Abstract
Emerging evidence indicates the association between an unhealthy gut and chronic diseases. A healthy gut comprises an intact gut epithelium and balanced gut microbes. Diet is one of the critical factors that modulate gut health by positively or negatively affecting the intestinal barrier and gut microbes. Blueberries are an excellent source of health-promoting bioactive components, and this systematic review was conducted to evaluate the effect of dietary blueberries on gut health. A literature search was conducted on PubMed/MEDLINE, Scopus, Web of Science, and Embase databases to review relevant studies published between 2011–2022 according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The Systematic Review Centre for Laboratory Animal Experimentation’s (SYRCLE) risk-of-bias tool was used for methodological quality assessments. Sixteen studies included from four countries were reviewed and the results were synthesized narratively. Our data analysis indicates that blueberry supplementation improves gut health by improving intestinal morphology, reducing gut permeability, suppressing oxidative stress, ameliorating gut inflammation, and modulating the composition and function of gut microbes. However, there are significant knowledge gaps in this field. These findings indicate that further studies are needed to establish the beneficial effects of blueberries on gut health.
Keywords: gut microbiota, intestinal barrier, gut inflammation, anthocyanins, oxidative stress, short chain fatty acids, gut morphology
1. Introduction
Emerging evidence indicates the importance of gut health in preventing several chronic diseases such as Alzheimer’s, cardiovascular disease, diabetes, inflammatory bowel disease, and colon cancer [1]. A healthy gut is characterized by an intact gut epithelium with a thick mucus layer [formed by tightly connected intestinal epithelial cells (IEC)] and balanced gut microbes [1, 2]. The intestinal barrier regulates barrier permeability (by tight-junctional proteins and formation of a mucus layer), acts as a defense barrier against foreign substances (bacteria, toxins, and allergens), and is a modulator of the intestinal immune and inflammatory response (through secretion of cytokines, chemokines, antimicrobial peptides, and mucins) [2]. Any disruption in these factors leads to reduced tight junctions, a thinner mucus layer, gut inflammation, and dysbiosis (a shift in gut microbial balance) that are reported in the pathogenesis of intestinal disorders [1, 2]. Hence, targeting gut health may be a potential strategy to prevent and/or treat many chronic diseases.
Diet is one of the critical factors capable of modulating gut health. Different food patterns are associated with physical and biochemical changes in the gut, including gut inflammation and permeability [3]. For example, consumption of a high-fat diet (HFD) changes the gut microbial composition that subsequently alters the production of gut microbial metabolites such as short-chain fatty acids (SCFA), lipopolysaccharide (LPS), and bile acids [4]. These changes affect satiety, food efficiency, lipid metabolism, intestinal permeability, and low-grade inflammation of the host leading to an increased risk of developing various chronic diseases [5]. But consuming fruits and vegetables beneficially modulates the gut microbes and reduces the risk of chronic diseases [4–6]. Blueberries contribute significantly to the human diet and are an excellent source of health-promoting bioactive components such as anthocyanins. Anthocyanins have been widely reported for their benefits against endotoxemia, inflammation, and obesity through modulating the intestinal microbiota [7, 8].
Anthocyanins are glycosidic compounds made up of a glycan component (monosaccharide) and a non-glycan component (anthocyanidin) [9]. Cyanidin, malvidin, peonidin, and delphinidin are the major anthocyanidins found in blueberries [10]. The metabolism of anthocyanins in the gastrointestinal tract starts from the oral cavity [11]. Anthocyanins are partially hydrolyzed into aglycons in the oral cavity, a portion of anthocyanins is absorbed in the stomach (by specific transporters), and intact anthocyanins are absorbed in the small intestine (by the action of enzymes and transporters) [11]. Gut microbes play a critical role in the metabolism of anthocyanins in the large intestine [12]. Microbial glycosidases act on the glycosidic bond of anthocyanins to release the anthocyanidin from the carbohydrate component [13]. The gut microbiota uses these monosaccharides as an energy source, and the anthocyanins act as a prebiotic [14]. Additionally, microbial hydrolases act on the anthocyanidins to produce metabolites such as phenolic acids which are more easily absorbable [15]. Blueberry bioactive components are extensively metabolized by intestinal microbiota in humans suggesting that microbiota-induced metabolites might mediate the health beneficial effects of dietary blueberry [15]. Studies from our lab and others indicate that dietary blueberries impact gut health by improving gut dysbiosis [15, 16].
The present systematic review highlights the recent developments in our understanding of the effects of dietary blueberries on gut health with special emphasis on the current knowledge on the effect of dietary blueberries on intestinal morphology, gut permeability, oxidative stress, gut inflammation, and gut microbes. In addition, knowledge gaps and challenges in this field, and future direction will be discussed.
2. Methods
2.1. Study selection
Two research questions guided this systematic review: (1) “What is the impact of dietary blueberries on gut health in preclinical models?”. (2) “What are the possible molecular mechanisms involved in the beneficial effects of dietary blueberries on gut health?”. This review was developed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [17].
2.2. Eligibility criteria
The present review article was developed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines, which guide studies’ selection, screening, and eligibility. The participants, intervention comparators, outcomes, and study design (PICOS) criteria adopted in this study are shown in Table 1. Duplicate studies were excluded, and the search and screening for titles and abstracts were carried out by the authors, in pairs, according to the inclusion criteria (Table 1) [17].
Table 1.
PICOS criteria for study inclusion
| Parameter | Inclusion criteria |
|---|---|
| Model | Preclinical model (mice and rat) |
| Intervention | Blueberry supplemented diet |
| Comparison | Negative control (without the intervention) |
| Outcomes | Gut inflammation and oxidative stress, gut permeability, composition of gut microbes, short chain fatty acids and histological parameters of gut. |
| Study design | Experimental controlled studies |
2.3. Search strategy
Five authors (CMDL, ARC, KAD, LFM, and SMSP) independently searched for original articles using the following electronic databases: PubMed/MEDLINE (https://www.ncbi.nlm.nih.gov/pubmed), Scopus (https://www.scopus.com/home.uri), Web of Science (https://www.webofknowledge.com), and Embase (https://www.embase.com). The descriptors were structured based on search filters built for four domains: (i) blueberry, (ii) intestine, (iii) microbiota, and (iv) gut inflammation. The PubMed/MEDLINE platform filters were constructed using a hierarchical distribution of the MeSH terms (Medical Subject Headings) and by the algorithm TIAB (Title and Abstract). These filters were adapted for research in the Scopus platform, Web of Science, and Embase; however, the filter for the original article was provided by the Scopus platform (Supplementary Table S1). The search strategies were limited by the last ten years (2011 – 2022). Only articles published in English were considered in this review. The bibliographic search was performed on January 25th, 2023. The authors (CMDL, ARC, KAD, LFM, and SMSP) selected eligible studies following the analysis based on titles and abstracts. The level of agreement among these reviewers was assessed using kappa (0.925). The information was extracted independently and analyzed separately. Letters, reviews, book chapters, abstracts, unpublished articles, case studies, in vitro studies, human interventions, studies that focused on organs other than the intestine, studies conducted with other types of berries or other associate compounds (example: green tea), and studies that evaluated the use of blueberries on diseases (example: cancer, diabetes, colitis) were excluded. Selections were then compared, and differences were resolved in consultation with another reviewer (PVAB).
2.4. Data extraction and quality assessment
After selecting the studies, the data of the publications were extracted using standardized information such as an author’s name, year of publication, the country where the study was conducted, animal model (age, sex, number of animals per group), study protocol, follow-up, intervention characteristics (dosage and form of blueberries), and outcomes (primary and secondary). After the data extraction step, the researchers compared the data to ensure integrity and reliability.
2.5. Study risk-of-bias assessment
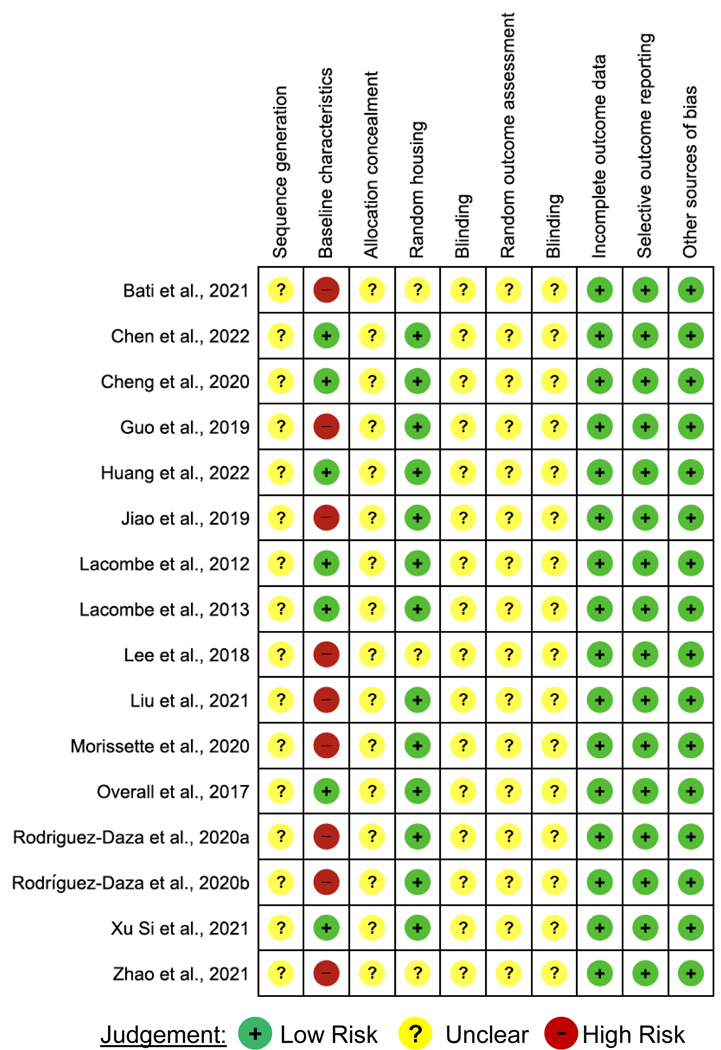
The methodological quality of the included studies was assessed. The risk of bias was verified using the Systematic Review Center for Laboratory Animal Experimentation Risk of Bias (SYRCLE RoB) tool, which helped to identify study quality and measure the bias in research involving animal studies [18]. The SYRCLE RoB toll considers ten entries that are related to six types of bias: selection bias, performance bias, detection bias, attrition bias, reporting bias, and others. For each included study, the six bias types were classified as “high” [+], “low” [−] or “unclear” [?] (Figure 2).
Fig. 2.
Risk of Bias Assessment Evaluated according to the SYstematic Review Centre for Laboratory Animal Experimentation (SYRCLE) Risk of Bias Tool.
