Abstract
Background:
Mucosal-associated invariant T cells (MAITs) are markedly reduced in patients with alcohol-associated liver disease (ALD); however, the potential mechanism underlying MAITs’ loss remains elusive. Hence, we aimed to explore what induced MAITs’ loss and its clinical significance.
Methods:
The characteristics of pyroptotic MAITs were evaluated in a cohort of patients with ALD, including 41 patients with alcohol-associated liver cirrhosis (ALC) and 21 patients with ALC complicated with severe alcoholic hepatitis (ALC + SAH).
Results:
In patients with ALD, blood MAITs were significantly decreased, hyperactivated, and displayed enhanced cell death through pyroptosis. The frequencies of pyroptotic MAITs increased with disease severity in patients with ALC and patients with ALC + SAH. These frequencies were negatively associated with the frequencies of MAITs and positively correlated with the levels of MAITs’ activation, plasma levels of intestinal fatty acid-binding protein (a marker of intestinal enterocyte damage), soluble CD14, lipopolysaccharide-binding protein, and peptidoglycan recognition proteins (surrogate markers of microbial translocation). Pyroptotic MAITs were also found in the liver of patients with ALD. Interestingly, MAITs underwent further activation and pyroptosis in vitro under stimulation by Escherichia coli or direct bilirubin. Notably, blocking IL-18 signaling reduced the activation and frequencies of pyroptotic MAITs.
Conclusions:
The loss of MAITs in patients with ALD is, at least in part, due to cell death from pyroptosis and is associated with the severity of ALD. Such increased pyroptosis may be affected by dysregulated inflammatory responses to intestinal microbial translocation or direct bilirubin.
INTRODUCTION
Alcohol-associated liver disease (ALD) is a progressive cause of various illnesses worldwide.1 Alcohol abstinence and symptomatic support are available; however, there are no specific and effective treatment options for ALD. Clinically, ALD patients are at high risk for decompensation of liver function, and ~2% of patients are predisposed to HCC per year.2 Therefore, there is an urgent need to explore new immunopathogenesis mechanisms during ALD progression for the development of clinical treatment strategies.
Mucosal-associated invariant T cells (MAITs), newly identified innate-like T lymphocytes, have been reported to play a critical role in antimicrobial immunity. Typically, MAITs express a semi-invariant T-cell receptor (TCR) alpha chain (Va7.2-Ja33) paired with a restricted number of Vβ chains.3,4 In healthy people, MAITs constitute up to 38% of T cells in the liver, 17% in the intestinal mucosa, and 6% in peripheral blood or the lung and are found less in other tissues.5 MAITs can be activated in a TCR-dependent manner by recognizing microorganism-derived riboflavin intermediate metabolites (5-OP-RU and 5-OE-RU) present in the major histocompatibility complex-I molecule (MR1) of antigen-presenting cells. In addition, MAITs can also be activated by several cytokines in a TCR/MR1-independent manner, including IL-12, IL-15, IL-18, and type-I interferons.6,7 On activation, MAITs are capable of secreting various effector molecules, such as IFN-γ, TNF α, and granzymes.8 In addition, they express a variety of tissue repair-related genes in concert with microbial infection exposure or an inflammatory immune response.9
Some properties of MAITs in liver disease have been investigated. It is noteworthy that MAITs were decreased in both peripheral blood and liver tissue, regardless of pathogen exposure, in most liver diseases, which has corresponding clinical significance. In patients with chronic HBV infection, MAITs were decreased, which was inversely associated with serum direct bilirubin (DBIL) levels and correlated with disease progression.10 The MAITs’ count was further decreased in patients with HBV-related liver failure and was positively correlated with their overall survival rate.11 In patients with chronic HCV infection, intrahepatic MAITs decrease with the severity of liver inflammation and fibrosis stage.12 Notably, the frequencies of MAITs could be partially improved in patients with HCV infection, whereas circulating MAITs in patients with HCV/HIV coinfection failed to recover even through effective antiviral therapy.12,13 A profound loss of MAITs was also observed in patients with autoimmune liver disease and NAFLD.14,15 Recovery of MAITs’ frequencies was observed after ursodeoxycholic acid treatment for 6 months or lifestyle intervention.16,17 In HBV-related HCC and cholangiocarcinoma, both the frequencies and absolute numbers of MAITs were markedly decreased and probably occurred in response to the increased bacterial burden within the tumor microenvironment, whereas high tumor-infiltrating MAITs were closely linked to unfavorable clinical outcomes.18,19 Collectively, the decrease in MAITs is related to the progression of chronic liver disease.
In patients with ALD, decreased MAITs’ frequencies were also observed, which could be regarded as an indicator of the severity of liver cirrhosis.20 Furthermore, abnormalities in MAITs cannot be completely restored through long-term alcohol abstinence in patients with ALD.21 Riva et al.22 also suggested that the depletion of MAITs may not be the direct effect of alcohol on MAITs due to the lack of MAITs’ loss after ethanol treatment in vitro but rather may be associated with long-term exposure to cytokines or bacterial antigens derived from intestinal microorganisms. Indeed, some studies have demonstrated that intestinal microbiota disorder is implicated in liver damage and the pathogenesis of ALD.23,24 A higher risk for ALD progression mainly results from the alterations in microbiota composition, destruction of the intestinal mucosal barrier, and the immune response activated by the transfer of microbial products to the liver. MAITs, as a pivotal part of liver antibacterial host defense, are bidirectional communication between intestinal microorganisms and ALD progress. Therefore, it is reasonable to speculate that the loss of MAITs is a direct consequence, at least in part, of microbial translocation; however, the specific immunological mechanisms underlying MAITs’ loss are poorly understood and warrant further exploration to provide evidence for preventing ALD progression.
METHODS
Clinical participants
Patients with ALD (n = 62), who have a long history of excessive alcohol consumption (>40 g/d, over 5 y) together with the indication of liver cirrhosis diagnosed using ultrasonography, MRI, CT techniques, or liver biopsy, were enrolled in the study based on diagnostic criteria.25 These patients with ALD were further divided into 2 groups, including patients with alcohol-associated liver cirrhosis (ALC) (n = 41) and patients with ALC + severe alcoholic hepatitis (SAH) (n = 21) based on Maddrey discriminant function (MDF) scores of more than 32. Individuals with chronic viral hepatitis, autoimmune liver disease, DILI, liver cancer, and other severe coexisting diseases, such as serious cardiovascular and cerebrovascular diseases, were excluded. Age-matched and sex-matched healthy donors are considered as healthy controls (HCs) (n=38). Participants were recruited from the Fifth Medical Center of the Chinese PLA General Hospital, and signed informed consent was obtained from each participant. Ethical approvals were obtained from The First Affiliated Hospital of Zhengzhou University and the Fifth Medical Center of the Chinese PLA General Hospital, and the study was performed in accordance with the 2013 Declaration of Helsinki. The clinical characteristics of the participants are summarized in Table 1.
