Abstract
Purpose:
To analyze the topographic distribution of neovascularization (NV) and capillary nonperfusion (CNP) using ultra-wide field fluorescein angiography (UWFFA) in patients with proliferative diabetic retinopathy (PDR).
Methods:
This was a prospective, single-center, observational study in which all patients who presented between March 2019 and December 2020 and satisfied the inclusion criteria were recruited. In our study, patients with treatment-naïve PDR without any fibrovascular proliferation underwent UWFFA. The images were analyzed qualitatively for the topographic distribution of NV and the CNP area was quantified. The number of lesions picked by UWFFA was compared with 7 standard field (7SF) image using overlay of 7SF. The main outcome measure was characteristics of neovascularization, such as the number, location, and area of CNP, measured using UWFFA, which was considered with 95% confidence intervals (CI).
Results:
Two hundred and fifty-three eyes of 187 patients with a mean age of 56.03 ± 8 years were included. Mean neovascularization elsewhere (NVE) was 2.91 ± 3.43. Maximum NVEs were seen in the superotemporal (ST; 0.9 ± 1.13) quadrant, followed by the inferotemporal (IT; 0.7 ± 1.08), inferonasal (IN; 0.66 ± 1.02) and superonasal (SN; 0.66 ± 1.01) quadrants. Maximum CNP area was seen in the SN (13.75 ± 8.83 disc diameter square [DD2]) quadrant, followed by the IN (13.48 ± 8.59 DD2), IT (11.34 ± 8.37 DD2), and ST (11.3 ± 8.34 DD2) quadrants. Mean CNP area was maximum in patients with only neovascularization of disc (NVD; 64.99 ± 41.47 DD2), followed by both NVD and NVE (61.37 ± 35.61 DD2), and was minimum in patients with only NVE (36.44 ± 22.03 DD2). Eighty-one (32%) eyes out of 253 had NVE and 189 (75%) out of 253 had CNP area outside 7SF (overlay) of Early Treatment Diabetic Retinopathy Study (ETDRS).
Conclusion:
Diabetic NV lesions and CNP areas are distributed asymmetrically throughout the retina and are not restricted to the posterior pole. Compared to conventional 7SF imaging, UWFFA reveals significantly more retinal vascular pathology in patients with PDR.
Keywords: Capillary nonperfusion, neovascularization, neovascularization at disc, proliferative diabetic retinopathy, ultra-wide field fluorescein angiography
Proliferative diabetic retinopathy (PDR) is characterized by development of neovascularization elsewhere (NVE) or neovascularization at the optic disc (NVD). These may lead to vitreous hemorrhage and tractional retinal detachment and are the most common causes of severe vision loss in diabetes mellitus (DM). Prompt detection of NVE/NVD during routine screening is essential for timely management and preservation of vision. Early Treatment Diabetic Retinopathy Study (ETDRS) 7 standard field (7SF) has been the conventional gold standard used in clinical and research settings for the diagnosis of diabetic retinopathy (DR). Regular screening with clinical examination and color fundus imaging alone may not be enough for the detection of neovascularization (NV). Subtle vascular changes such as an early NV may be easily missed even by an expert ophthalmologist. Fluorescein angiography may be used to supplement the screening protocol in patients with PDR as it can clearly demonstrate late leakage from NV as well as differentiate between other vascular abnormalities such as intraretinal microvascular abnormality. Adequate knowledge of topographic distribution of NV may help the clinician in early detection of PDR. Topographic distribution of NV in PDR has been studied in the past using color fundus images and conventional fluorescein angiography. This protocol is, however, limited by inability to visualize far periphery, which is the site of involvement in many diseases including DR. Wide-angle camera systems developed later enlarged field of view to 120° to 148°.[1] Latest generation of Optos ultra-wide field (UWF) imaging system gives a 200° field of view for clinical picture and angiography and is even useful in small pupil and hazy media due to unique placement of its ellipsoid mirrors.[2] This technology provides for more accurate and complete documentation of retinal characteristics and pathologies in DR.