3. Results
3.1. Study characteristics
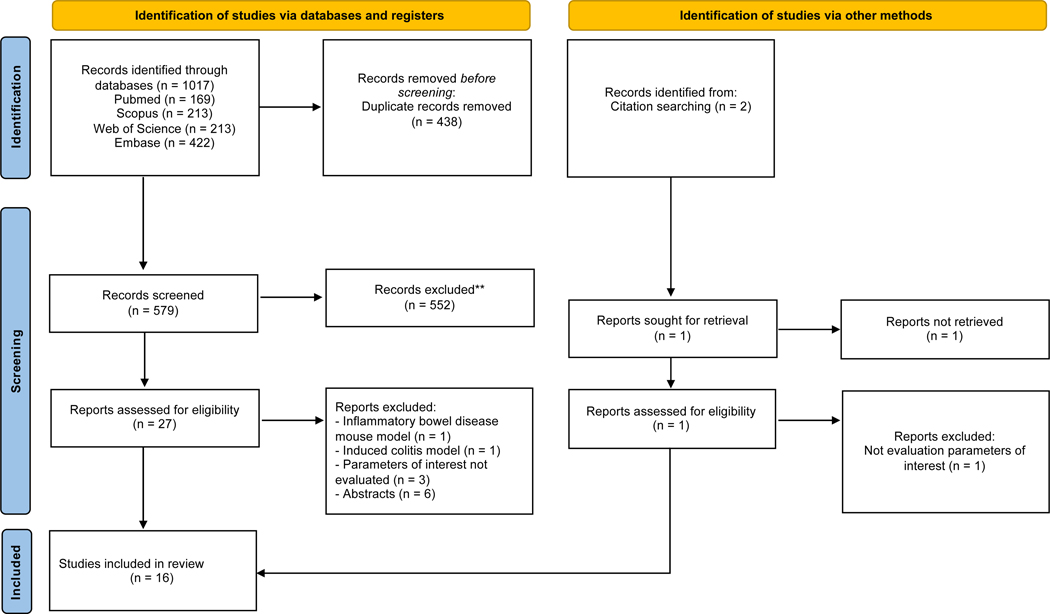
The flowchart with the number of selected and excluded articles in each stage was built according to the PRISMA guidelines (Figure 1). After searching PubMed, Scopus, Web of Science, and Embase, we have identified 1017 articles. We have posteriorly excluded 438 duplicates and then excluded 552 articles after reading the titles and abstracts. We also excluded studies that involve inflammatory mouse disease mouse model mdr1a−/− or induced colitis model (2 manuscripts), did not assess parameters of interest (3 manuscripts) and do not have full texts (6 manuscripts). We have performed the citation search to identify other relevant studies but none met the eligibility criteria. Finally, we have included 16 articles for this systematic review.
Fig. 1.
PRISMA Flowchart Depicting Study Search and Selection Process.
3.2. Description of included studies
The studies included in this article [n=16] were performed in four different countries that include China [n=7] [19–25], USA [n=4] [16, 26–28], Canada [n=3] [29–31], Ukraine [n=1] [32] and Sweden [n=1] [33]. Regarding the animal model used in the studies, 11 of them were performed with mice [19–23, 25, 28–31, 33] and 5 were performed with rats [16, 24, 26, 27, 32]. Most studies used male animals [n=14] [16, 19–33] and two used both male and female animals [24, 32]. The initial age of the animals ranged from 1 week to 24 months old, although one study did not mention this information [16]. The studies’ main characteristics were chronologically organized by the publication year, starting with the first published and shown in Table 2.
Table 2.
Characteristics of animal studies
| Country | Intervention Group | Control Group | Form of Blueberry | Blueberry dosage | Mode of Administration | Ref |
|---|---|---|---|---|---|---|
| USA | Diet AIN93 + LWB powder | AIN-93 | Powder | 8% w/w LWB | Blueberry supplemented diet | [26] |
| USA | Diet AIN93 + LWB powder | AIN-93 | Powder | 8% w/w LWB | Blueberry supplemented diet | [64] |
| USA | ○ LFD (10% fat) ○ HFD (60% fat) ○ Blackberry (BB) ○ Black currant (BC) ○ Black raspberry (BR) ○ Blueberry (BL) ○ Concord grape (CG) ○ Maqui berry (MB) |
LFD and HFD | Powder | 400 mg/g Total anthocyanins | Blueberry supplemented diet | [28] |
| USA | ○ Blue berry powder + LFD (10% fat) ○ Blueberry powder + HFD (45% fat) |
LFD and HFD | Powder | 10 g Freeze-dried blueberry powder/100 g. The blueberry powder was a Tifblue/Rubel 50/50 blend with 38.39 mg phenolics/g and 21.34 mg anthocyanins/g. Selection of 10% blueberry concentration was based on previously published studies | Blueberry supplemented diet | [16] |
| China | Blueberry polyphenol extract + HFD | HFD | Extract | 200 mg/kg | Blueberry in drinking water | [20] |
| China | ○ Study 1: Blueberry extract + HFD ○ Study 2: Blueberry extract + Control diet ○ Study 3: Blueberry extract + HFD and Blueberry extract + HFD + antibiotic |
Study 1: Control diet and HFD Study 2: Control diet Study 3: Control diet, HFD, HFD + antibiotic |
Extract | 5 gL-1 BE in drinking water (219.24mg of anthocyanin/g BE) | Blueberry in drinking water | [19] |
| Canada | ○ WBE + HFHS ○ Anthocyanins and phenolic of WBE + HFHS ○ PACs oligomers, phenolic acids and flavonols of WBE + HFHS ○ PACs polymers of WBE + HFHS |
CHOW and HFHS (65% fat) | Extract and fraction | WBE: 200 mg/kg – 17 mg polyphenols/day (1.68 mg/kg of anthocyanin) Anthocyanins and phenolic acids: 32 mg/kg PACs oligomers, phenolic acids and flavonols: 53 mg/kg PACs polymers: 37 mg/kg |
Oral gavage | [30] |
| China | ○ Fermented blueberry pomace (FBP) + Control diet ○ Fermented blueberry pomace (FBP) + HFD |
Control diet and HFD | Pomace | 4% or 8% (w/w) FBP-supplementation | Oral gavage | [21] |
| Canada | Blueberry powder (BB) + HFHS Blueberry anthocyanin-rich fraction (ANT) + HFHS Proanthocyanidin-rich fraction (PAC) + HFHS |
HFHS | Powder | 4% (w/w) | Blueberry supplemented diet | [29] |
| Canada | Blueberry powder or blueberry fibrous residue + HFHS | HFHS | Powder and fibrous residue | 200 mg/kg BW (1.94 mg/kg BW of anthocyanin) | Blueberry supplemented diet | [31] |
| China | Blueberry polyphenol extract + Standard diet | Standard diet | Extract | 200 mg/kg | Blueberry supplemented diet | [24] |
| China | Blueberry anthocyanin-rich extract + HFD | HFD | Extract | 100 and 200 mg/kg | Blueberry supplemented diet | [23] |
| Ukraine | Blueberry juice + Control group | Standard vivarium diet | Juice | 0.5mL/day | Oral gavage | [32] |
| China | Blueberry extract + HFD | HFD | Extract | 1% or 2% blueberry extract | Blueberry supplemented diet | [22] |
| China | ○ Blueberry (BLUB) ○ Black berry (BLAB) ○ Black goji berry (BGJB) Mulberry (MULB) ○ Red Chinese bayberry (RCBB) ○ Raspberry (RASB) ○ Red goji berry (RGJB) Strawberry (STRB) ○ White Chinese bayberry (WCBB) |
Distilled water | Extract | 50 mg/kg-1 | Oral gavage | [25] |
| Sweden | ○ Lingonberry + HFD ○ Bilberry + HFD ○ Cloudberry + HFD ○ Blackcurrant + HFD ○ Sea buckthorn + HFD ○ Blueberry + HFD |
LFD and HFD | Fine powder | 6% (w/w) on a dry weight basis | Rodent diet pellets | [33] |
BE: blueberry extract; BW: body weight; CHOW: standard chow diet; HFD: high fat diet; HFHS: high-fat high-sucrose diet; LFD: low fat diet; LWB: lowbush wild blueberry; PACs: oligomeric proanthocyanidins; WBE: blueberry extract.
3.3. Key findings on the impact of dietary blueberries on gut health
In this systematic review, the impact of dietary blueberries on gut health was assessed based on (i) intestinal morphology and permeability, (ii) intestinal inflammation and oxidative stress, and (iii) gut microbiota (microbial composition, short-chain fatty acids, and microbial metabolic pathways) which are summarized in Table 3.
Table 3.
