Abstract
Ferroptosis is a unique cell death modality triggered by iron-dependent lipid peroxidation, with cysteine metabolism and glutathione-dependent antioxidant defence responses as the primary triggering mechanisms. Ferroptosis is an independent tumour suppression mechanism and has been implicated in various disorders. In tumourigenesis, ferroptosis plays a dual role in promoting and inhibiting tumours. P53, NFE2L2, BAP1, HIF, and other tumour suppressor genes regulate ferroptosis, releasing damage-associated molecular patterns or lipid metabolites to influence cellular immune responses. Ferroptosis is also involved in tumour suppression and metabolism. The combination of amino acid, lipid, and iron metabolism is involved in the initiation and execution of ferroptosis, and metabolic regulatory mechanisms also play roles in malignancies. Most investigations into ferroptosis in gastric cancer are concentrated on predictive models, not the underlying processes. This review investigates the underlying mechanisms of ferroptosis, tumour suppressor genes, and the tumour microenvironment.
Keywords: ferroptosis, gastric cancer, metabolic regulation, tumour microenvironment, tumour suppressor genes
Gastric cancer is a heterogeneous disease characterized by numerous genetic mutations and epigenetic alterations that disrupt the balance between oncogenic and tumour suppressor pathways. This imbalance causes gastric cancer cell proliferation and cancer cell death. Ferroptosis is a controlled form of cell death that differs morphologically, biochemically, and genetically from apoptosis, necrosis, and necroptosis. It is characterized by iron-dependent reactive oxygen species (ROS) generation, lipid peroxidation, and iron accumulation (Chen et al., 2021a). Ferroptosis-related genes can be classified into three categories: drivers that promote ferroptosis, suppressors that inhibit ferroptosis, and markers that regulate ferroptosis. These genes regulate ferroptosis and other cellular processes (Zhou and Bao, 2020). Ferroptosis may play a role in tumour formation and the resistance of certain cancers, including gastric cancer, to medications, and therefore targeting ferroptosis may be an effective therapeutic strategy for gastric cancer (Alvarez et al., 2017; Zhang et al., 2020a). Hence, this review focuses on the correlation between ferroptosis and gastric cancer from several perspectives, including ferroptosis mechanisms, tumour suppressor genes, the tumour microenvironment (TME), and metabolic regulation.
Ferroptosis is a novel form of cell death
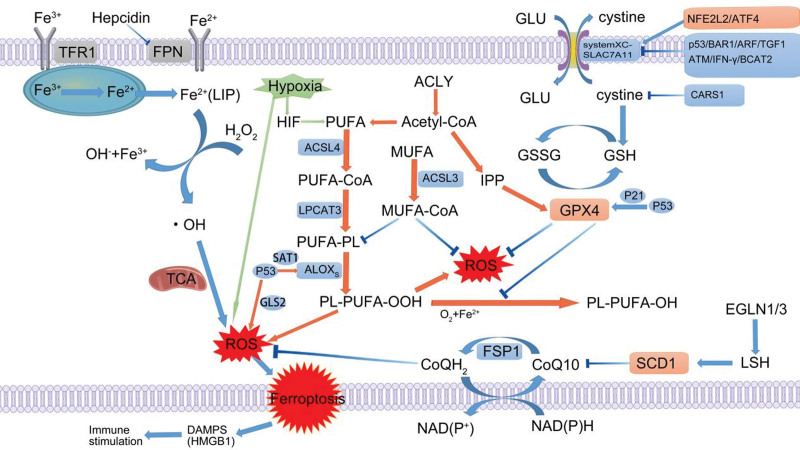
Ferroptosis mechanisms that have been discovered thus far are primarily based on cysteine metabolism and glutathione-lysine antioxidant defence (Table 1 and Fig. 1). Cysteine, a critical amino acid for cell viability, generally enters cells in dimerized and oxidized forms. The glutamate/cystine antiporter (System XC-), a cystine-glutamate antiporter encoded by the SLC7A11 gene, which encodes the System XC-substrate-specific subunit that mediates the uptake of extracellular cystine in exchange for intracellular glutamate release (Lin et al., 2020). SLCA711 expression is closely controlled by oncoproteins [such as nuclear factor erythroid 2-related factor 2 (NRF2)] and tumour suppressors (including p53, BAP1, and ARF). Rastin was first described as a System XC inhibitor that efficiently inhibits cellular cystine absorption and depletes glutathione, which is a typical ferroptosis inducer (Yagoda et al., 2007). Glutathione is also an essential cellular antioxidant that maintains cellular redox equilibrium and protects cells from oxidative damage (Ursini and Maiorino, 2020). Inactivation of the glutathione-dependent phospholipid peroxidase GPX4 leads to an imbalance in the antioxidant defence system, leaving cells vulnerable to lipid peroxidation and ultimately leading to ferroptosis (Forcina and Dixon, 2019). In addition, the cysteine-tRNA synthetase CARS has been identified as essential for ferroptosis (Hayano et al., 2016). CARS inhibition led to increased transcriptional upregulation of serine metabolism and transsulfuration pathway components, making methionine available for cysteine production. This process maintained the stability of cysteine and glutathione levels. ACSL4 is also a crucial component in ferroptosis, contributing to the oxidation of membrane phospholipids, which may influence ferroptosis activation (Feng et al., 2021). FSP1 is a ferroptosis inhibitor that exerts effects independent of the canonical GPX4 signalling pathway. A study found that the myristoylation of FSP1 was essential to the ability of FSP1 to inhibit ferroptosis. FSP1 prevents ferroptosis by lowering CoQ10 levels and preventing lipid oxidation (Bersuker et al., 2019; Doll et al., 2019). Hence, ferroptosis is related to various pathophysiological processes and diseases, including cancer, type II diabetes, cardiovascular disease, and neurodegeneration (Stockwell et al., 2017; Sha et al., 2021). Furthermore, because tumour cells with specific oncogenic mutations are highly susceptible to ferroptosis, inducing ferroptosis in these tumour cells might result in a robust therapeutic response.
Table 1.
The action pathway of ferroptosis core gene
| Suppressor | Driver | |
|---|---|---|
| Iron absorption and efflux | Tfr1, Tfr2, DMT1 | |
| Iron storage | Ferritin | |
| Ferriregulatory protein | FBXL5 | IRP1, IRP2 |
| Heat shock protein | HSF-1, HSBP1, HSPA5 | |
| Iron autophagy regulatory factor | NCOA4 | |
| Redox-regulated | NRF2, GPX4, FSP1, HO-1 NQO1, CISD1 |
ALOXs, PEBP1, NOXs DPP4/CD26, VDAC2/3 |
| Glutathione steady-state regulation | SLC7A11, MUC1, GCLC | SLC1A5, GLS2, CARS, CHAC1 |
| Lipid metabolism regulation | LSH/HELLS, SCD, FADS2 | ACSL4, ACSL3, LPCAT3, CS |
| Regulation of glucose metabolism | PHGDH, G6PD, ME1 | PHKG2 |
| The mevalerate pathway | HMGCR | SQLE, SQS |
| Transcription factor | STAT3, ATF4, HIF1A | ATF3, BACH1, P53, ATF4, HIF1A, ZEB1, NRF2, YAP/TAZ |
DPP4, dipeptidyl peptidase 4, LSH, lymphoid-specific helicase.
Fig. 1.