Our study aims to analyze the topographic distribution of NV and capillary nonperfusion (CNP) areas using UWF fluorescein angiography (UWFFA).
Methods
Study design
This prospective observational study was conducted at a tertiary eye center in North India between March 2019 and December 2020. The study was done in accordance with the tenets of Declaration of Helsinki, and written informed consent was obtained from all the participants. Approval for the study was obtained from the institutional ethics board before patient enrollment (reference number IECPG-17/23.11.2019, RT-04/dated 02.28.2019).
Participants
Patients of type 1 or 2 DM with treatment-naïve PDR undergoing UWFFA were included in the study. Patients with any other ocular disease and media opacity such as cataract, vitreous hemorrhage, and advanced fibrovascular proliferation that preclude precise ocular and UWFFA examinations were excluded from the study.
Patient assessment
A detailed record of all the patients, including history, systemic and ocular examination, was maintained. Those meeting the inclusion criteria underwent UWFFA on either Optos 200Tx (Optos plc, Dunfermline, UK) or Optos California (Optos plc).
Image analysis
Angiographs were analyzed independently by two trained readers for characteristics including retinal NV and CNP areas. The magnification was set at 1.5 for all images. Graders were allowed to adjust brightness and contrast to optimize visualization of CNPs. Horizontal and vertical lines intersecting at the optic disc were overlaid on the UWFFA images to divide the image into superotemporal (ST), inferotemporal (IT), superonasal (SN), and inferonasal (IN) quadrants. NV was defined as a focal area of characteristic appearance of fine loops or network of vessels with leakage in the late phase. NVD was diagnosed when new vessels were located either on or within one disc diameter (DD) of the optic disc. If NV was further than 1 DD from the disc, it was classified as NVE. Areas of NVE were graded as separate foci if there was no contiguous leakage on early UWFFA frames, no NV connecting them, and there was a separation of at least a half DD. Large NVEs that spanned multiple quadrants were assigned to the quadrant containing the center of the lesion. Any NV in the macular area was termed as neovascularization at the macula (NVM). CNP was defined as any area of image appearing dark due to lack of fluorescein-filled capillaries. Border of CNPs was manually delineated using ImageJ version 1.49b (US National Institutes of Health, Bethesda, MD, USA). For CNP areas, DD was chosen to be the reference unit and the area was calculated in DD2.
For comparison with ETDRS 7SF, images were transferred to Adobe software (Adobe Systems, Inc, San Jose, CA, USA). Using the protocol described in ETDRS, 30° circles were combined to create a digital 7SF template.[3] This template was then overlaid on the UWFFA image to identify the potential viewable area of 7SF, and a final image was then evaluated.
Statistical analysis
Presentation of the categorical variables was done in the form of number and percentage (%). On the other hand, presentation of the continuous variables was done as mean ± standard deviation and median values. Comparison of the variables which were quantitative in nature was done using repeated measure analysis of variance (ANOVA), and post hoc comparison was done by Bonferroni correction. Data entry was done in the Microsoft Excel spreadsheet, and the final analysis was done with the use of Statistical Package for Social Sciences (SPSS) software version 21.0. For statistical significance, a P- value of less than 0.05 was considered as significant.
Results
A total of 253 eyes of 187 patients were included in the study, of which only four patients were of type 1 DM and the rest had type 2 DM. One eye of 121 (64.7%) patients and both eyes of 66 patients were included. Forty-eight patients (25.6%) were females and 139 (74.4%) were males. Of the 121 patients in whom PDR was found in one eye, 103 had right eye involvement and the rest had left eye involvement. The mean age at the time of diagnosis of PDR in our study was 56.03 ± 8 years. Four patients were less than 40 years of age, 45 patients were between 41 and 50 years of age, 92 patients were between 51 and 60 years of age, and 46 patients were more than 60 years of age. The mean vision was 0.14 ± 0.13 in decimal notation. Mean intraocular pressure was 16.47 ± 4.48 mmHg. The mean systolic blood pressure was 146.3 ± 13.23 mmHg and the mean diastolic blood pressure was 91.53 ± 6.98 mmHg. The mean glycated hemoglobin (HbA1c) was 8.5%, and the mean duration of diabetes was 10.93 ± 2.61 years.
