ABSTRACT
Introduction
Hip fractures are associated with significant morbidity and mortality. This study assessed the feasibility of smartwatches supporting rehabilitation post-surgical fixation.
Methods
This UK-based non-randomised intervention study recruited patients who had sustained a hip fracture (age ≥65 and Abbreviated Mental Test Score ≥8/10), following surgical fixation, at one hospital to the intervention group, and at a second hospital to a usual care group. The intervention group received a smartwatch (Fitbit Charge 4) and app (CUSH Health©). Feasibility measures included retention and completion of outcome measures.
Results
Between November 2020 and November 21, 66 participants were recruited (median age 78 (IQR 74–84)). The intervention cohort were younger, with no significant differences in frailty or multi-morbidity between the cohorts. Hospital stay was shorter in the intervention cohort (10 days (7–16) versus 12 (10–18), p=0.05). There were 15 falls-related readmissions in the control cohort, including 11 fractures, with none in the intervention cohort (p=0.016). In the intervention group, median daily step counts increased from 477 (320–697) in hospital, to 931 (505–1238) 1 week post-discharge, to 5,352 (3,552–7,944) at 12-weeks (p=0.001). Of the intervention cohort, 12 withdrew.
Conclusion
This study found that smartwatch-supported rehabilitation was feasible in this cohort. A significant proportion of patients either chose not to participate or withdrew; such a decrease in participants must be addressed to avoid digital exclusion. Falls and fracture-related readmissions were more frequent at the control site compared with the intervention site.
KEYWORDS: smartwatches, hip fracture, rehabilitation, digital health
Introduction
Hip fractures (of the proximal femur) are a significant burden to individuals and society, globally affecting 14.2 million adults in 2019, an increase of 92% since 1990.1 In the UK in 2019 there were 67,302 hip fracture admissions, accounting for one and a half million hospital bed days.2 By 2033, it is projected that there will be 100,000 cases a year, costing the NHS £3.6 billion–5.6 billion.3 Associated morbidity and mortality is considerable, with a 20–25% 1-year mortality rate.4,5 Only around half recover to prefracture levels of mobility, up to 60% of those previously independent require long-term assistance, and 31% of people admitted from home do not return to their original residence.2,6,7
A UK-wide audit revealed that only 20% of hospitals achieved continuity of rehabilitation as patients transitioned home.8 However, there is no consensus on what constitutes optimal rehabilitation.9 A James Lind Alliance report (2018) identified understanding the best in-hospital and out-of-hospital rehabilitation approaches for recovery as the top research priorities.10
Wearable smartwatches and mobile applications (apps), have gained popularity in healthcare and wider society. However, evidence of their efficacy in supporting older people's rehabilitation is scarce, with no studies reporting use of smartwatches.11 Older people have previously been stigmatised in their ability to engage with, and adapt to, healthcare technology.12 However, given worldwide population ageing, the inclusion of older people in healthcare digital research is crucial.13 Trials studying the effects of rehabilitation after a hip fracture are also lacking.14 The usefulness of using novel methods when evaluating effects of rehabilitation, such as data captured with wearable devices, has been acknowledged.15 Nevertheless, few studies have assessed the acceptability of such devices in the acute setting.16 A 2022 review of patients sustaining a hip fracture and the use of technology to aid recovery found no interventional studies using wearables,17 although several studies have looked at the feasibility of technology use in hospital.18–23 To our knowledge, no study has reported the potential role of smartwatches to support tailoring of rehabilitation in the context of a hip fracture in hospital and then continuing at home. If successful, such technology could assist the continuity of rehabilitation between hospital and the community and minimise the economic burden of rehabilitation.
The Hospital-to-Home (H2H) study aimed to assess the feasibility, during the Coronavirus 2019 (COVID-19) pandemic, of remote rehabilitation (using smartwatches and an app, CUSH Health©) in patients following a hip fracture, compared with usual care. This was a direct response to the pandemic, during which time social distancing was associated with attenuated access to direct health and social care. The acceptability assessment was framed by the technology acceptance model (TAM).24 Objectives were to assess: (i) the recruitment and retention of patients; (ii) whether the intervention components were feasible to deliver and acceptable to staff and patients; and (iii) whether data could be extracted from the smartwatches. This report provides data on the first 6 months of follow-up.
