Abstract
Three clinical isolates, Enterobacter cloacae EC1562 and EC1563 and Citrobacter freundii CFr564, displayed an aminoglycoside resistance profile evocative of low-level 6′-N acetyltransferase type II [AAC(6′)-II] production, which conferred reduced susceptibility to gentamicin but not to amikacin or isepamicin. Aminoglycoside acetyltransferase assays suggested the synthesis in the three strains of an AAC(6′) which acetylated amikacin practically as well as it acetylated gentamicin in vitro. Both compounds, however, as well as isepamicin, retained good bactericidal activity against the three strains. The aac genes were borne by conjugative plasmids (pLMM562 and pLMM564 of ca. 100 kb and pLMM563 of ca. 20 kb). By PCR mapping and nucleotide sequence analysis, an aac(6′)-Ib gene was found in each strain upstream of an ant(3")-I gene in a sulI-type integron. The size of the AAC(6′)-Ib variant encoded by pLMM562 and pLMM564, AAC(6′)-Ib7, was deduced to be 184 (or 177) amino acids long, whereas in pLMM563 a 21-bp duplication allowing the recruitment of a start codon resulted in the translation of a variant, AAC(6′)-Ib8, of 196 amino acids, in agreement with size estimates obtained by Western blot analysis. Both variants had at position 119 a serine instead of the leucine typical for the AAC(6′)-Ib variants conferring resistance to amikacin. By using methods that predict the secondary structure, these two amino acids appear to condition an α-helical structure within a putative aminoglycoside binding domain of AAC(6′)-Ib variants.
Bacterial resistance to the aminoglycoside group of antibiotics in the clinical setting is predominantly due to drug-modifying enzymes: acetyltransferases, nucleotidyltransferases, and phosphotransferases. Their production can be inferred from the susceptibility profiles of the host strains, from substrate profiles, or from DNA-DNA hybridization patterns (34). Aminoglycoside-resistant strains most often emerge after acquisition of plasmid-borne enzyme-encoding genes (9). Many of these genes may be carried on mobile genetic elements including integrons and transposons (14, 25, 39), and these may aid in the dissemination of drug resistance.
Among the acetyltransferases, four families whose members acetylate amino groups at the 1, 2′, 3, or 6′ position have been identified (36). All members of the 6′-N-acetyltransferase [AAC(6′)] family acetylate kanamycin, tobramycin, netilmicin, and sisomicin. When the members of AAC(6′) are of type I [AAC(6′)-I], they also modify amikacin and, to a lesser degree, isepamicin but not gentamicin C1. At least 13 distinct genes, designated aac(6′)-Ia through aac(6′)-Il (8, 15, 20, 21, 36, 37) and aacA7 (5), are now known to encode such enzymes. When the members of AAC(6′) are of type II [AAC(6′)-II], they modify gentamicin but not amikacin and isepamicin. Presently, at least two distinct AAC(6′)-II-encoding genes, aac(6′)-IIa and aac(6′)-IIb, are known (35, 36).
Recently, it was demonstrated that different modifications of the amino acid sequence of the AAC(6′) proteins influence their enzymatic activities. Rather et al. (31), studying AAC(6′)-I and AAC(6′)-II proteins, identified amino acids responsible for the differences in substrate specificity and assigned a decisive role to the amino acid at position 119, where a leucine was correlated with amikacin resistance and a serine was correlated with gentamicin resistance. More subtle differences in the relative acetylation efficiencies of AAC(6′)-Ib enzymes have been related to N-terminal size variations in in vitro truncated enzymes (3, 4). Similar variations occur in naturally produced Ib-type acetyltransferases (10, 22, 23, 43), and these variations probably account for the heterogeneities observed in clinical isolates (42). Three clinical isolates of Citrobacter freundii or Enterobacter cloacae were found, upon inspection of their antibiograms, to be resistant to netilmicin and tobramycin, to have intermediate susceptibility to gentamicin, and to be susceptible to amikacin and isepamicin. This aminoglycoside resistance profile was evocative of a low-level production of an AAC(6′)-II, which is not known to occur in these species. In this report, we present a characterization of the AAC(6′) variants produced by the three bacterial isolates, based on substrate profiles and immunoblot analysis and on the nucleotide sequence of the corresponding genes. We also examined the consequences of the altered substrate specificity of these enzymes on the bactericidal activities of gentamicin, amikacin, and isepamicin against the acetyltransferase-producing strains.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The relevant characteristics of the bacterial strains and plasmids used in this study are listed in Table 1. E. cloacae EC1562 and EC1563 and C. freundii CFr564 were isolated at the Saint-Louis Hospital in Paris, France. Recipient strains were Escherichia coli HB101 and DH5α for bacterial conjugation (1) and E. coli JM101 for transfection with M13 bacteriophage vectors. Cultures were grown on Mueller-Hinton (MH) agar or in MH broth (Sanofi Diagnostics Pasteur); however, E. coli JM101 was grown in 2× yeast tryptone broth (Difco).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Resistance phenotypea or derivation | Source or reference |
|---|---|---|
| Strain | ||
| E. coli | ||
| HB101 | Strr | 24 |
| JM101 | 24 | |
| DH5α | Nalr | Clonetech |
| E. cloacae | ||
| ECl562(pLMM562) | Netr Tobr Genr [AAC(6′)-Ib7]b Strr Spcr Sulr, clinical isolate | This study |
| ECl563(pLMM563) | Netr Tobr Genr [AAC(6′)-Ib8] Strr Spcr Sulr Cmpr Tetr, clinical isolate | This study |
| C. freundii CFr564(pLMM564) | Netr Tobr Genr [AAC(6′)-Ib7] Strr Spcr Suli, clinical isolate | This study |
| P. aeruginosa PAO 38 | leu-38 Strr | 30 |
| Plasmid | ||
| pAZ505 | 1.5-kb fragment [AAC(6′)-Ib] cloned in pBR322 | 43 |
| pLMM6 | Ca. 100-kb plasmid [AAC(6′)-Ib2] from KPn88 | 4 |
| pSCH884 | 24-kb plasmid [AAC(6′)-IIa] from SCH80090884 | 35 |
Abbreviations: Cmp, chloramphenicol; Gen, gentamicin; Net, netilmicin; Str, streptomycin; Spc, spectinomycin; Sul, sulfonamide (Suli, reduced susceptibility to Sul); Tet, tetracycline; Tob, tobramycin.
