Abstract
A simple method for complete genome radiolabelling is described, involving long-wave UV exposure of agarose-embedded chromosomal DNA and [α-32P]dCTP incorporation mediated by the Klenow fragment. Experiments on the budding yeast genome show that the labelling procedure can be coupled with two new two-dimensional pulsed field gel electrophoresis (2D-PFGE) protocols of genome analysis: (i) the KARD (karyotype and restriction display)-PFGE which provides a complete view of the fragments resulting from a single restriction of the whole genome and (ii) the DDIC (double digestion of isolated chromosome)-PFGE which is the eukaryotic counterpart of complete/complete 2D-PFGE in bacterial genomics.
INTRODUCTION
Pulsed-field gel electrophoresis (PFGE) techniques are particularly useful for separating chromosome-sized DNA molecules of small eukaryotic genomes, as first demonstrated for the 13.5-Mb nuclear genome of the yeast Saccharomyces cerevisiae (1,2). In addition, the development of two-dimensional (2D)-PFGE procedures combined with in-gel restriction rapidly led to the construction of a physical map of the circular chromosome in bacterial species such as Mycoplasma mobile (3), Pseudomonas aeruginosa PAO (4) or Anabaena sp. (5). The methodology involved two successive steps of PFGE separation of DNA fragments arising from either partial then complete digestion of the prokaryotic genome by a restriction endonuclease (‘partial/complete 2D-PFGE’), or complete digestions by two different enzymes (‘complete/complete 2D-PFGE’). However, 2D-PFGE protocols suitable for studying the physical organisation of the more complex nuclear genome in eukaryotic organisms have been rarely reported. The genome of several unicellular parasites including the malaria parasite Plasmodium falciparum has been claimed to be analysable through chromosome fractionation in the first dimension followed by in-gel restriction enzyme digestion then separation of resulting fragments in the second dimension (6). Used in combination with Southern-blot hybridisation, this 2D-PFGE procedure was successfully applied to the study of a large gene family dispersed on Plasmodium chromosomes. However, to our knowledge, its potential use for fingerprinting a whole nuclear genome through the resolution of all the restriction fragments produced by a rare cutter and the applicability of complete/complete 2D-PFGE to the mapping of eukaryotic linear chromosomes have not been assessed.
We were specifically interested by 2D-PFGE protocols for investigating chromosomal organisation and karyotype polymorphism in the intracellular eukaryotic parasite Encephalitozoon cuniculi of which the haploid genome size is <3 Mb (7,8). As this small genome consists of chromosomes within a size range amenable to PFGE separation and rarely cut by restriction enzymes with 8- or 6-bp recognition sites, the success of 2D-PFGE strategies was mainly dependent on DNA visualisation. In this paper, the genome of the yeast S.cerevisiae has been used as a model to develop an efficient in-gel procedure for radioactive labelling of chromosomal DNA, which is compatible with restriction and 2D-PFGE analyses. The expected yeast restriction fragments were separated after either digestion of all chromosomes by a single restriction enzyme or digestion of individual chromosomes by two different restriction enzymes. It is hoped that the two proposed 2D-PFGE methods will facilitate investigations of intra- and inter-species genomic variability in the highly diversified lineages of unicellular eukaryotes.
MATERIALS AND METHODS
UV nicking of agarose-embedded DNA and in-gel enzymatic reaction
Saccharomyces cerevisiae strain YPH80 embedded in 1% low-melting point agarose was supplied in a GelSyringe™ dispenser (New England Biolabs, Beverly, MA) with DNA at 3 mg.ml–1. For UV nicking, agarose slices containing chromosomal DNA were exposed over a UV transilluminator (312 nm, 8000 µW.cm–2, Bioblock Scientific) for various times (from 5 to 150 s). In-gel enzymatic reactions were performed after six 15-min washings with High-salt Restriction (HR) buffer (10 mM Tris–HCl pH 7.9 at 25°C, 100 mM NaCl, 10 mM MgCl2, 1 mM DTT and 20 µg.ml–1 BSA) at room temperature. Five µCi of [α-32P]dCTP (Amersham, Saclay, France) were added to either 400 µl (for labelling the whole genome before fractionation or individual chromosomes) or 3–4 ml (for labelling a lane spanning the electrophoretic karyotype) of HR buffer including 16 U of E.coli DNA polymerase I Klenow fragment (USB, Cleveland, OH). The reaction was done at 30°C for 3 h. For restriction enzyme digestion, gel slices were washed six times for 15 min in TE buffer (10 mM Tris–HCl pH 7.4, 1 mM EDTA) and digestion was performed overnight at 37°C in HR buffer, using either 80 U of MluI or 50 U of BssHII (Pharmacia Biotech Europe, Saclay, France).
