Abstract
Purpose:
The aim of this study was to explore types of Descemet membrane detachment (DMD) after ocular surface burns by anterior segment optical coherence tomography.
Methods:
This is a pilot, case series, observational study. Patients with DMD after ocular surface burns were enrolled. Ophthalmologic examinations were performed in all patients including slit-lamp photography and anterior segment optical coherence tomography.
Results:
Three types of DMDs in 9 eyes of 9 patients with ocular surface burns were identified depending on the detachment components involved with the pre-Descemet layer (PDL). Type A was referred as a taut chord that the PDL and Descemet membrane (DM) detached simultaneously but were remained attached to each other, while type B was identified as a wavy line separated from the stroma by a dark slit that demonstrated the detachment of DM from the PDL and stroma. Type C was defined as the DM detached with or without PDL but they were separated from each other. We found that DM and PDL were detached simultaneously in most condition, with type A in 4 cases, type C in 5 cases, and type B in only 1 case.
Conclusions:
Our study demonstrated 3 types of DMDs after ocular surface burns and revealed that the limbal involvement and retrocorneal exudations may give clues to DMD in the corresponding areas. DMDs may be neglected for long in patients with extensive limbal involvement in early stages and also play an important role in unstable ocular surface condition until the late stages of conjunctivalization after ocular surface burns.
Key Words: anterior segment optical coherence tomography, ocular surface burns, Descemet membrane detachment
Descemet membrane detachment (DMD) is defined as separation of Descemet membrane (DM) from the corneal stroma that occurs after cataract surgery most commonly. It is a potentially vision-threatening complication and patients generally present with corneal edema and diminution of visions. Major risk factors include advanced age, preexisting endothelial diseases, and intraoperative factors. Untreated or unsettled DMDs may lead to corneal decompensation and opacification.1
In addition to iatrogenic etiology, ocular trauma such as ocular chemical burns can also lead to DMD.2–4 Ocular surface burns are emergencies that can suddenly lead to severe corneal edema resulting in persistent corneal opacity.5,6 In patients with ocular surface burns, attention of ophthalmologists is often drawn to the emergency treatment and severe injury of ocular surface. Furthermore, DMD has been neglected because it cannot be fully observed through the cloudy cornea by routine ophthalmic examination. DMD is a rare complication, and it is very difficult to be detected during routine examinations and to be treated timely, especially in the cases of refractive media opacity. Undiagnosed DMDs may be neglected in patients with extensive ocular surface burns and may play an important role in persistent unstable ocular surface and late conjunctivalization and scarring in such patients. Few reports have demonstrated DMD after ocular surface burns, including hydrogen peroxide, sodium cyanide, and caustic soda injury.2–4 Recently, Dua et al7 proposed a new classification and gave a new description of DMD by optical coherence tomography (OCT), which takes the pre-Descemet layer (PDL) into account. The new findings by OCT images had changed the conventional insights into DMD.7
In this article, we assessed eyes with DMD after ocular surface burns by anterior segment OCT (AS-OCT) to confirm the existence of PDL involvement and aimed to explore the effect of limbal involvement, retrocorneal exudations, and iris involvement on DMD.
METHODS
In this retrospective study, 9 eyes of 9 patients with ocular surface burns were enrolled from January 2018 to February 2022 in Peking University Third Hospital and were confirmed with DMD by AS-OCT. Following the principles of Declaration of Helsinki, written informed consent was obtained from all participants after explaining the nature and consequences of ocular surface chemical burns with approval from our institutional review committee.
The injury severity was graded according to the Dua classification. Ocular surface burns were classified into 6 grades according to the Dua classification. Grade III was defined as 3 to 6 clock hours of limbal involvement and 30% to 50% conjunctival involvement while grade VI was identified as total limbus (12 clock hours) involved and total conjunctiva (100%) involved.8
Slit-lamp biomicroscopy was used to note the extent of conjunctival involvement (percentage) and limbal involvement in clock hours. All cases were examined to assess the range.