NVE characteristics
NVEs were observed in 201 out of 253 eyes with PDR. Both NVD and NVE were observed in 81 eyes, and 120 eyes had only NVE with no concurrent NVD. In these 120 eyes, a single site of NVE was seen in 48 eyes (40%), whereas 72 (60%) had multiple sites. The total number of NVEs in all 201 eyes was 736. Mean number of NVEs (in 253 eyes) was 2.91 ± 3.43. Maximum number of NVEs was seen in the ST quadrant with a mean of 0.9 ± 1.13, followed by the IT (0.7 ± 1.08), IN (0.66 ± 1.02), and SN (0.66 ± 1.01) quadrants [Fig. 1]. There was a nasotemporal asymmetry in the distribution of NVEs, as 54.75% of lesions were located in the temporal hemisphere and 45.24% were located in the nasal hemisphere. The distribution of NVE in the superior and inferior hemispheres was 53.39% and 46.60% lesions, respectively.
Figure 1.
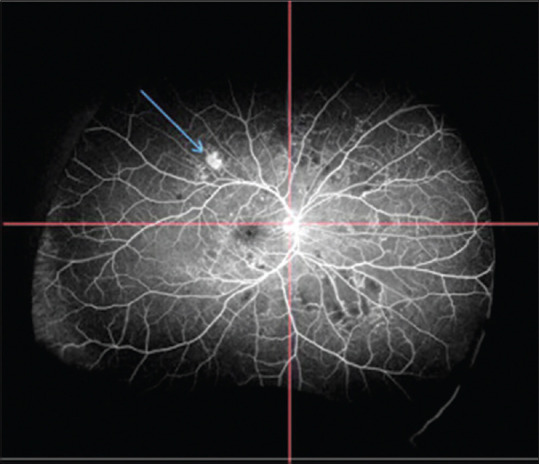
UWFA image of the patient divided into four quadrants showing NVE in the superotemporal quadrant. NVE = neovascularization elsewhere
NVD and NVM
NVD was observed in 133 (56.57%) of the 253 eyes with PDR. In 52 (20.55%) of these eyes, there was no concurrent NVE, whereas 81 eyes had both NVD and NVE. NV at the macula (NVM) was seen in 15 (5.93%) out of 253 eyes with PDR.
CNP characteristics
Mean area of CNP in 253 eyes was 49.86 ± 32.97 DD2. Maximum area of CNP was seen in the SN quadrant (13.75 ± 8.83 DD2), followed by the IN (13.48 ± 8.59 DD2), IT (11.34 ± 8.37 DD2), and ST (11.3 ± 8.34 DD2) quadrants [Fig. 2].
Figure 2.