PFGE conditions
PFGE of CHEF (contour-clamped homogeneous electric field) type was performed with the Gene Navigator™ system (Pharmacia Biotech Europe) and 1.2% agarose (Seakem GTG, FMC Bioproducts, Rockland, ME) gels (15 × 15 cm). Saccharomyces cerevisiae YPH80 molecular karyotype was resolved after a 24-h run at 12°C in 0.5× TBE, using 190 V and pulses of 90 s. These conditions were maintained for the first dimension of KARD (karyotype and restriction display)-PFGE. Separation of restriction fragments in second-dimension KARD-PFGE or first-dimension DDIC (double digestion of isolated chromosome)-PFGE step was performed at 8°C, 230 V, with 2-s pulses for 6 h then 10-s pulses for 10 h, in 0.5× TBE. Second-dimension DDIC-PFGE conditions were 6 h at 8°C, 450 V, 0.45-s pulses, 0.15× TBE.
Staining, drying, blotting and autoradiography of electrophoresis gels
After electrophoresis, and when necessary, gels were stained in a 0.5 µg.ml–1 ethidium bromide solution and photographed on a UV transilluminator. For autoradiography, gels were either dried or blotted. Drying was performed by putting the gel on two pieces of Whatmann“ 3MM filter paper for 1.5 h in a Gel Slab Dryer (Model 224, Bio-Rad, Ivry, France). Before transfer, DNA was depurinated (20 min in 0.25 M HCl), denaturated (0.4 M NaOH, 0.8 M NaCl) and neutralised (0.5 M Tris–HCl pH 7.4, 0.8 M NaCl). Standard Southern blotting was done on Hybond-N+ nylon membrane (Amersham) using 5× SSC (9) and followed by UV crosslinking of transferred DNA. Autoradiographs were obtained with Biomax™ MS film (Kodak, Rochester, NY), after a 2-h to 2-day exposure between two intensifying screens at – 80°C.
RESULTS
In-gel radiolabelling of large DNA molecules
The frequently used fluorescent dye ethidium bromide can detect ~10 ng/band. The visualisation of a 2-kb restriction fragment from a 20-Mb genome should therefore require a DNA load of 10 ng × (20 000/2) = 100 µg, which is beyond the amounts leading to well resolved bands in usual agarose matrices. To overcome this problem, we developed a labelling protocol based on the incorporation of [α-32P]dCTP using the Klenow fragment of the E.coli DNA polymerase I. As the Klenow fragment retains a significant activity in basic restriction buffers, the selected buffer was the HR buffer known to be compatible with BssHII and MluI enzymes used in further PFGE experiments. When applied to large molecules, the labelling procedure must be performed on DNA embedded in agarose gel. The UV light was tested as a single-strand nicking agent, because it was relatively easier to set up than a procedure involving DNase I.
Thin slices of a Yeast Chromosome PFGE Marker (S.cerevisiae YPH80) were exposed to UV light at 312 nm for different times on a transilluminator and the labelling was carried out in HR buffer supplemented with [α-32P]dCTP. Chromosomes were CHEF-fractionated. After staining with ethidium bromide, gels were examined under UV and vacuum-dried for subsequent autoradiography. Autoradiographs show clearly that the radioactivity incorporation rate has increased with UV exposure time (data not shown). This indicates that long-wave UV irradiation of chromosomal DNA is favourable to a nucleotide exchange reaction mediated by the Klenow fragment. No significant DNA degradation occurred up to 60-s UV irradiation.
Whole genome typing by KARD-PFGE
Conceivably, a genomic fingerprint can be achieved by means of a 2D technique involving the resolution of the molecular karyotype in the first dimension (Fig. 1A). After excision of an agarose lane containing the different chromosomes and incubation with a restriction enzyme, the second-dimension electrophoretic step will ensure the separation of restriction fragments. The two coordinates of the final pattern are therefore the chromosome size (or number) and restriction fragment size. To distinguish this procedure from those based on the 2D-analysis of two successive restriction digests, we propose the designation ‘karyotype and restriction display, 2D-PFGE’. The acronym KARD-PFGE is suggestive of the obtaining of a genomic ‘identity card’.