TABLE 1.
Clinical characteristics of study participants
| Characteristics | HC (n = 38) | ALC (n = 41) | ALC + SAH (n = 21) |
|---|---|---|---|
| Age, y, n (%) | 40 (32, 53) | 47 (40, 52) | 44 (38, 49) |
| Male/female | 35/0 | 42/0 | 21/0 |
| White Blood Cell, × 109/L | 5.11 (3.83, 5.81) | 3.65 (2.81, 4.9) | 8.32 (3.92, 12.11) |
| Neutrophils, × 109/L | 2.21 (2.08, 3.32) | 2.10 (1.58, 3.34) | 6.45 (2.47, 9.56) |
| Lymphocytes, × 109/L | 1.12 (0.86, 1.67) | 0.93 (0.62, 1.22) | 1.19 (0.92, 1.77) |
| Monocytes, × 109/L | 0.27 (0.21, 0.39) | 0.36 (0.27, 0.47) | 0.60 (0.39, 0.94) |
| Platelet, × 109/L | 248 (137, 283) | 74.5 (59.25, 97.25) | 83 (51, 166.5) |
| Hemoglobin, g/L | 136 (127, 149) | 106.5 (85.25, 128.25) | 90 (83.50, 102.5) |
| Prothrombin time, s | 12.6 (11.9, 13.6) | 14.75 (13.48, 15.75) | 19.5 (18.55, 24.2) |
| International normalized ratio | 0.96 (0.92, 1.07) | 1.33 (1.19, 1.45) | 1.75 (1.60, 2.3) |
| Total bilirubin, μmol/L | 12.7 (8.7, 17.9) | 27.00 (16.6, 40.93) | 153.80 (95.85, 310.15) |
| Direct bilirubin, μmol/L | 4.3 (2.7, 5.4) | 11.7 (6.7-23.93) | 123 (61.2–227.8) |
| Alanine aminotransferase, U/L | 19 (14, 28) | 21.00 (14.75, 27.25) | 26 (17, 36) |
| Aspartate transaminase, U/L | 21 (12, 27) | 34 (28, 50.25) | 59.00 (41, 97) |
| Albumin, g/L | 44 (39, 48) | 29 (26, 33.25) | 28.00 (25.5, 31.5) |
| Gamma-glutamyl transferase, U/L | 24 (16, 27) | 54 (25, 126.75) | 64 (31.5, 264.5) |
| Creatinine, μmol/L | 73 (63, 87) | 72 (65.75, 84) | 75.00 (65, 101) |
| MDF Score | — | 14 (9, 23) | 46 (35, 71.5) |
| MELD Score | — | 5 (2.5, 8) | 16 (13, 21) |
| Child-Pugh Score | — | 8 (7, 9) | 11 (11, 13) |
Note: Data are presented as median (interquartile range) or number (percent).
Abbreviations: ALC, alcohol-associated liver cirrhosis; ALC + SAH, ALC, complicated with severe alcoholic hepatitis; HC, healthy control; MDF, Maddrey discriminant function; MELD, model for end-stage liver disease.
Flow cytometric analysis
The MR1 5-OP-RU APC-labeled tetramer was kindly provided by the American National Institutes of Health Tetramer Facility. Other fluorescence-conjugated reagents and antibodies were purchased from BD Biosciences and BioLegend, including anti-CD3-BV510, anti-TCR Vα7.2-BV421, anti-CD161-PE, anti-CD4-Percp/Cyanine5.5, anti-CD8-APC/Cy7, anti-CD38-BV785, anti-HLA-DR-Percp/Cyanine5.5, anti-PD-1-BV421, anti-CCR7-BUV395, anti-CD45RA-BV605, anti-Bcl-2-AF647, anti-CD57-Percp/Cyanine5.5, anti-CD49d-BV510, anti-Ki-67-BV786, and anti-Cyclin D1-FITC. The FAM-FLICA caspase-1 reagent (Bio-Rad) was used to measure the pyroptosis rate of MAITs. Fixable viability stain 700 (BD Biosciences) was used to distinguish viable from nonviable peripheral blood mononuclear cells (PBMCs).
Imaging flow cytometry analysis
PBMCs (1 × 106) were first stained with FLICA caspase-1 at 37 °C for 1 hour, followed by MR1 5-OP-RU tetramer staining at 4 °C for 40 minutes and incubation with anti-CD3 and anti-CD161 at 4 °C for 30 minutes. A single staining tube was used as a control to determine the threshold for the staining channel. Stained samples were fixed in 50-µL 1% paraformaldehyde (Sigma-Aldrich), and 50,000 single cells per sample were acquired and imaged under ×40 magnification on an Amnis ImageStreamX Mark II Flow Cytometer (Luminex). Graphs were generated using the IDEAS 6.2 software.
Stimulation assay
PBMCs were suspended in RPMI 1640 medium (Sigma-Aldrich) mixed with 10% fetal bovine serum (Sigma-Aldrich) and 1% 100-IU/mL penicillin and streptomycin (4A Biotech) and then treated in round bottom 96 well plates (1 × 106 cells/200 µL/well) at 37 °C and 5% CO2 as follows: (1) medium only for 24 or 72 hours; (2) 1% PFA-fixed Escherichia coli (E. coli) DH5α (10 bacteria per cell) for 24 hours in the absence or the presence of anti-IL-18Rα antibody (20 µg/mL, R&D Systems); and (3) conjugated bilirubin (100 µmol/L, Cayman) for 72 hours.