Key findings on the effects of blueberries on gut health in preclinical models
| Animal Model | Study Protocol | Duration of Intervention | Effect of blueberries on gut health |
Ref | |
|---|---|---|---|---|---|
| Primary Outcome | Secondary Outcome | ||||
| Sprague-Dawley rats Male Age: 3 Weeks |
AIN-93 Diet (n=4) AIN93 Diet supplemented with 8% lowbush wild blueberry powder (w/w) (n=5) |
6 Weeks | ↑ Protein families involved with amino acid metabolism, 2,4-dienoyl-CoA reductase, metal ion binding, glutamate synthase, REDOX homeostasis, and aryl transferases. ↓ Protein families involved with integrase/recombinase, reverse transcriptase, and transposon/transposase. |
[26] | |
| Sprague-Dawley rats Male Age: 3 Weeks |
AIN-93 Diet (n=4) AIN93 Diet supplemented with 8% lowbush wild blueberry powder (w/w) (n=5) |
6 Weeks | ↑ Abundance of the phylum Actinobacteria, the order Actinomycetales and family Bifidobacteriaceae and Coriobacteriaceae, ORFs assigned to KEGG category xenobiotics biodegradation and metabolism. ↓ Abundance of the genera Lactobacillus and Enterococcus, number of ORFs assigned to the bacterial invasion of epithelial cells. |
[64] | |
| C57BL/6J mice Male Age: 6 Weeks |
Low fat diet (n=12) High fat diet (n=56) Obese mice were further randomized to control high fat diet (HFD, n=8) or berry-supplemented treatment groups normalized to 400 mg/g total anthocyanins. |
12 Weeks | ↑ Firmicutes, Bacteroidetes, Actinobacteria ↓ Anthocyanin content of the feces |
↑ Lean body and water mass, insulin sensitivity ↓ Body weight, fat body mass ↔ Food intake, blood glucose levels |
[28] |
| Wistar rats Male |
Low fat diet (LF) (n=8) High fat diet (HF) (n=8) High fat diet supplemented with 10% blueberry powder (w/w) (HF_BB) (n=8) |
8 Weeks | ↔ Abundance and ratio of the main phyla Firmicutes and Bacteriodetes (HF vs. LF), IL-1B (ileum), IL-6 (ileum), CD11d (ileum), CD6 (ileum) ↓ Firmicutes and Bacteriodetes abundance (HF_BB vs. HF and LF) ↑ Proteobacteria (HF_BB vs. HF and LF) ↑ Fusobacteria (HF_BB vs. HF and LF) ↑ Bacilli (class) (HF_BB vs. HF and LF) ↑ Porphyromonadaceae (family) (HF_BB vs. HF and LF) ↓ Butyrate (HF_BB vs. LF) ↓ TNF-A (ileum) (HF_BB and LF vs. HF) ↑ Acetate (HF_BB vs. HF and LF) ↑ Propionate (HF_BB vs. LF) ↑ Gpr43 and Defb2 gene expression (HF_BB vs. LF) ↑ Villus length (HF_BB and LF vs. HF) ↑ Goblet cells/crypt (HF_BB vs. HF) ↑ Muc2 gene expression (HF_BB vs. HF) ↑ Glp1 gene expression (LF and HF_BB vs. HF) |
↔ Body weight, mesenteric fat, epididymal fat, AUC, IL-6 (adipose tissue), CD68 (adipose tissue), phosphorylation of NF-κB p65 in adipose tissue ↑ Retroperitoneal fat (HF_BB vs. LF) ↑ Gene expression of PPARD (HF_BB vs. HF) ↑ Gene expression of PPARD (HF_BB and LF vs. HF) ↑ Urine F2-isoprostanes (HF_BB and LF vs. HF) ↑Liver fat droplets (HF_BB and HF vs. LF) ↓ Blood glucose (min. 15) (HF_BB vs. LF) ↓ Serum insulin (min. 30) (HF_BB and LF vs. HF) ↓ TNF-A and IL-1B (adipose tissue) (HF_BB and LF vs. HF) ↓ Hepatic p-IRS1 (Ser307) to IRS1 ratio (HF_BB and LF vs. HF) ↓ CD11D (adipose tissue) (HF_BB vs. LF and HF) ↓ Serum LBP in adipose tissue (HF_BB vs. HF) ↓Liver MDA (HF_BB vs. HF) |
[16] |
| C57BL/6J Mice Male Age: 4 Weeks |
Normal fat diet (n=6) High fat diet (HFD) (n=6) HFD supplemented with blueberry polyphenol extract (200 mg/kg body weight/day) (HFD + PPE) (n=6) |
12 Weeks | ↔OTUs and species richness (HFD vs. HFD+PPE) ↓ The Simpson diversity (HFD+PPE vs. HFD) ↑ Bifidobacterium, Desulfovibrio, Adlercreutzia, Helicobacter, and Flexispira (HFD+PPE vs. HFD) ↓ Adlercreutzia and Prevotella (HFD+PPE vs. HFD) ↔ Bacterial taxa |
↓Body weight, weight gain and food intake (HFD+PPE vs. HFD) ↓FER compared to HFD ↔ TG, AST, ALT, and leptin (serum) ↓ LDL-C (HFD+PPE vs. HFD) ↑ HDL-C (HFD+PPE vs. HFD) ↓T-CHO in (HFD+PPE vs. HFD) ↓Hepatic PPARy, FAS, SREBP-1 (HFD+PPE vs. HFD) ↑ Hepatic CPT1 and PPARɑ (HFD+PPE vs. HFD) ↓EWAT PPARy, FAS, SREBP-1, aP2, GAPDH, GLUT4 (HFD+PPE vs. HFD) ↑Hepatic and EWAT p-AMP/total AMPK ratio (HFD+PPE vs. HFD) |
[20] |
|
Study 1: C57BL/6 mice Male Age: 1– 3 Weeks |
Study 1: (n=9–12/group) G1: CHOW 1 (standard chow diet) G2: HFD 1 (high fat diet, 60% fat) G3: CBE (CHOW + 5 gL−1 blueberry extract in drinking water) G4: BE 1 (HFD + 5 gL−1 blueberry extract in drinking water) |
Study 1:
15 Weeks |
Study 1: ↑ mRNA expression of occludin, TJP1, MUC2 levels ↓ mRNA levels TLR4, IL-6, TNF-ɑ in the colon and ileum ↑ Akkermansia and Bifidobacterium ↓ Desulfovibrio and Bilophila genera ↑ Abundance of the significant taxa ↑ Ratio Bacteroidetes to Firmicutes |
Study 1: ↓ Weight gain (BE1 vs. HFD 1) ↔ Energy intake, water intake ↓ Body fat, liver fat induced by HFD ↓ Hepatic and plasmatic TG ↓ ALT and HDL ↑ Energy expenditure, core body temperature ↓ mRNA levels LPS, IL-6, TNFɑ in plasma ↓ Plasma leptin ↑ Glucose tolerance and insulin sensitivity ↓ Expression of CCAAT in the iWAT and mRNA of proteins linked to lipolysis, FXR, SHP, TGR5 ↓ Plasma BAs |
[19] |
|
Study 2:
C57BL/ KsJ db/db mice Male Age: 21 Days |
Study 2: (n=10/group) G1: CHOW 2 (standard chow diet) G2: BE 2 (CHOW + 5 gL−1 blueberry extract in drinking water) |
Study 2:
10 Weeks |
Study 2: ↑ Diversity of the GM ↓ Abundance of Proteobacteria ↑ Ratio Bacteroidetes to Firmicutes |
Study 2: ↓ Weight gain ↔ Energy intake ↓ TG, colesterol, leptin ↑ Energy expenditure ↑ Hepatic steatosis, systemic inflammation, fat deposition ↑ Glucose and lipid metabolism ↓ Plasma BAs |
|
|
Study 3: C57BL/6 mice Male Age: 8 Weeks |
Study 3: (n=10–12/group) G1: CHOW 3 (standard chow diet) G2: HFD 3 (high fat diet, 60% fat) G3: BE 3 (HFD + 5 gL−1 blueberry extract in drinking water) G4: Abx (HFD + 200uL PBS containing antibiotic) G5: ABE (HFD + 5 gL−1 blueberry extract in drinking water + 200uL PBS containing antibiotic) |
Study 3:
15 Weeks |
Study 3: ↓ Fecal anthocyanin content (BE vs. ABE) |
Study 3: ↔ Weight gain ↔ TG, hepatic fat, cholesterol ↔ Metabolic disease, systemic inflammation and glucose metabolism ↔ Plasma BA pool size and composition, FXR, TGR5 |
|
| C57BL/6J mice Male Age: 6 Weeks |
(n=12/ Group) Standard chow diet (n=12) High-fat high-sucrose diet (HFHS) (n=12) HFHS diet supplemented with wild blueberry extract (200 mg/kg/day equivalent to 17 mg polyphenols) (WBE) HFHS diet supplemented with anthocyanins and phenolic acids (32 mg/kg) (F1) HFHS diet supplemented with anthocyanins and phenolic acids (32 mg/kg) (F1) G5: F2 (PACs oligomers, phenolic acids and flavonols, 53mg/kg) + HFHS G6: F3 (PACs polymers, 37mg/kg) + HFHS oligomeric proanthocyanidins (PACs) |
8 Weeks | ↑ Mucus layer thickness (colon) (WBE and BPFs) ↔ Crypt’s depth (WBE, BPF) and total goblet cells (WBE, F1 and F2) ↔ Firmicutes/ Bacteroidetes ratio ↑ Number of total goblet cells (GC) (at F3 group) ↑ Adlercreutzia equolifacens (WBE e F2) ↑ Mix of neutral and acidic mucins (BPFs) ↑ Neutral mucin-filled GC proportion (WBE) ↓ Proportion of mucin-unfilled GC (WBE and BPFs) ↔ mRNA of ZO-1 and occludin ↔ α-diversity index, species abundance and Shannon’s diversity index, β-diversity of the gut microbiota ↑ Family Coriobacteriaceae, S24–7, Verrucomicrobia and the order Clostridiales (WBE and BPFs) ↓ Unassigned genus of the family S24–7 (WBE) ↑ A. muciniphila at feces (F2 group) ↑ A. equolifaciens at feces (F2, WBE) |
↔ Total energy intake ↔ Visceral mass, fasting glycemia, HOMA-IR index ↓ AUC of the OGTT (WBE e F3) |
[30] |
| C57BL/6 mice Male Age: 5 weeks |
(n=7/ Group) G1: C (control) G2: CL (C with 4% fermented blueberry pomace (FBP) supplementation) G3: CH (C with 8% FBP-supplementation) G4: HFD (high fat diet) G5: HFDL (HFD with 4% FBP-supplementation) G6: HFDH (HFD with 8% FBP-supplementation) |
5 Weeks | ↑ Villus length (HFDL and HFDH vs. HFD) ↑ Ratio of villus length to crypt depth (HFDL and HFDH vs. HFD) ↑ Claudin-4 mRNA level (HFDL and HFDH vs. HFD) ↑ Occludin mRNA level (HFDL and HFDH vs. HFD) ↑ Goblet cells (HFDL and HFDH vs. HFD) ↑ mRNA expression of ZO-1 (HFDL and HFDH vs. HFD) ↑ Claudin-1 mRNA level (HFDH vs. HFDL and HFD) ↑E-cadherin mRNA level (HFDH vs. HFDL vs. HFD) ↑ Muc 2 mRNA level (HFDH vs. HFDL vs. HFD) ↓ Crypt depth (CL vs. C) ↑ T-AOC (ileum) (HFDH vs. HFD) ↑ CAT (ileum) (HFDH vs. HFDL vs. HFD) ↑ SOD (ileum) (HFDH vs. HFDL) ↓ MDA (ileum) (HFDH vs. HFD) |
↓ TNF-α (serum) (HFDL vs. HFD) ↑ IL-10 level (serum) (HFDH vs. HFDL vs. HFD) ↓NF-κB mRNA level (HFDH vs. HFDL and HFD) ↓ protein level of NF-κB p-P65 (HFDH vs. HFD and HFDL) ↓ MPO activity in small intestine tissue (HFDL and HFDH vs. HFD) ↓ MLCK mRNA level (HFDH vs. HFDL and HFD) ↓Protein level of p-MLC (HFDH vs. HFDL vs. HFD) ↓ Final body weight (HFDL and HFDH vs. HFD) ↓ Abdominal fat index (HFDL and HFDH vs. HFD) ↑ GSH (liver) (HFDH vs. HFD and HFDL) ↑ T-AOC (liver) (HFDH and HFDL vs. HFD) ↑ CAT (liver) (HFDH vs. HFDL and HFD) ↑ SOD (liver) (HFDH and HFDL vs. HFD) |