Antibiotic susceptibility testing.
Disk diffusion tests were performed on MH agar. The aminoglycoside disks were generously provided by Schering-Plough Research Institute. Testing for resistance to mercuric ions was carried out as described previously (17). The MICs of the aminoglycosides were determined, after serial twofold dilution of the antibiotics, on MH agar with inocula of 105 to 106 CFU/ml. The following antibiotics were generously provided by the indicated companies: amikacin and kanamycin, Bristol-Myers Squibb; gentamicin, isepamicin, netilmicin, 2′-N-ethylnetilmicin, 6′-N-ethylnetilmicin, and sisomicin, Schering-Plough Research Institute; tobramycin, Eli Lilly & Company.
Bacterial killing kinetics.
Killing curves were established with gentamicin, amikacin, and isepamicin at drug concentrations fourfold higher than the MICs. One milliliter of an overnight culture in MH broth was inoculated into 9 ml of fresh broth, and the mixture was incubated on a shaker at 37°C for 3 to 4 h to reach an optical density at 650 nm of ca. 0.4. The bacterial suspension was adjusted to between 105 and 106 CFU/ml, and 9.9 ml of MH broth containing the antibiotic to be tested was inoculated with 100 μl of the bacterial suspension. Samples were removed at timed intervals and were serially diluted 10-fold with broth. Aliquots of 100 μl were plated in duplicate onto MH agar. The plates were incubated overnight at 37°C, and the number of colonies was counted. The lower limit of detection was ca. 10 CFU/ml.
Assay of aminoglycoside acetyltransferase activity.
Acetylating activity was assayed by the phosphocellulose paper-binding assay described previously (13) by using [1-14C]acetyl coenzyme A (0.6 GBq/mmol; Amersham International) as the cofactor. To prepare crude cell extracts containing the enzyme, bacteria were grown overnight in L broth, harvested by centrifugation, and disrupted by sonication in ice-cold buffer (50 mM Tris HCl, 50 mM NH4Cl, 10 mM MgCl2, 10 mM β-mercaptoethanol [pH 7.5]). The supernatant, obtained after centrifugation at 100,000 × g for 45 min, was used for the assay.
Immunoblot analysis.
Cell extracts were subjected to electrophoresis on sodium dodecyl sulfate-containing polyacrylamide gels (19). After electrotransfer of the proteins to polyvinylidene difluoride membranes (Millipore), the acetyltransferases were revealed with an anti-AAC(6′)-Ib rabbit antiserum and peroxidase-labelled anti-rabbit immunoglobulin G as described previously (42).
DNA-DNA hybridization.
Small-scale preparations of plasmid DNA were obtained as described previously (18) and were analyzed by agarose (0.8%) gel electrophoresis. For Southern blot analysis and hybridization, plasmid DNA was transferred to Gene-Screen plus filters (NEN Research Products) and hybridized with [32P]dCTP-labelled probes by using the Multiprime labelling kit, as recommended by the manufacturer (Amersham International). Autoradiography was carried out with Kodak X-Omat AR film.
DNA amplification by PCR.
An internal probe for the aac(6′)-Ib gene was prepared by amplification of a 535-bp fragment from plasmid pAZ505 by PCR with the oligonucleotide primers AN4 (5′-CGCGCGGATCCAAAGTTAGGCATCACA-3′) combined with AC6 (5′-ACCTGTACAGGCTGGAC-3′), corresponding to nucleotide positions 378 to 393 and 915 to 899, respectively, of the aac(6′)-Ib gene (43). For nucleotide sequence determination, a fragment extending from the integrase gene to the ant(3")-I gene was amplified from plasmids pLMM562, pLMM563, and pLMM564 by using the oligonucleotide primers AN1 (5′-CTGTTCGTTCGTAAGC-3′) corresponding to nucleotide positions 1046 to 1062 of the integrase gene (2) and AC2 (5′-GCGCGCAAGCTTGCGGAGCCGTACAAATG-3′) complementary to positions 1139 to 1122 of the ant(3")-I gene (43). Amplification by PCR was performed in 100 μl of a reaction mixture containing 10 mM Tris HCl (pH 8.3); 50 mM KCl; 1.5 mM MgCl2; 0.1 mM (each) dATP, dTTP, dGTP, and dCTP; 0.5 U of Taq DNA polymerase (Boehringer Mannheim); and 10 ng of template DNA. The mixture was overlaid with mineral oil and heated to 92°C for 4 min, followed by 40 cycles of 1 min at 92°C, 1 min at 55°C, and 1 min at 72°C.
Nucleotide sequence determination.
