Abstract
Background:
There is a bidirectional link between diabetes and periodontal disease. Control of active periodontal infection is an essential requisite to maintain optimal oral and systemic health in diabetic patients. The purpose of this study was to evaluate the efficacy of subgingival irrigation and powered toothbrush as home care maintenance protocol in type 2 diabetic patients with active periodontal disease compared to routine oral hygiene.
Materials and Methods:
Forty (n = 40) diabetic (HbA1c >7%) patients were enrolled in this parallel, examiner-blind, interventional clinical trial. Patients were randomized into two groups: Group A (sonic toothbrush and irrigation with water twice daily) or Group B (manual toothbrush and mouth rinsing with 0.12% Chlorhexidine gluconate (CHX) twice daily)). All patients received Phase I therapybefore the start of the study. Clinical parameters (plaque index [PI], gingival index [GI], oral hygiene index [OHI], pocket depth [PD], clinical attachment level [CAL], and bleeding index [BI]) were assessed at baseline, 1, 2, and 4 months. Levels of C-reactive protein (CRP), HbA1c, and interleukin (IL)-1 β were assessed at baseline and 4 months only. Verbal and written instructions were provided to each subject specific to their intervention allocation. Descriptive, parametric, and nonparametric analyses were used where appropriate.
Results:
Sixteen (n = 16) patients in Group A and fifteen (n = 15) patients in Group B completed the 4-month study. Both groups showed a significant difference in BI, PD, CAL, and HbA1c from baseline to 4 months. There were no differences within groups for OHI, GI, or PI and CRP, IL-1 β. The results are based on an underpowered study due to the drop out of 9 patients reducing the number below the needed 19 patients per group based on the power analysis.
Conclusion:
Results from this study provide information for future studies on self-care regimens for individuals living with Type 2 diabetes.
Keywords: Chlorhexidine digluconate, diabetes, gingival crevicular fluid, hyperglycemia, periodontitis, subgingival irrigation
INTRODUCTION
Periodontitis is a polymicrobial, multifactorial host-mediated inflammatory condition that affects supporting tissues of the periodontium. Diabetes mellitus is a heterogeneous syndrome with impaired glucose tolerance and impaired lipid and carbohydrate metabolism. Both of these chronic multifactorial conditions share a bidirectional relationship and modify host immune response by elevating pro-inflammatory cytokines, upregulating inflammatory cells, and finally initiating damage to tissue architecture.[1-4]
Chronic periodontal condition, like any other infection caused by Gram-negative bacteria adds on to the systemic inflammatory condition in diabetics and leads to exacerbate insulin resistance in the body. This perpetuated hyperglycemic state, caused by periodontal inflammation, is known to further worsen the glycemic status and enhances the associated complications of diabetes.[5-8]
Several systematic reviews from the literature indicate that periodontal therapy has an impact on HbA1c levels in blood, thus improving the metabolic control of the patient.[9-11] Diabetes and periodontal disease are both chronic diseases, so in addition to the active therapy of the disease conditions, a consistent maintenance therapy is imperative for the overall health of the patient. Since diabetic patients are always at increased risk of disease progression; hence, these patients demand particularly an effective supportive periodontal therapy.[12,13] During the maintenance phase, mechanical home-care methods, i.e., brushing of the teeth have long been considered as the best method for maintaining oral hygiene by the patients. However, mechanical methods are only reasonably effective in various inaccessible areas of the mouth, i.e., in deep developmental grooves, furcations, interproximal areas, malposed teeth, bridge work, orthodontic appliances, etc., Chemical plaque control/antimicrobials methods have been reported as an effective adjunct to mechanical methods in these clinical situations.[14,15] Chemical antiplaque agents being effective against pathogens modify the oral environment and inhibit plaque growth and plaque-associated inflammation. Chlorhexidine digluconate (CHX) is a time-tested oral antiseptic for chemical plaque control for five decades. CHX has a broad bactericidal and bacteriostastic spectrum with high substantivity up to 12 h.[14,15] When CHX is used as a mouthrinse, it has minimal effect on subgingival plaque because it does not penetrate pockets to any significant extent as rinsing delivers the solution to only 4% of pocket depth (PD).[16,17]
Gingival irrigation with different delivery tips has repeatedly been shown to. Reduce clinical signs of inflammation and host modulation.[12,18,19] Evidence from literature depicts that a standard jet tip penetrates 29% to 71% depth in pockets up to 7 mm and 44% to 68% in pockets ≥7 mm.[20,21] However, the subgingival jet tip showed delivery of 90% in pockets ≤6 mm and 64% in pockets ≥7 mm.
Presently, the “Standard of Care” for periodontitis patients with diabetes is nonsurgical therapy followed by surgical therapy depending on the severity of the disease. However, this particular group of patients, because of their systemic limitations, more often than not, is not recommended for elective surgery on an immediate basis. Presently, there is no standard recommended protocol on which these patients should be put on till the time they are deemed fit for surgery.
Hence, it was hypothesized that the use of home irrigation with a subgingival jet tip with water, along with a powered toothbrush, twice daily as an adjunct after scaling and root planning in type 2 diabetic patients suffering from periodontal disease should help in controlling active disease state in diabetic patients as compared to diabetic patients on routine home care comprising chlorhexidine gluconate rinses (0.12%) combined with manual tooth brushing twice daily. The use of water as the solution with the oral irrigator was based on the results reported by Al-Mubarak et al.[12] and Cutler et al.[18] who found a significant reduction in clinical parameters and pro-inflammatory mediators in diabetic or periodontal patients compared to routine oral hygiene.
The present study aimed to evaluate the efficacy of subgingival home irrigation using water along with powered toothbrushes (performed twice a day) as home care maintenance regimen on clinical and biochemical parameters in diabetic patients with chronic periodontitis.
MATERIALS AND METHODS
This study was conducted following Good Clinical Practice guidelines and ethical principles of the Declaration of Helsinki for medical research involving the human participants act (WMO), and applicable local regulations in the area. The study was approved by “Panjab University Institutional Ethics Committee (PUIEC), Panjab University, Chandigarh. (PUIEC/2018/125/A-1/29/10) The study has been registered in clinical trial registry, India –CTRI/ 2019/07/020444.