[21] |
| C57BL/6J mice Male Age: 8 weeks |
G1: Chow (n=14); G2: HFHS: High-fat,high-sucrose diet (n=13); G3: BB-HFHS: High-fat, high-sucrose + whole blueberry powder (n=14); G4: ANT-HFHS: High-fat, high-sucrose diet + blueberry anthocyanin-rich fraction (n=14); G5: PAC-HFHS: High-fat, high-sucrose diet + proanthocyanidin-rich fraction (n =13) |
20 Weeks | ↓ Lachnospiraceae bacterium Choco86, Ruminococcus, Blautia hansenii and Blautia sp. N6H1–15 (BB-HFHS and ANT-HFHS vs. HFHS). ↑Turicibacter sp. H121 abundance (BB-HFHS vs. HFHS) ↑ Muribaculum intestinale abundance (PAC-HFHS vs. HFSH) ↓Acetic and propionic acids (BB-HFHS vs. HFHS) ↓Valeric acid (BB-HFHS and ANT-HFHS vs. HFHS) ↓ Isobutyric and isovaleric acid BB-HFHS, ANT-HFHS and PAC vs. HFHS) ↔ Fecal Butyric Acid |
↓ Weight gain (PAC-HFHS VC HFHS) ↔ Food intake, IWAT, MWAT, lean mass, energy expenditure, glycaemia, insulinemia 6h fasting, C-Peptide, liver weight, TBARS, liver TG, liver cholesterol, AST, ALT ↔ IL-2, IL-6, TNF-a, MCP-1, INFy ↓HOMA-IR (PAC-HFHS vs. HFHS) ↓ Insulin (ANT-HFHS and PAC-HFHS vs. HFHS) |
[29] |
| C57BL/6J Male mice Age: 6 weeks |
(n=12/ Group) G1: CT (standard chow diet) G2: HFHS (high-fat high-sucrose diet) G3: HFHS + BP (high-fat high-sucrose diet + blueberry powder) G4: HFHS + BF (high-fat high-sucrose diet + blueberry fibrous residue) |
8 Weeks | ↑ Mucus thickness (BP vs. HFHS) ↔ Crypt depth, number of Goblet cells, types of mucin ↑ Microbial richness relative ↑ Gut microbiota diversity ↑ Verrucomicrobia (BP group) ↓ Firmicutes (BP group) ↔ Abundance of pathobionts, taxa at the family level (BP group) ↑ Akkermansiaceae (BP group) ↑ Eggerthellaceae and Coriobacteriales_Incertae_Sedis (BP group) ↑ A. muciniphila (BP group) ↓ Romboutsia, Ruminiclostridium, and Oscillibacter ↑ Raxa Lachnospiraceae_NK4A136_group e Acetatifactor ↑ Polysaccharide-degrading taxa such as Clostridium_senso_stricto1, Muribaculaceae, and Roseburia ↑ Pathways of metabolism of cofactors and vitamins, lipid metabolism and DNA replication and repair (BP group) |
↔ Body weight ↑ EWAT (BF group) ↑ TG (BF vs. HFHS) ↔ Glycemia, HOMA-IR ↑ Fasting insulin (BF vs. HFHS) ↔ Cecum weight |
[31] |
| Sprague-Dawley rats Age: 21 days |
(n=48/Group) G1: Standard diet (AIN-93G) G2: Standard diet + 200 mg/kg blueberry polyphenol extract |
2 Weeks | ↑SOD jejunal, CAT ileal, T-AOC jejunal and ileal ↓MDA jejunal ↔ SOD and MDA ileum, CAT jejunum ↓IL-1 and IFN-y (jejunum and ileum) ↔ IL-6 and TNF-a (jejunum and ileum) ↓ Keap1 jejunal and ileal ↑ Nrf2 jejunal and ileal ↑mRNA of mTOR, S6K1, 4EBP1 jejunal and ileal ↔HO-1 jejunal and ileal |
↔ Growth performance | [24] |
| C57BL/6 mice Male Age: 4 weeks |
(n=6/ Group) G1: ND (normal diet) G2: HFD (high fat diet, 53.8% basic feed, 21% lard oil, 20% saccharose, 5% cholesterol, and 0.2% sodium cholate) G3: BAE100 (HFD and 100 mg/kg body weight of blueberry anthocyanin-rich extract) G4: BAE200 (HFD and 200 mg/kg body weight of blueberry anthocyanin-rich extract) |
8 Weeks |
↓Staphylococcus (BAE100 and BAE200) ↑Ruminiclostridium (BAE100 and BAE200) ↑Bacteroidetes/Firmicutes (BAE100) ↑ Bifidobacterium (BAE200) ↑ Lactobacillus (BAE200) ↑ Roseburia (BAE200) ↑ Faecalibaculum (BAE200) ↑ Parabacteroides (BAE200) ↑ Acetate (BAE200 vs. BAE100 vs. HFD) ↑ Butyrate (BAE200 vs. BAE100 vs. HFD) ↑ Propionate (BAE200 vs. BAE100 vs. HFD) |
↓ Serum concentrations of phospholipids with PUFA (BAE100 and BAE200 vs. HFD) ↑ Liver T-AOC (BAE200 vs. BAE100 vs. HFD) ↑ Liver SOD activity (BAE200 vs. BAE100 vs. HFD) ↑Liver GSH-Px (BAE200 vs. BAE100 vs. HFD) ↑ USFA/SFA (BAE200 vs. BAE100 vs. HFD) |
[23] |
| Outbred laboratory rats Male and female Age: 22–24 months |
(n=12/ Group) G2: blueberry juice; G9: control group (standard vivarium diet food) |
12 Weeks | ↓ Klebsiella pneumoniae, Morganella morganii, E. coli, Actinomyces naeslundii, and Bacteroides ↔ B. subitillis ↓ Streptococcus parvulus ↑ E. faecalis and staphylococci |
↓ Body weight, total lipid, cholesterol, glucose, calcium levels ↔ LDL, triglycerides, blood urea |
[32] |
| C57BL/6J mice Male Age: 5 weeks |
(n= 9/ Group) G1: LFD (Low fat diet - 10% kcal FAT) G2: HFD (High fat diet - 60% kcal FAT) G3: HFD + BL (High fat + 1% blueberry extract) G4: HFD + BH (High fat + 2% blueberry extract) |
24 Weeks | ↑ SCFA (BL and BH vs. HFD) ↔ Firmicutes/Bacteroidetes ratio ↑ Bacteroidetes (BH group) ↓ Abundance of Rikenellaceae (BL and BH vs. HFD) ↓ Abundance of Streptococcaceae (BH group) ↓ Relative abundance of Allobaculum, Anaerotruncus, Intestinimonas, Oscillibacter, Ruminiclostridium, and norank_f_Bacteroidales_S24–7_group ↓ Abundance of Rikenella ↑ Abundance of Peptoclostridium ↑ Functions such as general function prediction only, lipid transport and metabolism, cell motility, RNA processing and modification ↑ Abundance of metabolic pathways related of basic metabolism (BH group) |
↓ Weight gain induced by HFD ↔ energy intake ↓ Accumulation of white adipose tissue ↓ Plasmatic TC ↑ HDL/TC ratio (BL group) ↓ Plasma LPS (BH group) ↔TNFɑ, MCP-1, IL-1β ↓ Liver weight, hepatic total lipids ↔ Hepatic cholesterol |
[22] |
| Male mice Age: 6 weeks |
(n=7/ Group) G1: CON (distilled water) G4: BLUB (blueberry) |
2 Weeks | ↑ SOD activity e AOC (colon) ↔ MDA e GSH content, CAT e GSH-Px activity (colon) ↑ Prevotella, Clostridium_III, Clostridium_XVIII, Intestinimonas, Ruminococcus and Barnesiella ↓ Escherichia, Klebsiella, Proteus, Blautia, Enterococcus, Staphylococcus, Mucispirillum, Acinetobacter and Clostridium_XIVa |
↓ Weight gain, food intake and liver index ↓ CAT activity (serum) ↔ AOC, MDA and GSH content (serum) ↔ SOD (liver) ↑ SOD activity (serum) |
[25] |
| C57BL/6J mice Male Age: 22 weeks |
(n= 15/Group) G1: LFD (Low fat diet - 10% kcal FAT) G2: HFD (High fat diet - 60% kcal FAT) G3: HFD + Blueberry (High fat + 6% blueberry powder) |
16 Weeks | ↑ Shannon diversity (G3 vs. G2) ↑ Total OTUs richness (G3 vs. G2) ↑ Firmicutes (G3 vs. G2) ↓ Proteobacteria (G3 vs. G2) ↑ Unclassified genus from Clostridiales, Lachnospiraceae (G3 vs. G2) ↓ Unclassified genus from Ruminococcaceae, Desulfovibrionaceae (G3 vs. G2) ↔ Akkermansia muciniphila |
↔ Body weight, energy intake, fat pad weight | [33] |
4EBP1: eukaryotic initiation factor 4E-binding protein 1; AIN93: nutritional standard; ALT: alanine aminotransferase; AMP: adenosine mono phosphate; AMPK: AMP-activated protein kinase; aP2: adipocyte-specific acid binding protein; AST: aspartate transaminase; AUC: area under curve; CAT: catalase; iWAT; FXR and SHP; CPT1: Carnitine palmitoyl transferase I; Defb2: defensin beta 2; eWAT: epididymal white adipose tissue; FAS: fatty acid synthase; FER: food efficiency ratio; GAPDH: Glyceraldehyde-3-Phosphate Dehydrogenase; Glp1: Glucagon-like peptide-1; GLUT4: glucose transporter 4; Gpr43: G-protein-coupled receptor 43; GSH-Px: liver glutathione peroxidase; HDL-C: high-density lipoprotein cholesterol; HFD: high fat diet; HO-1: heme oxygenase-1; HOMA-IR: Homeostasis Model Assessment-Insulin Resistance; IFN-γ: interferon-γ; IL-1: interleukin-1; IL-10: interleukin-10; IL-2: interleukin-2; IL-6: interleukin-6; iWAT: inguinal white adipose tissue; Keap1: Kelch-like ECH-associated protein 1; KEGG: Kyoto Encyclopaedia of Gene and Genome; LBP: Adipocyte lipopolysaccharide-binding protein; LDH: lactate dehydrogenase; BA: bile acids; LDL-C: low-density lipoprotein cholesterol; LWB: lowbush wild blueberry; MCP-1: monocyte chemoattractant protein-1; MDA: malondialdehyde; MLCK: myosin light chain kinase; MPO: myeloperoxidase; mTOR: mammalian target of rapamycin; Muc2: mucin 2; mWAT: mesenteric white adipose tissue; NFκB: nuclear factor kappa B; Nrf2: nuclear factor-E2-related factor 2; ORFs: open reading frames; OTUs: operational taxonomic units; p-IRS1: Phospho-insulin receptor substrate 1; p-MLC: phospho-myosin light chain 2; PPAR-d: peroxisome proliferator-activated receptor delta; PPARɑ: Peroxisome proliferator-activated receptor ɑ; PPARy: peroxisome proliferator-activated receptor y; PUFA: Polyunsaturated fatty acids; S6K1: ribosomal p70 S6 kinase; SFA: saturated fatty acids; SOD: superoxide dismutase; SREBP-1: sterol regulatory element-binding protein 1; T-AOC: total antioxidant capacity; T-CHO: serum total cholesterol; TBARS: thiobarbituric acid reactive substances; TG: triglycerides; TNF-α: tumor necrosis factor-α; USFA: unsaturated fatty acid; ZO-1: Zonula occludens-1.