Limbal and conjunctival involvement in the acute stage was significant for the prognosis of ocular surface burns. To match the injury severity of Dua grading system, the ranges of DMD (degrees), ranges of anterior synechiae of iris (degrees), and features of DMD were evaluated by high-resolution AS-OCT scans in vertical and horizontal directions, with 5 eyes by the AS-OCT CASIA2 (wavelength nanometer, Tomey CASIA2) and 4 eyes by the RTVue (wavelength 840 ± 10 nm, Optovue, Inc, Fremont, CA). The DMDs are divided into 3 types: type A: a thick hyperreflective strand in the anterior chamber, which is straight and like the chord of a circle, showing detachment of DM and PDL from the posterior stroma. Type B: an undulating hyperreflective parallel line, representing DM detachment from the PDL. Type C: 2 separated lines, a mixture of the above 2 lines. DM and PDL detach from the posterior stroma and separate from each other.7 All data were evaluated by the same researcher.
RESULTS
Demographic Characteristics
The mean age of 9 patients was 48.6 ± 11.7 years (range 32–70 years), with no women included. Nine patients (9 eyes) suffered with severe ocular surface burns, in which 7 cases were alkali burns, 1 case was acid burn, and 1 case was thermal burn. According to the Dua classification, 7 eyes (77.8%) had grade VI ocular surface burns and 2 eyes (22.2%) had grade III burns. The mean interval between the initial examination and the injury was 57 ± 63.6 (1–196) days, with a median time of 39.5 (10.75, 84.75) days. Patients with DMD had an average range of 302.2 ± 94.18 degrees in limbal involvement and a mean range of 142.8 ± 99.4 degrees in DMD. Anterior synechiae of iris were found in 7 eyes with an average range of 165.7 ± 140.2 degrees. In addition, 7 eyes were found to be complicated with exudation in the anterior chamber. Anterior chamber inflammation with hypopyon or fibrin clot was detected in several patients through the mildly cloudy cornea. The exudation shows a clump-like reflection with low to moderate intensity illustrated by OCT in patients with media opacity (Table 1).
TABLE 1.
Demographic Characteristics and Types of DMD
| Patients No. | Age | Sex | Etiology | Dua Grading | Limbal Involvement (Degrees) | Type of DMD | Interval Between Injury and DMD (d) | Range of DMD (Degrees) | Range of Anterior Synechiae of Iris (Degrees) | Exudation |
| 1 | 48 | Male | Alkali burn | IV | 360 | A | 1 | 360 | 0 | Yes |
| 2 | 50 | Male | Alkali burn | IV | 270 | A | 44 | 135 | 45 | No |
| 3 | 50 | Male | Alkali burn | IV | 360 | B | 4 | 225 | 0 | No |
| 4 | 46 | Male | Alkali burn | III | 130 | C | 48 | 45 | 45 | No |
| 5 | 60 | Male | Alkali burn | IV | 360 | A | 196 | 135 | 180 | Yes |
| 6 | 32 | Male | Alkali burn | III | 160 | A and C | 31 | 160 | 80 | Yes |
| 7 | 34 | Male | Alkali burn | IV | 360 | C | 35 | 90 | 90 | Yes |
| 8 | 47 | Male | Acid burn | IV | 360 | C | 97 | 45 | 360 | Yes |
| 9 | 70 | Male | Thermal burn | IV | 360 | C | Not available | 90 | 360 | Yes |
Discovery interval (days): time from injury to the discovery of DMD.
Two DMD types were discovered in 1 eye of patient 6, and they were divided into 2 parts to analyze. Patient 5 suffered with alkali burn and underwent cataract phacoemulsification surgery and was included in the series given the following considerations: 1) DMD occurred in the inferotemporal quadrant, which was not in the corresponding site of incisions made in cataract surgery at 2-o'clock and 11-o'clock positions and 2) DMD was observed 7 months after cataract surgery, but no detachment was found in the perioperative period.