Figure 1.
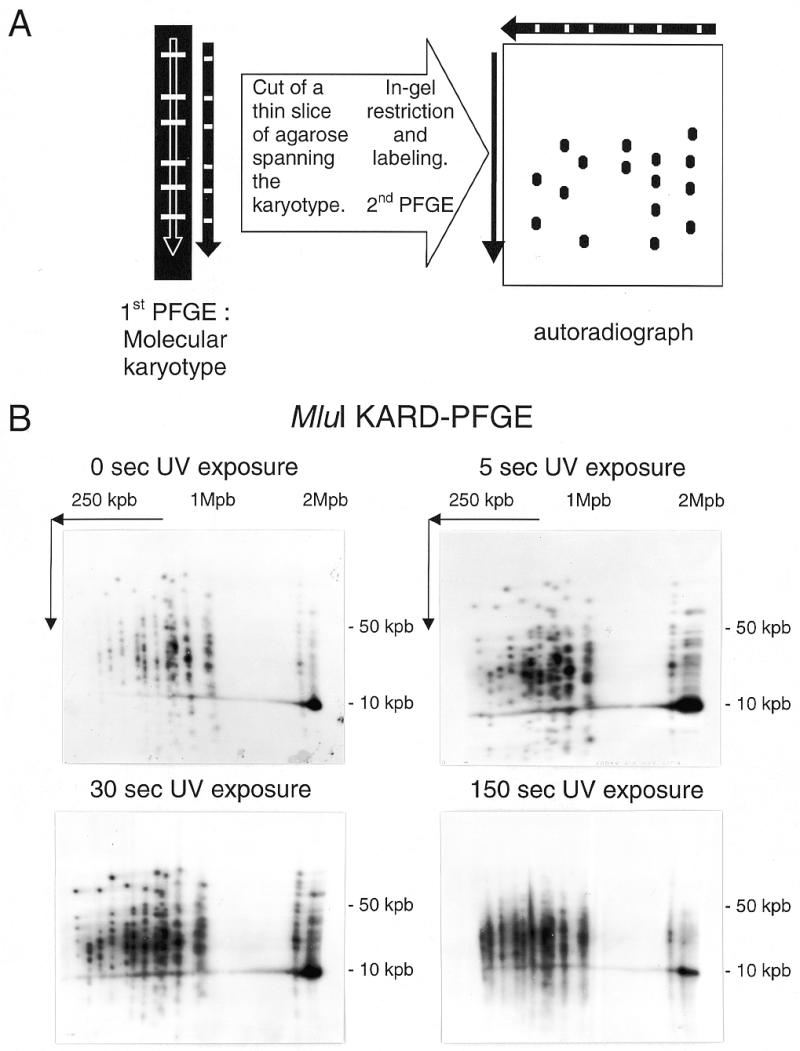
KARD-PFGE. (A) Principle of the procedure. A complete view of the DNA fragments resulting from a single in-gel restriction is obtained for every chromosome. (B) Effect of UV exposure time on the MluI-KARD-PFGE pattern of the S.cerevisiae YPH80 genome.
The KARD-PFGE method coupled with DNA radiolabelling was tested with the yeast genome. The lanes of separated chromosomes were blindly cut then exposed to long-wave UV for different times. The DNA of each slice was digested overnight with the endonuclease MluI and radiolabelling was done as described above, a few hours before the second PFGE. Examples of patterns of labelled restriction fragments are presented in Figure 1B. The MluI-KARD-PFGE autoradiograph corresponding to 5-s UV exposure gives a good picture of the restricted genome. About 150 fragments can be differentiated, their length ranging from <2 kb to >100 kb. For chromosome I, the sum of the estimated sizes of seven individual fragments (3, 9, 23, 31, 41, 46 and 72 kb) attains 226 kb. This fits with the chromosome size and thus confirms the achievement of complete digestion. In addition, most fragments are similar to those expected from the fully sequenced chromosome I in the reference S.cerevisiae strain (sequences available at the Stanford University website, http://genome-www.stanford.edu/Saccharomyces ). A very intense spot migrated at 9 kb in the lane of the largest chromosome (XII) is at the origin of a horizontal smear. This reflects numerous MluI fragments of identical size which likely derive from tandemly arranged rDNA units in the intact chromosome XII. Accordingly, a MluI site can be found within each rDNA unit of the reference strain and the interval between two MluI sites attains 9137 bp. The global restriction pattern was not significantly changed up to 30-s UV exposure. In contrast, as illustrated by the autoradiograph obtained for 150-s UV exposure, smearing related to chromosomal degradation is evident when UV irradiation lasts >1 min.