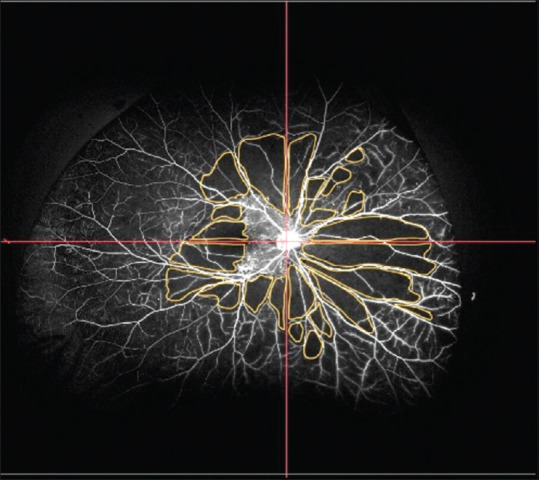
Quadrant-wise distribution of CNP areas in the UWFA images calculated using ImageJ (total CNP area: 62.13 DD2). CNP = capillary nonperfusion, DD2 = disc diameter square
Comparison of CNP area between NVD and NVE on UWFA
In patients with only NVD, the mean CNP area was higher in all four quadrants compared to the patients in whom only NVE was detected. Mean CNP area was maximum in patients with only NVD (64.99 ± 41.47 DD2), followed by patients with both NVD and NVE (61.37 ± 35.61 DD2), and was minimum in patients with only NVE (36.44 ± 22.03 DD2). This difference was statistically significant with P < 0.0001 [Fig. 3].
Figure 3.
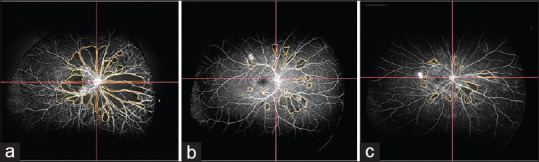
Total CNP areas in patients with NVD alone (62.13 DD2) (a), CNP areas in patient with NVD and NVE (29.32 DD2) (b), and CNP areas in patients with NVE only (16.58 DD2) (c). CNP = capillary nonperfusion, DD2 = disc diameter square, NVD = neovascularization of disc
Comparison of UWF image with 7SF (overlay) image
Eighty-one (32%) eyes out of 253 had NVE and 189 (75%) out of 253 had CNP area outside 7SF (overlay) of ETDRS. Mean CNP area outside ETDRS 7SF (overlay) was 23.47 ± 25.49 DD2. UWFFA was capable of capturing 24% more NVE and 37% more CNP area than the overlaid 7SF [Fig. 4].
Figure 4.
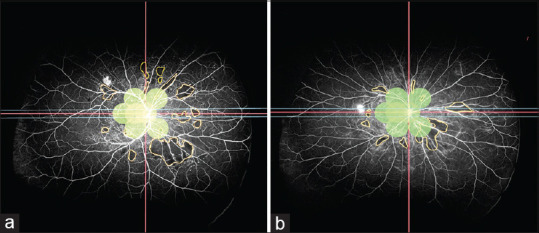
NVE and CNP areas outside 7 standard ETDRS 30° field calculated after overlapping the 7SF ETDRS field on the UWFA image. (a) 1 NVE with CNP area outside 7SF: 22.17 DD2. (b) 1 NVE with CNP area outside 7SF: 12.61 DD2. 7SF = 7 standard field, CNP = capillary nonperfusion, DD2 = disc diameter square, ETDRS = Early Treatment Diabetic Retinopathy Study, NVE = neovascularization elsewhere
Discussion
Early detection and management remains the cornerstone for avoiding complications such as vitreous hemorrhage and tractional retinal detachment secondary to NV in DR. The detection could be easier and more sensitive if the possible sites of NV are known. In the first ever study describing the topographic distribution of NVEs in PDR, Taylor and Dobree[4] used color photographs from the Zeiss fundus camera and found that maximum number of NVEs lie along the ST veins. Using ETDRS protocol, Feman et al.[5] found NVEs in 282 eyes of 189 patients and again reported maximum number of NV in the ST quadrant. Jansson et al.[6] used a digital fundus camera with 50°–60° fields of view and obtained six mydriatic photographs of each eye and created a montage. They found that the majority of NVEs are located IN to the optic disc and along the superior vascular arcades, while NVD had a predilection for ST rim of the optic disc. However, these studies were limited by small field and lack of fluorescein angiography, which can lead to missing peripheral and small NVEs, respectively.
