Abstract
Osteoarthritis impairs the functions of various joints, such as knees, hips, hands and spine, which causes pain, swelling, stiffness and reduced mobility in joints. Multiple factors, including age, joint injuries, obesity, and mechanical stress, could contribute to osteoarthritis development and progression. Evidence has demonstrated that genetics and epigenetics play a critical role in osteoarthritis initiation and progression. Noncoding RNAs (ncRNAs) have been revealed to participate in osteoarthritis development. In this review, we describe the pivotal functions and molecular mechanisms of numerous lncRNAs in osteoarthritis progression. We mention that long noncoding RNAs (lncRNAs) could be biomarkers for osteoarthritis diagnosis, prognosis and therapeutic targets. Moreover, we highlight the several compounds that alleviate osteoarthritis progression in part via targeting lncRNAs. Furthermore, we provide the future perspectives regarding the potential application of lncRNAs in diagnosis, treatment and prognosis of osteoarthritis.
Keywords: osteoarthritis, noncoding RNA, lncRNA, biomarkers, treatment
Introduction
Osteoarthritis (OA) is a common arthritis and often impairs the functions of joints, including knees, hips, hands and spine (Martel-Pelletier et al., 2016). Osteoarthritis patients exhibit various symptoms, such as pain, swelling, stiffness, and reduced mobility in joints due to the gradual breakdown and loss of cartilage in joints (Thakur et al., 2014). Osteoarthritis develops partly because of age, joint injuries, repetitive use of joints, mechanical stress, and obesity (Loeser et al., 2016; Wakale et al., 2023). In clinic, physical examination and imaging tests (X-rays and MRI) in combination with medical history are helpful for osteoarthritis diagnosis (Chalian et al., 2023; Nevalainen et al., 2023). In general, osteoarthritis was divided into four stages according to Kellgren-Lawrence classification based on radiological diagnostic criteria (Kellgren and Lawrence, 1957). Osteoarthritis patients with each stage exhibit different symptoms, joint space narrowing, cartilage loss, bone changes, and osteophyte formation (Mahmoudian et al., 2021; Szponder et al., 2022). So far, treatments mainly manage the symptoms and improve the life quality of osteoarthritis patients. Pain relievers and nonsteroidal anti-inflammatory drugs (NSAIDs) reduce pain and inflammation in osteoarthritis (Ouyang et al., 2023). For example, acetaminophen, ibuprofen, naproxen, corticosteroid injection, and hyaluronic acid injection, have been used to reduce pain and inflammation in osteoarthritis (Shentu et al., 2022; Goyal et al., 2023; Idres and Samaan, 2023; Richard et al., 2023). Exercise, weight management, and physical therapy are useful for osteoarthritis patients (Spanoudaki et al., 2023). In addition, assistive devices such as braces and canes are helpful to support joints. Joint replacement could be a useful approach for severe osteoarthritis patients (Postler et al., 2023). A healthy weight, regular exercise, and using protective techniques to protect joints can prevent and retard osteoarthritis development and progression (Mo et al., 2023; Young et al., 2023).
Genetic changes and epigenetics could involve in osteoarthritis development and progression (Hodgkinson et al., 2022; Nunez-Carro et al., 2023; Tonutti et al., 2023). For example, cellular signaling pathways have been reported to regulate osteoarthritis progression (Zhang et al., 2023a; Ruan et al., 2023; Zheng et al., 2023). In recent years, noncoding RNAs (ncRNAs) have been revealed to participate in osteoarthritis development and progression (Xie et al., 2020). It is well known that ncRNAs belong to a class of RNA molecules that do not encode proteins (Winkle et al., 2021). However, ncRNAs have diverse functions in regulation of gene expression and cellular processes (Liu and Shang, 2022). The common types of ncRNAs include microRNA (miRNA), transfer RNA (tRNA), ribosomal RNA (rRNA), long ncRNA (lncRNA), small interfering RNA (siRNA) and circular RNA (circRNA) (Eddy, 2001; Matera et al., 2007; Mattick et al., 2023). It has been shown that miRNA can bind to specific mRNA molecules and cause the inhibition of translation or degradation, leading to regulation of gene expression. LncRNAs with longer than 200 nucleotides have been identified to involve in diverse diseases via regulating gene expression and chromatin remodeling, including cancer, neurodegenerative diseases and cardiovascular disorders (Jiang et al., 2020; Cheng et al., 2022; Gareev et al., 2022; Sufianova et al., 2022; Xie et al., 2022). LncRNAs have gained significant attention and play crucial regulatory roles in osteoarthritis (Chen et al., 2017; Abbasifard et al., 2020; Tu et al., 2020; Wang et al., 2022; Okuyan and Begen, 2022). In this review, we will describe the role of numerous lncRNAs in osteoarthritis development and progression. We also mention lncRNAs as biomarkers for osteoarthritis progression. Moreover, we highlight the compounds that attenuate osteoarthritis progression via targeting lncRNAs. Furthermore, we provide the future perspectives regarding the application of lncRNAs in diagnosis, treatment and prognosis of osteoarthritis.
Microarray and bioinformatics analyses for measuring lncRNAs in osteoarthritis
Liu et al. found that lncRNAs that are related to cartilage injury induced the degradation of chondrocyte extracellular matrix in osteoarthritis (Liu et al., 2014). By microarray and qPCR assays, this study found up to 152 lncRNAs (82 upregulated and 70 downregulated lncRNAs) to be differentially expressed between OA and normal cartilage. Depletion of cartilage injury-related lncRNAs (lncRNA-CIR) by siRNA increased collagen and aggrecan formation and decreased the expression of MMP13 and ADAMTS5, two matrix-degrading enzymes. Upregulation of lncRNA-CIR caused the opposite phenotype (Liu et al., 2014). Another group also used microarray analysis to screen the expression profile of lncRNAs in OA cartilage and normal cartilage. In this study, 73 lncRNAs were upregulated and 48 lncRNAs were downregulated in OA compared with normal cartilage (Xing et al., 2014). RT-PCR validated the six lncRNAs with upregulation in OA, including H19, CTD-2574D22.4, HOTAIR, PMS2L2, RP11-445H22.4 and GAS5. The mRNA levels of BMP-2, ADAMTS5, MMP-9, and MMP-13 were increased in OA (Xing et al., 2014). Fu and coworkers used microarray approach and provided expression profiles of lncRNAs in cartilage from knee OA patients (Fu et al., 2015). In this work, 3,007 upregulated lncRNAs and 1707 downregulated lncRNAs were discovered in OA cartilage compared with normal cartilage. Moreover, 2,136 mRNAs were upregulated and 2,241 mRNAs were downregulated in OA samples (Fu et al., 2015).
By an analysis of 19 samples from knee OA patients, 580 dysregulated lncRNAs were discovered. Four lncRNAs, SNHG5, ZFAS1, GAS5, and DANCR could be important in OA cartilage (Xiao et al., 2019). One study by Zhang et al. performed lncRNA and mRNA microarray to explore the expression files in chondrocytes from three OA patients and four healthy people in Northwest Chinese Han population (Zhang et al., 2020). Total 990 lncRNAs (660 upregulated and 324 downregulated) and 546 mRNAs (419 upregulated and 127 downregulated) were identified in OA tissues compared with normal controls. Moreover, lncRNA CTD-2184D3.4, ENST00000564198.1, and ENST00000520562.1 could control the mRNA expression levels of SPC24, GALM, and ZNF345 in OA (Zhang et al., 2020). By comprehensive analysis of mRNA and lncRNA, Luo et al. revealed different features of OA between Han population and Tibetan patients. Specifically, 117 lncRNAs (49 upregulated and 68 downregulated) and 297 mRNAs (158 upregulated and 139 downregulated) had differently expressed in the cartilage of Tibetans compared with those of Han patients (Luo et al., 2021). RNA-sequencing was performed to identify the changed expression of mRNA and lncRNAs in between 5 osteoarthritic synovium samples and 5 healthy tissues. 17 lncRNAs and 384 mRNAs were differentially expressed in OA synovium compared with healthy controls. Moreover, these differential expression of lncRNAs regulate OA progression via immune response-related pathways through lncRNA-mRNA network (Xiang et al., 2019a).
LncRNAs regulate osteoarthritis progression
One study reported that 51 lncRNAs were upregulated and 56 lncRNAs were downregulated in the damaged cartilage tissues. LncRNA-MSR, one TMSB4 pseudogene, was elevated in the damage tissues and was upregulated in mechanical stress-stimulated chondrocytes. LncRNA-MSR competed with miRNA-152 and governed the expression of TMSB4 in chondrocytes. Hence, lncRNA-MSR upregulation enhanced cartilage degradation and initiated pathological changes (Liu et al., 2016). In the following paragraphs, we will discuss the functions and mechanisms of lncRNAs in regulation of osteoarthritis progression.
LncRNA SNHGs
LncRNA SNHG1 (small nucleolar RNA host gene 1) has been reported to repress miR-16-5p-mediated p38 MAPK and NF-κB pathways, such as p-p65, ERK1/2 and p-p38, leading to attenuation of IL-1β-mediated osteoarthritis (Lei et al., 2019). LncRNA SNHG1 reduced the expression of several cytokines in chondrocytes, such as NO, COX-2, IL-6, PGE2, i-NOS, and THF-a. LncRNA SNHG1 also alleviated the expression of MMPs, aggrecan, collagen, and ADAMTs in chondrocytes (Lei et al., 2019). Wang et al. reported that lncRNA SNHG1 reduced cell apoptosis and inflammation via influencing the miR-195/IKK-α axis in chondrocyte (Wang et al., 2023). LncRNA SNHG5 increased proliferation of chondrocyte via targeting miR-26a/SOX2 axis in OA (Shen et al., 2018). LncRNA SNHG5 was downregulated in OA tissues. Overexpression of lncRNA SNHG5 promoted cell proliferation and migration in chondrocyte. Moreover, lncRNA SNHG5 targeted the miR-26a and then upregulated the expression of SOX2 in chondrocyte, leading to promotion of proliferation and migration of chondrocyte cells (Shen et al., 2018). Yue and coworkers found that SNHG5 blocked IL-1β-induced OA and protected chondrocytes via changing miR-181a-5p and TGFBR3 pathway (Yue et al., 2021). Jiang and colleagues observed that SNHG5 targeted miR-10a-5p and H3F3B, resulting in inhibition of apoptosis and promotion of chondrocyte proliferation in OA (Jiang et al., 2021).
LncRNA SNHG7 sponged the miR-34a-5p and upregulated SYVN1 expression, contributing to promotion of proliferation, and inhibition of apoptosis and autophagy in osteoarthritis (Tian et al., 2020). In addition, lncRNA SNHG7 attenuated miR-214-5p-mediated PPARGC1B pathway and led to inhibition of IL-1β-induced osteoarthritis via suppression of NLRP3 inflammasome and apoptosis (Xu et al., 2021). LncRNA SNHG9 suppressed cell apoptosis via downregulation of miR-34a by methylation in chondrocyte, and the expression of lncRNA SNHG9 was decreased in osteoarthritis (Zhang et al., 2020b). LncRNA SNHG12 enhanced the progression of osteoarthritis via inhibition of miR-16–5p and suppression of chondrocyte proliferation, induction of apoptosis and inflammation and ECM degradation (Yang et al., 2022). Suppression of lncRNA SNHG14 blocked FSTL-1-induced NLRP3 and TLR4/NF-kappaB pathways via regulation of miR-214–3p, conferring to inhibition of osteoarthritis (Wang et al., 2021a). LncRNA SNHG15 sponged miR-141–3p and elevated the expression of BCL2L13, resulting in prevention of osteoarthritis progression (Zhang et al., 2020). Similarly, one group found that lncRNA SNHG15 regulated ECM homeostasis and attenuated the progression of osteoarthritis (Chen et al., 2020a). Fan and colleagues reported that lncRNA SNHG16 accelerated osteoarthritis occurrence via interaction with miR-373–3p (Fan et al., 2021). In summary, lncRNA SNHGs regulate occurrence and progression of osteoarthritis (Table 1).
TABLE 1.
LncRNA SNHGs in regulation of osteoarthritis.
| LncRNA | miRNA | Targets | Functions | Ref |
|---|---|---|---|---|
| SNHG1 | miR-16–5p | P38 MAPK, NF-κB | Attenuation of osteoarthritis | Lei et al. (2019) |
| SNHG1 | miR-195 | IKK-α | Reduces apoptosis and inflammation | Wang et al. (2023a). |
| SNHG5 | miR-26a | SOX2 | Increases proliferation of chondrocyte | Shen et al. (2018) |
| SNHG5 | miR-181a-5p | TGFBR3 | Blocks IL-1β-induced osteoarthritis | Yue et al. (2021) |
| SNHG5 | miR-10a-5p | H3F3B | Inhibition of apoptosis and promotion of proliferation | Jiang et al. (2021) |
| SNHG7 | miR-34a-5p | SYVN1 | Promotes proliferation, inhibits apoptosis and autophagy | Tian et al. (2020) |
| SNHG7 | miR-214–5p | PPARGC1B | Inhibits IL-1β-induced osteoarthritis | Xu et al. (2021) |
| SNHG9 | miR-34a | N/A | Suppresses cell apoptosis | Zhang et al. (2020b) |
| SNHG12 | miR-16–5p | N/A | Induces apoptosis, inflammation, ECM degradation, inhibits proliferation | Yang et al. (2022a) |
| SNHG14 | miR-214–3p | FSTL-1, NLRP3, NF-κB | Inhibits osteoarthritis | Wang et al. (2021a) |
| SNHG15 | miR-141–3p | BCL2L13 | Prevents osteoarthritis progression | Zhang et al. (2020c) |
| SNHG16 | miR-373–3p | N/A | Accelerates osteoarthritis occurrence | Fan et al. (2021a) |
LncRNA HOTAIR
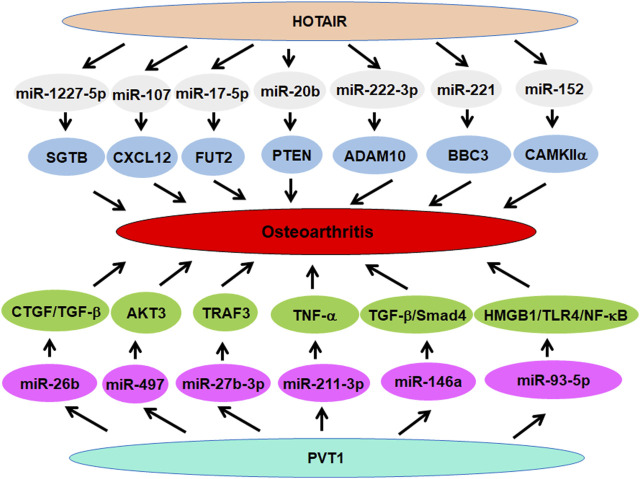
LncRNA HOTAIR (HOX transcript antisense RNA) promoted ADAMTS-5 expression in osteoarthritic articular chondrocytes (Dou et al., 2017). LncRNA HOTAIR was highly expressed in human OA cartilage. TNF-α (tumor necrosis factor) increased the expression of HOTAIR in OA chondrocytes. Knockdown of HOTAIR reduced the expression of ADAMTS-5, while upregulation of HOTAIR elevated ADAMTS-5 expression levels in OA chondrocytes. HOTAIR regulated the ADAMTS-5 mRNA stability in OA articular chondrocytes (Dou et al., 2017). Hu et al. found that HOTAIR facilitated osteoarthritis progression via regulation of miR-17–5p, fucosyltransferase 2 (FUT2) and β-catenin (Hu et al., 2018). Higher expression of HOTAIR was linked to chondrocyte apoptosis, ECM degradation and modified Mankin scale. HOTAIR interacted with miR-17–5p and upregulated FUT2 expression, leading to regulating wnt/β-catenin pathway. Moreover, HOTAIR aggravated chondrocyte apoptosis and ECM degradation (Hu et al., 2018). Overexpression of HOTAIR induced apoptosis of chondrocytes and caused IL-1β-mediated MMP upregulation in temporomandibular joint OA (Zhang et al., 2016). It is recognized that lncRNA HOTAIR-involved Wnt/β-catenin pathway regulated the pathogenesis of cartilage damage via modulation of MMP-13 (Zhou et al., 2019). Upregulation of HOTAIR induced an increase in apoptosis rates and reduced the viability of chondrocytes via sponging miR-130a-3p to inhibit autophagy in chondrocytes in knee OA, which was accompanied by the downregulation of Bcl-2 and survivin and upregulation of cleavage of caspase 3 and Bax expression (He and Jiang, 2020). Chen et al. reported that HOTAIR accelerated OA progression via sponging miR-20b and upregulating PTEN (Chen et al., 2020b).