Diagram of the core regulatory mechanism of tumor ferroptosis (green is the effect of hypoxia on ferroptosis, orange is the effect of lipid metabolism on ferroptosis). ACLY, ATP citrate lyase; ACSL4, acyl-CoA synthetase long-chain family member 4; AGEs, advanced glycation end products; ALOXS, arachidonate lipoxygenase; ARF, cyclin-dependent kinase inhibitor 2A; BCAT2, branched chain amino acid transaminase 2; CARS1, cysteinyl-tRNA synthetase; CRC, colorectal cancer; DAMPS, damage- associated molecular patterns; DPP4, dipeptidyl peptidase 4; DUSP1, dual-specificity phosphatase 1; EGLN1, egl-9 family hypoxia-inducible factor 1/3; EGLN1/3, Egl-9 family hypoxia-inducible factor 1/3; EMT, epithelial–mesenchymal transition; FASN, fatty acid synthase; Fe2+, ferrous iron; Fe3+, ferric iron; FPN, ferroportin; FSP1, ferroptosis suppressor protein 1; GC, gastric cancer; GLS2, glutaminase 2; Glu, glutamate; GPX4, glutathione peroxidase; GSH, l-glutathione; GSSG, l-glutathione oxidized; HIF, hypoxia- inducible factor; IFN-γ, interferon-γ; IPP, intracisternal A particle-promoted polypeptide; LIP, labile iron pool; LPCAT3, lysophosphatidylcholine acyltransferase 3; LSH, lymphoid tissue-specific helicase; MUFA, monounsaturated fatty acid; NFE2L2, nuclear factor, erythroid 2-like 2; NOX4, NADPH oxidase 4; PUFA, polyunsaturated fatty acid; PUFA-PL, phospholipid with polyunsaturated fatty acid; RLS, reactive lipid species; ROS, reactive oxygen species; SCD1, steroyl CoA desaturase; SLC7A11, solute carrier family 7 member 11; SNPs, single-nucleotide polymorphisms; TANs, tumour-associated neutrophils; TCA, mitochondrial tricarboxylic acid cycle; TFR1, transferrin receptor 1; TME, tumour microenvironment; TRAIL, TNF-related apoptosis-inducing ligand; TXNIP, thioredoxin-interacting protein; VCAM-1, vascular cell adhesion molecule-1.
Ferroptosis is a nontumourigenic tumour suppression mechanism
Ferroptosis is a tumour-suppressing process that may influence numerous biological features of cancers, including the epithelial–mesenchymal transition (EMT), immunological responses, genomic alterations, disease progression, and drug resistance (Granofszky et al., 2018; Chen et al., 2021b). High iron levels in cancer cells enhance the proliferative and anabolic/metabolic activities of the cancer cells, and sensitivity to ferroptosis induction is associated with an increased iron requirement (Battaglia et al., 2020). Tumour cells alter ferroptosis sensitivity by changing iron consumption patterns, autophagy, and mitochondrial iron levels (Battaglia et al., 2020; Liu et al., 2020). Mesenchymal dedifferentiated cancer cells and EMT/metastasis-prone cancer cells were identified as relatively susceptible to ferroptosis (Viswanathan et al., 2017; Guan et al., 2021). These findings demonstrate that ferroptosis activation in tumour cells, as mediated through, for example, GPX4 and ACSL4, lowers the tumour necrosis rate and invasiveness; however, the physiological role of ferroptosis in tumour suppression and the genetic mechanisms involved in overcoming this tumour-suppressing process remain unknown.
Ferroptosis and tumour suppressor genes
The p53 tumour suppressor pathway is inactivated in cancer (Kastenhuber and Lowe, 2017). Many p53 functions, including its involvement in cell cycle arrest, senescence, and apoptosis, have been linked to tumour suppression, including ferroptosis-mediated through regulation of SLC7A11 transcription (Kang et al, 2019). Thus, ferroptosis may be necessary for tumour suppression, particularly in the absence of modulators of cell cycle arrest, senescence, or apoptosis. In addition to regulating SLC7A11, p53 can induce SAT1 expression, enhancing ALOX15 activity, which modifies tumour cell sensitivity to ferroptosis (Ou et al., 2016). PTGS2 and CBS, two ferroptosis markers, have also been identified as p53 target genes (Enache et al., 2018); however, p53 can also inhibit ferroptosis in certain cancer cells by regulating p21 or dipeptidyl peptidase 4 (DPP4) expression (Xie et al., 2017; Venkatesh et al, 2020). The cell cycle regulatory protein p21 is an essential p53 target gene because it can slow the cell cycle and initiate the replacement of the raw materials needed to generate nucleic acids, NADPH, and glutathione, preventing ferroptosis (Venkatesh et al, 2020). The p53 protein may also directly bind DPP4 in the nucleus, preventing it from binding NOX1 in the cytoplasm and reducing the ferroptosis rate (Xie et al., 2017). These findings suggest the complicated roles played by ferroptosis in cancer, highlighting the need for further research. P53 is not the sole tumour suppressor that regulates ferroptosis. Similar to p53, BAP1 is commonly deleted or mutated in human malignancies (Carbone et al., 2020). BAP1, a tumour suppressor, has been shown to reduce SLC7A11 expression and promote ferroptosis by regulating H2A ubiquitination at the SLC7A11 promoter (Zhang et al., 2019).
NFE2L2 is a master regulator of oxidative stress signalling and exhibits a dual function in tumour development. Low constitutive NFE2L2 activity favours early carcinogenesis, whereas high constitutive NFE2L2 activity promotes tumour progression and therapeutic resistance (Dodson et al., 2019).
NFE2L2 expression in cancer cells is regulated by KEAP1-mediated protein degradation and the transcriptional modulation of oncogene signalling pathways such as KRAS-BRAF-MYC (Rojo de la Vega et al., 2018). The NFE2L2 signalling pathway has been demonstrated to inhibit ferroptosis by transactivating cytoprotective genes linked to iron metabolism (SLC40A1, MT1G, HMOX1, and FTH1) and glutathione metabolism (SLC7A11, GCLM, and CHAC1) and genes encoding ROS detoxification enzymes (TXNRD1, AKR1C1, AKR1C2, SESN2, GSTP1, and NQO1) (Anandhan et al., 2020). NFE2L2 promotes SLC7A11 expression and activity, whereas TP53, BAP1, and BECN1 exert the opposite effects (Sun et al., 2016). During ferroptosis, glutathione levels are altered through this dual-regulation mechanism. Other glutathione sources include the transsulfuration pathway, which is inhibited by aminoacyl-tRNA synthetases such as CARS1. Notably, single-nucleotide polymorphisms in CARS1 (rs384490, rs729662, rs2071101, and rs7394702) increase the risk of gastric cancer (Tian et al., 2017).
HIF promotes tumour growth and treatment resistance (Lee et al, 2021). HIF1 and EPAS1 expression is upregulated in many cancers and is linked to poor patient outcomes (Ivan and Kaelin, 2017). HIF seems to exhibit a dual function in cancer cell ferroptosis regulation. On the one hand, iron phagocytosis is inhibited by hypoxia, while mitochondrial ferritin expression is increased (Fuhrmann et al., 2020). On the other hand, EPAS1 activation increases lipid- and iron-regulator gene expression in tumours, making these tumours more susceptible to ferroptosis and increasing ROS levels via irreversible cysteine oxidation (Singhal et al., 2021). Therefore, effective control of HIF-mediated signalling is essential for maintaining lipid homeostasis and regulation of ferroptosis.
