Abstract
Background:
Extreme temperature events, including extreme cold, are becoming more frequent worldwide, which might be harmful to pregnant women and cause adverse birth outcomes. We aimed to investigate the association between exposure to low ambient temperature in pregnant women and adverse birth outcomes, such as preterm birth, low birth weight, and stillbirth, and to summarize the evidence herein.
Methods:
Relevant studies were searched in PubMed, Cochrane, and Embase electronic databases until November 2021. Studies involving low ambient temperature, preterm birth, birth weight, and stillbirth were included. The guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analyses were followed to conduct this study risk of bias and methods for data synthesis.
Results:
A total of 34 studies were included. First, pregnant women exposed to low ambient temperature had an increased risk of preterm birth (risk ratio [RR] 1.08; 95% confidence interval [CI] 1.04–1.13). Subgroup analyses revealed that exposure during late pregnancy was more likely to induce preterm birth. In addition, only pregnant women exposed to <1st percentile of the mean temperature suffered increased risk of preterm birth. Moreover, pregnant women living in medium or hot areas were more prone to have preterm births than those in cold areas when exposed to low ambient temperatures. Asians and Blacks were more susceptible to low ambient temperatures than Caucasians. Second, pregnant women exposed to low ambient temperature had an increased risk of low birth weight (RR 1.07; 95% CI 1.03–1.12). Third, pregnant women had an increased risk of stillbirth while exposed to low ambient temperature during the entire pregnancy (RR 4.63; 95% CI 3.99–5.38).
Conclusions:
Exposure to low ambient temperature during pregnancy increases the risk of adverse birth outcomes. Pregnant women should avoid exposure to extremely low ambient temperature (<1st percentile of the mean temperature), especially in their late pregnancy. This study could provide clues for preventing adverse outcomes from meteorological factors.
Registration:
No. CRD42021259776 at PROSPERO (https://www.crd.york.ac.uk/PROSPERO/).
Keywords: Ambient temperature, Pregnancy, Preterm birth, Birth weight, Stillbirth
Introduction
Climate change is a challenging problem in the 21st century. Extreme weather events, including extremely high and low ambient temperatures, have increased as a consequence of climate change.[1–3] The frequent occurrence of temperature extremes has raised concerns about the impact on public health, particularly maternal and infant health.[4] Because of poor thermoregulation, pregnant women are more susceptible to the additional burden of temperature changes that might affect fetal development.[5,6] Several studies have indicated that extreme ambient temperature exposure could compromise thermoregulation and further lead to increased levels of inflammatory cytokines.[7,8] Meanwhile, the disturbance in the maternal endocrine system caused by temperature changes might affect the maternal–fetal transmission of hormones.[2,9]
In recent decades, many studies have investigated the effects of ambient temperatures and birth outcomes. The hazards of high-temperature exposure on birth outcomes have been evaluated in a systematic review and meta-analysis, which showed that high ambient temperature exposure during pregnancy is associated with a high risk for preterm birth, low birth weight (LBW), and stillbirth.[6] However, the effects of extremely low ambient temperature exposure during pregnancy on the adverse birth outcomes have not been explored by meta-analysis. It is generally considered that global warming will reduce exposure to extremely low ambient temperatures to some extent. However, the frequency of extremely low ambient temperatures has also increased in the last three decades according to a report from US National Oceanic and Atmospheric Administration.[10] This increasing frequency is due to global warming, which has destroyed temperature stability and caused abnormal atmospheric circulation.[11] Several studies have reported that low ambient temperature exposure could jeopardize fetal development.[12–16] Animal studies found that although the fetal temperature is kept constant inside the uterus, cold-induced thermoregulation may increase maternal blood viscosity and cause vasoconstriction.[17] These responses may lead to reduced blood flow to the placenta, thereby affecting fetal growth.[18] Furthermore, extremely low ambient temperature elevated maternal stress hormone levels, which led to preterm birth after being transmitted to the fetus.[12]
Understanding the effect of meteorological factors, including extremely low ambient temperature, on neonatal outcomes could help in the development of health promotion strategies. Therefore, we conducted a systematic review and meta-analysis to examine the association between extremely low ambient temperature exposure in pregnancy and adverse birth outcomes. Moreover, subgroup analyses were performed to identify potential factors such as susceptible time windows, different exposure levels to low ambient temperature, and different climate types and ethnicities.
Methods
Registration
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines and was prospectively registered in PROSPERO (No. CRD42021259776; https://www.crd.york.ac.uk/PROSPERO/).
Information sources and search strategies
We performed systematic literature searches of PubMed, Cochrane, and Embase electronic databases. The search was performed until November 2021. A structured search strategy was designed including the following Medical Subject Headings (MeSH) search terms: “Premature Birth”, or text words “Premature Births”, “Preterm Birth”, “Preterm Births”, “Premature Birth” OR MeSH term “Birth Weight”, or text words “Birth Weights”, “Birth weight” OR MeSH term “Stillbirth”, or text words “Stillbirth”, “Fetal death”, AND MeSH term “Cold Temperature”, or text words “Cold”, “Cold temperature”, “Ambient temperature”, “Season” AND MeSH term “Pregnancy”, or text words “Pregnancy”, “Pregnant”. The search strategy was adapted for the other electronic databases used. Additionally, we searched the reference lists of the included studies to identify eligible studies.
Eligibility criteria
The eligibility criteria were studies assessing the association between exposure to low ambient temperature during pregnancy and LBW (<2500 g), preterm birth (<37 gestational weeks), or stillbirth (death of fetus >20 gestational weeks). There were no restrictions on the date of publication. Narrative reviews, animal research, case reports, comments, or editorials were excluded. Eligible studies were limited to English-language articles.
Study selection process
One investigator performed the database search and screened for duplicates. After removing duplicates, two investigators (Tiechao Ruan [TR] and Yan Yue [YY]) screened the titles and abstracts of all records and assessed the full text of the eligible articles.
Data collection
Information on study design/methodology, name of the author, year of publication, place of study, sample size, exposure details (the degree and duration of low ambient temperature in pregnancy), effect sizes of LBW, preterm birth, stillbirth, and confounding variables were extracted from the included studies. Data extraction was conducted by two independent reviewers (TR and YY).