HOTAIR promoted cartilage degradation by suppression of Wnt inhibitory factor 1 (WIF-1) expression via enhancing histone H3K27 trimethylation in WIF-1 promoter, resulting in activation of the Wnt/β-catenin pathway in OA (Yang et al., 2020). Osteopontin induced the expression of HOTAIR in the primary chondrocytes. Overexpression of HOTAIR and osteopontin promoted chondrocyte proliferation, whereas downregulation of HOTAIR reduced proliferation of chondrocyte cells, suggesting that HOTAIR could involve in OA via influencing cell proliferation (Liang et al., 2021). HOTAIR induced inflammation and LPS-induced chondrocyte apoptosis through influencing miR-1227–5p and increasing the expression of small glutamine rich tetratricopeptide repeat containing beta (SGTB) in OA (Wang et al., 2021b). Lu et al. observed that knockdown of HOTAIR blocked OA chondrocyte injury via modulation of miR-107 and CXCL12 pathway (Lu et al., 2021). IL-1β-mediated cell apoptosis, oxidative stress, inflammatory response, and ECM degradation were blocked by inhibition of HOTAIR in chondrocytes by affecting miR-222–3p and ADAM10 axis (Wang et al., 2021). Similarly, HOTAIR promoted mechanical stimulation-mediated apoptosis via regulation of miR-221 and BBC3 pathway (Zheng et al., 2021). In addition, dysregulation of HOTAIR in craniosynostosis led to impaired osteoclast differentiation via changing miR-152-CAMKIIα pathway (Dong et al., 2022). HOTAIR expression was positively associated with TNF-α, hs-CRP, IgA, total cholesterol (TC), and VAS (visual analog scale) score (Chen et al., 2023). Upregulation of HOTAIR repressed cell proliferation, increased the expression of TNF-α, p-PI3K, and p-AKT, attenuated PTEN and IL-10 expression in OA chondrocytes after stimulation by OA PBMCs (peripheral blood mononuclear cells) (Chen et al., 2023). Altogether, HOTAIR plays an essential role in OA progression (Figure 1).
FIGURE 1.
The role of lncRNA HOTAIR and lncRNA PVT1 in osteoarthritis.
LncRNA PVT1
LncRNA PVT1 expression was higher in OA chondrocytes than that in normal chondrocytes. Downregulation of PVT1 reduced the apoptosis of OA chondrocytes, while normal chondrocytes increased apoptosis after PVT1 upregulation. PVT1 induced apoptosis via sponging miR-488–3p in OA (Li et al., 2017). Zhao et al. found that PVT1 depletion abolished the suppression of IL-1β on aggrecan and collagen II expression, and reduced IL-1β-mediated induction of MMPs, such as MMP-3, MMP-9 and MMP-13. PVT1 downregulation abrogated the IL-1β-stimulated production of inflammatory cytokines, such as PGE2, TNF-α, IL-6, NO, and IL-8. Molecular mechanism revealed that PVT1 sponged miR-149 to perform regulate metabolic dysfunction in OA chondrocytes (Zhao et al., 2018). PVT1 regulated hyperglycemia-mediated collagen degradation via sponge of miR-26b and regulation of CTGF/TGF-β signaling pathway (Ding et al., 2020). In line with this study, PVT1 sponged miR-140 and induced chondrocyte ECM degradation in IL-1β-treated chondrocytes (Yao et al., 2022). One study showed that depletion of PVT1 blocked IL-1β-mediated injury by targeting miR-27b-3p and TRAF3 in chondrocytes (Lu et al., 2020).
Another study reported that PVT1 upregulated TNF-α in synoviocytes and increased apoptosis of chondrocyte via sponge of miR-211–3p (Xu et al., 2020). Similarly, depletion of exosome-mediated lncRNA PVT1 regulated the HMGB1/TLR4/NF-κB pathway by sponging miR-93–5p, resulting in LPS-mediated OA progression (Meng et al., 2020). In addition, PVT1 inhibited miR-146a and activated TGF-β/SMAD4 signaling, leading to promotion of cartilage degradation in diabetic OA mice (Wang et al., 2021). LncRNA PVT1 and GAS5 (growth arrest specific 5) regulated each other to govern chondrocyte apoptosis in osteoarthritis (Cai et al., 2022). PVT1 upregulation induced apoptosis, while GAS5 upregulation reduced apoptosis in chondrocytes induced by LPS, indicating that PVT1 and GAS5 have a negative feedback loop (Cai et al., 2022). Recently, PVT1 regulated miR-497/AKT3 pathway and influenced the function of osteoarthritis cells via regulation of proliferation, apoptosis and ECM degradation in chondrocytes (Xu J. et al., 2022). Taken together, PVT1 participates in the development and progression of osteoarthritis (Figure 1).
LncRNA MEG3
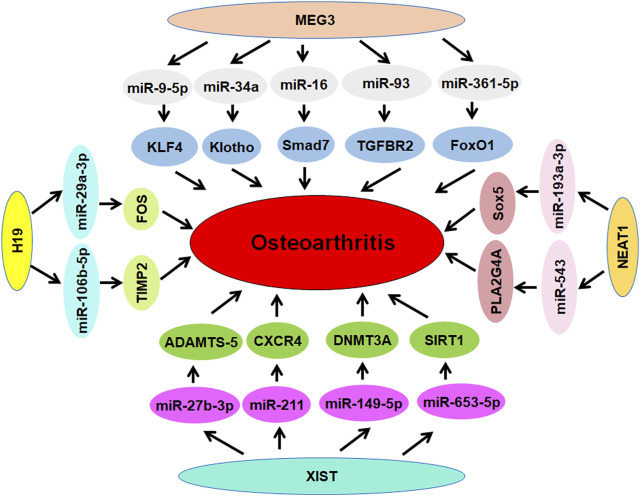
The expression of lncRNA maternally expressed gene 3 (MEG3) was decreased in patients with osteoarthritis compared with healthy cartilage samples by real-time RT-PCR. The expression of lncRNA MEG3 was inversely correlated with VEGF levels, which was measured by ELISA in cartilage tissues. This study indicated that lncRNA MEG3 could participate in osteoarthritis development via the modulation of angiogenesis (Su et al., 2015). Similarly, one group also found that lncRNA MEG3 was downregulated in rat osteoarthritis cartilage tissues. Inhibition of MEG3 enhanced proliferation and reduced apoptosis in IL-1β-mediated rat chondrocytes. MEG3 inhibited the expression of miR-16 and elevated SMAD7 expression in IL-1β-treated chondrocytes. Hence, inhibition of MEG3 could cause osteoarthritis progression via targeting miR-16/SMAD7 axis (Xu and Xu, 2017). Methylene blue, an inhibitor of peripheral nerve axons to alleviate pain, has been shown to increase the expression of lncRNA MEG3 and decrease P2X purinoceptor 3 (P2X3) protein levels. Methylene blue increased the expression of IL-6, IL-8, IL-1β and TNFα. Methylene blue could relieve the inflammation and pain via promotion of lncRNA MEG3 in osteoarthritis (Li et al., 2018a). Chen et al. reported that lncRNA MEG3 attenuated the ECM degradation of chondrocytes and induced proliferation and inhibited apoptosis of chondrocytes through modulation of miR-93 and TGFBR2 pathways in osteoarthritis (Chen et al., 2021). In line with this report, lncRNA MEG3 reduced apoptosis and induced proliferation of chondrocytes via influencing miR-361–5p/FoxO1 axis in osteoarthritis (Wang et al., 2019). Notably, lncRNA MEG3 retarded chondrogenic differentiation of synovium-derived mesenchymal stem cells (SMSCs) via suppression of TRIB2 by methyltransferase EZH2 (You et al., 2019). LncRNA MEG3 reduced chondrocyte impairment by IL-1β-mediated inflammation due to governing miR-9-5p/KLF4 axis (Huang et al., 2021). In addition, one report showed that lncRNA MEG3 controlled osteoarthritis progression via affecting miR-34a/Klotho axis (Xiong et al., 2022). LncRNA MEG3 is critically involved in osteoarthritis development and progression (Figure 2).
FIGURE 2.
The role of lncRNAs MEG3, H19, NEAT1, XIST in osteoarthritis.
LncRNA H19
Several studies have demonstrated that lncRNA H19 regulates osteoarthritis progression. For example, depletion of lncRNA H19 reduced LPS-mediated damage via regulation of miR-130a in osteoarthritis (Hu et al., 2019). LncRNA H19 exhibited the diagnostic value in the blood of osteoarthritis (Zhou et al., 2020). Peripheral blood of osteoarthritis patients had an increased expression of lncRNA H19, which was linked to the occurrence and development of osteoarthritis (Zhou et al., 2020). LncRNA H19 expression was elevated in osteoarthritis samples. Overexpression of lncRNA H19 enhanced apoptosis and decreased the proliferation in IL-1β-mediated chondrocytes via sponging miR-106a-5p in osteoarthritis (Zhang et al., 2019). LncRNA H19 regulated cartilage matrix degradation and calcification via interaction with miR-140–5p in osteoarthritis (Yang et al., 2020). Exosomal lncRNA H19 regulated the progression of osteoarthritis via governing the miR-106b-5p/TIMP2 axis (Tan et al., 2020). In addition, lncRNA H19 gene polymorphisms were associated with risk of osteoarthritis in a Chinese Han population (Wang et al., 2020). Umbilical cord blood MSCs produced lncRNA H19 controlled central sensitization of pain via targeting miR-29a-3p/FOS pathway in osteoarthritis (Yang et al., 2021). LncRNA H19 relieved inflammation via influencing TP53, IL-38, and IL-36 receptors in osteoarthritis (Zhou et al., 2022). Motion-mediated posttraumatic osteoarthritis was abrogated by moderate-intensity treadmill running due to upregulation of lncRNA H19 expression (Zhou. et al., 2021). Hence, lncRNA H19 influences osteoarthritis progression (Figure 2).
LncRNA XIST
LncRNA XIST was highly expressed in cartilage tissues of osteoarthritis patients. Downregulation of lncRNA XIST inhibited IL-1β-suppressed proliferation and induced apoptosis in chondrocytes. XIST can sponge miR-211 and upregulate the expression of CXCR4, leading to modulation of chondrocyte proliferation and apoptosis via targeting MAPK signaling pathway (Li et al., 2018). Methylation of TIMP-3 promoter was accelerated by lncRNA XIST, contributing to collagen degradation in osteoarthritic chondrocytes in patients with tibial plateau fracture (Chen et al., 2019). Sun et al. reported that miR-142–5p prevented osteoarthritis progression due to the interaction between lncRNA XIST and miR-142–5p (Sun et al., 2020). Similarly, lncRNA XIST protected chondrocytes from IL-1β-mediated injury through sponging miR-653–5p and upregulating SIRT1 (Lian and Xi, 2020). In line with this finding, lncRNA XIST conferred to osteoarthritis development by influencing miR-149–5p and DNMT3A pathways (Liu et al., 2020a). Chondrogenic differentiation of SMSCs was governed by lncRNA XIST through interaction with miR-27b-3p and upregulation of ADAMTS-5 (Zhu et al., 2021). In addition, YY1 increased the expression of lncRNA XIST, resulting in suppression of cartilage differentiation of BMSCs via interacting with TAF15 to maintain the stability of FUT1 protein (He et al., 2022). Interestingly, lncRNA XIST interacted with miR-150–5p and affected monocyte adherence, suggesting that lncRNA XIST might be useful for osteoarthritis treatment (Wang et al., 2022). In conclusion, lncRNA XIST regulates progression of osteoarthritis (Figure 2).
LncRNA MALAT1
LncRNA MALAT1 regulated PI3K/AKT pathway via targeting miR-127–5p and controlled osteopontin (OPN)-induced cell proliferation in human chondrocytes (Liang et al., 2018). MALAT1 targeted the JNK pathway and reduced apoptosis and matrix metabolism disorder in articular chondrocytes with IL-β-mediated inflammation (Gao et al., 2019). Zhang et al. found that MALAT1 influenced miR-150–5p and AKT3 pathways and accelerated osteoarthritis development (Zhang et al., 2019). Li et al. reported that MALAT1 sponged miR-146a and modulated PI3K/Akt/mTOR pathway, leading to regulation of chondrocyte proliferation after LPS treatment (Li et al., 2020). Liu and coworkers observed that MALAT1 sponged miR-145 and elevated ADAMTS5, resulting in regulation of IL-β-mediated viability and cartilage matrix degradation in osteoarthritis (Liu et al., 2019). MALAT1 was found to regulate the inflammatory synovial fibroblast phenotype in obese patients with osteoarthritis (Nanus et al., 2020). One group validated the expression and function of MALAT1 in osteoblasts from osteoarthritis patients (Alnajjar et al., 2021). MALAT1 from MSCs-derived extracellular vesicles blocked cartilage degradation and attenuated inflammation in osteoarthritis (Pan et al., 2021). Another group reported that miR-124–3p impaired MALAT1 stability and led to suppression of chondrocyte pytoptosis and inhibition of cartilage injury in osteoarthritis (Rozi et al., 2022). Altogether, MALAT1 controls osteoarthritis development and progression (Figure 3).
FIGURE 3.
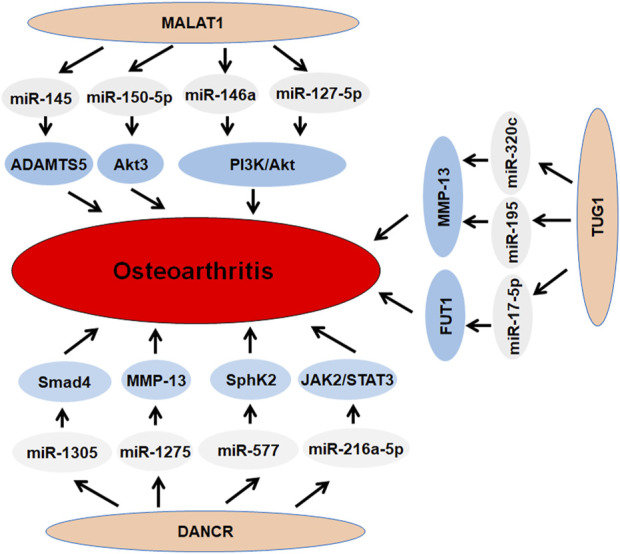
The role of lncRNAs MALAT1, TUG1, DANCR in osteoarthritis.
LncRNA NEAT1
LncRNA NEAT1 regulated cartilage matrix degradation via targeting miR-193a-3p and SOX5 pathways in osteoarthritis (Liu F. et al., 2020). Inhibition of lncRNA NEAT1 reduced apoptosis, and inflammation, and extracellular matrix degradation, while inhibiting the expression of MMP-3, MMP-13, and ADAMTS-5. Additionally, it increased Col2a1 and ACAN expression in chondrocytes (Liu et al., 2020). One group showed that lncRNA NEAT1 increased chondrocyte proliferation and reduced apoptosis via inhibition of miR-16–5p in osteoarthritis (Li. et al., 2020). Another group reported that lncRNA NEAT1 governed miR-543/PLA2G4A axis and led to inhibition of chondrocyte proliferation and induction of apoptosis in osteoarthritis (Xiao et al., 2021). Overexpression of lncRNA NEAT1 alleviated the expression of Bcl-2 and p-Akt1, elevated the expression of MMP-3, MMP-9, MMP-13, IL-6 and IL-8 in chondrocytes (Xiao et al., 2021). Wang et al. reported that NEAT1 sponged miR-378 and regulated LPS-mediated articular chondrocytes, resulting in influencing the osteoarthritis development (Wang et al., 2022). Moreover, achyranthes bidentata polysaccharides (ABPS) increased the expression of lncRNA NEAT1 and decreased the miR-377–3p expression, leading to attenuation of endoplasmic reticulum in osteoarthritis (Fu et al., 2022). Therefore, lncRNA NEAT1 affects osteoarthritis development (Figure 2).