Ferroptosis and the tumour microenvironment
Ferroptosis promotes and suppresses tumour growth by releasing damage-associated molecular patterns (DAMPs) into the TME and activating immune responses to trigger ferroptosis (Murao et al., 2021). DAMPs may cause immunogenic cell death, boosting antitumour immunity; however, DAMPs stimulate inflammatory responses that promote tumour development, indicating that ferroptosis influences the TME and that cancer cells may regulate ferroptosis (Sun et al., 2020a). In pancancer analyses, tumours sensitive to ferroptosis exhibited more CD8 + T cells (Tang et al., 2020). CD8 + T lymphocytes may secrete interferon γ to inhibit SLC3A2 and SLC7A11 expression and cystine absorption by tumour cells (Wang et al., 2019). Immune cells such as T cells, B cells, and macrophages undergo ferroptosis under specific circumstances, controlling tumour immunity. Reduced production of cytotoxic cytokines due to ferroptosis driven by T-cell CD36 expression and coupled with anti-PD-1 leads to diminished T-cell antitumour effects (Ma et al., 2021).
HMGB1 is released by ferroptotic cancer cells and promotes inflammatory responses in macrophages by binding to advanced glycation end products (AGEs). This ferroptosis-mediated inflammatory response is limited by the genetic and pharmacological inhibition mediated by the HMGB1-AGE pathway. Furthermore, pancreatic cancer cells may secrete exosomes carrying KRAS-G12D, and these exosomes are engulfed by macrophages. The uptake of AGEs leads to M2 macrophage polarization and tumour growth promotion (Wen et al., 2019; Dai et al., 2020). In addition to the aforementioned proteins, a complex network of nonproteinaceous DAMPs (such as ATP, host DNA, and lipid mediators) may be produced during ferroptosis and influence antitumour immunity (Shi et al., 2022). The long-term effects of ferroptosis on tumour immunity depend on interactions between cancer cells and various immune cell subsets. For example, the lymphatic system promotes tumour spreading by boosting ACSL3-dependent monounsaturated fatty acid (MUFA) synthesis (Quan et al., 2021); however, the role played by neutrophils in cancer remains controversial (Shaul and Fridlender, 2019; Yee et al., 2020). Early tumour development attracts neutrophils to damaged tissues, creating a positive feedback loop and leading to tumour necrosis (Yee et al., 2020). In contrast, neutrophils in various cancers are called tumour-associated neutrophils and are closely associated with poor prognosis in various advanced cancers, such as gastric cancer and colorectal cancer (CRC) (Mizuno et al., 2019; Wang et al., 2020b).
Neutrophils can promote tumour growth by releasing cytokines that affect the extracellular matrix, stimulating angiogenesis and regulating the behaviours of other inflammatory cells. They can also absorb and store iron and export proteins. Neutrophil cytotoxicity may lead to anticancer effects. Studies on neutrophil-induced cytotoxicity revealed that neutrophil-derived ROS, TNF-related apoptosis-inducing ligand, and hydrogen peroxide are critical for neutralizing granulocyte-mediated tumour cell death (Yuan et al., 2018); however, not all mature neutrophils can induce cell death. High-density mature neutrophils can lead to tumour cell death, but low-density or immature neutrophils exert no impact (Sagiv et al., 2015). Activated mature neutrophils promote ferroptosis by transporting myeloperoxidase-containing granules into tumour cells (Yee et al., 2020).
Ferroptosis is related to tumour suppression and metabolism
Recent studies have shown that ferroptosis involves pathways linking tumour suppression and metabolism. The initiation and execution of ferroptosis depend on the interaction between amino acid metabolism, lipid metabolism, and iron metabolism pathways, and metabolic regulators also play roles in tumours.
Ferroptosis and amino acid metabolism
Amino acids are essential substances metabolized by almost all types of cells. Recent tumour metabolomic research has linked numerous amino acids to cancer growth and diverse metabolic signatures in tumour cells, indicating their possible roles as biomarkers and keys to disease aetiologies. Wiggins et al. (2015) observed that gastric cancer patients presented with lower tyrosine, phenylalanine, and tryptophan levels. Citrulline, valine, tryptophan, and histidine levels were considerably reduced in gastric cancer patients, whereas increased levels of isoleucine and lysine were observed (Miyagi et al., 2011). Jing et al. (2018) discovered that gastric cancer patients presented with lower glutamine, histidine, arginine, and tryptophan levels and higher ornithine concentrations, and these markers showed excellent specificity and sensitivity for use in discriminating gastric cancer from stomach ulcers. Specifically, leucine, threonine, and serine are potential biomarkers for early gastric cancer (Liu et al., 2018).
Furthermore, arginine has been reported to be a prognostic factor for gastric cancer (Shi et al., 2021). Tumour cells overexpress amino acid transporters to accommodate increases in amino acid requirements. The cationic amino acid transporter PQLC2 is involved in the cysteine depletion process and is considered a possible therapeutic target in gastric cancer (Jeung et al., 2019).
In ferroptosis, cystine is transported by system XC- to produce glutathione. Recent research based on CRISPR/Cas9 editing revealed a previously unknown ferroptosis inhibitor, BCAT2, which controls intracellular glutamate levels, activates selective antagonistic system Xc inhibition and protects liver and pancreatic cancer cells against ferroptosis in vitro and in vivo (Wang et al., 2021a). ATF4, a ferroptosis inhibitor, has also been identified as a promoter of gastric cancer progression, possibly by regulating amino acid metabolism and autophagy pathways (Wang et al., 2021b).
Ferroptosis and lipid metabolism
The regulation of lipid metabolism, such as lipid uptake, synthesis, and hydrolysis, is essential for maintaining cellular homeostasis. Lipid production, storage, and degradation are closely linked to ferroptosis and lipid peroxidation (Röhrig and Schulze, 2016). Moreover, tumour cells also activate adipogenesis to maintain their high metabolic demands (Snaebjornsson et al., 2020). Therefore, lipid metabolism plays a role in ferroptosis and carcinogenesis.
Lipid peroxides form some of the ROS found in all living organisms and are the principal inducers of ferroptosis (Latunde-Dada, 2017). In the presence of ferrous iron, LPO is transformed into alkoxy radicals, which then combine with polyunsaturated fatty acids (PUFAs) to initiate a lipid radical chain reaction resulting in hydroxy fatty acid or reactive hazardous aldehyde production. These reactive lipid species may induce toxicity, stimulate lipid peroxidation, alter critical proteins, or drive cell death signalling cascades (Higdon et al., 2012). Exogenous MUFAs and PUFAs regulate intracellular lipid peroxidation and hence influence cell ferroptosis susceptibility (Yang et al., 2016). Exogenous MUFAs may displace PUFAs from phospholipids in the plasma membrane, reducing their oxidation susceptibility (Magtanong et al., 2019).
Cancer cell lipid metabolism is dynamically interconnected to the TME (Vriens et al., 2019). Oncogenic signalling and lipid metabolism promote cancer cell growth, survival, proliferation, migration, invasion, and metastasis. Induction of extracellular fatty acid transporters (CD36, SLC27, FABPs) (Su and Abumrad, 2009) and stromal cells (adipocytes and fibroblasts) may encourage tumour cells to take up extracellular fatty acids and trigger mitotic signalling (Ladanyi et al., 2018; Auciello et al., 2019). Cachexia cancer cells quickly release factors that cause excessive fatty acid oxidation (FAO) in human myotubes, causing oxidative stress, p38 activation, and decreased muscle growth (Fukawa et al., 2016). High levels of saturated membrane lipids due to lipid accumulation in gastric cancer patients protect cancer cells from ROS-induced damage (Kopecka et al., 2020).
