Abstract
Objective:
To estimate associations of HIV-status and antiretroviral (ART) regimen with gestational diabetes (GDM) and postpartum glucose metabolism.
Design:
Prospective cohort study
Methods:
We enrolled pregnant persons living with HIV(PHIV) and without HIV in Cape Town, South Africa who were ≥18 years of age at 24-28 weeks gestation and followed up to 26 months postpartum. Participants were tested for GDM in pregnancy and for diabetes postpartum using a 75 g 2-hour oral glucose tolerance test and diagnosed via WHO criteria. We estimated associations of HIV-status and ART regime (efavirenz (EFV) vs dolutegravir (DTG)) with GDM and postpartum impaired glucose metabolism using multivariable log binomial or linear regression models.
Results:
Among 397 participants (median age 30 (IQR 25,34; n=198 without HIV, n=199 PHIV), the prevalence of GDM was 6.0% (9.0 PHIV vs 3.0% without HIV). In multivariable analyses, PHIV were at higher risk of GDM (RR 3.9 95% CI 1.4, 10.7) after adjustment for pre-pregnancy BMI and other confounders. GDM risk did not differ by ART regimen (unadjusted prevalence 8.1% DTG vs 5.6% EFV, adjusted RR 1.1, 95% CI 0.2, 6.6). Few participants had diabetes, impaired glucose tolerance, or impaired fasting glucose postpartum (n=13, 6%) with no differences by HIV or ART status.
Conclusions:
In a setting of universal GDM testing, PHIV had an increased risk of impaired glucose metabolism during pregnancy but not postpartum. Among PHIV, GDM risk was similar regardless of EFV or DTG use. Given concerns about DTG and weight gain, diabetes risk should continue to be monitored.
Keywords: HIV, gestational diabetes, diabetes, antiretroviral therapy, glucose metabolism, adverse birth outcome, dolutegravir
Introduction
Gestational diabetes (GDM) is characterized by hyperglycemia first detected or diagnosed during pregnancy.[1] GDM complicates an estimated 10-14% of pregnancies globally[2] and is associated with an increased risk of adverse birth outcomes, including large-for-gestational age (LGA) infants and cesarean delivery, as well as an increased risk of progressing to type 2 diabetes mellitus (DM) postpartum.[3-6] Pregnant persons with HIV (PHIV) may be particularly at high risk for GDM or DM due to persistent HIV-associated inflammation and antiretroviral therapy (ART)-specific effects which influence insulin sensitivity and glucose homeostasis.[7, 8] However, little data on GDM or postpartum DM risk are available for PHIV in low- and middle-income countries (LIMCs), where the burden of HIV is highest.[2]
For PHIV, an increased risk of GDM has most commonly been associated with protease-inhibitor (PI)-based ART.[9-12] However recommendations for first line ART have shifted away from PI-based and non-nucleoside reverse-transcriptase inhibitor(NNRTI)- based ART, in favor of integrase strand-inhibitors (INSTIs). In 2019 the WHO recommended dolutegravir (DTG), an INSTI, as first line therapy for all PHIV.[13] Following this recommendation, South Africa began initiating or switching pregnant PHIV from efavirenz(EFV)-based ART to DTG-based ART. DTG has been associated with weight gain, including in pregnancy, and reports of hyperglycemia in non-pregnant adults.[14-16] Currently, there are no data on HIV, ART and GDM risk in pregnancy or postpartum DM risk among pregnant PHIV in South Africa following the rollout of DTG.
To address this gap, we conducted a prospective cohort study among pregnant PHIV and without HIV in Cape Town, South Africa. The study took place during the roll out of DTG in South Africa, and therefore included participants on both EFV and DTG. We evaluated differences in GDM risk in pregnancy and glucose metabolism postpartum by HIV status and ART regimen, and report on associations of GDM with the risk of adverse birth outcomes.
Methods
Study setting and design
Data come from the Cardiometabolic Health in Pregnancy (CAMP) study, a prospective cohort study which enrolled consecutive pregnant persons with and without HIV, who were ≥18 years of age and presented for antenatal care (ANC) in Gugulethu, Cape Town between November 2019 and June 2022. Participants were enrolled at 24-28 weeks gestation (baseline) and completed a follow-up postpartum visit, planned at 6 months postpartum. Due to COVID-19 pandemic disruptions, postpartum visits took place between 6-32 months postpartum (median 9.6 months, IQR 6.8-12.3 months). We enrolled equal numbers of persons without HIV and PHIV, with no restriction on timing of ART initiation for PHIV. All participants were tested for GDM at 24-28 weeks gestation and for impaired glucose metabolism postpartum using a 75 g 2-hour oral glucose tolerance test (OGTT) after an overnight fast. Information on birth outcomes was abstracted from medical records.[17-19] Ethics approval for the CAMP study was provided by the University of Cape Town’s Human Research Ethics Committee (protocols 486 and 505).
Gugulethu is a peri-urban community in Cape Town with a population of approximately 300,000, characterized by high levels of poverty and HIV among pregnant persons.[19-21] Access to antenatal care is nearly universal (95%) and same-day initiation of ART is provided at no cost as a part of routine antenatal care at all public-sector clinics.[20, 22] In June 2019, South Africa transitioned from initiating PHIV on EFV-based ART (tenofovir 300 mg + emtricitabine 200 mg/lamivudine 300 mg + efavirenz 600 mg) to DTG-based ART (tenofovir 300mg + lamivudine 300mg/emtricitabine 200mg + dolutegravir 50mg (TLD)).[23, 24] Both regimens are available as a fixed-dose combination pill taken once daily.[23]
Study population
Participants with frank diabetes at baseline, based on the 2h OGTT and WHO criteria[25] (n=3), were excluded from all analyses. We included all PHIV and without HIV in the analysis of GDM (n=397) and all participants who completed a postpartum study visit and did not seroconvert to HIV (n=1) in the analysis of postpartum glucose impairment (n=292, 74%). To understand the effect of initiating DTG or EFV on glucose metabolism, primary comparisons by ART regimen were restricted to PHIV on post-conception EFV or DTG. We included all PHIV on EFV- or DTG-based ART in a sensitivity analysis. Participants with an unknown pregnancy outcome (n= 13) or stillbirth (n=2) were excluded from the analysis of birth outcomes (n=385 live births).
Exposures and Outcomes
The exposures of interest were HIV status and ART regimen, collected via self-report at baseline and confirmed via medical records. Outcomes included GDM, diagnosed according to WHO criteria as one or more of the following: fasting plasma glucose ≥5.1-6.9 mmol/l [92-125 mg/dL], 1-hour (h) plasma glucose ≥10.0 mmol/l [180 mg/dL], or 2h plasma glucose ≥8.5-11.0 mmol/l [153-199 mg/dL].[25] Plasma glucose levels at fasting, 1h, and 2hs were also assessed. Postpartum impaired glucose metabolism was categorized as: impaired fasting glucose (IFG; fasting glucose 6.1-6.9mmol/l [110-125 mg/dL] and 2h glucose <7.8mmol/l [140 mg/dL]), impaired glucose tolerance (IGT; fasting glucose <7.0mmol/l [126 mg/dL] and 2h glucose ≥7.8- <11.1 mmol/l [140-200 mg/dL]) or DM (fasting glucose ≥7.0mmol/l [126 mg/dL] or 2h glucose ≥11.1mmol/l [200 mg/dL]) according to WHO criteria.[26] Due to few postpartum events, IFG, IGT, and DM were collapsed into a binary measure of ‘any impaired glucose metabolism’ versus ‘none’. Insulin resistance (evaluated as Homeostatic Model Assessment for Insulin resistance (HOMA-IR)[27] and sensitivity (Matsuda Index)[28] were considered as additional outcomes.