Multiplex immunofluorescence measurement
The tyramide signal amplification technique and Akoya Opal 7-color fluorescence staining kit (Akoya Biosciences) were used to detect the distribution of MAITs in the liver. The control liver tissue (n = 3) procured from deceased donors deemed acceptable for liver transplantation, and liver tissues (n = 4) from patients with ALC, who underwent liver transplantation, were obtained from the Department of Organ Transplantation, Tianjin First Central Hospital. Briefly, liver tissue sections (5 µm) were incubated with purified primary antibodies: anti-CD3 (EP41, 1:300, Zhongshan Golden Bridge Biotechnology), anti-TCR Vα7.2 (REA179, 1:500, Miltenyi Biotec), anti-IL-18Rα (polyclonal, 1:200, R&D Systems), and anticleaved gasdermin D (Asp275, 1:500, Abcam) for 60 minutes at 37 °C and then washed with antimouse/rabbit secondary antibodies labeled with horseradish peroxidase (HRP) staining (10 min, 37 °C). After signal amplification with ×1 tyrosine signal amplification solution and DAPI staining, all slides were scanned and imaged using a Vectra 3 Multispectral Imaging System (Akoya Biosciences) at ×10–40 magnification. The density information of TCR Vα7.2+IL-18+ cells and TCR Vα7.2+IL-18+GSDMD+ cells was summarized in 3 representative visual fields selected from each slide. The data were analyzed using the Inform Version 2.5.0 Image Analysis Software.
ELISA
Plasma concentrations of soluble CD14 (sCD14), lipopolysaccharide-binding protein (LBP), peptidoglycan recognition proteins (PGRPs), intestinal fatty acid-binding protein (I-FABP), and IL-18 were detected using human sCD14 (R&D Systems), human LBP (Wuhan Boster Biological Technology Co., Ltd.), human PGRPs (Wuhan Fine Biotech Co., Ltd.), FABP2/I-FABP (R&D Systems), and IL-18 (R&D Systems) ELISA kits according to the manufacturer’s instructions, and plasma samples were diluted 1:500, 1:1000, 1:2, 1:10, and 1:20, respectively.
Statistical analysis
All statistical data were calculated and analyzed using the IBM SPSS Statistics version 21. The distribution characteristics of the data were evaluated using the Kolmogorov-Smirnov normality test. For the 2 groups of non-normally distributed variables, nonparametric Mann-Whitney U tests were applied. The Wilcoxon signed-rank test was used for matched pairs. Correlations between variables were assessed using Spearman rank correlation test. Statistical significance was set at p < 0.05. *p < 0.05, **p < 0.01, and ***p < 0.001.
RESULTS
The frequencies of MAITs decreased with severity of ALD
We first assessed the frequencies of MAITs (within total CD3+ T cells) in peripheral blood and observed that the frequencies of MAITs were profoundly reduced in the ALD group compared with those in the HCs (Figure 1A, B). Further analysis showed that the frequencies of MAITs in the ALC + SAH group were significantly lower than that in the ALC group (Figure 1B). Importantly, immunofluorescence imaging revealed a decreased density of MAITs (TCR Vα7.2+ IL-18+) in the liver tissues of patients with ALD than in HCs (Figure 1C, Supplemental Figure S1A, http://links.lww.com/HC9/A281). Correlation analysis revealed that the frequencies of MAITs were negatively correlated with severe clinical indicators, such as MDF, the model for end-stage liver disease (MELD), and Child-Pugh scores (Figure 1D–F). These data suggest that a decrease in MAITs is associated with the severity and prognosis of ALD.
FIGURE 1.
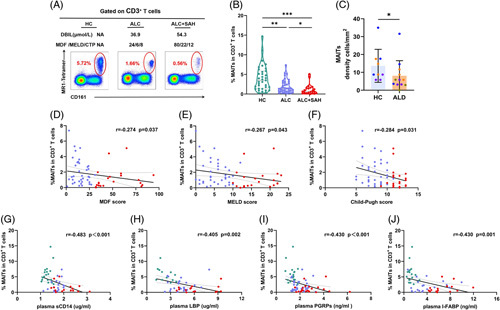
The MAITs’ frequencies decreased with severity of ALD. (A) Representative FACS plots of circulating MAITs’ frequencies (defined as 5-OP-RU/MR1 tetramer+ CD161hi) gated on CD3+ T cells from 1 HC, 1 patient with ALC, and 1 patient with ALC + SAH. (B) Violin plots showing MAITs’ frequencies of peripheral blood from HCs, patients with ALC, and patients with ALC + SAH detected by flow cytometry. The frequencies of MAITs in the ALC + SAH group were significantly lower than that in the ALC group. (C) Summary of density information of TCR Vα7.2+ IL-18+ cells in the liver tissues of HCs and patients with ALD. The density of TCR Vα7.2+ IL-18+ cells in the liver tissues of patients with ALD was significantly lower than that in the HCs. (D–F) Correlation analysis of MAITs’ frequencies and MDF, MELD, and Child-Pugh scores calculated in patients with ALC and patients with ALC + SAH. MAITs’ frequencies were negatively correlated with MDF, MELD, and Child‐Pugh scores in patients with ALC and patients with ALC + SAH. (G–J) Correlation analysis of MAITs’ frequencies and plasma levels of sCD14, LBP, PGRPs, and I-FABP in HCs, patients with ALC, and patients with ALC + SAH. MAITs’ frequencies were negatively correlated with plasma levels of sCD14, LBP, PGRPs, and I-FABP in HCs, patients with ALC, and patients with ALC + SAH. *p < 0.05, **p < 0.01, and ***p < 0.001, (B, C) Nonparametric Mann-Whitney U tests. (D–J) Spearman rank correlation test. Abbreviations: ALC, alcohol-associated liver cirrhosis; ALC + SAH, ALC complicated with severe alcoholic hepatitis; ALD, alcohol-associated liver disease; CTP, Child‐Pugh scores; HC, healthy control; I-FABP, intestinal fatty acid-binding protein; LBP, lipopolysaccharide-binding protein; MAITs, mucosal-associated invariant T cells; MDF, Maddrey discriminant function; MELD, model for end-stage liver disease; PGRPs, peptidoglycan recognition proteins; TCR, T-cell receptor.
We next detected the levels of sCD14, LBP, PGRPs, and surrogate markers of microbial translocation and found that their level was significantly increased in patients with ALD compared with that in HCs, especially in patients with ALC + SAH (Supplemental Figure S3A–C, http://links.lww.com/HC9/A282). Correlation analysis revealed that the frequencies of MAITs were negatively correlated with plasma sCD14, LBP, and PGRPs levels in patients with ALD (Figure 1G–I). Given that MAITs play a pivotal role in the repair of mucosal barriers, we further measured the levels of I-FABP, a marker of intestinal mucosa damage, and found that I-FABP levels were higher in patients with ALD than in HCs. Notably, the levels of I-FABP were significantly higher in patients with ALC + SAH than in those with ALC (Supplemental Figure S3D,http://links.lww.com/HC9/A282). Correlation analysis showed that the frequencies of MAITs were negatively correlated with plasma I-FABP levels in patients with ALD (Figure 1J). These data provide links between MAITs’ loss and microbial translocation in patients with ALD.
