Abstract
The ability of transition metal chitosan complexes (TMCs) of varying valence and charge to selectively adsorb As(III) and As(V) over their strongest adsorptive competitor, phosphate is examined. Fe(III)-chitosan, Al(III)-chitosan, Ni(II)-chitosan, Cu(II)-chitosan, and Zn(II)-chitosan are synthesized, characterized via Attenuated Total Reflectance-Fourier Transform Infrared spectroscopy (ATR-FTIR) and X-ray Diffractometry (XRD), and their selective sorption capabilities towards As(III) and As(V) in the presence of phosphate are evaluated. It was found that the stability of the metal-chitosan complexes varied, with Al(III)- and Zn(II)-chitosan forming very unstable complexes resulting in precipitation of gibbsite, and Wulfingite and Zincite, respectively. Cu(II)-, Ni(II)-, and Fe(III)-chitosan formed a mixture of monodentate and bidentate complexes. The TMCs which formed the bidentate complex (Cu(II)-, Ni(II)-, and Fe(III)-) showed greater adsorption capability for As(V) in the presence of phosphate. Using the binary separation factor , it can be shown that only Fe(III)-chitosan is selective for As(V) and As(III) over phosphate. Density Functional Theory (DFT) modeling and extended X-ray adsorption fine structure (EXAFS) determined that Fe(III)-chitosan and Ni(II)-chitosan adsorbed As(V) and As(III) via inner-sphere complexation, while Cu(II)-chitosan formed mainly outer-sphere complexes with As(V) and As(III). These differences in complexation likely result in the observed differences in selective adsorption capability towards As(V) and As(III) over phosphate. It is hypothesized that the greater affinity of Fe(III)- and Ni(II)-chitosan towards As(V) and As(III) compared to Cu(II)-chitosan is due to their forming less-stable, more reactive chitosan complexes as predicted by the Irving Williams Series.
1. Introduction
Efficient removal of toxic and carcinogenic inorganic pollutants such as arsenic (As) from drinking water is a topic of environmental and societal concern. Arsenic found in drinking water can be from either anthropogenic sources such as mining, agriculture, and coal combustion, or be released naturally from volcanic eruptions and weathering of arsenic bearing rocks.1–3 As of 2006, over 140 million people in 50 countries, including the United States, are at risk from elevated arsenic levels in groundwater and groundwater-irrigated-crops.4,5
Arsenic predominantly exists in aqueous environments as two oxyanions of varying oxidation state, arsenate (As(V)) and arsenite (As(III)). Arsenate is negatively charged (H2AsO4− / HAsO42−) at common environmental pH (5–7), while arsenite is usually present as neutrally charged H3AsO3. As a result of this neutral charge, As(III), which is the more toxic form of arsenic, tends to form weaker outer-sphere complexes with adsorbents, making it more difficult to remove.6–8 Therefore, it is a common pre-treatment step for As(III) to be oxidized to As(V) prior to removal,9–12 often via photo-oxidation with n-TiO2 and UV light.6,9–12 While photo-oxidation is an effective means of oxidizing As(III) to As(V), incorporation of UV light into treatment systems can be costly and pose a challenge to the ability to treat large volumes of water quickly at scale.12
Once As(III) is photo-oxidized to As(V), it is then more easily removed via adsorption, an attractive removal option due to the high removal efficiencies, ease of use, and regeneration potential of most adsorbent materials.2,13,14 However, on traditional adsorbents, co-existing oxyanions (e.g. phosphate, sulfate, nitrate, bicarbonate, and silicate), often compete with arsenic for non-specific adsorption sites due to their similar chemical structures and adsorptive mechanisms.9,12,15,16 Often, these competitive oxyanions also tend to occur at concentrations 10–100 fold higher than arsenic, further hindering effective arsenic removal.16 Of these competitive oxyanions, phosphate, widely considered to be the strongest adsorptive competitor with arsenic,15–21 has been shown to reduce arsenic removal efficiency by greater than 90%.16,20
Design of a selective adsorbent, one which favorably removes the target contaminant (e.g. As) over competing background oxyanions (e.g. P), promotes target contaminant adsorption and/or hinders competitive oxyanion adsorption.16 Adsorptive selectivity can be realized through a variety of mechanisms that exploit differences in chemical structure or adsorptive behavior between target and competitive oxyanions. Some examples include differences in complexation type, Lewis acid/base hardness, charge density, and size/shape between target contaminants and competitive ions.15,16 Selective adsorption enables increased material and resource efficiency as the adsorption effectiveness towards the target contaminant is greatly improved.9,12,16
Selective adsorbents for arsenic have been previously developed, but mainly for competition between As(V) and weakly competitive sulfate, nitrate, and bicarbonate,16,22–24 rather than the strongest adsorptive competitor with both As(V) and As(III), phosphate.16,25,26 However, previous research has found that selectivity for As(V) over phosphate can be developed in Cu(II)-chitosan-based adsorbents.9,12,15 In this material, as Cu(II) binds to the amine groups of chitosan, the Cu(II)-chitosan complex gains cationic character that can electrostatically attract oxyanions.9,12,15,22,27 Depending on the loading of Cu(II), as well as the pH of the system, Cu(II)-chitosan will form two different complexes. In the monodentate complex, Cu(II) binds with a single amine group on chitosan, and in the bidentate complex Cu(II), which only forms along with the monodentate complexes above pH 5.5 and 0.25 mol Cu/mol chitosan, binds with two amine groups and crosslinks the chitosan.15 It was found that the bidentate Cu(II)-chitosan complex selected for As(V) over phosphate, and was hypothesized that this selectivity was driven electrostatically.9,12,15
Phosphate and As(V) are strong adsorptive competitors due to their similar Lewis Base Hardness,28 pKa’s,17,29 tetrahedral geometry,19 and tendency to form strong inner-sphere complexes with traditional adsorbents.16,30 However, As(V) and phosphate do differ in their size, charge density, and polarizability, with the larger size of As(V) leading to lower charge density and higher polarizability.9,12,15–17 As copper binds to chitosan, it creates a complex with cationic character.9,12,15 In the monodentate complex, it was hypothesized that the positive charge of copper is more concentrated and thus is more similar to the partial positive charge on P and the partial negative charge on O within phosphate.12,15 In contrast, in the bidentate complex, the positive charge of copper is dispersed over a larger area so is more diffuse/polarizable, and more similar to the partial positive charge on As and the partial negative charge on O in H2AsO4−. Thus, As(V) is electrostatically preferred over phosphate.9,12,15
While the Cu(II)-chitosan complex was successful at developing selectivity for As(V) over P, it had very low sorption potential for As(III). Therefore, a multifunctional version of this adsorbent was created which incorporated nano titanium dioxide (n-TiO2) into the Cu(II)-chitosan complex in order to photo-oxidize As(III) to As(V) in UV light.9,12 This version of the adsorbent showed successful simultaneous photo-oxidation of As(III) to As(V) and selective adsorption of As(V) over phosphate, and higher arsenic removal capacity than Cu(II)-chitosan.9,12 Although this adsorbent was effective at both oxidation of As(III), to As(V) and selective adsorption of As(V), elimination of a the need for As(III) photo-oxidation by increasing sorptive potential towards both As(III) and As(V), could enhance ease of use in flow-through systems.12
Therefore, in this study we explore other transition metal chitosan complexes (TMCs) as alternatives to Cu(II)-chitosan in order to assess their potential to selectively adsorb both As(V) and As(III) over phosphate, without the need for UV photo-oxidation. Fe(III)-chitosan, Al(III)-chitosan, Ni(II)-chitosan, and Zn(II)-chitosan were synthesized and compared to Cu(II)-chitosan. These different transition metals form complexes of varying stability with chitosan based on the Irving-Williams Series which predicts the stability of metal-polymer complexes.31,32 Thus, these TMCs should have varying affinity for selective adsorption of As(V) and As(III) over phosphate.
Previous studies have explored Fe(III)-chitosan as an adsorbent for Cr(VI), perchlorate, and arsenic.33–37 However, these studies were mainly focused on sorption of induvial contaminants, not selective adsorption in competitive systems. Additionally, these adsorbents also contained glutaraldehyde as an additional cross-linker, which has been shown to lower adsorption capacity due to the binding of glutaraldehyde to the amine groups of chitosan, and also has toxicity concerns.38,39 No study has yet systematically examined the potential of chitosan-complexes of varying valence and charge as use for selective adsorbents for As(III) and As(V) over their strongest competitor, phosphate.
2. Materials and methods
2.1. Standards and reagents
Beads of Cu(II)-chitosan, Fe(III)-chitosan, Zn(II)-chitosan, Al(III)-chitosan and Cu(II)-n-TiO2-chitosan were synthesized using the following materials. Chitosan flakes (TCI America, 200–500 mPa-s, 0.5% in acetic acid) were mixed with metal ions in the form of the following nitrate salts: Cu(NO3)2-3H2O for Cu(II), (Sigma Aldrich, ACS reagent grade), Fe(NO3)3-9H2O for Fe(III) (Sigma Aldrich, ACS reagent grade), Ni(NO3)2-6H2O for Ni(II) (Sigma Aldrich, trace metal grade), Zn(NO3)2-6H2O for Zn(II) (Sigma Aldrich, ACS reagent grade), and Al(NO3)3-9H2O for Al (III) (Sigma Aldrich, ACS reagent grade).9,15 HNO3 and NaOH used in the synthesis were prepared from concentrated stock solutions (HNO3, Fisher Scientific, trace metal grade) (NaOH, JT Baker, ACS reagent grade).
Stock solutions (100 ppm) of As(V) and As(III) were prepared from Na2HAsO4–7H2O (Fisher Scientific, >99.5%) and NaAsO2 (Fluka, >99.0%) respectively, dissolved in DI water. Phosphate solution was prepared from NaH2PO4 - H2O (JT Baker, ACS reagent grade), phosphoric acid (Sigma Aldrich, trace metal grade), and DI water.9,15 NaCl solutions were prepared from NaCl salt (Sigma Aldrich, ACS reagent grade). HCl dilutions were prepared from concentrated HCl (JT Baker, ACS reagent grade). Acetate buffer pH 6 was prepared using glacial acetic acid (Fisher Scientific, ACS reagent grade) and anhydrous sodium acetate anhydrous (CH3COONa) (JT Baker, ACS reagent grade). All solutions were prepared from stock immediately before use.
2.2. Adsorbent synthesis
Transition metal cross-linked chitosan formulations were synthesized by adapting the procedures of Yamani et al. (2016) and Pincus et al. (2018).9,12,15 Briefly, using Cu(II)-chitosan as an example, Cu(NO3)2-3H2O was added to 0.1 M HNO3 and stirred until dissolved completely. Then chitosan was added and the mixture stirred for 24 hours. The resulting gel was loaded into syringes with 18G needles and a syringe pump was used to push droplets into 0.1 M NaOH where the droplets would then solidify as Cu(II)-chitosan beads. Next, the solidified beads were rinsed until filtrate was of neutral pH and then air-dried. Fe(NO3)3-9H2O, Ni(NO3)2-6H2O, Zn(NO3)2-6H2O, and Al(NO3)3-9H2O were used to synthesize Fe(III)-chitosan, Ni(II)-chitosan, Zn(II)-chitosan, and Al(III)-chitosan, respectively. All metals were added in equimolar ratios. Neat chitosan beads were synthesized by omitting the addition of any transition metals in the above procedure.