LncRNA DANCR
LncRNA DANCR has been reported to promote proliferation and differentiation of chondrogenesis of synovium-derived mesenchymal stem cells (SMSCs) (Zhang et al., 2017a). LncRNA DANCR upregulation increased proliferation and chondrogenesis of SMSCs via interaction with myc, Smad3, and STAT3 mRNA to influence their stability. DANCR increased the expression of Smad3 and STAT3, leading to activation of chondrogenesis of SMSCs (Zhang et al., 2017a). SOX4 was found to promote chondrogenic differentiation and proliferation of SMSCs by upregulation of lncRNA DANCR (Zhang et al., 2015). Moreover, lncRNA DANCR modulated miR-1305/Smad4 axis to enhance chondrogenic differentiation of SMSCs (Zhang et al., 2017b). Furthermore, DANCR accelerated chondrogenesis via modulation of miR-1275 and MMP-13 in SMSCs (Fang et al., 2019). LncRNA DANCR sponged miR-577 and elevated SphK2 expression, contributing to promotion of proliferation of chondrocytes and induction of apoptosis in osteoarthritis development (Fan et al., 2018). Moreover, lncRNA DANCR influenced chondrocyte proliferation and apoptosis through regulation of miR-216a-5p, JAK2 and STAT3 pathways in osteoarthritis (Zhang et al., 2018). These findings indicate that lncRNA DANCR plays an essential role in osteoarthritis progression (Figure 3).
LncRNA TUG1
LncRNA taurine upregulated gene 1 (TUG1) has been found to be elevated in cartilages of osteoarthritis patients compared with normal cartilages. IL-1β and TNF-α induced the expression of lncRNA TUG1 in chondrocytes. Upregulation of lncRNA TUG1 suppressed the miR-195 expression and inhibited the expression of collagen and aggrecan, whereas lncRNA TUG1 overexpression promoted the expression of MMP-13, indicating that TUG1 might promote degradation of chondrocyte extracellular matrix in osteoarthritis by regulation of miR-195 and MMP-13 (Tang et al., 2018). Li et al. found that depletion of lncRNA TUG1 reduced the expression of MMP-13 and induced the expression of collagen II and aggrecan in IL-1β-treated chondrocyte. Moreover, TUG1 targeted miR-17–5p and elevated the expression of fucosyltransferase 1 (FUT1). Silencing of TUG1 repressed osteoarthritis progression via downregulation of FUT1 by inhibition of miR-17–5p, which was due to promotion of viability and inhibition of apoptosis and ECM degradation in chondrocytes (Li et al., 2020c). Duan et al. reported that LncRNA TUG1 levels and loci at rs5749201, rs7284767 and rs886471 were correlated with knee osteoarthritis development (Duan et al., 2021). In addition, one study revealed that lncRNA TUG1 governed ECM degradation of chondrocytes in osteoarthritis via control of miR-320c/MMP-13 pathway (Han and Liu, 2021). Taken together, lncRNA TUG1 could be associated with osteoarthritis pathogenesis (Figure 3).
Other lncRNAs
Accumulating evidence indicated that besides the abovementioned lncRNAs, numerous other lncRNAs regulate osteoarthritis development and progression (Table 2). For instance, lncRNA AC006064.4-201 destabilized CDKN1B mRNA by interaction with PTBP1, which led to alleviation of cartilage senescence and protection of osteoarthritis (Shen et al., 2023). LncRNA WDR11-AS1 can bind to RNA-binding protein PABPC1 and then increase the SOX9 stabilization, resulting in facilitating extracellular matrix synthesis in osteoarthritis (Huang et al., 2023). LncRNA NAV2-AS5 alleviated chondrocyte inflammation via inhibition of miR-8082 and upregulation of TNFAIP3 interacting protein 2 (TNIP2) in osteoarthritis (Wang et al., 2023). LncRNA TM1-3P affected miR-144–3p and ONECUT2 and influenced apoptosis, inflammation, and proliferation of fibroblasts in osteoarthritis (Yi et al., 2022). LncRNA PMS2L2 suppressed proliferation of chondrocyte via promotion of miR-34a expression in osteoarthritis (Yang et al., 2022). LncRNA MINCR blocked progression of osteoarthritis through upregulation of BMPR2 expression by sponging miR-146a-5p (Li et al., 2022). LncRNA CRNDE relieved the progression of osteoarthritis via upregulation of dapper antagonist of catenin-1 (DACT1) by epigenetic modification and inactivation of Wnt/β-catenin pathway (Zhang et al., 2022). LncRNA Gm37494 relieved chondrocyte injury by interacting with miR-181a-5p and upregulating GABRA1 expression in osteoarthritis (Yuan et al., 2022). LncRNA LEMD1-AS1 alleviated chondrocyte inflammation by upregulation of PGAP1 via sponging miR-944 in osteoarthritis (Li et al., 2022).
TABLE 2.
Numerous lncRNAs regulate osteoarthritis progression.
| LncRNA | miRNA | Targets | Functions | Ref |
|---|---|---|---|---|
| NAV2-AS5 | miR-8082 | TNIP2 | Alleviates chondrocyte inflammation | Wang et al. (2023b) |
| TM1-3P | miR-144–3p | ONECUT2 | Influences apoptosis, inflammation and proliferation | Yi et al. (2022) |
| PMS2L2 | miR-34a | N/A | Suppresses proliferation of chondrocyte | Yang et al. (2022b) |
| MINCR | miR-146a-5p | BMPR2 | Blocks progression of osteoarthritis | Li et al. (2022a) |
| Gm37494 | miR-181a-5p | GABRA1 | Relieves chondrocyte injury | Yuan et al. (2022) |
| LEMD1-AS1 | miR-944 | PGAP1 | Alleviates chondrocyte inflammation | Li et al. (2022b) |
| POU3F3 | miR-29a-3p | FOXO3 | Relieves osteoarthritis pathogenesis | Shi et al. (2022) |
| MCM3AP-AS1 | miR-149–5p | Notch1 | Promotes osteoarthritis progression | Xu et al. (2022b) |
| LINC00473 | miR-424–5p | LY6E | Exacerbates osteoarthritis progression, induces apoptosis, proinflammatory cytokine production | Fan et al. (2021b) |
| LINC01385 | miR-140–3p | TLR4 | Promotes osteoarthritis progression | Wang et al. (2021e) |
| MIR22HG | miR-9-3p | ADAMTS5 | Facilitates osteoarthritis | Long et al. (2021) |
| CASC19 | miR-152–3p | DDX6 | Enhances apoptosis, induces proinflammatory cytokine production | Zhou et al. (2021b) |
| FGD5-AS1 | miR-302d-3p | TGFBR2 | Inhibits osteoarthritis development | Yang et al. (2021b) |
| KCNQ1OT1 | miR-211–5p | TCF4 | Promotes osteoarthritis | Aili et al. (2021) |
| OIP5-AS1 | miR-29b-3p | PGRN | Aggravates osteoarthritis progression | Zhi et al. (2021) |
| HOTTIP | miR-455–3p | CCL3 | Causes cartilage degradation | Mao et al. (2019) |
| HOTTIP | miR-663a | Fyn | Accelerates osteoarthritis | He et al. (2021b) |
| RMRP | miR-206 | CDK9 | Induces apoptosis, inhibits proliferation | Lu et al. (2020b) |
| LOXL1-AS1 | miR-423–5p | KDM5C | Enhances osteoarthritis | Chen et al. (2020c) |
| LINC00511 | miR-150–5p | SP1 | Affects proliferation and apoptosis | Zhang et al. (2020d) |
| IGHCγ1 | miR-6891–3p | TLR4 | Regulates macrophage inflammation | Zhang et al. (2020e) |
| LINC00623 | miR-101 | HRAS | Regulates senescence and apoptosis | Lu et al. (2020c) |
| NKILA | miR-145 | SP1, NF-kB | Inhibits apoptosis, induces proliferation | Xue et al. (2020) |
| MFI2-AS1 | miR-130a-3p | TCF4 | Promotes LPS-induced osteoarthritis | Luo et al. (2020) |
| PART1 | miR-373–3p | SOX4 | Impacts proliferation and apoptosis | Zhu and Jiang (2019) |
| PART1 | miR-590–3p | TGFBR2, Smad3 | Regulates viability, apoptosis | Lu et al. (2019a) |
| TNFSF10 | miR-376–3p | FGFR1 | Governs osteoarthritis progression | Huang et al. (2019a) |
| KLF3-AS1 | miR-206 | GIT1 | Regulates proliferation and apoptosis | Liu et al. (2018) |
| FOXD2-AS1 | miR-206 | CCND1 | Controls proliferation of chondrocytes | Cao et al. (2018a) |
LncRNA ZNFX1 antisense 1 (ZFAS1) reduced anti-oxidative stress via sponge of miR-1323 in osteoarthritis (Gu et al., 2022). LncRNA PILA enhanced the activity of PRMT1 and activated NF-κB pathway in osteoarthritis (Tang et al., 2022). LncRNA PRNCR1 affected apoptosis and proliferation of synoviocytes via binding with miR-377–3p in osteoarthritis (Wang G. et al., 2022). LncRNA POU3F3 relieved osteoarthritis pathogenesis via interaction with miR-29a-3p and upregulation of FOXO3 (Shi et al., 2022). LncRNA MCM3AP-AS1 promoted progression of osteoarthritis via modulation of miR-149–5p and Notch1 pathways (Xu et al., 2022). LINC00265 sponged miR-101–3p and regulated proliferation, inflammation and apoptosis of chondrocytes in osteoarthritis (Zou et al., 2021). LINC00473 exacerbated progression of osteoarthritis by enhancement of proinflammatory cytokine production and induction of chondrocyte apoptosis via influencing the miR-424–5p/LY6E axis (Fan et al., 2021). LINC01385 downregulation abolished progression of osteoarthritis via sponging miR-140–3p and increasing TLR4 expression (Wang Z. et al., 2021). LncRNA THUMPD3-AS1 accelerated inflammatory response and promoted chondrocyte proliferation in osteoarthritis (Wang et al., 2021). Downregulation of HOTAIRM1-1 promoted osteoarthritis development via regulation of miR-125b in cartilage tissues (Liu et al., 2021). LncRNA MIR22HG facilitated osteoarthritis progression via targeting miR-9-3p/ADAMTS5 axis (Long et al., 2021). LncRNA FER1L4 governed the expression of IL-6 and regulated osteoarthritis in chondrocyte cells (He et al., 2021). LncRNA CASC19 exacerbated osteoarthritis development via modulation of miR-152–3p and DDX6, which enhanced chondrocytes apoptosis and induced proinflammatory cytokine production (Zhou et al., 2021).
LncRNA FGD5-AS1 controlled miR-302d-3p and TGFBR2 expression and caused inhibition of osteoarthritis development (Yang et al., 2021). Silencing of lncRNA KCNQ1OT1 blocked the progression of osteoarthritis via changing the miR-211–5p/TCF4 axis (Aili et al., 2021). LncRNA OIP5-AS1 sponged the miR-30a-5p and affected the function of chondrocytes in osteoarthritis (Qin et al., 2021). Similarly, another study found that depletion of OIP5-AS1 affected miR-29b-3p/PGRN axis and aggravated osteoarthritis development (Zhi et al., 2021). LncRNA HOTTIP sponged miR-455–3p and increased the expression of CCL3 and led to degradation of cartilage (Mao et al., 2019). Moreover, HOTTIP was reported to control miR-663a/Fyn-related kinase axis and accelerate osteoarthritis progression (He et al., 2021). Depletion of lncRNA RMRP repressed apoptosis and induced proliferation of osteoarthritis chondrocytes via inhibition of miR-206 and upregulation of CDK9 (Lu et al., 2020). LncRNA PCAT-1 modulated the expression of miR-27b-3p and changed apoptosis of chondrocytes in osteoarthritis (Zhou et al., 2021). LncRNA MIR4435-2HG suppressed the expression of miR-510–3p and attenuated osteoarthritis progression (Liu et al., 2020c). Chen et al. found that lncRNA LOXL1-AS1 modulated the miR-423–5p/KDM5C pathway and resulted in osteoarthritis progression, which could be activated by JUND (Chen et al., 2020). Zhang et al. found a positive feedback loop between LINC00511, miR-150–5p and SP1, which affected chondrocyte apoptosis and proliferation in osteoarthritis (Zhang et al., 2020). LncRNA IGHCγ1 reduced the expression of miR-6891–3p and upregulated TLR4 expression levels, contributing to regulation of macrophage inflammation in osteoarthritis (Zhang et al., 2020). LncRNA CTBP1-AS2 promoted the miR-130a gene methylation and led to suppression of chondrocyte proliferation in osteoarthritis (Zhang et al., 2020f). LncRNA BLACAT1 was discovered to govern differentiation of bone marrow stromal stem cells via sponging miR-142–5p in osteoarthritis (Ji et al., 2020). IL-1β-induced degradation of ECM was modulated by LINC00623 due to regulation of miR-101 and HRAS, leading to regulation of senescence and apoptosis of osteoarthritis chondrocytes (Lu et al., 2020). Downregulation of lncRNA NKILA enhanced apoptosis and reduced proliferation of chondrocytes by targeting miR-145, SP1 and NF-κB in osteoarthritis (Xue et al., 2020).
LncRNA CASC2 can be regulated by miR-93–5p and affect chondrocyte apoptosis in osteoarthritis (Sun et al., 2020). Li and coworkers found that lncRNA ANRIL regulated apoptosis and proliferation of osteoarthritis synoviocytes and governed osteoarthritis progression (Li et al., 2019). Downregulation of lncRNA MFI2-AS1 reduced LPS-mediated osteoarthritis progression via impacting the miR-130a-3p/TCF4 axis (Luo et al., 2020). LncRNA CAIF attenuated LPS-mediated IL-6 upregulation via suppression of miR-1246 in osteoarthritis (Qi et al., 2019). LncRNA PART1 impacted miR-373–3p/SOX4 axis and affected proliferation and apoptosis of chondrocytes as well as ECM degradation in osteoarthritis (Zhu and Jiang, 2019). LncRNA PART1 sponged miR-590–3p and regulated TGFBR2/Smad3, resulting in regulation of viability and apoptosis of chondrocytes in osteoarthritis (Lu et al., 2019). LncRNA MIR4435-2HG was dissected to regulate proliferation and apoptosis of chondrocyte in osteoarthritis (Xiao et al., 2019). LncRNA TNFSF10 impacted the miR-376–3p/FGFR1 pathway and led to osteoarthritis progression (Huang et al., 2019). One positive association between lncRNA ANCR and TGF-β1 was reported in osteoarthritis patients (Li et al., 2019). LncRNA DILC was identified to regulate the expression of IL-6 in chondrocytes and participated in osteoarthritis (Huang et al., 2019). Similarly, lncRNA CASC2 regulated the expression of IL-17 and modulated apoptosis and proliferation of chondrocytes in osteoarthritis (Huang et al., 2019). LncRNA FOXD2-AS1 facilitated proliferation of chondrocytes via suppression of miR-27a-3p in osteoarthritis (Wang et al., 2019b). LncRNA TM1P3 mediated ECM degradation of chondrocytes and took part in osteoarthritis progression (Li et al., 2019). LINC00341 blocked osteoarthritis progression via enhancement of chondrocyte survival (Yang et al., 2019). Inhibition of LOC101928134 reduced the synovial hyperplasia and cartilage degradation of osteoarthritis rats via activation of JAK/STAT pathway by promotion of IFNA1 expression (Yang et al., 2019).
Lu et al. reported that cell apoptosis of chondrocytes was governed by lncRNA-CIR in osteoarthritis (Lu et al., 2019). LncRNA-CIR modulated autophagy and accelerated degeneration of articular cartilage in osteoarthritis (Wang et al., 2018). Moreover, lncRNA CIR sponged miR-27b and accelerated ECM degradation of chondrocytes in osteoarthritis (Li et al., 2017). LncRNA KLF3-AS1 affected miR-206 and GIT1 expressions in osteoarthritis, contributing to MSC-derived exosome-mediated promotion of proliferation and inhibition of apoptosis of chondrocytes (Liu et al., 2018). LncRNA-p21 served as a sponge of miR-451 and led to induction of apoptosis of chondrocytes in osteoarthritis (Tang et al., 2018). LncRNA GACAT3 governed IL-6/STAT3 pathway and induced proliferation of synoviocytes in osteoarthritis (Li et al., 2018c). LncRNA FOXD2-AS1 sponged miR-206 and upregulated CCND1 expression, governing proliferation of chondrocyte in osteoarthritis (Cao et al., 2018a). LncRNA FAS-AS1 was dissected to accelerate the ECM degradation of cartilage in osteoarthritis (Zhu et al., 2018). One study identified the critical role of lncRNA ZFAS1 in regulation of migration, apoptosis and proliferation of chondrocytes in osteoarthritis (Ye et al., 2018). Depletion of lncRNA RP11-445H22.4 reduced LPS-mediated injuries via modulation of miR-301a in osteoarthritis (Sun et al., 2018). LncRNA UFC1 facilitated proliferation of chondrocytes due to sponging miR-34a in osteoarthritis (Zhang et al., 2016). Taken together, lncRNAs play an essential role in osteoarthritis progression.