Methods
Ethics
Ethical approval was given by the Hampshire Research Ethics Committee (18/SC/0513/AM04 IRAS Project ID 247109). General Data Protection Regulation (GDPR) compliance for data security was applied. The app (through CUSH health©) did not collect IP addresses or Global Positioning System (GPS) data.
The H2H is a feasibility non-randomised prospective 12-month intervention study (intervention and control cohorts) (see supplementary material S1 for the Consort Checklist). Feasibility indicators included recruitment, retention, delivery of the intervention and collecting of outcomes, to inform future randomised controlled trials (RCT). This study was a rapid response to the COVID-19 pandemic to maximise engagement with rehabilitation in the context of restricted hospital and community services. Two TAM factors, ‘perceived usefulness’ and ‘ease of use’, predict technology acceptance and usage behaviour and were explored through feedback attained during participation.24
Source of data and participants
Data were collected prospectively for patients with a traumatic hip fracture (operated on) at two non-specialised hospital sites in Sussex, UK. The intervention arm was at one hospital, which on average manages 450 hip fractures per annum. At the control hospital (400 hip fractures per annum), participants were recruited by a research assistant, independent of the intervention team, for observational data. There was no overlap of staffing, who were blinded to the outcomes of the two groups. Exclusions were cognitive dysfunction (Abbreviated Mental Test Score (AMTS) <8/10),25 age <65 and admission from nursing or residential care. Eligible patients were approached at the first appropriate time during the postoperative period when they had regained capacity (typically during the first few days post operation). Participants were provided with the opportunity to ask questions and discuss the study with the researcher, family and carers, as required. All patients gave written consent to participate.
Intervention
Baseline data were collected for participants, including demographics (age and sex), clinical frailty scale score,26 Nottingham hip fracture score27 and number of comorbidities. Informed by baseline physical function, individualised exercise plans were supplemented by a smartwatch to enable the clinical team to track recovery of the patients following surgery and to iteratively develop the rehabilitation pathway. The intervention group received a Fitbit smartwatch Charge 4 (Fitbit Inc, San Francisco, CA, USA) with a digital display and an app. This has a screen enabling self-monitoring of real-time physical activity levels. The device requires synching with a smartphone or tablet device. Participants without either used a relative's device to synch data. Participants were instructed to wear their device continuously throughout the day and shown how to upload data to the app using Bluetooth wireless technology. The app displayed collated activity in an easy-to-view format and provided access to graded exercise videos. Simple instructions were delivered to titrate up activity using step counts, in consult with the clinical team. While on the ward, troubleshooting took place by the research team. Videos (exercises, yoga and Pilates) could be accessed through the app. On hospital discharge, the remote rehabilitation pathway continued, supported by the smartwatch data, for 3 months. Standardised structured weekly phone calls were provided (by a physiotherapist or physiotherapy associate practitioner) to allow reporting of technical issues and to tailor support.
Outcome measures
The primary outcomes measured were acceptability of using the smartwatch (wear time and percentage who reported positive use of the device) and feasibility of the intervention. This was assessed through: recruitment rates, retention, intervention delivery, ability to extract data from the smartwatch and the app, looking for correlations with standard core outcome measures and investigating whether the activity data could identify inactive patients. A threshold of 80% is frequently considered an appropriate cut-off for assessing measures of acceptability and feasibility.28 However, in an older hip fracture cohort starting an intervention in an acute care setting, low rates of around 50% have been reported, a cut-off that was used in this study.23
The study aimed to collect information recommended by the UK hip fracture core outcome set,29 (mortality, pain, daily activity, mobility and Health-related quality of life (HRQoL), though the EQ5D-5L rather than the EQ5D-3L was used.30 The Physical Activity Scale for the Elderly (PASE) was also collected.31
Length of hospital stay and number of readmissions (including readmission related to falls and further fractures) were collected for both groups. Data extracted from the smartwatch included step counts and hours of use. Feedback during the study was gained from focus groups and from asking for reasons for study withdrawal.