The restriction enzyme BssHII was also tested. The BssHII-KARD-PFGE autoradiograph for 5-s UV exposure provides a new fingerprint of the yeast genome (Fig. 2). Thus, it seems reasonable to think that the KARD-PFGE procedure can be used with any restriction enzyme able to work in agarose gel with HR buffer. Capillary neutral transfer on nylon membranes was done overnight. As shown in Figure 2, the relative intensities of the spots in the autoradiograph of the blot are similar to those in the autoradiograph of the dried gel, even for fragments encompassing 100 kb.
Figure 2.
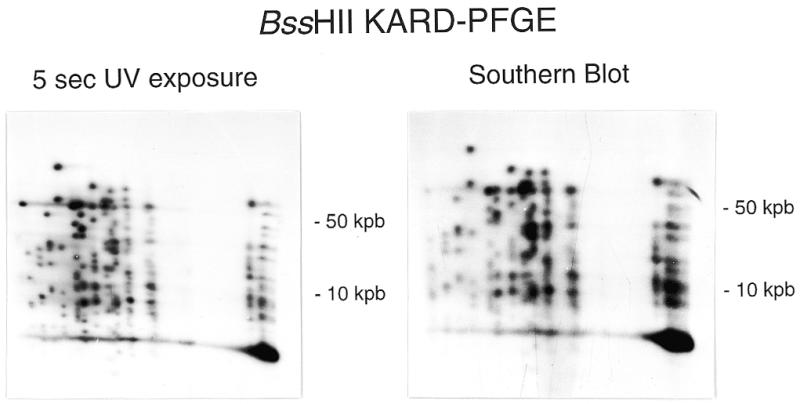
BssHII-KARD-PFGE pattern of the S.cerevisiae YPH80 genome after a short-duration UV irradiation. Comparison of autoradiographs obtained after gel drying (left) or Southern blotting (right).
Mapping of individual chromosome by DDIC-PFGE
As the nuclear genome comprises several chromosomes, a prerequisite to the application of a mapping procedure similar to complete/complete 2D-PFGE (10) is the isolation of each chromosome. This can theoretically be achieved through a first PFGE leading to the molecular karyotype followed by the excision of individual chromosomal bands. The whole procedure therefore implies three electrophoretic steps (Fig. 3A). As the two steps of 2D-PFGE correspond to the separation of restriction fragments yielded by the double digestion of an isolated chromosome, the abbreviation DDIC-PFGE is proposed.
Figure 3.
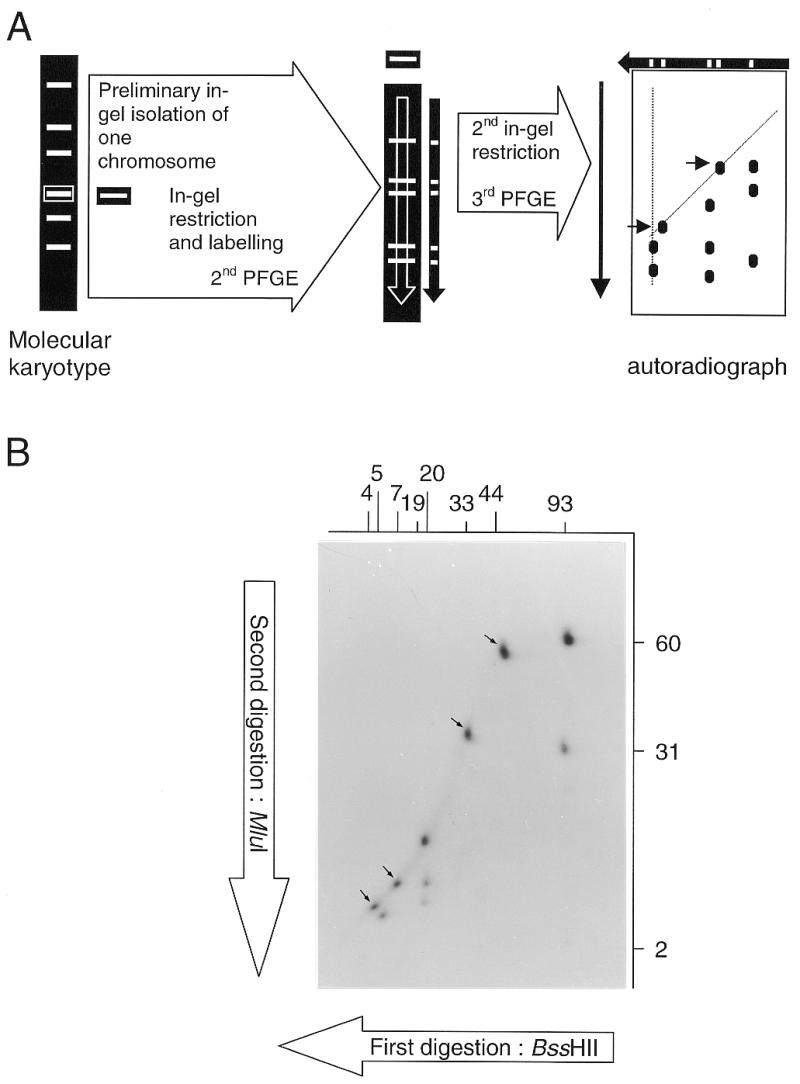
DDIC-PFGE. (A) Principle of the procedure. Separated DNA fragments arise from two successive in-gel restrictions of an individual chromosome. (B) Autoradiograph obtained after BssHII–MluI DDIC-PFGE of the chromosome I of S.cerevisiae YPH80. The first enzyme (BssHII) gives fragments (sizes indicated in kb above the autoradiograph) as determined by KARD-PFGE (see Fig. 2). The second enzyme (MluI) generates new fragments of which the sizes are indicated in kb at the right of the autoradiograph. Arrows show spots corresponding to fragments devoid of any restriction site for the second enzyme.
Increasing DNA losses through the successive manipulations and difficulties for visualising the smallest fragments were predictable. Radiolabelling of UV-irradiated DNA was tested again. After usual ethidium-bromide staining of the gel for molecular karyotype, agarose slices containing individual chromosomal bands were cut quickly over the UV-transilluminator. Chromosomal DNA was radioactively labelled