LncRNAs as biomarkers for osteoarthritis progression
Wu et al. identified exosomal mRNA, lncRNA and circRNA signatures in an OA synovial fluid-exosomal study (Wu et al., 2022). This work reported that 196 lncRNAs, 98 circRNAs, and 52 mRNAs were differentially expressed between healthy control and OA synovial exosomes. Moreover, 45 lncRNAs, 34 circRNAs, and 22 miRNAs were associated with the PI3K/Akt and autophagy pathways, which were linked to 7 mRNAs and might contribute to OA pathological process (Wu et al., 2022). One group identified that the expression of exosomal lncRNA PCGEM1 was higher in early OA than normal controls, and higher in late-stage OA than in early OA, suggesting that synovial fluid-derived exosomal lncRNA PCGEM1 could be a useful biomarker for the different stages of OA (Zhao and Xu, 2018). LncRNA Nespas was reported to be associated with osteoarthritis pathogenesis by upregulation of ACSL6, indicating that Nespas could act as a prognostic biomarker (Park et al., 2019). LncRNA HOTTIP was upregulated in the processes of endochondral ossification and osteoarthritis pathogenesis. HOTTIP might be a potential predictive biomarker for osteoarthritis (Kim et al., 2013). In addition, lncRNA DANCR was elevated in osteoarthritis patients and was validated as a useful biomarker and treatment target for osteoarthritis (Zhang et al., 2018). LncRNA TNFSF10 was validated as a novel potential biomarker for osteoarthritis progression (Huang et al., 2019). One study suggested that lncRNA SNHG5 might be a promising biomarker for osteoarthritis treatment (Jiang et al., 2021). Another study used RNA sequencing and found LINC00167 as a novel diagnosis biomarker for osteoarthritis (Jiang et al., 2020). LncRNA HOTAIR was highly expressed in osteoarthritis chondrocytes, suggesting that it could be a biomarker for osteoarthritis (Chen et al., 2023). Altogether, lncRNAs could act as biomarkers for osteoarthritis diagnosis and prognosis.
Compounds target lncRNAs to regulate osteoarthritis progression
In recent years, some compounds have been identified to alleviate osteoarthritis progression (Wu et al., 2023). Baicalin, a flavonoid isolated from the roots of Scutellaria baicalensis Georgi (Lamiaceae), has been primarily used in traditional Chinese Medicine (Srinivas, 2010). Baicalin has been reported to treat different diseases via exerting its various functions, such as anticancer, antioxidant, antiviral, anti-inflammatory effects (Li et al., 2021; Ganguly et al., 2022; Song et al., 2023; Wang and Li, 2023). Baicalin has shown its protective functions in OA pathological process. For example, baicalin prevented endplate chondrocyte apoptosis via suppression of H2O2-mediated oxidative stress (Pan et al., 2017). Baicalin reduced IL-1β-induced inflammatory response in human chondrocytes (Xing et al., 2017). Baicalin inhibited the expression of miR-126 and reduced IL-1β-triggered inflammatory injury in chondrocytes (Yang et al., 2018). In line with this report, Baicalin reduced muscular oxidative stress and alleviated joint pain and muscle dysfunction in OA rat model (Chen et al., 2018). One study showed that Baicalin modulated endoplasmic reticulum stress and protected chondrocytes from OA (Cao et al., 2018b). Another study demonstrated that baicalin blocked IL-1β-stimulated apoptosis and ECM degradation via activation of autophagy by targeting miR-766–3p and AIFM1 pathway, leading to protection of OA chondrocytes (Li et al., 2020d). Baicalin activated HIF-1α and increased extracellular matrix synthesis in chondrocytes (Wang et al., 2020). Moreover, baicalin enhanced the extracellular matrix synthesis and elevated chondrocyte viability via modulation of TGF-β/Smad3 pathway in chondrocytes (Wang et al., 2021). Recently, baicalin alleviated IL-1β-mediated OA chondrocytes damage via promotion of mitophagy (He and He, 2023). Notably, baicalin exerted therapeutic effects by inhibiting the expression of lncRNA HOTAIR, decreasing the protein levels of p-PI3K and p-AKT, and increasing the protein levels of PTEN, APN, and ADIPOR1 (Chen et al., 2023). Hence, baicalin could be a useful agent to protect OA.
Schisantherin A attenuated IL-1β-mediated inflammation via inactivation of NF-κB and MAPKs in chondrocytes (Liao et al., 2016). One study showed that protectin DX repressed IL-1β-involved inflammation and ameliorated osteoarthritis development via regulation of the AMPK and NF-κB pathways in chondrocytes (Piao et al., 2020). Salvianolic acid B reduced IL-1β-mediated inflammatory cytokine production in chondrocytes in osteoarthritis and protected osteoarthritis progression (Lou et al., 2017). Daurisoline activated the PI3K/Akt/mTOR axis and led to inhibition of H2O2-mediated autophagy in chondrocytes (Zhang et al., 2023b). Oroxin B suppressed the PI3K/Akt/mTOR pathway and induced autophagy and anti-inflammation, leading to inhibition of osteoarthritis progression (Lu et al., 2022). Icariin, a kind of flavonoid compound, promoted HIF-1α in chondrocytes and accelerated cartilage repair (Wang et al., 2016). Resveratrol was reported to regulate the lncRNA MALAT1 and miR-9/NF-κB axis and retard osteoarthritis progression (Zhang et al., 2020). Kaempferol reduced the functions of lncRNA XIST and miR-130a/STAT3 on ECM degradation and inflammation in osteoarthritis (Xiao et al., 2021). Hence, compounds could attenuate osteoarthritis progression via targeting lncRNA expressions.
Conclusion and perspectives
In summary, numerous lncRNAs play a critical regulatory role in osteoarthritis development and progression. Multiple lncRNAs could be useful for acting biomarkers for diagnosis, prognosis and therapeutic targets. Further understanding the functions and molecular mechanism of lncRNAs in osteoarthritis is necessary to improve the therapeutic outcome of osteoarthritis patients. Without a doubt, several issues should be mentioned to better understand the role of ncRNAs in osteoarthritis. Besides lncRNAs, miRNAs, and circRNAs have also been reported to participate in osteoarthritis progression. It is known that circRNAs form a covalently closed loop and regulate gene expression, leading to governing cellular processes. Accumulating evidence has suggested that circRNAs play a critical role in osteoarthritis initiation and progression (Wu and Zou, 2021; Wang et al., 2023; Li and Lu, 2023; Xue et al., 2023).
Xiang et al. used RNA sequencing and revealed the circular RNA expression profiles in osteoarthritic synovium (Xiang et al., 2019b). By an integrated bioinformatics analysis, 120 circRNAs were differentially expressed in OA synovium. Five decreased circRNAs and one increased circRNAs were confirmed by qRT-PCR approach (Xiang et al., 2019b). One study identified circRNA expression profile of articular chondrocytes using IL-1β-induced osteoarthritis in mice (Zhou et al., 2018). Another study screened differentially expressed circRNAs of cartilages in patients with osteoarthritis (Wang et al., 2019c). In addition, lncRNAs are involved in rheumatoid arthritis development (Lao and Xu, 2020). For example, lncRNA SNHG1 interacted with PTBP1 (polypyridine tract-binding protein 1) and promoted rheumatoid synovial proliferation and invasion (Liu F. et al., 2021). One lncRNA can regulate the expression of the other lncRNA to regulate osteoarthritis. For instance, lncRNA PACER overexpression suppressed apoptosis of chondrocyte. PACER upregulation led to inhibition of HOTAIR. HOTAIR upregulation induced chondrocyte apoptosis. Consistently, plasma PACER was decreased in osteoarthritis patients, whereas plasma HOTAIR was increased in OA samples. PACER governed chondrocyte apoptosis via inhibition of HOTAIR in osteoarthritis (Jiang et al., 2019).
In this review, we summarize the functions and mechanisms of numerous lncRNAs in governing OA pathogenesis. It is unclear which lncRNA plays the most important role in regulating OA development and progression. Which lncRNAs are key biomarkers for diagnosis and prognosis of OA patients? Which strategy is a best approach for targeting lncRNAs in OA treatment? It is necessary to explore whether anti-inflammatory drugs in combination with lncRNA inhibitors or activators would bring a better benefit for OA patients. Altogether, in-depth investigations are required to determine the molecular mechanisms of lncRNAs-mediated osteoarthritis development and progression.
Funding Statement
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Author contributions
XZ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Writing–original draft. QL: Formal Analysis, Investigation, Methodology, Resources, Software, Writing–original draft. JZ: Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Writing–original draft. CS: Data curation, Formal Analysis, Investigation, Software, Writing–original draft. ZH: Data curation, Formal Analysis, Methodology, Resources, Writing–original draft. JW: Data curation, Formal Analysis, Methodology, Software, Writing–original draft. LS: Investigation, Project administration, Resources, Software, Supervision, Writing–original draft. WL: Conceptualization, Software, Supervision, Validation, Writing–review and editing. JH: Investigation, Supervision, Validation, Visualization, Writing–review and editing. PW: Supervision, Validation, Visualization, Writing–review and editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Abbasifard M., Kamiab Z., Bagheri-Hosseinabadi Z., Sadeghi I. (2020). The role and function of long non-coding RNAs in osteoarthritis. Exp. Mol. Pathol. 114, 104407. 10.1016/j.yexmp.2020.104407 [DOI] [PubMed] [Google Scholar]
- Aili D., Wu T., Gu Y., Chen Z., Wang W. (2021). Knockdown of long non-coding RNA KCNQ1OT1 suppresses the progression of osteoarthritis by mediating the miR-211-5p/TCF4 axis in vitro . Exp. Ther. Med. 21 (5), 455. 10.3892/etm.2021.9886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alnajjar F. A., Sharma-Oates A., Wijesinghe S. N., Farah H., Nanus D. E., Nicholson T., et al. (2021). The expression and function of metastases associated lung adenocarcinoma transcript-1 long non-coding RNA in subchondral bone and osteoblasts from patients with osteoarthritis. Cells 10 (4), 786. 10.3390/cells10040786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L., Huang N., Zhang X., Wu S., Wang L., Ke Q. (2022). Long non-coding RNA plasmacytoma variant translocation 1 and growth arrest specific 5 regulate each other in osteoarthritis to regulate the apoptosis of chondrocytes. Bioengineered 13 (5), 13680–13688. 10.1080/21655979.2022.2063653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., Zhang Y., Wang T., Li B. (2018a). Endoplasmic reticulum stress is involved in baicalin protection on chondrocytes from patients with osteoarthritis. Dose Response 16 (4), 1559325818810636. 10.1177/1559325818810636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L., Wang Y., Wang Q., Huang J. (2018b). LncRNA FOXD2-AS1 regulates chondrocyte proliferation in osteoarthritis by acting as a sponge of miR-206 to modulate CCND1 expression. Biomed. Pharmacother. 106, 1220–1226. 10.1016/j.biopha.2018.07.048 [DOI] [PubMed] [Google Scholar]
- Chalian M., Roemer F. W., Guermazi A. (2023). Advances in osteoarthritis imaging. Curr. Opin. Rheumatol. 35 (1), 44–54. 10.1097/BOR.0000000000000917 [DOI] [PubMed] [Google Scholar]
- Chen D. S., Cao J. G., Zhu B., Wang Z. L., Wang T. F., Tang J. J. (2018). Baicalin attenuates joint pain and muscle dysfunction by inhibiting muscular oxidative stress in an experimental osteoarthritis rat model. Arch. Immunol. Ther. Exp. Warsz. 66 (6), 453–461. 10.1007/s00005-018-0518-6 [DOI] [PubMed] [Google Scholar]
- Chen H., Yang S., Shao R. (2019). Long non-coding XIST raises methylation of TIMP-3 promoter to regulate collagen degradation in osteoarthritic chondrocytes after tibial plateau fracture. Arthritis Res. Ther. 21 (1), 271. 10.1186/s13075-019-2033-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., Fang H., Xu N. (2020c). LncRNA LOXL1-AS1 is transcriptionally activated by JUND and contributes to osteoarthritis progression via targeting the miR-423-5p/KDM5C axis. Life Sci. 258, 118095. 10.1016/j.lfs.2020.118095 [DOI] [PubMed] [Google Scholar]
- Chen K., Zhu H., Zheng M. Q., Dong Q. R. (2021). LncRNA MEG3 inhibits the degradation of the extracellular matrix of chondrocytes in osteoarthritis via targeting miR-93/TGFBR2 Axis. Cartilage 13 (2), 1274S–1284S. 10.1177/1947603519855759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. K., Yu X. H., Yang W., Wang C., He W. S., Yan Y. G., et al. (2017). lncRNAs: novel players in intervertebral disc degeneration and osteoarthritis. Cell Prolif. 50 (1), e12313. 10.1111/cpr.12313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Liu J., Sun Y., Wen J., Zhou Q., Ding X., et al. (2023). Correlation analysis of differentially expressed long non-coding RNA HOTAIR with PTEN/PI3K/AKT pathway and inflammation in patients with osteoarthritis and the effect of baicalin intervention. J. Orthop. Surg. Res. 18 (1), 34. 10.1186/s13018-023-03505-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Guo H., Li L., Bao D., Gao F., Li Q., et al. (2020a). Long non-coding RNA (lncRNA) small nucleolar RNA host gene 15 (SNHG15) alleviates osteoarthritis progression by regulation of extracellular matrix homeostasis. Med. Sci. Monit. 26, e923868. 10.12659/MSM.923868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Zhang L., Li E., Zhang G., Hou Y., Yuan W., et al. (2020b). Long-chain non-coding RNA HOTAIR promotes the progression of osteoarthritis via sponging miR-20b/PTEN axis. Life Sci. 253, 117685. 10.1016/j.lfs.2020.117685 [DOI] [PubMed] [Google Scholar]
- Cheng Y., Wu X., Xia Y., Liu W., Wang P. (2022). The role of lncRNAs in regulation of DKD and diabetes-related cancer. Front. Oncol. 12, 1035487. 10.3389/fonc.2022.1035487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L. B., Li Y., Liu G. Y., Li T. H., Li F., Guan J., et al. (2020). Long non-coding RNA PVT1, a molecular sponge of miR-26b, is involved in the progression of hyperglycemia-induced collagen degradation in human chondrocytes by targeting CTGF/TGF-beta signal ways. Innate Immun. 26 (3), 204–214. 10.1177/1753425919881778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C., Liu X., Li J., Lan D., Zheng S. (2022). Dysregulation of the HOTAIR-miR-152-camkiiα Axis in craniosynostosis results in impaired osteoclast differentiation. Front. Genet. 13, 787734. 10.3389/fgene.2022.787734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou P., Hu R., Zhu W., Tang Q., Li D., Li H., et al. (2017). Long non-coding RNA HOTAIR promotes expression of ADAMTS-5 in human osteoarthritic articular chondrocytes. Pharmazie 72 (2), 113–117. 10.1691/ph.2017.6649 [DOI] [PubMed] [Google Scholar]