UWF imaging has been shown to be superior to 7SF photography for evaluating DR in clinical settings.[7,8,9,10] In addition, UWFFA assists in clear delineation of CNP areas and differentiating NV from other DR lesions such as intraretinal microvascular abnormalities. Therefore, we evaluated the eyes with treatment-naïve PDR with UWFFA for the presence of NV and CNP areas. To study topographic distribution, we divided the image with its center at the optic disc instead of macula, unlike most previous studies.[6] The optic disc is a more appropriate center for the division of NVE and CNP areas into quadrants since it is the entry point for the blood vessels that supply the retina and is a true vascular center. Since DR is a vascular disease, using a vascular center should be more appropriate. Taking the macula as the center can lead to false localization of the retinal area between the optic disc and the macula as nasal. Furthermore, to see if UWFFA is actually helpful in detecting additional NV and CNP areas, which would be missed otherwise, we overlapped ETDRS 7SF over UWFFA images and evaluated the number of NV and CNP areas lying beyond the scope of ETDRS 7SF.
While our study was underway, a retrospective study by Russel et al.[11] also evaluated the distribution of diabetic NV on UWFFA using similar protocol for Quadrant division and found that NVE was most prevalent in the ST quadrant. However, unlike our study, it was a retrospective study with a single grader using simulated widefield Optical coherence tomography angiography (OCTA) field of view. Also, distribution of CNP areas and benefit of UWF imaging over ETDRS 7SF was not evaluated in their paper.
Majority of the eyes in our study showed multiple NVEs (60%), which might indicate that NV starts simultaneously at different locations in an ischemic retina. In contrast to our study, Feman et al.[5] found a single site in 230 eyes (81.6%) and multiple sites in 52 eyes (18.4%). This discrepancy may be because patients tend to report later in developing countries compared to those in the developed world.
In our study, there was a nasotemporal asymmetry in the distribution of the NVEs, as 55% of lesions were located in the temporal and 45% in the nasal hemisphere. This was similar to data by Jansson et al.[6] (60% temporally and 40% nasally). The possible hypothesis for this could be because of differential vitreous degeneration and retinal thickness in the temporal and nasal hemispheres. The distribution of NVE in the superior and inferior hemispheres was 53% and 47%, respectively, which was similar to that reported by Jansson et al.[6] (54% lesions in the superior hemisphere and 46% lesions in the inferior hemisphere).
Our study reported higher prevalence of NVD compared to the aforementioned studies [Table 1]. This discrepancy could be related to the different study populations and the periods in which the studies were conducted. Also, the study population reported by Jansson et al.[6] consisted of patients with early PDR identified by screening, whereas the populations described by Taylor and Dobree,[4] Russell et al.,[11] and our study represented patients presenting for routine clinical care and not an organized screening program and may be more likely to have a relatively advanced stage of PDR.
Table 1.
Comparison of NVE only, NVD only, and both NVD and NVE in our study with previous studies
| Study | NVD only (%) | NVE only (%) | Both NVD and NVE (%) |
|---|---|---|---|
| Taylor and Dobree[4] | 16.3 | 27 | 56.7 |
| Jansson et al.[6] | 5 | 63 | 32 |
| Russel et al.[11] | 10 | 50 | 40 |
| Present study | 21 | 47 | 32 |
NVD=neovascularization of disc, NVE=neovascularization elsewhere
Determining the area of CNP accurately may help in planning more conservative management approaches such as targeted laser photocoagulation or selective scatter photocoagulation and also assist in determining more appropriate follow-up intervals for these patients by identifying the degree of nonperfusion.[12,13]
In our study, UWFFA was capable of capturing 24% of total NVEs and 37% more CNP area than the overlaid 7SF. Only one previous study by Wessel et al.[8] compared UWFA images with overlapping ETDRS 7SF. They reported that UWFFA demonstrated retinal pathology (including nonperfusion and NV) not evident in a 7SF overly in 10% of eyes. They showed 3.9 times more nonperfusion and 1.9 times more NV on UWFA compared to ETDRS 7SF. Price et al.[7] graded the severity of DR and assigned a higher retinopathy level to 19% of the images in the UWF view compared to the corresponding ETDRS 7SF view. Similarly, Silva et al.[10] showed that additional peripheral lesions identified by UWF images resulted in a more severe assessment of DR in 10% of eyes than was suggested by the lesions within the ETDRS fields. They also found one-third of total NVE lesions lying outside of the ETDRS 7SF. Talks et al.[9] showed that Optomap UWFFA images detected approximately 30% more peripheral NV compared to standard two-field imaging in patients referred from a UK DR screening service. Findings of these studies along with those of our study suggest that UWFFA is a preferred modality for assessment of PDR.