Study risk of bias assessment
Two reviewers (TR and YY) independently assessed the risk of bias in all selected studies using the Newcastle–Ottawa scale. Study quality was then classified into three grades: low (0–3), moderate (4–6), and high (7–9).
Statistical analysis
Data from selected studies were extracted into Excel (Microsoft, Washington, USA) and imported to Stata 12 software (StataCorp, College Station, TX, USA) and Review Manager 5.3 (Cochrane Collaboration, Oxford, UK) to conduct meta-analyses. We pooled the risk ratios (RRs) to assess the association between low ambient temperature exposure during pregnancy and LBW, preterm birth, and stillbirth. The I2 statistic and Q test for each analysis were used to quantify inconsistency across studies and describe the percentage of variability in effect estimates that may be caused by heterogeneity rather than sampling error. A random-effects model was used to screen for significant heterogeneity. Sensitivity analysis was conducted to assess the robustness of the associations by omitting one study each time. Publication bias was assessed by performing a funnel plot as well as Egger's and Begg's tests. Moreover, subgroup analyses were conducted by stratifying different time windows of exposure, different exposure levels to low temperature, climate types, and ethnicities. According to the information in the original studies, the time windows were categorized into eight subgroups: preconception (the month before conception), first trimester (1–13 gestational weeks), second trimester (14–26 gestational weeks), third trimester (27 gestational weeks to delivery), the month before delivery, the week before delivery, and the day of delivery. The exposure levels were divided into four subgroups based on the original studies: mean temperature <1st, <5th, <10th, and <25th percentiles during pregnancy. The climate types were divided into three subgroups: cold area, medium area, and hot area based on the annual monthly average temperature and maximum and minimum temperatures.[19,20] Moreover, different ethnicities were stratified, including Asians, Caucasians, and Blacks. The definitions of extremely low ambient temperature were divided into five subgroups, including exposure level of daily mean temperature, daily minimum temperature, daily maximum temperature, cold spells, and 2 days/month increase in the number of days at extremely cold, to find the source of heterogeneity. Furthermore, the different methodological models were also stratified to explore their influence on the association between low temperature and birth outcomes.
Results
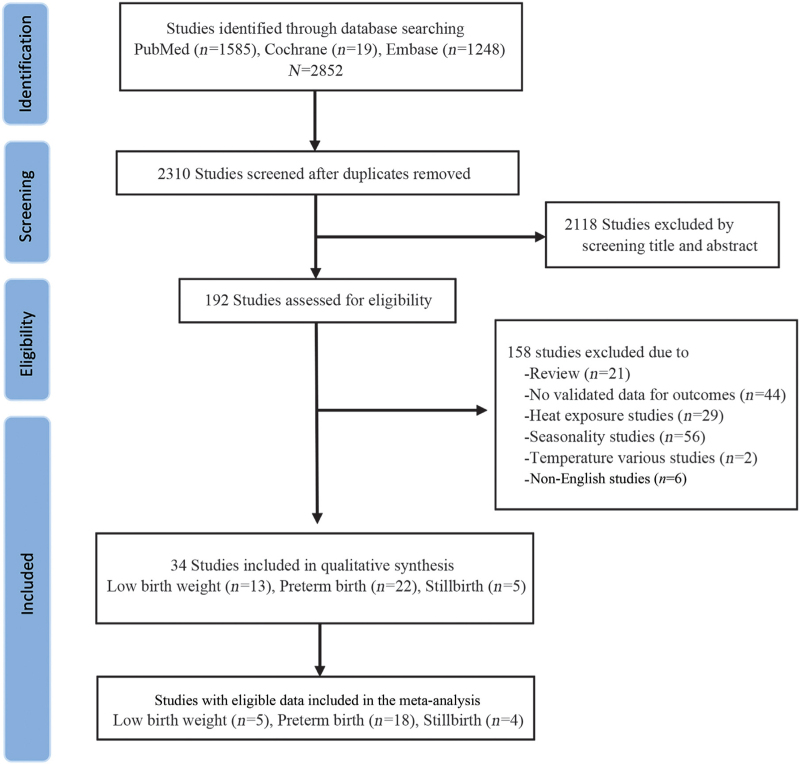
The study selection process is shown in Figure 1. A total of 2852 articles matched the search terms. After removing 542 duplicates, the titles and abstracts of 2310 articles were screened for eligibility, and the full texts of 192 articles were reviewed. Finally, 34 studies were included in the systematic review of adverse birth outcomes: 22 for preterm birth,[12,13,15,20–38] including 13 studies for LBW,[12,14,34,36,39–47] and five for stillbirth.[12,25,34,48,49] Among the 34 studies, five reported more than one outcome, 24 of which were included in the meta-analysis with eligible data [Figure 1].
Figure 1.
PRISMA flowchart. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
The main characteristics of the included studies are shown in [Supplementary Table 1]. These 34 studies were conducted in 16 countries, including countries in Asia (16 studies), Europe (seven studies), North America (seven studies), Australia (three studies), and South America (one study). Among the 34 studies, 24 studies had high methodological quality and 10 studies had moderate methodological quality [Supplementary Table 2].