The PCR-generated products were electrophoresed on agarose (1.2%) gels and purified by using the Gene Clean II kit (Bio 101, Inc.). After cloning into M13mp18 and M13mp19 and transfection into E. coli JM101, the nucleotide sequences of both strands were determined by the dideoxy chain-termination method by using the T7 sequencing kit (Pharmacia) and [33P]dATP (11,100 GBq/mol; NEN) according to the recommendations of the supplier. The nucleotide sequences were verified in a second set of independent experiments in which the PCR products were sequenced directly by using deazanucleotides and a thermal cycle DNA sequencing system (fmol Sequencing; Promega).
Prediction of secondary protein structure.
The secondary protein structure was predicted by following a procedure which defines a consensus derived from four different methods (11, 12).
Nucleotide sequence accession number.
The nucleotide sequences of the aac(6′)-Ib genes of pLMM562 and pLMM563 have been assigned EMBL accession numbers Y11946 and Y11947, respectively.
RESULTS
Resistance patterns and antimicrobial activities.
According to the inhibition zone diameters observed by the disk diffusion method, the clinical isolates ECl562, ECl563, and CFr564 were resistant to kanamycin, tobramycin, netilmicin, and 2′-N-ethylnetilmicin, moderately resistant to gentamicin, and susceptible to 6′-N-ethylnetilmicin, amikacin, and isepamicin (Table 2 and data not shown). The three strains were also resistant to mercuric chloride, streptomycin, and spectinomycin. Strains ECl562 and ECl563 were additionally resistant to sulfonamides, as was CFr564, but less so. The transconjugants of E. coli HB101 carrying plasmid pLMM562, pLMM563, or pLMM564 (see below) expressed the same resistance phenotype as the clinical strains, in which a diameter smaller around the 2′-N-ethylnetilmicin disk than around the 6′-N-ethylnetilmicin disk (15 to 20 mm and greater than 33 mm, respectively) and a diameter of greater than 35 mm around the fortimicin disk indicated aminoglycoside AAC(6′) activity (38). A moderately decreased susceptibility to gentamicin was evocative of low-level AAC(6′)-II activity. However, after determination of the MICs, resistance to gentamicin was not obvious. The MICs of gentamicin and amikacin were equally low for the wild strains and their transconjugants (2 and 2 μg/ml and 4 and 4 μg/ml, respectively) (Table 2).
TABLE 2.
Susceptibilities of bacterial strains to selected aminoglycosides
| Strain (plasmid) | MIC (μg/ml)a
|
|||||
|---|---|---|---|---|---|---|
| KAN | TOB | NET | GEN | AMI | ISE | |
| E. cloacae | ||||||
| ECl562(pLMM562) | 64 | 16 | 8 | 2 | 2 | 0.5 |
| ECl563(pLMM563) | 128 | 16 | 16 | 4 | 4 | 1 |
| C. freundii CFr564 (pLMM564) | 32 | 8 | 4 | 2 | 2 | 0.5 |
| P. aeruginosa PAO28 (pSCH884) | >256 | >256 | >256 | 128 | 4 | 4 |
| E. coli | ||||||
| HB101 | 1 | 0.25 | 0.12 | 0.25 | 0.5 | 0.25 |
| HB101(pLMM562) | 64 | 4 | 2 | 1 | 1 | 0.25 |
| HB101(pLMM563) | 128 | 8 | 8 | 2 | 1 | 0.5 |
| HB101(pLMM564) | 32 | 4 | 2 | 2 | 1 | 0.25 |
| DH5α(pAZ505) | 128 | 128 | 64 | 1 | 32 | 8 |
KAN, kanamycin; TOB, tobramycin; NET, netilmicin; GEN, gentamicin; AMI, amikacin; ISE, isepamicin.
The killing kinetics obtained with gentamicin, amikacin, and isepamicin at four times their MICs for the respective strains and their transconjugants are presented in Fig. 1. They showed that gentamicin, like amikacin and isepamicin, had bactericidal activity within 18 h against CFr564 and ECl562, and no bacterial regrowth was observed. All three drugs had lower bactericidal activities against ECl563, and regrowth occurred after about 6 h in the presence of gentamicin and amikacin but not in the presence of isepamicin.
FIG. 1.
In vitro killing kinetics for the three clinical strains and their respective transconjugants producing AAC(6′)-Ib variants. (A) ECl562; (B) ECl563; (C) CFr564; (D) HB101(pLMM562); (E) HB101(pLMM563); (F) HB101(pLMM564). The concentrations (in micrograms per milliliter) of amikacin (Ami), isepamicin (Ise), and gentamicin (Gen) used were four times the MICs for the respective strains.
Characteristics of the aminoglycoside acetyltransferases.
The phosphocellulose paper binding assay confirmed that aminoglycoside resistance was due to acetyltransferase production in the three clinical strains and their transconjugants, and the modification of 2′-N-ethylnetilmicin but not 6′-N-ethylnetilmicin confirmed that the enzymes modified the 6′-amino group. With kanamycin acetylation set at 100% (ca. 20,000 cpm) and the values averaged from three independent experiments, the percentage of acetylation with the other aminoglycosides was as follows: tobramycin, 47 to 66%; netilmicin, 44 to 59%; sisomicin, 46 to 65%; gentamicin, 84 to 92%; amikacin, 75 to 87%; and isepamicin, 43 to 56%. This substrate profile differed from those typically observed for AAC(6′)-Ib (42) or AAC(6′)-IIa (35, 36), in that it was intermediate between the profiles for the two enzymes. The substrate profiles obtained by this assay did not allow the discrimination between the acetyltransferases produced by the three strains.
