Abstract
Purpose:
We described the impact of alcohol use on longitudinal engagement in HIV care including loss to follow-up, durability of viral suppression, and death.
Methods:
We followed a cohort of 1,781 people with HIV from enrolled in care at one of seven US clinics, 2011–2019 through 102 months. We used a multistate, time-varying Markov process and restricted mean time to summarize engagement in HIV care over follow-up according to baseline self-reported alcohol use (none, moderate, or unhealthy).
Results:
Our sample (86% male, 54% white) had median age of 35 years. Over 102 months, people with no, moderate, and unhealthy alcohol use averaged 62.3, 61.1, and 59.5 months virally suppressed, respectively. People who reported unhealthy or moderate alcohol use spent 5.1 (95%CI: 0.8, 9.3) and 7.6 (95%CI: 3.1, 11.7) more months lost to care than non-drinkers. Compared to no use, unhealthy alcohol use was associated with 3.4 (95%CI: −5.6, −1.6) fewer months in care, not virally suppressed. There were no statistically significant differences after adjustment for demographic and clinical characteristics.
Conclusions:
Moderate or unhealthy drinking at enrollment in HIV care was associated with poor retention in care. Alcohol use was not associated with time spent virally suppressed.
Keywords: Alcohol use, Competing risks, Descriptive, HIV care, Multistate model, Survival, Viral suppression
People with HIV (PWH) who adhere to antiretroviral therapy (ART) and maintain a suppressed HIV viral load experience reduced morbidity and mortality and are unable to transmit their infection[1–3]. Yet some PWH do not initiate ART, are not retained in care, or do not maintain a suppressed viral load – key steps in the HIV “care continuum”[4,5]. Unhealthy alcohol use is common among PWH and has been associated with poorer retention in HIV care and poorer viral suppression in some studies[6–11].
However, prior studies have generally measured HIV outcomes using period prevalence (i.e., the proportion “in” a state in a calendar year out of the people who are alive and in care or in the study sample in that year). Period prevalence studies do not account for loss to follow-up or death as competing risks for viral suppression such that we might see “improvements” in viral suppression over time that result from emigrative selection bias[12,13] as the sickest individuals drop out of the sample or target population. Additionally, period prevalence studies classify people as “virally suppressed” or not and “retained” or not based on a single viral load value (usually the last one in the year) and ignore transitions between care continuum stages during the year, which can misclassify people whose viral suppression is not durable and who are at highest risk of loss to care and death[14]. The longitudinal care continuum has been proposed as an alternative to prevalence care continuums [15,16]. In contrast to the prevalence care continuum which describes the proportion of the population in each state of the care continuum across a period of time (perhaps inappropriately assigning them to a group), the longitudinal care continuum describes the distribution of person-time across the states of the care continuum across a (usually longer) study period, accounting for transitions between states (sometimes termed “churn”)[16–19].
Because we anticipate that the risk of viral non-suppression estimated from prevalence care continuums might be understated for PWH who drink alcohol at unhealthy levels due to selection bias and misclassification – that alcohol use might be associated with “churn” – we describe the longitudinal care continuum for a cohort of PWH engaged in routine HIV care according to their self-reported baseline alcohol use. Our objective was to identify the degree to which alcohol use at enrollment in HIV care is a marker of subsequent engagement in care and viral suppression.
Methods
Study sample:
The Centers for AIDS Research (CFAR) Network of Integrated Clinical Systems (CNICS) is a cohort of adults aged 18 years and older who enrolled in HIV care (eligible if they attended ≥2 clinic visits in a year) at eight CFAR-affiliated medical centers in the United States[20]. Briefly, clinical visits, laboratory results, prescribed medication, diagnoses, and demographic information are extracted from electronic medical records for all patients who consent to share their data. Additionally, a subset of patients complete patient-reported outcome (PROs) surveys on tablet computers approximately every 4–6 months in conjunction with routine clinical visits.
For this analysis, we included PWH who were ART-naïve at enrollment into HIV care at one of the seven sites that collected PROs during the study period, January 1, 2011-June 30, 2019 (or the sitespecific date through which data were complete) and who completed at least one AUDIT-C survey on a PRO within one year after enrollment. ART-naïve was defined as having no evidence of any antiretroviral prescription prior to enrollment date and no evidence of viral suppression (defined as HIV RNA ≤400 copies/mL) on the viral load measurement closest to enrollment date within the window six months prior to, and up to one month after enrollment.
Covariates:
Demographic covariates included: present gender, age, race, ethnicity, HIV acquisition risk factors,, and year of enrollment into CNICS. HIV risk factors are self-reported at enrollment as the most likely source of patients’ HIV infection and include heterosexual sex, injection drug use (IDU), and being a man who has sex with men (MSM); risk factors were not mutually exclusive. Clinical covariates included: baseline CD4 cell count, HIV RNA, weight, height, serum creatinine, hemoglobin, alanine transaminase (ALT), aspartate aminotransferase (AST), platelet count, white blood cell count, albumin, and hepatitis C virus (HCV) infection (defined as a positive HCV antibody or RNA test without subsequent evidence of HCV treatment and cure). Lab values were measured six months prior to and up to one month after enrollment. If there were multiple values, we used the one nearest enrollment. Clinical covariates were summarized by the Veterans Aging Cohort Study (VACS) 2.0 index, which is predictive of mortality and other clinical outcomes[21–23].
Exposure:
Alcohol consumption was collected using the Alcohol Use Disorders Identification Test Consumption (AUDIT-C) questions[24,25], which ask about alcohol use over the past year. We used the first PRO questionnaire within one year after enrollment to classify individuals into groups based on standard cut-offs and present gender: 1) no use (AUDIT-C=0); 2) moderate use (AUDIT-C>0 and <3 for women or AUDIT-C>0 and <4 for men); and 3) unhealthy use (AUDIT-C≥3 for women or AUDIT-C≥4 for men).
Outcomes:
We followed patients from CNICS enrollment until death or the administrative end of follow-up at 102 months (8.5 years) or June 30, 2019, whatever came first. We used dates of clinic visits, CD4 cell counts, viral load tests, and death to categorize follow-up time into the following states: 1) in care, not initiated on ART; 2) lost to clinic before ART initiation; 3) dead before ART initiation; 4) in care, initiated on ART, virally unsuppressed; 5) in care, initiated on ART, virally suppressed; 6) lost to clinic after ART initiation; and 7) dead after ART initiation (Figure 1). At the start of follow-up, everyone was classified as in care, not on ART (based on our study eligibility criteria). Retention “in care” was defined as having at least one HIV primary care visit, CD4 count, or viral load in the prior 12 months. Lost to clinic included persons who were lost to HIV care and persons who transferred their HIV care to another clinic; we could not disambiguate these two outcomes based on our data. Individuals who returned to care without an HIV viral load after being lost to care were considered virally unsuppressed upon return. Viral suppression was defined as having most recent viral load ≤400 copies/mL. Dates of death were obtained from clinic sources and regular matches against the Social Security Death Index.