Preterm birth
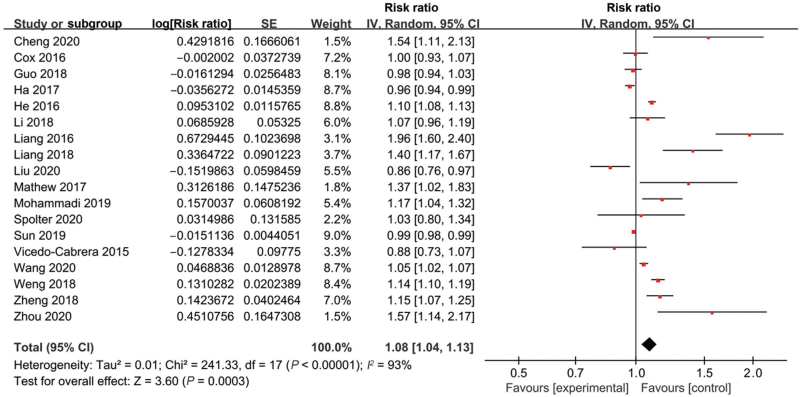
A total of 22 studies explored the association between preterm birth and low ambient temperature exposure during pregnancy [Supplementary Table 1]. Most studies (16/22) found that maternal exposure to low temperature increased the risk of preterm birth.[12,13,15,21–26,28,29,32–35,37] However, four studies documented that low ambient temperature was a protective factor,[20,27,31,38] and two studies showed no significant difference.[30,36] We conducted a meta-analysis on 18 studies with eligible data. The overall RR of the associations was 1.08 (95% confidence interval [CI] 1.04 to 1.13; I2 = 93%, Supplementary Table 3, Figure 2). Next, we evaluated the possibility of publication bias. The funnel plot resembles an asymmetrical distribution [Supplementary Figure 1]. Egger's test (P = 0.024), but not Begg's test (P = 0.472), showed publication bias. Therefore, we used the trim and fill method to evaluate the robustness of the results. The trimming estimator found that three missing studies could be attributed to publication bias. After filling the missed studies, the pooled effect size was also significant with an RR of 1.05 (95% CI 1.01–1.10), which suggested that the potential publication bias did not influence the effect on the pooled result [Supplementary Table 4]. Moreover, a sensitivity analysis was conducted to assess the robustness of the results, which showed that the RR was still robust by removing a single study each time [Supplementary Figure 2].
Figure 2.
Forest plot depicting the meta-analysis examining the association between low ambient temperature and preterm birth. CI: Confidence interval; SE: Standard error.
Additionally, subgroup analysis was conducted to identify the possible factors that influence the association between low ambient temperature and preterm birth caused by different time windows of exposure, exposure levels, ethnicity, and climate patterns. First, pregnant women exposed to low ambient temperature during the third trimester, the birth week, and at birth had an increased risk of preterm birth with RR of 1.16 (95% CI 1.12–1.20, I2 = 39%), 1.09 (95% CI 1.03–1.16, I2 = 83%), and 1.34 (95% CI 1.15–1.57, I2 = 95%), respectively [Table 1, Supplementary Figure 3]. However, no significant association was found during other exposure periods [Table 1, Supplementary Figure 3]. Second, with respect to the different exposure levels to low ambient temperatures, pregnant women with an exposure level <1st percentile of the mean temperature resulted in a significant RR of 1.14 (95% CI, 1.06–1.23; I2 = 77%), but this effect was not significant at other exposure levels [Table 1, Supplementary Figure 4]. As for the different climate types, exposure to medium or hot areas was associated with an increased risk for preterm birth with RR 1.15 (95% CI 1.02–1.30, I2 = 80%) and RR 1.09 (95% CI 1.00–1.18, I2 = 97%), respectively. However, no association was found in those living in cold areas (RR 0.96, 95% CI 0.92–1.01; I2 = 77%) [Table 1, Supplementary Figure 5]. Finally, as for ethnicities, an increased risk of preterm birth was found in Asians and Blacks with RR 1.13 (95% CI 1.06–1.20, I2 = 90%) and RR 1.37 (95% CI 1.02–1.83), respectively. However, no significant difference was found in Caucasians with RR 1.02 (95% CI 0.96–1.08, I2 = 60%) [Table 1, Supplementary Figure 6].
Table 1.
Subgroup analysis for maternal extreme low ambient temperature exposure and preterm birth.
| Subgroup | N of study | Pooled RR (95% CI) | I2 (%) |
| Exposure period | |||
| Preconception | 4 | 1.03 (0.89, 1.20) | 83 |
| First trimester | 6 | 0.99 (0.95, 1.04) | 89 |
| Second trimester | 6 | 0.98 (0.93, 1.03) | 92 |
| Third trimester | 5 | 1.16 (1.12, 1.20) | 39 |
| Entire pregnancy | 3 | 1.09 (0.99, 1.20) | 77 |
| Birth month | 7 | 1.15 (1.00, 1.33) | 89 |
| Birth week | 9 | 1.09 (1.03, 1.16) | 83 |
| At birth | 5 | 1.34 (1.15, 1.57) | 95 |
| Temperature grade of exposure (%) | |||
| <1st | 9 | 1.14 (1.06, 1.23) | 77 |
| <5th | 8 | 1.05 (0.99, 1.11) | 95 |
| <10th | 3 | 1.13 (0.94, 1.36) | 92 |
| <25th | 3 | 1.07 (0.77, 1.48) | 86 |
| Climate | |||
| Cold area | 4 | 0.96 (0.92, 1.01) | 77 |
| Medium area | 7 | 1.15 (1.02, 1.30) | 80 |
| Hot area | 8 | 1.09 (1.00, 1.18) | 97 |
| Race | |||
| Asian | 10 | 1.13 (1.06, 1.20) | 90 |
| Black | 1 | 1.37 (1.02, 1.83) | – |
| Caucasian | 6 | 1.02 (0.96, 1.08) | 60 |
| Type of exposure | |||
| Exposure levels of Tmean | 14 | 1.07 (1.02, 1.12) | 94 |
| Exposure levels of Tmin | 2 | 1.13 (0.84, 1.53) | 77 |
| Exposure levels of Tmax | 1 | 0.97 (0.86, 1.09) | – |
| Cold spell | 1 | 1.40 (1.17, 1.67) | – |
| 2 days/mouth increase of extreme cold | 1 | 1.15 (1.07, 1.25) | – |
| Methodological model | |||
| Distributed lag non-linear model | 9 | 1.15 (1.03, 1.30) | 91 |
| Cox proportional hazard regression | 4 | 1.07 (1.03, 1.11) | 62 |
| Logistic regression model | 2 | 1.06 (0.91, 1.24) | 91 |
| Generalized additive model | 1 | 1.57 (1.14, 2.17) | – |
| Chi-squared test | 1 | 1.14 (1.10, 1.19) | – |
| Poisson regression | 1 | 0.96 (0.94, 0.99) | – |
CI: Confidence interval; RR: Risk ratio; Tmean: Daily mean temperature; Tmin: Daily minimum temperature; Tmax: Daily maximum temperature; −: Not available.