Baseline characteristics for the two groups are presented using median (with interquartile range (IQR)) for continuous data (Table 1). For continuously distributed outcomes, differences were tested using Mann–Whitney U and Chi-square tests for categorical variables. Values across time were compared by Wilcoxon signed ranks and Friedman tests. A formal sample size was not calculated for this feasibility study, with a target of 25–50 patients per site, similar in size to recommended for such studies.32 Data were analysed using SPSS software (V.27).
Table 1.
Demographics and comorbidities of study participantsa
| Demographics and comorbidities | Intervention group (n=26) | Control group (n=40) | p-value |
|---|---|---|---|
| Female sex | 81% | 75% | 0.77 |
| Age | 75 (72–77) | 81 (77–87) | <0.001 |
| Multiple comorbidities | 69% | 73% | 0.79 |
| Clinical frailty scale | 3 (2–4) | 4 (3–4) | 0.10 |
| Nottingham Hip Fracture Score | 4 (3–5) | 5 (4–5) | 0.03 |
| Hypertension | 35% | 48% | 0.32 |
| Chronic kidney diseaseb | 12% | 10% | 1.0 |
| Chronic respiratory disease | 4% | 15% | 0.23 |
| Vascular | 4% | 0 | – |
| Cerebrovascular disease | 4% | 13% | 0.39 |
| Atrial fibrillation | 13% | 5% | 0.38 |
| Diabetes | 8% | 15% | 0.46 |
aData are percentages or median (interquartile range).
bEstimated glomerular filtration rate <60 mL).
Results
Enrolment
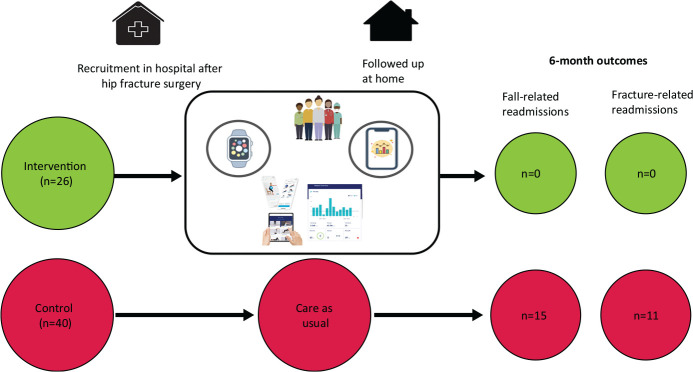
Between November 2020 and November 2021, 117 patients were screened and 66 were recruited to the study (n=26 at the intervention site). Fig 1 summarises the study intervention.
Fig 1.
Summary of the intervention. Patients in the intervention arm were recruited in hospital, set up with the smartwatch and app, and then had remote (telephone) follow-up at home.
Exclusions (n=51) were: acute or chronic confusion (n=10); acute complex medical problems (n=10); aversion to, or lack of, technology (n=15); ‘overwhelmed’ by the situation (n=5); or administrative (n=7, no staff, patient away from ward or discharged before potential recruitment). Over the first 6 months, 12 (46%) of the intervention group withdrew.
The intervention cohort were younger (median 75 years (IQR 72–77) versus 81 (77–87), p<0.001) (Table 1). There was no significant difference between the intervention and control sites for gender (81% versus 75% female, p=0.77), frailty scores (Clinical Frailty Scale (CFS) 3 (2–4) versus 4 (2–4), p=0.10) and multi-morbidity (69% versus 73%, odds ratio (OR) 0.85; 95% confidence interval (CI) 0.29–2.5, p=0.79).
Median length of stay in hospital was shorter in the intervention group (10 days (7–16) versus 12 (10–18), p=0.05). During follow-up, one participant at the intervention site and five at the control site died. There were five readmissions at the intervention site (occupying a total of 29 days in hospital) compared with 11 individuals (21 episodes) at the control site (362 days in hospital). None of reasons for the readmissions of five individuals at the intervention site were considered related to the original admission (being abdominal pain, Crohn's flare, fast atrial fibrillation, angina and a tongue bleed), compared with nine at the control site (p=0.009). A fall was the cause of a readmission in eight participants (accounting for 15 readmissions) at the control site (p=0.018). These included seven individual patients with a fracture (a total 11 fracture-related admissions) (p=0.037).
At 3 months on the EuroQol 5-dimensional outcomes questionnaire (EQ-5D-5L), the intervention group had a higher mobility score (p=0.046), with no significant differences in the other domains or overall score, compared with the control group.