2.3. Adsorbent characterization
To identify presence of crystalline materials within TMCs, a Rigaku SmartLab X-ray Diffractometer (XRD) operating with a k-beta filter at a voltage of 40 kV, current of 44 mA, ISL of 5 mm, IS of 2/3°, RS1 of 20 mm, and RS2 of 20 mm was used. Prior to analysis, the adsorbents were ground to <5 μm using a mortar and pestle and loaded onto a zero-background slide holder. Samples were scanned continuously from 10 to 90 °2θ with a step of 0.5° at a rate of 10°/min. Spectra were analyzed and peaks identified using Rigaku PDXL software.
Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR) was used in order to characterize the type of cross-linking by the transition metals in the TMCs. A Thermo Fisher Nicolet iS50 FTIR fit with a zinc selenide crystal ATR attachment, KBr splitter, and a DLa-TGS detector was used. Data were collected over the range of 600 to 4000 cm−1 as an average of 200 scans. 100 scans were collected as background before the loading of each sample. Spectra were analyzed using OMNIC FTIR analysis software.
2.4. Adsorption Experiments
The arsenic removal capacity of TMCs was examined through week-long batch incubations.6,9–12,15,40 The mass of adsorbent added was calculated to maintain a constant total mass of chitosan.9,15 The adsorbents were added to 40 mL of solution in a 50-mL polypropylene Falcon tube. The following systems conditions were analyzed: As(V), As(V)+P, As(III), and As(III)+P. In each system condition the initial concentration of arsenic was varied and duplicates were made of each sample with a corresponding black. When phosphate was present, the initial concentration was 16 ppm. A 25 mM acetate buffer was added to maintain pH 6, removing it as a confounding variable.9,12,15 An additional adsorption test was conducted to evaluate As(V) removal performance in a challenging freshwater matrix using NSF 53 Challenge Water spiked with 50 ppb As(V).41 Initial concentrations were 183 ppm HCO3−, 40 ppm Ca2+, 71 ppm Cl−, 1 ppm F−, 12 ppm Mg2+, 8.9 ppm NO3−, 0.12 ppm PO43−, 20 ppm SiO2, 89 ppm Na+, 48 ppm SO42−, and 50 ppb As(V). In this incubation, no acetate buffer was added, pH was adjusted to 7.5 +/− 0.15, and the incubation was conducted for four days. Results of this procedure can be found within the SI (Figure S1).
Samples were incubated at 150 rpm and 25 °C for one week in VWR shaking incubators. Next, samples were filtered via a 0.22 μm PVDF filter and diluted/acidified using 1% nitric acid. A Perkin Elmer DRC-e inductively coupled plasma-mass spectrometry (ICP-MS) was used to analyze the samples. A Scott Crossflow nebulizer with mixing tee, Argon plasma, and germanium and scandium internal standards were utilized during the analysis.9,15 Triplicate analyses were performed with a repeating standard and blank every ten samples to track instrument stability.
2.5. DFT Modeling and Calculations
All calculations were conducted using the Gaussian 16 suite of programs,42 as it contains all the tools necessary for the prediction of molecular properties, simulation of materials structure, and study of interactions and evaluation of binding energies. Modeling was based on the use of Density Functional Theory (DFT), implemented in Gaussian 16, which combines relatively low computational cost and good accuracy. DFT methods have been successfully used for several decades to predict a range of molecular properties, structural features of materials, interaction energies, reaction mechanisms and thermochemistry parameters.43,44 Ground-state geometries of all molecules were optimized using ωB97XD45 density functional and def2TZVP46 basis set in a vacuum, which is a standard procedure for DFT calculations. A pruned integration grid with 99 radial shells and 590 angular points (ultrafine grid, default in Gaussian 16) was used for all the calculations. Optimized geometries were then used to evaluate the interaction energies between metal-chitosan complex and oxyanions using counterpoise method to account for Basis Set Superposition Error (BSSE) as implemented in Gaussian 16.
2.6. XAS spectroscopy
X-ray Adsorption Spectroscopy (XAS) was utilized to gain further insight into the binding mechanism of arsenic to TMCs. X-ray Adsorption Near Edge Spectroscopy (XANES) and Extended X-ray Adsorption Fine Structure (EXAFS) were both analyzed, results of XANES can be found within SI (Figures S2–S4). Samples were prepared by incubating following the sample procedure as described in Section 2.4, with the exception that initial concentrations were 44 ppm As and 56 ppm P so as to ensure full saturation of the adsorbent with arsenic and phosphate, resulting in a good signal to noise ratio during XANES and EXAFS analysis. No acetate buffer was used to maintain pH. Instead, a background electrolyte of 0.01 M NaCl was added and pH was adjusted to 6 ± 0.15 daily using 0.1 M NaOH and 0.1 M HCl. Following incubation, the beads were frozen until time of analysis. At time of analysis, the frozen beads were sealed in Kapton tape with care taken to minimize pinholes. Liquid samples were analyzed in Kapton capillaries.
XAS spectra were collected at the As K-edge (11867 eV) at the Inner Shell Spectroscopy beamline (8-ID) at the National Synchrotron Light Source II (NSLS II) at Brookhaven National Laboratory and the As K-edge at beamline 9-BM at the Advanced Photon Source (APS) at Argonne National Laboratory. As K-edge spectra were calibrated to the Au L-edge (11919 eV) using reference foils measured with each sample.47 Multiple scans were collected and averaged to improve the signal to noise ratio. At NSLS II, the electron storage ring was operating at 3 GeV with a beam current of 400 mA. Spectra were acquired in fluorescence mode at room temperature using a cryogenically cooled Si (111) Double Crystal monochromator and a Passivated, Implanted, Planar Silicon (PIPS) detector. A germanium Z-1 filter was used to suppress the elastic scattering on the detector. At APS, the electron storage ring was operating at 7 GeV with a beam current of 100 mA. Samples were kept at 20 K using a liquid helium flow cryostat and spectra acquired in florescence mode using a 4-element Vortex Silicon Drift Detector (SDD). XANES data reduction and analysis were performed using the Athena program,48 and EXAFS data reduction and analysis was performed using the Athena49 and Artemis48 programs. For full procedure of EXAFS analysis and fitting please refer to the SI.
3. Results and Discussion
3.1. Adsorbent Characterization
FTIR was utilized in order to assess the type of complexation of the TMCs (Figure 1). FTIR analysis of chitosan beads not cross-linked with metals corresponds well with literature findings of chitosan.15,50–53
Figure 1.
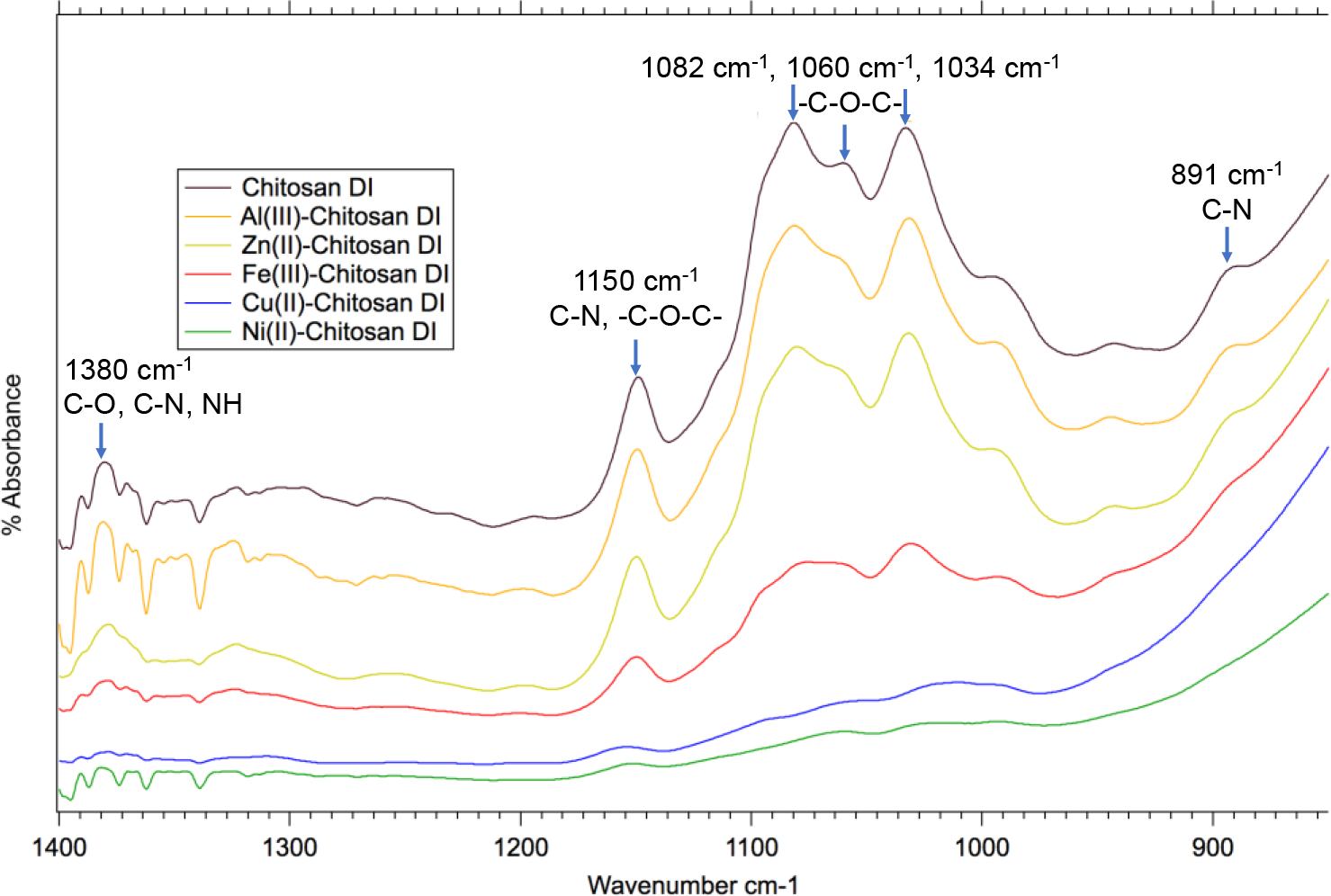
FTIR spectra of neat chitosan beads, Al(III)-chitosan, Zn(II)-chitosan, Fe(III)-chitosan, Cu(II)-chitosan, and Ni(II)-chitosan saturated with DI water. The decrease in intensity of the marked peaks indicate changes in C-N, C-O, and -O-C-O- stretching vibrations within chitosan.