Different definitions used for analyzing low ambient temperature exposure were reported in the included studies, including daily mean temperature, daily minimum temperature, daily maximum temperature, cold spells, and 2 days/month increase in extreme cold. We carried out a subgroup analysis based on the five definitions. Studies using a definition of daily mean temperature (14 studies), cold spell (1 study), or 2 days/month increase in extreme cold (1 study) had significant RRs of 1.07 (95% CI 1.02–1.12, I2 = 94%), 1.40 (95% CI 1.17–1.67), and 1.15 (95% CI 1.07–1.25), respectively [Supplementary Figure 7]. However, no significant association was found in studies using daily minimum temperature (two studies) or daily maximum temperature (one study) with RRs of 1.13 (95% CI 0.84–1.53) and 0.97 (95% CI 0.86–1.09), respectively [Supplementary Figure 7]. Moreover, different methodological models (six models) were used in the included studies. The subgroup analysis found that studies using four of the models showed an increased risk of preterm birth with pooled RRs of 1.15 (95% CI 1.03–1.30), 1.07 (95% CI 1.03–1.11), 1.57 (95% CI 1.14–2.17), and 1.14 (95% CI 1.10–1.19), respectively. However, an increased risk was not found in studies using logistic regression or Poisson models [Supplementary Figure 8].
Birth weight
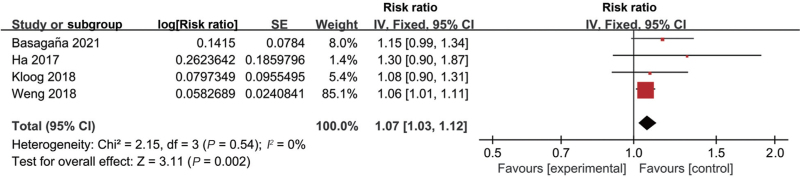
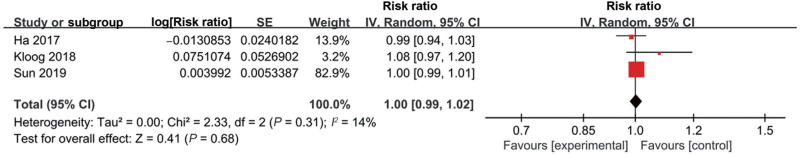
Thirteen studies explored the association between low ambient temperature and birth weight [Supplementary Table 1]. Eleven studies documented that exposure of pregnant women to low ambient temperature was associated with LBW,[14,34,36,40–47] while two studies showed no significant association.[12,39] Among these 13 studies, five of them had eligible data for conducting meta-analysis.[34,44–47] Two main outcomes, including LBW (four studies)[34,44,45,47] and small for gestational age (SGA) (three studies),[44–46] were documented in the original studies. The meta-analyses showed a significant association between low ambient temperature exposure and LBW (RR 1.07, 95% CI 1.03–1.12, I2 = 0%) [Figure 3, Supplementary Table 3] but not SGA (RR 1.00, 95% CI 0.99–1.02, I2 = 14%) [Figure 4, Supplementary Table 3].
Figure 3.
Forest plot depicting the meta-analysis examining the association between low ambient temperature and low birth weight. CI: Confidence interval; SE: Standard error.
Figure 4.
Forest plot depicting the meta-analysis examining the association between low ambient temperature and small for gestational age. CI: Confidence interval; SE: Standard error.
Subgroup analysis was performed by stratifying the different time windows of exposure. As for LBW, only exposure of pregnant women to low temperature during the third trimester (RR 1.22, 95% CI 1.11–1.33, I2 = 0%), the week before delivery (RR 1.06, 95% CI 1.01–1.11), and the entire pregnancy (RR 1.67, 95% CI 1.07–2.60, I2 = 97%), but not for other time windows [Table 2, Supplementary Figure 9], was associated with LBW. As for SGA, there was no association between low ambient temperature and SGA at any time window of exposure [Table 2, Supplementary Figure 10]. Moreover, four studies also reported continuous data on birth weight [Supplementary Table 5]. Three of them with large sample sizes showed that low ambient temperature exposure significantly reduced birth weight,[39,46,47] whereas just one of the four studies showed a non-significant association.[36] In the study, the result showed a large standard deviation, indicating the number of included cases was rather limited, which may have caused the non-significant result. Due to the limited number of studies and high heterogeneity, the meta-analysis for continuous data could not be conducted.
Table 2.
Subgroup analysis of different exposure time and low birth weight and small for gestational age.
| Subgroup analysis | N of study | Pooled RR (95% CI) | I2 (%) |
| Low birth weight | |||
| Preconception | 1 | 0.96 (0.83, 1.11) | – |
| First trimester | 2 | 0.99 (0.91, 1.08) | 0 |
| Second trimester | 2 | 1.09 (0.96, 1.23) | 50 |
| Third trimester | 2 | 1.22 (1.11, 1.33) | 0 |
| Entire pregnancy | 2 | 1.67 (1.07, 2.60) | 97 |
| A week before delivery | 1 | 1.06 (1.01, 1.11) | – |
| Small for gestational age | |||
| Preconception | 1 | 0.98 (0.92, 1.04) | – |
| First trimester | 3 | 1.01 (0.97, 1.05) | 53 |
| Second trimester | 3 | 1.01 (1.00, 1.03) | 0 |
| Third trimester | 3 | 1.01 (0.92, 1.12) | 89 |
| Entire pregnancy | 3 | 1.04 (0.96, 1.12) | 87 |
CI: Confidence interval; RR: Risk ratio.