The study was conducted at Dr. Harvansh Singh Judge Institute of Dental Sciences and Hospital, Panjab University, Chandigarh. Patients referred to the department of periodontics with Type 2 diabetes were informed of the study background, objectives, duration, and requirements of the study. If they wished to participate they were screened and if they met the criteria, read and signed a consent form were enrolled in the study. Patients were assigned a unique identifier to ensure anonymity.
This was a single-center, randomized, parallel, two groups, examiner-blind, interventional clinical trial. Randomization was done by the statistician using the coin-flipping method. The study coordinator (AJ) was responsible for allocation concealment. No stratification was applied. The examiner (VG), team members (JG, VK, MM, MG, DML) and recorder (JK) were blinded to the randomized allocation of the subjects. Any documents that contain the information was kept away from the examiner and team and secured in a locked location. Patients were told not to discuss their regimen with the examiner and team. After going through the literature and setting Type 1 error at 0.05 and power of study at 80%, the sample size in order to detect a difference of 12.4% between experimental and control groups in the percentage of bleeding on probing was estimated to be 19 in each group.
Forty (n = 40) diabetic patients with HbA1c >7% and generalized chronic periodontitis clinically diagnosed with the presence of probing PD of ≥5 mm that bleed on probing, and clinical attachment loss of 4 mm or more in at least two different quadrants were considered for the study. Patients had to be under a doctor’s care for their diabetes of >1 year, taking the same oral-hypoglycemic agents or insulin injections in combination or separately over the past 6 months, and informed of proper diet recommendations. Patients were otherwise systemically healthy, between the age of 30 and 60 years, had no history of allergy to chlorhexidine (CHX), and were on medication that would interfere with the study (nonsteroidal, anti-inflammatory drugs, anticoagulants, or antibiotics in the past 4 months, immunosuppressive drugs or ascorbic acid supplements) or had any periodontal therapy in the 6 monthsbefore the study. Pregnant or lactating women, smokers, alcoholics, and anyone with a severe chronic disease (cardiovascular, renal, hepatic, and immunological disorders) were excluded from the study. A complete medical history and full mouth clinical examination was performed.
Group A (Test/Experimental group): Subgingival home irrigation with 0.06% chlorhexidine gluconate along with a powered toothbrush (both performed twice daily).
Group B (Control group): Chlorhexidine gluconate rinses (0.12%) along with manual toothbrushing (both performed twice daily).
They were instructed to carry out the home care regimen according to the group allocated.
Study products were dispensed by one of the study member clinicians. Patients in study group A were provided with a water flosser with the sonic brush (Waterpik CC-01 Complete Care 9.0) All Patients in group B were provided a standard manual toothbrush (Oral-B Indicator Plus–Procterand Gamble, São Paulo, SP, and Brazil) and standard fluoride toothpaste (Colgate® Cavity Protection toothpaste, Colgate-Palmolive, colgate. com).(0.12%) mouthrinse (Hexidine EP, ICPA, India). CHX is a broad-spectrum bactericidal and bacteriostatic topical agent with high substantivity up to 12 h. It is a positively charged molecule, readily absorbed on a negatively charged surface such as intraoral mucosal membranes, tongue, salivary pellicle, biofilm components, including bacteria. The study subjects were instructed to use 15 ml of 0.12% CHX as an oral rinse for 1 min twice daily 10 min after brushing.[22]
The oral hygiene index-simplified (OHI-S),[23] plaque index (PI),[24] gingival index (GI),[24] gingival bleeding index (BI),[25] PD,[26] and clinical attachment level (CAL)[26] were recorded at baseline, 1st month, 2nd month and 4th month as primary outcome measurements. OHI-S recorded debris (DI-S) and calculus (CI-S) with the help of explorer running around the surface area of the index tooth (16, 11, 26, 36, 31, 41). Scores obtained were added and divided by the total number of index teeth to get the final score.[23]
For assessment of plaque, four gingival areas (mesiobuccal, midbuccal, distobuccal, lingual) per index tooth (16, 12, 24, 36, 32, 44) were examined with the help of dental explorer. PI was assessed by obtaining scores per gingival site were summed and divided by the total number of index tooth to give the final score.[24] GI assessment was carried out with the help of manual periodontal probe at four gingival sites per index tooth (16, 12, 24, 36, 32, 44) for the assessment of bleeding. In case index tooth was found missing, then full mouth GI scoring was done. The scores obtained from four areas per index tooth were summed and divided by the total number of index tooth to give the final score.[24]
Gingival bleeding index was performed by gently probing the sulcus, and moving the probe horizontally along the pocket wall in a stroking motion on labial and lingual marginal gingiva as well as mesial and distal papillary gingiva around each tooth using a William’s periodontal probe. Within the period of 10 s, if bleeding occurred a positive finding was recorded. The number of positive sites for bleeding was recorded and then expressed as a percentage of the number of sites examined.[25] PD was recorded by William’s periodontal probe, keeping it parallel to the long axis of tooth and walking it circumferentially in the sulcus around the tooth. Measurements were recorded at six sites (mesiobuccal, midbuccal, distobuccal, mesiolingual, midlingual, and distolingual) per tooth. PD was calculated as the distance of probe penetration up to the base of the pocket from the gingival margin. PDs around each tooth were added and divided by the total teeth examined to give the mean PD of the subject.[26]
For CAL, first gingival margin level (GML) was recorded with a William probe. GML was measured as the distance between the gingival margin and a fixed reference point on the tooth, i.e., CEJ at six sites (mesiobuccal, midbuccal, distobuccal, mesiolingual, midlingual, and distolingual) per tooth. GML was subtracted from PD to get CAL of each tooth. Measurements of each tooth were added and divided by the total teeth examined to get mean CAL s of the subject.[26]
An assessment of tooth staining was also done using modified Lobene Stain Index, recording intensity and area affected by staining. Stain levels were calculated using intensity scores and I × A scores. The modified Lobene staining index was assessed in 4th month in both groups for intergroup comparison only at the end of the study.[27]
HbA1c and C-reactive protein (CRP) were assessed as secondary outcome measures at baseline and 4 month visits in venous blood samples. The flexor surface of the patient’s nondominant arm was prepped with an alcohol swab. Using a disposable syringe, 2 ml of venous blood was drawn from the antecubital fossa into the syringe. The blood sample was sent to the laboratory for the measurement of CRP and blood glucose levels (HbA1c) using a reference analyzer.