Feasibility indicators
Of the screened patients, 56% were recruited to the study, exceeding the 50% threshold. Of those recruited to the intervention, all were successfully set up with smartwatches and the intervention was started with initial smartwatch data extracted from all participants. The activity data were shown at 2 weeks to identify inactive patients (those with step counts <2000) who remained less active at 3 months. A threshold of >50% for retention was met (54%, n=14/26).
The study collected information on the reasons for participant dropouts and focus group feedback (three in total, each up to 1 h, 12 participants), summarised in Table 2. At the intervention site, there was no significant difference in comorbidities, age or CFS score. However, all of the drop-outs were female participants (p=0.042). All of the ongoing users in the feedback sessions reported ease of use and that the technology was useful to them. By contrast, those who has dropped out reported difficulties with the technology, which they did not feel was of use. The videos (physiotherapy, yoga and Pilates) associated with the app were accessed 684 times by the 26 participants (range 0–64).
Table 2.
Reasons for dropouts from the study and focus group feedback
| Reason for drop-outs | Focus group feedback |
|---|---|
| ‘[I] struggled to use watch and laptop’ ‘[I] found it all confusing’ ‘Watch not working intermittently’ ‘Finding all the technology overwhelming’ ‘Felt stressed – I would be happy to look at videos in a few weeks, not at the moment. [I'm] very happy to continue receiving phone calls and completing questions’ ‘I'm doing okay & I'm not interested in the tech as [I'm] getting on with own physio’ |
‘I found a lot of the paper resources overwhelming when I first went home, so just used the videos’ ‘I used the videos every day and found it really useful’ ‘Having the option of different levels [of exercise] made me feel like I was progressing’ ‘I never imagined having one of these watches, but I'm addicted to it now!’ ‘Having different videos was nice to have some variation in what exercise I did’ ‘I always did the exercises in the morning as it gave me the confidence to get up and about’ ‘I was expecting to get back to normal quicker, but having something to work on helped me keep my motivation’ ‘I never got any input from community teams, so I was very reliant on the videos’ ‘I didn't realise how hard I would find going home, I didn't know if I was progressing like I should’ ‘I've had two previous hip operations and my recovery and pain was much better this time’ |
Step count data
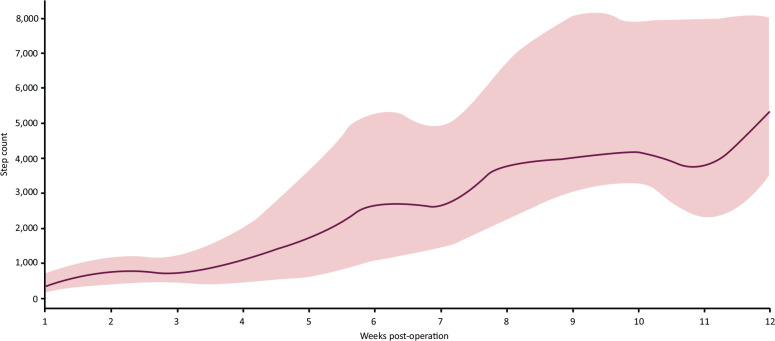
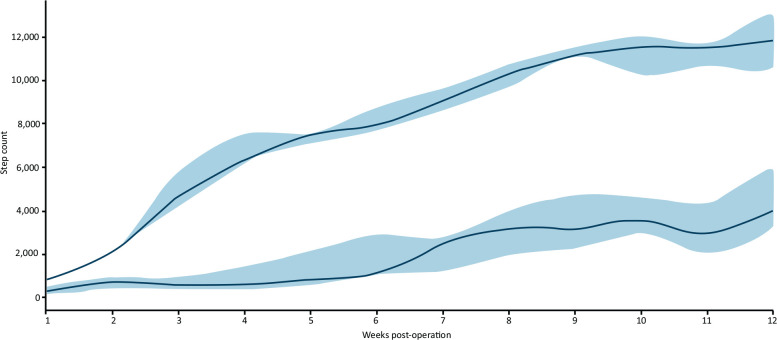
Overall median daily step counts increased significantly from 477 (320–697) in hospital, to 931 (505–1238) 1 week post discharge, to 5,352 (3,552–7,944) at 12 weeks (p=0.001) (Fig 2). Those managing >2,000 steps at 2 weeks had a significantly higher step count at 12 weeks (p=0.013) (Fig 3).