Stillbirth
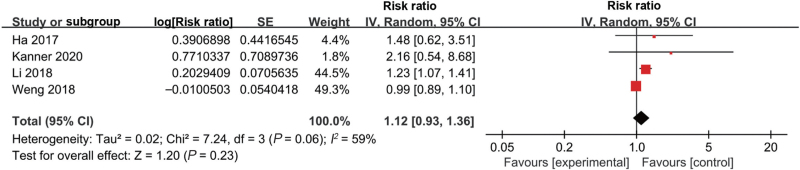
Five studies investigated the association between low ambient temperature and stillbirth [Supplementary Table 1]. Four of them showed an association between the exposure of pregnant women to low ambient temperature and the risk of stillbirth,[12,25,48,49] while the remaining one showed no significant association.[34] Four of the five studies had eligible data for conducting meta-analysis and showed no significant difference (RR 1.12, 95% CI 0.93–1.36, I2 = 59%) [Supplementary Table 3, Figure 5].[25,34,48,49] After conducting a subgroup analysis based on the different exposure windows, we found that low ambient temperature exposure during the entire pregnancy was associated with stillbirth (RR 4.63, 95% CI 3.99–5.38, I2 = 0%), but the association was not noted for other exposure time windows [Table 3, Supplementary Figure 11].
Figure 5.
Forest plot depicting the meta-analysis examining the association between low ambient temperature and stillbirth. CI: Confidence interval; SE: Standard error.
Table 3.
Subgroup analysis of different exposure time windows and stillbirth.
| Subgroup | No. study | Pooled RR (95% CI) | I2 (%) |
| Preconception | 1 | 1.21 (0.97, 1.50) | – |
| First trimester | 2 | 1.02 (0.80, 1.29) | 40 |
| Second trimester | 2 | 1.08 (0.80, 1.46) | 70 |
| Third trimester | 1 | 1.53 (0.83, 2.82) | – |
| A week before delivery | 2 | 1.05 (0.98, 1.12) | 49 |
| Entire pregnancy | 2 | 4.63 (3.99, 5.38) | 0 |
CI: Confidence interval; RR: Risk ratio.
Discussion
This systematic review and meta-analysis explored the association between exposure of pregnant women to low ambient temperatures and adverse birth outcomes. A total of 34 studies involving >79 million births were included. We found that exposure of pregnant women to low ambient temperature was associated with an increased risk of adverse birth outcomes, including preterm birth, LBW, and stillbirth.
Subgroup analyses for low ambient temperature and preterm births identified several influencing factors. Regarding different exposure levels, exposure to temperature <1st percentile of the mean was associated with preterm birth. An exposure level <5th percentile was non-significant but was very close to having a significant difference (RR 1.05, 95% CI 0.99–1.11). Therefore, a sensitivity analysis was performed to test the robustness of exposure level for <5th, which showed a significant difference by excluding the study of Liu et al[27]. This suggested that the non-significant result was not robust, and an exposure level <5th could also be a risk factor for preterm birth. Additionally, the original studies reported that the risk of adverse birth outcomes rose as ambient temperature fell,[12,40,42,43] similar to the finding that the hazard for preterm birth was increased when the temperature rose.[6] Therefore, the risk of ambient temperature exposure during preterm birth may present as a V-like shape. As for the different climate types, the association between low ambient temperature and preterm birth was significant in hot and medium areas, but not in cold areas. This may be due to the weaker adaptability of pregnant women in hot and medium areas than in cold areas. As for different exposure time windows, preterm birth was more likely to occur when pregnant women were exposed to low ambient temperatures during late pregnancy. This might be because both the mother and fetus are more susceptible to extremely low ambient temperature during that period.[6] Recent studies in mice have shown that low-temperature exposure during late pregnancy-induced placental apoptosis by activating nuclear factor kappa-B signaling pathway, which jeopardized placenta growth.[50] Furthermore, low-temperature exposure during the last trimester elevated maternal stress hormone levels such as corticotropin-releasing hormone and induced stress-related anxiety in pregnant women.[51] In addition, stress-related anxiety during the third trimester was more likely to induce preterm births than in the other trimesters.[52] Hence, it appears that the late pregnancy is a period of susceptibility to preterm birth due to low temperature. Therefore, it is important for pregnant women to avoid extremely low ambient temperature exposure, especially late in their pregnancy. Among the different ethnic groups, Asian pregnant women were more susceptible to extremely low ambient temperature than Caucasians. In addition, Black pregnant women had the possibility of a higher risk of preterm birth. However, the reasons are not clear. More studies are needed to confirm this possibility since only one original study was included. This is similar to previous findings that Asian and Black pregnant women were more likely to have adverse birth outcomes when exposed to high ambient temperature.[53,54]
Our meta-analyses showed that extremely low ambient temperature exposure could increase the risk of LBW. A significant association was found in the subgroup of extremely low ambient temperature exposure during late pregnancy, but not in the subgroups of the first and second trimesters. This is similar to other studies which reported environmental factors such as air pollution exposure during the late pregnancy is more likely to cause LBW compared with other trimesters.[55] This may be due to fact that the last trimester is a period of accelerated fetal and placental growth.[56,57] Different from the increased risk of LBW, we found that the risk of SGA was not increased when exposed to extremely low ambient temperature. This may be because LBW, but not SGA, is closely related to preterm birth. Therefore, the increased risk of LBW could be associated with the increased risk of preterm birth. The non-significant association found in SGA suggested that the impact of extremely low ambient temperature exposure on birth weight might be indirect. The major causes of SGA are maternal health status, placental insufficiency, and fetal genetic factors. There are no studies identified the exact mechanism by which low ambient temperature during pregnancy causes SGA. However, because the number of included studies on SGA is still limited to determine a robust conclusion, further studies with large samples are still needed.
Regarding the risk of stillbirth, we found that pregnant women exposed to extremely low temperatures throughout pregnancy were associated with a high risk for stillbirth (RR 4.63, 95% CI 3.99–5.38, I2 = 0%). Although only two studies were included in the analysis, the number of participants was >33,000, which provided good reliability for the results. Because stillbirth is the most serious outcome among the adverse birth outcomes, the potential danger should raise concerns. Regarding the association between temperature exposure and stillbirth, it is not clear why extremely low-temperature exposure could lead to stillbirth. It has been reported that extremely low ambient temperature exposure during pregnancy could induce structural and organic anomalies in fetal mice,[57] which may suggest a risk of stillbirth.
This systematic review and meta-analysis has several merits. First, the eligible studies were searched from three main databases to reduce the loss of inclusion. Second, information on extremely low ambient temperature exposure was collected from meteorological data. In contrast, maternal information and birth outcomes were collected from electronic medical records. These data collection methods reduced recall bias to a large extent. Third, the information on the included participants was obtained from national databases, which provided a representative population sample and a large sample size. Finally, most of the included studies (24/33) had high methodological quality, and some of them (9/33) had moderate, which produced high reliability for conducting the meta-analysis.