Lipid metabolism genes may play roles in cancer promotion. Reduced production of ATP citrate lyase, which is a catalyst for the first reaction in de-novo fat synthesis, decreases the viability of tumour cells and limits their proliferation, invasion, and metastasis (Khwairakpam et al., 2020). Inhibition of the fatty acid synthase gene reduces the fatty acid synthesis rate and increases malonyl-CoA build-up, which inhibits carnitine palmitoyltransferase-mediated FAO. Furthermore, FAO may lead to ATP and NADPH production, eliminating potentially toxic lipids and causing subsequent cell cycle arrest and tumour cell apoptosis (Carracedo et al., 2013; Schroeder et al., 2021). Sun et al. (2020b) found that overexpression of fat cell differentiation-related proteins may induce cellular hypoxia, decreasing the ferroptosis rate and increasing gastric cancer cell proliferation and apoptosis.
The ACSL protein family is involved in critical lipid metabolism pathways such as peroxisome adipocytokine signalling pathway. Furthermore, the PPAR signalling pathway regulates fatty acid metabolism. Notably, GPX4 has been found to activate NRF2 and inhibit the expression of vascular cell adhesion molecule-1, contributing to tumour metastasis and angiogenesis (Song and Long, 2020). The iron-dependent enzymes Egl-9 family hypoxia-inducible factor 1/3 and c-Myc directly induce the expression of chromatin remodelling factor lymphoid-specific helicase (LSH) by inhibiting HIF-1α. Increasing the LSH levels can upregulate the expression of genes involved in lipid metabolism, such as SCD1 and FADS2, and reduce the ferroptosis rate by inhibiting the accumulation of lipid peroxides and intracellular iron (Jiang et al., 2017).
Ferroptosis and iron metabolism
Iron is an essential element in all living organisms. The capacity of iron to be oxidized and reduced makes it ideal as an electron transporter and a cofactor in various biochemical reactions, including DNA synthesis, mitochondrial respiration, host defence responses, and cell signalling. Moreover, disrupted iron redox may lead to ROS formation, adversely affecting genome stability and inducing malignant transformation. Therefore, abnormal iron metabolism plays a significant role in the development and growth of tumours (Yang et al., 2016; Wang et al., 2018). Iron homeostasis in the TME or in tumour cells is related to tumour development (Hassannia et al., 2019).
Substantial oxidative stress disrupts the regulatory function of ferritin, causing the release of large quantities of iron, which leads to excessive iron build-up in cells and low ferroportin levels. Iron-dependent lipid peroxidation is promoted by high TfR expression levels (Verma et al., 2020). Intracellular ROS may be produced in several ways. The Fenton reaction, in which ferrous iron reacts with hydrogen peroxide to generate hydroxyl radicals that participate in the ROS reaction, is a crucial regulator of ferritin degradation and TfR1 expression during ferroptosis. ROS-induced autophagy is another critical regulator of ferritin degradation and TfR1 expression during ferroptosis (Park and Chung, 2019). Iron is also an essential cofactor of several ROS-producing enzymes (Liu et al., 2022).
In addition to the involvement of iron metabolism alteration in ferroptosis, genes that regulate iron metabolism can regulate ferroptosis. Hepcidin is a gene crucial for regulating iron metabolism and is mainly expressed in cells that have been exposed to iron, such as hepatocytes, intestinal epithelial cells, and macrophages in the reticuloendothelial system. Hepcidin binds to transferrin (ferroportin) on the cell surface, causing transferrin breakdown and reducing iron production.
Ferroportin levels are quickly stabilized when hepcidin levels are low and iron is transferred to plasma. When hepcidin expression is high, ferroportin is inactivated, and iron absorption into plasma is inhibited. As a result, elevated hepcidin levels have been reported in several tumour types, and hepcidin inhibits ferroportin-mediated iron export (Ginzburg, 2019). By establishing a ferroportin-deficient mouse model to mimic hepcidin hyperactivation in CRC, Schwartz et al. (2021) discovered that ferroportin-deficient individuals and tumour tissue exhibited considerably increased iron reserves. This study shows that intestinal epithelial hepcidin-ferroportin signalling is essential for CRC development and progression.
Ferroptosis and gastric cancer
In addition to the aforementioned interactions involved in ferroptosis and tumours, ferroptosis may play a role in the occurrence and development of gastric cancer mediated through Helicobacter pylori infection and gastric cancer-related molecules (Lopez and Skaar, 2018).
Ferroptosis and H. pylori infection
More than 60% of gastric cancers are closely related to infection with H. pylori, a gram-negative, active, microaerophilic, and spiral-shaped bacterium (Matsunaga et al., 2018). The only natural host of H. pylori is the human stomach, where it causes gastric cancer by producing specific toxic factors such as cytotoxin-related gene A, vacuolar cytotoxin A, and outer membrane protein. The WHO has classified H. pylori as a Grade 1 carcinogen (Crowe, 2019). An imbalance in iron metabolism is currently thought to induce ferroptosis and increase the risk of gastric cancer (Wei et al., 2020); however, multiple mechanisms may link iron metabolism, H. pylori infection, and cancer development; whether H. pylori is the primary cause of the underlying aberrant iron metabolism in gastric cancer is debated. H. pylori infection accelerates carcinogenesis by increasing pathogen virulence (Noto et al., 2013) and is related to iron shortage, a higher plasma sTfR level, and a higher sTfR/log ferritin ratio (Enko et al., 2019). Iron shortage enhances H. pylori cag pathogenicity island expression coupled with toxin VacA released by H. pylori to induce transmutation. Because ferritin receptors are mislocalized in the basolateral membrane, not the apical surface, H. pylori receive iron from transferrin to survive (Flores et al., 2017). In addition, the interplay of dyslipidaemia with H. pylori infection can promote gastric cancer progression by inducing TH17 cell differentiation and activation (Liu et al., 2017). Although the association between H. pylori, gastric cancer, and ferroptosis is widely acknowledged, further study is needed to understand the relevant processes.
Ferroptosis-related molecules and gastric cancer prognosis
Recent research shows that ferroptosis is linked to the development of gastric cancer, but the possible mechanism of ferroptosis in gastric cancer cells has not been fully explained (Table 2). Studies have shown that four gastric cancer cell lines (AGS, SNU-1, Hs-746 T, and HGC-27 cells) are all sensitive to erastin (Wang et al., 2021c). Moreover, ferroptosis is the primary form of erastin-induced gastric cancer cell death (Hao et al., 2017). Xiao et al. (2021) found that gastric cancer-associated TMEs can be classified into EMT-like, microsatellite instability-like, and metabolite-like types. In each subtype, a different set of enriched pathways is activated, and a different survival rate is associated with each type. Therefore, the identification of gastric cancer subtypes with varying ferroptosis rates has important clinical implications (Xiao et al., 2021). In addition, some genes have been found to play essential roles in regulating ferroptosis in gastric cancer. For example, CDO1 (Hao et al., 2017) and ALOX15 (Zhang et al., 2020a) can promote the ferroptosis of human gastric cancer cells, while SCD1 (Wang et al., 2020a), PLIN2 (Sun et al., 2020b), and GDF15 (Chen et al., 2020a) inhibit ferroptosis of human gastric cancer cells. Notably, ferroptosis-related genes were discovered as indicators of gastric cancer diagnosis and prognosis in prognostic models (Huo et al., 2021; Jiang et al., 2021; Liu et al., 2021b, 2021c; Wang et al., 2021d).
Table 2.