The frequencies of pyroptotic MAITs increased with severity of ALD
We used imaging flow cytometry (IFCM) to determine the characteristics of pyroptotic MAITs. As shown in Figure 2A, the FLICA caspase-1+ MAITs exhibited increased cell volume swelling. Interestingly, the frequencies of pyroptotic MAITs in the peripheral blood of patients with ALD were significantly higher than those of HCs (Figure 2A, B). Furthermore, the frequencies of pyroptotic MAITs in patients with ALC + SAH were significantly higher than those in patients with ALC (Figure 2B). We also found a higher density of pyroptotic MAITs (TCR Vα7.2+IL-18+GSDMD+) in the liver tissues of patients with ALD than in HCs (Figure 2C, Supplemental Figure S1B, http://links.lww.com/HC9/A281). Importantly, the frequencies of pyroptotic MAITs were significantly negatively correlated with the frequencies of MAITs (Figure 2D). We also found that the frequencies of pyroptotic MAITs were positively correlated with the MDF, MELD, and Child-Pugh scores (Figure 2E–G). These results indicate that increased pyroptotic MAITs might contribute to MAITs’ loss and are related to the severity and prognosis of ALD.
FIGURE 2.
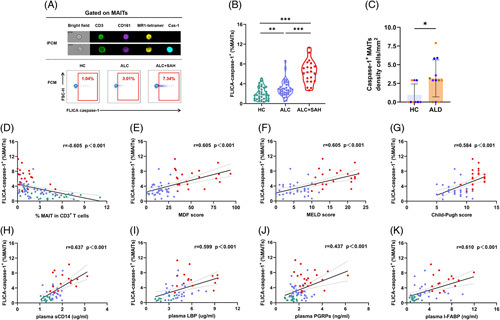
The pyroptotic MAITs’ frequencies increased with severity of ALD. (A) Representative IFCM image of pyroptotic MAITs from 1 patient with ALC and FACS plots of pyroptotic MAITs’ frequencies from 1 HC, 1 patient with ALC, and 1 patient with ALC + SAH. Pyroptotic MAITs were recognized by CD3+CD161+MR1 tetramer+caspase-1+. (B) Violin plots showing pyroptotic MAITs’ frequencies of peripheral blood from HCs, patients with ALC, and patients with ALC + SAH detected by flow cytometry. Pyroptotic MAITs’ frequencies of peripheral blood from patients with ALC and patients with ALC + SAH were significantly higher than that in the HCs. (C) Summary of density information of TCR vα7.2+ IL-18+ GSDMD+ cells in the liver tissues of HCs and patients with ALD. The density of TCR vα7.2+ IL-18+ GSDMD+ cells in the liver tissues of patients with ALD was significantly higher than that in the HCs. (D) Correlation analysis of pyroptotic MAITs’ frequencies and MAITs’ frequencies. The frequencies of pyroptotic MAITs were negatively correlated with the frequencies of MAITs. (E–G) Correlation analysis of pyroptotic MAITs’ frequencies and MDF, MELD, and Child‐Pugh scores calculated in patients with ALC and patients with ALC + SAH. The frequencies of pyroptotic MAITs were positively correlated with MDF, MELD, and Child‐Pugh scores. (H–K) Correlation analysis of pyroptotic MAITs’ frequencies and plasma levels of sCD14, LBP, PGRPs, and I-FABP in HCs, patients with ALC, and patients with ALC + SAH. The frequencies of pyroptotic MAITs were positively correlated with plasma levels of sCD14, LBP, PGRPs, and I-FABP in HCs, patients with ALC, and patients with ALC + SAH. *p < 0.05, **p < 0.01, and ***p < 0.001. (B, C) Nonparametric Mann-Whitney U tests. (D–K) Spearman rank correlation test. Abbreviations: ALC, alcohol-associated liver cirrhosis; ALC + SAH, ALC complicated with severe alcoholic hepatitis; ALD, alcohol-associated liver disease; HC, healthy control; I-FABP, intestinal fatty acid-binding protein; IFCM, imaging flow cytometry; LBP, lipopolysaccharide-binding protein; MAITs, mucosal-associated invariant T cells; MDF, Maddrey discriminant function; MELD, model for end-stage liver disease; MR1, major histocompatibility complex-I molecule; PGRPs, peptidoglycan recognition proteins; TCR, T-cell receptor.
Notably, the frequencies of pyroptotic MAITs were positively correlated with the levels of plasma sCD14, LBP, PGRPs, and I-FABP (Figure 2H–K), suggesting that MAITs’ pyroptosis is closely associated with the degree of antimicrobial immune response and intestinal damage.
The frequencies of pyroptotic MAITs increased with the activation and exhaustion levels of MAITs in patients with ALD
Flow cytometry results showed that the frequencies of CD38, HLA-DR, and PD-1 expressed on MAITs were remarkably augmented in patients with ALD compared with HCs, especially in patients with ALC + SAH (Figure 3A, B). Notably, the mean fluorescence intensity (MFI) of CD38, HLA-DR, and PD-1 on MAITs was significantly higher in patients with ALC + SAH than in patients with ALC (Figure 3C), indicating that the expression of these markers was also higher in a per-cell basis in severe ALD. These data suggest that MAITs’ activation and exhaustion levels correlate with ALD severity. Interestingly, the frequencies of MAITs were inversely correlated with both the frequencies and MFI of CD38, HLA-DR, and PD-1 on MAIT cells (Figure 3D). Further analysis revealed that the frequencies of pyroptotic MAITs were positively correlated with the frequencies and MFI of CD38, HLA-DR, and PD-1 on MAITs (Figure 3E). Notably, the frequencies of CD38 and HLA-DR expression in FLICA caspase-1+ MAITs were higher than those in FLICA caspase-1− MAITs (Figure 3F). These results highlight a close correlation between pyroptotic MAITs and their activation or exhaustion levels.
FIGURE 3.