Correlation Between Limbal Involvement, Anterior Synechiae of Iris, and DMD
Limbal involvement was evaluated in 9 eyes, among which 3 eyes had type 1 DMD, 1 eye had type 2 DMD, and 3 eyes had type 3 DMD. The range of limbal involvement after ocular surface burns was larger than that of DMD. DMDs were detected in 51.9% areas of limbal involvement in affected eyes. However, 94.4% areas of identified DMD showed limbal damage. Basically, the appearance of DMD is almost always accompanied with limbal involvement, while not all areas of limbal injury were accompanied with DMD. Especially, DMD areas were totally consistent with limbal involvement areas in 3 patients. Anterior synechiae of iris were discovered in 7 of 9 eyes where DMD occurred in 73.2% of areas. The focal areas in 3 patients were completely overlapped (Table 2).
TABLE 2.
Correlation of DMD, Limbal Involvement, and Anterior Synechiae of Iris
| Patients No. | Type of DMD | Limbal Involvement (Degrees) | Range of DMD (Degrees) | Overlapping Range 1 (Degrees) | Overlapping Range 1/Limbal Involvement Range (%) | Overlapping Range 1/DMD Range (%) | Range of Anterior Synechiae of Iris (Degrees) | Overlapping Range 2 (Degrees) | Overlapping Range 2/Range of Anterior Synechiae of Iris (%) |
| 1 | A | 360 | 360 | 360 | 100 | 100 | 0 | — | — |
| 2 | A | 270 | 135 | 60 | 22.2 | 44.4 | 45 | 45 | 100 |
| 3 | B | 360 | 225 | 225 | 62.5 | 100 | 0 | — | 0 |
| 4 | C | 130 | 45 | 45 | 34.6 | 100 | 45 | 45 | 100 |
| 5 | A | 360 | 135 | 135 | 37.5 | 100 | 180 | 135 | 75 |
| 6 | A | 80 | 80 | 80 | 100 | 100 | 0 | — | — |
| 6 | C | 80 | 80 | 80 | 100 | 100 | 80 | 80 | 100 |
| 7 | C | 360 | 90 | 90 | 25 | 100 | 90 | 90 | 100 |
| 8 | C | 360 | 45 | — | — | — | 360 | 45 | 12.5 |
| 9 | C | 360 | 90 | 90 | 25 | 100 | 360 | 90 | 25 |
Overlapping range 1 = overlapping range between limbal involvement and DMD.
Overlapping range 2 = overlapping range between anterior synechiae of iris and DMD.
Types of DMD Evaluated by AS-OCT
In the 9 eyes with ocular surface burns, the presence of DMD was clearly verified by AS-OCT. The DMD was categorized based on the involvement of the PDL and DM. Three types of DMD were discovered, which were as follows: 1) type A DMD referred as detachment of both DM and PDL from the posterior stroma, identified as a thick and straight strand like the chord of a circle in the anterior chamber by AS-OCT (Fig. 1A, B, C); 2) type B DMD was termed as DM detachment from the PDL and stroma that appeared as a wavy line separated from the posterior cornea by a dark slit by AS-OCT (Fig. 1D, E, F); and 3) type C DMD presented with 2 separated retrocorneal lines by AS-OCT (Fig. 1G, H, I), illustrating the detachment of DM and PDL and their separation from each other.
FIGURE 1.