Prognostic model results associated with ferroptosis in gastric cancer
| Author | Gene | |
|---|---|---|
| Wang C | SLC1A5, ANGPTL4, CGAS | Yang et al. (2018) |
| Chen L | GABARAPL1, ZFP36, DUSP1, TXNIP, NNMT, MYB, PSAT1, CXCL2 | Lopes et al. (2017) |
| Wang F | AKAP12, DUSP1, EFNA3, LOX, PIM1, SERPINE1, STC1, ZFP36 | Peng et al. 2020 |
| Liu G | TCFBR1, MYB, NFE2L2, ZFP36, TF, SLC1A5, NF2, NOX4 | Teng et al. (2018) |
| Huo J | NOX4, NOX5, GLS2, MYB, TGFBR1, NF2, AIFM2, ZFP36, SLC1A4, TXNIP, CXCL2, HAMP, SP1 | Schröder et al. (2020) |
DUSP1, dual-specificity phosphatase 1; NOX4, NADPH oxidase 4; TXNIP, thioredoxin-interacting protein.
Three separate studies revealed that the expression levels of ZFP36 and MYB are reliable predictors of clinical outcomes. ZFP36, also known as tristetraprolin, is an RNA-binding protein that downregulates TWIST1 and SNAI1 expression to initiate the mesenchymal-epithelial transition (Yoon et al., 2016). ZFP36 binds to PRC1 mRNA, limiting tumour development and enhancing 5-Fu sensitivity by downregulating PRC1 expression (Chen et al., 2020b). ZFP36 also protects cells against ferroptosis by regulating cellular responses to oxidative stress (Zhang et al., 2020b).
MYB is a well characterized proto-oncogene protein that controls various signalling pathways involved in cell differentiation, survival, and proliferation and plays a critical role in carcinogenesis in multiple cancer types (Fry and Inoue, 2019). MYB expression in gastric cancer cells can be targeted and inhibited by miR-139-5p, promoting gastric cancer cell proliferation and metastasis (Xie et al., 2021). Exosomes transport miR-130a from gastric cancer cells to vascular cells by targeting c-MYB in vivo and in vitro to enhance angiogenesis and tumour progression (Yang et al., 2018). Furthermore, c-Myb promotes the transcription of CDO1 and the expression of GPX4 during ferroptosis (Hao et al., 2017).
Dual-specificity phosphatase 1 (DUSP1) is an oncogene, and increasing evidence suggests that it is involved in tumour cell proliferation, differentiation, transformation, cell cycle arrest, and apoptosis by regulating the MAPK signalling pathway (Lopes et al., 2017; Peng et al., 2020). The expression of DUSP1 is more significant in the early stages of gastric cancer than in the late stages of the disease. Furthermore, it has been closely associated with the drug resistance of gastric cancer cells (Teng et al., 2018).
Thioredoxin-interacting protein (TXNIP) is a metabolic protein involved in redox homeostasis and several biological processes, making it a tumour suppressor in many malignancies, although it is expressed at low levels in numerous human cancers and tumours (Schröder et al, 2020). TXNIP overexpression reduces tumour growth and metastasis in transplant models (Park et al., 2018), while TXNIP deficiency has been shown to promote gastric cancer development through ROS signalling (Lee et al., 2010). NADPH oxidase 4 (NOX4) controls the generation of ROS and lipid peroxides. NOX4 expression has been linked to the aetiology, development, and prognosis of several cancer types. For example, overexpression of NOX4 promotes CRC progression and is associated with poor prognosis (Lin et al., 2017). The NOX4 gene is also often deleted in individuals with liver cancer, indicating that NOX4 may reduce tumour growth by mediating TGF-1-induced apoptosis (Herranz-Itúrbide et al., 2021). Although the mechanism of NOX4 action in gastric cancer is still unclear, studies have shown that NOX4 can significantly promote the proliferation and invasion of gastric cancer cells (Gregg et al., 2014).
The degree of information on identified ferroptosis-related genes varies greatly due to differences in the databases and assessment methodologies used in individual studies. Therefore, whether these ferroptosis-related genes can be used to diagnose or predict the prognosis of gastric cancer in the early stages remains unclear.
Summary
Ferroptosis, an iron-dependent controlled form of cell death caused by lipid peroxide build-up, is linked to several disorders and exhibits a tumour suppressor function. Many studies have linked ferroptosis to tumour incidence, growth, invasion, and metastasis, particularly in TME and immunotherapy studies. Gastric cancer is one of the most prevalent digestive tract cancers, and the role played by ferroptosis in gastric cancer incidence and progression remains unknown. This article summarizes the correlations identified between ferroptosis and gastric cancer by describing related mechanisms and their relationships with the TME and metabolic regulation, which contribute to future research on the mechanism of ferroptosis in gastric cancer tissue.
Acknowledgements
Writing – review and editing: H.W. Project administration: L.W. All authors have read and agreed to the published version of the manuscript.
Conflicts of interest
There are no conflicts of interest.
References
- Alvarez SW, Sviderskiy VO, Terzi EM, Papagiannakopoulos T, Moreira AL, Adams S, et al. (2017). NFS1 undergoes positive selection in lung tumors and protects cells from ferroptosis. Nature 551:639–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anandhan A, Dodson M, Schmidlin CJ, Liu P, Zhang DD. (2020). Breakdown of an ironclad defense system: the critical role of NRF2 in mediating ferroptosis. Cell Chem Biol 27:436–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auciello FR, Bulusu V, Oon C, Tait-Mulder J, Berry M, Bhattacharyya S, et al. (2019). A stromal lysolipid-autotaxin signaling axis promotes pancreatic tumor progression. Cancer Discov 9:617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia AM, Chirillo R, Aversa I, Sacco A, Costanzo F, Biamonte F. (2020). Ferroptosis and cancer: mitochondria meet the ‘iron maiden’ cell death. Cells 9:1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH, et al. (2019). The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 575:688–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone M, Harbour JW, Brugarolas J, Bononi A, Pagano I, Dey A, et al. (2020). Biological mechanisms and clinical significance of BAP1 mutations in human cancer. Cancer Discov 10:1103–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carracedo A, Cantley LC, Pandolfi PP. (2013). Cancer metabolism: fatty acid oxidation in the limelight. Nat Rev Cancer 13:227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Qiao L, Bian Y, Sun X. (2020a). GDF15 knockdown promotes erastin-induced ferroptosis by decreasing SLC7A11 expression. Biochem Biophys Res Commun 526:293–299. [DOI] [PubMed] [Google Scholar]
- Chen W, Chen M, Zhao Z, Weng Q, Song J, Fang S, et al. 2020b). ZFP36 binds with PRC1 to inhibit tumor growth and increase 5-fu chemosensitivity of hepatocellular carcinoma. Front Mol Biosci 7:126. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chen X, Yu C, Kang R, Kroemer G, Tang D. (2021a). Cellular degradation systems in ferroptosis. Cell Death Differ 28:1135–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Kang R, Kroemer G, Tang D. (2021b). Broadening horizons: the role of ferroptosis in cancer. Nat Rev Clin Oncol 18:280–296. [DOI] [PubMed] [Google Scholar]
- Crowe SE. (2019). Helicobacter pylori infection. N Engl J Med 380:1158–1165. [DOI] [PubMed] [Google Scholar]