Ours is the only study to date that has evaluated the relative quadrant-wise distribution of CNP areas in cases of PDR [Fig. 2]. The finding in our study that CNP areas are more in the nasal hemisphere and the number of NVEs are more in the temporal hemisphere could mean that vascular endothelial growth factor (VEGF) produced somewhere else in the fundus can lead to NV at some other site. Silva et al.[10] found that changes in retinal microvasculature were found in the upper temporal quadrant in the early stage of the disease, but the lower temporal quadrant, which is responsible for supplying the macula, was spared. They proposed that this difference was most likely due to a differential regulation of blood flow. It is our contention that the difference in RNFL thickness and vessel caliber in different quadrants of the retina and differential blood flow makes some vessels more prone to development of NVE.
Higher mean CNP area in patients with NVD compared to those without them could suggest that the presence of NVD indicates a more advanced stage of PDR. This has been suggested in earlier studies as well.[14,15,16] Shimizu et al.[14] showed that nonperfusion became more extensive in the group with NVD than with retinal NV using FA montage technique covering an area of 130°, while Deckert et al.[15] demonstrated better prognosis in patients with NVE than NVD. However, ours is the only study to date that has evaluated the relative quadrant-wise distribution of CNP areas in cases of PDR.
There are multiple limitations to our study. UWFFA images suffer from spherical distortions and warping in the periphery since a 3D structure is read in a 2D form. To perform statistics on the number of NVE in equal areas of the fundus, we divided the image with the center at the optic disc. This assumption that each quadrant or hemisphere comprises an equal area of the ocular fundus might lead to erroneous results. The clinical estimation of the CNP area using the optic DD as a reference may also lead to an inaccurate measurement of the most peripherally located lesions. Also, our study does not directly compare an actual 7SF angiography with an actual UWFFA in the same eye. Rather, using software, we overlaid an idealized, simulated 7SF on an actual UWFA. Traditional 7SF may have yielded different findings than single images with 7SF overlay.
Conclusion
Diabetic NV lesions and CNP areas are distributed asymmetrically throughout the retina. NVEs are maximum in the ST quadrant, and the CNP area is maximum in the SN quadrant. Topographic distribution of NVE gives us the preferential sites of new vessel formation. This is important in early detection of NV and its early treatment, particularly in the very early stages of PDR. UWFFA is an important tool for precise quantification and characterization of nonperfused areas in PDR and may be preferred over 7SF of ETDRS.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Pomerantzeff O. Equator-plus camera. Invest Ophthalmol. 1975;14:401–6. [PubMed] [Google Scholar]
- 2.Shoughy S, Arevalo JF, Kozak I. Update on wide- and ultra-widefield retinal imaging. Indian J Ophthalmol. 2015;63:575–81. doi: 10.4103/0301-4738.167122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Early treatment diabetic retinopathy study design and baseline patient characteristics. ETDRS report number 7. Ophthalmology. 1991;98((5 Suppl)):741–56. doi: 10.1016/s0161-6420(13)38009-9. [DOI] [PubMed] [Google Scholar]