using the Klenow fragment and overnight digested with the first restriction enzyme. The fragments were separated in the first dimension under conditions identical to the second dimension of KARD-PFGE. An agarose lane containing the restriction profile was blindly cut, overnight digested with the second enzyme, then subjected to the second dimension step. Autoradiography was performed after gel drying. Figure 3B illustrates a typical DDIC-PFGE pattern obtained for the chromosome I of S.cerevisiae YPH80, digested first with BssHII then with MluI, hence the designation ‘BssHII–MluI DDIC-PFGE’. The eight BssHII fragments previously identified by KARD-PFGE can be recognised along eight vertical axes, for which n fragments are expected to correspond to n – 1 MluI sites per BssHII fragment. The principle of the analysis is well described by Bautsch (11) and requires the complementary restriction separation for a full mapping (MluI–BssHII DDIC-PFGE in the present case). For example, the vertical axis corresponding to the 93-kb BssHII fragment comprises three MluI restriction fragments (2, 31 and 60 kb). The 93-kb BssHII fragment therefore contains two MluI sites. As the 2-kb MluI fragment is also found after MluI KARD-PFGE, it must belong to the 93-kb BssHII fragment. One MluI site must be located 31 kb from the first BssHII site. The second MluI site must be 2 kb further, i.e. 60 kb from the other BssHII site.
DISCUSSION
The method here described for labelling large DNA molecules such as yeast chromosomes has been shown to be useful for detecting restriction fragments on 2D-PFGE gels. The reaction mediated by the Klenow fragment occurs in the presence of a single radioactive nucleotide and a buffer compatible with restriction enzymes. The low incorporation level observed in the absence of UV treatment may reflect nucleotide replacement at the chromosomal ends. The increased incorporation during UV exposure is suggestive of the creation of new sites favourable to an exchange reaction. Some single-strand DNA breaks are also assumed to be induced during electrophoresis. Agarose-embedded genomic DNA may be exposed to long-wave UV for up to 30 s without entailing perceptible alterations. The major advantage of the procedure lies in its sensitivity compared to ethidium bromide staining. Conceivably, the smaller DNA amount required for molecular karyotyping should facilitate genomic studies of eukaryotic cells for which culture procedures are lacking or very expensive.