- Dai E, Han L, Liu J, Xie Y, Zeh HJ, Kang R, et al. (2020). Ferroptotic damage promotes pancreatic tumorigenesis through a TMEM173/STING-dependent DNA sensor pathway. Nat Commun 11:6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson M, Castro-Portuguez R, Zhang DD. (2019). NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol 23:101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll S, Freitas FP, Shah R, Aldrovandi M, da Silva MC, Ingold I, et al. (2019). FSP1 is a glutathione-independent ferroptosis suppressor. Nature 575:693–698. [DOI] [PubMed] [Google Scholar]
- Enache AO, Stepan AE, Mărgăritescu C, Pătraşcu V, Ciurea RN, Simionescu CE, et al. (2018). Immunoexpression of p53 and COX-2 in basal cell carcinoma. Rom J Morphol Embryol 59:1115–1120. [PubMed] [Google Scholar]
- Enko D, Wagner H, Kriegshäuser G, Wögerer J, Halwachs-Baumann G, Schnedl WJ, et al. (2019). Iron status determination in individuals with Helicobacter pylori infection: conventional vs. new laboratory biomarkers. Clin Chem Lab Med 57:982–989. [DOI] [PubMed] [Google Scholar]
- Feng J, Lu PZ, Zhu GZ, Hooi SC, Wu Y, Huang X-W, et al. (2021). ACSL4 is a predictive biomarker of sorafenib sensitivity in hepatocellular carcinoma. Acta Pharmacol Sin 42:160–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores SE, Aitchison A, Day AS, Keenan JI. (2017). Helicobacter pylori infection perturbs iron homeostasis in gastric epithelial cells. PLoS One 12:e0184026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcina GC, Dixon SJ. (2019). GPX4 at the crossroads of lipid homeostasis and ferroptosis. Proteomics 19:e1800311. [DOI] [PubMed] [Google Scholar]
- Fry EA, Inoue K. (2019). c-MYB and DMTF1 in cancer. Cancer Invest 37:46–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann DC, Mondorf A, Beifuß J, Jung M, Brüne B. (2020). Hypoxia inhibits ferritinophagy, increases mitochondrial ferritin, and protects from ferroptosis. Redox Biol 36:101670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukawa T, Yan-Jiang BC, Min-Wen JC, Jun-Hao ET, Huang D, Qian C-N, et al. (2016). Excessive fatty acid oxidation induces muscle atrophy in cancer cachexia. Nat Med 22:666–671. [DOI] [PubMed] [Google Scholar]
- Ginzburg YZ. (2019). Hepcidin-ferroportin axis in health and disease. Vitam Horm 110:17–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granofszky N, Lang M, Khare V, Schmid G, Scharl T, Ferk F, et al. (2018). Identification of PMN-released mutagenic factors in a co-culture model for colitis-associated cancer. Carcinogenesis 39:146–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg JL, Turner RM, 2nd, Chang G, Joshi D, Zhan Y, Chen L, et al. (2014). NADPH oxidase NOX4 supports renal tumorigenesis by promoting the expression and nuclear accumulation of HIF2α. Cancer Res 74:3501–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan D, Li C, Li Y, Li Y, Wang G, Gao F, et al. (2021). The DpdtbA induced EMT inhibition in gastric cancer cell lines was through ferritinophagy-mediated activation of p53 and PHD2/hif-1α pathway. J Inorg Biochem 218:111413. [DOI] [PubMed] [Google Scholar]
- Hao S, Yu J, He W, Huang Q, Zhao Y, Liang B, et al. (2017). Cysteine dioxygenase 1 mediates erastin-induced ferroptosis in human gastric cancer cells. Neoplasia 19:1022–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassannia B, Vandenabeele P, Vanden Berghe T. (2019). Targeting ferroptosis to iron out cancer. Cancer Cell 35:830–849. [DOI] [PubMed] [Google Scholar]
- Hayano M, Yang WS, Corn CK, Pagano NC, Stockwell BR. (2016). Loss of cysteinyl-tRNA synthetase (CARS) induces the transsulfuration pathway and inhibits ferroptosis induced by cystine deprivation. Cell Death Differ 23:270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herranz-Itúrbide M, López-Luque J, Gonzalez-Sanchez E, Caballero-Díaz D, Crosas-Molist E, Martín-Mur B, et al. (2021). NADPH oxidase 4 (Nox4) deletion accelerates liver regeneration in mice. Redox Biol 40:101841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higdon A, Diers AR, Oh JY, Landar A, Darley-Usmar VM. (2012). Cell signalling by reactive lipid species: new concepts and molecular mechanisms. Biochem J 442:453–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo J, Wu L, Zang Y. (2021). Eight-gene prognostic signature associated with hypoxia and ferroptosis for gastric cancer with general applicability. Epigenomics 13:875–890. [DOI] [PubMed] [Google Scholar]
- Ivan M, Kaelin WG., Jr (2017). The EGLN-HIF O2-sensing system: multiple inputs and feedbacks. Mol Cell 66:772–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeung YJ, Lee K, Lee HJ, Kim E, Son MJ, Ahn J, et al. (2019). Cationic amino acid transporter PQLC2 is a potential therapeutic target in gastric cancer. Cancer Sci 110:1453–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Mao C, Yang R, Yan B, Shi Y, Liu X, et al. (2017). EGLN1/c-Myc induced lymphoid-specific helicase inhibits ferroptosis through lipid metabolic gene expression changes. Theranostics 7:3293–3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Yan Q, Xie L, Xu S, Jiang K, Huang J, et al. (2021). Construction and validation of a ferroptosis-related prognostic model for gastric cancer. J Oncol 2021:6635526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing F, Hu X, Cao Y, Xu M, Wang Y, Jing Y, et al. (2018). Discriminating gastric cancer and gastric ulcer using human plasma amino acid metabolic profile. IUBMB Life 70:553–562. [DOI] [PubMed] [Google Scholar]
- Kang R, Kroemer G, Tang D. (2019). The tumor suppressor protein p53 and the ferroptosis network. Free Radic Biol Med 133:162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastenhuber ER, Lowe SW. (2017). Putting p53 in context. Cell 170:1062–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khwairakpam AD, Banik K, Girisa S, Shabnam B, Shakibaei M, Fan L, et al. (2020). The vital role of ATP citrate lyase in chronic diseases. J Mol Med (Berl) 98:71–95. [DOI] [PubMed] [Google Scholar]
- Kopecka J, Trouillas P, Gašparović AC, Gazzano E, Assaraf YG, Riganti C. (2020). Phospholipids and cholesterol: inducers of cancer multidrug resistance and therapeutic targets. Drug Resist Updat 49:100670. [DOI] [PubMed] [Google Scholar]
- Ladanyi A, Mukherjee A, Kenny HA, Johnson A, Mitra AK, Sundaresan S, et al. (2018). Adipocyte-induced CD36 expression drives ovarian cancer progression and metastasis. Oncogene 37:2285–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latunde-Dada GO. (2017). Ferroptosis: role of lipid peroxidation, iron and ferritinophagy. Biochim Biophys Acta Gen Subj 1861:1893–1900. [DOI] [PubMed] [Google Scholar]
- Lee JH, Jeong EG, Choi MC, Kim S-H, Park J-H, Song S-H, et al. (2010). Inhibition of histone deacetylase 10 induces thioredoxin-interacting protein and causes accumulation of reactive oxygen species in SNU-620 human gastric cancer cells. Mol Cells 30:107–112. [DOI] [PubMed] [Google Scholar]
- Lee SH, Golinska M, Griffiths JR. (2021). HIF-1-independent mechanisms regulating metabolic adaptation in hypoxic cancer cells. Cells 10:2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin XL, Yang L, Fu SW, Lin W-F, Gao Y-J, Chen H-Y, et al. (2017). Overexpression of NOX4 predicts poor prognosis and promotes tumor progression in human colorectal cancer. Oncotarget 8:33586–33600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Wang C, Liu G, Bi C, Wang X, Zhou Q, et al. (2020). SLC7A11/xCT in cancer: biological functions and therapeutic implications. Am J Cancer Res 10:3106–3126. [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wang H, Chen G, Yang M, Wu ZX, Ericksen RE, et al. (2017). The synergy of Helicobacter pylori and lipid metabolic disorders in induction of Th17-related cytokines in human gastric cancer. J Cancer Metastasis Treat 3:169–176. [Google Scholar]