Fig 2.
Step count data of intervention cohort. Red line indicates median (interquartile range) of step counts up to week 12 post-hip fracture operation.
Fig 3.
Comparison of participants with step counts at 2 weeks of more or less than 2000 steps. Blue line indicates >2000 steps at 2 weeks; green line indicates <2000 steps. Data shown are median (interquartile range).
Discussion
To our knowledge, this is the first study to report on the feasibility of using smartwatch technology in rehabilitation following a hip fracture. This study demonstrated the feasibility of technology to support older adults on their transition from hospital to home. The intervention enabled presentation of activity data remotely to the clinical team to inform rehabilitation. Many healthcare systems have human resources challenges that integrated digital technology could supplement. Older adults were recruited in an acute care setting during the peak of the second COVID-19 wave to affect the UK. In these challenging conditions, 54% of recruited participants continued the intervention to 6 months. Reasons for dropout included participants struggling to use the technology and not coping with their overall situation, following a significant medical problem. Of interest, all of the participants who dropped out were female. This warrants further exploration in a future study. Promising early data on hospital readmissions, in particular on falls and fractures, suggests these could be relevant outcome measures for a future RCT.
The higher number of readmissions at the control site (11 of the 40 participants, accumulating 21 episodes) could be explained, in part, by recruitment of an older cohort. However, this further emphasises the significant issues faced by this population following the index admission. Step count data provided objective evidence of increasing activity from hospital to home. Patients less active at 2 weeks post discharge (achieving >2,000 steps/day) remained significantly less active at 12 weeks than those who were active at 2 weeks. This detailed information could be used by clinical teams when planning further rehabilitation interventions. The focus groups provided further understanding of the challenges faced by the participants and their experience of the technology. Several themes supported further exploration of the technology in this cohort, including regular use of the videos and views from people who had not previously used such technology. Second, having the option of different levels of exercise encouraged progress. Third, the group reported how doing the regular exercises gave them the confidence to increment their activities and keep themselves motivated in the context of often little formal community support.
Comparison with existing literature
Previous studies have explored device accuracy,19 used accelerometers to define upright time,18 physical activity in hospital,21,22 and electronic delivery of physiotherapy information.33 In a UK observational study Armitage et al (2020), recruited 29 participants (from 125 eligible patients) and used a accelerometer.23 Withdrawals were similar to the presented study (48%, n=14). As with the study by Armitage et al, reasons for withdrawal in our study included participation burden, in the context of a significant medical problem. However, a second theme our participants reported was struggling to use, or fix, the technology, when issues occurred, which is a factor in Davis' technology acceptance model.24
Strengths and limitations
This feasibility study reports significant new knowledge around supporting with technology the journey of patients with hip fracture from hospital to home. Recruiting patients contemporaneously from a site in the same region allowed for comparisons, including subsequent readmissions and accessing of community physiotherapy sessions. The study demonstrated feasibility of setting up older people with smartwatches. This provided both the participant and clinical team with feedback during their recovery following surgery. The study excluded the large proportion of the hip fracture population who have concomitant cognitive impairment, which limits generalisability. The median age of participants at the intervention site (75 (72–77)) was also lower than that at the control site and the median age of European hip fracture populations (82 (75–87)).34 A multi-site RCT would have provided more robust findings, but the resources to deliver this during the pandemic were not available. Although the study initially aimed to recruit 25–50 participants, with the large surge of the COVID second wave placing unprecedented pressures on frontline staff, we limited recruitment numbers, although these were similar to previous feasibility work.23 Despite these challenges, recruitment and feasibility measures were met, suggesting that such work is feasible outside the pandemic.
Future directions
Digital healthcare is growing and an increasing proportion of older adults have internet access with devices including tablets, smartphones and/or a smartwatches.35,36 Use of such devices could be further explored in adequately powered RCTs to assess clinical and economic efficacy. The pandemic attenuated access to in-person healthcare, while activity levels reduced in older adults.37,38 Digital solutions offer benefits improving self-management, saving time and costs. However, digital exclusion must be addressed. Older people are still less likely to have access to, or use, the internet,35 and intervention-generated inequalities through digital exclusion could disadvantage older people.39 Acceptability of the intervention for staff and participants is the focus of ongoing qualitative work from our group that will add further in-depth information to this presented work.