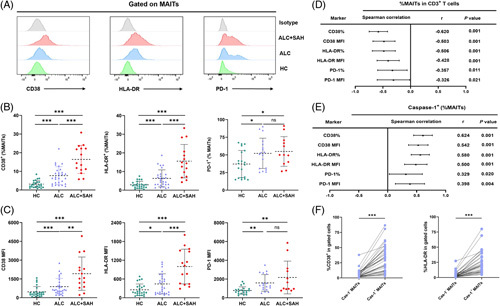
The pyroptotic MAITs’ frequencies increased with activation and exhaustion levels of MAITs in patients with ALD. (A) Representative offset plots of CD38, HLA-DR, and PD-1 expressed on MAITs in HC, ALC, ALC + SAH, and isotype. (B, C) The frequencies and MFI of CD38, HLA-DR, and PD-1 on MAITs from HCs, patients with ALC, and patients with ALC + SAH detected by flow cytometry. The frequencies and MFI of CD38, HLA-DR, and PD-1 on MAITs from patients with ALC and patients with ALC + SAH were significantly higher than that in the HCs. (D) Forest map of Spearman correlation between MAITs’ frequencies and the expression levels or MFI of CD38, HLA-DR, and PD-1 on MAITs in HCs, patients with ALC, and patients with ALC + SAH. The frequencies of MAITs were negatively associated with expression levels or MFI of CD38, HLA-DR, and PD-1 in HCs, patients with ALC, and patients with ALC + SAH. (E) Forest map of the Spearman correlation between pyroptotic MAITs’ frequencies and the expression levels or MFI of CD38, HLA-DR, and PD-1 on MAITs in HCs, patients with ALC, and patients with ALC + SAH. The frequencies of pyroptotic MAITs were positively associated with expression levels or MFI of CD38, HLA-DR, and PD-1 in HCs, patients with ALC, and patients with ALC + SAH. (F) Summary of CD38 or HLA-DR level expressed on caspase-1- MAITs and caspase-1+ MAITs. The level of CD38 or HLA-DR expressed in caspase-1+ MAITs was significantly higher than that in caspase-1− MAITs. *p < 0.05, **p < 0.01, and ***p < 0.001. (B, C, F) Nonparametric Mann-Whitney U tests. (D, E) Spearman rank correlation test. Abbreviations: ALC, alcohol-associated liver cirrhosis; ALC + SAH, ALC complicated with severe alcoholic hepatitis; ALD, alcohol-associated liver disease; HC, healthy control; MAITs, mucosal-associated invariant T cells; MFI, mean fluorescence intensity; PD-1, programmed cell death-1.
To further enrich the understanding of the MAITs’ immunophenotype, we analyzed the expression of CD45RA, CCR7, and immune senescence-related markers, CD57, Ki-67, Cyclin-D1, and CD49d. Results indicated that circulating MAITs mainly displayed a CD45RA-CCR7- effector memory phenotype and expressed low levels of CD57, Ki-67, Cyclin-D1, and CD49d (Supplemental Figure S2, http://links.lww.com/HC9/A283).
Exposure to E. coli-induced MAITs’ activation and pyroptosis
Monocytes and macrophages are involved in the pathophysiology of cirrhosis and are linked to microbial translocation during ALD progression.26 Therefore, we analyzed the correlation between MAITs’ frequencies and monocytes number and found that MAITs’ frequencies were negatively correlated with monocytes number, whereas pyroptotic MAITs’ frequencies were positively correlated with monocytes number (Figure 4A).
FIGURE 4.
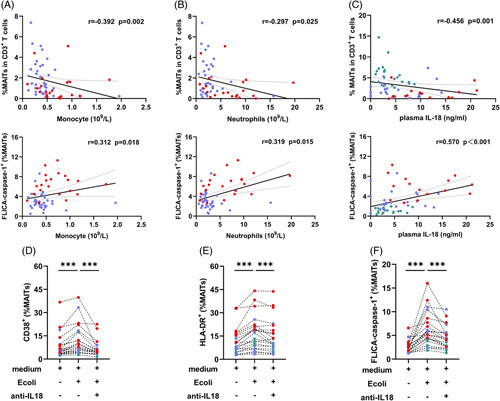
Exposure to E. coli-induced MAITs’ activation and pyroptosis. (A–C) Correlation analysis of MAITs’ frequencies or pyroptotic MAITs’ frequencies and absolute number of plasma monocytes (109/L), neutrophils (109/L), and plasma IL-18 level in patients with ALC and patients with ALC + SAH. Absolute number of plasma monocytes (109/L), neutrophils (109/L), and plasma IL-18 level were negatively associated with MAITs’ frequencies and positively associated with pyroptotic MAITs’ frequencies in patients with ALC and patients with ALC + SAH. (D–F) PBMCs from HCs, patients with ALC, and patients with ALC + SAH were treated with medium only, E. coli, and E. coli combined with anti-IL-18 antibody for 24 hours, respectively. The levels of CD38, HLA-DR, and FLICA caspase-1 expressed on MAITs were determined by flow cytometry. The levels of CD38, HLA-DR, and caspase-1 expressed by MAITs significantly increased after E. coli stimulation. However, they were significantly reduced after IL-18R blockade compared with that in cells without blockade. ***p < 0.001. (A–C) Spearman rank correlation test. (D–F) Wilcoxon signed-rank test. Abbreviations: ALC, alcohol-associated liver cirrhosis; ALC + SAH, ALC complicated with severe alcoholic hepatitis; HC, healthy control; MAITs, mucosal-associated invariant T cells; PBMC, peripheral blood mononuclear cells.
Neutrophil infiltration in the liver is a prominent sign of alcoholic hepatitis and immune defense against intestinal microbial translocation.27 We also found that MAITs’ frequencies were negatively correlated with the neutrophils number. Meanwhile, pyroptotic MAITs’ frequencies were positively correlated with neutrophils number (Figure 4B).
Interestingly, the levels of plasma IL-18, mainly secreted by activated innate immune cells, in patients with ALC were significantly higher than those in HCs, and it was further increased in patients with ALC + SAH (Supplemental Figure S3E, http://links.lww.com/HC9/A282). Impressively, the levels of plasma IL-18 were positively correlated with the frequencies of MAITs yet negatively correlated with the frequencies of pyroptotic MAITs (Figure 4C). These results suggest that plasma IL-18 levels are involved in the activation and pyroptosis of MAITs.