Figure 1.
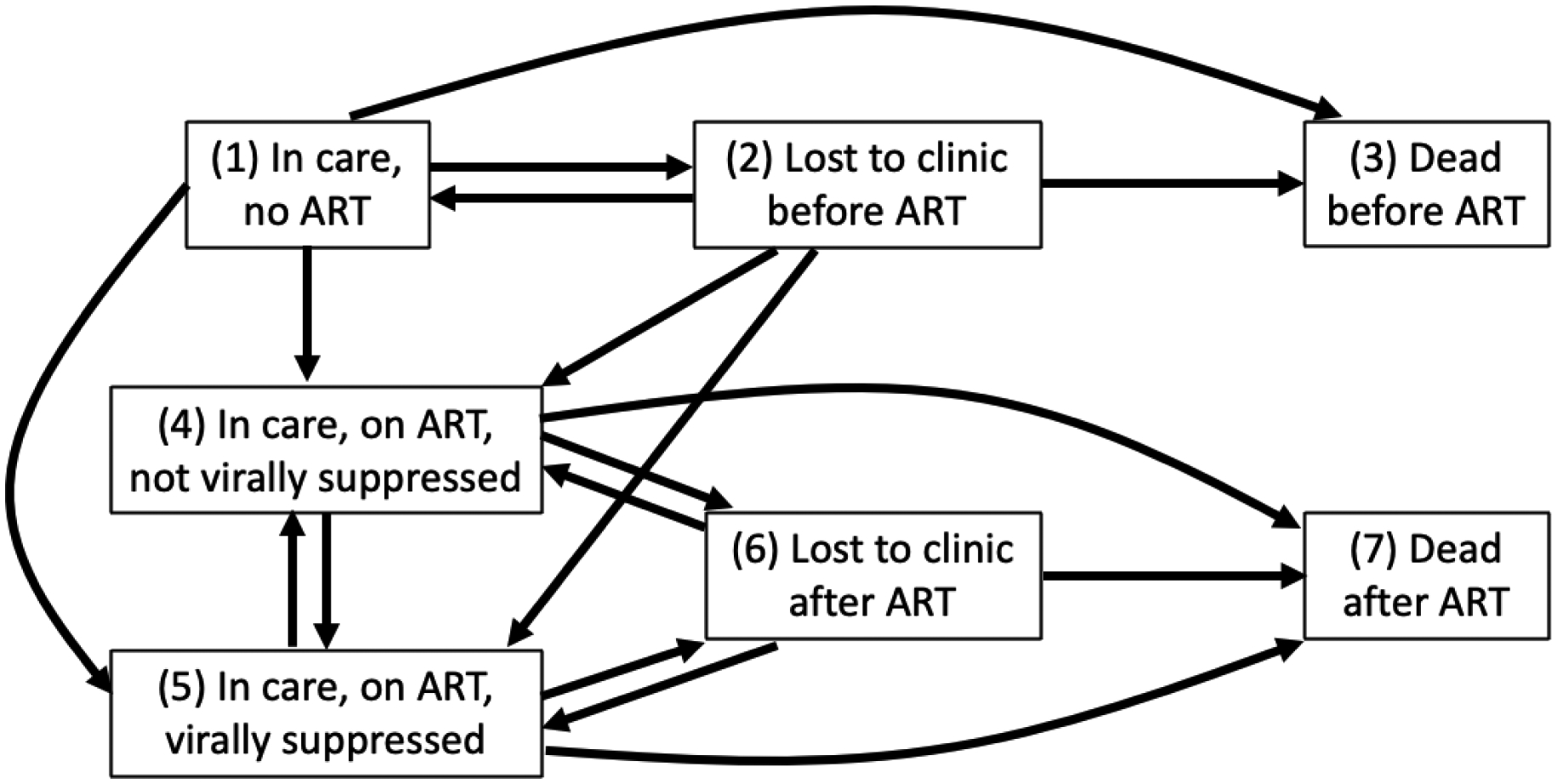
Multistate model of the HIV care continuum and valid transition. “In care” was defined as having at least one HIV primary care visit, CD4 count, or viral load in the prior 12 months. “Lost to clinic” is its complement. Viral suppression was defined as most recent viral load ≤400 copies/mL.
Antiretroviral therapy (ART) initiation was defined as initiating ≥3 antiretroviral medications on the same day or initiating an approved 2-drug regimen.
Statistical Analysis:
Within strata of alcohol use group, we used a multistate, time-varying Markov process (Xt)t≥0 (where X indexes the states and t indexes time since enrollment) with a finite state space 𝒮={1,2,3,4,5,6,7} (7 states as defined above) to estimate state occupancy probabilities for each of the seven states at all times from enrollment until 102 months of follow-up. To do so, we estimated 7 × 7 square matrices of transition probabilities denoted by P(s, t) for pairs of transition times (months) s and t. The transition probabilities are Plj(s, t) = P(Xt = j|XS = l), s ≤ t, l, j ∈ 𝒮, the probability that the Markov process is in state j at time t given it was in state l at time s. We were interested in the transition probability matrix P(0, t) conditional on the process starting at time s = 0. Because everyone in our study starts in state 1 at time 0 our transition probabilities simplify to the vector P1j(0, t) = P(Xt = j|X0 = 1), 0 ≤ t, j ∈ 𝒮, which is the first row of matrix P(0, t).
We built up the P(0, t) using transition hazards. The transition hazards of the multistate model are defined as αlj(t)dt = P(X(t+dt)− = j|Xt− = l), l, j ∈ 𝒮,l ≠ j where dt is an infinitesimal time and t − denotes time immediately prior to time t. The l → j transition hazard at t is the number of l → j transitions at time t divided by total individuals at risk in state l right before t. The cumulative transition hazards are . We estimated these conditional transition probabilities and cumulative transitions hazards non-parametrically using Aalen-Johansen estimators[26–28]. P(0, t) can be estimated by a finite product of matrices over all K distinct transition times that partition [0, t] such that 0 = t0 < t1 < ⋯ < tK−1 < tK = t and where I is the 7 × 7 identity matrix and the ljth element of matrix ΔA(tk) is Alj(tk) − Alj(tk−1). The diagonal elements are computed as .