The supragingival plaque was removed from the test area and was isolated with cotton rolls and dried with a gentle stream of air. Gingival crevicular fluid (GCF) was collected from the deepest periodontal pocket by placing a calibrated Hirschmann’s micro capillary pipette outside the margin of gingival sulcus for 60 s. Using a calibrated Hirschmann’s micro-capillary pipettes.[28] Samples that contained blood or plaque were discarded. 2 μl of collected GCF sample was collected, and stored at −70°C till the day of biochemical assessment. The concentrations of interleukin (IL)-1 β were determined using commercially available enzyme-linked immunosorbent assays (Weldon biotech, India) according to the manufacturers’ instructions.
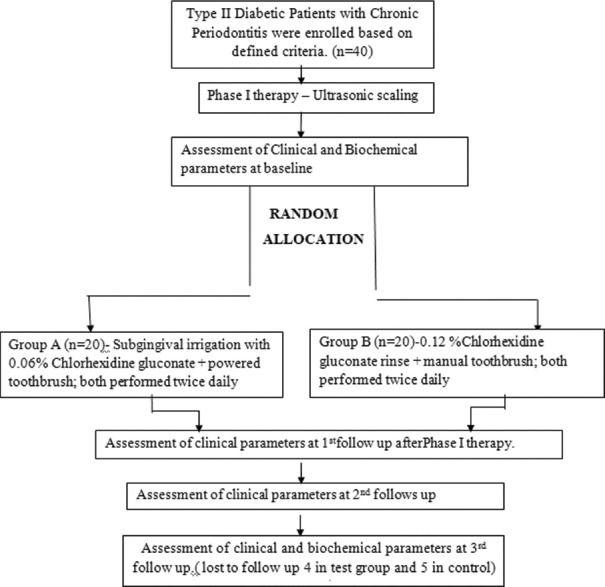
Figure 1 shows the study procedure from recruitment to the end of the study. A medical history was completed and reviewed. Intra- and extra-oral examinations were performed, and inclusion and exclusion criteria were reviewed at the screening appointment. If the patient met the criteria and was willing to participate, they were enrolled in the study and scheduled for 4 visits.
Figure 1.
Flow chart depicting chronological order of procedures done during study period. n – number of study subjects
All study participants received scaling and root planning, i.e., Phase I therapy. Baseline parameters were assessed 1 week following Phase I therapy and the patients were randomly assigned into two groups. Data were collected at baseline, 1 month, 2 months, and 4 months and documented on case report forms. All the biochemical parameters were assessed as secondary outcome measures at two follow-ups only, i.e., the baseline and the end of the study period, i.e., 4th month. Subjects were told to not use any other oral hygiene devices or agents during the study.
If any further deterioration was seen in the periodontal attachment apparatus, as evidenced by the increase in attachment levels ≥2 mm during the course of the study, patient was exited from the study and referred for periodontal therapy.
Statistical analysis
Quantitative data were reported as mean ± standard deviation and were analyzed with an independent sample t-test for any significant differences at the baseline, i.e., outset of the study, if any. The pairwise inter-group comparison of clinical parameters for groups at different observational periods were estimated using Student Paired t-test. The intergroup comparison for all study parameters at different observational periods was analyzed using Mann–Whitney test. The correlations between study parameters were calculated using spearman correlation test. The trends of individual clinical parameters were analyzed with the general linear model. All the above-mentioned analysis was conducted using IBM Statistical Package for the Social Sciences version 22.0 software (IBM Corp.; Armonk, NY, USA). The P < 0.05 was considered as significant. There was no change to the planned study protocol.
RESULTS
Out of 40 patients included in the study, a total of nine patients (4 in the test group, 5 in the control group) were lost to follow-up due to transfer to another city and concurrent flare-up of dental emergencies. The baseline data of both study groups regarding demographic details and study parameters are summarized in Table 1. In Group A, a statistically significant difference was seen in pairwise comparisons of BI at 1st, 2nd, and 3rd follow-ups, respectively [Table 2; however Group B, such improvements were not observed in 1st follow-up, but in 2nd and 3rd follow-up intervals only [Table 3]. For PD and CAL, pairwise comparisons in Group A revealed a statistically significant difference from baseline to all-time points, Whereas, in Group B, a statistically significant difference was seen only at 3rd follow-up and for PD at 1st follow-up also [Tables 2 and 3]. No statistically significant difference was observed in pairwise comparison of OHI, PI, and GI in both groups at 1st, 2nd, and 3rd follow-ups respectively [Tables 2 and 3].
Table 1.