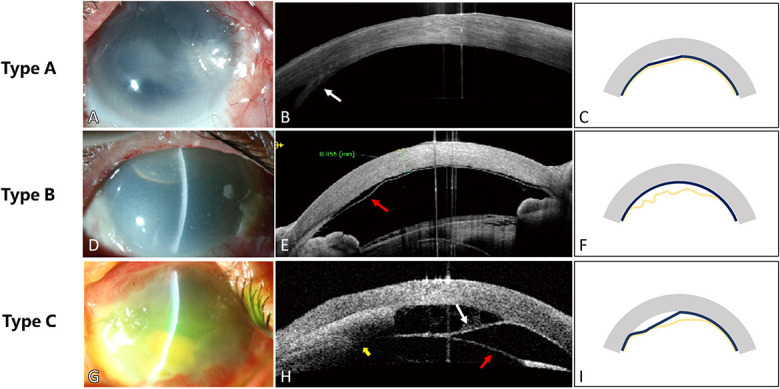
Three types of DMD after ocular surface burns. A, D, G, Limbal ischemia, corneal edema, and corneal clarity were common manifestations after acute ocular surface burns, obscuring the view of the details of iris and changes in the anterior chamber. AS-OCT (B, E, H) showed the eyes of different types of DMD. Schematic diagrams (C, F, I) illustrated the shape and involved layer in the 3 types. The gray stripe referred to the cornea, the dark blue line showed the pre-Descemet layer (PDL), and the yellow line showed DM. A thick hyperreflective strand was seen in the anterior chamber (white arrow in B), which was straight and chord-like, presenting detachment of both DM and PDL from the posterior stroma. An undulating hyperreflective parallel line (red arrow in E) representing DM detachment from the PDL and stroma could be seen in the anterior chamber, part of which was reattached to the PDL. Two separated lines showed DM detachment (white arrow in H) and PDL detachment (red arrow in H), respectively, with interlayer exudation behind the stroma (yellow arrow in H).
Among the 9 eyes, DMD of type A, B, and C were discovered in 3 eyes, 1 eye, and 4 eyes, respectively. In the eye of patient 6, both type A and type C DMDs were confirmed by OCT.
DMD in Patients After Gas Tamponade in the Anterior Chamber
Patient 4 suffered an accident by alkaline substance in battery. Patient 4 had grade III burn (based on the Dua classification) initially with corneal hydrops and epithelial defects and subsequently received amnion transplantation. Type C DMD ranging from 270 to 315 degrees was discovered 48 days after injury by AS-OCT, which overlapped with the epithelial defect area. Medical therapy including steroids, antibiotics, and epithelial repair drops continued to be used. However, the patient received gas tamponade in the anterior chamber and amniotic membrane transplantation immediately after wider DMD range was found 1 month later by AS-OCT. Corneal edema subsided quickly, corneal epithelial defects resolved, and visual acuity was improved to 0.15 gradually in subsequent follow-up along with the reattachment of DM and PDL (Fig. 2).
FIGURE 2.
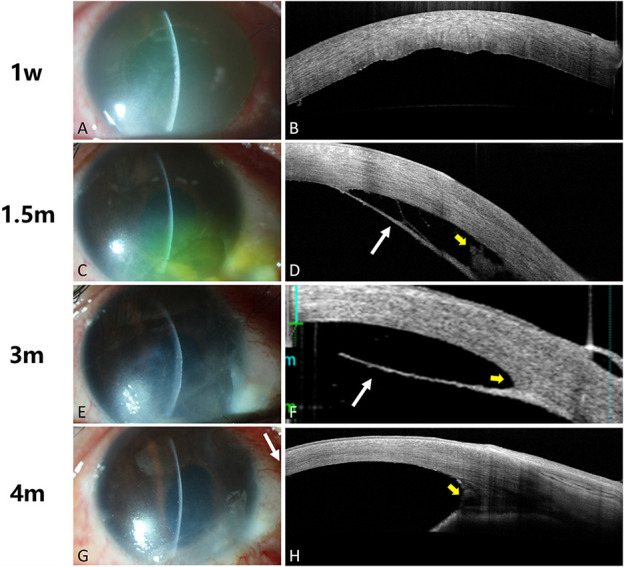
Reattachment of DM in patient 4 after gas tamponade in the anterior chamber. Slit-lamp illustration demonstrated that corneal edema in the inferonasal quadrant persisted (A, C, E) and clarified after gas tamponade in the anterior chamber (G). AS-OCT images showed DM folds at 1 week after burn (B), a taut straight hyperreflective strand at one and a half month after burn (marked with white arrow in D), and the strand range enlarged in 3 months (marked with white arrow in F). After gas tamponade in the anterior chamber, the strand was reattached to the posterior cornea (H). Note the interlayer exudations and exudations in the anterior chamber angle (marked with yellow arrow in D, F, H).