3.3.1. Effect of dietary blueberries on Intestinal morphology and gut permeability
Two studies [n=2; 12.5%] evaluated the effect of dietary blueberries on the morphology of intestinal crypts and villi. Blueberry supplementation restored gastrointestinal integrity and increased ileal villus length in HFD-fed mice [16]. Consistent with this study, fermented blueberry pomace (FBP) supplementation was shown to improve the integrity and orderly arrangements of villi in the HFD-fed mice that is associated with an increase in the villus length and the ratio of the villus to the crypt [21]. In addition, two studies [n=2; 12.5%] evaluated the crypt’s depth [30, 31]. However, no difference in crypt depth was found between HFD-fed animals supplemented with blueberries and the HFD control group in these studies.
Four studies [n = 4; 25%] indicated an alteration in intestinal permeability with dietary blueberry supplementation [19, 21, 23, 30]. Among these, three studies showed positive effects [19, 21, 23]. Guo et al. (2019) showed that blueberry supplementation increases mRNA expression of occludin and tight junction protein 1 (TJP1) both in the ileum and colon alleviating the increased permeability of the colon and distal ileum. Cheng et al. (2020) also observed that blueberry supplementation increases the mRNA expression of structural tight junctional proteins (ZO-1, claudin-1, claudin-4 and occludin), adherens junction protein (E-cadherin), and mucins as well as increased gut barrier function as compared to HFD-fed control group. Similarly, Si et al. (2021) observed that the administration of blueberry anthocyanin-rich extracts improves HFD-induced damage in colonic mucosal structure as shown by neatly arranged colonic epithelial cells and increased mucosal layer thickness. However, Rodríguez-Daza, Daoust, et al. (2020) did not observe a difference in the expression of ZO-1 and occludin mRNA in high-fat and high sucralose diet (HFHS) fed mice supplemented with or without blueberry. Cheng et al. (2020) showed an increased barrier function through NFκB and Myosin light-chain kinase (MLCK) signaling pathways with dietary supplementation of fermented blueberry pomace.
Three studies evaluated goblet cells [n = 3; 18.75%] [16, 30, 31]. Two indicated an increase in the number of goblet cells with blueberry supplementation [16, 30]. Lee et al. (2018) showed that the goblet cell number per crypt was significantly higher in the HFD-fed rats supplemented with blueberry compared to the HFD-fed rats. Similarly, Rodríguez-Daza et al. (2020a) showed an increased number of total mucin-secreting goblet cells in mice that received the blueberry fraction rich in polymeric (PACs) compared to the HFD control group. However, Rodríguez-Daza et al. (2020b) did not find differences in the number of goblet cells with blueberry supplementation.
Four studies [n=4; 25%] investigated mucus protein levels and mucus layer in the gut. Two showed that blueberry extract supplementation increases mucus protein (MUC2) levels in HFD-fed mice [16, 19]. Lee et al. (2018) reported an increase in gene expression of MUC2 in the ileum whereas Guo et al. (2019) reported an increase in MUC2 levels in the colon. Furthermore, two other studies observed that mice treated with blueberry polyphenols extract had a thicker and restored mucus layer as compared to the HFD group [n=2; 12.5%] [30, 31]. Finally, one study [n=1; 6.25%] used lipopolysaccharide (LPS)-binding protein (LBP) as a proxy to assess circulating LPS concentrations [16]. This study showed that blueberry supplementation significantly reduces circulating LBP in HFD-fed mice.
3.3.2. Effect of dietary blueberries on intestinal inflammation and oxidative stress
The markers related to intestinal inflammation or oxidative stress are reported in five studies [n=6; 37.5%] included in this review [16, 19, 21, 24, 25]. Two studies [n=2; 12.5%] found a significant increase in mRNA levels of inflammation-related genes in the gut of HFD-fed animals, which was normalized by the blueberry supplementation [16, 19]. Importantly, blueberries reduced the expression of tumor necrosis factor-α (TNF-α) in the colon and ileum. On the other hand, one study [n=1; 6.25%] did not find such an effect [24]. One study [n=1; 6.25%) found a reduction in the mRNA levels of interleukin 6 (IL-6) in animals that received blueberries [19], however, two other studies [n=2; 12.5%] did not find such an effect [16, 24]. Myeloperoxidase (MPO) at inflammatory sites is released into the extracellular space after phagocyte activation and induces damage to the host tissue. Cheng et al. (2020) found that mice MPO activity was significantly inhibited by fermented blueberry pomace supplementation.
To assess the effects of dietary blueberries on intestinal antioxidant status, two studies [n=2; 12.5%] evaluated oxidative enzymes in the ileum and jejunum [21, 24]. Both studies found catalase (CAT) and total antioxidant capacity (T-AOC) increase in the ileum of the experimental animals with blueberry supplementation. One study observed an increase in SOD with a reduction in ileal MDA [21] though another study did not observe this effect [24]. Zhao et al. (2021) further evaluated the antioxidant status in the jejunum and found that blueberry treatment increased SOD and T-AOC, whereas it reduced jejunal MDA. Further, blueberry supplementation markedly down-regulated Keap1 gene transcription and up-regulated the mRNA abundance of Nrf2, mTOR, S6K1 and 4EBP1 in the jejunum and ileum compared with the control group. Furthermore, one study [n=1; 6.25%] assessed the colonic antioxidant status through levels of T-AOC, GSH, T-SOD, CAT, GSH-PX, and MDA [25]. This study observed an increase in the levels of SOD and AOC in the colon compared with the control group following the intake of blueberry extract.
3.3.3. Effect of dietary blueberries on gut microbiota
3.3.3.1. Microbial composition
The reviewed experimental studies overall demonstrated an improved gut microbiota in animals fed blueberry. One study [n=1; 6.25%] showed a significant reduction in the abundance of the genera Lactobacillus and Enterococcus in mice fed dietary blueberries [27]. However, another study [n=1; 6.25%] showed an increase in the abundance of Lactobacillus, Roseburia, Faecalibaculum, and Parabacteroides with blueberry supplementation [23]. Three studies [n=3; 18.75%] observed an expansion of Bifidobacterium in mice fed blueberries [19, 20, 23]. Guo et al. (2019) observed an increase in the abundance of Akkermansia along with a significant decrease in the Desulfovibrio and Bilophila in the blueberry group. Further, blueberry extract administration increased the diversity of the gut microbiota and decreased the abundance of Proteobacteria. Huang et al. (2022) [n=1; 6.25%] also observed a decrease in Proteobacteria in the blueberry powder group, as well as a significant decrease in the unclassified genera from Desulfovibrionaceae and Ruminococcaceae families, and an increase in the unclassified genera from Clostridiales and Lachnospiraceae families. The abundance of Klebsiella pneumoniae and Morganella morganii decreased significantly with 0.5 mL/day blueberry juice treatment for 12 weeks in one study [n=1; 6.25%] [32]. Additionally, the abundance of Actinomyces naeslundii and Bacteroides significantly decreased whereas E. faecalis and staphylococci were increased under the influence of a blueberry juice-based diet. Two studies [n=2; 12.5%] showed a decreased abundance of Streptococcaceae after blueberry supplementation [22, 32]. One study [n=1; 6.25%] showed that the prebiotic-type selective effects on Akkermansiaceae and Coriobacteriales_Incertae_Sedis were enhanced by blueberry powder supplementation, but this result was not triggered by their fibrous fractions [31]. While one study [n=1; 6.25%] found a significant increase in the relative abundance of Actinobacteria [27], another study [n=1; 6.25%] showed that the supplementation with blueberries decreased the level of this phylum [20]. A favored in the family Coriobacteriaceae, S24–7, Verrucomicrobia and the order Clostridiales in the groups fed with wild blueberry polyphenols extract (WBE) and blueberry polyphenolic fractions (BPFs), and also an increase proportion of Adlercreutzia equolifacens in the mice treated with WBE and oligomeric PACs fractions (BPF 2), compared to the group that received the HFHS diet, was observed in one study [30] [n=1; 6.25%]. Finally, one study [29] [n= 1; 6.25%] showed that a high-fat, high-sucrose diet supplemented with whole blueberry powder modestly affects the gut microbiota with an increase in the abundance of Turicibacter sp. H121. Another study [n=1; 6.25] demonstrated that oral gavage with extract of blueberry positively regulated bacteria that were SCFAs-producing, including Prevotella and Barnesiella. Unexpectedly, the growth of Blautia and Clostridium_XIVa was inhibited in the blueberry group compared with the control group [25].
3.3.3.2. Microbial metabolic pathways
Three studies reported the modulation of microbial metabolic pathways with blueberry supplementation. In one study [n=1; 6.25%], deep whole genome sequencing (WGS) showed that dietary blueberries increase the abundance of microbial protein families that are involved in amino acid metabolism, metal ion binding, and REDOX homeostasis [26]. Further, protein families involved with integrase/recombinase, reverse transcriptase, and transposon/transposase were at a lower abundance in the blueberry group. Two studies [n=2; 12.5%] evaluated the Kyoto Encyclopedia of Gene and Genome (KEGG) pathways to understand the effect of blueberries on microbial function [22, 27]. Lacombe et al. (2013) showed that the blueberry diet impacts ~9% of the KEGG metabolic pathways. Specifically, xenobiotic metabolism, benzoate degradation, and glycosaminoglycan degradation were increased, whereas bacterial invasion of epithelial cells was decreased (8-fold) with dietary blueberry. In addition, another study showed an increase in basic metabolism with blueberry extract supplementation. One study [n=1; 6.25%] determined the microbial protein functions using Clusters of Orthologous Groups of proteins (COG) analysis [22]. In this study, 1% blueberry extract feeding showed a difference in lipid transport and metabolism, cell motility, and RNA processing and modification. Further, the 2% blueberry extract feeding group showed differences in functions, including inorganic ion transport and metabolism, translation, ribosomal structure and biogenesis, amino acids transport, and energy production and conversion. In one study [n=1; 6.25%], the metagenomic analysis revealed that the pathway related to β-lactam resistance was increased in mice fed high-fat and high-sugar diets supplemented with blueberries. However, the pathways related to pantothenate and CoA biosynthesis and the pool of carbon per folate were increased in mice fed a high-fat and high-sugar diet [29]. Further, pantothenate and CoA biosynthesis and one carbon pool by folate were associated with Akkermansia muciniphila and Bacteroides thetaiotaomicron respectively. The pathway related to β-lactam was associated with Eubacterium plexicaudatum. In this study, an increase in alanine, aspartate, and glutamate metabolism was associated with Adlercreutzia in HFHS-fed mice and an increase in oxidative phosphorylation for those fed proanthocyanidin-HFHS. However, such a difference was detected when pathway analysis was performed between HFHS-fed mice and anthocyanidin-HFHS-fed mice.