We hypothesized that continuous exposure to intestinal microorganisms induces MAITs’ pyroptosis. To test this hypothesis, we incubated PBMCs from HCs and patients with ALD with E. coli and measured the levels of MAITs’ activation and pyroptosis. The results showed that the frequencies of CD38 and HLA-DR expressed by MAITs significantly increased after E. coli stimulation (Figure 4D, E). Moreover, MAITs showed significantly elevated levels of FLICA caspase-1 expression in response to stimulation with E. coli compared with those in medium only (Figure 4F).
We also analyzed the effect of blocking IL-18/IL-18R interactions on activation and pyroptosis in MAITs. As expected, MAITs showed a marked decrease in activation marker expression after IL-18R blockade compared with that in cells without blockade (Figure 4D–E). This suggests that MAITs stimulated by IL-18 may be involved in the activation of MAITs in patients with ALD. Similarly, the levels of pyroptotic MAITs were also decreased after IL-18R blockade (Figure 4F), suggesting that activation-induced pyroptosis through IL-18 contributes to the loss of MAITs in patients with ALD.
Exposure to DBIL-induced MAITs’ activation and pyroptosis
Exposure to DBIL can lead to MAITs’ death, but the underlying mechanism is not clear.10 Our data showed that the frequencies of MAITs were negatively correlated with plasma levels of DBIL (Figure 5A), and the frequencies of pyroptotic MAITs were positively correlated with plasma levels of DBIL in patients with ALD (Figure 5B). This suggests that DBIL may be involved in the induction of MAITs’ activation and pyroptosis. Therefore, we incubated PBMC from HCs, patients with ALC, and patients with ALC + SAH with DBIL. We found that exposure to DBIL resulted in a significant increase in the expression of CD38 and HLA-DR in MAITs (Figure 5C, D). Similarly, the frequencies of FLICA caspase-1+ MAITs increased significantly after DBIL stimulation compared with that in cells without stimulation (Figure 5E). These data indicate that elevated DBIL could cause MAITs’ activation and pyroptosis, thus contributing to MAITs’ loss in patients with ALD. Notably, the levels of CD38, HLA-DR, and FLICA caspase-1 on MAITs in patients with ALD were higher than those in HCs after DBIL stimulation, especially in patients with ALC + SAH (Figure 5C–E).
FIGURE 5.
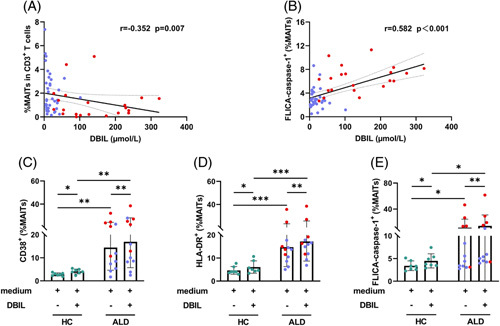
Exposure to DBIL-induced MAITs’ activation and pyroptosis. (A, B) Correlation analysis of MAITs’ frequencies or pyroptotic MAITs’ frequencies and plasma DBIL level in patients with ALC and patients with ALC + SAH. The frequencies of MAITs were negatively correlated with plasma levels of DBIL, and the frequencies of pyroptotic MAITs were positively correlated with plasma levels of DBIL. (C–E) PBMCs from HCs, patients with ALC, and ALC + SAH were treated with medium only and DBIL for 72 hours, respectively. Frequencies of CD38, HLA-DR, and FLICA caspase-1 expressed on MAITs were determined by flow cytometry. The levels of CD38, HLA-DR, and caspase-1 expressed by MAITs significantly increased after DBIL stimulation. The levels of CD38, HLA-DR, and FLICA caspase-1 on MAITs in patients with ALD were higher than those in HCs after DBIL stimulation. *p < 0.05, **p < 0.01, and ***p < 0.001. (A, B) Spearman rank correlation test. (C–E) Wilcoxon signed-rank test and nonparametric Mann-Whitney U tests. Abbreviations: ALC, alcohol-associated liver cirrhosis; ALC + SAH, ALC complicated with severe alcoholic hepatitis; ALD, alcohol-associated liver disease; DBIL, direct bilirubin; HC, healthy control; MAITs, mucosal-associated invariant T cells; PBMC, peripheral blood mononuclear cells.
DISCUSSION
The misadjustment of the immunological response caused by long-term exposure to antigens or metabolites from intestinal microorganisms might be the basis of the occurrence of ALD and a major cause of ALD progression.23 MAITs are key immune regulators of intestinal microbiota and have been reported to play a pivotal role in ALD immunopathogenesis.22,28 Nevertheless, recent studies have found that the frequencies of MAITs in patients with ALD are significantly reduced, but the potential mechanisms of MAITs’ loss remain elusive. Here, we evaluated the characteristics of MAITs according to the degree of microbial translocation and the severity of ALD, and further investigated the mechanisms underlying MAITs’ loss in ALD.
The occurrence and development of ALD are caused by many factors. First, intestinal leakage attributed to chronic alcohol consumption enhances susceptibility to ALD.29 We observed that both I-FABP, an index of gut injury, sCD14, secreted by monocytes and macrophages in response to the gut-derived microbiome, and LBP and PGRPs, surrogate markers of microbial translocation, were significantly increased in patients with ALD. This suggests that downstream of gut leakage may play an important role in our enrolled patients with ALD. Interestingly, we observed a negative correlation between the frequencies of MAITs and the levels of I-FABP, sCD4, and LBP and PGRPs, suggesting that the decreased frequencies of MAITs probably weaken the defense against microbial infections. Second, activated monocytes and neutrophils are believed to contribute to liver injury by secreting various proinflammatory cytokines during ALD.30 We also found a negative correlation between the frequencies of MAITs and increased numbers of monocytes and neutrophils in patients with ALD. Importantly, MDF, MELD, and Child-Pugh scores are usually used to evaluate the severity and prognosis of ALD,2 and our data showed negative correlations between MAITs’ frequencies and these scores. In combination with previous studies showing that the frequencies of MAITs were decreased in patients with ALD,22 our data support the concept that MAITs may actively participate in ALD disease progression.
Although the precise mechanism of MAITs’ loss in patients with ALD remains unknown, we found that MAITs in patients with ALD expressed high levels of FLICA caspase-1, a molecule that mediates pyroptosis.31 Importantly, increased pyroptotic MAITs’ frequencies were negatively correlated with decreased MAITs’ frequencies, suggesting that pyroptosis of MAITs might be responsible for MAITs’ loss in patients with ALD. It will be of interest to make a comparison of pyroptosis with other forms of cell death (apoptosis, necroptosis, etc.) for the diminution of circulating MAITs in advanced forms of ALD.