Patient 3 had suffered grade VI alkali burn (based on the Dua classification), and type B DMD was observed at the injury day and was back in situ after gas tamponade the next day. Medical therapy including steroids, antibiotics, and epithelial repair drops continued to be used. However, recurrent DMD was detected again in 10 days and 18 days after injury, respectively, and gas tamponade in the anterior chamber was instituted each time promptly. However, in patient 3, the corneal epithelial defects continued and conjunctivalization progressed quickly after initial severe injury (Fig. 3).
FIGURE 3.
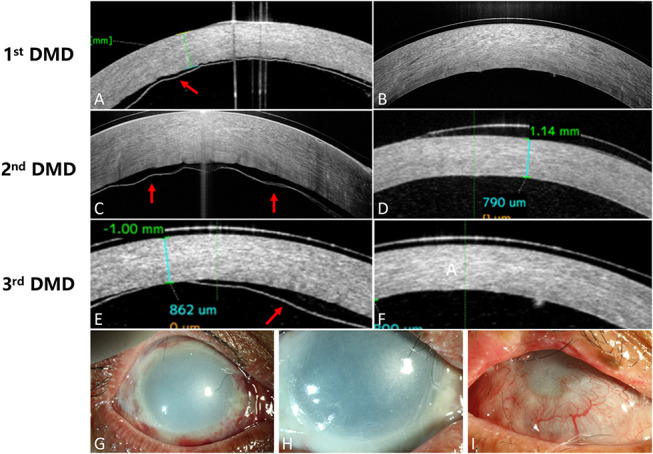
Recurrent DMD in patient 3 after gas tamponade in the anterior chamber. A–F, Recurrent DMD was discovered by AS-OCT in patient 3 after 3 times of prompt gas tamponade in the anterior chamber. Either primary DMD or recurrent DMD shared similar performance, representing wavy lines separated from the cornea by a dark slit (marked with red arrow in D, F, H). Slit-lamp illustration demonstrated that the gray cornea with severe edema (G) and alleviated edema (H), denoting the presence of DMD and resumption of DM after gas tamponade in the anterior chamber. I, Patient 3 presented extensive ocular surface conjunctivalization 5 months after burn.
Corneal epithelial defects persisted in patient 4 with relatively mild burns before the gas tamponade in the anterior chamber and did not improve until the reattachment of DM. The epithelial defects and subsequent resolution seemed to be affected by the attachment of DM. Because of initial severe damage to the limbus, conjunctivalization progressed in patient 3 rapidly. In addition to destruction of limbal cells, the long-time failure of reattachment of DM seemed to be responsible for the poor outcome of ocular surface to some extent in patient 3.
DMD in the Patient with Extensive Ocular Surface Conjunctivalization
Patient 1 suffered caustic soda burns with Dua grading VI. DMD and corneal epithelial exfoliation were observed soon on the injury day, and amniotic membrane transplantation was performed in the second day, combined with gas tamponade in the anterior chamber (Fig. 4). Although DM was reattached timely after surgery, patient had been presented with persistent epithelial defects, exudation in the anterior chamber, and recurrent DMD in the consecutive follow-ups. Repeated amniotic membrane transplantation and temporary tarsorrhaphy were performed to promote epithelial healing.
FIGURE 4.