3.3.3.3. Microbial metabolite short chain fatty acids (SCFA)
Animals fed a high-fat diet decrease the production of total SCFA compared to the low-fat diet fed mice. Two studies [n=2; 12.5%] indicated that the supplementation of blueberry anthocyanin extracts reverses this decrease in SCFA [22, 23]. In addition, one study [n=1; 6.25%] showed that blueberry supplementation was associated with higher concentrations of acetate and propionate, whereas the lower concentration of butyrate as compared to the control group [16]. This is also associated with a 3-fold increase in the gene expression of SCFA receptor Gpr43. However, another study [n=1; 6.25%] demonstrated that mice fed an HFHS diet supplemented with blueberry for 12 weeks had lower fecal concentrations of acetic and propionic acids than the HFHS control without changes in butyrate concentration [29]. Furthermore, the concentration of valeric acid was lower in the feces of mice fed HFHS containing whole blueberry powder or anthocyanidin-rich extract. In contrast, the levels of the BCFAs isobutyric acid and isovaleric acid were decreased in the feces of mice receiving whole blueberry powder, anthocyanidin or proanthocyanidin-rich extract compared to control HFHS [29].
3.4. Risk of bias
In all the studies that were included in this systematic review [n=16], the baseline characteristics (sex, age, and initial weight of animals) were completed in five studies [n=7; 43.75%]. In none of the studies, the allocation of animals was described in detail. Three studies did not mention if the animal allocation to treatment groups was performed randomly [n=3, 18.75%]. Furthermore, none of the studies reported blinding the investigators involved in the research (Figure 2).
4. Discussion
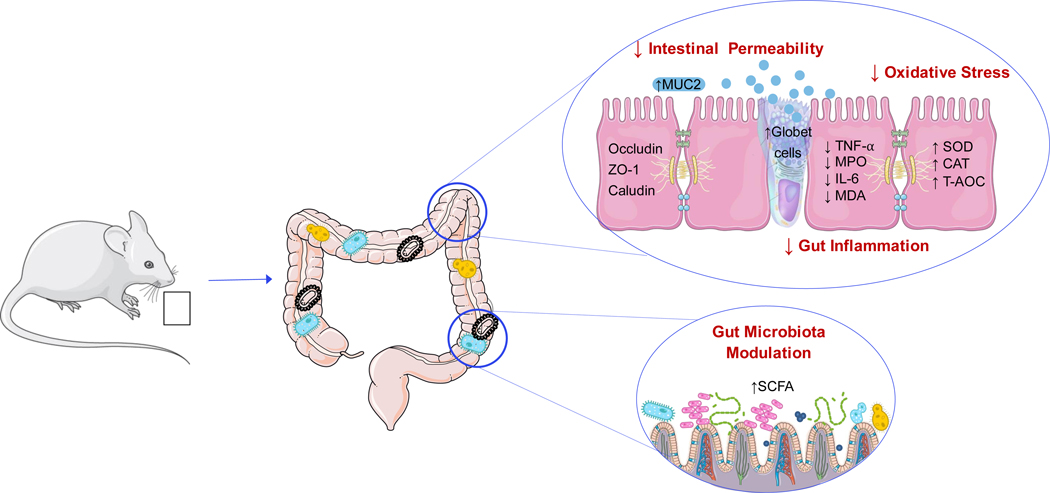
The present systematic review evaluates the effects of blueberries on gut health focusing on studies conducted in murine models. Evidence shows that dietary blueberries improve gut health by acting on multiple targets such as (i) improving intestinal morphology, (ii) reducing gut permeability, (iii) modulating oxidative stress, (iv) suppressing gut inflammation, and (v) modulating composition and function of gut microbes (Fig. 3).
Fig. 3.
Possible Mechanisms for the Beneficial Effects of Blueberries on Gut Health.1
1CAT: catalase; IL-6: interleukin-6; MDA: Malondialdehyde; MPO: Myeloperoxidase; MUC2: Mucin 2; SCFA: short chain fatty acids; SOD: Superoxide dismutase; T-AOC: Total Antioxidant Capacity; TNF-α: Tumor necrosis factor alpha; ZO-1: Zonula occludens.
The effect of dietary blueberries on intestinal epithelial morphology was determined by assessing intestinal crypts and villi. A compartmentalized crypt-villus structure is a key factor for maintaining intestinal homeostasis and the functional capacity of the tissue. Hence, a compromised crypt-villus negatively impacts gut health [34]. Dietary blueberry was shown to increase ileal villus length, improve villi arrangement, increase the ratio of the villus to the crypt, and restore gastrointestinal integrity in mice fed HFD without altering the crypt’s depth [16, 30, 31]. These intestinal morphology changes induced by blueberry anthocyanins could result in a better absorptive function, improving absorption surface, brush border enzyme expression, and nutrient transport [35].
The intestinal barrier depends on interactions among barrier components, including the mucus layer, immunoglobulin A, antibacterial peptides, and intercellular tight junctions [36]. The tight junctions complex are cells attached by a protein complex and constitute the major determinant of the intestinal physical barrier, regulating the paracellular permeability of water, ions, and macromolecules [37]. The tight junctions structure consists of transmembrane proteins, such as claudins, occluding, and zonula occludens. Damage to these proteins increases the paracellular permeability resulting in the permeation of pro-inflammatory molecules, forcible activation of immune cells and inflammation [36–38]. The beneficial effect of blueberries on intestinal permeability was reported in HFD-fed mice [19, 21, 23]. These studies demonstrate an increase in the expression of structural tight junctional proteins, adherens junction protein, and mucins which are associated with an increase in mucosal thickness and gut barrier function. However, blueberry supplementation was ineffective in improving the expression of junctional proteins such as ZO-1 and occludin in mice fed high fat and high sucralose diet (HFHS) [30]. The discrepancy could be due to the difference in the dietary model (HFD vs. HFHS) used to induce damage to the colon. The possible mechanism suggested for the effect of blueberry on gut barrier function is nuclear factor-kappa B (NFκB) and myosin light-chain kinase (MLCK) signaling pathways [21]. Anthocyanins exhibit anti-inflammatory as shown by a decrease in the gene expression of pro-inflammatory cytokines that damage intestinal barriers such as TNF-α. This effect is mediated through the inhibition of NFκB via regulation of the I-Kappa-B-alpha (IκBα) phosphorylation [35, 39].
Goblet cells in the IEC are responsible for the synthesis and secretion of mucins which is an important component of the mucus layer of ICE [40]. Mucins are highly glycosylated proteins that provide luminal protection of the gastrointestinal tract [16]. Hence, qualitative and quantitative changes in mucin are associated with several gastrointestinal conditions including inflammatory bowel disease, colorectal cancer, and enteric infections [40]. Blueberry increased goblet cells in animals fed HFD [16, 30]. Blueberry also increased mucus protein MUC2 (the primary glycoprotein of the gastrointestinal mucus layer) and established a thicker mucus layer in animals fed HFD [16, 19, 30, 31]. These studies indicate the beneficial effects of blueberries on the mucus layer.
The gut is a key resource of reactive oxygen species as the intestinal tract is frequently exposed to foreign substances and microbial pathogens. The gut is vulnerable to damage from oxidative stress, which can lead to intestinal dysfunction and subsequent impairment of nutrient metabolism [41]. Thus, maintaining the proper functioning of the antioxidant defense system in the gut is essential. Indeed, pathogen sensing and intestinal homeostasis are important physiological functions of the intestine. An imbalanced reactive oxygen species and the resulting oxidative stress play a critical role in intestinal injury that activates intestinal barrier dysfunction leading to gut inflammation. This is implicated in several complications including inflammatory bowel diseases, enteric infections, and colorectal cancer [42]. The effect of dietary blueberries on intestinal antioxidant status was determined by assessing antioxidative enzymes, Nrf, and antioxidant capacity in the ileum and jejunum. Blueberry supplementation increases antioxidant enzymes (catalase and superoxide dismutase), Nrf2, and total antioxidant capacity in experimental animals [21, 24]. Malondialdehyde (MDA) is one of the lipid peroxidation products widely used as an indicator of cellular injury and a biomarker of oxidative stress [43]. Blueberry supplementation reduces MDA in jejunum indicating that blueberry can suppress lipid peroxidation [21, 24]. Myeloperoxidase (MPO) catalyzes the production of a number of reactive oxidant species. MPO at the inflammatory sites is released into the extracellular space after phagocyte activation and induces damage to the host tissue. Blueberry was shown to inhibit MPO [21]. Nrf2 is considered an important nuclear transcription factor that accelerates the transcription of endogenous antioxidant enzyme genes by promoting the binding of the antioxidant response element in the promoter region of the antioxidant enzyme genes [44]. Blueberry supplementation was shown to upregulate Nrf2 transcription in the jejunum and ileum [24]. This finding indicates that blueberry supplementation can increase jejunal and ileal antioxidant capacity by regulating the Nrf2-Keap1 signaling pathway. The beneficial effects of the blueberry on antioxidant enzymes may be partially attributable to the upregulation of Nrf2 signaling. These studies indicate the beneficial effects of dietary blueberries on oxidative stress in the gut.
Cytokines play important roles in the regulation of intestinal epithelial homeostasis during gut inflammation. IL-6, interferon-γ (IFN-γ) and TNF-α are known to negatively regulate the barrier and self-renewal properties of the intestinal epithelium, thus modulating epithelial homeostasis and exacerbating mucosal inflammation [45]. Blueberry supplementation was shown to decrease the pro-inflammatory factors such as TNF-α, IL-6, and IFN-y in the colon and ileum of HFD-fed animals [19]. These findings suggest that blueberry supplementation may protect the intestine from oxidative stress and associated inflammation. However, another study did not report such an effect of blueberry on oxidative stress and gut inflammation [24].
The human microbiota is a complex ecosystem that colonizes the gastrointestinal tract with a greater distribution in the colon (1014 cells per gram of feces). This ecosystem consists of bacteria (more than 1000 identified species), archaea, fungi, and viruses that are 1.3 times larger than the number of eukaryotic cells in the human body [46]. Diet influences the development, composition, and function of the gut microbiota [15, 47, 48]. Studies included in this systematic review have demonstrated that blueberry polyphenols modulate the composition of gut microbiota at different taxonomic levels in HFD-fed animals. Among the bacterial phyla known for being most prevalent and responsible for more than 160 species are Actinobacteria, Akkermansia, Bacteroidetes, Firmicutes, and Proteobacteria [49]. Actinobacteria play a key role in maintaining gut homeostasis despite it representing a small percentage of gut microbes [50]. An increase in Proteobacteria indicates gut dysbiosis and an unstable microbial community [51]. Blueberry supplementation increases the diversity of gut microbes with an increased abundance of Akkermansia and decreased abundance of Proteobacteria indicating dietary blueberries may improve gut dysbiosis [19, 27]. Blueberry supplementation also alters the gut microbial composition at the genus and species levels. Blueberry supplementation increases the abundance of Bifidobacterium, Faecalibaculum, Lactobacillus, Parabacteroides, and Roseburia whereas it reduces the abundance of Desulfovibrio and Bilophila in experimental animals [19, 20, 23]. Bifidobacterium and Lactobacillus are commensal bacteria associated with beneficial effects for the host such as reduced low-grade inflammation and improved gut barrier function [15]. The improved intestinal environment with dietary blueberries may be attributed to the increased Bifidobacterium and Lactobacillus [23]. Faecalibaculum, Parabacteroides, and Roseburia are involved in producing SFCA [23]. Specifically, Roseburia produces butyrate and is considered the main reason for significantly enhanced butyrate in blueberries-treated mice [23]. Further, blueberries reduce the relative abundance of Enterococcus faecalis [27]. Enterococcus faecalis was shown to induce irritable bowel syndrome (IBS) in IL-10 gene-deficient mice suggesting that certain enteric microbes tend to be more opportunistic and induce colon inflammation [52]. Desulfovibrio is an H2S-producing gut bacteria [19, 53]. Excess H2S can reduce the disulfide bonds in the mucous network and increase the permeability of the intestine, thus contributing to the transposition of bacteria and their metabolites such as LPS that can trigger a systematic inflammatory response [54, 55]. Blueberry supplementation significantly reduced Desulfovibrio in HFD-fed mice [19].