MluI and BssHII fragments from the Saccharomyces genome have been visualised after separation by a two-step electrophoresis procedure. All the restriction fragments derived from each chromosome can be viewed on a single KARD-PFGE autoradiograph. Thus, a KARD-PFGE fingerprint should be obtainable from any eukaryotic organism harbouring a relatively small genome of which the different chromosomes can be separated by PFGE, especially from various protists and fungi. Some DNA macro-modifications (insertion/deletion/translocation) entailing variations in fragment size and number can be easily allocated to individual chromosomes. In E.cuniculi, two co-migrating heterologous chromosomes as well as two homologous chromosomes differing in size have been clearly distinguished using KARD-PFGE (12). Various restriction enzymes may be assessed to increase the discrimination power and therefore characterise more rapidly the variable regions of a genome. The typing and mapping of genomic regions could be performed through the combined use of KARD-PFGE and hybridisation. We have ascertained that DNA fragments resolved by KARD-PFGE are efficiently transferred onto nylon membranes after conventional treatments.
Similar to complete/complete 2D-PFGE for mapping bacterial genomes (13), the DDIC-PFGE separation of restriction fragments combined with DNA radiolabelling and autoradiography is quite suitable for investigating small eukaryotic genomes. The excision of chromosomal bands from an ethidium bromide-stained molecular karyotype can be simply performed over a UV transilluminator. DDIC-PFGE may serve to construct a physical map of any PFGE-resolved eukaryotic chromosome as well as YAC clones. We have succeeded in localising 139 BssHII and MluI sites among the 11 chromosomes of the E.cuniculi genome (12). To get a better separation and size determination, ethidium bromide can be extracted before restriction (6), labelling and PFGE. A sensitive non-radioactive protocol such as silver staining (14) should deserve the attention for further testing its suitability for the detection of DNA fragments on KARD- and DDIC-PFGE gels.
Acknowledgments
ACKNOWLEDGEMENTS
The authors thank B. Chebance and R. Guerry for their technical assistance. J.-F.B. is very grateful to M. Barbazanges for his helpful support and efficient contribution in KARD-PFGE development.
REFERENCES
- 1.Schwartz D.C. and Cantor,C.R. (1984) Cell, 37, 67–75. [DOI] [PubMed] [Google Scholar]
- 2.Chu G., Vollrath,D. and Davis,R.W. (1986) Science, 234, 1582–1585. [DOI] [PubMed] [Google Scholar]
- 3.Bautsch W. (1988) Nucleic Acids Res., 16, 11461–11467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Römling U., Grothues,D., Bautsch,W. and Tümmler,B. (1989) EMBO J., 8, 4081–4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bancroft I., Wolk,C.P. and Oren,E.V. (1989) J. Bacteriol., 171, 5940–5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernandez-Rivas R. and Scherf,A. (1997) Mem. Inst. Oswaldo Cruz, 92, 815–819. [DOI] [PubMed] [Google Scholar]
- 7.Biderre C., Pages,M., Méténier,G., Canning,E.U. and Vivares,C.P. (1995) Mol. Biochem. Parasitol., 74, 229–231. [DOI] [PubMed] [Google Scholar]
- 8.Biderre C., Mathis,A., Deplazes,P., Weber,R., Méténier,G. and Vivares,C.P. (1999) Parasitology, 118, 439–445. [DOI] [PubMed] [Google Scholar]
- 9.Sambrook J., Fritsch,E.F. and Maniatis,T. (1989) Molecular Cloning: A Laboratory Manual, 2nd Edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 10.Römling U., Grothues,D., Heuer,T. and Tümmler,B. (1992) Electrophoresis, 13, 626–631. [DOI] [PubMed] [Google Scholar]
- 11.Bautsch W. (1994) Mol. Biotechnol., 2, 29–44. [DOI] [PubMed] [Google Scholar]
- 12.Brugère J.-F., Cornillot,E., Méténier,G., Bensimon,A. and Vivarès,C.P. (2000) Nucleic Acids Res., 28, 2026–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Römling U. and Tümmler,B. (1991) Nucleic Acids Res., 19, 3199–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prieto C.C., Leonardelli,R.I. and Zalazar,F.E. (1997) Anal. Biochem., 252, 15–18. [DOI] [PubMed] [Google Scholar]