These transition probabilities P1j(0, t) are equivalent to marginal state occupancy probabilities P(Xt = j), the probability of being in state j at time t. We represented the estimated state occupancy probabilities over time as a set of stacked curves[15,16]. The area between the stacked curves or, equivalently, the area under each curve graphed separately, represents the estimated 102-monthrestricted mean months spent in each state[29–31]. We computed restricted mean time using Riemann sums, and calculated differences in 102-month-restricted mean months in each care continuum state for all pairwise alcohol group comparisons. To get 95% confidence intervals (CI) we selected the 2.5th and 97.5th percentile of 500 estimates based on analyses (exactly as described above) from 500 unrestricted resamples of individuals in our original dataset[37].
Because our analysis is descriptive, we highlight unadjusted results[32–34]. To aid in interpretation, we also present results adjusted for site, gender, race, HIV risk factor and VACS 2.0 score[21,22]. We used inverse probability of exposure weighting for adjustment[35,36]. For the 20% of patients with ≥1 missing baseline laboratory value, we multiply imputed 10 datasets using all covariates and exposure above and then averaged across all 10 datasets for the point estimate. We took 500 bootstrap resamples of the data within each multiply-imputed dataset and computed 5000 estimates over all resamples and multiply-imputed dataset. The 95% CIs were then the 2.5th and the 97.5th percentile of the 5000 estimates.
All statistical analyses were conducted in R version 3.6.2[38], using the packages etm v0.6–2.1[39], tidyverse v1.3–0[40], and DescTools v0.99.28[41].
Results
The study sample included 1,781 PWH, and was majority men (86%), white (54%), and MSM (71%). Median age was 35 years (interquartile range [IQR]: 28, 46). Median CD4 cell count was 367 (IQR: 182, 551). Baseline prevalence of unhealthy alcohol use was 38%; 37% reported moderate alcohol use, and 25% reported no use in the past year. No alcohol use was more common among women, older patients, and patients with heterosexual sex or IDU as HIV risk factors. Unhealthy alcohol use was more common among younger patients and white patients (Table 1).
Table 1.
Characteristics (number and percent unless otherwise specified) of antiretroviral therapy-naïve persons who enrolled in the Center for AIDS Clinical Research Network of Integrated Clinical Systems, 2011–2019, and who self-reported alcohol consumption within 1 year of enrollment, stratified by alcohol usea
| Baseline alcohol use | Total | |||
|---|---|---|---|---|
| No use | Moderate | Unhealthy | ||
| N | 448 | 662 | 671 | 1781 |
| Demographics | ||||
| Men (gender) | 348 (78) | 585 (88) | 589 (88) | 1522 (86) |
| Ageb | 41 (30, 51) | 35 (27, 45) | 32 (27, 43) | 35 (28, 46) |
| Race | ||||
| Black | 181 (40) | 267 (40) | 187 (28) | 635 (36) |
| White | 233 (52) | 316 (48) | 410 (61) | 959 (54) |
| Other | 34 (7) | 79 (12) | 74 (11) | 187 (11) |
| Hispanic ethnicity | 75 (17) | 79 (12) | 134 (20) | 288 (16) |
| HIV Risk Factor | ||||
| Heterosexual | 144 (32) | 121 (18) | 107 (16) | 372 (21) |
| IDU | 34 (8) | 15 (2) | 28 (4) | 77 (4) |
| MSM | 205 (46) | 470 (71) | 479 (71) | 1154 (65) |
| MSM + IDU | 36 (8) | 38 (6) | 36 (5) | 110 (6) |
| Other/Unknown | 29 (7) | 18 (3) | 21 (3) | 68 (4) |
| Calendar year of enrollmentb | 14 (12, 15) | 13 (12, 15) | 14 (12, 15) | 13 (12, 15) |
| Clinical variables | ||||
| Viral load [log10 copies/mL] | 4.7 (4.1, 5.2) | 4.7 (4.1, 5.2) | 4.6 (4.0, 5.1) | 4.6 (4.1, 5.1) |
| Missing | 32 (7) | 35 (5) | 44 (7) | 111 (6) |
| CD4 cell countb | 304 (109, 527) | 369 (180, 548) | 391 (237, 558) | 367 (182, 551) |
| Missing | 25 (6) | 32 (5) | 31 (5) | 88 (5) |