Conclusions
In this feasibility study, a smartwatch and app supported remote rehabilitation pathway-enabled presentation of activity data to the clinical team. To our knowledge, this is the first to recruit patients following hip fracture to a pathway in hospital and to continue their rehabilitation remotely via support through technology. The contemporaneous control cohort had a high burden of hospital readmissions, which emphasises the importance of exploring ways to optimise the recovery of this population. A significant proportion of participants did not complete the intervention, which must be addressed when considering embarking on further study. Future work could explore the extent to which technology can help improve clinical outcomes and to which populations this is applicable.
Funding
The smartwatches used in this study were donated to the Research Department of University Hospitals Sussex by the League of Friends charity; an Individual development award from NIHR KSS ARC and Innovate UK supported delivery of the study.
Supplementary material
Additional supplementary material may be found in the online version of this article at www.rcpjournals.org/content/futurehosp. S1. Consort Checklist.
Conflicts of interest
SF, founder of CUSH Health©, had no role in the analysis of the results.
References
- 1.Johnell O, Kanis J. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 2006;17:1726–33. [DOI] [PubMed] [Google Scholar]
- 2.Royal College of Physicians . National Hip Fracture Database Annual Report 2019. London: RCP, 2019. [Google Scholar]
- 3.White S, Griffiths R. Projected incidence of proximal femoral fracture in England: a report from the NHS Hip Fracture Anaesthesia Network (HIPFAN). Injury 2011;42:1230–33. [DOI] [PubMed] [Google Scholar]
- 4.Klop C, Welsing PM, Cooper C, et al. Mortality in British hip fracture patients, 2000-2010: a population-based retrospective cohort study. Bone 2014;66:171–7. [DOI] [PubMed] [Google Scholar]
- 5.Bergh C, Möller M, Ekelund J, et al. 30-day and 1-year mortality after skeletal fractures: a register study of 295,713 fractures at different locations. Acta Orthop 2021;92:739–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dyer SM, Crotty M, Fairhall N, et al. A critical review of the long-term disability outcomes following hip fracture. BMC Geriatr 2016;16:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magaziner J, Hawkes W, Hebel JR, et al. Recovery from hip fracture in eight areas of function. J Gerontol A Biol Sci Med Sci 2000;55:M498–507. [DOI] [PubMed] [Google Scholar]
- 8.RCP . Recovering after a hip fracture: helping people understand physiotherapy in the NHS. London: RCP, 2017. [Google Scholar]
- 9.Chen B, Hu N, Tan J-H. Efficacy of home-based exercise programme on physical function after hip fracture: a systematic review and meta-analysis of randomised controlled trials. Int Wound J 2020;17:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez MA, Arnel L, Gould J, et al. Research priorities in fragility fractures of the lower limb and pelvis: a UK priority setting partnership with the James Lind alliance. BMJ Open 2018;8:e023301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kraaijkamp JJM, van Dam van Isselt EF, Persoon A, et al. eHealth in geriatric rehabilitation: systematic review of effectiveness, feasibility, and usability. J Med Internet Res 2021;23:e24015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDonough CC. The effect of ageism on the digital divide among older adults. J Gerontol Geriatr Med 2016;2:008. [Google Scholar]
- 13.Mannheim I, Schwartz E, Xi W, et al. Inclusion of older adults in the research and design of digital technology. Int J Environ Res Public Health 2019;16:3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Handoll HH, Sherrington C, Mak JC. Interventions for improving mobility after hip fracture surgery in adults. Cochrane Database Syst Rev 2011;2011:CD001704. [DOI] [PubMed] [Google Scholar]
- 15.Jansen C-P, Klenk J, Nerz C, et al. Association between everyday walking activity, objective and perceived risk of falling in older adults. Age Ageing 2021;50:1586–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sprogis SK, Currey J, Considine J. Patient acceptability of wearable vital sign monitoring technologies in the acute care setting: a systematic review. J Clin Nurs 2019;28:2732–44. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Yang M, Ge Y, et al. The role of digital health for post-surgery care of older patients with hip fracture: a scoping review. Int J Med Inform 2022;160:104709. [DOI] [PubMed] [Google Scholar]