The greatest indicator of the formation of the bidentate complex is a decrease in peak intensity for peaks associated with C-O, and C-N bonds.15 These peaks include 891 cm−1 for C-N stretching vibrations,51 1034 cm−1, 1060 cm−1, 1082 cm−1, for -C-O-C- glyosidic linkages and C-O alcohol stretching vibrations,15,50 1150 cm−1 for C-N and -C-O-C- stretching vibrations,15,50,51,54 1370–1380 cm−1 for C-N, C-O, and N-H stretching vibrations,15,55,56 (Figure 1) and the broad band at 3349 for N-H and O-H stretching vibrations (Figure S5).50,52 FTIR analysis of TMCs indicate that Cu(II)-chitosan, and Ni(II)-chitosan form bidentate chitosan complexes as indicated by a decrease in intensity in all of these characteristic chitosan peaks.15,51,53 This decreased intensity is likely due to the complexation of the amine groups restricting vibrations in the C-N and C-O bonds within chitosan.15 Fe(III)-chitosan exhibits less of a decrease in peak intensity than Ni(II)-chitosan and Cu(II)-chitosan, but still a significant decrease compared to the neat chitosan beads. This could suggest that either the Fe(III)-chitosan is forming less of the bidentate-chitosan complex or that less rigid bidentate complexes are being formed compared to Cu(II) and Ni(II). Unlike the other TMCs, Zn(II)-chitosan and Al(III)-chitosan do not exhibit a decrease in peak intensity, suggesting that they do not form bidentate metal-chitosan complexes.
Previous research has found oversaturation of the TMC complex with copper results in Cu no longer forming bidentate complexes and instead precipitating as CuO.9,12,15 Thus, XRD analysis was performed on TMCs in order to identify any crystalline precipitates which may be leading to a lack of bidentate complex formation (Figure 2). Crystalline precipitates were identified in Al(III)-chitosan and Zn(II)-chitosan, but not in Fe(III)-chitosan, Cu(II)-chitosan, and Ni(II)-chitosan. The peak observed in Al(III)-chitosan at 20.5 °2θ identifies Al2O3 (gibbsite) as the mineral precipitate.57 Both Zn(OH)2 (Wulfingite) and ZnO (Zincite) were identified as precipitates in Zn(II)-chitosan.58 The peak at 19.7 °2θ corresponds with Zn(OH)2 and the peaks at 31.7 °2θ, 34.3 °2θ, 36.2 °2θ, 47.4 °2θ, 56.4 °2θ, 62.8 °2θ, 66.4 °2θ, 67.9 °2θ, and 69.0 °2θ correspond with ZnO.58 In this study, the loading of Cu was lowered to 0.4 g Cu(NO3)2-3H2O / 1 g chitosan, the threshold for copper precipitates forming, thus no CuO peaks are observed in Figure 2.9,12 All TMCs were made with an equimolar ratio of metal nitrate added in order to lower the potential for metal precipitates forming. Thus, the formation of Zn and Al precipitates forming may suggest that they have a lower tendency to form a bidentate-chitosan complex, even at lower loadings of Zn and Al, agreeing with FTIR results (Figure 1).
Figure 2.
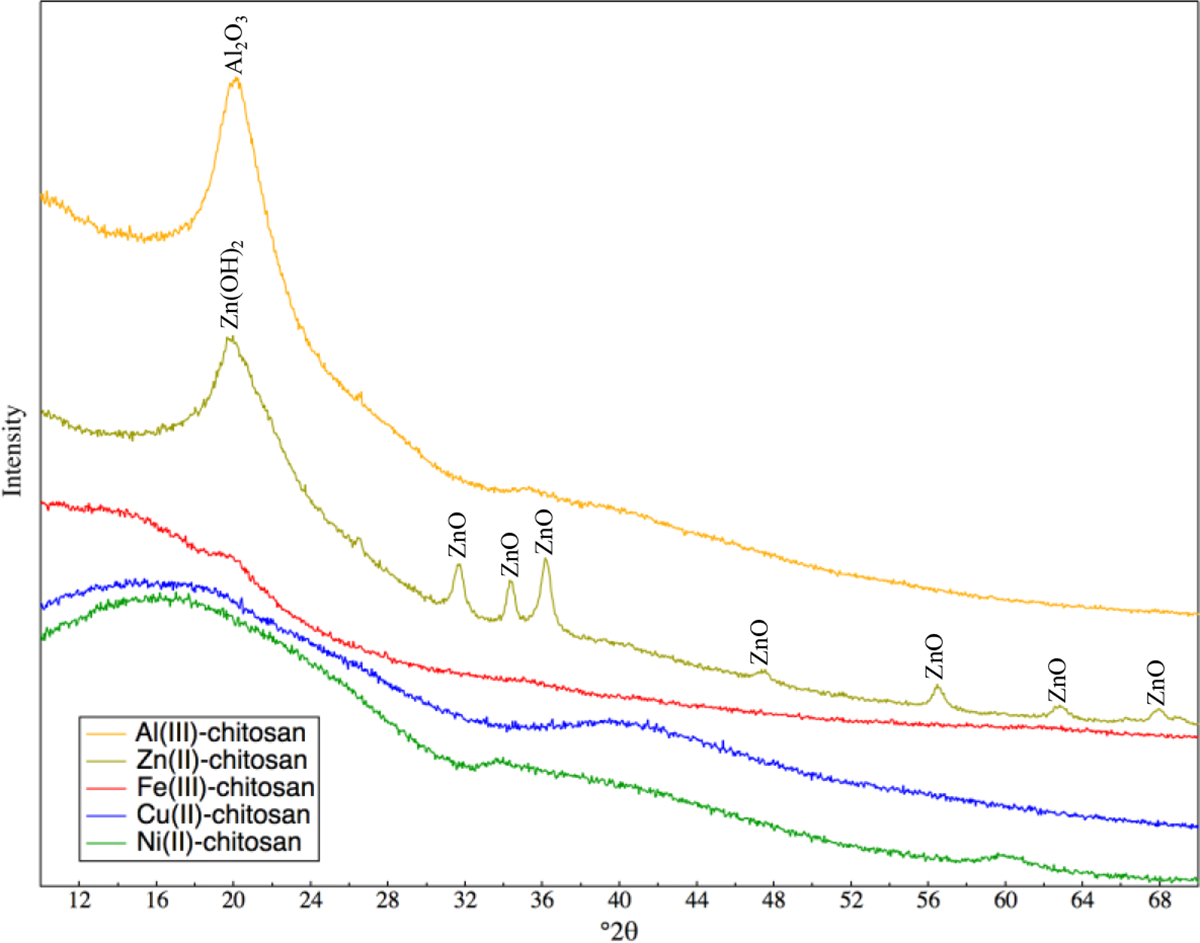
XRD spectra of Al(III)-chitosan, Zn(II)-chitosan, Fe(III)-chitosan, Cu(II)-chitosan, and Ni(II)-chitosan. Labels show peak positions of crystalline phases identified in Al(III)-chitosan and Zn(II)-chitosan.
3.2. Assessment of Selective Arsenic Removal Performance by TMCs
In order to assess the arsenic removal performance by TMCs, adsorption isotherms were generated for each TMC in four systems conditions As(V) (Figure S6), As(V)+P (Figure 3A), As(III) (Figure S7), and As(III)+P (Figure 3B). Fe(III)-chitosan was the adsorbent with the highest capacity for arsenic removal overall. While Cu(II)-chitosan performed better than Ni(II) chitosan at As(V) removal with/without P present, it was less capable of removing As(III) singularly and with phosphate present.
Figure 3.
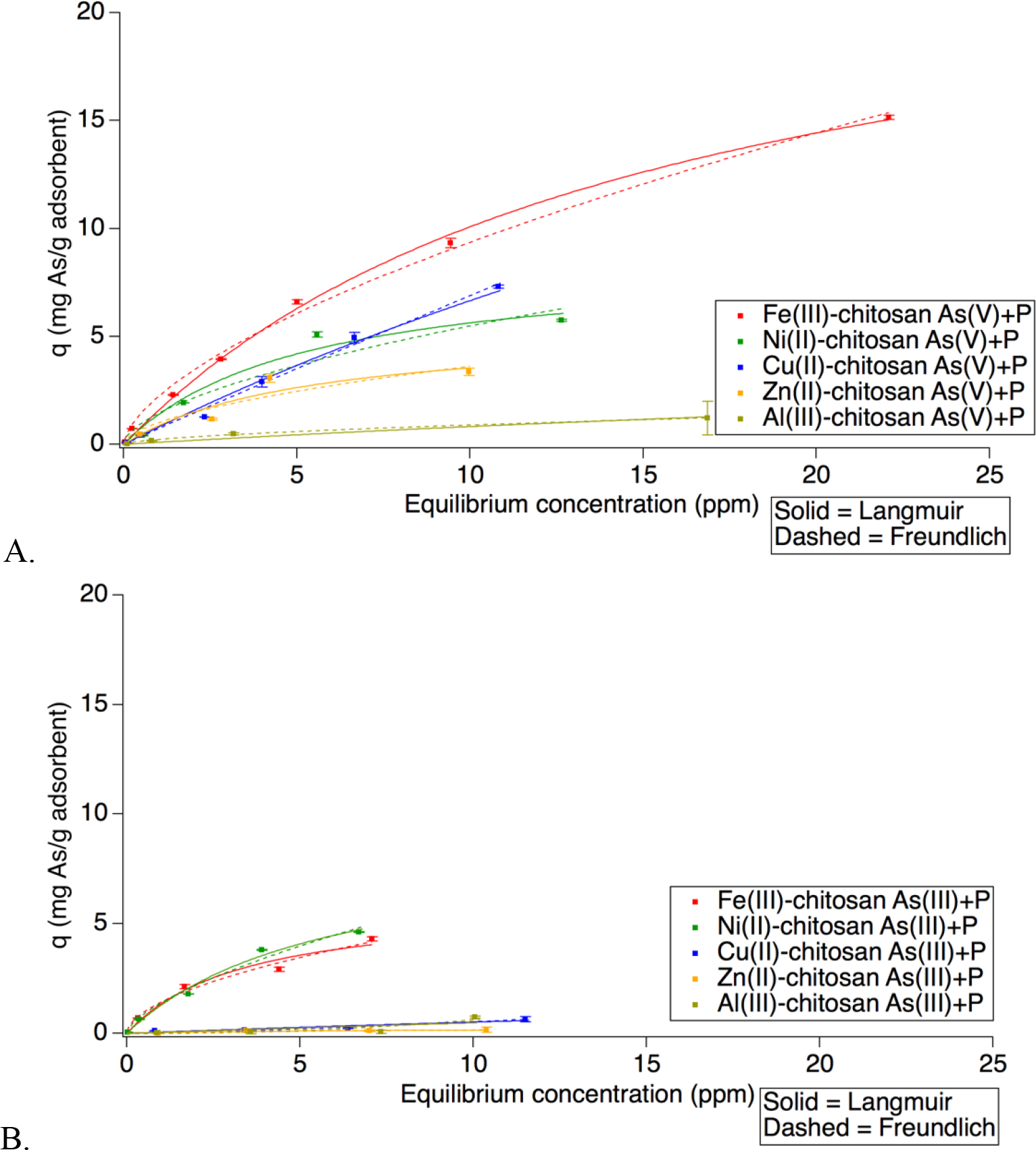
Adsorption isotherms for As removal by Fe(III)-chitosan, Ni(II)-chitosan, Cu(II)-chitosan, Zn(II)-chitosan, and Al(III)-chitosan in As(V) with phosphate present (A) and As (III) with phosphate present (B). Initial concentrations were 1, 4, 8, and 12 ppm and 0.25 mM acetate buffer pH 6, for all systems except the following: Cu As(V)+P (100 ppb, 20 ppm added), Ni As(V)+P (20 ppm added), Ni As(III)+P (50 ppb added), Fe As(V)+P (100 ppb, 20 ppm, 40 ppm added, and Fe As(III)+P (50 ppb added). Phosphate concentration was 16 ppm where noted. Langmuir fits indicated with solid lines and Freundlich fits with dashed lines.