- Liu J, Lin S, Li Z, Zhou L, Yan X, Xue Y, et al. (2018). Free amino acid profiling of gastric juice as a method for discovering potential biomarkers of early gastric cancer. Int J Clin Exp Pathol 11:2323–2336. [PMC free article] [PubMed] [Google Scholar]
- Liu J, Kuang F, Kroemer G, Klionsky DJ, Kang R, Tang D. (2020). Autophagy-dependent ferroptosis: machinery and regulation. Cell Chem Biol 27:420–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Kang R, Tang D. (2022). Signaling pathways and defense mechanisms of ferroptosis. FEBS J 22:7038–7050. [DOI] [PubMed] [Google Scholar]
- Liu G, Ma JY, Hu G, Jin H. (2021). Identification and validation of a novel ferroptosis-related gene model for predicting the prognosis of gastric cancer patients. PLoS One 16:e0254368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SJ, Yang YB, Zhou JX, Lin Y-J, Pan Y-L, Pan J-H. (2021c). A novel ferroptosis-related gene risk signature for predicting prognosis and immunotherapy response in gastric cancer. Dis Markers 2021:2385406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes LJS, Tesser-Gamba F, Petrilli AS, de Seixas Alves MT, Garcia-Filho RJ, Toledo SRC. (2017). MAPK pathways regulation by DUSP1 in the development of osteosarcoma: potential markers and therapeutic targets. Mol Carcinog 56:1630–1641. [DOI] [PubMed] [Google Scholar]
- Lopez CA, Skaar EP. (2018). The impact of dietary transition metals on host-bacterial interactions. Cell Host Microbe 23:737–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Xiao L, Liu L, Ye L, Su P, Bi E, et al. (2021). CD36-mediated ferroptosis dampens intratumoral CD8+ T cell effector function and impairs their antitumor ability. Cell Metab 33:1001–1012.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magtanong L, Ko PJ, To M, Cao JY, Forcina GC, Tarangelo A, et al. (2019). Exogenous monounsaturated fatty acids promote a ferroptosis-resistant cell state. Cell Chem Biol 26:420–432.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga S, Nishiumi S, Tagawa R, Yoshida M. (2018). Alterations in metabolic pathways in gastric epithelial cells infected with Helicobacter pylori. Microb Pathog 124:122–129. [DOI] [PubMed] [Google Scholar]
- Miyagi Y, Higashiyama M, Gochi A, Akaike M, Ishikawa T, Miura T, et al. (2011). Plasma free amino acid profiling of five types of cancer patients and its application for early detection. PLoS One 6:e24143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno R, Kawada K, Itatani Y, Ogawa R, Kiyasu Y, Sakai Y. (2019). The role of tumor-associated neutrophils in colorectal cancer. Int J Mol Sci 20:529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murao A, Aziz M, Wang H, Brenner M, Wang P. (2021). Release mechanisms of major DAMPs. Apoptosis 26:152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noto JM, Gaddy JA, Lee JY, Piazuelo MB, Friedman DB, Colvin DC, et al. (2013). Iron deficiency accelerates Helicobacter pylori-induced carcinogenesis in rodents and humans. J Clin Invest 123:479–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou Y, Wang SJ, Li D, Chu B, Gu W. (2016). Activation of SAT1 engages polyamine metabolism with p53-mediated ferroptosis responses. Proc Natl Acad Sci U S A 113:E6806–E6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E, Chung SW. (2019). ROS-mediated autophagy increases intracellular iron levels and ferroptosis by ferritin and transferrin receptor regulation. Cell Death Dis 10:822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JW, Lee SH, Woo GH, Kwon H-J, Kim D-Y. (2018). Downregulation of TXNIP leads to high proliferative activity and estrogen-dependent cell growth in breast cancer. Biochem Biophys Res Commun 498:566–572. [DOI] [PubMed] [Google Scholar]
- Peng W, Zhang C, Peng J, Huang Y, Peng C, Tan Y, et al. (2020). Lnc-FAM84B-4 acts as an oncogenic lncRNA by interacting with protein hnRNPK to restrain MAPK phosphatases-DUSP1 expression. Cancer Lett 494:94–106. [DOI] [PubMed] [Google Scholar]
- Quan J, Bode AM, Luo X. (2021). ACSL family: the regulatory mechanisms and therapeutic implications in cancer. Eur J Pharmacol 909:174397. [DOI] [PubMed] [Google Scholar]
- Röhrig F, Schulze A. (2016). The multifaceted roles of fatty acid synthesis in cancer. Nat Rev Cancer 16:732–749. [DOI] [PubMed] [Google Scholar]
- Rojo de la Vega M, Chapman E, Zhang DD. (2018). NRF2 and the hallmarks of cancer. Cancer Cell 34:21–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv JY, Michaeli J, Assi S, Mishalian I, Kisos H, Levy L, et al. (2015). Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep 10:562–573. [DOI] [PubMed] [Google Scholar]
- Schröder J, Schumacher U, Böckelmann LC. (2020). Thioredoxin interacting protein (TXNIP) is differentially expressed in human tumor samples but is absent in human tumor cell line xenografts: implications for its use as an immunosurveillance marker. Cancers (Basel) 12:3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder B, Vander Steen T, Espinoza I, Venkatapoorna CMK, Hu Z, Silva FM, et al. (2021). Fatty acid synthase (FASN) regulates the mitochondrial priming of cancer cells. Cell Death Dis 12:977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz AJ, Goyert JW, Solanki S, Kerk SA, Chen B, Castillo C, et al. (2021). Hepcidin sequesters iron to sustain nucleotide metabolism and mitochondrial function in colorectal cancer epithelial cells. Nat Metab 3:969–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha W, Hu F, Xi Y, Chu Y, Bu S. (2021). Mechanism of ferroptosis and its role in type 2 diabetes mellitus. J Diabetes Res 2021:9999612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul ME, Fridlender ZG. (2019). Tumor-associated neutrophils in patients with cancer. Nat Rev Clin Oncol 16:601–620. [DOI] [PubMed] [Google Scholar]
- Shi L, Liu Y, Li M, Luo Z. (2022). Emerging roles of ferroptosis in the tumor immune landscape: from danger signals to antitumor immunity. FEBS J 13:3655–3665. [DOI] [PubMed] [Google Scholar]
- Shi LY, Wang YY, Jing Y, Xu M-H, Zhu Z-T, Wang Q-J. (2021). Abnormal arginine metabolism is associated with prognosis in patients of gastric cancer. Transl Cancer Res 10:2451–2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal R, Mitta SR, Das NK, Kerk SA, Sajjakulnukit P, Solanki S, et al. (2021). HIF-2α activation potentiates oxidative cell death in colorectal cancers by increasing cellular iron. J Clin Invest 131:e143691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaebjornsson MT, Janaki-Raman S, Schulze A. (2020). Greasing the wheels of the cancer machine: the role of lipid metabolism in cancer. Cell Metab 31:62–76. [DOI] [PubMed] [Google Scholar]
- Song X, Long D. (2020). Nrf2 and ferroptosis: a new research direction for neurodegenerative diseases. Front Neurosci 14:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, et al. (2017). Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171:273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Abumrad NA. (2009). Cellular fatty acid uptake: a pathway under construction. Trends Endocrinol Metab 20:72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R, et al. (2016). Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology 63:173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Chen P, Zhai B, Zhang M, Xiang Y, Fang J, et al. 2020a). The emerging role of ferroptosis in inflammation. Biomed Pharmacother 127:110108. [DOI] [PubMed] [Google Scholar]