Comparative analysis of the mean±standard deviation values of demographic data and study parameters at baseline in Group A and B
| Variables | Mean±SD values | Independent samples t-test | |||
|---|---|---|---|---|---|
|
|
|
||||
| Group A (16) | Group B (15) | Variance | F | Significance (two-tailed) | |
| Age | 50.13±6.6 | 55.07±9.83 | Equal variances assumed | 2.706 | 0.110 |
| Equal variances not assumed | 0.116 | ||||
| Duration of diabetes | 7.5±5.7 | 7.3±3.3 | Equal variances assumed | 4.138 | 0.906 |
| Equal variances not assumed | 0.905 | ||||
| OHI | 0.77±0.28 | 1.2±1.07 | Equal variances assumed | 4.909 | 0.015 |
| Equal variances not assumed | 0.017 | ||||
| PI | 1.01±0.34 | 1.13±0.2820 | Equal variances assumed | 8.150 | 0.134 |
| Equal variances not assumed | 0.130 | ||||
| GI | 1.16±0.24 | 1.20±0.22 | Equal variances assumed | 0.121 | 0.635 |
| Equal variances not assumed | 0.636 | ||||
| BI (%) | 51.6±20.8 | 48.8±21.5 | Equal variances assumed | 6.680 | 0.765 |
| Equal variances not assumed | 0.768 | ||||
| PD (mm) | 2.92±0.52 | 2.91±0.733 | Equal variances assumed | 0.029 | 0.388 |
| Equal variances not assumed | 0.392 | ||||
| CAL (mm) | 3.91±1.13 | 4.16±1.67 | Equal variances assumed | 0.645 | 0.389 |
| Equal variances not assumed | 0.395 | ||||
| HbA1c (%) | 8.72±1.43 | 9.3±1.61 | Equal variances assumed | 1.803 | 0.257 |
| Equal variances not assumed | 0.260 | ||||
| CRP (mg/l) | 2.67±3.11 | 2.79±2.14 | Equal variances assumed | 0.430 | 0.900 |
| Equal variances not assumed | 0.899 | ||||
| Il-1β (ng/ml) | 8.92±11.9 | 9.56±4.02 | Equal variances assumed | 1.935 | 0.946 |
| Equal variances not assumed | 0.946 | ||||
OHI – Oral hygiene index; PI – Plaque index; GI – Gingival index; BI – Bleeding index; PD – Pocket depth; CAL – Clinical attachment loss; HbA1c – Glycosylated hemoglobin; CRP – C-reactive protein; Il-1β – Interleukin-1β; SD – Standard deviation; F – Test statistic (F distribution)
Table 2.
Intragroup comparison of pairwise mean difference of clinical parameters in Group A at different times of observation
| Group | Time (I) | Time (J) | Mean difference (I-J) | SE | P |
|---|---|---|---|---|---|
| Group A | OH10 | OHI1 | −0.181 | 0.100 | 0.902 |
| OH12 | −0.288 | 0.129 | 0.421 | ||
| OHI3 | −0.400 | 0.125 | 0.059 | ||
| PI0 | PI1 | −0.074 | 0.030 | 0.242 | |
| PI2 | −0.069 | 0.033 | 0.514 | ||
| PI3 | −0.118 | 0.038 | 0.071 | ||
| GI0 | GI1 | 0.056 | 0.055 | 1.000 | |
| GI2 | 0.091 | 0.055 | 1.000 | ||
| GI3 | 0.117 | 0.064 | 0.882 | ||
| BI0 (%) | BI1 | 16.113* | 4.657 | 0.035 | |
| BI2 | 22.900* | 3.614 | 0.000 | ||
| BI3 | 29.094* | 4.077 | 0.000 | ||
| PD0 (mm) | PD1 | 0.610* | 0.145 | 0.007 | |
| PD2 | 0.787* | 0.148 | 0.001 | ||
| PD3 | 0.733* | 0.134 | 0.001 | ||
| CAL0 (mm) | CAL1 | 0.626* | 0.123 | 0.001 | |
| CAL2 | 0.784* | 0.129 | 0.000 | ||
| CAL3 | 0.711* | 0.115 | 0.000 |
*significance P <0.035, P <0.05 was considered as significant and value <0.001 was considered highly statistically significant. OHI – Oral hygiene index; PI – Plaque index; GI – Gingival index; BI – Bleeding index; PD – Pocket depth; CAL – Clinical attachment loss; SE – Standard error; P – Probability value
Table 3.
Intragroup comparison of pairwise mean difference of clinical parameters in Group B at different times of observation
| Group | Time (I) | Time (J) | Mean difference (I-J) | SE | P |
|---|---|---|---|---|---|
| Group B | OH10 | OHI1 | 0.215 | 0.232 | 1.000 |
| OH12 | 0.280 | 0.242 | 1.000 | ||
| OHI3 | 0.109 | 0.247 | 1.000 | ||
| PI0 | PI1 | 0.055 | 0.071 | 1.000 | |
| PI2 | 0.047 | 0.081 | 1.000 | ||
| PI3 | 0.046 | 0.076 | 1.000 | ||
| GI0 | GI1 | 0.085 | 0.047 | 0.896 | |
| GI2 | 0.126 | 0.056 | 0.423 | ||
| GI3 | 0.133 | 0.058 | 0.375 | ||
| Bl0 (%) | BI1 | 6.960 | 4.125 | 1.000 | |
| BI2 | 12.646* | 3.773 | 0.047 | ||
| BI3 | 17.849* | 3.438 | 0.001 | ||
| PD0 (mm) | PD1 | 0.362* | 0.087 | 0.009 | |
| PD2 | 0.393 | 0.130 | 0.092 | ||
| PD3 | 0.589* | 0.125 | 0.003 | ||
| CAL0 (mm) | CAL1 | 0.299 | 0.147 | 0.610 | |
| CAL2 | 0.333 | 0.233 | 1.000 | ||
| CAL3 | 0.671* | 0.163 | 0.010 |
* significance P < 0.010, P <0.05 was considered as significant and value<0.001 was considered highly statistically significant. OHI – Oral hygiene index; PI – Plaque index; GI – Gingival index; BI – Bleeding index; PD – Pocket depth; CAL – Clinical attachment loss; SE – Standard error; P – Probability value
For biochemical parameters, both the groups revealed statistically significant differences for HbA1c from baseline to 3rd follow-up and nonsignificant differences were revealed for CRP and IL-1 β [Table 4]. Table 5 shows the comparative analysis of the staining index in Group A and B and showed nonsignificant differences in both groups at 3rd follow-up, respectively.
Table 4.