We evaluated associations between GDM and several birth outcomes, including cesarean delivery (as indicated in medical records), infant birthweight (g), low birthweight (<2500 grams), high birthweight (>4000 grams), preterm birth (<37 completed weeks’ gestation), small for gestational age (SGA, birthweight <10th percentile for gestational age) and large for gestational age (LGA, birthweight >90th percentile for gestational age).[29] Gestational age at enrollment was determined primarily by ultrasound (360/400, 90%) at entry into antenatal care (mean gestational age 16 weeks’ (SD 5.7). In some cases last menstrual period and symphysis fundal height were used for women presenting later in pregnancy when ultrasound is less reliable.[30, 31] Gestational birth weight percentiles were defined using the INTERGROWTH-21 standards.[32, 33]
Covariates
At baseline, information on clinical, behavioral, and HIV disease (if applicable) characteristics was collected. We developed a composite socio-economic status (SES) score, based on current employment, education, housing type, and access to household assets, that was used to categorize participants into tertiles of ‘highest, ‘moderate’ or ‘lowest SES.[34] Alcohol use was measured using the 3-item Alcohol Use Disorders Identification Test-Consumption (AUDIT-C; range 0-12) and a score of ≥3 indicates hazardous drinking for women in the previous 12 months.[35] Pre-pregnancy body mass (BMI, kg/m2) was calculated based on self-reported pre-pregnancy weight[36] and categorized as underweight (<18.5 kg/m2), normal (18.5-24.9 kg/m2), overweight (25-29.9 kg/m2), obese (≥30 kg/m2). Physical activity was evaluated as the number of times and intensity of physical activity in a week. Household food security was assessed using adapted measures of the Household Food Insecurity Access Scale, Food and Nutrition Technical Assistance Project, and the Community Childhood Hunger Identification Project Index.[37] Tuberculosis status (defined as no previous, previous, or current tuberculosis treatment) was defined based on medical records. Blood pressure was evaluated by trained research assistants on seated participants using an Edan M3A Vital Signs Monitor. Four measures, at least 30 minutes apart, were taken and averaged for analyses. Lipid levels (total cholesterol, high- and low-density cholesterol, triglycerides) were evaluated using fasted blood samples.
Among PHIV, CD4 cell count and viral load information within 6 months of study enrollment (mean 2 months, SD 1.3) was abstracted from medical records. Timing of HIV diagnosis (during the current pregnancy or previously), ART regimen, and pre- or post-conception initiation of ART were assessed at baseline and confirmed in medical records.
Statistical Analysis
The goals of the analysis were to estimate the prevalence of GDM, to determine associations of HIV status with GDM and impaired glucose metabolism postpartum, and associations of GDM with adverse birth outcomes. Subgroup analyses among PHIV assessed associations of ART (DTG versus EFV) with GDM and postpartum glucose metabolism.
PHIV were overrepresented in our study population relative to the general population (50% versus 24%) in South Africa.[21] Thus, we estimated the prevalence of GDM in the study population and weighted to represent the general population. We estimated associations between HIV status and ART regimen using Poisson models with robust variance estimators for binary outcomes (e.g. GDM) and linear regression for continuous outcomes (e.g. plasma glucose). Associations between GDM and birth outcomes were estimated using similar methods. All models were adjusted for a minimally sufficient adjustment set of confounders identified using directed acyclic graphs (Figure S1). Collinearity among potential confounders was evaluated and the covariate that predicted the outcome most strongly was selected (e.g. pre-pregnancy BMI and parity over age). Limited sample size precluded examining HIV as a potential effect measure modifier of GDM and adverse birth outcomes. However, we graphically examined differences in the risk of adverse birth outcomes by GDM and HIV status. Except for viral load (56%) and CD4 count (21%) which were available from medical records, missing data was limited (≤3%) and therefore all analyses were complete case. Statistical analyses were conducted in Stata version 15 (StataCorp, College Station, TX).
Results
We enrolled 397 participants (n=198 without HIV, n=199 PHIV) during pregnancy (median gestational age 26 weeks; IQR 24, 27). PHIV were slightly older (median age 31 versus 27 years) and had higher parity (median 3 versus 2), compared to those without HIV (Table 1). Over half of the cohort (52%) reported living with obesity pre-pregnancy and 17% reported food insecurity. PHIV were less likely to be living with obesity pre-pregnancy (48% versus 55%), but more likely to experience food insecurity (20% versus 14%) and to have low SES (40% versus 15%), compared to participants without HIV. PHIV were also more likely to have a family history of diabetes (13% versus 7%) and to report tuberculosis treatment (16% versus 5%). Blood pressure and lipid levels at enrollment were similar by HIV status.
Table 1.
Characteristics at 24-28 weeks gestation among 397 pregnant persons in Cape Town, South Africa, overall and by HIV status
| Without HIV- (n=198) | With HIV (n=199) | Total (n=397) | |
|---|---|---|---|
| Median, IQR | |||
| Age | 27 (24, 31) | 31 (27, 36) | 30 (25, 34) |
| Gestational age | 26 (24, 27) | 26 (24, 27) | 26 (24, 27) |
| Parity | 2 (1, 3) | 3 (2, 4) | 2 (2, 3) |
| Blood pressure, mm/Hg | |||
| Systolic | 113.5 (105.5, 120.8) | 111.5 (103.8, 119.8) | 112.3 (104.7, 120.1) |
| Diastolic | 67.0 (62.5, 71.3) | 66.8 (62.8, 73.0) | 67.0 (62.5, 72.1) |
| Lipids, mmol/l | |||
| Total cholesterol | 4.8 (4.2, 5.4) | 4.5 (3.9, 5.1) | 4.6 (4.0, 5.3) |
| LDL cholesterol | 2.4 (1.8, 2.9) | 2.1 (1.6, 2.6) | 2.3 (1.7, 2.8) |
| HDL cholesterol | 1.7 (1.4, 1.9) | 1.6 (1.4, 1.9) | 1.6 (1.4, 1.9) |
| Triglycerides | 1.5 (1.2, 1.8) | 1.6 (1.3, 2.0) | 1.5 (1.2, 1.9) |
| N(%) | |||
| Pre-pregnancy BMI category (m/kg2)1 | |||
| Underweight (<18.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Normal weight (18.5 - <25.0) | 37 (18.8) | 42 (21.0) | 79 (19.9) |
| Overweight (25.0 - <30.0) | 51 (25.9) | 61 (30.6) | 112 (28.3) |
| Obese (≥ 30.0) | 109 (55.3) | 96 (48.2) | 205 (51.8) |
| SES Category | |||
| Lowest | 50 (25.3) | 77 (39.7) | 127 (32.0) |
| Moderate | 58 (29.3) | 51 (25.6) | 109 (27.5) |
| Highest | 90 (45.4) | 71 (35.7) | 161 (40.5) |
| Marital status | |||