This study has a few limitations. First, the meta-analysis for preterm births showed high heterogeneity. Although we performed sensitivity and subgroup analyses to identify the possible sources of heterogeneity, it seemed it was not because of the different time windows, exposure levels, ethnicities, climate patterns, the definition of extremely low ambient temperature, or different methodological models. The variations in population characteristics, such as maternal demographic characteristics, economic level, and heating equipment used could be responsible for the heterogeneity. However, because lack of sufficient information to stratify these variations, it was difficult to trace the sources of heterogeneity. Secondly, different definitions of extremely low ambient temperature and different methodological models were used in the included studies. The differences may result in different exposure levels and durations of extremely low temperatures among studies. Although the subgroup analysis showed that the results did not differ greatly among different subgroups, it would be better to standardize the methodological difference in future studies so as to reduce potential heterogeneity. Finally, the number of original studies on LBW (four studies) and stillbirth (four studies) were relatively limited, although these studies each included large sample sizes of 2.3 million and 330,000, respectively. Further studies are required to confirm these findings.
In conclusion, pregnant women exposed to extremely low ambient temperature, especially in late pregnancy, have an increased risk of adverse birth outcomes. Pregnant women living in medium and hot areas, especially Asians and Blacks, might be more sensitive to low-temperature exposure. Stillbirth, the most serious adverse birth outcome, was significantly associated with extremely low ambient temperature exposure throughout pregnancy, which should be of great concern. These findings help in identifying environmental risk factors for birth outcomes and call attention to the need to avoid low-temperature exposure during pregnancy.
Funding
This work was supported by grants from the National Science Foundation of China (Nos. 82271749, 82201905, 81971433, 81971428, and 82171710), the National Key Research and Development Program of China (Nos. 2017YFA 0104200, and 2021YFC2701700), the Ministry of Education of China (No. IRT0935), the Science and Technology Bureau of Sichuan Province (Nos. 2021YJ0017, 2020YFS0041, 2020YJ0298, and 2020YJ0236), the Health Commission of Sichuan Province (No. 20PJ071), and the clinical discipline program (Neonatology) from the Ministry of Health of China (No. 1311200003303).
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Ruan T, Yue Y, Lu W, Zhou R, Xiong T, Jiang Y, Ying J, Tang J, Shi J, Wang H, Xiao G, Li J, Qu Y, Mu D. Association between low ambient temperature during pregnancy and adverse birth outcomes: A systematic review and meta-analysis. Chin Med J 2023;136:2307–2315. doi: 10.1097/CM9.0000000000002361
Tiechao Ruan and Yan Yue contributed equally to this work.
Supplemental digital content is available for this article.
References
- 1.Hoegh-Guldberg O, Jacob D, Taylor M, Guillén Bolaños T, Bindi M, Brown S, et al. The human imperative of stabilizing global climate change at 1.5°C. Science 2019; 365:eaaw6974.doi: 10.1126/science.aaw6974. [DOI] [PubMed] [Google Scholar]
- 2.Sandman CA, Glynn L, Schetter CD, Wadhwa P, Garite T, Chicz-DeMet A, et al. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): Priming the placental clock. Peptides 2006; 27:1457–1463. doi: 10.1016/j.peptides.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Li Z, Guo L, Sha Y, Yang K. Knowledge map and global trends in extreme weather research from 1980 to 2019: a bibliometric analysis. Environ Sci Pollut Res Int 2021; 28:49755–49773. doi: 10.1007/s11356-021-13825-6. [DOI] [PubMed] [Google Scholar]
- 4.Xiong T, Yue Y, Li WX, Choonara I, Qazi S, Chen HJ, et al. Effectiveness of azithromycin mass drug administration on trachoma: a systematic review. Chin Med J 2021; 134:2944–2953. doi: 10.1097/cm9.0000000000001717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paterson SK, Godsmark CN. Heat-health vulnerability in temperate climates: lessons and response options from Ireland. Global Health 2020; 16:1–17. doi: 10.1186/s12992-020-00554-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chersich MF, Pham MD, Areal A, Haghighi MM, Manyuchi A, Swift CP, et al. Associations between high temperatures in pregnancy and risk of preterm birth, low birth weight, and stillbirths: systematic review and meta-analysis. BMJ 2020; 371:m3811.doi: 10.1136/bmj.m3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becker JA, Stewart LK. Heat-related illness. Am Fam Physician 2011; 83:1325–1330. [PubMed] [Google Scholar]
- 8.Li H, Zhang Y, Li R, Wu Y, Zhang D, Xu H, et al. Effect of seasonal thermal stress on oxidative status, immune response and stress hormones of lactating dairy cows. Anim Nutr 2021; 7:216–223. doi: 10.1016/j.aninu.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chauhan NR, Kapoor M, Prabha Singh L, Gupta RK, Chand Meena R, Tulsawani R, et al. Heat stress-induced neuroinflammation and aberration in monoamine levels in hypothalamus are associated with temperature dysregulation. Neuroscience 2017; 358:79–92. doi: 10.1016/j.neuroscience.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 10.Kretschmer M, Coumou D, Agel L, Barlow M, Tziperman E, Cohen J. More-persistent weak stratospheric polar vortex states linked to cold extremes. J Bull Am Meteorol Soc 2018; 99:49–60. doi: 10.1175/bams-d-16-0259.1. [Google Scholar]