Mean±standard deviation values of study (biochemical) parameters at 3rd follow-up in Group A and B
| Group | Variable (mean±SD) | Time | Mean±SD | P |
|---|---|---|---|---|
| Group A | HbA1c0 (%) (8.727±1.433) | HbA1c3 | 7.69±1.32 | 0.003* |
| CRP0 (mg/l) (2.67±3.11) | CRP3 | 2.35±2.98 | 0.94 | |
| IL-1β0 (ng/ml) (8.925±11.9) | IL-1β3 | 30.75±51.67 | 0.884 | |
| Group B | HbA1c0 (%) (9.36±1.61) | HbA1c3 | 8.15±1.57 | 0.008* |
| CRP0 (mg/l) (2.799±2.14) | CRP3 | 2.72±2.27 | 0.793 | |
| IL-1β0 (ng/ml) (9.56±4.02) | IL-1β3 | 7.03±2.32 | 0.203 |
*significance P <0.003, P <0.05 was considered as significant and value<0.001 was considered highly statistically significant. HbA1c – Glycosylated hemoglobin; CRP – C reactive protein; Il-1β – Interleukin-1β; SD – Standard deviation; P – Probability value
Table 5.
Comparative analysis of staining index at 3rd follow-up in Group A and B
| Group | Mean±SD | P | |
|---|---|---|---|
| Stain index – 3rd Follow-up | Group A | 1.8725±5.49141 | 0.582 |
| Group B | 1.0567±1.45324 |
significance P <0.580, P <0.05 was considered as significant and value <0.001 was considered highly statistically significant. SD – Standard deviation; P – Probability value
Figures 2-7 present the observed trend of fluctuation in clinical parameter scores over the study period in both groups.
Figure 2.
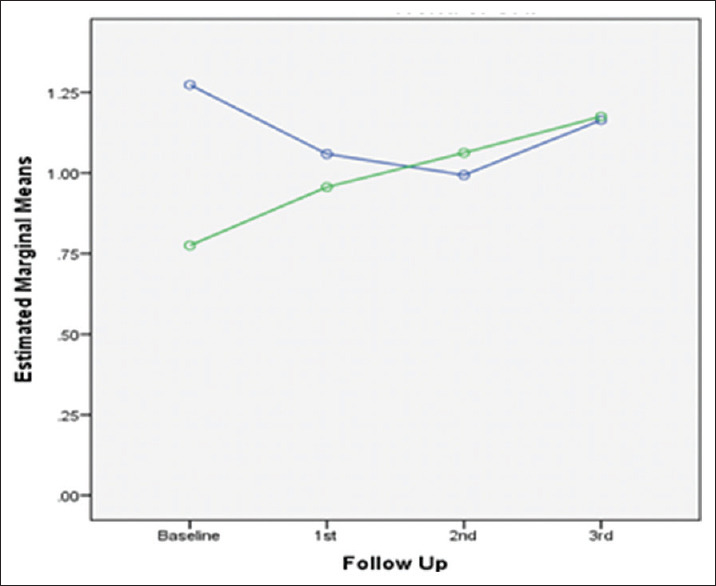
Trend of change in OHI over study period in both groups. OHI – Oral hygiene index
Figure 7.
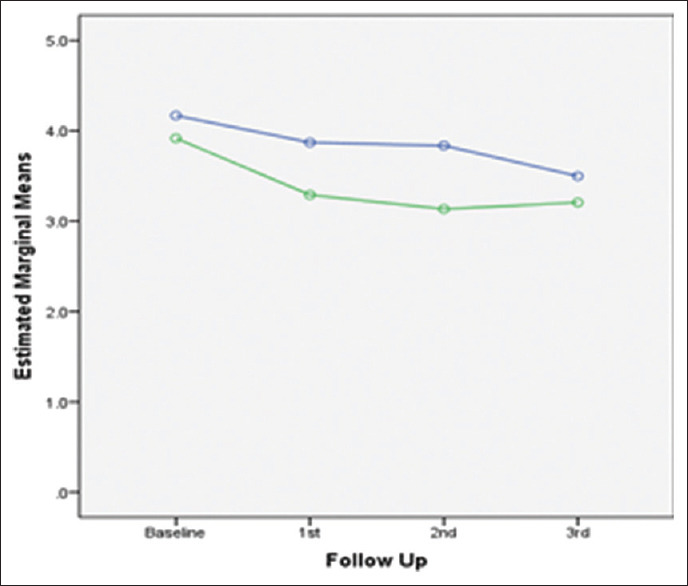
Trend of change in CAL over study period in both groups. CAL – Clinical attachment level
Figure 3.

Trend of change in PI over study period in both groups. PI – Plaque index
Figure 4.

Trend of change in GI over study period in both groups. GI – Gingival index
Figure 5.
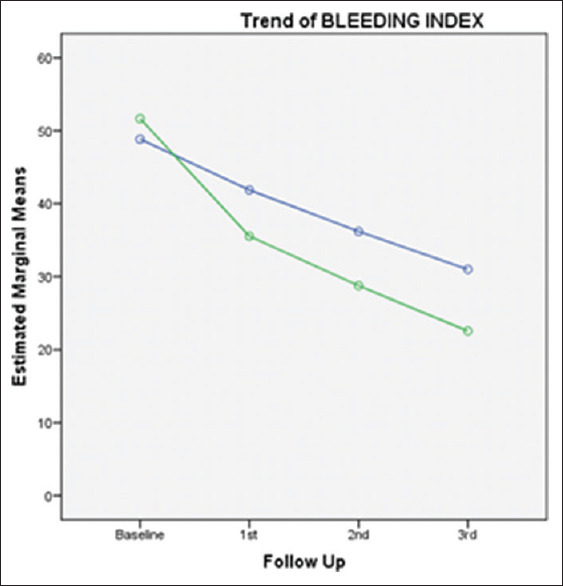
Trend of change in BI over study period in both groups. BI – Business intelligence
Figure 6.