The increased pyroptotic MAITs may be caused by downstream gut leakage, as demonstrated by the close correlation between MAITs and markers for gut damage and microbial translocation. Several mechanisms may be involved in the induction of MAITs’ pyroptosis. First, pyroptotic MAITs often suffer from persistent activation signaling. In fact, activated MAITs’ frequencies were increased in patients with ALD and correlated with pyroptotic MAITs’ frequencies. Pyroptotic MAITs had a higher activation phenotype than other MAITs. Second, there were higher levels of proinflammatory cytokines in patients with ALD, which may cause T-cell loss by inducing pyroptosis, because MAITs can be activated in the absence of TCR stimulation. Indeed, increased levels of IL-18 were observed in patients with ALD and may directly act on and exacerbate pyroptosis in MAITs. The abovementioned issue was further supported by the fact that MAITs were evidently activated and underwent pyroptosis after E. coli stimulation, whereas the effect was decreased after inhibiting IL-18, suggesting that IL-18 might be involved in the immunopathogenesis of ALD. Third, a previous study revealed that DBIL could stimulate MAITs’ activation and apoptosis, and we further found that DBIL directly promoted MAITs’ activation and pyroptosis. Thus, pyroptotic MAITs are directly induced by an inflammation-related environment in patients with ALD.
Taken together, ALC + SAH patients with severe liver damage showed an increased level of gut damage, elevated innate cell numbers, and excessive proinflammatory cytokines caused by intestinal microbiota. In addition, they showed a profound decrease in MAITs’ frequencies and an increased level of MAITs’ death through pyroptosis compared with those in patients with ALC. Mechanically, activation-induced MAITs’ pyroptosis occurs in response to proinflammatory innate cells and damaged hepatocytes, leading to MAITs’ loss and susceptibility to infection. Thus, the poor circulation loop of MAITs’ pyroptosis links MAITs’ loss with gut damage and liver disease severity.
Supplementary Material
Acknowledgments
AUTHOR CONTRIBUTIONS
Ji-Yuan Zhang, Qing-Lei Zeng, Zheng-Sheng Zou: study concept and design; Zheng-Sheng Zou: experimental samples’ support: Li-Ping Zhang, Xing-Ran Zhai, Chun-Bao Zhou, Jin-Hong Yuan, Ye-Nv Ma, Zeng-Tao Yao, Hui-Fang Wang, Shuo Huang, and Wei-Zhe Li: sample processing and experiment performing: Li-Ping Zhang, Ji-Yuan Zhang, and Qing-Lei Zeng: data acquisition and analysis and drafting of the manuscript, and Fu-Sheng Wang, Ji-Yuan Zhang, Qing-Lei Zeng, Zheng-Sheng Zou, Yan-Mei Jiao, and Li-Ping Zhang: critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript to be published.
ACKNOWLEDGMENTS
The authors acknowledge the patients, study investigators, and coordinators for contributions to this study.
FUNDING INFORMATION
The study was supported by the National Natural Science Foundation of China (Nos. 81970517, 82271781, and 82270629).
CONFLICTS OF INTEREST
The authors have no conflicts to report.
DATA SHARING STATEMENT
All data generated or analyzed during this study are included in this published article.
Footnotes
Abbreviations: ALC, alcohol-associated liver cirrhosis; ALD, alcohol-associated liver disease; DBIL, direct bilirubin; HCs, healthy controls; HLA-DR, Human leukocyte antigen-DR; I-FABP, intestinal fatty acid-binding protein; IFCM, imaging flow cytometry; LBP, lipopolysaccharide-binding protein; MAITs, mucosal-associated invariant T cells; MDF, Maddrey discriminant function; MELD, model for endstageliver disease; MFI, mean fluorescence intensity; MR1, major histocompatibility complex-I molecule; PBMC, peripheral blood mononuclear cells; PD-1, programmed cell death-1; PGRPs, peptidoglycan recognition proteins; SAH, severe alcoholic hepatitis; sCD14, soluble CD14; TCR, T-cell receptor.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.hepcommjournal.com.
Contributor Information
Li-Ping Zhang, Email: zlp15272084015@163.com.
Hui-Fang Wang, Email: huifangwang1821@163.com.
Xing-Ran Zhai, Email: jiandanxiaozhai@163.com.
Chun-Bao Zhou, Email: chbzhou@aliyun.com.
Jin-Hong Yuan, Email: yuanjinhongli@163.com.
Ye-Nv Ma, Email: 1375328761@qq.com.
Zeng-Tao Yao, Email: public_302@126.com.
Shuo Huang, Email: huang2shuo@163.com.
Wei-Zhe Li, Email: lwzh0805@163.com.
Yan-Mei Jiao, Email: jiaoyanmei@sina.com.
Fu-Sheng Wang, Email: fswang302@163.com.
Zheng-Sheng Zou, Email: zszou302@163.com.
Ji-Yuan Zhang, Email: uniquezjy@163.com.
Qing-Lei Zeng, Email: zengqinglei2009@163.com.