- 18.Taraldsen K, Sletvold O, Thingstad P, et al. Physical behavior and function early after hip fracture surgery in patients receiving comprehensive geriatric care or orthopedic care—a randomized controlled trial. J Gerontol Series A Biol Sci Med Sci 2013;69A:338–45. [DOI] [PubMed] [Google Scholar]
- 19.Schmal H, Holsgaard-Larsen A, Izadpanah K, et al. Validation of activity tracking procedures in elderly patients after operative treatment of proximal femur fractures. Rehabil Res Pract 2018;2018:3521271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pol MC, ter Riet G, van Hartingsveldt M, et al. Effectiveness of sensor monitoring in a rehabilitation programme for older patients after hip fracture: a three-arm stepped wedge randomised trial. Age Ageing 2019;48:650–57. [DOI] [PubMed] [Google Scholar]
- 21.Benzinger P, Lindemann U, Becker C, et al. Geriatric rehabilitation after hip fracture. Z Gerontol Geriatr 2014;47:236–42. [DOI] [PubMed] [Google Scholar]
- 22.Willems E, Visschedijk J, Balen R, et al. Physical activity, physical function and fear of falling after hip fracture. J Orthop Res Physiother 2017;3:031. [Google Scholar]
- 23.Armitage LC, Chi Y, Santos M, et al. Monitoring activity of hip injury patients (MoHIP): a sub-study of the World Hip Trauma Evaluation observational cohort study. Pilot Feasibility Stud 2020;6:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis FD. A technology acceptance model for empirically testing new end-user information systems. Cambridge, MA; MIT: 1986. [Google Scholar]
- 25.Hodkinson HM. Evaluation of a mental test score for assessment of mental impairment in the elderly. Age Ageing 1972;1:23338. [DOI] [PubMed] [Google Scholar]
- 26.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005;173:489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maxwell M, Moran C, Moppett I. Development and validation of a preoperative scoring system to predict 30 day mortality in patients undergoing hip fracture surgery. Br J Anaesth 2008;101:511–17. [DOI] [PubMed] [Google Scholar]
- 28.Moore CG, Carter RE, Nietert PJ, et al. Recommendations for planning pilot studies in clinical and translational research. Clin Transl Sci 2011;4:332–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haywood KL, Griffin XL, Achten J, et al. Developing a core outcome set for hip fracture trials. Bone Joint J 2014;96-B:1016–23. [DOI] [PubMed] [Google Scholar]
- 30.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Washburn RA, Smith KW, Jette AM, et al. The physical activity scale for the elderly (PASE): Development and evaluation. J Clin Epidemiol 1993;46:15362. [DOI] [PubMed] [Google Scholar]
- 32.Teare MD, Dimairo M, Shephard N, et al. Sample size requirements to estimate key design parameters from external pilot randomised controlled trials: a simulation study. Trials 2014;15:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dallimore RK, Asinas-Tan ML, Chan D, et al. A randomised, double-blinded clinical study on the efficacy of multimedia presentation using an iPad for patient education of postoperative hip surgery patients in a public hospital in Singapore. Singapore Med J 2017;58:562–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baker PN, Salar O, Ollivere BJ, et al. Evolution of the hip fracture population: time to consider the future? A retrospective observational analysis. BMJ Open 2014;4:e004405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ofcom . Adults' Media Use & Attitudes report. London; Ofcom: 2020. [Google Scholar]
- 36.Age UK . Digital inclusion and older people – how have things changed in a Covid-19 world? London; Age UK: 2021. [Google Scholar]
- 37.PHE . Wider Impacts of COVID-19 on Physical Activity, Deconditioning and Falls in Older Adults. London; PHE: 2021. [Google Scholar]
- 38.Strain T, Sharp SJ, Spiers A, et al. Population level physical activity before and during the first national COVID-19 lockdown: A nationally representative repeat cross-sectional study of 5 years of Active Lives data in England. Lancet Reg Health Eur 2022;12:100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White M, Adams J, Heywood P. How and why do interventions that increase health overall widen inequalities within populations. Soc Inequality Public Health. 2009;65:82. [Google Scholar]