| Not married/cohabitating | 114 (57.6) | 112 (56.3) | 226 (56.9) |
| Married/cohabitating | 84 (42.4) | 87 (43.7) | 171 (43.1) |
| Primigravida | |||
| No | 147 (74.2) | 176 (88.4) | 323 (81.4) |
| Yes | 51 (25.8) | 23 (11.6) | 74 (18.6) |
| Alcohol use2 | |||
| Below threshold | 186 (93.9) | 187 (94.0) | 373 (94.0) |
| Hazardous drinking | 12 (6.1) | 12 (6.0) | 24 (6.0) |
| Food security3 | |||
| No perceived food insecurity | 170 (85.9) | 160 (80.4) | 330 (83.1) |
| Perceived food insecurity | 28 (14.1) | 39 (19.6) | 67 (16.9) |
| Physical activity | |||
| None | 103 (52.0) | 77 (38.7) | 180 (45.3) |
| 1-2 times/week | 51 (2.5.8) | 48 (24.1) | 99 (24.9) |
| 3-4 times/week | 35 (17.7) | 57 (28.6) | 92 (23.2) |
| >4 times/week | 9 (4.5) | 17 (8.5) | 26 (6.6) |
| Physical activity intensity (among women who engage in physical activity n=219) | |||
| Light | 80 (84.2) | 112 (91.8) | 192 (88.5) |
| Moderate | 10 (10.5) | 9 (7.4) | 19 (8.8) |
| Vigorous | 5 (5.3) | 1 (0.8) | 6 (2.8) |
| Family history of diabetes | |||
| No | 181 (92.8) | 169 (87.1) | 350 (90.0) |
| Yes | 14 (7.2) | 25 (12.9) | 39 (10.0) |
| Tuberculosis | |||
| No tuberculosis | 188 (95.0) | 170 (85.4) | 358 (90.2) |
| Previous tuberculosis | 10 (5.0) | 28 (14.1) | 38 (9.6) |
| Current tuberculosis | 0 (0.0) | 1 (0.5) | 1 (0.2) |
| Maternal HIV characteristics (N=199) | |||
| Preconception ART N=88 |
Postconception ART N=111 |
Participants with HIV N=199 |
|
| HIV diagnosis | N (%) | ||
| Before this pregnancy, but during another pregnancy | 47 (53.4) | 18 (16.2) | 65 (32.7) |
| Before this pregnancy, but not during another pregnancy | 41 (46.6) | 27 (24.3) | 68 (34.2) |
| During this pregnancy | 0 (0.0) | 65 (58.6) | 65 (32.7) |
| Perinatally infected | 0 (0.0) | 1 (0.9) | 1 (0.5) |
| ART regimen | |||
| Efavirenz based | 71 (80.6) | 36 (32.4) | 107 (53.8) |
| Dolutegravir based | 11 (12.5) | 74 (66.7) | 85 (42.7) |
| Other | 6 (6.8) | 1 (0.9) | 7 (3.5) |
| Viral load | |||
| Undetectable (<50 copies/ml) | 60 (84.5) | 2 (11.1) | 62 (69.7) |
| Detectable (≥50 copies/ml) | 11 (15.5) | 16 (88.9) | 27 (30.3) |
| CD4 count, cells/mm3 | |||
| ≤350 | 9 (12.0) | 38 (45.8) | 47 (29.8) |
| 351 - ≤500 | 23 (30.7) | 19 (22.9) | 42 (26.6) |
| >500 | 43 (57.3) | 26 (31.3) | 69 (43.7) |
BMI = body mass index; ART = antiretroviral therapy, GA= gestational age.
Based on WHO categories and self-reported pre-pregnancy weight.
Based on the AUDIT-C (range 0-12); a score of ≥3 indicates hazardous drinking.
Household food security was assessed using adapted measures of the Household Food Insecurity Access Scale, Food and Nutrition Technical Assistance Project, and the Community Childhood Hunger Identification Project Index’. Missing data: pre-pregnancy BMI n=1 (03%); family history of diabetes n=8 (2.0%); CD4 cell count n=41 (21%); viral load n=110 (55%).
Among PHIV, ART 44% initiated ART pre-conception (n=88; n=71 EFV, n=11 DTG, n=6 other) and 56% post-conception (n=111; n=36 EFV, n=74 DTG, n=1 other). Post-conception initiators were on ART an average of 6.7 weeks (SD 5.1) at baseline. Pre-pregnancy BMI was similar between those initiating DTV versus EFV (31 versus 29 kg/m2). About a third of PHIV with measures available had a CD4 count ≤350 cells/mm3 or a detectable viral load (≥50 copies/ml) at enrollment.
The unadjusted prevalence of GDM in the study population was 6.0% (9.0% PHIV vs 3.0% without HIV, Table 2) and 4.5% when weighted reflect the general population (Table S1). In multivariable analyses, PHIV were at higher risk of GDM (RR 3.9 95% CI 1.4, 10.7) after adjustment for pre-pregnancy BMI, family history of diabetes, and other confounders. This translates to a 6.1% (95% CI 1.1%, 11.1%) higher absolute risk of GDM for PHIV, relative to participants without HIV. The association between HIV status and GDM was similar when restricted to PHIV initiating post-conception ART only (unadjusted prevalence 7.2% PHIV vs 3.0% without HIV: adjusted RR 3.7, 95% CI 1.2, 11.6). Among the 18 PHIV who developed GDM, 9 (50%) were on pre-conception EFV, 2 on post-conception EFV (11%), 6 were on post-conception DTG (33%), and 1 was on an alternative pre-conception regimen (6%).
Table 2.
Glucose metabolism at 24-28 weeks gestation and up to 32 months postpartum, by HIV status and ART regimen among post-conception ART new
| Without HIV | With HIV | Full Cohort | Efavirenz | Dolutegravir | Post-conception ART | |||
|---|---|---|---|---|---|---|---|---|
| Pregnancy | Pregnancy | |||||||
| n=198 | n=199 | N=397 | n=36 | n=74 | N=110* | |||
| Mean (SD) | Mean difference (95% CI)1 | Mean (SD) | Mean difference (95% CI)1 | |||||
| Plasma Glucose, mmol/l | ||||||||
| Fasting | 4.1 (0.4) | 4.3 (0.5) | 0.2 (0.1, 0.3) | 4.4 (0.5) | 4.2 (0.6) | −0.2 (−0.4, 0.1) | ||
| 1-hour | 5.9 (1.4) | 5.9 (1.4) | −0.1 (−0.4, 0.2) | 5.7 (1.1) | 5.5 (1.3) | −0.4 (−0.9, 0.1) | ||
| 2-hours | 5.5 (1.1) | 5.5 (1.2) | −0.1 (−0.3, 0.2) | 5.6 (1.0) | 5.1 (1.0) | −0.5 (−1.0, −0.1) | ||
| N (%) | Risk Ratio (95% CI) | N (%) | Risk Ratio (95% CI) | |||||
| Gestational Diabetes | ||||||||
| No | 191 (97.0) | 181 (91.0) | 1.00 | 34 (94.4) | 68 (91.9) | 1.00 | ||
| Yes | 6 (3.0) | 18 (9.0) | 3.9 (1.4, 10.7) | 2 (5.6) | 6 (8.1) | 1.1 (0.2, 6.6)2 | ||
| Postpartum | Postpartum | |||||||
| n=142 | n=150 | N=292 | n=22 | n=60 | N=82 | |||
| Mean (SD) | Mean difference (95% CI)1 | Mean (SD) | Mean difference (95% CI)1 | |||||
| Plasma Glucose, mmol/l | ||||||||
| Fasting | 4.7 (0.6) | 4.7 (0.5) | 0.0 (−0.1, 0.2) | 4.7 (0.6) | 4.6 (0.5) | −0.1 (−0.4, 0.2) | ||
| 1-hour | 5.8 (1.3) | 5.5 (1.7) | −0.4 (−0.8, 0.0) | 5.6 (1.7) | 5.6 (1.6) | 0.0 (−0.9, 0.9) | ||
| 2-hours | 5.5 (1.2) | 5.3 (1.2) | −0.2 (−0.5, 0.1) | 5.3 (0.9) | 5.3 (1.1) | 0.1 (−0.4, 0.7) | ||
| HOMA-IR | 2.9 (2.2) | 2.5 (2.1) | 0.0 (−0.6, 0.4) | 2.5 (2.4) | 2.4 (1.8) | −0.2 (−1.2, 0.8) | ||
| Matsuda Index | 6.5 (4.6) | 8.0 (6.1) | 0.9 (−0.5, 2.3) | 8.4 (5.4) | 8.1 (6.8) | −1.2 (−4.7, 2.3) | ||
| N (%) | Risk Ratio (95% CI)1 | N (%) | Risk Ratio (95% CI)1 | |||||
| Glucose Metabolism | ||||||||
| Normal | 124 (93.2) | 137 (95.8) | 1.00 | 21(100.0) | 56 (96.6) | 1.00 | ||
| Any glucose metabolism impairment (combined) | 9 (6.8) | 6 (4.2) | 0.5 (0.2, 1.2) | 0 (0.0) | 2 (3.5) | --3 | ||
| Impaired fasting glucose | 1 (0.8) | 2 (1.4) | -- | 0 (0.0) | 1 (1.7) | -- | ||
| Impaired glucose tolerance | 6 (4.5) | 2 (1.4) | 0 (0.0) | 1 (1.7) | ||||
| Diabetes mellitus | 2 (1.5) | 2 (1.4) | 0 (0.0) | 0 (0.0) | ||||
Adjusted for: pre-pregnancy BMI, food insecurity status, family history of diabetes, tuberculosis status, physical activity frequency, parity, socioeconomic status.