The impact of dietary blueberries on microbial metabolite SCFA is also reported in several studies included in this systematic review. Blueberry anthocyanin was shown to reverse the decrease in SCFA caused by HFD feeding [22, 23]. The alterations in gut microbiota and SCFA production revealed the role of blueberry in improving the intestinal barrier action and the healthy colonic environment, which further benefits the lipid metabolism and the defensive capability of the whole body [23]. The gut microbiota can increase glucose uptake from the small intestine and directly produce SCFA such as acetate, propionate, and butyrate by energy metabolism following the fermentation of carbohydrates in the colon [56]. The increased SCFAs maintained the stable colon environment (such as pH) and further improved the colonic integrity and mucosa functions, preventing harmful bacteria and toxins from entering the bloodstream. Additionally, SCFAs could also participate in lipid metabolism and appetite regulation in peripheral organs and hypothalamus regions [57, 58]. However, modulation of the microbiota can modify the type and amount of SCFA produced. Blueberry supplementation was associated with higher concentrations of acetate and propionate in HFD-fed mice which is also associated with an increased expression of SCFA receptor Gpr43 [16]. However, blueberry supplementation reduced acetate and propionate in HFHS-fed mice [29]. The difference in dietary composition (HFD vs HFHS) may have influenced the observed results. In the case of obesity, butyrate and propionate have been classified as predominantly anti-obesogenic, while acetate exerts the opposite effect. This highlights the importance of analyzing the type of SCFA produced and not just the total SCFA [59]. These studies indicate that dietary blueberries may improve gut barrier integrity by modulating SCFA production.
Diet can modify not only the composition of gut microbes but also their metabolic potential [60]. The consumption of foods rich in polyphenols can cause a bioprotective action on the microbiota since it can increase beneficial microorganisms and act on catabolic processes. This will contribute to the bioavailability of nutrients that will act on DNA repair, chromosomal maintenance, reduction of formation of free radicals, and elimination of free radicals [61]. Indeed, these changes were observed in a study where the blueberry-enriched diet increased protein families involved in biosynthetic and metabolic processes of amino acids and redox homeostasis [26]. Further, dietary blueberry was able to modify metabolic pathways related to the metabolism of amino acids, carbohydrates, and xenobiotics, demonstrating that blueberries are a good source of energy collection for the intestinal microbiota, since this comes from the host`s food consumption [22, 27]. Blueberry consumption can affect important metabolic pathways through the activity of certain gut microbes [29]. Gut microbes ferment the fiber in blueberries, which can alter the activity of bacteria responsible for the degradation of polyphenols [6]. In addition, the metabolism of polyphenols can be reduced by the binding of fibers with proanthocyanidins, resulting in a decrease in the production of bioactive anti-inflammatory metabolites [62]. Thus, gut microbes play a key role in mediating the beneficial effects of dietary polyphenols. These studies suggest that the beneficial effects of dietary blueberries on gut health could possibly be mediated by modulating gut microbial metabolism.
5. Knowledge gaps, challenges and future direction
The studies included in this systematic review indicate the beneficial effects of dietary blueberries on gut health in murine models. However, there is a significant knowledge gap in this field, and further studies are needed to establish a scientific rationale for recommending dietary blueberries to improve gut health. (1) The bioactive components of blueberries, including anthocyanins, are greatly metabolized by gut microbes, and the bioactivities are possibly mediated through their circulating metabolites. Hence, studies are needed to identify the causal association between dietary blueberries, gut microbes, blueberry metabolites, and gut health. (2) Dose and time-dependent effects of dietary blueberries are needed to identify the optimum dosage and duration to improve gut health. (3) Dietary composition has a major impact on the metabolism of anthocyanins, and understanding the role of the food matrix on the bioactivities of anthocyanins is needed. (4) Future studies using shotgun metagenomics will help to identify the influence of dietary blueberries on gut microbial metabolism and its impact on the host’s gut health. (5) The transcriptomic, proteomic, and metabolomic studies will also help to demonstrate host-microbiome interaction in improving gut health. (6) Efforts toward personalized medicine will be helpful to promote the understanding of the gut microbiota’s functional metabolic capacity as a tool to design individualized treatments. (7) Murine models are a valuable tool to assess the role of diet on the gut microbiome and its impact on health. However, several factors, such as vendor and environment, affect the composition and functional potential of the gut microbes in animals [63]. This is one of the major challenges associated with the translatability of microbiome studies conducted in murine models.
6. Conclusion
Together, the studies discussed in this systematic review indicate that blueberries improve gut health possibly through modulating intestinal morphology, reducing gut permeability, modulating oxidative stress, ameliorating gut inflammation, and improving gut microbes. However, there are significant knowledge gaps in this field. Hence further studies are needed to establish the beneficial effects of blueberries on gut health.
Supplementary Material
Funding
Supported by research funds from the NIH/NCCIH: R01AT010247 and USDA/NIFA: 2019-67017-29253 (to P.V.A.B.).
ABBREVIATIONS
- BPFs
blueberry polyphenolic fractions
- CAT
catalase
- GSH-PX
glutathione peroxidase
- GSH
glutathione
- HFD
high-fat diet
- HFHS
high fat high sucrose diet
- ICE
intestinal epithelial cells
- IFN-γ
interferon-γ
- IL-6
interleukin-6
- IκBα
I-Kappa-B-alpha
- LBP
lipopolysaccharide (LPS)-binding protein
- LPS
lipopolysaccharide
- MDA
Malondialdehyde
- MLCK
Myosin light-chain kinase
- MPO
Myeloperoxidase
- MUC2
Mucin 2
- PICOS
participants, intervention comparators, outcomes, and study design
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- SCFA
short chain fatty acids
- SOD
Superoxide dismutase
- SYRCLE-RoB
Systematic Review Centre for Laboratory Animal Experimentation Risk of Bias
- SYRCLE
Systematic Review Centre for Laboratory Animal Experimentation
- T-AOC
Total Antioxidant Capacity
- TJP1
tight junctional protein 1
- TNF-α
Tumor necrosis factor alpha
- WBE
wild blueberry polyphenols extract
- ZO-1
Zonula occludens - 1
Biographies
Dr. Ceres Mattos Della Lucia

Dr. Ceres Mattos Della Lucia is an Adjunct Professor in the Department of Nutrition and Health at the University of Viçosa, Brazil. Dr. Della Lucia’s research is focused on the nutritional and functional value of foods and diets, working mainly on the following topics: development and validation of methods for analyzing vitamins and other bioactive compounds in foods, control of vitamin losses in fruits and vegetables, the impact of food fortification on population groups, new products development, and the association between phenolic compounds and gut microbiota.
Dr. Anandh Babu Pon Velayutham

Dr. Anandh Babu Pon Velayutham is an Associate Professor in the Department of Nutrition and Integrative Physiology at the University of Utah, USA. Dr. Velayutham’s research is focused on two main projects. (1) Identifying the vascular effects of dietary berries with special emphasis on the diet-derived microbial metabolites and the molecular mechanisms involved. These studies will provide key insights toward understanding the causal association between dietary berries, gut microbiome, berry-derived microbial metabolites, and vascular health. (2) Identifying the gut microbial taxonomic and functional aspects in adolescents with type 1 diabetes and their association with dietary and clinical factors.
Footnotes
Declarations
The authors declare that they have no competing interests. All authors have read and approved the submission of the manuscript and have provided consent for publication.