Comparing arsenic removal performance of the TMCs at 4 ppm As and 16 ppm P (when present) (Figure 4), Fe(III)-chitosan has the highest As removal performance of all the TMCs in each of the four systems conditions tested, removing 93% of As(V), 59% of As(V) with P present, 89% of As(III), and 53% of As(III) with P present, followed by, in order of decreasing arsenic removal performance: Ni(II)-chitosan, Cu(II)-chitosan, Zn(II)-chitosan, and Al(III)-chitosan. As(V) removal performance was also evaluated in NSF 53 Challenge Water spiked with 50 ppb As(V) at pH 7.5 (Figure S1).41 Within NSF 53 challenge water, only Fe(III)-chitosan and Ni(II)-chitosan were capable of removing As(V) beneath the 10 ppb limit set by the World Health Organization (WHO), with equilibrium concentrations of 3.9 ppb and 2.4 ppb.
Figure 4.
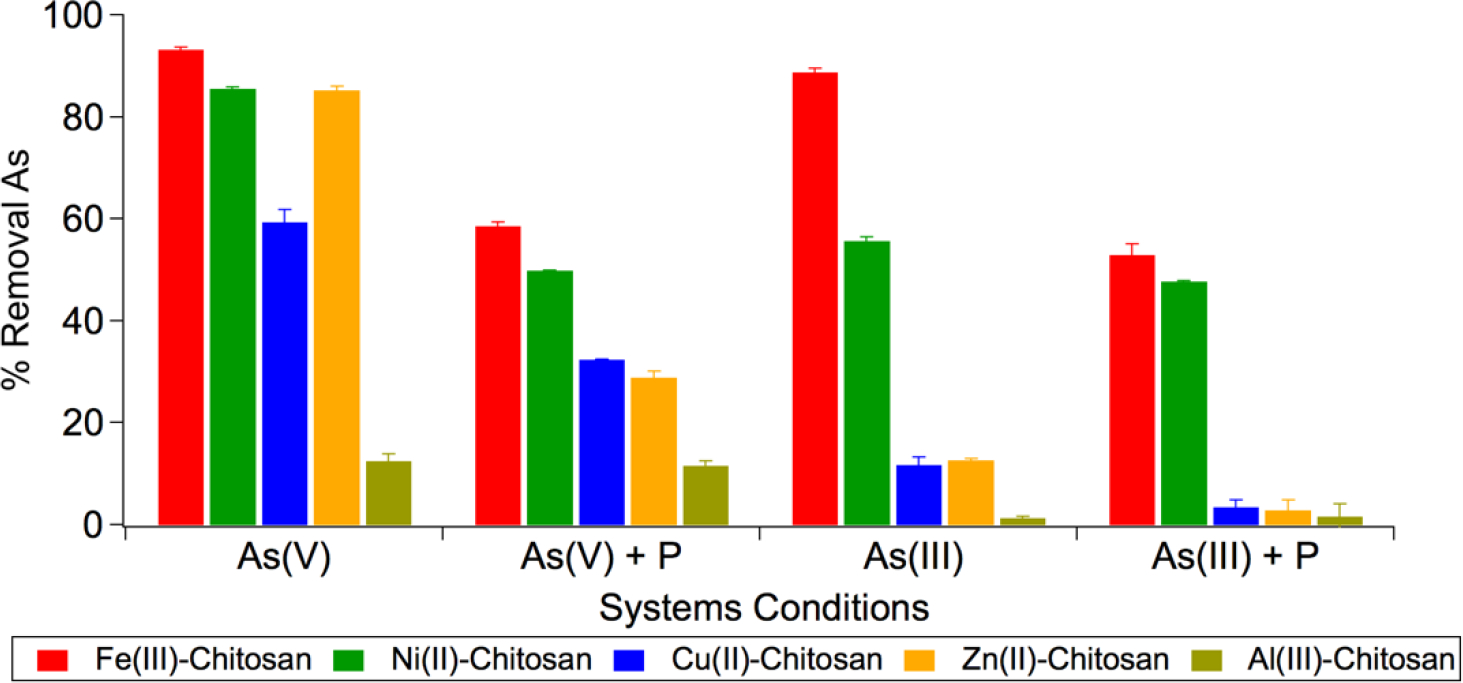
Comparison of arsenic removal performance by Fe(III)-chitosan, Ni(II)-chitosan, Cu(II)-chitosan, Zn(II)-chitosan, and Al(III)-chitosan in various systems conditions. Initial concentrations: 4 ppm As, 25 mM acetate buffer pH 6, and 16 ppm P where noted.
In order to quantify the degree of selectivity towards As(V) and As(III), the binary separation factor was used due to its ability to compare adsorption affinity between both the target and competitive oxyanions within the same system (Figure 5).15,22,27 The binary separation factor is a dimensionless metric which compares (equilibrium adsorption capacity) and (equilibrium concentration) values of both the target oxyanion, as well as the competitor to quantify selective adsorption preference:
where is the binary separation factor for the adsorption of target oxyanion, , in the presence of competitive oxyanion, is the equilibrium adsorption capacity of the target, is the equilibrium adsorption capacity of the competitor, is the equilibrium concentration of the competitive oxyanion in the mixed ion system, and is the equilibrium concentration of the target oxyanion in the mixed ion system.15,16,59 If , then is preferred and if , then is preferred.15 Using this metric, it can be shown that only Fe(III)-chitosan is selective for both As(V) () and As(III) over phosphate (). Interestingly Al(III)-chitosan was shown to be selective for As(V) over P (). However, if we consider the arsenic removal capability by this adsorbent (Figure 4), we can see that Al(III)-chitosan is ineffective at removing As(V). Thus, despite the high value, this would be a poor choice of selective adsorbent as it likely unable to remove As(V) beneath regulatory limits in most systems conditions.
Figure 5.
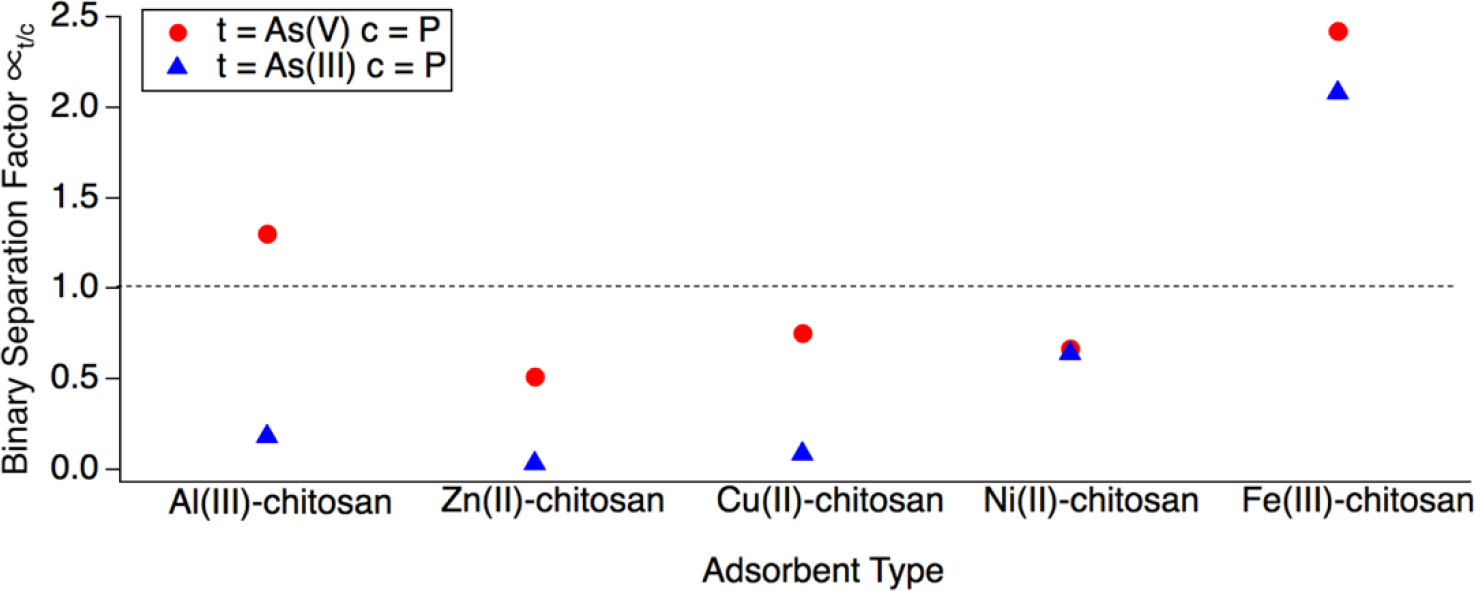
Binary separation factor where is the target contaminant and c is the competitor for the As(V)/P and As(III)/P systems for TMCs. Red circles show binary separation factors for As(V) selectivity and blue triangles for As(III) selectivity. Dashed line denotes where selectivity towards As(V) or As(V) is quantitatively defined by the binary separation factor (. Initial concentrations were 4 ppm As, 16 ppm P and 50 mM acetate buffer pH 6.
Overall, these results support the previous findings that TMCs with a bidentate metal-chitosan complex such as Fe(III)-, Ni(II), and Cu(II)-chitosan are more capable of selective adsorption of As(V) over phosphate due to their generally higher higher values.9,12,15 Of particular note is the superior performance of Fe(III)-chitosan and Ni(III)-chitosan at selective adsorption of As(III) over phosphate. This high As(III) removal performance, with and without phosphate present, is not observed for Cu(II)-, Zn(II)-, and Al(III)-chitosan. In the next section we will explore possible mechanistic explanations for the observed differences in As(V) and As(III) removal capacity between the TMCs.