- Duan J., Shen T., Dong H., Han S., Li G. (2021). Association of the expression levels of long-chain noncoding RNA TUG1 and its gene polymorphisms with knee osteoarthritis. Genet. Test. Mol. Biomarkers 25 (2), 102–110. 10.1089/gtmb.2020.0208 [DOI] [PubMed] [Google Scholar]
- Eddy S. R. (2001). Non-coding RNA genes and the modern RNA world. Nat. Rev. Genet. 2 (12), 919–929. 10.1038/35103511 [DOI] [PubMed] [Google Scholar]
- Fan G., Liu J., Zhang Y., Guan X. (2021b). LINC00473 exacerbates osteoarthritis development by promoting chondrocyte apoptosis and proinflammatory cytokine production through the miR-424-5p/LY6E axis. Exp. Ther. Med. 22 (5), 1247. 10.3892/etm.2021.10682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H., Ding L., Yang Y. (2021a). lncRNA SNHG16 promotes the occurrence of osteoarthritis by sponging miR-373-3p. Mol. Med. Rep. 23 (2), 117. 10.3892/mmr.2020.11756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X., Yuan J., Xie J., Pan Z., Yao X., Sun X., et al. (2018). Long non-protein coding RNA DANCR functions as a competing endogenous RNA to regulate osteoarthritis progression via miR-577/SphK2 axis. Biochem. Biophys. Res. Commun. 500 (3), 658–664. 10.1016/j.bbrc.2018.04.130 [DOI] [PubMed] [Google Scholar]
- Fang P., Zhang L. X., Hu Y., Zhang L., Zhou L. W. (2019). Long non-coding RNA DANCR induces chondrogenesis by regulating the miR-1275/MMP-13 axis in synovial fluid-derived mesenchymal stem cells. Eur. Rev. Med. Pharmacol. Sci. 23 (23), 10459–10469. 10.26355/eurrev_201912_19685 [DOI] [PubMed] [Google Scholar]
- Fu C., Qiu Z., Huang Y., Lin Q., Jin L., Tu H., et al. (2022). Achyranthes bidentata polysaccharides alleviate endoplasmic reticulum stress in osteoarthritis via lncRNA NEAT1/miR-377-3p pathway. Biomed. Pharmacother. 154, 113551. 10.1016/j.biopha.2022.113551 [DOI] [PubMed] [Google Scholar]
- Fu M., Huang G., Zhang Z., Liu J., Zhang Z., Huang Z., et al. (2015). Expression profile of long noncoding RNAs in cartilage from knee osteoarthritis patients. Osteoarthr. Cartil. 23 (3), 423–432. 10.1016/j.joca.2014.12.001 [DOI] [PubMed] [Google Scholar]
- Ganguly R., Gupta A., Pandey A. K. (2022). Role of baicalin as a potential therapeutic agent in hepatobiliary and gastrointestinal disorders: A review. World J. Gastroenterol. 28 (26), 3047–3062. 10.3748/wjg.v28.i26.3047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G. C., Cheng X. G., Wei Q. Q., Chen W. C., Huang W. Z. (2019). Long noncoding RNA MALAT-1 inhibits apoptosis and matrix metabolism disorder in interleukin-1β-induced inflammation in articular chondrocytes via the JNK signaling pathway. J. Cell Biochem. 120 (10), 17167–17179. 10.1002/jcb.28977 [DOI] [PubMed] [Google Scholar]
- Gareev I., Kudriashov V., Sufianov A., Begliarzade S., Ilyasova T., Liang Y., et al. (2022). The role of long non-coding RNA ANRIL in the development of atherosclerosis. Noncoding RNA Res. 7 (4), 212–216. 10.1016/j.ncrna.2022.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal B., Bishnoi S., Parveen S., Patel D., Yasmeen T. A., Tarekar A. (2023). Managing arthritis pain: medications and lifestyle changes. Georgian Med. News (339), 117–122. [PubMed] [Google Scholar]
- Gu Y., Wang G., Xu H. (2022). Long non-coding RNA ZNFX1 antisense 1 (ZFAS1) suppresses anti-oxidative stress in chondrocytes during osteoarthritis by sponging microRNA-1323. Bioengineered 13 (5), 13188–13200. 10.1080/21655979.2022.2074770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H., Liu L. (2021). Long noncoding RNA TUG1 regulates degradation of chondrocyte extracellular matrix via miR-320c/MMP-13 axis in osteoarthritis. Open Life Sci. 16 (1), 384–394. 10.1515/biol-2021-0037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B., Jiang D. (2020). HOTAIR-induced apoptosis is mediated by sponging miR-130a-3p to repress chondrocyte autophagy in knee osteoarthritis. Cell Biol. Int. 44 (2), 524–535. 10.1002/cbin.11253 [DOI] [PubMed] [Google Scholar]
- He J., He J. (2023). Baicalin mitigated IL-1β-Induced osteoarthritis chondrocytes damage through activating mitophagy. Chem. Biol. Drug Des. 101 (6), 1322–1334. 10.1111/cbdd.14215 [DOI] [PubMed] [Google Scholar]
- He J., Wang L., Ding Y., Liu H., Zou G. (2021a). lncRNA FER1L4 is dysregulated in osteoarthritis and regulates IL-6 expression in human chondrocyte cells. Sci. Rep. 11 (1), 13032. 10.1038/s41598-021-92474-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J. Y., Cheng M., Ye J. L., Peng C. H., Chen J., Luo B., et al. (2022). YY1-induced lncRNA XIST inhibits cartilage differentiation of BMSCs by binding with TAF15 to stabilizing FUT1 expression. Regen. Ther. 20, 41–50. 10.1016/j.reth.2022.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Gao K., Lu S., Wu R. (2021b). LncRNA HOTTIP leads to osteoarthritis progression via regulating miR-663a/Fyn-related kinase axis. BMC Musculoskelet. Disord. 22 (1), 67. 10.1186/s12891-020-03861-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkinson T., Kelly D. C., Curtin C. M., O'Brien F. J. (2022). Mechanosignalling in cartilage: an emerging target for the treatment of osteoarthritis. Nat. Rev. Rheumatol. 18 (2), 67–84. 10.1038/s41584-021-00724-w [DOI] [PubMed] [Google Scholar]
- Hu J., Wang Z., Shan Y., Pan Y., Ma J., Jia L. (2018). Long non-coding RNA HOTAIR promotes osteoarthritis progression via miR-17-5p/FUT2/β-catenin axis. Cell Death Dis. 9 (7), 711. 10.1038/s41419-018-0746-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Li S., Zou Y. (2019). Knockdown of LncRNA H19 relieves LPS-induced damage by modulating miR-130a in osteoarthritis. Yonsei Med. J. 60 (4), 381–388. 10.3349/ymj.2019.60.4.381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B., Yu H., Li Y., Zhang W., Liu X. (2019a). Upregulation of long noncoding TNFSF10 contributes to osteoarthritis progression through the miR-376-3p/FGFR1 axis. J. Cell Biochem. 120 (12), 19610–19620. 10.1002/jcb.29267 [DOI] [PubMed] [Google Scholar]
- Huang H., Yan J., Lan X., Guo Y., Sun M., Zhao Y., et al. (2023). LncRNA WDR11-AS1 promotes extracellular matrix synthesis in osteoarthritis by directly interacting with RNA-binding protein PABPC1 to stabilize SOX9 expression. Int. J. Mol. Sci. 24 (1), 817. 10.3390/ijms24010817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Liu L., Yang J., Ding J., Xu X. (2019b). lncRNA DILC is downregulated in osteoarthritis and regulates IL-6 expression in chondrocytes. J. Cell Biochem. 120 (9), 16019–16024. 10.1002/jcb.28880 [DOI] [PubMed] [Google Scholar]
- Huang T., Wang J., Zhou Y., Zhao Y., Hang D., Cao Y. (2019c). LncRNA CASC2 is up-regulated in osteoarthritis and participates in the regulation of IL-17 expression and chondrocyte proliferation and apoptosis. Biosci. Rep. 39 (5). 10.1042/BSR20182454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Chen D., Yan Z., Zhan J., Xue X., Pan X., et al. (2021). LncRNA MEG3 protects chondrocytes from IL-1β-induced inflammation via regulating miR-9-5p/KLF4 Axis. Front. Physiol. 12, 617654. 10.3389/fphys.2021.617654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idres F. A., Samaan M. (2023). Intra-articular platelet-rich plasma vs. corticosteroid injections efficacy in knee osteoarthritis treatment: A systematic review. Ann. Med. Surg. (Lond). 85 (2), 102–110. 10.1097/MS9.0000000000000106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y., Fang Q. Y., Wang S. N., Zhang Z. W., Hou Z. J., Li J. N., et al. (2020). Lnc-RNA BLACAT1 regulates differentiation of bone marrow stromal stem cells by targeting miR-142-5p in osteoarthritis. Eur. Rev. Med. Pharmacol. Sci. 24 (6), 2893–2901. 10.26355/eurrev_202003_20653 [DOI] [PubMed] [Google Scholar]
- Jiang H., Pang H., Wu P., Cao Z., Li Z., Yang X. (2021). LncRNA SNHG5 promotes chondrocyte proliferation and inhibits apoptosis in osteoarthritis by regulating miR-10a-5p/H3F3B axis. Connect. Tissue Res. 62 (6), 605–614. 10.1080/03008207.2020.1825701 [DOI] [PubMed] [Google Scholar]
- Jiang L., Zhou Y., Shen J., Chen Y., Ma Z., Yu Y., et al. (2020b). RNA sequencing reveals LINC00167 as a potential diagnosis biomarker for primary osteoarthritis: A multi-stage study. Front. Genet. 11, 539489. 10.3389/fgene.2020.539489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M., Liu J., Luo T., Chen Q., Lu M., Meng D. (2019). LncRNA PACER is down-regulated in osteoarthritis and regulates chondrocyte apoptosis and lncRNA HOTAIR expression. Biosci. Rep. 39 (6). 10.1042/BSR20190404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W., Xia J., Xie S., Zou R., Pan S., Wang Z. W., et al. (2020a). Long non-coding RNAs as a determinant of cancer drug resistance: towards the overcoming of chemoresistance via modulation of lncRNAs. Drug Resist Updat 50, 100683. 10.1016/j.drup.2020.100683 [DOI] [PubMed] [Google Scholar]
- Kellgren J. H., Lawrence J. S. (1957). Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 16 (4), 494–502. 10.1136/ard.16.4.494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Song J., Han J., Kim Y., Chun C. H., Jin E. J. (2013). Two non-coding RNAs, MicroRNA-101 and HOTTIP contribute cartilage integrity by epigenetic and homeotic regulation of integrin-α1. Cell Signal 25 (12), 2878–2887. 10.1016/j.cellsig.2013.08.034 [DOI] [PubMed] [Google Scholar]
- Lao M. X., Xu H. S. (2020). Involvement of long non-coding RNAs in the pathogenesis of rheumatoid arthritis. Chin. Med. J. Engl. 133 (8), 941–950. 10.1097/CM9.0000000000000755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei J., Fu Y., Zhuang Y., Zhang K., Lu D. (2019). LncRNA SNHG1 alleviates IL-1β-induced osteoarthritis by inhibiting miR-16-5p-mediated p38 MAPK and NF-κB signaling pathways. Biosci. Rep. 39 (9). 10.1042/BSR20191523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Sun Y., Wan Y., Wu X., Yang W. (2020b). LncRNA NEAT1 promotes proliferation of chondrocytes via down-regulation of miR-16-5p in osteoarthritis. J. Gene Med. 22 (9), e3203. 10.1002/jgm.3203 [DOI] [PubMed] [Google Scholar]
- Li D., Wang X., Yi T., Zhang L., Feng L., Zhang M., et al. (2022a). LncRNA MINCR attenuates osteoarthritis progression via sponging miR-146a-5p to promote BMPR2 expression. Cell Cycle 21 (22), 2417–2432. 10.1080/15384101.2022.2099191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Lian K., Mao J., Huang F., Zhang C., Zang J. (2022b). LncRNA LEMD1-AS1 relieves chondrocyte inflammation by targeting miR-944/PGAP1 in osteoarthritis. Cell Cycle 21 (19), 2038–2050. 10.1080/15384101.2022.2084294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Xie S., Li H., Zhang R., Zhang H. (2020a). LncRNA MALAT1 mediates proliferation of LPS treated-articular chondrocytes by targeting the miR-146a-PI3K/Akt/mTOR axis. Life Sci. 254, 116801. 10.1016/j.lfs.2019.116801 [DOI] [PubMed] [Google Scholar]
- Li K., Liang Y., Cheng A., Wang Q., Li Y., Wei H., et al. (2021). Antiviral properties of baicalin: A concise review. Rev. Bras. Farmacogn. 31 (4), 408–419. 10.1007/s43450-021-00182-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Lv G., Wang B., Kuang L. (2018b). The role of lncRNA XIST/miR-211 axis in modulating the proliferation and apoptosis of osteoarthritis chondrocytes through CXCR4 and MAPK signaling. Biochem. Biophys. Res. Commun. 503 (4), 2555–2562. 10.1016/j.bbrc.2018.07.015 [DOI] [PubMed] [Google Scholar]
- Li Q., Zhang Z., Guo S., Tang G., Lu W., Qi X. (2019b). LncRNA ANCR is positively correlated with transforming growth factor-β1 in patients with osteoarthritis. J. Cell Biochem. 120 (9), 14226–14232. 10.1002/jcb.28881 [DOI] [PubMed] [Google Scholar]
- Li X., Huang T. L., Zhang G. D., Jiang J. T., Guo P. Y. (2019a). LncRNA ANRIL impacts the progress of osteoarthritis via regulating proliferation and apoptosis of osteoarthritis synoviocytes. Eur. Rev. Med. Pharmacol. Sci. 23 (22), 9729–9737. 10.26355/eurrev_201911_19535 [DOI] [PubMed] [Google Scholar]
- Li X., Ren W., Xiao Z. Y., Wu L. F., Wang H., Guo P. Y. (2018c). GACAT3 promoted proliferation of osteoarthritis synoviocytes by IL-6/STAT3 signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 22 (16), 5114–5120. 10.26355/eurrev_201808_15705 [DOI] [PubMed] [Google Scholar]
- Li X., Tang C., Wang J., Guo P., Wang C., Wang Y., et al. (2018a). Methylene blue relieves the development of osteoarthritis by upregulating lncRNA MEG3. Exp. Ther. Med. 15 (4), 3856–3864. 10.3892/etm.2018.5918 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Li Y., Li S., Luo Y., Liu Y., Yu N. (2017a). LncRNA PVT1 regulates chondrocyte apoptosis in osteoarthritis by acting as a sponge for miR-488-3p. DNA Cell Biol. 36 (7), 571–580. 10.1089/dna.2017.3678 [DOI] [PubMed] [Google Scholar]
- Li Y., Li Z., Li C., Zeng Y., Liu Y. (2019c). Long noncoding RNA TM1P3 is involved in osteoarthritis by mediating chondrocyte extracellular matrix degradation. J. Cell Biochem. 120 (8), 12702–12712. 10.1002/jcb.28539 [DOI] [PubMed] [Google Scholar]
- Li Y. F., Li S. H., Liu Y., Luo Y. T. (2017b). Long noncoding RNA CIR promotes chondrocyte extracellular matrix degradation in osteoarthritis by acting as a sponge for mir-27b. Cell Physiol. Biochem. 43 (2), 602–610. 10.1159/000480532 [DOI] [PubMed] [Google Scholar]
- Li Z., Cheng J., Liu J. (2020d). Baicalin protects human OA chondrocytes against IL-1β-induced apoptosis and ECM degradation by activating autophagy via MiR-766-3p/AIFM1 Axis. Drug Des. Devel Ther. 14, 2645–2655. 10.2147/DDDT.S255823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Lu J. (2023). CircRNAs in osteoarthritis: research status and prospect. Front. Genet. 14, 1173812. 10.3389/fgene.2023.1173812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Wang J., Yang J. (2020c). TUG1 knockdown promoted viability and inhibited apoptosis and cartilage ECM degradation in chondrocytes via the miR-17-5p/FUT1 pathway in osteoarthritis. Exp. Ther. Med. 20 (6), 154. 10.3892/etm.2020.9283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian L. P., Xi X. Y. (2020). Long non-coding RNA XIST protects chondrocytes ATDC5 and CHON-001 from IL-1β-induced injury via regulating miR-653-5p/SIRT1 axis. J. Biol. Regul. Homeost. Agents 34 (2), 379–391. 10.23812/19-549-A-65 [DOI] [PubMed] [Google Scholar]