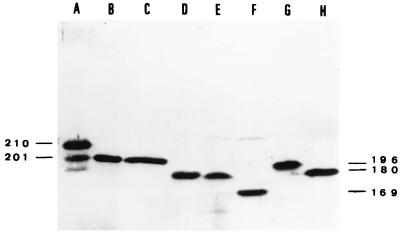
The cell extracts from the wild strains CFr564, ECl562, and ECl563 and their transconjugants were analyzed by immunoblotting with anti-AAC(6′)-Ib antibodies. All strains produced a protein which reacted with the antiserum (Fig. 2). The AAC(6′) from ECl563 had an apparent Mr of ca. 22,000, close to that of AAC(6′)-Ib with 200 amino acids (43), but those from ECl562 and CFr564 were clearly smaller, with Mr values of ca. 20,000 or ca. 178 amino acids, as estimated by comparison with AAC(6′)-Ib variants truncated in vitro (4).
FIG. 2.
Immunoblot of the AAC(6′)-Ib variants. Lane A, KPn88(pLMM6) producing AAC(6′)-Ib2 of 210 amino acids (4); lane B, DH5α(pAZ505) producing AAC(6′)-Ib of 200 amino acids (42); lane C, ECl563 producing AAC(6′)-Ib8; lane D, CFr564; lane E, ECl562 producing AAC(6′)-Ib7; lanes F, G, and H, in vitro-truncated AAC(6′)-Ib variants coded for by PCR-generated fragments derived from the natural plasmid pLMM6 and cloned into pBTac2 (3, 4). The numbers of amino acids of the reference variants are given at the margins: lane F, 169 amino acids; lane G, 196 amino acids; lane H, 180 amino acids.
Transfer of aminoglycoside resistance.
The aminoglycoside resistance determinants of the three clinical isolates were transferred by conjugation to E. coli HB101, and the transconjugants acquired the full resistance pattern of the donors (Table 2). Southern blot analysis and hybridization with an aac(6′)-Ib probe indicated that the aminoglycoside resistance determinants in ECl562 and CFr564 were borne by plasmids that were ca. 100 kb but that were not identical in size (pLMM562 and pLMM564, respectively) and by a ca. 20-kb plasmid, pLMM563, in ECl563 (data not shown).
Nucleotide sequences of the aac(6′) determinants and neighboring sequences.
Aminoglycoside resistance determinants encoding acetyltransferases and adenylyltransferases are often organized as gene cassettes in integrons (14). The association of the aac(6′) genes with resistance markers for mercuric chloride (mer) and sulfonamide (sulI) and also with markers for streptomycin and spectinomycin resistance [ant(3")-I], as is known to occur in integrons often carried by members of the Tn21 family of transposable elements (2, 25), suggested that the aac(6′) genes in the three clinical strains studied might be borne by an integron.
Using PCR mapping, we determined the order of the aminoglycoside resistance genes inserted between the 5′ and 3′ conserved integron segments. In all three strains we found an aac(6′)-I upstream of an ant(3")-I gene. The DNA fragments extending from nucleotide 1046 in the integrase gene (2) to nucleotide 1139 in ant(3")-I (43) were sequenced and found to be 980 nucleotides in length in pLMM562 and pLMM564 and 1,001 nucleotides in pLMM563.
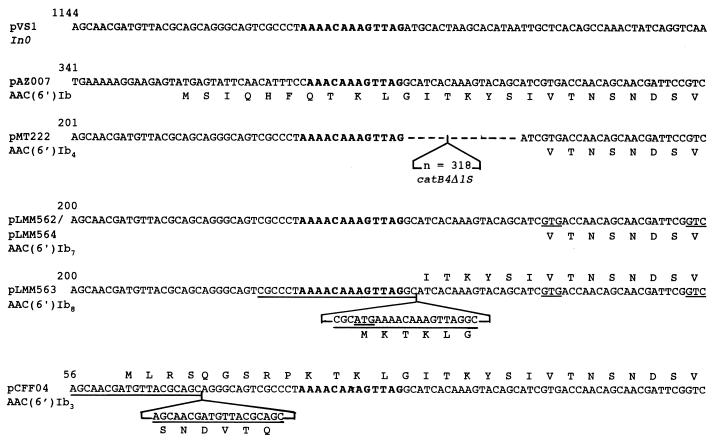
In pLMM562 and pLMM564 the nucleotide sequences were identical and contained an open reading frame (ORF) with several potential translation start codons. Translational initiation at the ATG codons at position 352 or 372 (cf. accession number Y11946) or the GTG codon at position 316 (Fig. 3) would yield proteins of 172, 165, or 184 amino acids, respectively, with calculated molecular sizes of 19.1, 18.3, or 22.4 kDa, respectively, values either smaller or larger than the estimated value. If the GTC at position 337 (Fig. 3) functioned as a start codon, a protein of 177 amino acids and 19.5 kDa, closest to the estimated size (Fig. 2), would be produced. The coding sequences for very similar aac(6′)-Ib genes, inserted in the same context and with the same ambiguity concerning the start codon, have been reported previously in Pseudomonas aeruginosa BM2556 (10) and Pseudomonas fluorescens BM2657 (22). In pLMM563, an ORF of 591 nucleotides with a potential ATG start codon at position 301 and a coding capacity of 196 amino acids was identified (cf. accession number Y11947). This was in reasonable agreement with the apparent size of the protein estimated by immunoblotting (Fig. 2). Upstream from the possible start codons of the aac(6′)-I genes of pLMM562 and pLMM564, in an otherwise similar nucleotide stretch, a close to perfect 21-bp duplication (three mismatches) was noted in pLMM563, and this duplication overlapped the recombinational hot spot (CTAAAACAAAGTTA) (6, 29, 32, 33) on both sides and contained an ATG in frame (cf. Fig. 3).