- 4.Taylor E, Dobree JH. Proliferative diabetic retinopathy. Site and size of initial lesions. Br J Ophthalmol. 1970;54:11–8. doi: 10.1136/bjo.54.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feman SS, Leonard-Martin TC, Semchyshyn TM. The topographic distribution of the first sites of diabetic retinal neovascularization. Am J Ophthalmol. 1998;125:704–6. doi: 10.1016/s0002-9394(98)00013-0. [DOI] [PubMed] [Google Scholar]
- 6.Jansson RW, Frøystein T, Krohn J. Topographical distribution of retinal and optic disc neovascularization in early stages of proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2012;53:8246–52. doi: 10.1167/iovs.12-10918. [DOI] [PubMed] [Google Scholar]
- 7.Price LD, Au S, Chong NV. Optomap ultrawide field imaging identifies additional retinal abnormalities in patients with diabetic retinopathy. Clin Ophthalmol Auckl NZ. 2015;9:527–31. doi: 10.2147/OPTH.S79448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wessel MM, Aaker GD, Parlitsis G, Cho M, D'Amico DJ, Kiss S. Ultra-wide-field angiography improves the detection and classification of diabetic retinopathy. Retina Phila Pa. 2012;32:785–91. doi: 10.1097/IAE.0b013e3182278b64. [DOI] [PubMed] [Google Scholar]
- 9.Talks SJ, Manjunath V, Steel DHW, Peto T, Taylor R. New vessels detected on wide-field imaging compared to two-field and seven-field imaging:Implications for diabetic retinopathy screening image analysis. Br J Ophthalmol. 2015;99:1606–9. doi: 10.1136/bjophthalmol-2015-306719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silva PS, Cavallerano JD, Sun JK, Soliman AZ, Aiello LM, Aiello LP. Peripheral lesions identified by mydriatic ultrawide field imaging:Distribution and potential impact on diabetic retinopathy severity. Ophthalmology. 2013;120:2587–95. doi: 10.1016/j.ophtha.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Russell JF, Flynn HW, Sridhar J, Townsend JH, Shi Y, Fan KC, et al. Distribution of diabetic neovascularization on ultra-widefield fluorescein angiography and on simulated widefield OCT angiography. Am J Ophthalmol. 2019;207:110–20. doi: 10.1016/j.ajo.2019.05.031. [DOI] [PubMed] [Google Scholar]
- 12.Nikkhah H, Ghazi H, Razzaghi MR, Karimi S, Ramezani A, Soheilian M. Extended targeted retinal photocoagulation versus conventional pan-retinal photocoagulation for proliferative diabetic retinopathy in a randomized clinical trial. Int Ophthalmol. 2018;38:313–21. doi: 10.1007/s10792-017-0469-7. [DOI] [PubMed] [Google Scholar]
- 13.Venkatesh P. Selective Scatter Photocoagulation (SSP) in Patients with Proliferative Diabetic Retinopathy without High Risk Characteristics. J Laser Opt Photonics. 2014;1:2. [Google Scholar]
- 14.Shimizu K, Kobayashi Y, Muraoka K. Midperipheral fundus involvement in diabetic retinopathy. Ophthalmology. 1981;88:601–12. doi: 10.1016/s0161-6420(81)34983-5. [DOI] [PubMed] [Google Scholar]
- 15.Deckert T, Simonsen SE, Poulsen JE. Prognosis of proliferative retinopathy in juvenile diabetics. Diabetes. 1967;16:728–33. doi: 10.2337/diab.16.10.728. [DOI] [PubMed] [Google Scholar]
- 16.Fan W, Nittala MG, Velaga SB, Hirano T, Wykoff CC, Ip M, et al. Distribution of nonperfusion and neovascularization on ultrawide-field fluorescein angiography in proliferative diabetic retinopathy (RECOVERY Study):Report 1. Am J Ophthalmol. 2019;206:154–60. doi: 10.1016/j.ajo.2019.04.023. [DOI] [PubMed] [Google Scholar]