- 11.Screen JA, Bracegirdle TJ, Simmonds I. Polar climate change as manifest in atmospheric circulation. Curr Climate Change Rep 2018; 4:383–395. doi: 10.1007/s40641-018-0111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruckner TA, Modin B, Vågerö D. Cold ambient temperature in utero and birth outcomes in Uppsala, Sweden, 1915-1929. Ann Epidemiol 2014; 24:116–121. doi: 10.1016/j.annepidem.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Cox B, Vicedo-Cabrera AM, Gasparrini A, Roels HA, Martens E, Vangronsveld J, et al. Ambient temperature as a trigger of preterm delivery in a temperate climate. J Epidemiol Community Health 2016; 70:1191–1199. doi: 10.1136/jech-2015-206384. [DOI] [PubMed] [Google Scholar]
- 14.Elter K, Ay E, Uyar E, Kavak ZN. Exposure to low outdoor temperature in the midtrimester is associated with low birth weight. Aust N Z J Obstet Gynaecol 2004; 44:553–557. doi: 10.1111/j.1479-828X.2004.00314.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhou G, Yang M, Chai J, Sun R, Zhang J, Huang H, et al. Preconception ambient temperature and preterm birth: a time-series study in rural Henan, China. Environ Sci Pollut Res Int 2021; 28:9407–9416. doi: 10.1007/s11356-020-11457-w. [DOI] [PubMed] [Google Scholar]
- 16.Xiong T, Chen P, Mu Y, Li X, Di B, Li J, et al. Association between ambient temperature and hypertensive disorders in pregnancy in China. Nat Commun 2020; 11:2925.doi: 10.1038/s41467-020-16775-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rintamaki H. Human responses to cold. Alaska Med 2007; 49:29–31. [PubMed] [Google Scholar]
- 18.Mongelli M. Factors influencing fetal growth. Ann Acad Med Singap 2003; 32:283–288. [PubMed] [Google Scholar]
- 19.Chen R-s, Kang E-s, Ji X-b, Yang J-p, Yang Y. Cold regions in China. Cold Regions Sci Technol 2006; 45:95–102. doi: 10.1016/j.coldregions.2006.03.001. [Google Scholar]
- 20.Guo T, Wang Y, Zhang H, Zhang Y, Zhao J, Wang Y, et al. The association between ambient temperature and the risk of preterm birth in China. Sci Total Environ 2018; 613-614:439–446. doi: 10.1016/j.scitotenv.2017.09.104. [DOI] [PubMed] [Google Scholar]
- 21.Liang Z, Wang P, Zhao Q, Wang BQ, Ma Y, Lin H, et al. Effect of the 2008 cold spell on preterm births in two subtropical cities of Guangdong Province, Southern China. Sci Total Environ 2018; 642:307–313. doi: 10.1016/j.scitotenv.2018.06.026. [DOI] [PubMed] [Google Scholar]
- 22.Cheng P, Peng L, Hao J, Li S, Zhang C, Dou L, et al. Short-term effects of ambient temperature on preterm birth: a time-series analysis in Xuzhou, China. Environ Sci Pollut Res Int 2021; 28:12406–12413. doi: 10.1007/s11356-020-11201-4. [DOI] [PubMed] [Google Scholar]
- 23.Ha S, Liu D, Zhu Y, Kim SS, Sherman S, Mendola P. Ambient temperature and early delivery of singleton pregnancies. Environ Health Perspect 2017; 125:453–459. doi: 10.1289/ehp97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He JR, Liu Y, Xia XY, Ma WJ, Lin HL, Kan HD, et al. Ambient temperature and the risk of preterm birth in Guangzhou, China (2001-2011). Environ Health Perspect 2016; 124:1100–1106. doi: 10.1289/ehp.1509778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li S, Chen G, Jaakkola JJK, Williams G, Guo Y. Temporal change in the impacts of ambient temperature on preterm birth and stillbirth: Brisbane, 1994-2013. Sci Total Environ 2018; 634:579–585. doi: 10.1016/j.scitotenv.2018.03.385. [DOI] [PubMed] [Google Scholar]
- 26.Liang Z, Lin Y, Ma Y, Zhang L, Zhang X, Li L, et al. The association between ambient temperature and preterm birth in Shenzhen, China: a distributed lag non-linear time series analysis. Environ Health 2016; 15:84.doi: 10.1186/s12940-016-0166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X, Xiao J, Sun X, Chen Q, Yao Z, Feng B, et al. Associations of maternal ambient temperature exposures during pregnancy with the risk of preterm birth and the effect modification of birth order during the new baby boom: a birth cohort study in Guangzhou, China. Int J Hyg Environ Health 2020; 225:113481.doi: 10.1016/j.ijheh.2020.113481. [DOI] [PubMed] [Google Scholar]
- 28.Mathew S, Mathur D, Chang AB, McDonald E, Singh GR, Nur D, et al. Examining the effects of ambient temperature on pre-term birth in central Australia. Int J Environ Res Public Health 2017; 14:147–159. doi: 10.3390/ijerph14020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohammadi D, Naghshineh E, Sarsangi A, Zare Sakhvidi MJ. Environmental extreme temperature and daily preterm birth in Sabzevar, Iran: a time-series analysis. Environ Health Prev Med 2019; 24:1–13. doi: 10.1186/s12199-018-0760-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spolter F, Kloog I, Dorman M, Novack L, Erez O, Raz R. Prenatal exposure to ambient air temperature and risk of early delivery. Environ Int 2020; 142:105824.doi: 10.1016/j.envint.2020.105824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun S, Weinberger KR, Spangler KR, Eliot MN, Braun JM, Wellenius GA. Ambient temperature and preterm birth: a retrospective study of 32 million US singleton births. Environ Int 2019; 126:7–13. doi: 10.1016/j.envint.2019.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vicedo-Cabrera AM, Olsson D, Forsberg B. Exposure to seasonal temperatures during the last month of gestation and the risk of preterm birth in Stockholm. Int J Environ Res Public Health 2015; 12:3962–3978. doi: 10.3390/ijerph120403962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang YY, Li Q, Guo Y, Zhou H, Wang QM, Shen HP, et al. Ambient temperature and the risk of preterm birth: a national birth cohort study in the mainland China. Environ Int 2020; 142:105851.doi: 10.1016/j.envint.2020.105851. [DOI] [PubMed] [Google Scholar]
- 34.Weng YH, Yang CY, Chiu YW. Adverse neonatal outcomes in relation to ambient temperatures at birth: a nationwide survey in Taiwan. Arch Environ Occup Health 2018; 73:48–55. doi: 10.1080/19338244.2017.1299084. [DOI] [PubMed] [Google Scholar]