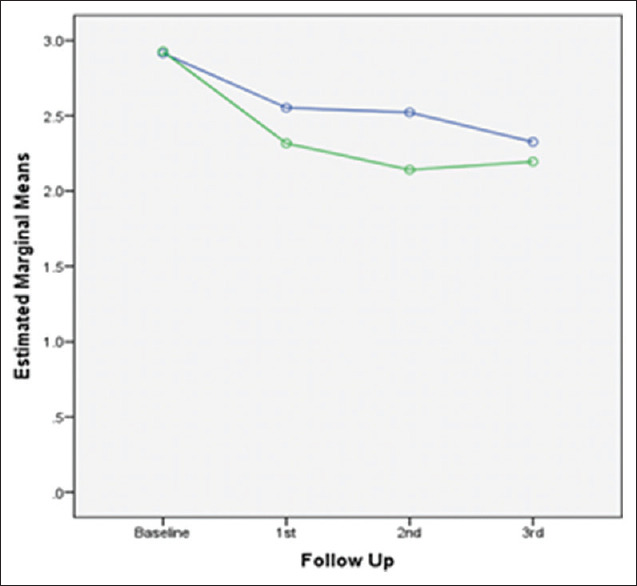
Trend of change in PD over study period in both groups. PD – Pocket depth
DISCUSSION
Periodontal disease and diabetes are chronic inflammatory conditions interacting in a bidirectional manner, adding severity to each other’s pathological mechanisms.[1-4]
The present study evaluated the efficacy of subgingival home irrigation using water along with powered toothbrushes (performed twice a day) as a home care maintenance regimen in diabetic patients with chronic periodontitis and the findings suggest that BI, PD, CAL, and HbA1c showed a statistically significant decrease in levels from baseline to 3rd follow-up with oral care regimen (experimental group).
BI scoring showed decreased levels in both groups during the course of the study. However, the improvement in levels was more evident in patients using water with sub-gingival home irrigation and powered toothbrush compared to patients using 0.12% CHX mouth rinsing [Tables 2 and 3]. These effects may be attributed to a better plaque control or reduction in inflammation in the subgingival home irrigation and powered toothbrush group owing to the additive benefits of two mechanical modes in action (brushing and irrigation), thus contributing to better scores in this group. These findings were consistent with the findings previously published by Al-Mubarak et al., who provided that the test group (Ultrasonic scaling and root planing, and subgingival water irrigation two times daily for 12 weeks) showed significant improvement in BOP, MGI at 6 and 12 weeks as compared to control group (Ultrasonic scaling and root planing plus normal hygiene instructions).[12] Subgingival irrigation disrupts the unattached plaque, thereby reducing toxicity of plaque, making plaque less pathogenic. Gingival inflammation reduction can also be imparted to the deeper penetration of fluid and thus diluting the pathogenic effects of subgingival plaque as additive effect.[28,29] In the present study, oral hygiene and plaque scores were reduced, but not statistically significant difference in improvement was seen in both groups. Chaves et al. reported that irrigation reduces the toxicity of the plaque with or without affecting the quantity of plaque accumulation.[19]
PD showed a highly statistically significant improvement from baseline to 3rd follow-up in patients using water sub-gingival home irrigation and powered toothbrush as maintenance regimen as compared to 0.12% CHX mouth rinsing group. Reduction in PD and CAL can be related to the fact that subgingival irrigation disturbs unattached plaque, thus reducing putative pathogens and their products, thereby reducing microbial load.[19] Sub-gingival irrigation is effective in delivering 90% solution to PD as compared to mouth rinsing, which delivers only 21% solution to PD.[16] Marked improvement in study parameters was observed in patients using water subgingival home irrigation and powered toothbrush as maintenance regimen, suggesting that subgingival irrigation helped to maintain better oral health, thus preventing deterioration of periodontium during the maintenance period. This may be attributed to the additional benefits of subgingival irrigators as they penetrate deeper pockets, thus diluting and removing bacterial toxins, and reducing bacterial counts.[30] Furthermore, bacterial cell disruption was evident using oral irrigators, as depicted by electron microscopic studies.[12] Furthermore, daily subgingival irrigation with water may have cumulative effects on the reduction of the depth of periodontal pockets.[31] These mechanisms are largely responsible for establishing/maintaining healthy periodontium.
The present study did not find a significant difference in the staining index at 4th month up in both groups. This is in agreement with studies by Lang and Ramseier-Grossmann, who reported the same while using different concentrations of CHX also with oral irrigators.[32] Furthermore, a systematic review by Van Strydonck et al. mentioned that CHX rinsing groups produced significantly more staining on teeth.[15] CHX is responsible for protein denaturation, catalysis of Maillard reactions, precipitation of dietary chromogens leading to the development of staining and also individual variations in developing stains play a role.[33]
In the case of HbA1c, there was a significant improvement in levels from baseline to 4th month in both groups [Tables 4 and 5], but more improvement was witnessed in patients using water subgingival home irrigation and powered toothbrush as maintenance regimen. This is in accordance with a study by Al-Mubarak et al., who showed numerical improvements in HbA1c in both groups from baseline to 12 weeks.[12] However, they did not reveal significant differences between the groups. A study by Navarro-Sanchez et al. found statistically significant improvements in HbA1c levels at 3 and 6 months follow-up in diabetic patients who underwent nonsurgical periodontal therapy.[5]
In the present study, CRP levels did not depict statistically significant improvements in both groups. A systematic review and meta-analysis by Demmer et al. depicted that anti-infective periodontal treatment results in short-term modest reductions (0.4 mg/L) in systemic CRP.[34] A study by Correa et al. did not find any statistically significant reductions in CRP levels at 3 months after periodontal therapy.[35] CRP being nonspecific marker of inflammation, it could have been impacted by any lifestyle habits, the emergence of other concurrent conditions/diseases in patients during the course of the study.
In the case of IL-1 β levels, a significant increase in levels was observed from baseline to 4th month in both groups. This is in agreement with the study by Goutoudi et al. that reported total IL-1 β amount was reduced, but concentration gradually increased at 6, 16, and 32 weeks after therapy in nondiseased and diseased sites. This increase in IL-1 β concentration could be attributed to the reduction of GCF volume as inflammation subsides following successful therapy.[29,36]
The study’s strength lies in the fact that it explored the role of a structured novel oral health care maintenance regimen in diabetic patients suffering from periodontal disease. At present, there is no standard recommended protocol for the maintenance of oral hygiene in diabetics. However, this specific population has paramount significance, as both diseases are highly prevalent and coexistent. Thus, these patients deserve a well-articulated maintenance protocol for long-term periodontal health, which made the premise of the current study. Yet there exists some limitations also, in the study. The results should be interpreted with caution owing to the drop out of nine patients reducing the power of study. Further, the baseline OHI scores differed significantly between the study groups, limiting the reliability of the findings.