- Liang J., Xu L., Zhou F., Liu A. M., Ge H. X., Chen Y. Y., et al. (2018). MALAT1/miR-127-5p regulates osteopontin (OPN)-Mediated proliferation of human chondrocytes through PI3K/akt pathway. J. Cell Biochem. 119 (1), 431–439. 10.1002/jcb.26200 [DOI] [PubMed] [Google Scholar]
- Liang Q., Asila A., Deng Y., Liao J., Liu Z., Fang R. (2021). Osteopontin-induced lncRNA HOTAIR expression is involved in osteoarthritis by regulating cell proliferation. BMC Geriatr. 21 (1), 57. 10.1186/s12877-020-01993-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao S., Zhou K., Li D., Xie X., Jun F., Wang J. (2016). Schisantherin A suppresses interleukin-1β-induced inflammation in human chondrocytes via inhibition of NF-κB and MAPKs activation. Eur. J. Pharmacol. 780, 65–70. 10.1016/j.ejphar.2016.03.032 [DOI] [PubMed] [Google Scholar]
- Liu C., Ren S., Zhao S., Wang Y. (2019). LncRNA MALAT1/MiR-145 adjusts IL-1β-induced chondrocytes viability and cartilage matrix degradation by regulating ADAMTS5 in human osteoarthritis. Yonsei Med. J. 60 (11), 1081–1092. 10.3349/ymj.2019.60.11.1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Feng X. X., Zhu S. L., Lin L., Huang H. Y., Zhang B. Y., et al. (2021b). Long non-coding RNA SNHG1 regulates rheumatoid synovial invasion and proliferation by interaction with PTBP1. Int. Immunopharmacol. 90, 107182. 10.1016/j.intimp.2020.107182 [DOI] [PubMed] [Google Scholar]
- Liu F., Liu X., Yang Y., Sun Z., Deng S., Jiang Z., et al. (2020b). NEAT1/miR-193a-3p/SOX5 axis regulates cartilage matrix degradation in human osteoarthritis. Cell Biol. Int. 44 (4), 947–957. 10.1002/cbin.11291 [DOI] [PubMed] [Google Scholar]
- Liu J., Shang G. (2022). The roles of noncoding RNAs in the development of osteosarcoma stem cells and potential therapeutic targets. Front. Cell Dev. Biol. 10, 773038. 10.3389/fcell.2022.773038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Hu X., Zhang X., Dai L., Duan X., Zhou C., et al. (2016). The TMSB4 pseudogene LncRNA functions as a competing endogenous RNA to promote cartilage degradation in human osteoarthritis. Mol. Ther. 24 (10), 1726–1733. 10.1038/mt.2016.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Zhang X., Dai L., Hu X., Zhu J., Li L., et al. (2014). Long noncoding RNA related to cartilage injury promotes chondrocyte extracellular matrix degradation in osteoarthritis. Arthritis Rheumatol. 66 (4), 969–978. 10.1002/art.38309 [DOI] [PubMed] [Google Scholar]
- Liu W. B., Li G. S., Shen P., Li Y. N., Zhang F. J. (2021a). Long non-coding RNA HOTAIRM1-1 silencing in cartilage tissue induces osteoarthritis through microRNA-125b. Exp. Ther. Med. 22 (3), 933. 10.3892/etm.2021.10365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Lin L., Zou R., Wen C., Wang Z., Lin F. (2018). MSC-derived exosomes promote proliferation and inhibit apoptosis of chondrocytes via lncRNA-KLF3-AS1/miR-206/GIT1 axis in osteoarthritis. Cell Cycle 17 (21-22), 2411–2422. 10.1080/15384101.2018.1526603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Liu K., Tang C., Shi Z., Jing K., Zheng J. (2020a). Long non-coding RNA XIST contributes to osteoarthritis progression via miR-149-5p/DNMT3A axis. Biomed. Pharmacother. 128, 110349. 10.1016/j.biopha.2020.110349 [DOI] [PubMed] [Google Scholar]
- Liu Y., Yang Y., Ding L., Jia Y., Ji Y. (2020c). LncRNA MIR4435-2HG inhibits the progression of osteoarthritis through miR-510-3p sponging. Exp. Ther. Med. 20 (2), 1693–1701. 10.3892/etm.2020.8841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeser R. F., Collins J. A., Diekman B. O. (2016). Ageing and the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 12 (7), 412–420. 10.1038/nrrheum.2016.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long H., Li Q., Xiao Z., Yang B. (2021). LncRNA MIR22HG promotes osteoarthritis progression via regulating miR-9-3p/ADAMTS5 pathway. Bioengineered 12 (1), 3148–3158. 10.1080/21655979.2021.1945362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou Y., Wang C., Zheng W., Tang Q., Chen Y., Zhang X., et al. (2017). Salvianolic acid B inhibits IL-1β-induced inflammatory cytokine production in human osteoarthritis chondrocytes and has a protective effect in a mouse osteoarthritis model. Int. Immunopharmacol. 46, 31–37. 10.1016/j.intimp.2017.02.021 [DOI] [PubMed] [Google Scholar]
- Lu C., Li Z., Hu S., Cai Y., Peng K. (2019a). LncRNA PART-1 targets TGFBR2/Smad3 to regulate cell viability and apoptosis of chondrocytes via acting as miR-590-3p sponge in osteoarthritis. J. Cell Mol. Med. 23 (12), 8196–8205. 10.1111/jcmm.14690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu G., Li L., Wang B., Kuang L. (2020c). LINC00623/miR-101/HRAS axis modulates IL-1β-mediated ECM degradation, apoptosis and senescence of osteoarthritis chondrocytes. Aging (Albany NY) 12 (4), 3218–3237. 10.18632/aging.102801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Wu Z., Xiong Y. (2021). Knockdown of long noncoding RNA HOTAIR inhibits osteoarthritis chondrocyte injury by miR-107/CXCL12 axis. J. Orthop. Surg. Res. 16 (1), 410. 10.1186/s13018-021-02547-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J. F., Qi L. G., Zhu X. B., Shen Y. X. (2020b). LncRNA RMRP knockdown promotes proliferation and inhibits apoptosis in osteoarthritis chondrocytes by miR-206/CDK9 axis. Pharmazie 75 (10), 500–504. 10.1691/ph.2020.0591 [DOI] [PubMed] [Google Scholar]
- Lu R., He Z., Zhang W., Wang Y., Cheng P., Lv Z., et al. (2022). Oroxin B alleviates osteoarthritis through anti-inflammation and inhibition of PI3K/AKT/mTOR signaling pathway and enhancement of autophagy. Front. Endocrinol. (Lausanne) 13, 1060721. 10.3389/fendo.2022.1060721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Yu Y., Yin F., Yang C., Li B., Lin J., et al. (2020a). Knockdown of PVT1 inhibits IL-1β-induced injury in chondrocytes by regulating miR-27b-3p/TRAF3 axis. Int. Immunopharmacol. 79, 106052. 10.1016/j.intimp.2019.106052 [DOI] [PubMed] [Google Scholar]
- Lu Z., Luo M., Huang Y. (2019b). lncRNA-CIR regulates cell apoptosis of chondrocytes in osteoarthritis. J. Cell Biochem. 120 (5), 7229–7237. 10.1002/jcb.27997 [DOI] [PubMed] [Google Scholar]
- Luo J., Luo X., Duan Z., Bai W., Che X., Shan Z., et al. (2021). Comprehensive analysis of lncRNA and mRNA based on expression microarray profiling reveals different characteristics of osteoarthritis between Tibetan and Han patients. J. Orthop. Surg. Res. 16 (1), 133. 10.1186/s13018-021-02213-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X., Wang J., Wei X., Wang S., Wang A. (2020). Knockdown of lncRNA MFI2-AS1 inhibits lipopolysaccharide-induced osteoarthritis progression by miR-130a-3p/TCF4. Life Sci. 240, 117019. 10.1016/j.lfs.2019.117019 [DOI] [PubMed] [Google Scholar]
- Mahmoudian A., Lohmander L. S., Mobasheri A., Englund M., Luyten F. P. (2021). Early-stage symptomatic osteoarthritis of the knee - time for action. Nat. Rev. Rheumatol. 17 (10), 621–632. 10.1038/s41584-021-00673-4 [DOI] [PubMed] [Google Scholar]
- Mao G., Kang Y., Lin R., Hu S., Zhang Z., Li H., et al. (2019). Long non-coding RNA HOTTIP promotes CCL3 expression and induces cartilage degradation by sponging miR-455-3p. Front. Cell Dev. Biol. 7, 161. 10.3389/fcell.2019.00161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel-Pelletier J., Barr A. J., Cicuttini F. M., Conaghan P. G., Cooper C., Goldring M. B., et al. (2016). Osteoarthritis. Nat. Rev. Dis. Prim. 2, 16072. 10.1038/nrdp.2016.72 [DOI] [PubMed] [Google Scholar]
- Matera A. G., Terns R. M., Terns M. P. (2007). Non-coding RNAs: lessons from the small nuclear and small nucleolar RNAs. Nat. Rev. Mol. Cell Biol. 8 (3), 209–220. 10.1038/nrm2124 [DOI] [PubMed] [Google Scholar]
- Mattick J. S., Amaral P. P., Carninci P., Carpenter S., Chang H. Y., Chen L. L., et al. (2023). Long non-coding RNAs: definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. 24 (6), 430–447. 10.1038/s41580-022-00566-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y., Qiu S., Sun L., Zuo J. (2020). Knockdown of exosome‑mediated lnc‑PVT1 alleviates lipopolysaccharide‑induced osteoarthritis progression by mediating the HMGB1/TLR4/NF‑κB pathway via miR‑93‑5p. Mol. Med. Rep. 22 (6), 5313–5325. 10.3892/mmr.2020.11594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo L., Jiang B., Mei T., Zhou D. (2023). Exercise therapy for knee osteoarthritis: A systematic review and network meta-analysis. Orthop. J. Sports Med. 11 (5), 23259671231172773. 10.1177/23259671231172773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanus D. E., Wijesinghe S. N., Pearson M. J., Hadjicharalambous M. R., Rosser A., Davis E. T., et al. (2020). Regulation of the inflammatory synovial fibroblast phenotype by metastasis-associated lung adenocarcinoma transcript 1 long noncoding RNA in obese patients with osteoarthritis. Arthritis Rheumatol. 72 (4), 609–619. 10.1002/art.41158 [DOI] [PubMed] [Google Scholar]
- Nevalainen M. T., Uusimaa A. P., Saarakkala S. (2023). The ultrasound assessment of osteoarthritis: the current status. Skelet. Radiol. 10.1007/s00256-023-04342-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez-Carro C., Blanco-Blanco M., Villagran-Andrade K. M., Blanco F. J., de Andres M. C. (2023). Epigenetics as a therapeutic target in osteoarthritis. Pharm. (Basel) 16 (2), 156. 10.3390/ph16020156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyan H. M., Begen M. A. (2022). LncRNAs in osteoarthritis. Clin. Chim. Acta 532, 145–163. 10.1016/j.cca.2022.05.030 [DOI] [PubMed] [Google Scholar]
- Ouyang Z., Dong L., Yao F., Wang K., Chen Y., Li S., et al. (2023). Cartilage-related collagens in osteoarthritis and rheumatoid arthritis: from pathogenesis to therapeutics. Int. J. Mol. Sci. 24 (12), 9841. 10.3390/ijms24129841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan C., Huang W., Chen Q., Xu J., Yao G., Li B., et al. (2021). LncRNA malat-1 from MSCs-derived extracellular vesicles suppresses inflammation and cartilage degradation in osteoarthritis. Front. Bioeng. Biotechnol. 9, 772002. 10.3389/fbioe.2021.772002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Chen D., Lu Q., Liu L., Li X., Li Z. (2017). Baicalin prevents the apoptosis of endplate chondrocytes by inhibiting the oxidative stress induced by H2O2. Mol. Med. Rep. 16 (3), 2985–2991. 10.3892/mmr.2017.6904 [DOI] [PubMed] [Google Scholar]
- Park S., Lee M., Chun C. H., Jin E. J. (2019). The lncRNA, Nespas, is associated with osteoarthritis progression and serves as a potential new prognostic biomarker. Cartilage 10 (2), 148–156. 10.1177/1947603517725566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao S., Du W., Wei Y., Yang Y., Feng X., Bai L. (2020). Protectin DX attenuates IL-1β-induced inflammation via the AMPK/NF-κB pathway in chondrocytes and ameliorates osteoarthritis progression in a rat model. Int. Immunopharmacol. 78, 106043. 10.1016/j.intimp.2019.106043 [DOI] [PubMed] [Google Scholar]
- Postler A. E., Lutzner C., Goronzy J., Lange T., Deckert S., Gunther K. P., et al. (2023). When are patients with osteoarthritis referred for surgery? Best. Pract. Res. Clin. Rheumatol., 101835. 10.1016/j.berh.2023.101835 [DOI] [PubMed] [Google Scholar]
- Qi K., Lin R., Xue C., Liu T., Wang Y., Zhang Y., et al. (2019). Long non-coding RNA (LncRNA) CAIF is downregulated in osteoarthritis and inhibits LPS-induced interleukin 6 (IL-6) upregulation by downregulation of MiR-1246. Med. Sci. Monit. 25, 8019–8024. 10.12659/MSM.917135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin G. H., Yang W. C., Yao J. N., Zhao Y., Wu X. J. (2021). LncRNA OIP5-AS1 affects the biological behaviors of chondrocytes of patients with osteoarthritis by regulating micro-30a-5p. Eur. Rev. Med. Pharmacol. Sci. 25 (3), 1215–1224. 10.26355/eurrev_202102_24825 [DOI] [PubMed] [Google Scholar]
- Richard M. J., Driban J. B., McAlindon T. E. (2023). Pharmaceutical treatment of osteoarthritis. Osteoarthr. Cartil. 31 (4), 458–466. 10.1016/j.joca.2022.11.005 [DOI] [PubMed] [Google Scholar]
- Rozi R., Zhou Y., Rong K., Chen P. (2022). miR-124-3p sabotages lncRNA MALAT1 stability to repress chondrocyte pyroptosis and relieve cartilage injury in osteoarthritis. J. Orthop. Surg. Res. 17 (1), 453. 10.1186/s13018-022-03334-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan X., Gu J., Chen M., Zhao F., Aili M., Zhang D. (2023). Multiple roles of ALK3 in osteoarthritis. Bone Jt. Res. 12 (7), 397–411. 10.1302/2046-3758.127.BJR-2022-0310.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H., Wang Y., Shi W., Sun G., Hong L., Zhang Y. (2018). LncRNA SNHG5/miR-26a/SOX2 signal axis enhances proliferation of chondrocyte in osteoarthritis. Acta Biochim. Biophys. Sin. (Shanghai). 50 (2), 191–198. 10.1093/abbs/gmx141 [DOI] [PubMed] [Google Scholar]
- Shen P., Gao J., Huang S., You C., Wang H., Chen P., et al. (2023). LncRNA AC006064.4-201 serves as a novel molecular marker in alleviating cartilage senescence and protecting against osteoarthritis by destabilizing CDKN1B mRNA via interacting with PTBP1. Biomark. Res. 11 (1), 39. 10.1186/s40364-023-00477-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shentu C. Y., Yan G., Xu D. C., Chen Y., Peng L. H. (2022). Emerging pharmaceutical therapeutics and delivery technologies for osteoarthritis therapy. Front. Pharmacol. 13, 945876. 10.3389/fphar.2022.945876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M., Sun M., Wang C., Shen Y., Wang Y., Yan S. (2022). Therapeutic potential of POU3F3, a novel long non-coding RNA, alleviates the pathogenesis of osteoarthritis by regulating the miR-29a- 3p/FOXO3 Axis. Curr. Gene Ther. 22 (5), 427–438. 10.2174/1566523222666220309150722 [DOI] [PubMed] [Google Scholar]
- Song S., Ding L., Liu G., Chen T., Zhao M., Li X., et al. (2023). The protective effects of baicalin for respiratory diseases: an update and future perspectives. Front. Pharmacol. 14, 1129817. 10.3389/fphar.2023.1129817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanoudaki M., Giaginis C., Mentzelou M., Bisbinas A., Solovos E., Papadopoulos K., et al. (2023). Sarcopenia and sarcopenic obesity and osteoarthritis: A discussion among muscles, fat, bones, and aging. Life (Basel) 13 (6), 1242. 10.3390/life13061242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas N. R. (2010). Baicalin, an emerging multi-therapeutic agent: pharmacodynamics, pharmacokinetics, and considerations from drug development perspectives. Xenobiotica 40 (5), 357–367. 10.3109/00498251003663724 [DOI] [PubMed] [Google Scholar]