FIG. 3.
Nucleotide sequences at the 5′-common-segment regions of integration of aac gene cassettes. These data were compiled from the sequences obtained from plasmids pVS1 (2), pCFF04 (23), pMT222 (41), and pAZ007 (43) and those analyzed here. Numbering of the nucleotides is according to the respective references. The conserved motif of the recombination sites is shown in boldface. The duplicated sequences and the possible start codons are underlined. Amino acids are designated by the single-letter code.
Downstream from the hot spot, the aac(6′)-Ib sequences of the three plasmids differed only slightly from that of the prototype aac(6′)-Ib sequence (43). All coded for a Ser119 instead of Leu and for Ser199 and Asp200, like the aac(6′)-Ib of plasmid pSa (40). Except for the shorter N termini, the deduced amino acid sequence of the pLMM562- and pLMM564-encoded variants, AAC(6′)-Ib7, possibly starting with Val18 or Val25, was identical to that of the prototype AAC(6′)-Ib (43). AAC(6′)-Ib8, assumed to be five amino acids shorter than AAC(6′)-Ib (43), had a Lys in the position corresponding to Gln7 of AAC(6′)-Ib (Fig. 3).
Partial secondary AAC(6′) structure predictions.
An in vitro-generated Leu119-to-Ser change in AAC(6′)-Ib, believed to have occurred in an aminoglycoside binding domain, has previously been associated with a slight reduction in local hydrophobicity (31). However, no structural data are available for AAC(6′) proteins or related enzymes, including the aminoglycoside binding domain(s). To support some speculation on the possible structural properties of such a domain, an array of secondary structure prediction methods (11, 12) was applied to the conserved region immediately surrounding position 119 (or its equivalent) in AAC(6′)-Ib and AAC(6′)-IIa variants. As shown in Fig. 4, the derived consensus clearly predicted the region surrounding Leu at this position as an α helix in AAC(6′)-Ib (amino acids 115 to 127), with a strong probability that the N-terminal portion of this helix is shortened in variants with Ser instead of Leu. Similarly, in AAC(6′)-IIa, the presence of the experimentally introduced Leu (31) favored the prediction of α-helix formation, but it was shorter than that in AAC(6′)-Ib, while the existence of the naturally occurring Ser did not favor such a prediction.
FIG. 4.
Partial secondary structure prediction for AAC(6′)-Ib variants based on the methods indicated at the left, as described by Gourgeon and Delage (11, 12). AAC(6′)-Ib (AMIR > GENR), sequence of AAC(6′)-Ib conferring resistance to amikacin but not to gentamicin (43); AAC(6′)-Ib (AMIR = GENR), sequence of AAC(6′)-Ib variants conferring similar low levels of resistance to gentamicin and amikacin in members of the family Enterobacteriaceae (this study); AAC(6′)-IIa (GENR > AMIR), AAC(6′)-IIa conferring resistance to gentamicin but not amikacin (35); AAC(6′)-IIa (AMIR > GENR), AAC(6′)-IIa variant obtained after replacement of Ser119 with Leu conferring resistance to amikacin but not gentamicin (31). Abbreviations: C, coil; E, β-sheet; H, helix; S, bend; T, turn.
DISCUSSION
An aminoglycoside resistance phenotype unusual in members of the family Enterobacteriaceae has been observed in two clinical isolates of E. cloacae and one clinical isolate of C. freundii. Inspection of the antibiogram suggested low-level production of an AAC(6′)-II which confers reduced susceptibility to gentamicin but not to amikacin or isepamicin. This type of enzyme typically occurs only in P. aeruginosa (35). Curiously, no difference between the MICs of gentamicin and amikacin for any of the three strains was observed. The filter binding assay of the aminoglycoside-modifying enzymes produced by the three clinical isolates and the E. coli transconjugants carrying the resistance genes of the clinical isolates indicated that the unusual phenotype was due to the synthesis of an AAC(6′) which acetylated amikacin as well as it acetylated gentamicin, but it acetylated isepamicin somewhat less. The three compounds retained good bactericidal activity against the clinical strains ECl562 and CFr564, while ECl563 was less well killed by gentamicin and amikacin, and there was no obvious difference between the killing kinetics for the clinical strains and the corresponding E. coli transconjugants (Fig. 1).