| Creatinine [mg/dL] | 0.9 (0.8, 1.0) | 0.9 (0.8, 1.0) | 0.9 (0.8, 1.0) | 0.9 (0.8, 1.0) |
| Missing | 59 (13) | 85 (13) | 82 (12) | 226 (13) |
| Hemoglobin [g/dL] | 13.2 (11.6, 14.4) | 14.0 (12.7, 15.0) | 14.3 (13.3, 15.2) | 14.0 (12.6, 15.0) |
| Missing | 65 (15) | 84 (13) | 92 (14) | 241 (14) |
| FIB-4 | 1.0 (0.7, 1.5) | 0.8 (0.6, 1.2) | 0.8 (0.6, 1.2) | 0.9 (0.6, 1.3) |
| Missing | 89 (20) | 142 (21) | 123 (18) | 354 (20) |
| BMI | 25.4 (22.6, 28.4) | 24.8 (22.2, 28.2) | 24.6 (22.2, 27.7) | 24.8 (22.3, 28.0) |
| Missing | 192 (43) | 314 (47) | 250 (37) | 756 (42) |
| Albumin [g/dl] | 4.1 (3.7, 4.4) | 4.2 (3.9, 4.5) | 4.4 (4.0, 4.6) | 4.2 (3.9, 4.5) |
| Missing | 63 (14) | 101 (15) | 105 (16) | 269 (15) |
| White blood cells [x 109/L] | 5.1 (3.9, 6.4) | 5.1 (4.0, 6.6) | 5.3 (4.2, 6.6) | 5.2 (4.1, 6.6) |
| Missing | 63 (14) | 79 (12) | 86 (13) | 228 (13) |
| eGFRb [mL/min] | 105 (87, 120) | 108 (96, 121) | 112 (97, 122) | 109 (95, 122) |
| Missing | 59 (13) | 85 (13) | 82 (12) | 226 (13) |
| VACS Index 2.0 | 68 (55, 82) | 60 (51, 75) | 57 (48, 67) | 61 (51, 74) |
| Missing | 229 (51) | 374 (56) | 335 (50) | 938 (53) |
Alcohol use categories were based on responses to the Alcohol Use Disorders Identification Test – Consumption questions (AUDIT-C) and standard cut-offs based on current gender: 1) no use (AUDIT-C=0); 2) moderate use (AUDIT-C>0 and <3 for women or AUDIT-C>0 and <4 for men); and 3) unhealthy use (AUDIT-C≥3 for women or AUDIT-C≥4 for men)
Median (interquartile range); 2-digit calendar year of enrollment
Patients were followed for a median of 4.7 years (IQR:2.9, 6.4). The proportions of the cohort estimated to be in each HIV care continuum state over time, stratified by baseline alcohol use, are presented in Figure 2. The majority of person-time was spent in care and virally suppressed and time spent in care and virally suppressed was similar across alcohol use groups. On average, this was 62.3 (95% CI: 58.3, 66.1) out of 102 months for people who reported no drinking at baseline, 61.1 (95% CI: 57.6, 64.2) months for people who reported moderate baseline drinking, and 59.5 (95% CI: 56.4, 62.7) months for people who reported unhealthy drinking at baseline. People who reported unhealthy drinking at baseline spent 6.2 fewer months in care than those who were not drinking at baseline (restricted mean months difference [RMMD]=−3.4, 95% CI: −5.6, −1.6 in care without viral suppression; RMMD=−2.8, 95% CI: −7.5, 2.3 in care with viral suppression). However, they were also less likely to die during follow-up (RMMD=−3.8, 95% CI: −5.7, −1.7 for death after ART initiation). In contrast, people with baseline unhealthy drinking spent 8.6 more months lost to clinic than baseline non-drinkers (RMMD=7.6, 95% CI: 3.1, 11.7 after ART initiation; RMMD=1.0, 95% CI: −0.6, 2.8 before ART initiation). Contrasts in time spent in each care continuum stage for moderate versus no baseline alcohol use followed a similar pattern. People who reported moderate versus unhealthy drinking did not differ significantly from one another in months spent in each state of the HIV care continuum over 102 months of follow-up (Table 2).
Figure 2.
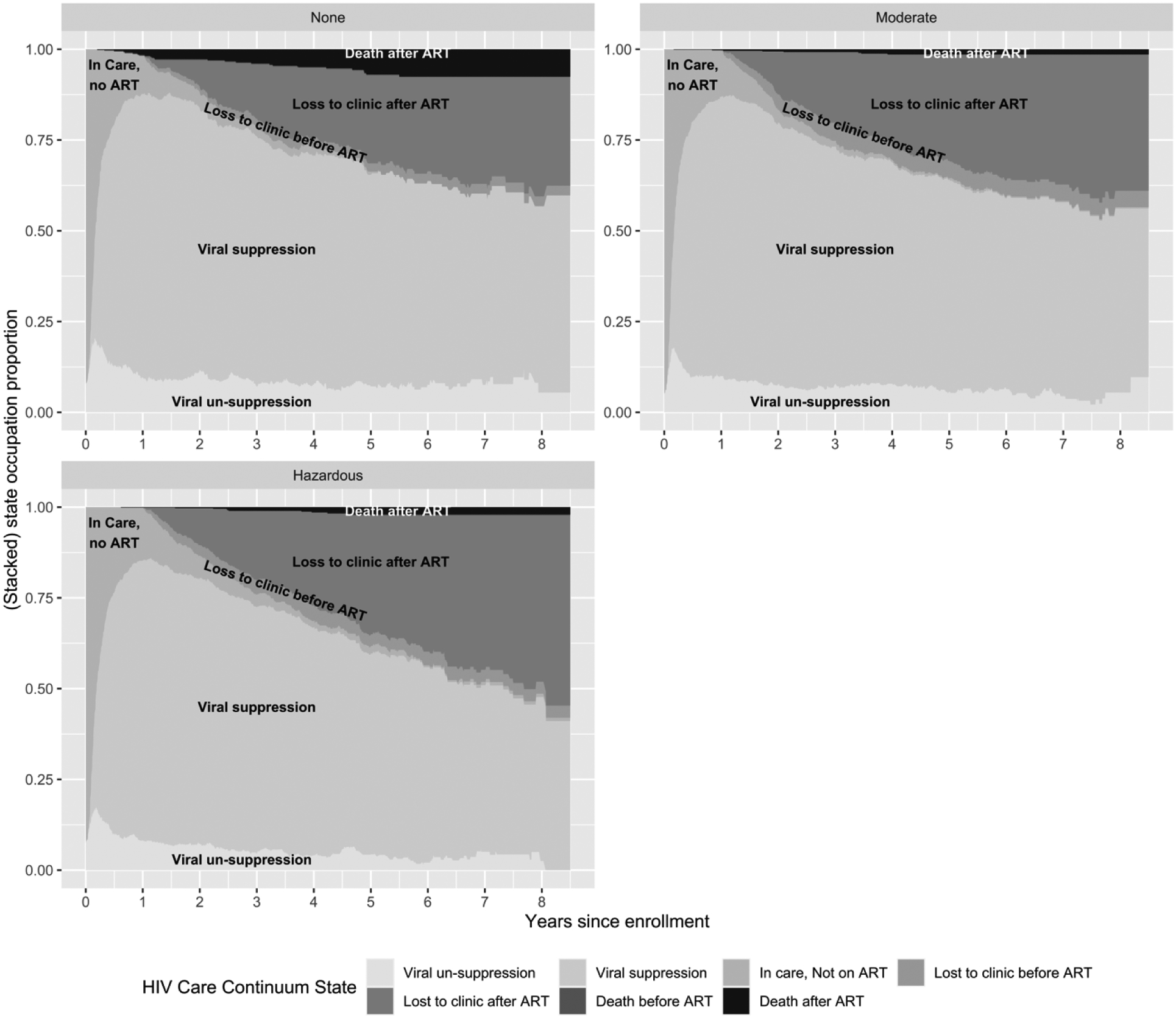
State occupation probabilities (proportion of the sample estimated to be in each HIV care continuum state) over 8.5 years of follow-up stratified by baseline alcohol use among 1781 antiretroviral therapy-naïve persons who enrolled in the Center for AIDS Clinical Research Network of Integrated Clinical Systems, 2011–2019, and who self-reported alcohol consumption within 1 year of enrollment
a Note that while the proportion of the cohort that experiences “Death before ART” is plotted in these figures, it is so small that is is not visible on the graphs
Table 2.