Thus, in future well-designed longitudinal studies inclusion of a large number of patients and longer follow-up visits are required to ascertain the current findings. Future research should be performed for evaluation of additional relevant blood parameters i.e., multi panel inflammatory markers and using antimicrobial agents along with sub-gingival delivery tip irrigation systems in diabetic patients. Furthermore, the present study aimed on clinical evaluation primarily, a more rigorous evaluation of other parameters of disease such as microbial or genetic markers, can be carried out to provide insight into specific domains of periodontal structures and healing after therapy.
CONCLUSION
Within the limitations of this study, the findings revealed a significant improvement in clinical periodontal status and glycemic control marker HbA1c in diabetic patients performing subgingival home irrigation using water and powered toothbrush. Although clinical and biochemical improvements were also observed in patients performing mouth rinsing using 0.12% chlorhexidine gluconate and manual tooth brushing (performed twice daily), statistically significant improvements were evident in patients performing subgingival home irrigation and powered toothbrush. The differences observed suggest that the test maintenance protocol, i.e., subgingival irrigation using water as irrigant with powered toothbrush is a better home oral care regimen in diabetic patients and can be opted as a maintenance protocol for better patient outcomes and quality of life for diabetic patients having periodontal disease. Results from this study provide information for future studies on self-care regimens for individuals living with type 2 diabetes.
Financial support and sponsorship
The study products were provided by Water Pik, Inc., Fort Collins, CO, USA. The study was conducted at the Department of Periodontology, Dr. HS Judge Institute of Dental Sciences, Punjab University, Chandigarh, India.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors duly acknowledge the support from Water Pik, Inc. for kindly providing the home irrigation device units for study subjects.
REFERENCES
- 1.Nassar H, Kantarci A, van Dyke TE. Diabetic periodontitis: A model for activated innate immunity and impaired resolution of inflammation. Periodontol 2000. 2007;43:233–44. doi: 10.1111/j.1600-0757.2006.00168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of severe periodontitis in 1990-2010: A systematic review and meta-regression. J Dent Res. 2014;93:1045–53. doi: 10.1177/0022034514552491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sonnenschein SK, Meyle J. Local inflammatory reactions in patients with diabetes and periodontitis. Periodontol 2000. 2015;69:221–54. doi: 10.1111/prd.12089. [DOI] [PubMed] [Google Scholar]
- 4.Mealey BL, Ocampo GL. Diabetes mellitus and periodontal disease. Periodontol 2000. 2007;44:127–53. doi: 10.1111/j.1600-0757.2006.00193.x. [DOI] [PubMed] [Google Scholar]
- 5.Navarro-Sanchez AB, Faria-Almeida R, Bascones-Martinez A. Effect of non-surgical periodontal therapy on clinical and immunological response and glycaemic control in type 2 diabetic patients with moderate periodontitis. J Clin Periodontol. 2007;34:835–43. doi: 10.1111/j.1600-051X.2007.01127.x. [DOI] [PubMed] [Google Scholar]
- 6.Haraszthy VI, Zambon JJ, Trevisan M, Zeid M, Genco RJ. Identification of periodontal pathogens in atheromatous plaques. J Periodontol. 2000;71:1554–60. doi: 10.1902/jop.2000.71.10.1554. [DOI] [PubMed] [Google Scholar]
- 7.Ebersole JL, Cappelli D. Acute-phase reactants in infections and inflammatory diseases. Periodontol 2000. 2000;23:19–49. doi: 10.1034/j.1600-0757.2000.2230103.x. [DOI] [PubMed] [Google Scholar]
- 8.Garcia RI, Henshaw MM, Krall EA. Relationship between periodontal disease and systemic health. Periodontol 2000. 2001;25:21–36. doi: 10.1034/j.1600-0757.2001.22250103.x. [DOI] [PubMed] [Google Scholar]
- 9.Janket SJ, Wightman A, Baird AE, Van Dyke TE, Jones JA. Does periodontal treatment improve glycemic control in diabetic patients?A meta-analysis of intervention studies. J Dent Res. 2005;84:1154–9. doi: 10.1177/154405910508401212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teeuw WJ, Gerdes VE, Loos BG. Effect of periodontal treatment on glycemic control of diabetic patients: A systematic review and meta-analysis. Diabetes Care. 2010;33:421–7. doi: 10.2337/dc09-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Botero JE, Rodríguez C, Agudelo-Suarez AA. Periodontal treatment and glycaemic control in patients with diabetes and periodontitis: An umbrella review. Aust Dent J. 2016;61:134–48. doi: 10.1111/adj.12413. [DOI] [PubMed] [Google Scholar]
- 12.Al-Mubarak S, Ciancio S, Aljada A, Mohanty P, Ross C, Dandona P. Comparative evaluation of adjunctive oral irrigation in diabetics. J Clin Periodontol. 2002;29:295–300. doi: 10.1034/j.1600-051x.2002.290404.x. [DOI] [PubMed] [Google Scholar]
- 13.Renvert S, Persson GR. Supportive periodontal therapy. Periodontol 2000. 2004;36:179–95. doi: 10.1111/j.1600-0757.2004.03680.x. [DOI] [PubMed] [Google Scholar]
- 14.Van Strydonck DA, Timmerman MF, Van der Velden U, Van der Weijden GA. Plaque inhibition of two commercially available chlorhexidine mouthrinses. J Clin Periodontol. 2005;32:305–9. doi: 10.1111/j.1600-051X.2005.00681.x. [DOI] [PubMed] [Google Scholar]