Nucleotide sequence analysis of the genes responsible for the particular phenotype of aminoglycoside susceptibility of E. cloacae and C. freundii showed that they were closely related, downstream from the recombinational hot spot (29, 33) (Fig. 3), to aac(6′)-Ib (43). Comparison of the deduced amino acid sequences suggested that the two enzymes described here might be variants of an AAC(6′)-Ib such as that coded for by plasmid pSA, even though its sequence has been established only partially (40). All three acetyltransferases have the amino acids Ser119, Ser199, and Asp200 in common, at variance with the amino acids in AAC(6′)-Ib (43), to which the amino acid numbering refers (36). With plasmid pSA initially described in 1968 (44), it is conceivable that these variants predate those from more recent clinical isolates which confer resistance to amikacin (10, 23, 43). The amino acid at position 119 has been found to be critical functionally in that a Leu-to-Ser switch at this position was responsible for the loss of amikacin resistance and the acquisition of gentamicin resistance (31). Despite the differences in substrate specificity, the AAC(6′)-Ib variants are fully functional enzymes, which implies that the Leu119-to-Ser change does not fundamentally alter global protein folding. It has been suggested that the presence of a free amino group in gentamicin as opposed to a hydroxy-amino-butyl group in amikacin at position 1 might be a critical difference for revealing the substrate specificities of the AAC(6′)-I and AAC(6′)-II enzymes and that both amino groups could interact with Ser119 (27). While the Leu-to-Ser change has been predicted to result in a local change of hydrophobicity (31), the methods for predicting secondary structure applied here reveal the possibility that the putative binding domain or part of it contains an α-helical structure and that a Leu119-to-Ser change reduces the probability of such a secondary structure (Fig. 4). Taken together, these observations raise the possibility that the presence of serine, a small (73 Å3) polar amino acid capable of establishing hydrogen bonding, or leucine, a larger (124 Å3) hydrophobic amino acid, at position 119 (or its equivalent) also conditions the local conformation of the putative aminoglycoside binding domain (or part of it) in AAC(6′)-I and AAC(6′)-II enzymes.
Many of the aminoglycoside acetyl- and adenylyltransferase genes are localized in integrons within Tn21-like elements. There they constitute individual mobile units, called gene cassettes, that can be inserted into and excised from the integron by site-specific recombination catalyzed by IntI (7, 26, 39). In this study, the aac(6′)-Ib cassettes found in members of the family Enterobacteriaceae, like those found in Pseudomonas species (10, 22), were inserted adjacent to the 5′-conserved segment of an integron in a Tn21-like element. In pAZ007 (43) and Tn1331 (28), the aac(6′)-Ib gene was inserted as a cassette in a Tn3-type transposon, together with the ant(3")-I gene, and its expression resulted from the fusion, at the recombinational hot spot, of its ORF with the ORF of the TEM-1 β-lactamase (43). Comparison of the published 5′ sequences flanking the aac(6′)-Ib cassette junctions reveals that this region displays considerable genetic plasticity. The minor variations in the molecular weights of the AAC(6′)-Ib proteins observed previously (42) appear to be due to variations in the N-terminal sequences, which in turn depend upon the generation or placement in phase of translational start codons by nucleotide rearrangements resulting from the insertional events. In most instances, the putative start codon lies downstream from the hot spot, and its distance to the cassette junction is quite short. On the other hand, a tandem duplication of 19 bp “moved” the start codon for the AAC(6′)-Ib variants encoded by pCFF04 and pMG7 (23) [like for AAC(3)-I (45), for which it was confirmed by N-terminal sequencing (16)] to 54 bp upstream from the cassette boundary, leading to the synthesis of AAC(6′) proteins of a predicted length of 210 amino acids. A novel configuration was observed in the gene coding for the AAC(6′)-Ib8 variant, in which a 21-bp duplication overlapped the recombinational hot spot with the creation of a possible start codon (A302TG) (Fig. 3) for a deduced protein of 196 amino acids. When the insertion of the aac(6′)-Ib cassette occurred without apparent sequence rearrangements, as in pLMM562 and pLMM564, translation initiation could occur at G316TG, yielding a protein of 184 amino acids, in modest agreement with the estimation from the immunoblot (Fig. 2) or maybe, in better agreement, at G337TC, although this codon is not known to serve in the initiation of translation. Whichever the codon used, neither is preceded by an apparent ribosome binding site.
The diversity of the genetic rearrangements associated with aac(6′)-Ib cassette insertions in transposon-borne integrons related to or derived from In0 (Fig. 3) is indicative of rather flexible structural requirements for the N terminus of AAC(6′)-Ib variants which may be a factor contributing to their predominance among the aminoglycoside-resistant members of the family Enterobacteriaceae.
ACKNOWLEDGMENTS
This study was funded in part by a grant (CRI 95 06 01) from the Institut National de la Santé et de la Recherche Médicale, Paris, France.
We are grateful to G. Miller for communicating initial hybridization results and helpful discussions and to P. H. Lagrange for support. We thank C. Harcour for secretarial assistance.
REFERENCES
- 1.Ausubel F M, Brent F, Kingston R E, Moore D D, Smith J A, Seidman J G, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1993. [Google Scholar]
- 2.Bissonnette L, Roy P H. Characterization of In0 of Pseudomonas aeruginosa plasmid pVS1, an ancestor of integrons of multiresistance plasmids and transposons of gram-negative bacteria. J Bacteriol. 1992;174:1248–1257. doi: 10.1128/jb.174.4.1248-1257.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bordon-Pallier F, Collatz E. Program and abstracts of 32nd Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1992. Structural and functional analysis of naturally occurring variant of AAC(6′)-Ib and in vitro truncated derivatives. abstr. 440; p. 194. [Google Scholar]
- 4.Bordon-Pallier, F. Unpublished results.