3.3. Investigation of Arsenic Adsorption Mechanism
EXAFS analysis and DFT modeling were utilized in order to investigate the complexation of As(III) and As(V) by TMCs. DFT models were created in order to estimate adsorption behavior of As(V), As(III), and phosphate with Fe(III)-, Ni(II)-, and Cu(II)-chitosan. As the exact structure of metal-bound chitosan polymers is still not known, and because of the limitations of the DFT methodology regarding the size of the studied system, we have suggested a model of only the immediate surroundings of the metal center where oxyanion binding is expected to happen, specifically the two monomer chitosan units coordinated in a bidentate complex with the metal center, M (Cu, Ni, Fe), through amino groups, and additional hydroxyl groups. This model structure was chosen to best mimic experimentally observed coordination of the metal and its steric environment in the metal-activated chitosan polymer. The oxyanion molecule (As(III), As(V), P) was then placed in the vicinity of the metal center, and the geometry of the system was left to optimize to the potential energy. Theoretical structures of optimized geometries for these model “cross-linked monomer” systems with As(V) and As(III) (Figures 6 and 7, respectively) are used for the EXAFS fittings.
Figure 6.
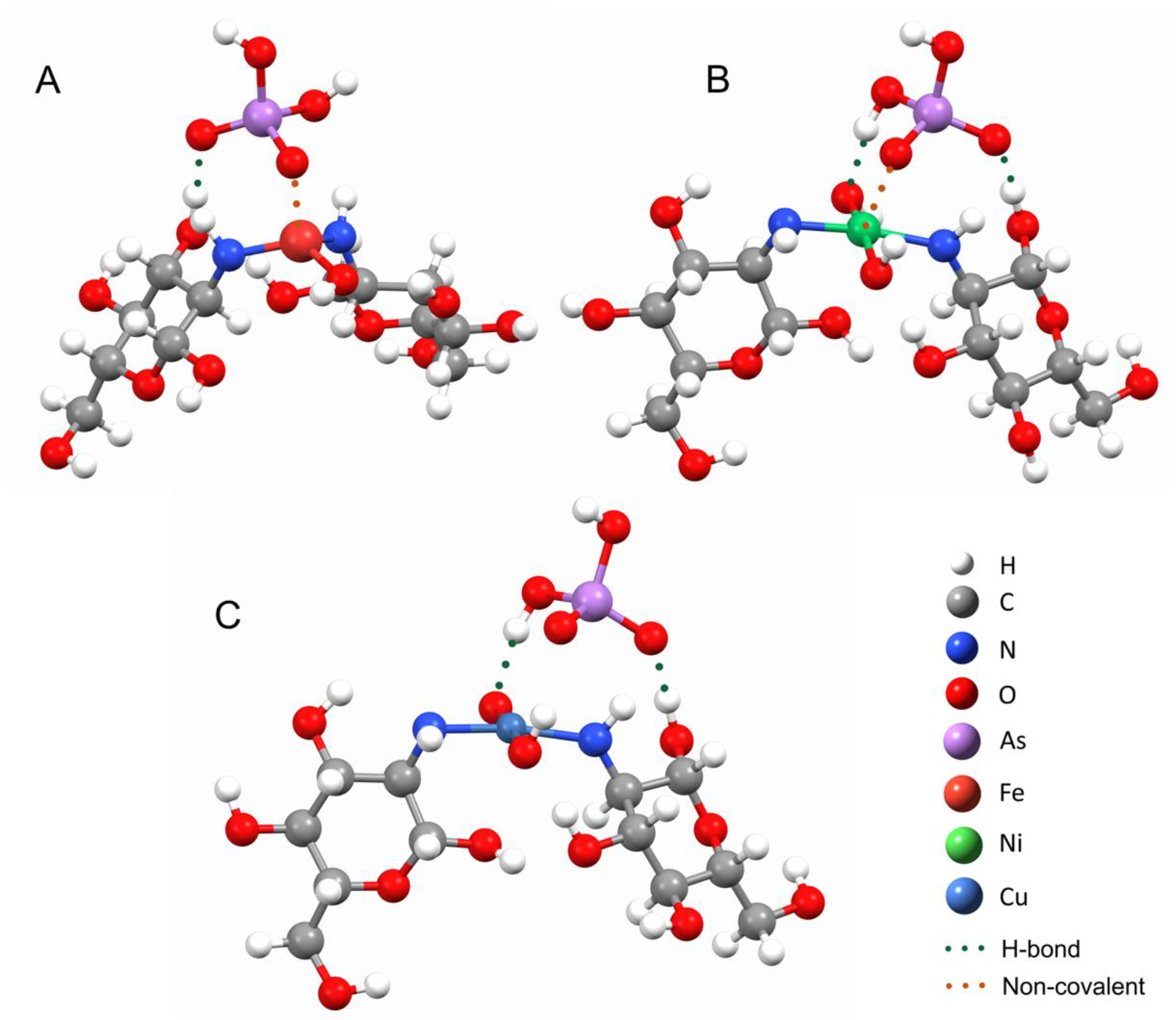
DFT optimized geometries of As(V) adsorption by Fe(III)-chitosan (Figure 6a), Ni(II)-chitosan (Figure 6b) and Cu(II)-chitosan (Figure 6C). Each element in the structure is represented with a different color with while for H, gray for C, blue for N, red for O, purple for As, light red for Fe, green for Ni, and light blue for Cu. Green dots depict hydrogen bonding interactions between As(V) and the metal-chitosan complex. Orange dots indicate inner-sphere non-covalent interactions between As(V) and the metal-chitosan complex.
Figure 7.
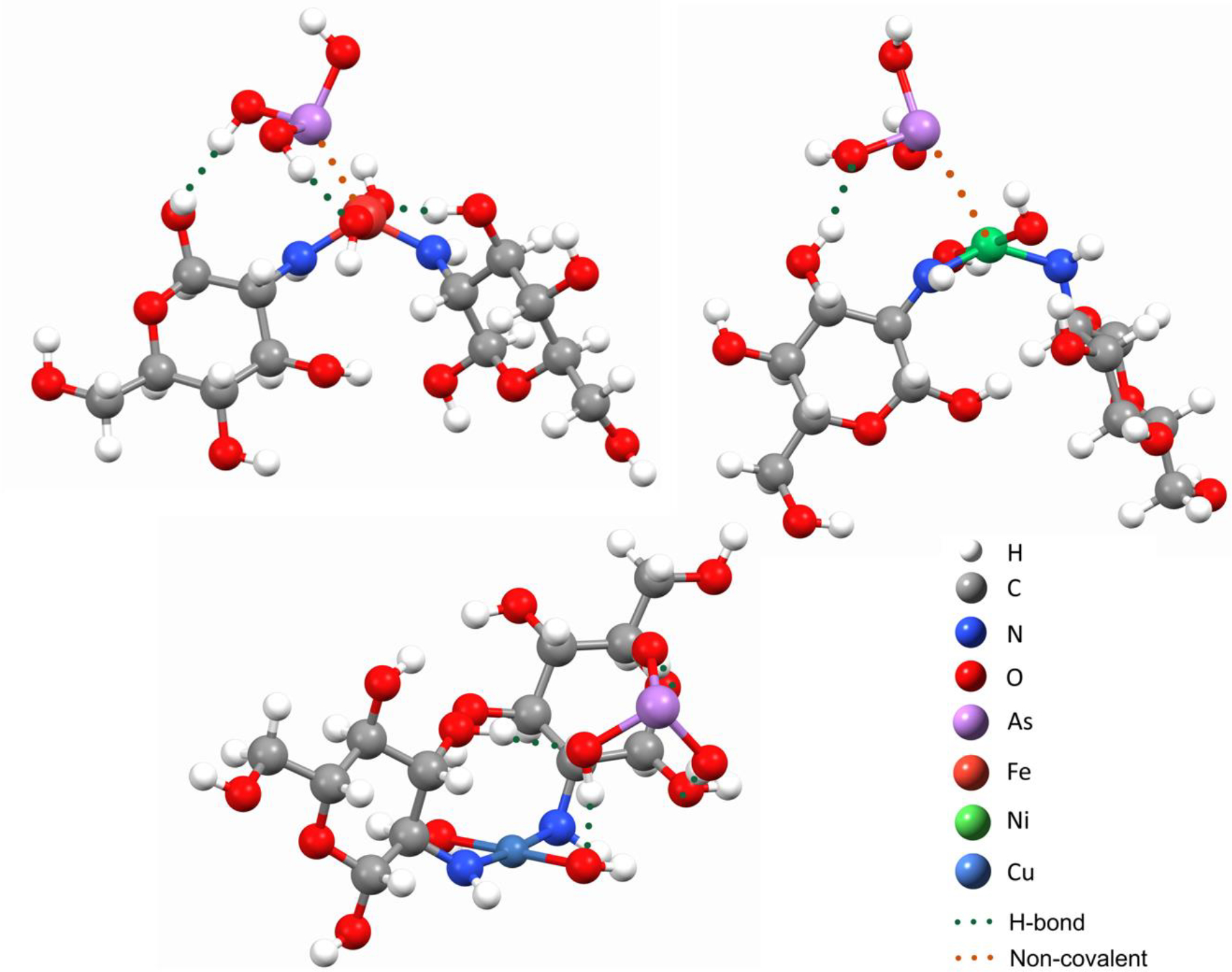
DFT optimized geometries of As(III) adsorption by Fe(III)-chitosan (Figure 7a), Ni(II)-chitosan (Figure 7b) and Cu(II)-chitosan (Figure 7C). Each element in the structure is represented with a different color with while for H, gray for C, blue for N, red for O, purple for As, light red for Fe, green for Ni, and light blue for Cu. Green dots depict hydrogen bonding interactions between As(III) and the metal-chitosan complex. Orange dots indicate inner-sphere non-covalent interactions between As(III) and the metal-chitosan complex.
The As K-edge was utilized in order to investigate arsenic complexation mechanism. Adsorption of As(V) and As(III) with and without phosphate present to Fe(III)-chitosan, Ni(II)-chitosan, and Cu(II)-chitosan was analyzed. Due to the lack of formation of a bidentate metal-cross-linked complex in Zn(II)-chitosan and Al(III)-chitosan (Figure 1), these TMCs were not analyzed via EXAFS. While adsorption of As(III) onto Cu(II)-chitosan with/without P was analyzed, due to the low amount of As(III) adsorbed by Cu(II)-chitosan (Figure 4), EXAFS spectra could not be reliably obtained due to the high amount of noise relative to the EXAFS signal. Fe, Ni, and Cu K-edge spectra were also collected for EXAFS analysis, however, the symmetric planar geometry of the Fe(III)-, Ni(II)-, and Cu(II)-chitosan complexes results in an unusually high multiple scattering contribution to the EXAFS spectra.60 These multiple scattering path contributions occur at the same radial distance as the expected binding of arsenic, preventing investigation of As binding mechanism through these metal adsorption edges.
The EXAFS spectra in space (Fourier transforms of the functions) of As(V) (Figure 7a) and As(III) (Figure 7b) adsorbed by TMCs were analyzed and the parameters obtained by fitting the theoretical paths generated from the DFT models to the experimental spectra can be found within the Supplemental Information (Table S1). Overall, reasonably good fits (defined as an R factor of <0.05) were found for all experimental spectra indicating that the DFT models agree with the experimental data from EXAFS. At times during the fitting, the delta R (change in interatomic distance), was around 0.2 Å for second shell fittings, this could be in part due to the use of the “cross-linked monomer” model which does not account for the steric hindrance of the full chitosan polymer on As(III) or As(V).