102-month (8.5-year) restricted mean number of months, and differences in restricted mean months (95% confidence intervals),a spent in each of the HIV care continuum stages by baseline alcohol use among 1781 antiretroviral therapy-naïve persons who enrolled in the Center for AIDS Clinical Research Network of Integrated Clinical Systems, 2011–2019, and who self-reported on their alcohol consumption within 1 year of enrollment
| Alcohol Use | Difference | |||||
|---|---|---|---|---|---|---|
| HIV care continuum stages | None | Moderateb | Unhealthyb | Moderate – None | Unhealthy – None | Unhealthy – Moderate |
| In care, not ART initiate | 5.1 (4.3, 5.9) | 5.4 (4.7, 6.3) | 6.6 (5.6, 7.8) | 0.3 (−0.8, 1.5) | 1.5 (0.1, 2.9) | 1.2 (−0.3, 2.6) |
| Lost to clinic before ART initiation | 2.1 (0.8, 3.2) | 3.9 (2.6, 5.3) | 3.1 (1.8, 4.5) | 1.8 (0.0, 3.8) | 1.0 (−0.6, 2.8) | −0.8 (−2.7, 0.9) |
| Dead before ART initiation | 0.2 (0.0, 0.7) | c | 0.1 (0.0, 0.4) | −0.2 (−0.7, 0.0) | −0.1 (−0.7, 0.4) | 0.1 (0.0, 0.4) |
| In care, not virally suppressed | 8.9 (7.3, 10.8) | 7.2 (5.9, 8.6) | 5.4 (4.4, 6.6) | −1.7 (−4.0, 0.5) | −3.4 (−5.6, −1.6) | −1.8 (−3.5, 0.1) |
| In care, virally suppressed | 62.3 (58.3, 66.1) | 61.1 (57.6, 64.2) | 59.5 (56.4, 62.7) | −1.2 (−6.0, 3.6) | −2.8 (−7.5, 2.3) | −1.6 (−6.1, 3.0) |
| Lost to clinic after ART initiation | 18.3 (15.2, 21.6) | 23.4 (20.6, 26.2) | 25.9 (23.3, 28.8) | 5.1 (0.8, 9.3) | 7.6 (3.1, 11.7) | 2.5 (−1.7, 6.9) |
| Dead after ART initiation | 5.1 (3.3, 6.9) | 1.1 (0.4, 1.8) | 1.3 (0.6, 2.2) | −4.0 (−6.1, −1.9) | −3.8 (−5.7, −1.7) | 0.2 (−0.8, 1.4) |
Abbreviations: ART, antiretroviral therapy
Difference estimates may differ slightly from direct subtractions of alcohol use estimates due to rounding
Moderate alcohol consumption defined as AUDIT-C score >0 and <3 for women and >0 and <4 for men; unhealthy alcohol consumption defined as AUDIT-C score ≥3 for women and ≥4 for men.
Among patients in the Moderate alcohol use group at baseline, there were no deaths prior to ART initiation (no transition to this state).
After adjustment, people with unhealthy alcohol use still spent more time lost to clinic (4.3 months) and less time retained in care (−3.7 months) than people with no baseline alcohol use, but the association between alcohol use and time spent in each care continuum stage was weaker and no associations were statistically significant or particularly clinically meaningfully (Table 3).
Table 3.
Adjusteda 102-month restricted mean number of months, and differences in restricted mean months (95% confidence intervals),b spent in each of the HIV care continuum stages by baseline alcohol use among 1781 antiretroviral therapy-naïve persons who enrolled in the Center for AIDS Clinical Research Network of Integrated Clinical Systems, 2011–2019, and who self-reported alcohol consumption within 1 year of enrollment
| Alcohol Use | Difference | |||||
|---|---|---|---|---|---|---|
| HIV care continuum stages | None | Moderatec | Unhealthyc | Moderate – None | Unhealthy – None | Unhealthy – Moderate |
| In care, not ART initiated | 5.7 (4.5, 6.9) | 5.6 (4.9, 6.7) | 6.1 (5.3, 7.4) | −0.1 (−1.5, 1.7) | 0.4 (−0.9, 2.3) | 0.5 (−0.8, 1.9) |
| Lost to clinic before ART initiation | 2.2 (0.8, 3.7) | 4.1 (2.7, 5.9) | 2.7 (1.6, 4) | 1.9 (−0.1, 4.2) | 0.5 (−1.3, 2.4) | −1.5 (−3.5, 0.4) |
| Dead before ART initiation | 0.2 (0, 0.5) | d | 0.1 (0, 0.4) | −0.2 (−0.5, 0.0) | 0.0 (−0.5, 0.3) | 0.1 (0.0, 0.4) |
| In care, not virally suppressed | 8.2 (6, 9.7) | 7.2 (5.9, 8.7) | 6 (4.8, 7.2) | −0.9 (−3.0, 1.7) | −2.2 (−4.1, 0.3) | −1.2 (−3.2, 0.5) |
| In care, virally suppressed | 61.7 (58.3, 67) | 59.7 (55.8, 62.9) | 60.3 (56.7, 63.6) | −2.0 (−9.1, 2.2) | −1.5 (−8.0, 3.2) | 0.5 (−4.1, 5.8) |
| Lost to clinic after ART initiation | 20.6 (16.3, 24.1) | 23.8 (20.8, 26.8) | 24.9 (21.9, 28.1) | 3.3 (−1.4, 8.7) | 4.3 (−0.2, 9.7) | 1.1 (−3.1, 5.5) |
| Dead after ART initiation | 3.5 (2, 5.1) | 1.5 (0.6, 3) | 2 (0.6, 3.3) | −2.0 (−3.8, 0.1) | −1.5 (−3.7, 0.4) | 0.5 (−1.6, 2.0) |
Abbreviations: ART, antiretroviral therapy; Mod., moderate
Adjusted for site, gender, race, HIV risk factor and VACS 2.0 score. The VACS 2.0 score incorporates age, sex, body mass index, CD4 cell count, estimated glomerular filtration rate (calculated from serum creatinine, age, sex, and race), HIV RNA, hemoglobin, alanine transaminase, aspartate aminotransferase, platelet count, white blood cell count, albumin, and hepatitis C virus infection.
Difference estimates may differ slightly from direct subtractions of alcohol use estimates due to rounding
Moderate alcohol consumption defined as AUDIT-C score >0 and <3 for women and >0 and <4 for men; unhealthy alcohol consumption defined as AUDIT-C score ≥3 for women and ≥4 for men.