Adjusted for: pre-pregnancy BMI, family history of diabetes, physical activity frequency, parity, socioeconomic status due to model convergence issues.
Cannot be estimated. Missing data: Pregnancy fasting glucose n=2, 1-hour glucose n=3, 2-hour glucose n=2; Postpartum: fasting glucose n=17, 1-hour glucose n=19, 2-hour glucose n=18, HOMA-IR n=17, Matsuda index n=22.
When considering plasma glucose levels, PHIV had higher fasting plasma glucose (unadjusted mean 4.3 versus 4.1 mmol/l, adjusted mean difference (MD) 0.2 95% CI 0.1, 0.3), but no differences in 1- and 2-hour glucose levels (Table 2; Figure S2). Among the 24 participants with GDM, GDM was diagnosed primarily based solely on elevated fasting plasma glucose (19/24 79%, mean 5.5 (SD 0.6), rather than 1-hour (mean 7.4, SD 2.7) or 2-hour (mean 6.8, SD 1.8) plasma glucose.
Among participants on post-conception ART, the risk of GDM was similar (unadjusted prevalence 8.1% DTG versus 5.6% EFV, adjusted RR 1.1, 95% CI 0.2, 6.6). However, those initiating EFV tended to have slightly higher plasma glucose levels, with the largest difference at 2h post-glucose load (5.6 versus 5.1 mmol/l) (Table 2; Figure S2). In a sensitivity analysis including pre- and post-conception ART initiators, 10.1% of EFV users developed GDM compared to 7.1% of DTG users (adjusted RR 0.6, 95% CI 0.2, 1.6).
Of the 24 participants who developed GDM, 15 (65%) were living with obesity pre-pregnancy (Table 3). Pre-pregnancy obesity was not associated with GDM overall or when stratified by HIV status; however, confidence intervals were wide due to the small number of events. For both PHIV and without HIV, the highest proportion of GDM diagnoses occurred among participants who were living with obesity pre-pregnancy.
Table 3.
Associations between pre-pregnancy BMI category and gestational diabetes (GDM), overall and by HIV status.
| Without HIV N=198 |
With HIV N=199 |
Full Cohort N=397 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| No GDM N=191 |
GDM N=6 |
Risk Ratio | No GDM N=181 |
GDM N=18 |
Risk Ratio | No GDM N=372 |
GDM N=24* |
Risk Ratio | |
| N (%) | (95% CI) | N (%) | (95% CI) | N (%) | (95% CI) | ||||
| Pre-pregnancy BMI (m/kg2) | |||||||||
| Underweight (<18.5) | 0 (0.0) | 0 (0.0) | -- | 0 (0.0) | 0 (0.0) | -- | 0 (0.0) | 0 (0.0) | -- |
| Normal weight (18.5 - <25.0) | 36 (97.3) | 1 (2.7) | 1.00 | 39 (92.9) | 3 (7.1) | 1.00 | 75 (94.9) | 4 (5.0) | 1.00 |
| Overweight (25.0 - <30.0) | 49 (98.0) | 1 (2.0) | 0.74 (0.05, 11.53) | 58 (95.1) | 3 (4.9) | 0.69 (0.15, 3.26) | 107 (96.4) | 4 (3.6) | 0.71 (0.18, 2.77) |
| Obese (≥ 30.0) | 106 (97.2) | 3 (2.8) | 1.01 (0.11, 9.54) | 84 (87.5) | 12 (12.5) | 1.75 (0.52, 5.89) | 190 (92.7) | 15 (7.3) | 1.45 (0.49, 4.23) |
1 person with without HIV, but with GDM was missing pre-pregnancy BMI.
Among 385 live births, 16% of infants were preterm, 13% low birthweight, 4% high birthweight, 8% were SGA, 15% LGA and 20% of deliveries were by emergency cesarean section (Table 4). After adjustment for confounders, participants with GDM delivered at slightly earlier gestation age (MD −1.0 week, 95% CI −2.0, −0.0). Participants with GDM were more likely to have a LGA (RR 2.40, 95 %CI 1.23, 4.66), preterm birth (RR 2.44, 95% CI 1.24, 4.81), and an emergency cesarean (RR 2.37, 95% CI 1.26, 4.46). In exploratory analyses among participants with GDM, there was little difference in LGA or preterm birth risk by HIV status (Figure 1). The risk of an emergency cesarean section was higher among participants with GDM and HIV, compared to those with GDM but without HIV.
Table 4.
Associations between gestational diabetes in pregnancy and birth outcomes among 385 live births.
| Overall | Gestational Diabetes (GDM) | ||||
|---|---|---|---|---|---|
| Median (IQR) | Without GDM n=385 (94%) |
With GDM n=24 (6%) |
Mean Difference (95% CI)1 |
||
| Birthweight (grams) | 3160 (2800, 3520) | 3160 (2800, 3520) | 3250 (2880, 3550) | 8.0 (−1245.3, 261.3) | |
| Gestational age | 39 (37, 39) | 39 (37, 40) | 38 (36, 39) | −1.0 (−2.0, −0.0) | |
| N (%) | RR 95% CI1 | ||||
| Preterm Birth | 63 (16.4) | 55 (15.4) | 7 (30.4) | 2.44 (1.24, 4.81) | |
| Low birthweight | 49 (12.7) | 44 (12.3) | 4 (17.4) | 1.70 (0.68, 4.23) | |
| High birthweight | 16 (4.2) | 14 (3.9) | 2 (8.7) | 3.38 (0.76, 14.95) | |
| Small for gestational age | 30 (7.8) | 27 (7.6) | 2 (8.7) | 1.22 (0.29, 5.12) | |
| Large for gestational age | 58 (15.1) | 51 (14.3) | 7 (30.4) | 2.40 (1.23, 4.66) | |
| Emergency Cesarean | 74 (19.9) | 64 (18.6) | 9 (39.1) | 2.37 (1.26, 4.46) | |
Preterm birth: birth <37 weeks’ gestation. Low birthweight: <2500 grams. Small for gestational age: birthweight <10th percentile for gestational age. Large for gestational age: birthweight> 90th percentile for gestational age. Missing Data: 3 people missing GDM; 1 person missing BP; 1 missing birthweight; 8 missing emergency c-section outcome. RRs estimated using Poisson models with robust variance estimators.