- 35.Zheng X, Zhang W, Lu C, Norbäck D, Deng Q. An epidemiological assessment of the effect of ambient temperature on the incidence of preterm births: identifying windows of susceptibility during pregnancy. J Therm Biol 2018; 74:201–207. doi: 10.1016/j.jtherbio.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 36.Ngo NS, Horton RM. Climate change and fetal health: the impacts of exposure to extreme temperatures in New York City. Environ Res 2016; 144:158–164. doi: 10.1016/j.envres.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 37.Yackerson N, Piura B, Sheiner E. The influence of meteorological factors on the emergence of preterm delivery and preterm premature rupture of membrane. J Perinatol 2008; 28:707–711. doi: 10.1038/jp.2008.69. [DOI] [PubMed] [Google Scholar]
- 38.Muresan D, Staicu A, Zaharie G, Marginean C, Rotar IC. The influence of seasonality and weather changes on premature birth incidence. Clujul Med 2017; 90:273–278. doi: 10.15386/cjmed-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Molina O, Saldarriaga V. The perils of climate change: in utero exposure to temperature variability and birth outcomes in the Andean region. Econ Hum Biol 2017; 24:111–124. doi: 10.1016/j.ehb.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 40.Murray LJ, O’Reilly DPJ, Betts N, Patterson CC, Davey Smith G, Evans AE. Season and outdoor ambient temperature: effects on birth weight. Obstet Gynecol 2000; 96:689–695. doi: 10.1016/S0029-7844(00)01022-X. [DOI] [PubMed] [Google Scholar]
- 41.Pereira G, Cook A, Haggar F, Bower C, Nassar N. Seasonal variation in fetal growth: accounting for sociodemographic, biological, and environmental exposures. Am J Obstet Gynecol 2012; 206:e1–e7. 74.e1-7. doi: 10.1016/j.ajog.2011.07.038. [DOI] [PubMed] [Google Scholar]
- 42.Lawlor DA, Leon DA, Davey Smith G. The association of ambient outdoor temperature throughout pregnancy and offspring birthweight: findings from the Aberdeen Children of the 1950s cohort. BJOG 2005; 112:647–657. doi: 10.1111/j.1471-0528.2004.00488.x. [DOI] [PubMed] [Google Scholar]
- 43.Poeran J, Birnie E, Steegers EA, Bonsel GJ. The impact of extremes in outdoor temperature and sunshine exposure on birth weight. J Environ Health 2016; 78:92–100. [PubMed] [Google Scholar]
- 44.Ha S, Zhu Y, Liu D, Sherman S, Mendola P. Ambient temperature and air quality in relation to small for gestational age and term low birthweight. Environ Res 2017; 155:394–400. doi: 10.1016/j.envres.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kloog I, Novack L, Erez O, Just AC, Raz R. Associations between ambient air temperature, low birth weight and small for gestational age in term neonates in southern Israel 11 Medical and Health Sciences 1117 Public Health and Health Services. Environ Health 2018; 17:1–9. doi: 10.1186/s12940-018-0420-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun S, Spangler KR, Weinberger KR, Yanosky JD, Braun JM, Wellenius GA. Ambient temperature and markers of fetal growth: a retrospective observational study of 29 Million U.S. Singleton Births. Environ Health Perspect 2019; 127:67005.doi: 10.1289/ehp4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Basagaña X, Michael Y, Lensky IM, Rubin L, Grotto I, Vadislavsky E, et al. Low and high ambient temperatures during pregnancy and birth weight among 624,940 singleton term births in Israel (2010-2014): an investigation of potential windows of susceptibility. Environ Health Perspect 2021; 129:107001.doi: 10.1289/ehp8117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ha S, Liu D, Zhu Y, Soo Kim S, Sherman S, Grantz KL, et al. Ambient temperature and stillbirth: a multi-center retrospective cohort study. Environ Health Perspect 2017; 125:067011.doi: 10.1289/ehp945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kanner J, Williams AD, Nobles C, Ha S, Ouidir M, Sherman S, et al. Ambient temperature and stillbirth: risks associated with chronic extreme temperature and acute temperature change. Environ Res 2020; 189:109958.doi: 10.1016/j.envres.2020.109958. [DOI] [PubMed] [Google Scholar]
- 50.Lian S, Guo J, Wang L, Li W, Wang J, Ji H, et al. Impact of prenatal cold stress on placental physiology, inflammatory response, and apoptosis in rats. Oncotarget 2017; 8:115304–115314. doi: 10.18632/oncotarget.23257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin Y, Hu W, Xu J, Luo Z, Ye X, Yan C, et al. Association between temperature and maternal stress during pregnancy. Environ Res 2017; 158:421–430. doi: 10.1016/j.envres.2017.06.034. [DOI] [PubMed] [Google Scholar]
- 52.Khalesi ZB, Bokaie M. The association between pregnancy-specific anxiety and preterm birth: a cohort study. Afr Health Sci 2018; 18:569–575. doi: 10.4314/ahs.v18i3.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Basu R, Malig B, Ostro B. High ambient temperature and the risk of preterm delivery. Am J Epidemiol 2010; 172:1108–1117. doi: 10.1093/aje/kwq170. [DOI] [PubMed] [Google Scholar]
- 54.Rammah A, Whitworth KW, Han I, Chan W, Hess JW, Symanski E. Temperature, placental abruption and stillbirth. Environ Int 2019; 131:105067.doi: 10.1016/j.envint.2019.105067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen G, Guo Y, Abramson MJ, Williams G, Li S. Exposure to low concentrations of air pollutants and adverse birth outcomes in Brisbane, Australia, 2003-2013. Sci Total Environ 2018; 622-623:721–726. doi: 10.1016/j.scitotenv.2017.12.050. [DOI] [PubMed] [Google Scholar]
- 56.Theiler K. The House Mouse: Atlas of Embryonic Development. Springer Science & Business Media 2013. [Google Scholar]
- 57.Mayvaneh F, Entezari A, Sadeghifar F, Baaghideh M, Guo Y, Atabati A, et al. Exposure to suboptimal ambient temperature during specific gestational periods and adverse outcomes in mice. Environ Sci Pollut Res Int 2020; 27:45487–45498. doi: 10.1007/s11356-020-10416-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.