- 5.Bunny K L, Hall R M, Stokes H W. New mobile gene cassettes containing an aminoglycoside resistance gene, aacA7, and a chloramphenicol resistance gene, catB3, in an integron in pBH301. Antimicrob Agents Chemother. 1995;39:686–693. doi: 10.1128/AAC.39.3.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collis C M, Grammatiticopoulos G, Briton J, Stokes H W, Hall R M. Site specific insertion of gene cassettes into integrons. Mol Microbiol. 1993;9:41–52. doi: 10.1111/j.1365-2958.1993.tb01667.x. [DOI] [PubMed] [Google Scholar]
- 7.Collis C M, Hall R M. Site-specific deletion and rearrangement of integron insert genes catalysed by the integron DNA integrase. J Bacteriol. 1992;174:1574–1585. doi: 10.1128/jb.174.5.1574-1585.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costa Y, Galimand M, Leclercq R, Duval J, Courvalin P. Characterization of the chromosomal aac(6′)-Ii gene specific for Enterococcus faecium. Antimicrob Agents Chemother. 1993;37:1896–1903. doi: 10.1128/aac.37.9.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies J, Smith D I. Plasmid determined resistance to antimicrobial agents. Annu Rev Microbiol. 1978;32:469–518. doi: 10.1146/annurev.mi.32.100178.002345. [DOI] [PubMed] [Google Scholar]
- 10.Galimand M, Lambert T, Gerbaud G, Courvalin P. Characterization of the aac(6′-Ib gene encoding an aminoglycoside 6′-N-acetyltransferase in Pseudomonas aeruginosa BM2656. Antimicrob Agents Chemother. 1993;37:1456–1462. doi: 10.1128/aac.37.7.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gourgeon C, Delage G. A self optimised prediction method for protein secondary structure prediction. Protein Eng. 1994;7:157–164. doi: 10.1093/protein/7.2.157. [DOI] [PubMed] [Google Scholar]
- 12.Gourgeon C, Delage G. SOPMA: significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Comput Appl Biosci. 1995;11:681–684. doi: 10.1093/bioinformatics/11.6.681. [DOI] [PubMed] [Google Scholar]
- 13.Haas M J, Dowding J E. Aminoglycoside-modifying enzymes. Methods Enzymol. 1975;43:611–628. doi: 10.1016/0076-6879(75)43124-x. [DOI] [PubMed] [Google Scholar]
- 14.Hall R M, Collis C M. Mobile gene cassettes and integrons: capture and spread of genes by site-specific recombination. Mol Microbiol. 1995;15:593–600. doi: 10.1111/j.1365-2958.1995.tb02368.x. [DOI] [PubMed] [Google Scholar]
- 15.Hannecart-Pokorni E, Depuydt F, De Wit L, Van Bossuyt E, Content J, Vanhoof R. Characterization of the 6′-N-aminoglycoside acetyltransferase gene aac(6′)-Il associated with a sulI-type integron. Antimicrob Agents Chemother. 1997;41:314–318. doi: 10.1128/aac.41.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsiang M W, White T J, Davies J E. NH2-terminal sequence of the aminoglycoside acetyltransferase(3)-I mediated by plasmid RIP135. FEBS Lett. 1978;92:97–99. doi: 10.1016/0014-5793(78)80730-3. [DOI] [PubMed] [Google Scholar]
- 17.Jacoby G A, Sutton L, Knobel L, Mammen P. Properties of IncP-2 plasmids of Pseudomonas spp. Antimicrob Agents Chemother. 1983;24:168–175. doi: 10.1128/aac.24.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kado C I, Liu S T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981;145:1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.Lambert T, Gerbaud G, Galimand M, Courvalin P. Characterization of Acinetobacter haemolyticus aac(6′)-Ig gene encoding an aminoglycoside 6′-N-acetyltransferase which modifies amikacin. Antimicrob Agents Chemother. 1993;37:2093–2100. doi: 10.1128/aac.37.10.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lambert T, Gerbaud G, Courvalin P. Characterization of the chromosomal aac(6′)-Ij gene of Acinetobacter sp. 13 and the aac(6′)-Ih plasmid gene of Acinetobacter baumannii. Antimicrob Agents Chemother. 1994;38:1883–1889. doi: 10.1128/aac.38.9.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambert T, Ploy M C, Courvalin P. A spontaneous point mutation in the aac(6′)-Ib′ gene results in altered substrate specificity of aminoglycoside 6′-N-acetyltransferase of a Pseudomonas fluorescens strain. FEMS Microbiol Letters. 1994;115:297–304. doi: 10.1111/j.1574-6968.1994.tb06654.x. [DOI] [PubMed] [Google Scholar]
- 23.Mabilat C, Lourençao-Vital J, Goussard S, Courvalin P. A new example of physical linkage between Tn1 and Tn21: the antibiotic multiple-resistance region of plasmid pCFF04 encoding extended-spectrum β-lactamase TEM-3. Mol Gen Genet. 1992;235:113–121. doi: 10.1007/BF00286188. [DOI] [PubMed] [Google Scholar]
- 24.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 25.Martinez E, de la Cruz F. Genetic elements involved in Tn21 site specific integration: a novel mechanism for the dissemination of antibiotic resistance genes. EMBO J. 1990;9:1275–1281. doi: 10.1002/j.1460-2075.1990.tb08236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez E, de la Cruz F. Transposon Tn21 encodes a RecA- independent site-specific integration system. Mol Gen Genet. 1988;211:320–325. doi: 10.1007/BF00330610. [DOI] [PubMed] [Google Scholar]
- 27.Miller G H, Sabatelli F J, Naples L, Hare R S, Shaw K J. The changing nature of aminoglycoside resistance mechanisms and the role of isepamicin. A new broad-spectrum aminoglycoside. J Chemother. 1995;7:S31–S44. [PubMed] [Google Scholar]