- 15.Van Strydonck DA, Slot DE, Van der Velden U, Van der Weijden F. Effect of a chlorhexidine mouthrinse on plaque, gingival inflammation and staining in gingivitis patients: A systematic review. J Clin Periodontol. 2012;39:1042–55. doi: 10.1111/j.1600-051X.2012.01883.x. [DOI] [PubMed] [Google Scholar]
- 16.Braun RE, Ciancio SG. Subgingival delivery by an oral irrigation device. J Periodontol. 1992;63:469–72. doi: 10.1902/jop.1992.63.5.469. [DOI] [PubMed] [Google Scholar]
- 17.Pitcher GR, Newman HN, Strahan JD. Access to subgingival plaque by disclosing agents using mouthrinsing and direct irrigation. J Clin Periodontol. 1980;7:300–8. doi: 10.1111/j.1600-051x.1980.tb01972.x. [DOI] [PubMed] [Google Scholar]
- 18.Cutler CW, Stanford TW, Abraham C, Cederberg RA, Boardman TJ, Ross C. Clinical benefits of oral irrigation for periodontitis are related to reduction of pro-inflammatory cytokine levels and plaque. J Clin Periodontol. 2000;27:134–43. doi: 10.1034/j.1600-051x.2000.027002134.x. [DOI] [PubMed] [Google Scholar]
- 19.Chaves ES, Kornman KS, Manwell MA, Jones AA, Newbold DA, Wood RC. Mechanism of irrigation effects on gingivitis. J Periodontol. 1994;65:1016–21. doi: 10.1902/jop.1994.65.11.1016. [DOI] [PubMed] [Google Scholar]
- 20.Boyd RL, Hollander BN, Eakle WS. Comparison of a subgingivally placed cannula oral irrigator tip with a supragingivally placed standard irrigator tip. J Clin Periodontol. 1992;19:340–4. doi: 10.1111/j.1600-051x.1992.tb00656.x. [DOI] [PubMed] [Google Scholar]
- 21.Eakle WS, Ford C, Boyd RL. Depth of penetration in periodontal pockets with oral irrigation. J Clin Periodontol. 1986;13:39–44. doi: 10.1111/j.1600-051x.1986.tb01412.x. [DOI] [PubMed] [Google Scholar]
- 22.Bescos R, Ashworth A, Cutler C, Brookes ZL, Belfield L, Rodiles A, et al. Effects of Chlorhexidine mouthwash on the oral microbiome. Sci Rep. 2020;10:5254. doi: 10.1038/s41598-020-61912-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greene JC, Vermillion JR. The simplified oral hygiene index. J Am Dent Assoc. 1964;68:7–13. doi: 10.14219/jada.archive.1964.0034. [DOI] [PubMed] [Google Scholar]
- 24.Löe H. The gingival index, the plaque index and the retention index systems. J Periodontol. 1967;38:l610–6. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 25.Ainamo J, Bay I. Problems and proposals for recording gingivitis and plaque. Int Dent J. 1975;25:229–35. [PubMed] [Google Scholar]
- 26.Armitage GC. The complete periodontal examination. Periodontol 2000. 2004;34:22–33. doi: 10.1046/j.0906-6713.2002.003422.x. [DOI] [PubMed] [Google Scholar]
- 27.Macpherson LM, Stephen KW, Joiner A, Schäfer F, Huntington E. Comparison of a conventional and modified tooth stain index. J Clin Periodontol. 2000;27:854–9. doi: 10.1034/j.1600-051x.2000.027011854.x. [DOI] [PubMed] [Google Scholar]
- 28.Griffiths GS. Formation, collection and significance of gingival crevice fluid. Periodontol 2000. 2003;31:32–42. doi: 10.1034/j.1600-0757.2003.03103.x. [DOI] [PubMed] [Google Scholar]
- 29.Goutoudi P, Diza E, Arvanitidou M. Effect of periodontal therapy on crevicular fluid interleukin-1beta and interleukin-10 levels in chronic periodontitis. J Dent. 2004;32:511–20. doi: 10.1016/j.jdent.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Flemmig TF, Newman MG, Doherty FM, Grossman E, Meckel AH, Bakdash MB. Supragingival irrigation with 0.06% chlorhexidine in naturally occurring gingivitis I. 6 month clinical observations. J Periodontol. 1990;61:112–7. doi: 10.1902/jop.1990.61.2.112. [DOI] [PubMed] [Google Scholar]
- 31.Walsh M, Heckman B, Leggott P, Armitage G, Robertson PB. Comparison of manual and power toothbrushing, with and without adjunctive oral irrigation, for controlling plaque and gingivitis. J Clin Periodontol. 1989;16:419–27. doi: 10.1111/j.1600-051x.1989.tb01670.x. [DOI] [PubMed] [Google Scholar]
- 32.Lang NP, Ramseier-Grossmann K. Optimal dosage of chlorhexidine digluconate in chemical plaque control when applied by the oral irrigator. J Clin Periodontol. 1981;8:189–202. doi: 10.1111/j.1600-051x.1981.tb02030.x. [DOI] [PubMed] [Google Scholar]
- 33.Lakhani N, Vandana KL. Chlorhexidine –An insight. Int J Adv Res. 2016;4:1321–8. [Google Scholar]
- 34.Demmer RT, Trinquart L, Zuk A, Fu BC, Blomkvist J, Michalowicz BS, et al. The influence of anti-infective periodontal treatment on C-reactive protein: A systematic review and meta-analysis of randomized controlled trials. PLoS One. 2013;8:e77441. doi: 10.1371/journal.pone.0077441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Correa FO, Gonçalves D, Figueredo CM, Bastos AS, Gustafsson A, Orrico SR. Effect of periodontal treatment on metabolic control, systemic inflammation and cytokines in patients with type 2 diabetes. J Clin Periodontol. 2010;37:53–8. doi: 10.1111/j.1600-051X.2009.01498.x. [DOI] [PubMed] [Google Scholar]
- 36.Gamonal J, Acevedo A, Bascones A, Jorge O, Silva A. Levels of interleukin-1 beta, -8, and -10 and RANTES in gingival crevicular fluid and cell populations in adult periodontitis patients and the effect of periodontal treatment. J Periodontol. 2000;71:1535–45. doi: 10.1902/jop.2000.71.10.1535. [DOI] [PubMed] [Google Scholar]