotides (PTOs) with sense and G-quartet control sequences also did not alter cell proliferation, whereas a 20mer antisense PTO with a sequence identical to our unmodified antisense ODN reduced the proliferation rate. Therefore, these observations support our theory that it was mainly inhibition of c-Myc protein that resulted in the lower cell proliferation in the present study.
Acknowledgments
ACKNOWLEDGEMENTS
The authors would like to thank Mr H. Schewe and Mr M. Ruppel from the Botanic Institute of the Johann Wolfgang Goethe-University of Frankfurt for their assistance with the electron microscopy. Financial support by the Willkomm Stiftung (Frankfurt am Main, Germany) is gratefully acknowledged.
REFERENCES
- 1.Shi Y., Hutchinson,H.G., Hall,D.J. and Zalewski,A. (1993) Circulation, 88, 1190–1195. [DOI] [PubMed] [Google Scholar]
- 2.Edgington S.M. (1993) Biotechnology, 11, 787–792. [DOI] [PubMed] [Google Scholar]
- 3.Kaddurah-Daouk R., Greene,J.M., Baldwin,A.S.,Jr and Kingston,R.E. (1987) Genes Dev., 1, 347–357. [DOI] [PubMed] [Google Scholar]
- 4.Shi Y., Glynn,J.M., Guilbert,L.J., Cotter,T.G., Bissonnette,R.P. and Green,D.R. (1992) Science, 257, 212–214. [DOI] [PubMed] [Google Scholar]
- 5.Bissonnette R.P., Echeverri,F., Mahboubi,A. and Green,D.R. (1992) Nature, 359, 552–554. [DOI] [PubMed] [Google Scholar]
- 6.Holt J.T., Redner,R.L. and Nienhuis,A.W. (1988) Mol. Cell. Biol., 8, 963–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prendergast G.C. and Cole,M.D. (1989) Mol. Cell. Biol., 9, 124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson L.A., Smith,L.J., Fontaine,M.P., Kay,H.D., Mountjoy,C.P. and Pirruccello,S.J. (1995) Ann. Thorac. Surg., 60, 1583–1591. [DOI] [PubMed] [Google Scholar]
- 9.Iguchi-Ariga S.M., Itani,T., Kiji,Y. and Ariga,H. (1987) EMBO J., 6, 2365–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanamaru T., Takagi,T., Takakura,Y. and Hashida,M. (1998) J. Drug Target., 5, 235–246. [DOI] [PubMed] [Google Scholar]
- 11.Gryaznov S., Skorski,T., Cucco,C., Nieborowska-Skorska,M., Chiu,C.Y., Lloyd,D., Chen,J.K., Koziolkiewicz,M. and Calabretta,B. (1996) Nucleic Acids Res., 24, 1508–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helene C. (1991) Eur. J. Cancer, 27, 1466–1471. [DOI] [PubMed] [Google Scholar]
- 13.Zelphati O., Imbach,J.L., Signoret,N., Zon,G., Rayner,B. and Leserman,L. (1994) Nucleic Acids Res., 22, 4307–4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Capaccioli S., Di Pasquale,G., Mini,E., Mazzei,T. and Quattrone,A. (1993) Biochem. Biophys. Res. Commun., 197, 818–825. [DOI] [PubMed] [Google Scholar]
- 15.Zelphati O. and Szoka,F.C., Jr (1996) Pharm. Res., 13, 1367–1372. [DOI] [PubMed] [Google Scholar]
- 16.Zobel H.P., Kreuter,J., Werner,D., Noe,C.R., Kumel,G. and Zimmer,A. (1997) Antisense Nucleic Acid Drug Dev., 7, 483–493. [DOI] [PubMed] [Google Scholar]
- 17.Bertling W.M., Gareis,M., Paspaleeva,V., Zimmer,A., Kreuter,J., Nurnberg,E. and Harrer,P. (1991) Biotechnol. Appl. Biochem., 13, 390–405. [PubMed] [Google Scholar]
- 18.Bunnell B.A., Askari,F.K. and Wilson,J.M. (1992) Somat. Cell Mol. Genet., 18, 559–569. [DOI] [PubMed] [Google Scholar]
- 19.Kulka M., Smith,C.C., Aurelian,L., Fishelevich,R., Meade,K., Miller,P.S. and Pop,T.O. (1989) Proc. Natl Acad. Sci. USA, 86, 6868–6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mosmann T. (1983) J. Immunol. Methods, 65, 55–63. [DOI] [PubMed] [Google Scholar]
- 21.Sorgi F.L., Bhattacharya,S. and Huang,L. (1997) Gene Ther., 4, 961–968. [DOI] [PubMed] [Google Scholar]
- 22.Gao X. and Huang,L. (1996) Biochemistry, 35, 1027–1036. [DOI] [PubMed] [Google Scholar]
- 23.Degols G., Leonetti,J.P., Mechti,N. and Lebleu,B. (1991) Nucleic Acids Res., 19, 945–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gosule L.C. and Schellman,J.A. (1978) J. Mol. Biol., 121, 311–326. [DOI] [PubMed] [Google Scholar]
- 25.Wilson R.W. and Bloomfield,V.A. (1979) Biochemistry, 18, 2192–2196. [DOI] [PubMed] [Google Scholar]
- 26.Milligan J.F., Matteucci,M.D. and Martin,J.C. (1993) J. Med. Chem., 36., 1923–1937. [DOI] [PubMed] [Google Scholar]
- 27.Bergan R., Connell,Y., Fahmy,B. and Neckers,L. (1993) Nucleic Acids Res., 21, 3567–3573. [DOI] [PMC free article] [PubMed] [Google Scholar]