- 28.Nobuta K, Tolmasky M E, Crosa L M, Crosa J H. Sequencing and expression of the 6′-N-acetyltransferase gene of transposon Tn1331 from Klebsiella pneumoniae. J Bacteriol. 1988;170:3769–3773. doi: 10.1128/jb.170.8.3769-3773.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ouellette M, Bissonnette L, Roy P H. Precise insertion of antibiotic resistance determinants into Tn21-like transposons: nucleotide sequence of the OXA-1 β-lactamase gene. Proc Natl Acad Sci USA. 1987;84:7378–7382. doi: 10.1073/pnas.84.21.7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pemberton J M, Holloway B W. Chromosome mapping in Pseudomonas aeruginosa. Genet Res. 1972;19:251–260. doi: 10.1017/s0016672300014518. [DOI] [PubMed] [Google Scholar]
- 31.Rather P N, Munayyer H, Mann P A, Hare R S, Miller G H, Shaw K J. Genetic analysis of bacterial acetyltransferases: identification of amino acids determining the specificities of the aminoglycoside 6′-N-acetyltransferase Ib and IIa proteins. J Bacteriol. 1992;174:3196–3203. doi: 10.1128/jb.174.10.3196-3203.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Recchia G D, Stokes H W, Hall R M. Characterization of specific and secondary recombination sites recognized by the integron integrase. Nucleic Acids Res. 1994;22:2071–2078. doi: 10.1093/nar/22.11.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt F R J, Nucken E J, Henschke R B. Structure and function of hot spots providing signals for site-directed specific recombination and gene expression in Tn21 transposons. Mol Microbiol. 1989;3:1545–1555. doi: 10.1111/j.1365-2958.1989.tb00140.x. [DOI] [PubMed] [Google Scholar]
- 34.Shaw K J, Hare R S, Sabatelli F J, Rizzo M, Cramer C A, Naples L, Kocsi S, Munayyer H, Mann P, Miller G H, Verbist L, Van Landuyt H, Glupczynski Y, Catalano M, Wolo M. Correlation between aminoglycoside resistance profiles and DNA hybridization of clinical isolates. Antimicrob Agents Chemother. 1991;35:2253–2261. doi: 10.1128/aac.35.11.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shaw K J, Kramer C A, Rizzo M, Mierzwa R, Gewain K, Miller G H, Hare R S. Isolation, characterization, and DNA sequence analysis of an aac(6′)-II gene from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1989;33:2052–2062. doi: 10.1128/aac.33.12.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaw K J, Rather P N, Hare R S, Miller G H. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol Rev. 1993;57:138–163. doi: 10.1128/mr.57.1.138-163.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaw K J, Rather P N, Sabatelli F J, Mann P, Munayyer H, Mierzwa R, Petrikkos G L, Hare R S, Miller G H, Bennett P, Downey P. Characterization of the chromosomal aac(6′)-Ic gene from Serratia marcescens. Antimicrob Agents Chemother. 1992;36:1447–1455. doi: 10.1128/aac.36.7.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimizu K, Kumada T, Hsieh W, Chung H, Chong Y, Hare R S, Miller G H, Sabatelli F J, Howard J. Comparison of aminoglycoside resistance patterns in Japan, Formosa, and Korea, Chile, and the United States. Antimicrob Agents Chemother. 1985;28:282–288. doi: 10.1128/aac.28.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stokes H W, Hall R M. A novel family of potentially mobile DNA elements encoding site-specific gene-integration functions: integrons. Mol Microbiol. 1989;3:1669–1683. doi: 10.1111/j.1365-2958.1989.tb00153.x. [DOI] [PubMed] [Google Scholar]
- 40.Tait R, Rempel H, Rodriguez R L, Kado C I. The aminoglycoside-resistance operon of the plasmid pSa nucleotide sequence of the streptomycin-spectinomycin resistance gene. Gene. 1985;36:97–104. doi: 10.1016/0378-1119(85)90073-3. [DOI] [PubMed] [Google Scholar]
- 41.Toriya M, Sakakibara M, Matsustita K, Morohoshi T. Nucleotide sequence of aminoglycoside 6′-N-acetyltransferase [AAC(6′)] determinant from Serratia sp45. Chem Pharm Bull. 1992;40:2473–2477. doi: 10.1248/cpb.40.2473. [DOI] [PubMed] [Google Scholar]
- 42.Tran Van Nhieu G, Bordon F, Collatz E. Incidence of an aminoglycoside 6′-N-acetyltransferase, AAC (6′)-1b, in amikacin-resistant clinical isolates of gram-negative bacilli, as determined by DNA-DNA hybridization and immunoblotting. J Med Microbiol. 1992;36:83–88. doi: 10.1099/00222615-36-2-83. [DOI] [PubMed] [Google Scholar]
- 43.Tran Van Nhieu G, Collatz E. Primary structure of an aminoglycoside 6′-N-acetyltransferase, AAC(6′)-4, fused in vivo with the signal peptide of the Tn3-encoded β-lactamase. J Bacteriol. 1987;169:5708–5714. doi: 10.1128/jb.169.12.5708-5714.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watanabe T, Furuse C, Sakaizumi S. Transduction of various R-factors by phage P22 in Salmonella typhimurium. J Bacteriol. 1968;96:1791–1795. doi: 10.1128/jb.96.5.1791-1795.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wohlleben W, Arnold W, Bissonnette L, Pelletier A, Tangay A, Roy P H, Gamboa G C, Barry G F, Aubert E, Davies J, Kagan S A. On the evolution of Tn21-like multiresistance transposons: sequence analysis of the gene (aacC1) for gentamicin acetyltransferase-3-I (AAC(3)-I), another member of the Tn21-based expression cassette. Mol Gen Genet. 1989;217:202–208. doi: 10.1007/BF02464882. [DOI] [PubMed] [Google Scholar]