REFERENCES
- 1. Wang FS, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of China. Hepatology. 2014;60:2099–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seitz HK, Bataller R, Cortez-Pinto H, Gao B, Gual A, Lackner C, et al. Alcoholic liver disease. Nat Rev Dis Primers. 2018;4:16. [DOI] [PubMed] [Google Scholar]
- 3. Porcelli S, Yockey CE, Brenner MB, Balk SP. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4-8- alpha/beta T cells demonstrates preferential use of several V beta genes and an invariant TCR alpha chain. J Exp Med. 1993;178:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tilloy F, Treiner E, Park SH, Garcia C, Lemonnier F, de la Salle H, et al. An invariant T cell receptor alpha chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted alpha/beta T cell subpopulation in mammals. J Exp Med. 1999;189:1907–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Constantinides MG, Belkaid Y. Early-life imprinting of unconventional T cells and tissue homeostasis. Science. 2021;374:eabf0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Corbett AJ, Eckle SB, Birkinshaw RW, Liu L, Patel O, Mahony J, et al. T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature. 2014;509:361–5. [DOI] [PubMed] [Google Scholar]
- 7. Ussher JE, Bilton M, Attwod E, Shadwell J, Richardson R, de Lara C, et al. CD161++ CD8+ T cells, including the MAIT cell subset, are specifically activated by IL-12+IL-18 in a TCR-independent manner. Eur J Immunol. 2014;44:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jeffery HC, van Wilgenburg B, Kurioka A, Parekh K, Stirling K, Roberts S, et al. Biliary epithelium and liver B cells exposed to bacteria activate intrahepatic MAIT cells through MR1. J Hepatol. 2016;64:1118–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hinks TSC, Marchi E, Jabeen M, Olshansky M, Kurioka A, Pediongco TJ, et al. Activation and in Vivo evolution of the MAIT cell transcriptome in mice and humans reveals tissue repair functionality. Cell Rep. 2019;28:3249–62.e3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu Y, Zhu P, Wang W, Tan X, Liu C, Chen Y, et al. Mucosal-associated invariant T cell dysregulation correlates with conjugated bilirubin level in chronic HBV infection. Hepatology. 2021;73:1671–87. [DOI] [PubMed] [Google Scholar]
- 11. Xue H, Li H, Ju LL, Han XD, Cheng TC, Luo X, et al. Mucosal-associated invariant T cells in hepatitis B virus-related liver failure. World J Gastroenterol. 2020;26:4703–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bolte FJ, O’Keefe AC, Webb LM, Serti E, Rivera E, Liang TJ, et al. Intra-hepatic depletion of mucosal-associated invariant T cells in hepatitis C virus-induced liver inflammation. Gastroenterology. 2017;153:1392–403.e1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cannizzo ES, Cerrone M, Merlini E, van Wilgenburg B, Swadling L, Ancona G, et al. Successful direct-acting antiviral therapy in HIV/HCV co-infected patients fails to restore circulating mucosal-associated invariant T cells. Eur J Immunol. 2019;49:1127–29. [DOI] [PubMed] [Google Scholar]
- 14. Böttcher K, Rombouts K, Saffioti F, Roccarina D, Rosselli M, Hall A, et al. MAIT cells are chronically activated in patients with autoimmune liver disease and promote profibrogenic hepatic stellate cell activation. Hepatology. 2018;68:172–86. [DOI] [PubMed] [Google Scholar]
- 15. Hegde P, Weiss E, Paradis V, Wan J, Mabire M, Sukriti S, et al. Mucosal-associated invariant T cells are a profibrogenic immune cell population in the liver. Nat Commun. 2018;9:2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jiang X, Lian M, Li Y, Zhang W, Wang Q, Wei Y, et al. The immunobiology of mucosal-associated invariant T cell (MAIT) function in primary biliary cholangitis: Regulation by cholic acid-induced Interleukin-7. J Autoimmun. 2018;90:64–75. [DOI] [PubMed] [Google Scholar]
- 17. Naimimohasses S, O’Gorman P, Wright C, Ni Fhloinn D, Holden D, Conlon N, et al. Differential effects of dietary versus exercise intervention on intrahepatic MAIT cells and histological features of NAFLD. Nutrients. 2022;14:2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Duan M, Goswami S, Shi JY, Wu LJ, Wang XY, Ma JQ, et al. Activated and exhausted MAIT cells foster disease progression and indicate poor outcome in hepatocellular carcinoma. Clin Cancer Res. 2019;25:3304–16. [DOI] [PubMed] [Google Scholar]
- 19. Zimmer CL, Filipovic I, Cornillet M, O’Rourke CJ, Berglin L, Jansson H, et al. Mucosal-associated invariant T-cell tumor infiltration predicts long-term survival in cholangiocarcinoma. Hepatology. 2022;75:1154–68. [DOI] [PubMed] [Google Scholar]
- 20. Zhang Y, Fan Y, He W, Han Y, Bao H, Yang R, et al. Persistent deficiency of mucosa-associated invariant T (MAIT) cells during alcohol-related liver disease. Cell Biosci. 2021;11:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li W, Lin EL, Liangpunsakul S, Lan J, Chalasani S, Rane S, et al. Alcohol abstinence does not fully reverse abnormalities of mucosal-associated invariant T cells in the blood of patients with alcoholic hepatitis. Clin Transl Gastroenterol. 2019;10:e00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Riva A, Patel V, Kurioka A, Jeffery HC, Wright G, Tarff S, et al. Mucosa-associated invariant T cells link intestinal immunity with antibacterial immune defects in alcoholic liver disease. Gut. 2018;67:918–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: pathophysiological basis for therapy. J Hepatol. 2020;72:558–577. [DOI] [PubMed] [Google Scholar]
- 24. Maccioni L, Gao B, Leclercq S, Pirlot B, Horsmans Y, De Timary P, et al. Intestinal permeability, microbial translocation, changes in duodenal and fecal microbiota, and their associations with alcoholic liver disease progression in humans. Gut Microbes. 2020;12:1782157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. EASL clinical practical guidelines: management of alcoholic liver disease. J Hepatol. 2012;57:399–420. [DOI] [PubMed] [Google Scholar]
- 26. Rainer F, Horvath A, Sandahl TD, Leber B, Schmerboeck B, Blesl A, et al. Soluble CD163 and soluble mannose receptor predict survival and decompensation in patients with liver cirrhosis, and correlate with gut permeability and bacterial translocation. Aliment Pharmacol Ther. 2018;47:657–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ren R, He Y, Ding D, Cui A, Bao H, Ma J, et al. Aging exaggerates acute-on-chronic alcohol-induced liver injury in mice and humans by inhibiting neutrophilic sirtuin 1-C/EBPα-miRNA-223 axis. Hepatology. 2022;75:646–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Atif M, Warner S, Oo YH. Linking the gut and liver: crosstalk between regulatory T cells and mucosa-associated invariant T cells. Hepatol Int. 2018;12:305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Arab JP, Martin-Mateos RM, Shah VH. Gut-liver axis, cirrhosis and portal hypertension: the chicken and the egg. Hepatol Int. 2018;12:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li S, Tan HY, Wang N, Feng Y, Wang X, Feng Y. Recent insights into the role of immune cells in alcoholic liver disease. Front Immunol. 2019;10:1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xia P, Xing XD, Yang CX, Liao XJ, Liu FH, Huang HH, et al. Activation-induced pyroptosis contributes to the loss of MAIT cells in chronic HIV-1 infected patients. Mil Med Res. 2022;9:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