DMD in patient 1 with extensive ocular surface conjunctivalization after alkali burn. A, Slit-lamp illustration demonstrated an unstable ocular surface with partial corneal epithelial defects, extensive ocular surface conjunctivalization, and severe neovascularization obscured the central cornea. B–D, AS-OCT images showed the exudations (marked with yellow arrow in B) in the retrocorneal space of DMD and subtle DMD of type A (marked with white arrow in C and D) given the shadow effect by the hyperreflective conjunctival epithelium.
DISCUSSION
The pre-Descemet layer (PDL) has been clearly demonstrated as an acellular and strong layer existing between deep corneal stroma and DM by the Anwar big bubble technique in vivo and ex vivo.9–11 Dua et al11 then described a new classification of DMD by AS-OCT based on the PDL. It was found that detachment of DM occurred not only in the interface of stroma but also in the PDL layer. Three types of DMDs were depicted in 23 patients diagnosed with DMD after cataract surgery, failed corneal transplants, acute hydrops, and descemetocele.
In this study, DMDs in patients with ocular surface burns were categorized into 3 types based on the new classification by Dua. Type A referred as a taut chord that the PDL and DM detached together but remained attached to each other, while type B was identified as a wavy line separated from the stroma by a dark slit that demonstrated the only detachment of DM from the PDL and the stroma. Type C was defined as DM detached with or without PDL but they were separated from each other. The result demonstrated that DM and PDL detached simultaneously in most condition, with type A in 4 cases, type C in 5 cases, and type B in only 1 case.
Previous studies have proposed several possible underlying mechanisms of DMD. Zhang et al2 reported 2 cases and believed the mechanism of DMD was that the presence of an inflammatory component caused adhesions and traction of iris to DM. Najjar reported 2 cases of DMD associated with hyphema after corneal alkali burn and presumed that it was the retrocorneal membrane that pulled DM down and ruptured new blood vessels separated potential space between DM and stroma.4 In our study, retrocorneal interlayer exudation and anterior synechiae of iris were found in 6 and 7 eyes, respectively, and 3 cases showed overlapping in the corresponding areas between DMD and exudation, which is consistent with the previous study related to the adhesion mechanism.
Our results also depicted that where limbal ischemia occurs, DMD may also occur simultaneously because 94.4% DMD areas are located in the limbal involvement area. In addition, severe limbal involvement was frequently accompanied by severe inflammatory reactions in the anterior chamber. The intralayer exudations were discovered in 2 eyes with Dua grading IV and type C DMD, which suggested the unsourced intralayer exudations may play an important role in the onset of DMD.
We also found that the opaque cornea caused by edema, scarring, and conjunctivalization hindered the early detection and diagnosis of DMD. Patient 1 presented with whole 360-degree limbal involvement in the acute stage and extensive conjunctivalization and severe neovascularization in the late stage of chemical burn. Persistent epithelial defects cannot be fully recovered despite repeated ocular surface surgeries and growth factor medication for a stable ocular surface. However, the AS-OCT detected the clear DMD underneath the fibrovascularization of ocular surface which completely obscured the changes in the retrocorneal space. In addition to the limbal stem cell defects, long-term dislocation and inadequacy of the corneal endothelium may negatively affect corneal epithelial recovery. The long ignored DMD through the opaque cornea may cause long-term poor prognosis.
Type B DMD was found in patient 3. The image showed an undulating hyperreflective parallel line in anterior chamber. Although the influence of gas tamponade in the anterior chamber in its configuration should be taken into consideration, gas tamponade in the anterior chamber cannot change the length of the “hyperreflective line.”