The first shell for EXAFS spectra analyzed corresponds to the As-O bond within the As(V) and As(III) structure.61–65 Given the known structures of As(V) where As is bound to four oxygens in a tetrahedral conformation, and As(III) where As is bound to three Os in a trigonal pyramid conformation, and conformation from XANES analysis that no oxidation state changes occurred upon adsorption of As to the TMCs (Fig. S2–S4), the coordination number (N) was set as 4 for As(V) and 3 for As(III) for all fits. The interatomic distance (R) was found to be 1.70–1.71 Å for all As(V) samples, and 1.78–1.80 Å for all As(III) samples, the values of which agree with those found within the literature.61–65
Second shell fits were obtained for all samples analyzed which correspond to As-O/N and As-M (where M is the metal cross-linker (e.g. Fe)) complexation interactions.64,66 Due to O and N having backscattering cross-sections that are very close, EXAFS is unable to distinguish between coordination of As with N and O.36 For Fe(III)-chitosan As(V), the second shell was found to be generated by As-Fe scattering with an R of 3.31 Å and N of 1.5 and As-O/N interactions with an N of 6 at an R of 3.59 Å. Similarly, Ni(II)-chitosan As(V) had a second shell of As-Ni scattering with an R of 2.99 Å and an N of 1.5 and As-O/N with an R of 2.89 Å and an N of 6. Both of these results suggest that As(V) is forming inner-sphere complexes with Fe, and Ni,63,65,66 as well as hydrogen bonding with the amine and/or hydroxyl groups of the chitosan backbone. In contrast, the second shell of Cu(II)-chitosan only shows As-O/N interactions with an R of 2.95 Å and an N of 8 which suggests that As(V) coordinates via hydrogen bonding with the amines and/or hydroxyls of chitosan, however does not form an inner-sphere complex with Cu.66 These results may explain the higher As(V) removal performance by Fe(III)- and Ni(II)-chitosan compared to Cu(II)-chitosan (Figure 4).
For As(III)-adsorption, the second shell fit of Fe(III)-chitosan showed As-Fe interactions with an R of 2.36 Å and an N of 1.5 and As-O/N interactions with an N of 4 (1 at 2.93 Å and 3 at 3.30 Å). As(III) adsorption to Ni(II)-chitosan showed As-Ni coordination with an N of 1.5 at 3.37 Å and with O/N with an N of 4 at 2.84 Å. These results suggest that, similar to As(V), As(III) interacts with Fe(III)- and Ni(II)-chitosan through hydrogen bonding with the chitosan backbone as well as inner-sphere complexation with the transition metal cross-linker.36,63–66 Due to the low As(III) removal capacity in Cu(II)-chitosan (Figures 3 and 4), no EXAFS spectra were obtained for As(III) removal by Cu(II)-chitosan. However, the DFT models show that As(III) forms a hydrogen bond network with the hydroxyls of the chitosan backbone, but does not form inner-sphere complexes with the Cu(II) (Figure 6C), similar to As(V) removal by Cu(II)-chitosan (Figure 7C).
Generally, better fits were obtained for As(III) and As(V) adsorption to TMCs without phosphate present, which is expected since the DFT models used for the fitting did not include phosphate with the As(V) or As(III) ions within the same structure. However, the best fits of the spectra for both As(III)+P and As(V)+P were obtained with the same N as was used for fitting As(III) and As(V) (Table S1). The fits also had very little change in R with the addition of phosphate (Table S1). The relatively small amount of change in the fits of the EXAFS spectra between adsorption of As(III) and As(V) to Fe(III)- and Ni(II)-chitosan with and without phosphate suggests that the complexation mechanism did not change significantly with the addition of phosphate. This supports the finding that Fe(III)-chitosan and Ni(II)-chitosan are selective for both As(V) and As(III) over phosphate.
It is hypothesized that Fe(III)-chitosan and Ni(II)-chitosan show greater adsorptive affinity towards As(V) and As(III) than Cu(II)-chitosan due to their forming less-stable transition metal-chitosan complexes. The Irving Williams Series, which dictates the stability of metal-polymer complexes, predicts that Cu(II)-chitosan will form the most stable chitosan complex followed by Ni(II) and then Fe(III).31,32 This high stability could lead to lower reactivity, and thus, a lower tendency to adsorb arsenic.31,32 Therefore, the greater instability of the Fe(III)- and Ni(II)-chitosan complexes may increase their reactivity and adsorptive affinity towards arsenic, leading to their generally forming inner-sphere, rather than outer-sphere, complexes.
Overall, these results demonstrate the successful development of a selective adsorbent for As(III) and As(V) over phosphate. Variation of transition metal within the TMC strongly controls selective adsorption performance towards both As(V) and As(III). In order to achieve favorable selective adsorption performance, the TMC must form a bidentate-chitosan complex, must be effective at removing arsenic and ineffective at removing P, and must also adsorb As via inner-sphere complexation with the transition metal. Of the adsorbents tested, only Fe(III)-chitosan satisfied these criteria resulting in its superior selective arsenic removal capability compared to Ni(II)-chitosan, Cu(II)-chitosan, Zn(II)-chitosan, and Al(III)-chitosan. The ability to remove arsenic beneath regulatory limits in NSF 53 challenge water (Fig. S1) demonstrates the robust capabilities of this adsorbent materials to remove arsenic selectively. The results of this study present an opportunity to use the knowledge gained to develop adsorbents selective for arsenic over other significant competitors such as silicate and bicarbonate, as well as to other target oxyanion contaminants of interest including hexavalent chromium and selenium.16
Supplementary Material
Figure 8.
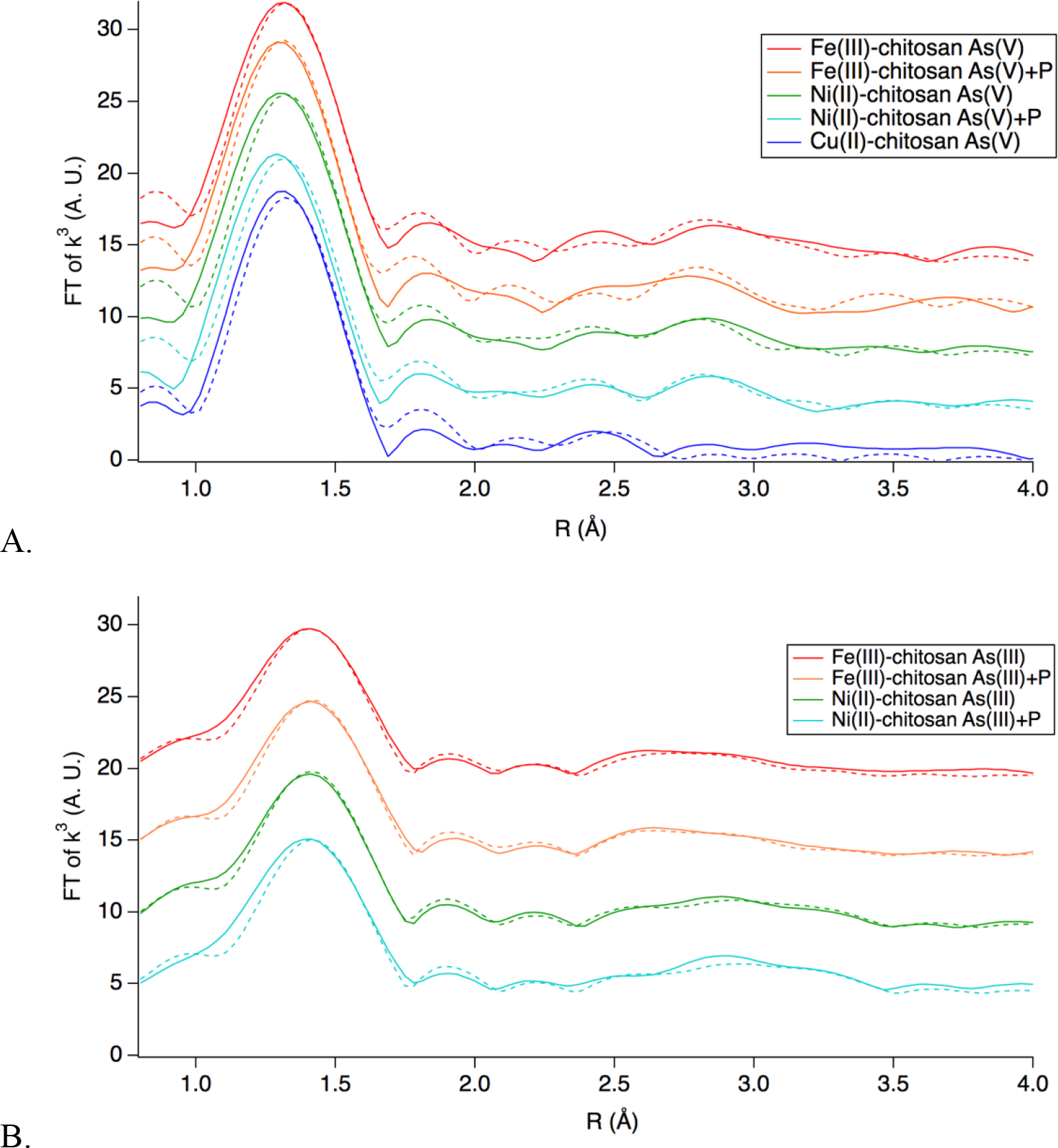
a. As-K edge EXAFS (Fourier transforms of the functions) of As(V) adsorption to Fe(III)-chitosan and Ni(II)-chitosan with and without phosphate present, As(V) adsorption without phosphate present to Cu(II)-chitosan. b. As K-edge EXAFS of As(III) adsorption to Fe(III)-chitosan and Ni(II)-chitosan with and without phosphate present. Solid line indicate experimental data and dashed lines the fit to DFT calculated theoretical standards. Initial concentrations were 56 ppm As and 0.01 M NaCl, 56 ppm P, when present.