Adjusted for: pre-pregnancy BMI, food insecurity status, family history of diabetes, tuberculosis status, physical activity frequency, parity, socioeconomic status.
Adjusted for: pre-pregnancy BMI, food insecurity status, tuberculosis status, physical activity frequency, parity, socioeconomic status.
Figure 1.
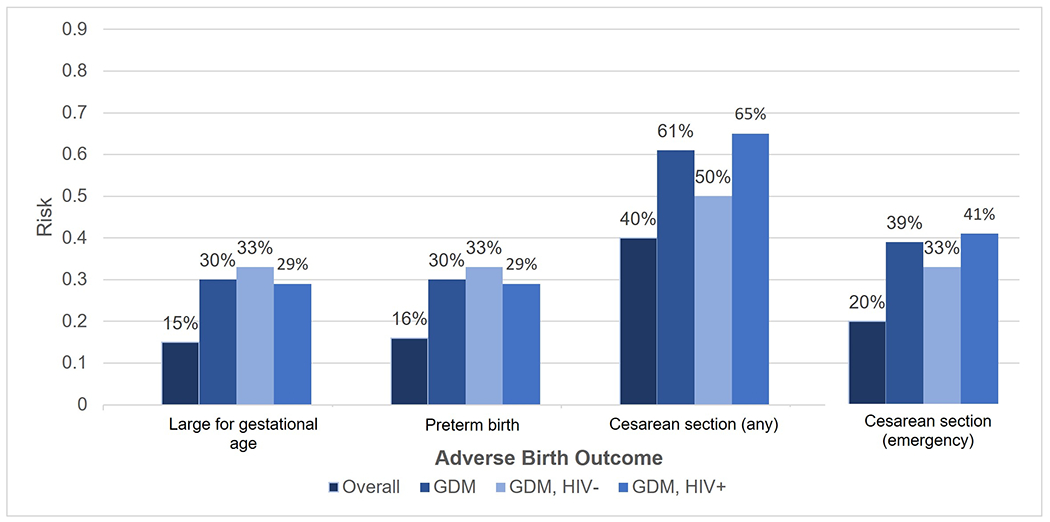
Risk of an adverse birth outcomes overall, among women with gestational diabetes (GDM) and among participants with GDM by HIV status
In the postpartum period, only 5% (n=15) of participants had any form of impaired glucose metabolism (Table 2). Of the 24 participants with GDM, 17 (71%) had a postpartum visit and 3 (18%) had DM; none had IFG or IGT. Of the 4 cases of DM postpartum, 3 (75%) were among participants with GDM. Postpartum, there were no differences in impaired glucose metabolism, insulin resistance or insulin sensitivity by HIV status or ART regimen.
Discussion
In a cohort of pregnant persons with and without HIV, 6.0% were diagnosed with GDM. Compared to those without HIV, PHIV were at an increased risk of GDM, after accounting for pre-pregnancy BMI and other important confounders. Among post-conception ART initiators, the risk of GDM was similar regardless of EFV or DTG use. Participants with GDM were at an increased risk of an emergency cesarean and a LGA or preterm infant. In exploratory analyses, the risk of LGA and preterm birth did not meaningfully differ by HIV status among participants with GDM, but the risk of an emergency cesarean was highest for participants with GDM and HIV. The risk of postpartum impaired glucose metabolism did not differ by HIV or ART status.
Estimates of GDM vary widely globally, in part due to differences in screening guidelines and diagnostic criteria.[1, 25, 38-40] Across much of sub-Saharan Africa, GDM screening is risk-based and screening for diabetes outside of pregnancy varies, making it challenging to estimate GDM prevalence and distinguish GDM from pre-existing DM.[41] Our observed GDM prevalence of 6.0% in the cohort (4.5% general population) was lower than estimates from several meta-analyses in Africa (range 9%-14%)[42-44] and in South Africa (9.1%).[45] Among PHIV, 9% developed GDM, the same as a recent study in Botswana[46], but higher than GDM estimates among PHIV in Rwanda, Kenya, and a recent meta-analysis of PHIV in Africa (3.2%).[47-49] These differences may reflect differences in study population, GDM screening and diagnosis, or the increasing prevalence of GDM among pregnant persons.[3]
Our study is among the first to identify a higher risk of GDM among PHIV in a setting of universal GDM screening and ART. A recent study in India found an increased risk of GDM among PHIV (13.9% vs 6.5% HIV-uninfected participants) primarily on NNRTI-based ART.[50] In our study, PHIV were nearly 4 times as likely to develop GDM, compared to participants without HIV, controlling for important risk factors including pre-pregnancy BMI and family history of diabetes. No evidence of an association between HIV and GDM has been reported in several other studies.[10, 12, 48, 51, 52] A recent study in Botswana, which used the same GDM screening and diagnostic criteria and had a similar study population, did not observe an association between HIV and GDM (adjusted OR 0.83, 95% CI 0.37, 1.85).[46] These differing results may be driven by the lower prevalence of GDM among participants without HIV in our study (3.0% versus 7.4% in the Botswana study), rather than PHIV (9.0% in both studies).
We found a similar risk of GDM among women initiating EFV- versus DTG-based ART. To our knowledge, only one other study has examined associations of EFV versus DTG in pregnancy with GDM and found a lower risk of GDM among women on DTG (6.1% versus 13.5% EFV; adjusted OR 0.40 95% CI 0.18, 0.92). We did not observe evidence of a protective association between DTG and GDM, possibly due to limited sample size. However, we did observe higher levels of plasma glucose in participants initiating EFV, particularly at 2h post-glucose load, and a higher risk of GDM among EFV users (10.1% vs 7.1% on DTG), when all ART users were included. GDM has most commonly been associated with PI-based, rather than NNRTI-based, ART.[9-12] However, NNRTIs, including EFV, are associated with mitochondrial toxicity which may contribute to abnormalities in adipose tissue and inflammatory pathways linked to GDM and diabetes risk.[53, 54] The relationship between DTG and glucose metabolism is less clear. In treatment experienced non-pregnant PHIV, switching to DTG, particularly from a PI-boosted regimen, has been associated with no change[55, 56] improvements in insulin sensitivity,[57, 58] an increased risk of hyperglycemia[14, 15] and diabetes in PHIV initiating ART.[16] DTG has been linked to weight gain in and outside of pregnancy,[59-61] which over time, could influence GDM or diabetes risk.[62, 63] As DTG use expands, ongoing studies of ART regimen, GDM, and progression to postpartum DM are needed.
As previously reported in South Africa, GDM was diagnosed in this study primarily due to higher fasting plasma glucose.[45] While the OGTT is the gold standard of GDM screening, universal OGTT screening presents operational challenges.[64, 65] Fasting plasma glucose is not as accurate as an OGTT in diagnosing GDM,[66-68] but is operationally more efficient than an OGTT and predicts adverse birth outcomes,[69] although not as well as in conjunction with 1h and 2h hyperglycemia.[70] If the finding of higher fasting plasma glucose leading to GDM is replicated in larger cohorts in LMICs, additional research may consider whether fasting plasma glucose could be part of a GDM screening algorithm in LMICs to improve GDM screening coverage.