- Su W., Xie W., Shang Q., Su B. (2015). The long noncoding RNA MEG3 is downregulated and inversely associated with VEGF levels in osteoarthritis. Biomed. Res. Int. 2015, 356893. 10.1155/2015/356893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sufianova G., Shumadalova A., Wenhao Y., Gareev I. (2022). Long non-coding RNAs as biomarkers and therapeutic targets for ischemic stroke. Noncoding RNA Res. 7 (4), 226–232. Epub 20220910. 10.1016/j.ncrna.2022.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun P., Wu Y., Li X., Jia Y. (2020a). miR-142-5p protects against osteoarthritis through competing with lncRNA XIST. J. Gene Med. 22 (4), e3158. 10.1002/jgm.3158 [DOI] [PubMed] [Google Scholar]
- Sun T., Yu J., Han L., Tian S., Xu B., Gong X., et al. (2018). Knockdown of long non-coding RNA RP11-445H22.4 alleviates LPS-induced injuries by regulation of MiR-301a in osteoarthritis. Cell Physiol. Biochem. 45 (2), 832–843. 10.1159/000487175 [DOI] [PubMed] [Google Scholar]
- Sun Y., Kang S., Pei S., Sang C., Huang Y. (2020b). MiR93-5p inhibits chondrocyte apoptosis in osteoarthritis by targeting lncRNA CASC2. BMC Musculoskelet. Disord. 21 (1), 26. 10.1186/s12891-019-3025-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szponder T., Latalski M., Danielewicz A., Krac K., Kozera A., Drzewiecka B., et al. (2022). Osteoarthritis: pathogenesis, animal models, and new regenerative therapies. J. Clin. Med. 12 (1), 5. 10.3390/jcm12010005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan F., Wang D., Yuan Z. (2020). The fibroblast-like synoviocyte derived exosomal long non-coding RNA H19 alleviates osteoarthritis progression through the miR-106b-5p/TIMP2 Axis. Inflammation 43 (4), 1498–1509. 10.1007/s10753-020-01227-8 [DOI] [PubMed] [Google Scholar]
- Tang L., Ding J., Zhou G., Liu Z. (2018b). LncRNA-p21 promotes chondrocyte apoptosis in osteoarthritis by acting as a sponge for miR-451. Mol. Med. Rep. 18 (6), 5295–5301. 10.3892/mmr.2018.9506 [DOI] [PubMed] [Google Scholar]
- Tang L. P., Ding J. B., Liu Z. H., Zhou G. J. (2018a). LncRNA TUG1 promotes osteoarthritis-induced degradation of chondrocyte extracellular matrix via miR-195/MMP-13 axis. Eur. Rev. Med. Pharmacol. Sci. 22 (24), 8574–8581. 10.26355/eurrev_201812_16620 [DOI] [PubMed] [Google Scholar]
- Tang S., Cao Y., Cai Z., Nie X., Ruan J., Zhou Z., et al. (2022). The lncRNA PILA promotes NF-κB signaling in osteoarthritis by stimulating the activity of the protein arginine methyltransferase PRMT1. Sci. Signal 15 (735), eabm6265. 10.1126/scisignal.abm6265 [DOI] [PubMed] [Google Scholar]
- Thakur M., Dickenson A. H., Baron R. (2014). Osteoarthritis pain: nociceptive or neuropathic? Nat. Rev. Rheumatol. 10 (6), 374–380. 10.1038/nrrheum.2014.47 [DOI] [PubMed] [Google Scholar]
- Tian F., Wang J., Zhang Z., Yang J. (2020). LncRNA SNHG7/miR-34a-5p/SYVN1 axis plays a vital role in proliferation, apoptosis and autophagy in osteoarthritis. Biol. Res. 53 (1), 9. 10.1186/s40659-020-00275-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonutti A., Granata V., Marrella V., Sobacchi C., Ragusa R., Sconza C., et al. (2023). The role of WNT and IL-1 signaling in osteoarthritis: therapeutic implications for platelet-rich plasma therapy. Front. Aging 4, 1201019. 10.3389/fragi.2023.1201019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu J., Huang W., Zhang W., Mei J., Zhu C. (2020). The emerging role of lncRNAs in chondrocytes from osteoarthritis patients. Biomed. Pharmacother. 131, 110642. 10.1016/j.biopha.2020.110642 [DOI] [PubMed] [Google Scholar]
- Wakale S., Wu X., Sonar Y., Sun A., Fan X., Crawford R., et al. (2023). How are aging and osteoarthritis related? Aging Dis. 14 (3), 592–604. 10.14336/AD.2022.0831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A., Hu N., Zhang Y., Chen Y., Su C., Lv Y., et al. (2019a). MEG3 promotes proliferation and inhibits apoptosis in osteoarthritis chondrocytes by miR-361-5p/FOXO1 axis. BMC Med. Genomics 12 (1), 201. 10.1186/s12920-019-0649-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Li J., Tian F. (2021a). Downregulation of lncRNA SNHG14 attenuates osteoarthritis by inhibiting FSTL-1 mediated NLRP3 and TLR4/NF-κB pathway through miR-124-3p. Life Sci. 270, 119143. 10.1016/j.lfs.2021.119143 [DOI] [PubMed] [Google Scholar]
- Wang B., Sun Y., Liu N., Liu H. (2021b). LncRNA HOTAIR modulates chondrocyte apoptosis and inflammation in osteoarthritis via regulating miR-1277-5p/SGTB axis. Wound Repair Regen. 29 (3), 495–504. 10.1111/wrr.12908 [DOI] [PubMed] [Google Scholar]
- Wang C. L., Peng J. P., Chen X. D. (2018). LncRNA-CIR promotes articular cartilage degeneration in osteoarthritis by regulating autophagy. Biochem. Biophys. Res. Commun. 505 (3), 692–698. 10.1016/j.bbrc.2018.09.163 [DOI] [PubMed] [Google Scholar]
- Wang D., Li Y. (2023). Pharmacological effects of baicalin in lung diseases. Front. Pharmacol. 14, 1188202. 10.3389/fphar.2023.1188202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Li C., Zhang X., Tang L., Li Y. (2022d). Long non-coding PRNCR1 regulates the proliferation and apoptosis of synoviocytes in osteoarthritis by sponging miR-377-3p. J. Orthop. Surg. Res. 17 (1), 238. 10.1186/s13018-022-03035-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Li J., Cheng Y., Yao J. (2020a). Association of long-chain noncoding RNA H19 and MEG3 gene polymorphisms and their interaction with risk of osteoarthritis in a Chinese han population. Genet. Test. Mol. Biomarkers 24 (6), 328–337. 10.1089/gtmb.2019.0230 [DOI] [PubMed] [Google Scholar]
- Wang H., Zhao J., Wang J. (2023c). Role of circular RNAs in osteoarthritis: update on pathogenesis and therapeutics. Mol. Genet. Genomics 298 (4), 791–801. 10.1007/s00438-023-02021-5 [DOI] [PubMed] [Google Scholar]
- Wang J., Luo X., Cai S., Sun J., Wang S., Wei X. (2021c). Blocking HOTAIR protects human chondrocytes against IL-1β-induced cell apoptosis, ECM degradation, inflammatory response and oxidative stress via regulating miR-222-3p/ADAM10 axis. Int. Immunopharmacol. 98, 107903. 10.1016/j.intimp.2021.107903 [DOI] [PubMed] [Google Scholar]
- Wang P., Liu J., Zhang S., Zhu P., Xiong X., Yu C., et al. (2021g). Baicalin promotes chondrocyte viability and the synthesis of extracellular matrix through TGF-β/Smad3 pathway in chondrocytes. Am. J. Transl. Res. 13 (9), 10908–10921. [PMC free article] [PubMed] [Google Scholar]
- Wang P., Wang Y., Ma B. (2023b). Long noncoding RNA NAV2-AS5 relieves chondrocyte inflammation by targeting miR-8082/TNIP2 in osteoarthritis. Cell Cycle 22 (7), 796–807. 10.1080/15384101.2022.2154554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Zhang F., He Q., Wang J., Shiu H. T., Shu Y., et al. (2016). Flavonoid compound icariin activates hypoxia inducible factor-1α in chondrocytes and promotes articular cartilage repair. PLoS One 11 (2), e0148372. 10.1371/journal.pone.0148372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Zhu P., Liu R., Meng Q., Li S. (2020b). Baicalin promotes extracellular matrix synthesis in chondrocytes via the activation of hypoxia-inducible factor-1α. Exp. Ther. Med. 20 (6), 226. 10.3892/etm.2020.9356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Deng F., Li J., Guo L., Li K. (2023a). The long non-coding RNA SNHG1 attenuates chondrocyte apoptosis and inflammation via the miR-195/IKK-α axis. Cell Tissue Bank. 24 (1), 167–180. 10.1007/s10561-022-10019-3 [DOI] [PubMed] [Google Scholar]
- Wang R., Shiu H. T., Lee W. Y. W. (2022a). Emerging role of lncRNAs in osteoarthritis: an updated review. Front. Immunol. 13, 982773. 10.3389/fimmu.2022.982773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Hu Y., Fang C., Zhu C. (2022c). 4cRNA NEAT1 sponge adsorption of miR-378 modulates activity of lipopolysaccharide-treated articular chondrocytes and influences the pathological development of osteoarthritis. Altern. Ther. Health Med. 28 (6), 103–111. [PubMed] [Google Scholar]
- Wang Y., Cao L., Wang Q., Huang J., Xu S. (2019b). LncRNA FOXD2-AS1 induces chondrocyte proliferation through sponging miR-27a-3p in osteoarthritis. Artif. Cells Nanomed Biotechnol. 47 (1), 1241–1247. 10.1080/21691401.2019.1596940 [DOI] [PubMed] [Google Scholar]
- Wang Y., Li T., Yang Q., Feng B., Xiang Y., Lv Z., et al. (2021f). LncRNA THUMPD3-AS1 enhances the proliferation and inflammatory response of chondrocytes in osteoarthritis. Int. Immunopharmacol. 100, 108138. 10.1016/j.intimp.2021.108138 [DOI] [PubMed] [Google Scholar]
- Wang Y., Wu C., Zhang F., Zhang Y., Ren Z., Lammi M. J., et al. (2019c). Screening for differentially expressed circular RNAs in the cartilage of osteoarthritis patients for their diagnostic value. Genet. Test. Mol. Biomarkers 23 (10), 706–716. 10.1089/gtmb.2019.0108 [DOI] [PubMed] [Google Scholar]
- Wang Y. H., Tsai C. H., Liu S. C., Chen H. T., Chang J. W., Ko C. Y., et al. (2022b). miR-150-5p and XIST interaction controls monocyte adherence: implications for osteoarthritis therapy. Front. Immunol. 13, 1004334. 10.3389/fimmu.2022.1004334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. Z., Yao L., Liang S. K., Ding L. B., Feng L., Guan J., et al. (2021d). LncPVT1 promotes cartilage degradation in diabetic OA mice by downregulating miR-146a and activating TGF-β/SMAD4 signaling. J. Bone Min. Metab. 39 (4), 534–546. 10.1007/s00774-020-01199-7 [DOI] [PubMed] [Google Scholar]
- Wang Z., Huang C., Zhao C., Zhang H., Zhen Z., Xu D. (2021e). Knockdown of LINC01385 inhibits osteoarthritis progression by modulating the microRNA-140-3p/TLR4 axis. Exp. Ther. Med. 22 (5), 1244. 10.3892/etm.2021.10679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkle M., El-Daly S. M., Fabbri M., Calin G. A. (2021). Noncoding RNA therapeutics - challenges and potential solutions. Nat. Rev. Drug Discov. 20 (8), 629–651. 10.1038/s41573-021-00219-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W., Zou J. (2021). Studies on the role of circRNAs in osteoarthritis. Biomed. Res. Int. 2021, 8231414. 10.1155/2021/8231414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Bian B., Lin Z., Wu C., Sun Y., Pan Y., et al. (2022). Identification of exosomal mRNA, lncRNA and circRNA signatures in an osteoarthritis synovial fluid-exosomal study. Exp. Cell Res. 410 (1), 112881. 10.1016/j.yexcr.2021.112881 [DOI] [PubMed] [Google Scholar]
- Wu Z., Yang Z., Liu L., Xiao Y. (2023). Natural compounds protect against the pathogenesis of osteoarthritis by mediating the NRF2/ARE signaling. Front. Pharmacol. 14, 1188215. 10.3389/fphar.2023.1188215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang S., Li Z., Bian Y., Weng X. (2019a). Identification of changed expression of mRNAs and lncRNAs in osteoarthritic synovium by RNA-sequencing. Gene 685, 55–61. 10.1016/j.gene.2018.10.076 [DOI] [PubMed] [Google Scholar]
- Xiang S., Li Z., Bian Y., Weng X. (2019b). RNA sequencing reveals the circular RNA expression profiles of osteoarthritic synovium. J. Cell Biochem. 120 (10), 18031–18040. 10.1002/jcb.29106 [DOI] [PubMed] [Google Scholar]
- Xiao K., Yang Y., Bian Y., Feng B., Li Z., Wu Z., et al. (2019a). Identification of differentially expressed long noncoding RNAs in human knee osteoarthritis. J. Cell Biochem. 120 (3), 4620–4633. 10.1002/jcb.27750 [DOI] [PubMed] [Google Scholar]
- Xiao P., Zhu X., Sun J., Zhang Y., Qiu W., Li J., et al. (2021a). LncRNA NEAT1 regulates chondrocyte proliferation and apoptosis via targeting miR-543/PLA2G4A axis. Hum. Cell 34 (1), 60–75. 10.1007/s13577-020-00433-8 [DOI] [PubMed] [Google Scholar]
- Xiao Y., Bao Y., Tang L., Wang L. (2019b). LncRNA MIR4435-2HG is downregulated in osteoarthritis and regulates chondrocyte cell proliferation and apoptosis. J. Orthop. Surg. Res. 14 (1), 247. 10.1186/s13018-019-1278-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y., Liu L., Zheng Y., Liu W., Xu Y. (2021b). Kaempferol attenuates the effects of XIST/miR-130a/STAT3 on inflammation and extracellular matrix degradation in osteoarthritis. Future Med. Chem. 13 (17), 1451–1464. 10.4155/fmc-2021-0127 [DOI] [PubMed] [Google Scholar]
- Xie F., Liu Y. L., Chen X. Y., Li Q., Zhong J., Dai B. Y., et al. (2020). Role of MicroRNA, LncRNA, and exosomes in the progression of osteoarthritis: A review of recent literature. Orthop. Surg. 12 (3), 708–716. 10.1111/os.12690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W., Chu M., Song G., Zuo Z., Han Z., Chen C., et al. (2022). Emerging roles of long noncoding RNAs in chemoresistance of pancreatic cancer. Semin. Cancer Biol. 83, 303–318. 10.1016/j.semcancer.2020.11.004 [DOI] [PubMed] [Google Scholar]
- Xing D., Gao H., Liu Z., Zhao Y., Gong M. (2017). Baicalin inhibits inflammatory responses to interleukin-1β stimulation in human chondrocytes. J. Interferon Cytokine Res. 37 (9), 398–405. 10.1089/jir.2017.0030 [DOI] [PubMed] [Google Scholar]
- Xing D., Liang J. Q., Li Y., Lu J., Jia H. B., Xu L. Y., et al. (2014). Identification of long noncoding RNA associated with osteoarthritis in humans. Orthop. Surg. 6 (4), 288–293. 10.1111/os.12147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong G., Wang S., Pan Z., Liu N., Zhao D., Zha Z., et al. (2022). Long non-coding RNA MEG3 regulates the progress of osteoarthritis by regulating the miR-34a/Klotho axis. Ann. Transl. Med. 10 (8), 454. 10.21037/atm-22-894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F., Hu Q. F., Li J., Shi C. J., Luo J. W., Tian W. C., et al. (2022b). SOX4-activated lncRNA MCM3AP-AS1 aggravates osteoarthritis progression by modulating miR-149-5p/Notch1 signaling. Cytokine 152, 155805. 10.1016/j.cyto.2022.155805 [DOI] [PubMed] [Google Scholar]
- Xu J., Fang X., Qin L., Wu Q., Zhan X. (2022a). LncRNA PVT1 regulates biological function of osteoarthritis cells by regulating miR-497/AKT3 axis. Med. Baltim. 101 (45), e31725. 10.1097/MD.0000000000031725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Pei Y., Lu J., Liang X., Li Y., Wang J., et al. (2021). LncRNA SNHG7 alleviates IL-1β-induced osteoarthritis by inhibiting miR-214-5p-mediated PPARGC1B signaling pathways. Int. Immunopharmacol. 90, 107150. 10.1016/j.intimp.2020.107150 [DOI] [PubMed] [Google Scholar]
- Xu J., Xu Y. (2017). The lncRNA MEG3 downregulation leads to osteoarthritis progression via miR-16/SMAD7 axis. Cell Biosci. 7, 69. 10.1186/s13578-017-0195-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K., Meng Z., Xian X. M., Deng M. H., Meng Q. G., Fang W., et al. (2020). LncRNA PVT1 induces chondrocyte apoptosis through upregulation of TNF-alpha in synoviocytes by sponging miR-211-3p. Mol. Cell Probes 52, 101560. 10.1016/j.mcp.2020.101560 [DOI] [PubMed] [Google Scholar]