Among patients in the Moderate alcohol use group at baseline, there were no deaths prior to ART initiation (no transition to this state).
Discussion
People who reported moderate or unhealthy drinking spent less time in care, on ART and more time lost to clinic across the first 102 months they were in HIV care compared to people who were not drinking at baseline. They also lived slightly longer. This may be because many of the people who reported no alcohol consumption may have been abstaining from alcohol due to an underlying health condition[11,42,43], which may have placed them at higher risk of mortality but made them more engaged in care due to additional care needs. Indeed, after adjusting for a robust set of clinical covariates, time spent in each care continuum state was neither clinically meaningfully nor statistically significantly different by baseline alcohol use.
While we did see patients with moderate or unhealthy alcohol use were more likely to be lost to care than patients with no alcohol use, we did not observe meaningful differences in the average time spent in different care continuum states between people who reported moderate versus unhealthy drinking, in contrast with some[6,10,44–47], but not all[48–50], prior studies that considered outcomes at only one time point. All current care continuums (including the longitudinal framework) measure loss to care (gaps in care) rather than poor engagement in care (e.g., less than one visit per year or missing visits without rescheduling) and poor engagement is more predictive of viral non-suppression and mortality than loss to care[51,52]. Alcohol use is associated poorer retention based different conceptualizations of retention in care including visit adherence and regular HIV primary care visits[53], but alcohol use is also associated with increased utilization of medical care in other studies[54]. Because our definition of care was based on healthcare utilization rather than missed visits, we likely overestimated total time “retained” and underestimated differences in the time spent engaged.
Compared to cross-sectional or prevalence care continuums, the longitudinal care continuum is more sensitive at detecting differences in time spent with a suppressed viral load, since transitions between suppressed and unsuppressed within a calendar year are captured by the longitudinal framework, but ignored in the prevalence framework[14]. However, the longitudinal framework also does not artificially inflate estimates of viral suppression by only calculating it among people who are retained in care. Thus, one explanation for why we did not observe large differences in the restricted mean time spent with a suppressed viral load associated with alcohol use could be that prior associations between alcohol use and viral suppression reported in cross-sectional studies were mostly driven by retention in care. That is, if patients who were lost to clinic were truly out of care and not silent transfers to another clinic, the differences we saw in time spent lost to clinic would be differences in time spent virally suppressed.
We observed a very high prevalence of alcohol use and unhealthy alcohol use at baseline. Unhealthy alcohol consumption among people with HIV is associated with many other comorbidities including liver disease, cardiovascular disease, premature aging, and metabolic complications[55–57]. Although we did not observe large disparities is HIV care outcomes for people consuming alcohol at moderate or unhealthy levels, reducing unhealthy alcohol consumption could improve health overall. Brief interventions to reduce alcohol use have had modest effects in this population[58,59]. Even increasing patients awareness of their alcohol consumption might improve health [59]. Furthermore, reductions in alcohol consumption short of abstinence may improve outcomes for people drinking at unhealthy levels[60].
A limitation of this work is our inability to distinguish between loss to clinic (possibly due to transfer to another clinic) and loss to care (and associated access to ART and clinical monitoring). However, while alcohol might influence loss to care, it is less likely to be associated with the probability of transferring care to another clinic and this should not dramatically alter our conclusions. Additionally, we only considered baseline alcohol use rather than time-updated alcohol use. Although alcohol consumption is fairly stable in this population[62], failure to account for time-updated alcohol use may have limited our ability to detect differences in HIV care engagement associated with recent alcohol use. However, because engagement in care might itself influence subsequent alcohol use, stratifying on baseline alcohol use provides information that is useful to target programmatic supports to patients upon enrollment or to allocate resources for retention and adherence support in the absence of interventions on alcohol use. As is appropriate for descriptive analyses[33,34], we have presented unadjusted results. Neither our unadjusted nor our adjusted results should be interpreted as the effect of drinking on HIV care continuum outcomes. Interventions on alcohol use may take many forms, and analyses intended to estimate causal effects should explicitly model the intervention to be implemented. Furthermore, our study sample was engaged in care (attended ≥2 clinic visits in a year) at the start of follow-up and our results may not generalize to PWH who never firmly link to care. Finally, future iterations of this type of investigation might consider different categorizations of alcohol use that incorporate history of alcohol use disorder given that, in prior work, alcohol use disorder appeared to interact with alcohol consumption to predict viral suppression[11].
In conclusion, any baseline alcohol use was weakly positively associated with the 102-month-restricted mean time spent alive, and negatively associated with time spent retained in clinic. People reporting moderate or unhealthy drinking at enrollment in HIV care could be targeted with interventions that promote retention in care.