In this cohort, postpartum impaired glucose metabolism was uncommon (5%), with the risk of postpartum DM highest among with GDM in pregnancy.[71, 72] The risk of any impaired glucose metabolism postpartum in this cohort did not differ by HIV or ART status. The low prevalence of postpartum glucose impairment in the presence of high levels of pre-pregnancy obesity but may reflect a different diabetes phenotype in persons of African ancestry.[73] Persons with GDM are at increased risk of progressing to impaired glucose metabolism or DM postpartum. However, to date no data is available on how HIV or ART may affect the risk of progression to postpartum DM for PHIV.[74] If HIV or ART are confirmed to influence GDM risk in larger cohorts, efforts to improve postpartum glucose mentalism screening will be important to prevent progression to DM for PHIV.
Our study has several strengths and limitations. Major strengths include universal GDM testing using WHO diagnostic criteria, robust control of confounders and risk factors for GDM, and the ability to evaluate associations between EFV and DTG and GDM. Limitations include the relatively small sample size of PHIV initiating ART, which may have resulted in limited power to detect differences between EFV and DTG users, the imprecision of associations of pre-pregnancy BMI with GDM due to limited GDM cases, postpartum loss to follow-up, and the large timespan among postpartum visits (6-32 months). Most pregnant persons with GDM return to euglycemia by 6-12 weeks postpartum, indicating that assessment after that time point is a reasonable indication of postpartum glucose metabolism. In addition, there were no important differences in sociodemographic characteristics between the full cohort and those with a postpartum visit (Table S2). Our study took place during the COVID-19 pandemic, but we did not have data on COVID-19 exposure or treatment. SARS-CoV-2 seroprevalence among pregnant women in South Africa is estimated at >60% and is typically asymptomatic but may increase the risk of low birthweight.[75-77]
Conclusion
In a setting of universal GDM screening, we observed a higher risk of GDM among PHIV, but no difference in GDM risk among participants initiating EFV- versus DTG-based ART or in postpartum glucose metabolism by HIV or ART status. GDM increased the risk of LGA, preterm birth and emergency cesarean section, with no appreciable difference in LGA or preterm birth risk by HIV status among participants with GDM. Improved screening for hyperglycemia in pregnancy in LMICs, and in particular among PHIV, is needed to improve clinical outcomes and monitor GDM risk over time. Given concerns about weight gain associated with DTG, glucose metabolism and GDM risk in pregnancy and progression to postpartum DM should continue to be monitored among pregnant PHIV.
Supplementary Material
Acknowledgements:
AMB and LM received funding for the study; AMB, HM, and LM were involved in data collection; AMB conducted the statistical analysis with input from HM and LM and drafted the paper. All authors assisted with the interpretation of the study findings and critically reviewed the manuscript.
Funding:
This work was supported by the Providence/Boston Center for AIDS Research (P30AI042853), the Population Studies and Training Center at Brown University (P2C HD041020), the Brown University School of Public Health, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK U01-DK-18-018/019) and the Fogarty International Center at the National Institutes of Health (R21TW011678) and the South African Medical Research Council.
References
- 1.Committee on Practice BO. ACOG Practice Bulletin No. 190: Gestational Diabetes Mellitus . Obstet Gynecol 2018; 131(2):e49–e64. [DOI] [PubMed] [Google Scholar]
- 2.Zhu Y, Zhang C. Prevalence of Gestational Diabetes and Risk of Progression to Type 2 Diabetes: a Global Perspective. Current diabetes reports 2016; 16(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang H, Li N, Chivese T, Werfalli M, Sun H, Yuen L, et al. IDF Diabetes Atlas: Estimation of Global and Regional Gestational Diabetes Mellitus Prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group’s Criteria. Diabetes Res Clin Pract 2022; 183:109050. [DOI] [PubMed] [Google Scholar]
- 4.Lowe LP, Metzger BE, Dyer AR, Lowe J, McCance DR, Lappin TR, et al. Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: associations of maternal A1C and glucose with pregnancy outcomes. Diabetes Care 2012; 35(3):574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Catalano PM, McIntyre HD, Cruickshank JK, McCance DR, Dyer AR, Metzger BE, et al. The hyperglycemia and adverse pregnancy outcome study: associations of GDM and obesity with pregnancy outcomes. Diabetes Care 2012; 35(4):780–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care 2002; 25(10):1862–1868. [DOI] [PubMed] [Google Scholar]
- 7.Hadigan C, Kattakuzhy S. Diabetes mellitus type 2 and abnormal glucose metabolism in the setting of human immunodeficiency virus. Endocrinol Metab Clin North Am 2014; 43(3):685–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubé MP. Disorders of glucose metabolism in patients infected with human immunodeficiency virus. Clin Infect Dis 2000; 31(6):1467–1475. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Tome MI, Ramos Amador JT, Guillen S, Solis I, Fernandez-Ibieta M, Munoz E, et al. Gestational diabetes mellitus in a cohort of HIV-1 infected women. HIV Med 2008; 9(10):868–874. [DOI] [PubMed] [Google Scholar]
- 10.Jao J, Wong M, Van Dyke RB, Geffner M, Nshom E, Palmer D, et al. Gestational diabetes mellitus in HIV-infected and -uninfected pregnant women in Cameroon. Diabetes Care 2013; 36(9):e141–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marti C, Pena JM, Bates I, Madero R, de Jose I, Pallardo LF, et al. Obstetric and perinatal complications in HIV-infected women. Analysis of a cohort of 167 pregnancies between 1997 and 2003. Acta Obstet Gynecol Scand 2007; 86(4):409–415. [DOI] [PubMed] [Google Scholar]
- 12.Soepnel LM, Norris SA, Schrier VJ, Browne JL, Rijken MJ, Gray G, et al. The association between HIV, antiretroviral therapy, and gestational diabetes mellitus. Aids 2017; 31(1):113–125. [DOI] [PubMed] [Google Scholar]
- 13.Organization WH. WHO recommends dolutegravir as preferred HIV treatment option in all populations. In; 2019. [Google Scholar]
- 14.Namara D, Schwartz JI, Tusubira AK, McFarland W, Birungi C, Semitala FC, et al. The risk of hyperglycemia associated with use of dolutegravir among adults living with HIV in Kampala, Uganda: A case-control study. Int J STD AIDS 2022; 33(14):1158–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lamorde M, Atwiine M, Owarwo NC, Ddungu A, Laker EO, Mubiru F, et al. Dolutegravir-associated hyperglycaemia in patients with HIV. The lancet HIV 2020; 7(7):e461–e462. [DOI] [PubMed] [Google Scholar]
- 16.Rebeiro PF, Rebeiro PF, Jenkins C, Bian A, Lake J, Lake J, et al. LB9. The Effect of Initiating Integrase Inhibitor-based vs. Non-Nucleoside Reverse Transcriptase Inhibitor-based Antiretroviral Therapy on Progression to Diabetes among North American Persons in HIV Care. Open forum infectious diseases 2019; 6(Supplement_2):S996–S997. [Google Scholar]