Acknowledgements
This work was supported by the NSF Nanosystems Engineering Research Center for Nanotechnology-Enabled Water Treatment (ERC-1449500). We would like to thank Dr. Min Li and the West Campus Materials Characterization Material Core at Yale and Dr. Jonas Karosas and the Yale Analytical and Stable Isotope Center for their help and use of XRD and ICP-MS, respectively. We also thank Dr. George E. Sterbinsky at APS 9-BM for use of the beamlines and assistance with our project. Additionally, we thank Mary Kate Mitchell Lane and Holly Rudel, for their help with this project. This research used the Inner Shell Spectroscopy beamline (8-ID) of the National Synchrotron Light Source II, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under Contract No. DE-SC0012704. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
References
- (1).Lata S; Samadder SR Removal of Arsenic from Water Using Nano Adsorbents and Challenges: A Review. J. Environ. Manage. 2016, 166, 387–406. 10.1016/j.jenvman.2015.10.039. [DOI] [PubMed] [Google Scholar]
- (2).Mohan D; Pittman CU Jr. Arsenic Removal from Water/Wastewater Using Adsorbents—A Critical Review. J. Hazard. Mater. 2007, 142 (1–2), 1–53. 10.1016/j.jhazmat.2007.01.006. [DOI] [PubMed] [Google Scholar]
- (3).Smedley PL; Kinniburgh DG A Review of the Source, Behaviour and Distribution of Arsenic in Natural Waters. Appl. Geochem. 2002, 17 (5), 517–568. 10.1016/S0883-2927(02)00018-5. [DOI] [Google Scholar]
- (4).Hossain MF Arsenic Contamination in Bangladesh—An Overview. Agric. Ecosyst. Environ. 2006, 113 (1–4), 1–16. 10.1016/j.agee.2005.08.034. [DOI] [Google Scholar]
- (5).Ahmad SA; Khan MH; Haque M Arsenic Contamination in Groundwater in Bangladesh: Implications and Challenges for Healthcare Policy. Risk Manag. Healthc. Policy 2018, 11, 251–261. 10.2147/RMHP.S153188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Yamani JS; Miller SM; Spaulding ML; Zimmerman JB Enhanced Arsenic Removal Using Mixed Metal Oxide Impregnated Chitosan Beads. Water Res. 2012, 46 (14), 4427–4434. 10.1016/j.watres.2012.06.004. [DOI] [PubMed] [Google Scholar]
- (7).Goldberg S; Johnston CT Mechanisms of Arsenic Adsorption on Amorphous Oxides Evaluated Using Macroscopic Measurements, Vibrational Spectroscopy, and Surface Complexation Modeling. J. Colloid Interface Sci. 2001, 234 (1), 204–216. 10.1006/jcis.2000.7295. [DOI] [PubMed] [Google Scholar]
- (8).Vahter M Species Differences in the Metabolism of Arsenic Compounds. Appl. Organomet. Chem. 1994, 8 (3), 175–182. 10.1002/aoc.590080304. [DOI] [Google Scholar]
- (9).Pincus LN; Melnikov F; Yamani JS; Zimmerman JB Multifunctional Photoactive and Selective Adsorbent for Arsenite and Arsenate: Evaluation of Nano Titanium Dioxide-Enabled Chitosan Cross-Linked with Copper. J. Hazard. Mater. 2018, 358, 145–154. 10.1016/j.jhazmat.2018.06.033. [DOI] [PubMed] [Google Scholar]
- (10).Miller SM; Spaulding ML; Zimmerman JB Optimization of Capacity and Kinetics for a Novel Bio-Based Arsenic Sorbent, TiO2-Impregnated Chitosan Bead. Water Res. 2011, 45 (17), 5745–5754. 10.1016/j.watres.2011.08.040. [DOI] [PubMed] [Google Scholar]
- (11).Miller SM; Zimmerman JB Novel, Bio-Based, Photoactive Arsenic Sorbent: TiO2-Impregnated Chitosan Bead. Water Res. 2010, 44 (19), 5722–5729. 10.1016/j.watres.2010.05.045. [DOI] [PubMed] [Google Scholar]
- (12).Pincus LN; Lounsbury AW; Zimmerman JB Toward Realizing Multifunctionality: Photoactive and Selective Adsorbents for the Removal of Inorganics in Water Treatment. Acc. Chem. Res. 2019, 52 (5), 1206–1214. 10.1021/acs.accounts.8b00668. [DOI] [PubMed] [Google Scholar]
- (13).Ahmaruzzaman M; Gupta VK Rice Husk and Its Ash as Low-Cost Adsorbents in Water and Wastewater Treatment. Ind. Eng. Chem. Res. 2011, 50 (24), 13589–13613. 10.1021/ie201477c. [DOI] [Google Scholar]
- (14).Lata S; Singh P; Samadder S Regeneration of Adsorbents and Recovery of Heavy Metals: A Review. Int. J. Environ. Sci. Technol. IJEST 2015, 12 (4), 1461–1478. 10.1007/s13762-014-0714-9. [DOI] [Google Scholar]
- (15).Yamani JS; Lounsbury AW; Zimmerman JB Towards a Selective Adsorbent for Arsenate and Selenite in the Presence of Phosphate: Assessment of Adsorption Efficiency, Mechanism, and Binary Separation Factors of the Chitosan-Copper Complex. Water Res. 2016, 88, 889–896. 10.1016/j.watres.2015.11.017. [DOI] [PubMed] [Google Scholar]
- (16).Pincus L; Rudel H; Petrovic P; Gupta S; Westerhoff P; Muhich C; Zimmerman JB Exploring the Mechanisms of Selectivity for Environmentally Significant Oxo-Anion Removal During Water Treatment: A Review of Common Competing Oxo-Anions and Tools for Quantifying Selective Adsorption. Environ. Sci. Technol. 2020, acs.est.0c01666. 10.1021/acs.est.0c01666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Awual Md. R.; Jyo A; El-Safty SA; Tamada M; Seko N A Weak-Base Fibrous Anion Exchanger Effective for Rapid Phosphate Removal from Water. J. Hazard. Mater. 2011, 188 (1), 164–171. 10.1016/j.jhazmat.2011.01.092. [DOI] [PubMed] [Google Scholar]
- (18).Paikaray S; Hendry MJ; Essilfie-Dughan J Controls on Arsenate, Molybdate, and Selenate Uptake by Hydrotalcite-like Layered Double Hydroxides. Chem. Geol. 2013, 345, 130–138. 10.1016/j.chemgeo.2013.02.015. [DOI] [Google Scholar]
- (19).Youngran J; FAN M; Van Leeuwen J; Belczyk JF Effect of Competing Solutes on Arsenic(V) Adsorption Using Iron and Aluminum Oxides. J. Environ. Sci. 2007, 19 (8), 910–919. 10.1016/S1001-0742(07)60151-X. [DOI] [PubMed] [Google Scholar]
- (20).Jain A; Loeppert RH Effect of Competing Anions on the Adsorption of Arsenate and Arsenite by Ferrihydrite. J. Environ. Qual. Madison 2000, 29 (5), 1422. [Google Scholar]
- (21).Manning BA; Goldberg S Modeling Competitive Adsorption of Arsenate with Phosphate and Molybdate on Oxide Minerals. Soil Sci. Soc. Am. J. 1996, 60 (1), 121–131. 10.2136/sssaj1996.03615995006000010020x. [DOI] [Google Scholar]
- (22).An B; Steinwinder TR; Zhao D Selective Removal of Arsenate from Drinking Water Using a Polymeric Ligand Exchanger. Water Res. 2005, 39 (20), 4993–5004. 10.1016/j.watres.2005.10.014. [DOI] [PubMed] [Google Scholar]
- (23).Awual MR; Hossain MA; Shenashen MA; Yaita T; Suzuki S; Jyo A Evaluating of Arsenic(V) Removal from Water by Weak-Base Anion Exchange Adsorbents. Environ. Sci. Pollut. Res. Int. 2013, 20 (1), 421–430. 10.1007/s11356-012-0936-7. [DOI] [PubMed] [Google Scholar]
- (24).Awual Md. R. El-Safty SA; Jyo A Removal of Trace Arsenic(V) and Phosphate from Water by a Highly Selective Ligand Exchange Adsorbent. J. Environ. Sci. 2011, 23 (12), 1947–1954. 10.1016/S1001-0742(10)60645-6. [DOI] [PubMed] [Google Scholar]
- (25).Costa ETS; Guilherme LRG; Lopes G; Lima JM; Curi N Competitive Sorption of Arsenate and Phosphate on Aluminum Mining By-Product. Water. Air. Soil Pollut. 2012, 223 (8), 5433–5444. 10.1007/s11270-012-1291-5. [DOI] [Google Scholar]
- (26).Chowdhury SR; Yanful EK Arsenic and Chromium Removal by Mixed Magnetite–Maghemite Nanoparticles and the Effect of Phosphate on Removal. J. Environ. Manage. 2010, 91 (11), 2238–2247. 10.1016/j.jenvman.2010.06.003. [DOI] [PubMed] [Google Scholar]
- (27).An B; Fu Z; Xiong Z; Zhao D; SenGupta AK Synthesis and Characterization of a New Class of Polymeric Ligand Exchangers for Selective Removal of Arsenate from Drinking Water. React. Funct. Polym. 2010, 70 (8), 497–507. 10.1016/j.reactfunctpolym.2010.01.006. [DOI] [Google Scholar]
- (28).Hawthorne FC A Bond-Topological Approach to Theoretical Mineralogy: Crystal Structure, Chemical Composition and Chemical Reactions. Phys. Chem. Miner. 2012, 39 (10), 841–874. 10.1007/s00269-012-0538-4. [DOI] [Google Scholar]
- (29).Yazdani M. (Roza); Bhatnagar A; Vahala R Synthesis, Characterization and Exploitation of Nano-TiO2/Feldspar-Embedded Chitosan Beads towards UV-Assisted Adsorptive Abatement of Aqueous Arsenic (As). Chem. Eng. J. 2017, 316, 370–382. 10.1016/j.cej.2017.01.121. [DOI] [Google Scholar]
- (30).Manceau A; Charlet L The Mechanism of Selenate Adsorption on Goethite and Hydrous Ferric Oxide. J. Colloid Interface Sci. 1994, 168 (1), 87–93. 10.1006/jcis.1994.1396. [DOI] [Google Scholar]
- (31).Henry WD; Zhao D; SenGupta AK; Lange C Preparation and Characterization of a New Class of Polymeric Ligand Exchangers for Selective Removal of Trace Contaminants from Water. React. Funct. Polym. 2004, 60, 109–120. 10.1016/j.reactfunctpolym.2004.02.016. [DOI] [Google Scholar]
- (32).Martin RB A Stability Ruler for Metal Ion Complexes. J. Chem. Educ. 1987, 64 (5), 402. 10.1021/ed064p402. [DOI] [Google Scholar]
- (33).Demarchi CA; Debrassi A; Magro JD; Nedelko N; Ślawska-Waniewska A; Dłużewski P; Greneche J-M; Rodrigues CA Adsorption of Cr(VI) on Crosslinked Chitosan–Fe(III) Complex in Fixed-Bed Systems. J. Water Process Eng. 2015, 7, 141–152. 10.1016/j.jwpe.2015.05.003. [DOI] [Google Scholar]