- Sun X, Yang S, Feng X, Zheng Y, Zhou J, Wang H, et al. 2020b). The modification of ferroptosis and abnormal lipometabolism through overexpression and knockdown of potential prognostic biomarker perilipin2 in gastric carcinoma. Gastric Cancer 23:241–259. [DOI] [PubMed] [Google Scholar]
- Tang R, Hua J, Xu J, Liang C, Meng Q, Liu J, et al. (2020). The role of ferroptosis regulators in the prognosis, immune activity and gemcitabine resistance of pancreatic cancer. Ann Transl Med 8:13471347.134711347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng F, Xu Z, Chen J, Zheng G, Zheng G, Lv H, et al. (2018). DUSP1 induces apatinib resistance by activating the MAPK pathway in gastric cancer. Oncol Rep 40:1203–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian T, Xiao L, Du J, Zhu X, Gu Y, Qin N, et al. (2017). Polymorphisms in CARS are associated with gastric cancer risk: a two-stage case-control study in the Chinese population. Gastric Cancer 20:940–947. [DOI] [PubMed] [Google Scholar]
- Ursini F, Maiorino M. (2020). Lipid peroxidation and ferroptosis: The role of GSH and GPx4. Free Radic Biol Med 152:175–185. [DOI] [PubMed] [Google Scholar]
- Venkatesh D, Stockwell BR, Prives C. (2020). p21 can be a barrier to ferroptosis independent of p53. Aging (Albany, NY) 12:17800–17814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma N, Vinik Y, Saroha A, Nair NU, Ruppin E, Mills G, et al. (2020). Synthetic lethal combination targeting BET uncovered intrinsic susceptibility of TNBC to ferroptosis. Sci Adv 6:eaba8968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan VS, Ryan MJ, Dhruv HD, Gill S, Eichhoff OM, Seashore-Ludlow B, et al. (2017). Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature 547:453–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriens K, Christen S, Parik S, Broekaert D, Yoshinaga K, Talebi A, et al. (2019). Evidence for an alternative fatty acid desaturation pathway increasing cancer plasticity. Nature 566:403–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Luo J, Zhang Z, Dong D, Shen Y, Fang Y, et al. (2018). Iron and magnetic: new research direction of the ferroptosis-based cancer therapy. Am J Cancer Res 8:1933–1946. [PMC free article] [PubMed] [Google Scholar]
- Wang W, Green M, Choi JE, Gijón M, Kennedy PD, Johnson JK, et al. (2019). CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 569:270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Shi M, Ji J, Cai Q, Zhao Q, Jiang J, et al. 2020a). Stearoyl-CoA desaturase 1 (SCD1) facilitates the growth and anti-ferroptosis of gastric cancer cells and predicts poor prognosis of gastric cancer. Aging (Albany NY) 12:15374–15391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhai J, Zhang T, Han S, Zhang Y, Yao X, et al. 2020b). Tumor-associated neutrophils can predict lymph node metastasis in early gastric cancer. Front Oncol 10:570113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Zhang Z, Tsai HI, Liu Y, Gao J, Wang M, et al. 2021a). Branched-chain amino acid aminotransferase 2 regulates ferroptosis cell death in cancer cells. Cell Death Differ 28:1222–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Lu Y, Wang H, Wu Y, Xu X, Li Y. (2021b). High ATF4 expression is associated with poor prognosis, amino acid metabolism, and autophagy in gastric cancer. Front Oncol 11:740120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang T, Zhang Y, Liu J, Song J, Han Y, et al. 2021c). CPEB1 enhances erastin-induced ferroptosis in gastric cancer cells by suppressing twist1 expression. IUBMB Life 73:1180–1190. [DOI] [PubMed] [Google Scholar]
- Wang F, Chen C, Chen WP, Li Z-L, Cheng H. (2021d). Development and validation of a novel ferroptosis-related gene signature for predicting prognosis and the immune microenvironment in gastric cancer. Biomed Res Int 2021:6014202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Gao X, Qin Y, Liu T, Kang Y. (2020). An iron metabolism-related SLC22A17 for the prognostic value of gastric cancer. Onco Targets Ther 13:12763–12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Q, Liu J, Kang R, Zhou B, Tang D. (2019). The release and activity of HMGB1 in ferroptosis. Biochem Biophys Res Commun 510:278–283. [DOI] [PubMed] [Google Scholar]
- Wiggins T, Kumar S, Markar SR, Antonowicz S, Hanna GB. (2015). Tyrosine, phenylalanine, and tryptophan in gastroesophageal malignancy: a systematic review. Cancer Epidemiol Biomarkers Prev 24:32–38. [DOI] [PubMed] [Google Scholar]
- Xiao S, Liu X, Yuan L, Chen X, Wang F. (2021). Expression of ferroptosis-related genes shapes tumor microenvironment and pharmacological profile in gastric cancer. Front Cell Dev Biol 9:694003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Zhu S, Song X, Sun X, Fan Y, Liu J, et al. (2017). The tumor suppressor p53 limits ferroptosis by blocking DPP4 activity. Cell Rep 20:1692–1704. [DOI] [PubMed] [Google Scholar]
- Xie Y, Rong L, He M, Jiang Y, Li H, Mai L, et al. (2021). LncRNA SNHG3 promotes gastric cancer cell proliferation and metastasis by regulating the miR-139-5p/MYB axis. Aging (Albany NY) 13:25138–25152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagoda N, von Rechenberg M, Zaganjor E, Bauer AJ, Yang WS, Fridman DJ, et al. (2007). RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature 447:864–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR. (2016). Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci U S A 113:E4966–E4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Zhang H, Ge S, Ning T, Bai M, Li J, et al. (2018). Exosome-derived miR-130a activates angiogenesis in gastric cancer by targeting C-MYB in vascular endothelial cells. Mol Ther 26:2466–2475. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yee PP, Wei Y, Kim SY, Lu T, Chih SY, Lawson C, et al. (2020). Neutrophil-induced ferroptosis promotes tumor necrosis in glioblastoma progression. Nat Commun 11:5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon NA, Jo HG, Lee UH, Park JH, Yoon JE, Ryu J, et al. (2016). Tristetraprolin suppresses the EMT through the down-regulation of Twist1 and Snail1 in cancer cells. Oncotarget 7:8931–8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Gajan A, Chu Q, Xiong H, Wu K, Wu GS. (2018). Developing TRAIL/TRAIL death receptor-based cancer therapies. Cancer Metastasis Rev 37:733–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Koppula P, Gan B. (2019). Regulation of H2A ubiquitination and SLC7A11 expression by BAP1 and PRC1. Cell Cycle 18:773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Deng T, Liu R, Ning T, Yang H, Liu D, et al. 2020a). CAF secreted miR-522 suppresses ferroptosis and promotes acquired chemo-resistance in gastric cancer. Mol Cancer 19:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Guo M, Li Y, Shen M, Kong D, Shao J, et al. 2020b). RNA-binding protein ZFP36/TTP protects against ferroptosis by regulating autophagy signaling pathway in hepatic stellate cells. Autophagy 16:1482–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N, Bao J. (2020). FerrDb: a manually curated resource for regulators and markers of ferroptosis and ferroptosis-disease associations. Database (Oxford) 2020:baaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]