- 17.Bengtson AM, Phillips TK, le Roux SM, Brittain K, Zerbe A, Madlala HP, et al. High blood pressure at entry into antenatal care and birth outcomes among a cohort of HIV-uninfected women and women living with HIV initiating antiretroviral therapy in South Africa. Pregnancy hypertension 2020; 23:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bengtson AM, Roux SMl, Phillips TK, Brittain K, Zerbe A, Malaba T, et al. High body mass in HIV+ & HIV- women and their HIV-uninfected infants in South Africa In: Conference on Retroviruses and Opportunistic Infections (CROI). Boston, MA; 2020. [Google Scholar]
- 19.Myer L, Phillips TK, Zerbe A, Brittain K, Lesosky M, Hsiao NY, et al. Integration of postpartum healthcare services for HIV-infected women and their infants in South Africa: A randomised controlled trial. PLoS Med 2018; 15(3):e1002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myer L, Phillips T, Manuelli V, McIntyre J, Bekker LG, Abrams EJ. Evolution of antiretroviral therapy services for HIV-infected pregnant women in Cape Town, South Africa. J Acquir Immune Defic Syndr 2015; 69(2):e57–e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Republic of South Africa DoS. Statistical Release In: Stats SA. Edited by Africa SS. Pretoria, South Africa: Repulblic of South Africa, Department of Statistics; 2021. [Google Scholar]
- 22.WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. In. Geneva, Switzerland: World Health Organization; 2013. [PubMed] [Google Scholar]
- 23.Langwenya N, Phillips TK, Brittain K, Zerbe A, Abrams EJ, Myer L. Same-day antiretroviral therapy (ART) initiation in pregnancy is not associated with viral suppression or engagement in care: A cohort study. J Int AIDS Soc 2018; 21(6):e25133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moorhouse MA, Carmona S, Davies N, Dlamini S, van Vuuren C, Manzini T, et al. Southern African HIV Clinicians Society Guidance on the use of dolutegravir in first-line antiretroviral therapy. Southern African journal of HIV medicine 2018; 19(1):917–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Organization WH. WHO recommendations on antenatal care for a positive pregnancy experience. In. Geneva, Switzerland: WHO; 2016. [PubMed] [Google Scholar]
- 26.World Health Organization. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia : report of a WHO/IDF consultation. In. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 27.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28(7):412–419. [DOI] [PubMed] [Google Scholar]
- 28.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999; 22(9):1462–1470. [DOI] [PubMed] [Google Scholar]
- 29.Organization WH. Global nutrition targets 2025: low birth weight policy brief (WHO/NMH/NHD/14.5). In. Geneva: World Health Organization; 2014. [Google Scholar]
- 30.Butt K, Lim K. Determination of gestational age by ultrasound. Journal of obstetrics and gynaecology Canada : JOGC = Journal d’obstetrique et gynecologie du Canada : JOGC 2014; 36(2):171–181. [DOI] [PubMed] [Google Scholar]
- 31.Malaba TR, Phillips T, Le Roux S, Brittain K, Zerbe A, Petro G, et al. Antiretroviral therapy use during pregnancy and adverse birth outcomes in South African women. Int J Epidemiol 2017; 46(5):1678–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Villar J, Cheikh Ismail L, Victora CG, Ohuma EO, Bertino E, Altman DG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 2014; 384(9946):857–868. [DOI] [PubMed] [Google Scholar]
- 33.Villar J, Giuliani F, Fenton TR, Ohuma EO, Ismail LC, Kennedy SH. INTERGROWTH-21st very preterm size at birth reference charts. Lancet 2016; 387(10021):844–845. [DOI] [PubMed] [Google Scholar]
- 34.Brittain K, Mellins CA, Phillips T, Zerbe A, Abrams EJ, Myer L, et al. Social Support, Stigma and Antenatal Depression Among HIV-Infected Pregnant Women in South Africa. AIDS Behav 2017; 21(1):274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med 1998; 158(16):1789–1795. [DOI] [PubMed] [Google Scholar]
- 36.Han E, Abrams B, Sridhar S, Xu F, Hedderson M. Validity of Self-Reported Pre-Pregnancy Weight and Body Mass Index Classification in an Integrated Health Care Delivery System. Paediatr Perinat Epidemiol 2016; 30(4):314–319. [DOI] [PubMed] [Google Scholar]
- 37.Labadarios D, McHiza ZJ, Steyn NP, Gericke G, Maunder EM, Davids YD, et al. Food security in South Africa: a review of national surveys. Bull World Health Organ 2011; 89(12):891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.American Diabetes A 13. Management of Diabetes in Pregnancy: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018; 41(Suppl 1):S137–S143. [DOI] [PubMed] [Google Scholar]
- 39.Metzger BE, Buchanan TA, Coustan DR, de Leiva A, Dunger DB, Hadden DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care 2007; 30 Suppl 2:S251–260. [DOI] [PubMed] [Google Scholar]
- 40.Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010; 33(3):676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adam S, Rheeder P. Screening for gestational diabetes mellitus in a South African population: Prevalence, comparison of diagnostic criteria and the role of risk factors. S Afr Med J 2017; 107(6):523–527. [DOI] [PubMed] [Google Scholar]
- 42.Natamba BK, Namara AA, Nyirenda MJ. Burden, risk factors and maternal and offspring outcomes of gestational diabetes mellitus (GDM) in sub-Saharan Africa (SSA): a systematic review and meta-analysis. BMC Pregnancy Childbirth 2019; 19(1):450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mwanri AW, Kinabo J, Ramaiya K, Feskens EJ. Gestational diabetes mellitus in sub-Saharan Africa: systematic review and metaregression on prevalence and risk factors. Trop Med Int Health 2015; 20(8):983–1002. [DOI] [PubMed] [Google Scholar]
- 44.Muche AA, Olayemi OO, Gete YK. Prevalence and determinants of gestational diabetes mellitus in Africa based on the updated international diagnostic criteria: a systematic review and meta-analysis. Arch Public Health 2019; 77:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Macaulay S, Ngobeni M, Dunger DB, Norris SA. The prevalence of gestational diabetes mellitus amongst black South African women is a public health concern. Diabetes Res Clin Pract 2018; 139:278–287. [DOI] [PubMed] [Google Scholar]
- 46.Mmasa KN, Powis K, Sun S, Makhema J, Mmalane M, Kgole S, et al. Gestational diabetes in women living with HIV in Botswana: lower rates with dolutegravir- than with efavirenz-based antiretroviral therapy. HIV Med 2021; 22(8):715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meharry PM, Tengera O, Rulisa S, Byambu AK, Nietert PJ, Byiringiro S, et al. Prevalence of gestational diabetes mellitus among women attending antenatal care at public health centers in Rwanda. Diabetes Res Clin Pract 2019; 151:252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pastakia SD, Kosgei WK, Christoffersen-Deb A, Kiragu B, Hector JN, Anusu G, et al. Risk of Dysglycemia in Pregnancy amongst Kenyan Women with HIV Infection: A Nested Case-Control Analysis from the STRiDE Study. J Diabetes Res 2021; 2021:8830048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Biadgo B, Ambachew S, Abebe M, Melku M. Gestational diabetes mellitus in HIV-infected pregnant women: A systematic review and meta-analysis. Diabetes Res Clin Pract 2019; 155:107800. [DOI] [PubMed] [Google Scholar]