- (34).dos Santos HH; Demarchi CA; Rodrigues CA; Greneche JM; Nedelko N; Ślawska-Waniewska A Adsorption of As(III) on Chitosan-Fe-Crosslinked Complex (Ch-Fe). Chemosphere 2011, 82 (2), 278–283. 10.1016/j.chemosphere.2010.09.033. [DOI] [PubMed] [Google Scholar]
- (35).Lv L; Xie Y; Liu G; Liu G; Yu J Removal of Perchlorate from Aqueous Solution by Cross-Linked Fe(III)-Chitosan Complex. J. Environ. Sci. 2014, 26 (4), 792–800. 10.1016/S1001-0742(13)60519-7. [DOI] [PubMed] [Google Scholar]
- (36).Shen C; Chen H; Wu S; Wen Y; Li L; Jiang Z; Li M; Liu W Highly Efficient Detoxification of Cr(VI) by Chitosan–Fe(III) Complex: Process and Mechanism Studies. J. Hazard. Mater. 2013, 244, 689–697. 10.1016/j.jhazmat.2012.10.061. [DOI] [PubMed] [Google Scholar]
- (37).Shinde RN; Pandey AK; Acharya R; Guin R; Das SK; Rajurkar NS; Pujari PK Chitosan-Transition Metal Ions Complexes for Selective Arsenic(V) Preconcentration. Water Res. 2013, 47 (10), 3497–3506. 10.1016/j.watres.2013.03.059. [DOI] [PubMed] [Google Scholar]
- (38).Zhang B; Chen N; Feng C; Zhang Z Adsorption for Phosphate by Crosslinked/Non-Crosslinked-Chitosan-Fe(III) Complex Sorbents: Characteristic and Mechanism. Chem. Eng. J. 2018, 353, 361–372. 10.1016/j.cej.2018.07.092. [DOI] [Google Scholar]
- (39).Sano LL; Krueger AM; Landrum PF Chronic Toxicity of Glutaraldehyde: Differential Sensitivity of Three Freshwater Organisms. Aquat. Toxicol. 2005, 71 (3), 283–296. 10.1016/j.aquatox.2004.12.001. [DOI] [PubMed] [Google Scholar]
- (40).Yamani JS; Lounsbury AW; Zimmerman JB Adsorption of Selenite and Selenate by Nanocrystalline Aluminum Oxide, Neat and Impregnated in Chitosan Beads. Water Res. 2014, 50, 373–381. 10.1016/j.watres.2013.10.054. [DOI] [PubMed] [Google Scholar]
- (41).Möller T; Sylvester P Effect of Silica and PH on Arsenic Uptake by Resin/Iron Oxide Hybrid Media. Water Res. 2008, 42 (6), 1760–1766. 10.1016/j.watres.2007.10.044. [DOI] [PubMed] [Google Scholar]
- (42).Frish MJ; Trucks GW; Schlegel HB; Scuseria GE; Robb MA; Cheeseman JR; Scalmani G; Barone V; Petersson GA; Nakatsuji H; Li X; Caricato M; Marenich AV; Bloino J; Janesko BG; Gomperts R; Mennucci B; Hratchian HP; Ortiz JV; Izmaylov AF; Sonnenberg JL; Williams-Young D; Ding F; Lipparini F; Egidi F; Goings J; Peng B; Petrone A; Henderson T; Ranasinghe D; Zakrzewski VG; Gao J; Rega N; Zheng G; Liang W; Hada M; Ehara M; Toyota K; Fukuda R; Hasegawa J; Ishida M; Nakajima T; Honda Y; Kitao O; Nakai H; Vreven T; Throssell K; Montgomery JA Jr.,; Peralta JE; Ogliaro F; Bearpark MJ; Heyd JJ; Brothers EN; Kudin KN; Staroverov VN; Keith TA; Kobayashi R; Normand J; Raghavachari K; Rendell AP; Burant JC; Iyengar SS; Tomasi J; Cossi M; Milliam JM; Klene M; Adamo C; Cammi R; Ochterski JW; Martin RL; Morokuma K; Farkas O; Foresman JB; Fox DJ Gaussian 16; Gaussian, Inc.: Wallingford CT, 2019. [Google Scholar]
- (43).Goerigk L; Mehta N A Trip to the Density Functional Theory Zoo: Warnings and Recommendations for the User*. Aust. J. Chem. 2019, 72 (8), 563–573. 10.1071/CH19023. [DOI] [Google Scholar]
- (44).Mardirossian N; Head-Gordon M Thirty Years of Density Functional Theory in Computational Chemistry: An Overview and Extensive Assessment of 200 Density Functionals. Mol. Phys. 2017, 115 (19), 2315–2372. 10.1080/00268976.2017.1333644. [DOI] [Google Scholar]
- (45).Chai J-D; Head-Gordon M Long-Range Corrected Hybrid Density Functionals with Damped Atom–Atom Dispersion Corrections. Phys. Chem. Chem. Phys. 2008, 10 (44), 6615–6620. 10.1039/B810189B. [DOI] [PubMed] [Google Scholar]
- (46).Weigend F; Ahlrichs R Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7 (18), 3297–3305. 10.1039/B508541A. [DOI] [PubMed] [Google Scholar]
- (47).Müller K; Ciminelli VST; Dantas MSS; Willscher S A Comparative Study of As(III) and As(V) in Aqueous Solutions and Adsorbed on Iron Oxy-Hydroxides by Raman Spectroscopy. Water Res. 2010, 44 (19), 5660–5672. 10.1016/j.watres.2010.05.053. [DOI] [PubMed] [Google Scholar]
- (48).Ravel B; Newville M ATHENA, ARTEMIS, HEPHAESTUS: Data Analysis for X-Ray Absorption Spectroscopy Using IFEFFIT. J. Synchrotron Radiat. 2005, 12 (4), 537–541. 10.1107/S0909049505012719. [DOI] [PubMed] [Google Scholar]
- (49).Newville M IFEFFIT : Interactive XAFS Analysis and FEFF Fitting. J. Synchrotron Radiat. 2001, 8 (2), 322–324. 10.1107/S0909049500016964. [DOI] [PubMed] [Google Scholar]
- (50).Pawlak A; Mucha M Thermogravimetric and FTIR Studies of Chitosan Blends. Thermochim. Acta 2003, 396 (1–2), 153–166. 10.1016/S0040-6031(02)00523-3. [DOI] [Google Scholar]
- (51).Li N; Bai R A Novel Amine-Shielded Surface Cross-Linking of Chitosan Hydrogel Beads for Enhanced Metal Adsorption Performance. Ind. Eng. Chem. Res. 2005, 44 (17), 6692–6700. 10.1021/ie050145k. [DOI] [Google Scholar]
- (52).Boggione MJ; Mahl CRA; Beppu MM; Farruggia B Synthesis and Characterization of Chitosan Membranes Functionalized with Amino Acids and Copper for Adsorption of Endoglucanase. Powder Technol. 2017, 315, 250–257. 10.1016/j.powtec.2017.04.014. [DOI] [Google Scholar]
- (53).Cheng Z; Liu X; Han M; Ma W Adsorption Kinetic Character of Copper Ions onto a Modified Chitosan Transparent Thin Membrane from Aqueous Solution. J. Hazard. Mater. 2010, 182 (1), 408–415. 10.1016/j.jhazmat.2010.06.048. [DOI] [PubMed] [Google Scholar]
- (54).Krishnapriya KR; Kandaswamy M A New Chitosan Biopolymer Derivative as Metal-Complexing Agent: Synthesis, Characterization, and Metal(II) Ion Adsorption Studies. Carbohydr. Res. 2010, 345 (14), 2013–2022. 10.1016/j.carres.2010.06.005. [DOI] [PubMed] [Google Scholar]
- (55).Horzum N; Demir MM; Nairat M; Shahwan T Chitosan Fiber-Supported Zero-Valent Iron Nanoparticles as a Novel Sorbent for Sequestration of Inorganic Arsenic. RSC Adv. 2013, 3 (21), 7828–7837. 10.1039/C3RA23454A. [DOI] [Google Scholar]
- (56).Boddu VM; Abburi K; Talbott JL; Smith ED; Haasch R Removal of Arsenic (III) and Arsenic (V) from Aqueous Medium Using Chitosan-Coated Biosorbent. Water Res. 2008, 42 (3), 633–642. 10.1016/j.watres.2007.08.014. [DOI] [PubMed] [Google Scholar]
- (57).Reddy TR; Thyagarajan K; Montero OA; Reddy SRL; Endo T X-Ray Diffraction, Electron Paramagnetic Resonance and Optical Absorption Study of Bauxite. J. Miner. Mater. Charact. Eng. 2014, 02 (02), 114–120. 10.4236/jmmce.2014.22015. [DOI] [Google Scholar]
- (58).Wang M; Zhou Y; Zhang Y; Hong Hahn S; Jung Kim E From Zn(OH) 2 to ZnO: A Study on the Mechanism of Phase Transformation. CrystEngComm 2011, 13 (20), 6024–6026. 10.1039/C1CE05502J. [DOI] [Google Scholar]
- (59).Li K; Li P; Cai J; Xiao S; Yang H; Li A Efficient Adsorption of Both Methyl Orange and Chromium from Their Aqueous Mixtures Using a Quaternary Ammonium Salt Modified Chitosan Magnetic Composite Adsorbent. Chemosphere 2016, 154, 310–318. 10.1016/j.chemosphere.2016.03.100. [DOI] [PubMed] [Google Scholar]
- (60).Muñoz-Páez A; Díaz-Moreno S; Sánchez Marcos E; Rehr JJ Importance of Multiple-Scattering Phenomena in XAS Structural Determinations of [Ni(CN)4]2-in Condensed Phases. Inorg. Chem. 2000, 39 (17), 3784–3790. 10.1021/ic000274n. [DOI] [PubMed] [Google Scholar]
- (61).Pena M; Meng X; Korfiatis GP; Jing C Adsorption Mechanism of Arsenic on Nanocrystalline Titanium Dioxide. Environ. Sci. Technol. 2006, 40 (4), 1257–1262. 10.1021/es052040e. [DOI] [PubMed] [Google Scholar]
- (62).Arai Y; Elzinga EJ; Sparks DL X-Ray Absorption Spectroscopic Investigation of Arsenite and Arsenate Adsorption at the Aluminum Oxide–Water Interface. J. Colloid Interface Sci. 2001, 235 (1), 80–88. 10.1006/jcis.2000.7249. [DOI] [PubMed] [Google Scholar]
- (63).Duarte G; Ciminelli VST; Dantas MSS; Duarte HA; Vasconcelos IF; Oliveira AF; Osseo-Asare K As(III) Immobilization on Gibbsite: Investigation of the Complexation Mechanism by Combining EXAFS Analyses and DFT Calculations. Geochim. Cosmochim. Acta 2012, 83, 205–216. 10.1016/j.gca.2011.12.019. [DOI] [Google Scholar]
- (64).Liu S; Jing C; Meng X Arsenic Re-Mobilization in Water Treatment Adsorbents under Reducing Conditions: Part II. XAS and Modeling Study. Sci. Total Environ. 2008, 392 (1), 137–144. 10.1016/j.scitotenv.2007.10.033. [DOI] [PubMed] [Google Scholar]
- (65).Ona-Nguema G; Morin G; Juillot F; Calas G; Brown GE EXAFS Analysis of Arsenite Adsorption onto Two-Line Ferrihydrite, Hematite, Goethite, and Lepidocrocite. Environ. Sci. Technol. 2005, 39 (23), 9147–9155. 10.1021/es050889p. [DOI] [PubMed] [Google Scholar]
- (66).He J; Bardelli F; Gehin A; Silvester E; Charlet L Novel Chitosan Goethite Bionanocomposite Beads for Arsenic Remediation. Water Res. 2016, 101, 1–9. 10.1016/j.watres.2016.05.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