Funding:
This study was supported by grants from the National Institutes of Health (U01 DA036935, U24 AA020801, P01 AA029544, K24 AA027483, and K01 AA028193).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CRediT authorship contribution statement:
Catherine R Lesko: conceptualization, writing – original draft, supervision, project administration; Jeanine S. Gnang: methodology, software, formal analysis, data curation; Anthony T. Fojo: writing – review & editing; Heidi E. Hutton: writing – review & editing; Mary E. McCaul: writing – review & editing; Joseph A. Delaney: writing – review & editing; Edward R. Cachay: resources, writing – review & editing; Kenneth H. Mayer: resources, writing – review & editing; Heidi M. Crane: resources, writing – review & editing; D. Scott Batey: writing – review & editing; Sonia Napravnik: resources, writing – review & editing; Katerina A. Christopoulos: resources, writing – review & editing; Bryan Lau: conceptualization, methodology, supervision, funding acquisition; Geetanjali Chander: conceptualization, writing – review & editing, supervision, project administration, funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- 1.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Insight Start Study Group, Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med 2015; 373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fauci AS, Redfield RR, Sigounas G, Weahkee MD, Giroir BP. Ending the HIV Epidemic: A Plan for the United States. JAMA 2019; 321:844–845. [DOI] [PubMed] [Google Scholar]
- 4.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clinical Infectious Diseases 2011; 52:793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenberg AE, Hader SL, Masur H, Young AT, Skillicorn J, Dieffenbach CW. Fighting HIV/AIDS in Washington, D.C. Health affairs 2009; 28:1677–87. [DOI] [PubMed] [Google Scholar]
- 6.Chander G, Lau B, Moore RD. Hazardous alcohol use: a risk factor for non-adherence and lack of suppression in HIV infection. Journal of acquired immune deficiency syndromes (1999) 2006; 43:411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crane HM, McCaul ME, Chander G, Hutton H, Nance RM, Delaney JAC, et al. Prevalence and Factors Associated with Hazardous Alcohol Use Among Persons Living with HIV Across the US in the Current Era of Antiretroviral Treatment. AIDS Behav 2017; 21:1914–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galvan FH, Bing EG, Fleishman JA, London AS, Caetano R, Burnam MA, et al. The prevalence of alcohol consumption and heavy drinking among people with HIV in the United States: results from the HIV Cost and Services Utilization Study. J Stud Alcohol 2002; 63:179–86. [DOI] [PubMed] [Google Scholar]
- 9.Hendershot CS, Stoner SA, Pantalone DW, Simoni JM. Alcohol use and antiretroviral adherence: review and meta-analysis. Journal of acquired immune deficiency syndromes (1999) 2009; 52:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lesko CR, Nance RM, Lau B, Fojo AT, Hutton HE, Delaney JAC, et al. Changing Patterns of Alcohol Use and Probability of Unsuppressed Viral Load Among Treated Patients with HIV Engaged in Routine Care in the United States. AIDS Behav 2021; 25:1072–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lesko CR, Hutton HE, Edwards JK, McCaul ME, Fojo AT, Keruly JC, et al. Alcohol Use Disorder and Recent Alcohol Use and HIV Viral Non-Suppression Among People Engaged in HIV Care in an Urban Clinic, 2014–2018. AIDS Behav Published Online First: 9 October 2021. doi: 10.1007/s10461021-03487-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howe CJ, Robinson WR. Survival-related selection bias in studies of racial health disparities: the importance of the target population and study design. Epidemiology (Cambridge, Mass) 2018; 29:521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howe CJ, Cole SR, Lau B, Napravnik S, Eron JJ Jr. Selection Bias Due to Loss to Follow Up in Cohort Studies. Epidemiology 2016; 27:91–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lesko CR, Chander G, Moore RD, Lau B. Variation in estimated viral suppression associated with the definition of viral suppression used. Aids 2020; 34:1519–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lesko CR, Edwards JK, Moore RD, Lau B. A longitudinal, HIV care continuum: 10-year restricted mean time in each care continuum stage after enrollment in care, by history of injection drug use. AIDS 2016; 30:2227–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haber NA, Lesko CR, Fox MP, Powers KA, Harling G, Edwards JK, et al. Limitations of the UNAIDS 90-90-90 metrics: a simulation-based comparison of cross-sectional and longitudinal metrics for the HIV care continuum. AIDS 2020; 34:1047–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Powers KA, Miller WC. Building on the HIV Cascade: A Complementary “HIV States and Transitions” Framework for Describing HIV Diagnosis, Care, and Treatment at the Population Level. J Acquir Immune Defic Syndr 2015; 69:341–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Powers KA, Samoff E, Weaver MA, Sampson LA, Miller WC, Leone PA, et al. Longitudinal HIV care trajectories in North Carolina. J Acquir Immune Defic Syndr 2017; 74:S88–S95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mugavero MJ, Amico KR, Horn T, Thompson MA. The State of Engagement in HIV Care in the United States: From Cascade to Continuum to Control. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America Published Online First: 23 June 2013. doi: 10.1093/cid/cit420 [DOI] [PubMed] [Google Scholar]
- 20.Kitahata MM, Rodriguez B, Haubrich R, Boswell S, Mathews WC, Lederman MM, et al. Cohort profile: the Centers for AIDS Research Network of Integrated Clinical Systems. International journal of epidemiology 2008; 37:948–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGinnis KA, Justice AC, Moore RD, Silverberg MJ, Althoff KN, Karris M, et al. Discrimination and Calibration of the Veterans Aging Cohort Study Index 2.0 for Predicting Mortality Among People With Human Immunodeficiency Virus in North America. Clinical Infectious Diseases 2022; 75:297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tate JP, Sterne JAC, Justice AC, Veterans Aging Cohort Study (VACS) and the Antiretroviral Therapy Cohort Collaboration (ART-CC). Albumin, white blood cell count, and body mass index improve discrimination of mortality in HIV-positive individuals. AIDS 2019; 33:903–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qian Y, Moore RD, Coburn SB, Davy-Mendez T, Akgün KM, McGinnis KA, et al. Association of the VACS Index With Hospitalization Among People With HIV in the NA-ACCORD. J Acquir Immune Defic Syndr 2022; 89:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Archives of internal medicine 1998; 158:1789–1795. [DOI] [PubMed] [Google Scholar]
- 25.Bradley KA, DeBenedetti AF, Volk RJ, Williams EC, Frank D, Kivlahan DR. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcoholism: Clinical and Experimental Research 2007; 31:1208–1217. [DOI] [PubMed] [Google Scholar]
- 26.Cole SR, Lau B, Eron JJ, Brookhart MA, Kitahata MM, Martin JN, et al. Estimation of the standardized risk difference and ratio in a competing risks framework: application to injection drug use and progression to AIDS after initiation of antiretroviral therapy. American journal of epidemiology 2015; 181:238–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borgan O Aalen-Johansen estimator. Encyclopedia of Biostatistics 1998; 1:5–10. [Google Scholar]
- 28.Aalen OO, Johansen S. Empirical Transition Matrix for Nonhomogeneous Markov-Chains Based on Censored Observations. Scand J Stat 1978; 5:141–150. [Google Scholar]
- 29.Calkins KL, Canan CE, Moore RD, Lesko CR, Lau B. An application of restricted mean survival time in a competing risks setting: comparing time to ART initiation by injection drug use. BMC Med Res Methodol 2018; 18:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Royston P, Parmar MK. Restricted mean survival time: an alternative to the hazard ratio for the design and analysis of randomized trials with a time-to-event outcome. BMC Med Res Methodol 2013; 13:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao L, Claggett B, Tian L, Uno H, Pfeffer MA, Solomon SD, et al. On the restricted mean survival time curve in survival analysis. Biometrics 2016; 72:215–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zalla LC, Martin CL, Edwards JK, Gartner DR, Noppert GA. A geography of risk: Structural racism and COVID-19 mortality in the United States. American Journal of Epidemiology 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaufman JS. Statistics, Adjusted Statistics, and Maladjusted Statistics. Am J Law Med 2017; 43:193–208. [DOI] [PubMed] [Google Scholar]
- 34.Lesko CR, Fox MP, Edwards JK. A framework for descriptive epidemiology. Am J Epidemiol 2022; :kwac115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology 2000; 11:561–70. [DOI] [PubMed] [Google Scholar]
- 36.Cole SR, Hernan MA. Constructing Inverse Probability Weights for Marginal Structural Models. American Journal of Epidemiology 2008; 168:656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Efron B, Tibshirani R. An introduction to the bootstrap. New York: Chapman & Hall; 1993. Publisher description http://www.loc.gov/catdir/enhancements/fy0730/93004489-d.html [Google Scholar]
- 38.R Core Team. R: A language and environment for statistical computing. https://www.R-project.org/
- 39.Allignol A, Schumacher M, Beyersmann J. Empirical Transition Matrix of Multi-State Models: The etm Package. Journal of Statistical Software 2011; 38:1–15. [Google Scholar]
- 40.Wickham H, Averick M, Bryan J, Chang W, McGowan LD, François R, et al. Welcome to the Tidyverse. Journal of Open Source Software 2019; 4:1686. [Google Scholar]
- 41.Signorell A, Aho K, Alfons A, Anderegg N, Aragon T, Arachchige C, et al. DescTools: Tools for Descriptive Statistics. 2021.https://CRAN.R-project.org/package=DescTools (accessed 28 Dec2021).