- 50.Chebrolu P, Alexander M, Bhosale R, Naik S, Patil N, Gupta A, et al. FACTORS ASSOCIATED WITH GESTATIONAL DIABETES IN HIV+ AND HIV– WOMEN IN PUNE, INDIA (Abstract 774). In: Converence on Retroviruses and Opportunistic Infections (CROI). Boston, MA; 2020. [Google Scholar]
- 51.Ørbaek M, Thorsteinsson K, Moseholm Larsen E, Katzenstein TL, Storgaard M, Johansen IS, et al. Risk factors during pregnancy and birth-related complications in HIV-positive versus HIV-negative women in Denmark, 2002-2014. HIV Med 2020; 21(2):84–95. [DOI] [PubMed] [Google Scholar]
- 52.Reitter A, Stücker AU, Linde R, Königs C, Knecht G, Herrmann E, et al. Pregnancy complications in HIV-positive women: 11-year data from the Frankfurt HIV Cohort. HIV Med 2014; 15(9):525–536. [DOI] [PubMed] [Google Scholar]
- 53.Blas-García A, Apostolova N, Ballesteros D, Monleón D, Morales JM, Rocha M, et al. Inhibition of mitochondrial function by efavirenz increases lipid content in hepatic cells. Hepatology 2010; 52(1):115–125. [DOI] [PubMed] [Google Scholar]
- 54.Karamchand S, Leisegang R, Schomaker M, Maartens G, Walters L, Hislop M, et al. Risk Factors for Incident Diabetes in a Cohort Taking First-Line Nonnucleoside Reverse Transcriptase Inhibitor-Based Antiretroviral Therapy. Medicine 2016; 95(9):e2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bagella P, Squillace N, Ricci E, Gulminetti R, De Socio GV, Taramasso L, et al. Lipid profile improvement in virologically suppressed HIV-1-infected patients switched to dolutegravir/abacavir/lamivudine: data from the SCOLTA project. Infect Drug Resist 2019; 12:1385–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ibrahim F, Samarawickrama A, Hamzah L, Vincent R, Gilleece Y, Waters L, et al. Bone mineral density, kidney function, weight gain and insulin resistance in women who switch from TDF/FTC/NNRTI to ABC/3TC/DTG. HIV Med 2021; 22(2):83–91. [DOI] [PubMed] [Google Scholar]
- 57.Calza L, Colangeli V, Borderi M, Coladonato S, Tazza B, Bon I, et al. Improvement in insulin sensitivity and serum leptin concentration after the switch from a ritonavir-boosted PI to raltegravir or dolutegravir in non-diabetic HIV-infected patients. The Journal of antimicrobial chemotherapy 2019; 74(3):731–738. [DOI] [PubMed] [Google Scholar]
- 58.van Wyk J, Ait-Khaled M, Santos J, Scholten S, Wohlfeiler M, Ajana F, et al. Brief Report: Improvement in Metabolic Health Parameters at Week 48 After Switching From a Tenofovir Alafenamide-Based 3- or 4-Drug Regimen to the 2-Drug Regimen of Dolutegravir/Lamivudine: The TANGO Study. J Acquir Immune Defic Syndr 2021; 87(2):794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.NAMSAL ANRS 12313 Study Group, Kouanfack C, Mpoudi-Etame M, Bassega PO, Eymard-Duvernay S, Leroy S, et al. Dolutegravir-Based or Low-Dose Efavirenz-Based Regimen for the Treatment of HIV-1. N Engl J Med 2019. [DOI] [PubMed] [Google Scholar]
- 60.Venter WDF, Moorhouse M, Sokhela S, Fairlie L, Mashabane N, Masenya M, et al. Dolutegravir plus Two Different Prodrugs of Tenofovir to Treat HIV. N Engl J Med 2019; 381(9):803–815. [DOI] [PubMed] [Google Scholar]
- 61.Caniglia EC, Shapiro R, Diseko M, Wylie BJ, Zera C, Davey S, et al. Weight gain during pregnancy among women initiating dolutegravir in Botswana. EClinicalMedicine 2020; 29-30:100615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.González-Cordón A, Assoumou L, Moyle G, Waters L, Johnson M, Domingo P, et al. Switching from boosted PIs to dolutegravir decreases soluble CD14 and adiponectin in high cardiovascular risk people living with HIV. The Journal of antimicrobial chemotherapy 2021; 76(9):2380–2393. [DOI] [PubMed] [Google Scholar]
- 63.Jung I, Tu-Sekine B, Jin S, Anokye-Danso F, Ahima RS, Brown TT, et al. Dolutegravir Suppresses Thermogenesis via Disrupting Uncoupling Protein 1 Expression and Mitochondrial Function in Brown/Beige Adipocytes in Preclinical Models. J Infect Dis 2022; 226(9):1626–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ramaiya KL, Swai AM, Mutabingwa TK, Mwanri AW, Kagaruki GB. Capacity and capability of Tanzania health facilities to diagnose and manage diabetes mellitus in pregnancy. Diabetes Res Clin Pract 2018; 145:119–129. [DOI] [PubMed] [Google Scholar]
- 65.Mutabazi JC, Enok Bonong PR, Trottier H, Ware LJ, Norris SA, Murphy K, et al. Integrating gestational diabetes and type 2 diabetes care into primary health care: Lessons from prevention of mother-to-child transmission of HIV in South Africa - A mixed methods study. PLoS One 2021; 16(1):e0245229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Agarwal MM, Dhatt GS, Punnose J, Koster G. Gestational diabetes in a high-risk population: using the fasting plasma glucose to simplify the diagnostic algorithm. Eur J Obstet Gynecol Reprod Biol 2005; 120(1):39–44. [DOI] [PubMed] [Google Scholar]
- 67.Agarwal MM, Dhatt GS, Punnose J. Gestational diabetes: utility of fasting plasma glucose as a screening test depends on the diagnostic criteria. Diabet Med 2006; 23(12):1319–1326. [DOI] [PubMed] [Google Scholar]
- 68.Agarwal MM, Dhatt GS. Fasting plasma glucose as a screening test for gestational diabetes mellitus. Arch Gynecol Obstet 2007; 275(2):81–87. [DOI] [PubMed] [Google Scholar]
- 69.Riskin-Mashiah S, Younes G, Damti A, Auslender R. First-trimester fasting hyperglycemia and adverse pregnancy outcomes. Diabetes Care 2009; 32(9):1639–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008; 358(19):1991–2002. [DOI] [PubMed] [Google Scholar]
- 71.Nicolaou V, Soepnel L, Huddle K, Klipstein-Grobusch K, Levitt NS, Norris SA. Cardiometabolic outcomes of women exposed to hyperglycaemia first detected in pregnancy at 3-6 years post-partum in an urban South African setting. PLoS One 2022; 17(2):e0263529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chivese T, Norris SA, Levitt NS. Progression to type 2 diabetes mellitus and associated risk factors after hyperglycemia first detected in pregnancy: A cross-sectional study in Cape Town, South Africa. PLoS Med 2019; 16(9):e1002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kibirige D, Lumu W, Jones AG, Smeeth L, Hattersley AT, Nyirenda MJ. Understanding the manifestation of diabetes in sub Saharan Africa to inform therapeutic approaches and preventive strategies: a narrative review. Clin Diabetes Endocrinol 2019; 5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hoffman RM, Newhouse C, Chu B, Stringer JSA, Currier JS. Non-communicable Diseases in Pregnant and Postpartum Women Living with HIV: Implications for Health Throughout the Life Course. Curr HIV/AIDS Rep 2021; 18(1):73–86. [DOI] [PubMed] [Google Scholar]
- 75.Nunes MC, Jones S, Strehlau R, Baba V, Ditse Z, da Silva K, et al. Antepartum SARS-CoV-2 infection and adverse birth outcomes in South African women. J Glob Health 2022; 12:05050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nunes MC, Jones S, Strehlau R, Baba V, Ditse Z, da Silva K, et al. Active Intrapartum SARS-CoV-2 Infection and Pregnancy Outcomes. Am J Perinatol 2022; 39(S 01):S42–S48. [DOI] [PubMed] [Google Scholar]
- 77.Sawry S, Le Roux J, Wolter N, Mbatha P, Bhiman J, Balkus J, et al. High prevalence of SARS-CoV-2 antibodies in pregnant women after the second wave of infections in the inner-city of Johannesburg, Gauteng Province, South Africa. Int J Infect Dis 2022; 125:241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