References
- [1].Vancamelbeke M, Vermeire S, The intestinal barrier: a fundamental role in health and disease. Expert Rev Gastroenterol Hepatol 2017, 11, 821–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Selvakumar D, Evans D, Coyte KZ, McLaughlin J, et al. , Understanding the development and function of the gut microbiota in health and inflammation. Frontline Gastroenterol 2022, 13, e13–e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rohr MW, Narasimhulu CA, Rudeski-Rohr TA, Parthasarathy S, Negative Effects of a High-Fat Diet on Intestinal Permeability: A Review. Adv Nutr 2020, 11, 77–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Olofsson LE, Backhed F, The Metabolic Role and Therapeutic Potential of the Microbiome. Endocrine reviews 2022, 43, 907–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Agus A, Clement K, Sokol H, Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut 2021, 70, 1174–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Edwards CA, Havlik J, Cong W, Mullen W, et al. , Polyphenols and health: Interactions between fibre, plant polyphenols and the gut microbiota. Nutr Bull 2017, 42, 356–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jamar G, Estadella D, Pisani LP, Contribution of anthocyanin-rich foods in obesity control through gut microbiota interactions. Biofactors 2017, 43, 507–516. [DOI] [PubMed] [Google Scholar]
- [8].Tian L, Tan Y, Chen G, Wang G, et al. , Metabolism of anthocyanins and consequent effects on the gut microbiota. Critical reviews in food science and nutrition 2019, 59, 982–991. [DOI] [PubMed] [Google Scholar]
- [9].Cutler BR, Petersen C, Anandh Babu PV, Mechanistic insights into the vascular effects of blueberries: Evidence from recent studies. Molecular nutrition & food research 2017, 61. [DOI] [PubMed] [Google Scholar]
- [10].Feliciano RP, Istas G, Heiss C, Rodriguez-Mateos A, Plasma and Urinary Phenolic Profiles after Acute and Repetitive Intake of Wild Blueberry. Molecules 2016, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gui H, Sun L, Liu R, Si X, et al. , Current knowledge of anthocyanin metabolism in the digestive tract: absorption, distribution, degradation, and interconversion. Critical reviews in food science and nutrition 2022, 1–14. [DOI] [PubMed] [Google Scholar]
- [12].Rodriguez-Mateos A, Ishisaka A, Mawatari K, Vidal-Diez A, et al. , Blueberry intervention improves vascular reactivity and lowers blood pressure in high-fat-, high-cholesterol-fed rats. The British journal of nutrition 2013, 109, 1746–1754. [DOI] [PubMed] [Google Scholar]
- [13].Kalt W, Anthocyanins and Their C(6)-C(3)-C(6) Metabolites in Humans and Animals. Molecules 2019, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Igwe EO, Charlton KE, Probst YC, Kent K, Netzel ME, A systematic literature review of the effect of anthocyanins on gut microbiota populations. J Hum Nutr Diet 2019, 32, 53–62. [DOI] [PubMed] [Google Scholar]
- [15].Petersen C, Bharat D, Wankhade UD, Kim JS, et al. , Dietary Blueberry Ameliorates Vascular Complications in Diabetic Mice Possibly through NOX4 and Modulates Composition and Functional Diversity of Gut Microbes. Molecular nutrition & food research 2022, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lee S, Keirsey KI, Kirkland R, Grunewald ZI, et al. , Blueberry Supplementation Influences the Gut Microbiota, Inflammation, and Insulin Resistance in High-Fat-Diet-Fed Rats. J Nutr 2018, 148, 209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Page MJ, McKenzie JE, Bossuyt PM, Boutron I, et al. , The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical research ed 2021, 372, n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, et al. , SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol 2014, 14, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Guo J, Han X, Tan H, Huang W, et al. , Blueberry Extract Improves Obesity through Regulation of the Gut Microbiota and Bile Acids via Pathways Involving FXR and TGR5. iScience 2019, 19, 676–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jiao X, Wang Y, Lin Y, Lang Y, et al. , Blueberry polyphenols extract as a potential prebiotic with anti-obesity effects on C57BL/6 J mice by modulating the gut microbiota. J Nutr Biochem 2019, 64, 88–100. [DOI] [PubMed] [Google Scholar]
- [21].Cheng Y, Wu T, Tang S, Liang F, et al. , Fermented blueberry pomace ameliorates intestinal barrier function through the NF-kappaB-MLCK signaling pathway in high-fat diet mice. Food & function 2020, 11, 3167–3179. [DOI] [PubMed] [Google Scholar]
- [22].Liu J, Hao W, He Z, Kwek E, et al. , Blueberry and cranberry anthocyanin extracts reduce bodyweight and modulate gut microbiota in C57BL/6 J mice fed with a high-fat diet. European journal of nutrition 2021, 60, 2735–2746. [DOI] [PubMed] [Google Scholar]
- [23].Si X, Bi J, Chen Q, Cui H, et al. , Effect of Blueberry Anthocyanin-Rich Extracts on Peripheral and Hippocampal Antioxidant Defensiveness: The Analysis of the Serum Fatty Acid Species and Gut Microbiota Profile. J Agric Food Chem 2021, 69, 3658–3666. [DOI] [PubMed] [Google Scholar]
- [24].Zhao F, Yan S, Tian M, Blueberry Polyphenol Extracts Enhance the Intestinal Antioxidant Capacity in Weaned Rats by Modulating the Nrf2-Keap1 Signal Pathway. Frontiers in physiology 2021, 12, 640737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chen J, Shu Y, Chen Y, Ge Z, et al. , Evaluation of Antioxidant Capacity and Gut Microbiota Modulatory Effects of Different Kinds of Berries. Antioxidants (Basel) 2022, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lacombe A, Li RW, Klimis-Zacas D, Kristo AS, et al. , Lowbush blueberries, Vaccinium angustifolium, modulate the functional potential of nutrient utilization and DNA maintenance mechanisms in the rat proximal colon microbiota. Functional Foods in Health and Disease 2012, 2, 228–241. [Google Scholar]
- [27].Lacombe A, Li RW, Klimis-Zacas D, Kristo AS, et al. , Lowbush wild blueberries have the potential to modify gut microbiota and xenobiotic metabolism in the rat colon. PloS one 2013, 8, e67497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Overall J, Bonney SA, Wilson M, Beermann A, et al. , Metabolic Effects of Berries with Structurally Diverse Anthocyanins. International journal of molecular sciences 2017, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Morissette A, Kropp C, Songpadith JP, Junges Moreira R, et al. , Blueberry proanthocyanidins and anthocyanins improve metabolic health through a gut microbiota-dependent mechanism in diet-induced obese mice. American journal of physiology 2020, 318, E965–E980. [DOI] [PubMed] [Google Scholar]
- [30].Rodriguez-Daza MC, Daoust L, Boutkrabt L, Pilon G, et al. , Wild blueberry proanthocyanidins shape distinct gut microbiota profile and influence glucose homeostasis and intestinal phenotypes in high-fat high-sucrose fed mice. Scientific reports 2020, 10, 2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Rodriguez-Daza MC, Roquim M, Dudonne S, Pilon G, et al. , Berry Polyphenols and Fibers Modulate Distinct Microbial Metabolic Functions and Gut Microbiota Enterotype-Like Clustering in Obese Mice. Frontiers in Microbiology 2020, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bati VV, Meleshko TV, Pallah OV, Zayachuk IP, Boyko NV, Personalised diet improve intestine microbiota and metabolism of obese rats. The Ukrainian Biochemical Journal 2021, 93, 77–92. [Google Scholar]
- [33].Huang F, Marungruang N, Kostiuchenko O, Kravchenko N, et al. , Identification of Nordic Berries with Beneficial Effects on Cognitive Outcomes and Gut Microbiota in High-Fat-Fed Middle-Aged C57BL/6J Mice. Nutrients 2022, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kwon O, Han TS, Son MY, Intestinal Morphogenesis in Development, Regeneration, and Disease: The Potential Utility of Intestinal Organoids for Studying Compartmentalization of the Crypt-Villus Structure. Front Cell Dev Biol 2020, 8, 593969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Verediano TA, Stampini Duarte Martino H, Dias Paes MC, Tako E, Effects of Anthocyanin on Intestinal Health: A Systematic Review. Nutrients 2021, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Witten J, Samad T, Ribbeck K, Selective permeability of mucus barriers. Curr Opin Biotechnol 2018, 52, 124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Suzuki T, Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci 2013, 70, 631–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hiippala K, Jouhten H, Ronkainen A, Hartikainen A, et al. , The Potential of Gut Commensals in Reinforcing Intestinal Barrier Function and Alleviating Inflammation. Nutrients 2018, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Grace MH, Esposito D, Dunlap KL, Lila MA, Comparative analysis of phenolic content and profile, antioxidant capacity, and anti-inflammatory bioactivity in wild Alaskan and commercial Vaccinium berries. J Agric Food Chem 2014, 62, 4007–4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Grondin JA, Kwon YH, Far PM, Haq S, Khan WI, Mucins in Intestinal Mucosal Defense and Inflammation: Learning From Clinical and Experimental Studies. Front Immunol 2020, 11, 2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE, Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiological reviews 2014, 94, 329–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wang Y, Chen Y, Zhang X, Lu Y, Chen H, New insights in intestinal oxidative stress damage and the health intervention effects of nutrients: A review. Journal of Functional Foods 2020, 75, 104248. [Google Scholar]
- [43].Cui X, Gong J, Han H, He L, et al. , Relationship between free and total malondialdehyde, a well-established marker of oxidative stress, in various types of human biospecimens. J Thorac Dis 2018, 10, 3088–3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Tonelli C, Chio IIC, Tuveson DA, Transcriptional Regulation by Nrf2. Antioxidants & redox signaling 2018, 29, 1727–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Andrews C, McLean MH, Durum SK, Cytokine Tuning of Intestinal Epithelial Function. Front Immunol 2018, 9, 1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sender R, Fuchs S, Milo R, Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell 2016, 164, 337–340. [DOI] [PubMed] [Google Scholar]
- [47].Valdes AM, Walter J, Segal E, Spector TD, Role of the gut microbiota in nutrition and health. BMJ (Clinical research ed 2018, 361, k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Petersen C, Wankhade UD, Bharat D, Wong K, et al. , Dietary supplementation with strawberry induces marked changes in the composition and functional potential of the gut microbiome in diabetic mice. J Nutr Biochem 2019, 66, 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Laterza L, Rizzatti G, Gaetani E, Chiusolo P, Gasbarrini A, The Gut Microbiota and Immune System Relationship in Human Graft-versus-Host Disease. Mediterr J Hematol Infect Dis 2016, 8, e2016025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Binda C, Lopetuso LR, Rizzatti G, Gibiino G, et al. , Actinobacteria: A relevant minority for the maintenance of gut homeostasis. Dig Liver Dis 2018, 50, 421–428. [DOI] [PubMed] [Google Scholar]
- [51].Shin NR, Whon TW, Bae JW, Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol 2015, 33, 496–503. [DOI] [PubMed] [Google Scholar]
- [52].Paturi G, Mandimika T, Butts CA, Zhu S, et al. , Influence of dietary blueberry and broccoli on cecal microbiota activity and colon morphology in mdr1a(−/−) mice, a model of inflammatory bowel diseases. Nutrition (Burbank, Los Angeles County, Calif 2012, 28, 324–330. [DOI] [PubMed] [Google Scholar]
- [53].Guo J, Han X, Zhan J, You Y, Huang W, Vanillin Alleviates High Fat Diet-Induced Obesity and Improves the Gut Microbiota Composition. Front Microbiol 2018, 9, 2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Cox AJ, West NP, Cripps AW, Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol 2015, 3, 207–215. [DOI] [PubMed] [Google Scholar]
- [55].Ijssennagger N, van der Meer R, van Mil SWC, Sulfide as a Mucus Barrier-Breaker in Inflammatory Bowel Disease? Trends Mol Med 2016, 22, 190–199. [DOI] [PubMed] [Google Scholar]
- [56].Portincasa P, Bonfrate L, Vacca M, De Angelis M, et al. , Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. International journal of molecular sciences 2022, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Lange KW, Omega-3 fatty acids and mental health. Global Health Journal 2020, 4, 18–30. [Google Scholar]
- [58].Tang W, Wang Y, Xu F, Fan W, et al. , Omega-3 fatty acids ameliorate cognitive dysfunction in schizophrenia patients with metabolic syndrome. Brain Behav Immun 2020, 88, 529–534. [DOI] [PubMed] [Google Scholar]
- [59].Davis HC, Can the gastrointestinal microbiota be modulated by dietary fibre to treat obesity? Irish Journal of Medical Science 2018, 187, 393–402. [DOI] [PubMed] [Google Scholar]
- [60].Okubo H, Nakatsu Y, Kushiyama A, Yamamotoya T, et al. , Gut Microbiota as a Therapeutic Target for Metabolic Disorders. Current medicinal chemistry 2018, 25, 984–1001. [DOI] [PubMed] [Google Scholar]
- [61].Marin L, Miguelez EM, Villar CJ, Lombo F, Bioavailability of dietary polyphenols and gut microbiota metabolism: antimicrobial properties. BioMed research international 2015, 2015, 905215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Le Bourvellec C, Bagano Vilas Boas P, Lepercq P, Comtet-Marre S, et al. , Procyanidin-Cell Wall Interactions within Apple Matrices Decrease the Metabolization of Procyanidins by the Human Gut Microbiota and the Anti-Inflammatory Effect of the Resulting Microbial Metabolome In Vitro. Nutrients 2019, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Zhang C, Franklin CL, Ericsson AC, Consideration of Gut Microbiome in Murine Models of Diseases. Microorganisms 2021, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Lacombe A, Tadepalli S, Hwang CA, Wu VC, Phytochemicals in lowbush wild blueberry inactivate Escherichia coli O157:H7 by damaging its cell membrane. Foodborne Pathog Dis 2013, 10, 944–950. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.