- 42.Marshall BDL, Tate JP, McGinnis KA, Bryant KJ, Cook RL, Edelman EJ, et al. Long-term alcohol use patterns and HIV disease severity. AIDS 2017; 31:1313–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crane HM, Nance RM, Merrill JO, Hutton H, Chander G, McCaul ME, et al. Not all non-drinkers with HIV are equal: demographic and clinical comparisons among current non-drinkers with and without a history of prior alcohol use disorders. AIDS Care 2017; 29:177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams EC, McGinnis KA, Edelman EJ, Matson TE, Gordon AJ, Marshall BDL, et al. Level of Alcohol Use Associated with HIV Care Continuum Targets in a National U.S. Sample of Persons Living with HIV Receiving Healthcare. AIDS Behav 2019; 23:140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Samet JH, Cheng DM, Libman H, Nunes DP, Alperen JK, Saitz R. Alcohol consumption and HIV disease progression. J Acquir Immune Defic Syndr 2007; 46:194–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barai N, Monroe A, Lesko C, Lau B, Hutton H, Yang C, et al. The Association Between Changes in Alcohol Use and Changes in Antiretroviral Therapy Adherence and Viral Suppression Among Women Living with HIV. AIDS Behav 2017; 21:1836–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cook R, Zhou Z, Kelso-Chichetto N, Janelle J, Morano J, Somboonwit C, et al. Alcohol consumption patterns and HIV viral suppression among persons receiving HIV care in Florida: an observational study. Addiction science & clinical practice 2017; 12:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalichman SC, Grebler T, Amaral CM, McNerney M, White D, Kalichman MO, et al. Viral suppression and antiretroviral medication adherence among alcohol using HIV-positive adults. International journal of behavioral medicine 2014; 21:811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Conen A, Wang Q, Glass TR, Fux CA, Thurnheer MC, Orasch C, et al. Association of Alcohol Consumption and HIV Surrogate Markers in Participants of the Swiss HIV Cohort Study. JAIDS Journal of Acquired Immune Deficiency Syndromes 2013; 64:472–478. [DOI] [PubMed] [Google Scholar]
- 50.Wu ES, Metzger DS, Lynch KG, Douglas SD. Association between alcohol use and HIV viral load. Journal of acquired immune deficiency syndromes (1999) 2011; 56:e129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mugavero MJ, Westfall AO, Zinski A, Davila J, Drainoni ML, Gardner LI, et al. Measuring retention in HIV care: the elusive gold standard. Journal of acquired immune deficiency syndromes 2012; 61:574–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lesko CR, Mugavero MJ, Shen NM, Fojo AT, Moore RD, Keruly JC, et al. Exploring definitions of retention in care for people living with HIV in the United States in the modern treatment era. AIDS 2022; 36:1181–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Monroe AK, Lau B, Mugavero MJ, Mathews WC, Mayer KC, Napravnik S, et al. Heavy Alcohol Use is Associated with Worse Retention in HIV Care. J Acquir Immune Defic Syndr 2016; 73:419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Azar MM, Springer SA, Meyer JP, Altice FL. A systematic review of the impact of alcohol use disorders on HIV treatment outcomes, adherence to antiretroviral therapy and health care utilization. Drug and Alcohol Dependence 2010; 112:178–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barve S, Kapoor R, Moghe A, Ramirez JA, Eaton JW, Gobejishvili L, et al. Focus on the liver: alcohol use, highly active antiretroviral therapy, and liver disease in HIV-infected patients. Alcohol Res Health 2010; 33:229–236. [PMC free article] [PubMed] [Google Scholar]
- 56.Williams EC, Hahn JA, Saitz R, Bryant K, Lira MC, Samet JH. Alcohol Use and Human Immunodeficiency Virus (HIV) Infection: Current Knowledge, Implications, and Future Directions. Alcohol Clin Exp Res 2016; 40:2056–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Justice A, Sullivan L, Fiellin D. HIV/AIDS, Comorbidity, and Alcohol. Alcohol Res Health 2010; 33:258–266. [PMC free article] [PubMed] [Google Scholar]
- 58.McCaul ME, Hutton HE, Cropsey KL, Crane HM, Lesko CR, Chander G, et al. Decreased Alcohol Consumption in an Implementation Study of Computerized Brief Intervention among HIV Patients in Clinical Care. AIDS Behav 2021; 25:4074–4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chander G, Hutton HE, Lau B, Xu X, McCaul ME. Brief Intervention Decreases Drinking Frequency in HIV-Infected, Heavy Drinking Women: Results of a Randomized Controlled Trial. J Acquir Immune Defic Syndr 2015; 70:137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gastfriend DR, Garbutt JC, Pettinati HM, Forman RF. Reduction in heavy drinking as a treatment outcome in alcohol dependence. Journal of Substance Abuse Treatment 2007; 33:71–80. [DOI] [PubMed] [Google Scholar]
- 61.Buskin SE, Kent JB, Dombrowski JC, Golden MR. Migration Distorts Surveillance Estimates of Engagement in Care: Results of Public Health Investigations of Persons Who Appear to be Out of HIV Care. Sexually transmitted diseases 2014; 41:35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bilal U, McCaul ME, Crane HM, Mathews WC, Mayer KH, Geng E, et al. Predictors of Longitudinal Trajectories of Alcohol Consumption in People with HIV. Alcohol Clin Exp Res 2018; 42:561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]