- Xue H., Yu P., Wang W. Z., Niu Y. Y., Li X. (2020). The reduced lncRNA NKILA inhibited proliferation and promoted apoptosis of chondrocytes via miR-145/SP1/NF-κB signaling in human osteoarthritis. Eur. Rev. Med. Pharmacol. Sci. 24 (2), 535–548. 10.26355/eurrev_202001_20030 [DOI] [PubMed] [Google Scholar]
- Xue Q., Huang Y., Chang J., Cheng C., Wang Y., Wang X., et al. (2023). CircRNA-mediated ceRNA mechanism in osteoarthritis: special emphasis on circRNAs in exosomes and the crosstalk of circRNAs and RNA methylation. Biochem. Pharmacol. 212, 115580. 10.1016/j.bcp.2023.115580 [DOI] [PubMed] [Google Scholar]
- Yang B., Xu L., Wang S. (2020b). Regulation of lncRNA-H19/miR-140-5p in cartilage matrix degradation and calcification in osteoarthritis. Ann. Palliat. Med. 9 (4), 1896–1904. 10.21037/apm-20-929 [DOI] [PubMed] [Google Scholar]
- Yang D. W., Zhang X., Qian G. B., Jiang M. J., Wang P., Wang K. Z. (2019b). Downregulation of long noncoding RNA LOC101928134 inhibits the synovial hyperplasia and cartilage destruction of osteoarthritis rats through the activation of the Janus kinase/signal transducers and activators of transcription signaling pathway by upregulating IFNA1. J. Cell Physiol. 234 (7), 10523–10534. 10.1002/jcp.27730 [DOI] [PubMed] [Google Scholar]
- Yang F., Zhao M., Sang Q., Yan C., Wang Z. (2022b). Long non-coding RNA PMS2L2 is down-regulated in osteoarthritis and inhibits chondrocyte proliferation by up-regulating miR-34a. J. Immunotoxicol. 19 (1), 74–80. 10.1080/1547691X.2022.2049664 [DOI] [PubMed] [Google Scholar]
- Yang Q., Li X., Zhou Y., Fu W., Wang J., Wei Q. (2019a). A LINC00341-mediated regulatory pathway supports chondrocyte survival and may prevent osteoarthritis progression. J. Cell Biochem. 120 (6), 10812–10820. 10.1002/jcb.28372 [DOI] [PubMed] [Google Scholar]
- Yang Q., Yao Y., Zhao D., Zou H., Lai C., Xiang G., et al. (2021a). LncRNA H19 secreted by umbilical cord blood mesenchymal stem cells through microRNA-29a-3p/FOS axis for central sensitization of pain in advanced osteoarthritis. Am. J. Transl. Res. 13 (3), 1245–1256. [PMC free article] [PubMed] [Google Scholar]
- Yang X., Chen H., Zheng H., Chen K., Cai P., Li L., et al. (2022a). LncRNA SNHG12 promotes osteoarthritis progression through targeted down-regulation of miR-16-5p. Clin. Lab. 68 (1). 10.7754/Clin.Lab.2021.210402 [DOI] [PubMed] [Google Scholar]
- Yang X., Zhang Q., Gao Z., Yu C., Zhang L. (2018). Baicalin alleviates IL-1β-induced inflammatory injury via down-regulating miR-126 in chondrocytes. Biomed. Pharmacother. 99, 184–190. 10.1016/j.biopha.2018.01.041 [DOI] [PubMed] [Google Scholar]
- Yang Y., Sun Z., Liu F., Bai Y., Wu F. (2021b). FGD5-AS1 inhibits osteoarthritis development by modulating miR-302d-3p/TGFBR2 Axis. Cartilage 13 (2), 1412S–1420S. 10.1177/19476035211003324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Xing D., Wang Y., Jia H., Li B., Li J. J. (2020a). A long non-coding RNA, HOTAIR, promotes cartilage degradation in osteoarthritis by inhibiting WIF-1 expression and activating Wnt pathway. BMC Mol. Cell Biol. 21 (1), 53. 10.1186/s12860-020-00299-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao N., Peng S., Wu H., Liu W., Cai D., Huang D. (2022). Long noncoding RNA PVT1 promotes chondrocyte extracellular matrix degradation by acting as a sponge for miR-140 in IL-1β-stimulated chondrocytes. J. Orthop. Surg. Res. 17 (1), 218. 10.1186/s13018-022-03114-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye D., Jian W., Feng J., Liao X. (2018). Role of long noncoding RNA ZFAS1 in proliferation, apoptosis and migration of chondrocytes in osteoarthritis. Biomed. Pharmacother. 104, 825–831. 10.1016/j.biopha.2018.04.124 [DOI] [PubMed] [Google Scholar]
- Yi Y., Yang N., Yang Z., Tao X., Li Y. (2022). LncRNA TM1-3P regulates proliferation, apoptosis and inflammation of fibroblasts in osteoarthritis through miR-144-3p/ONECUT2 Axis. Orthop. Surg. 14 (11), 3078–3091. 10.1111/os.13530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You D., Yang C., Huang J., Gong H., Yan M., Ni J. (2019). Long non-coding RNA MEG3 inhibits chondrogenic differentiation of synovium-derived mesenchymal stem cells by epigenetically inhibiting TRIB2 via methyltransferase EZH2. Cell Signal 63, 109379. 10.1016/j.cellsig.2019.109379 [DOI] [PubMed] [Google Scholar]
- Young J. J., Pedersen J. R., Bricca A. (2023). Exercise therapy for knee and hip osteoarthritis: is there an ideal prescription? Curr. Treatm Opt. Rheumatol. 9, 82–98. 10.1007/s40674-023-00205-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan A., Wu P., Zhong Z., He Z., Li W. (2022). Long non-coding RNA Gm37494 alleviates osteoarthritis chondrocyte injury via the microRNA-181a-5p/GABRA1 axis. J. Orthop. Surg. Res. 17 (1), 304. 10.1186/s13018-022-03202-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Y., Zhibo S., Feng L., Yuanzhang B., Fei W. (2021). SNHG5 protects chondrocytes in interleukin-1β-stimulated osteoarthritis via regulating miR-181a-5p/TGFBR3 axis. J. Biochem. Mol. Toxicol. 35 (10), e22866. 10.1002/jbt.22866 [DOI] [PubMed] [Google Scholar]
- Zhang C., Wang P., Jiang P., Lv Y., Dong C., Dai X., et al. (2016a). Upregulation of lncRNA HOTAIR contributes to IL-1β-induced MMP overexpression and chondrocytes apoptosis in temporomandibular joint osteoarthritis. Gene 586 (2), 248–253. 10.1016/j.gene.2016.04.016 [DOI] [PubMed] [Google Scholar]
- Zhang F., Lammi M. J., Tan S., Meng P., Wu C., Guo X. (2020a). Cell cycle-related lncRNAs and mRNAs in osteoarthritis chondrocytes in a Northwest Chinese Han Population. Med. Baltim. 99 (24), e19905. 10.1097/MD.0000000000019905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Wu Y., Xu D., Yan X. (2016b). Long noncoding RNA UFC1 promotes proliferation of chondrocyte in osteoarthritis by acting as a sponge for miR-34a. DNA Cell Biol. 35 (11), 691–695. 10.1089/dna.2016.3397 [DOI] [PubMed] [Google Scholar]
- Zhang G., Zhang H., You W., Tang X., Li X., Gong Z. (2020g). Therapeutic effect of Resveratrol in the treatment of osteoarthritis via the MALAT1/miR-9/NF-κB signaling pathway. Exp. Ther. Med. 19 (3), 2343–2352. 10.3892/etm.2020.8471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Li J., Shao W., Shen N. (2020f). LncRNA CTBP1-AS2 is upregulated in osteoarthritis and increases the methylation of miR-130a gene to inhibit chondrocyte proliferation. Clin. Rheumatol. 39 (11), 3473–3478. 10.1007/s10067-020-05113-4 [DOI] [PubMed] [Google Scholar]
- Zhang H., Li J., Shao W., Shen N. (2020b). LncRNA SNHG9 is downregulated in osteoarthritis and inhibits chondrocyte apoptosis by downregulating miR-34a through methylation. BMC Musculoskelet. Disord. 21 (1), 511. 10.1186/s12891-020-03497-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Chen S., Bao N., Yang C., Ti Y., Zhou L., et al. (2015). Sox4 enhances chondrogenic differentiation and proliferation of human synovium-derived stem cell via activation of long noncoding RNA DANCR. J. Mol. Histol. 46 (6), 467–473. 10.1007/s10735-015-9638-z [DOI] [PubMed] [Google Scholar]
- Zhang L., Sun X., Chen S., Yang C., Shi B., Zhou L., et al. (2017b). Long noncoding RNA DANCR regulates miR-1305-Smad 4 axis to promote chondrogenic differentiation of human synovium-derived mesenchymal stem cells. Biosci. Rep. 37 (4). 10.1042/BSR20170347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Yang C., Chen S., Wang G., Shi B., Tao X., et al. (2017a). Long noncoding RNA DANCR is a positive regulator of proliferation and chondrogenic differentiation in human synovium-derived stem cells. DNA Cell Biol. 36 (2), 136–142. 10.1089/dna.2016.3544 [DOI] [PubMed] [Google Scholar]
- Zhang L., Zhang P., Sun X., Zhou L., Zhao J. (2018). Long non-coding RNA DANCR regulates proliferation and apoptosis of chondrocytes in osteoarthritis via miR-216a-5p-JAK2-STAT3 axis. Biosci. Rep. 38 (6). 10.1042/BSR20181228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Sun J., Liang C., Gu B., Xu Y., Lu H., et al. (2020e). lncRNA IGHCγ1 acts as a ceRNA to regulate macrophage inflammation via the miR-6891-3p/TLR4 Axis in osteoarthritis. Mediat. Inflamm. 2020, 9743037. 10.1155/2020/9743037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Huang C. R., Pan S., Pang Y., Chen Y. S., Zha G. C., et al. (2020c). Long non-coding RNA SNHG15 is a competing endogenous RNA of miR-141-3p that prevents osteoarthritis progression by upregulating BCL2L13 expression. Int. Immunopharmacol. 83, 106425. 10.1016/j.intimp.2020.106425 [DOI] [PubMed] [Google Scholar]
- Zhang X., Liu X., Ni X., Feng P., Wang Y. U. (2019a). Long non-coding RNA H19 modulates proliferation and apoptosis in osteoarthritis via regulating miR-106a-5p. J. Biosci. 44 (6), 128. 10.1007/s12038-019-9943-x [DOI] [PubMed] [Google Scholar]
- Zhang Y., Dong Q., Sun X. (2020d). Positive feedback loop linc00511/miR-150-5p/SP1 modulates chondrocyte apoptosis and proliferation in osteoarthritis. DNA Cell Biol. 39 (9), 1506–1512. 10.1089/dna.2020.5718 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Liu D., Vithran D. T. A., Kwabena B. R., Xiao W., Li Y. (2023a). CC chemokines and receptors in osteoarthritis: new insights and potential targets. Arthritis Res. Ther. 25 (1), 113. 10.1186/s13075-023-03096-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Liu W., Liu Z., Liu Y. (2023b). Daurisoline attenuates H(2)O(2)-induced chondrocyte autophagy by activating the PI3K/Akt/mTOR signaling pathway. J. Orthop. Surg. Res. 18 (1), 248. 10.1186/s13018-023-03717-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Wang F., Chen G., He R., Yang L. (2019b). LncRNA MALAT1 promotes osteoarthritis by modulating miR-150-5p/AKT3 axis. Cell Biosci. 9, 54. 10.1186/s13578-019-0302-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Yang P., Wang C., Tian R. (2022). LncRNA CRNDE hinders the progression of osteoarthritis by epigenetic regulation of DACT1. Cell Mol. Life Sci. 79 (8), 405. 10.1007/s00018-022-04427-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Xu J. (2018). Synovial fluid-derived exosomal lncRNA PCGEM1 as biomarker for the different stages of osteoarthritis. Int. Orthop. 42 (12), 2865–2872. 10.1007/s00264-018-4093-6 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Zhao J., Guo X., She J., Liu Y. (2018). Long non-coding RNA PVT1, a molecular sponge for miR-149, contributes aberrant metabolic dysfunction and inflammation in IL-1β-simulated osteoarthritic chondrocytes. Biosci. Rep. 38 (5). 10.1042/BSR20180576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C., Chen J., Wu Y., Wang X., Lin Y., Shu L., et al. (2023). Elucidating the role of ubiquitination and deubiquitination in osteoarthritis progression. Front. Immunol. 14, 1217466. 10.3389/fimmu.2023.1217466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng T., Huang J., Lai J., Zhou Q., Liu T., Xu Q., et al. (2021). Long non-coding RNA HOTAIRincreased mechanical stimulation-induced apoptosis by regulating microRNA-221/BBC3 axis in C28/I2 cells. Bioengineered 12 (2), 10734–10744. 10.1080/21655979.2021.2003129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhi L., Zhao J., Zhao H., Qing Z., Liu H., Ma J. (2021). Downregulation of LncRNA OIP5-AS1 induced by IL-1β aggravates osteoarthritis via regulating miR-29b-3p/PGRN. Cartilage 13 (2), 1345S–1355S. 10.1177/1947603519900801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C., He T., Chen L. (2021b). LncRNA CASC19 accelerates chondrocytes apoptosis and proinflammatory cytokine production to exacerbate osteoarthritis development through regulating the miR-152-3p/DDX6 axis. J. Orthop. Surg. Res. 16 (1), 399. 10.1186/s13018-021-02543-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Gu M., Ma X., Wen L., Zhang B., Lin Y., et al. (2021c). Long non-coding RNA PCAT-1 regulates apoptosis of chondrocytes in osteoarthritis by sponging miR-27b-3p. J. Bone Min. Metab. 39 (2), 139–147. 10.1007/s00774-020-01128-8 [DOI] [PubMed] [Google Scholar]
- Zhou L., Wan Y., Cheng Q., Shi B., Zhang L., Chen S. (2020). The expression and diagnostic value of LncRNA H19 in the blood of patients with osteoarthritis. Iran. J. Public Health 49 (8), 1494–1501. 10.18502/ijph.v49i8.3893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., He X., Chen Z., Fan D., Wang Y., Feng H., et al. (2019). LncRNA HOTAIR-mediated Wnt/β-catenin network modeling to predict and validate therapeutic targets for cartilage damage. BMC Bioinforma. 20 (1), 412. 10.1186/s12859-019-2981-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Cao H., Wang M., Zou J., Wu W. (2021a). Moderate-intensity treadmill running relieves motion-induced post-traumatic osteoarthritis mice by up-regulating the expression of lncRNA H19. Biomed. Eng. Online 20 (1), 111. 10.1186/s12938-021-00949-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Li J., Xu F., Ji E., Wang C., Pan Z. (2022). Long noncoding RNA H19 alleviates inflammation in osteoarthritis through interactions between TP53, IL-38, and IL-36 receptor. Bone Jt. Res. 11 (8), 594–607. 10.1302/2046-3758.118.BJR-2021-0188.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Du D., Chen A., Zhu L. (2018). Circular RNA expression profile of articular chondrocytes in an IL-1β-induced mouse model of osteoarthritis. Gene 644, 20–26. 10.1016/j.gene.2017.12.020 [DOI] [PubMed] [Google Scholar]
- Zhu J. K., He T. D., Wei Z. X., Wang Y. M. (2018). LncRNA FAS-AS1 promotes the degradation of extracellular matrix of cartilage in osteoarthritis. Eur. Rev. Med. Pharmacol. Sci. 22 (10), 2966–2972. 10.26355/eurrev_201805_15051 [DOI] [PubMed] [Google Scholar]
- Zhu Y., Li R., Wen L. M. (2021). Long non-coding RNA XIST regulates chondrogenic differentiation of synovium-derived mesenchymal stem cells from temporomandibular joint via miR-27b-3p/ADAMTS-5 axis. Cytokine 137, 155352. 10.1016/j.cyto.2020.155352 [DOI] [PubMed] [Google Scholar]
- Zhu Y. J., Jiang D. M. (2019). LncRNA PART1 modulates chondrocyte proliferation, apoptosis, and extracellular matrix degradation in osteoarthritis via regulating miR-373-3p/SOX4 axis. Eur. Rev. Med. Pharmacol. Sci. 23 (19), 8175–8185. 10.26355/eurrev_201910_19124 [DOI] [PubMed] [Google Scholar]
- Zou H., Lu C., Qiu J. (2021). Long non-coding RNA LINC00265 promotes proliferation, apoptosis, and inflammation of chondrocytes in osteoarthritis by sponging miR-101-3p. Autoimmunity 54 (8), 526–538. 10.1080/08916934.2021.1978432 [DOI] [PubMed] [Google Scholar]