Three cases underwent gas tamponade in the anterior chamber. Patient 4 obtained acceptable prognosis, while other 2 eyes progressed to extensive conjunctivalization. For patient 3, a higher grade of burn was diagnosed, resulting in type B DMD in a large area next to the burns, and DM was hard to be reattached after gas tamponade in the anterior chamber. It could be inferred that DM along with PDL might both be involved in extensive injury so that DM was detached from PDL easily in early stage and hard to be reattached. In addition, persisted epithelial defects were observed in patients 1 and 3, while epithelial reconstruction occurred after reattachment of DMD in patient 4, which reminds us that unrepaired DMD might be associated with a poor epithelium. Meanwhile, patients with persisted epithelial defects should be checked carefully for DMD.
CONCLUSIONS
Our study showed that 3 types of DMD were detected by AS-OCT in patients with ocular surface burns with PDL involvement. The presence of limbal ischemia, iris synechiae, and exudations in the anterior chamber seem to play a great role in DMD after ocular surface burns that cannot be overlooked. Therefore, the use of AS-OCT could be a promising approach in patients with ocular surface burns for early detection and intervention to prevent and reduce the risk of long-term detachment of DM and improve the visual prognosis.
Footnotes
Supported by a grant from the National Natural Science Foundation of China (No. 81700799, 82070926).
The authors have no funding or conflicts of interest to disclose.
Y.-T. Li and W.-Y. Wu contributed equally to this study.
Contributor Information
Yuan-Ting Li, Email: Liyuanting_bjmu@126.com.
Wen-Yu Wu, Email: yywwy0704@163.com.
Jing-Yi Li, Email: li0921jingyi@163.com.
Szy-Yann Chan, Email: szyyann@163.com.
Marcus Ang, Email: drmarcusang@gmail.com.
REFERENCES
- 1.Singhal D, Sahay P, Goel S, et al. Descemet membrane detachment. Surv Ophthalmol. 2020;65:279–293. [DOI] [PubMed] [Google Scholar]
- 2.Zhang X, Jhanji V, Chen H. Tractional Descemet's membrane detachment after ocular alkali burns: case reports and review of literature. BMC Ophthalmol. 2018;18:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yuen HKL, Yeung BYM, Wong TH, et al. Descemet membrane detachment caused by hydrogen peroxide injury. Cornea. 2004;23:409–411. [DOI] [PubMed] [Google Scholar]
- 4.Najjar DM, Rapuano CJ, Cohen EJ. Descemet membrane detachment with hemorrhage after alkali burn to the cornea. Am J Ophthalmol. 2004;137:185–187. [DOI] [PubMed] [Google Scholar]
- 5.Ahmmed AA, Ting DSJ, Figueiredo FC. Epidemiology, economic and humanistic burdens of Ocular Surface Chemical Injury: a narrative review. Ocul Surf. 2021;20:199–211. [DOI] [PubMed] [Google Scholar]
- 6.Sha Z, Han-Ping X, Hong-Yan X. Clinical analysis of 135 patients with severe eye burn. Zhonghua Shao Shang Za Zhi. 2006;22:50–52. [PubMed] [Google Scholar]
- 7.Dua HS, Sinha R, D'souza S, et al. “Descemet membrane detachment”: a novel concept in diagnosis and classification. Am J Ophthalmol. 2020;218:84–98. [DOI] [PubMed] [Google Scholar]
- 8.Dua HS, King AJ, Joseph A. A new classification of ocular surface burns. Br J Ophthalmol. 2001;85:1379–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anwar M, Teichmann KD. Big-bubble technique to bare Descemet's membrane in anterior lamellar keratoplasty. J Cataract Refract Surg. 2002;28:398–403. [DOI] [PubMed] [Google Scholar]
- 10.Anwar M, Teichmann KD. Deep lamellar keratoplasty: surgical techniques for anterior lamellar keratoplasty with and without baring of descemet's membrane. Cornea. 2002;21:374–383. [DOI] [PubMed] [Google Scholar]
- 11.Dua HS, Faraj LA, Said DG, et al. Human corneal anatomy redefined: a novel pre-Descemet's layer (Dua's layer). Ophthalmology. 2013;120:1778–1785. [DOI] [PubMed] [Google Scholar]


