Abstract

Daidzein (DDZ) is a well-known nutraceutical supplement belonging to the class of isoflavones. It is isolated from various sources such as alfalfa, soybean, and red clover. It demonstrates a broad array of pharmacological/beneficial properties such as cardiovascular exercise, cholesterol reduction, and anticancer, antifibrotic, and antidiabetic effects, which make it effective in treating a wide range of diseases. Its structure and operation are the same as those of human estrogens, which are important in preventing osteoporosis, cancer, and postmenopausal diseases. It is thus a promising candidate for development as a phytopharmaceutical. Addressing safety, efficacy, and physicochemical properties are the primary prerequisites. DDZ is already ingested every day in varying amounts, so there should not be a significant safety risk; however, each indication requires a different dose to be determined. Some clinical trials are already being conducted globally to confirm its safety, efficacy, and therapeutic potential. Furthermore, as a result of its therapeutic influence on health, in order to establish intellectual property, patents are utilized. In light of the vast potential of eugenol, this review presents a detailed data collection on DDZ to substantiate the claim to develop it in the therapeutic category.
1. Introduction
In recent times, natural remedies have been considered as an important kind of approach for cures. Historically, medicinal plants have been utilized to both treat and stave off various illnesses. Natural products have been used for the treatment of different ailments since prehistoric times. According to a fossil study, the first or oldest evidence of the usage of plants as medicines dates back about 60 000 years.1 This offers a fresh perspective on how the knowledge of traditional medicinal plants is operating and how it might be applied to treat emerging and lifestyle disorders. However, there is growing support for incorporating traditional herbal medicine knowledge into the investigation of new therapeutic agents. Various computational methods for bioprospecting in the postgenomic era can be used to investigate the rich medicinal plant history.2 While many new medications have been created over the past 50 years utilizing high-throughput screening techniques and combinatorial chemistry, natural products and the chemicals produced from them have remained crucial elements in pharmacopoeias. Only a small number of the estimated 250 000–500 000 plant species currently in existence have been studied scientifically for bioactivities.3 Therefore, there is a lot of potential for new scientific discoveries to come from plants and other natural products, which may be used to learn a lot about unique chemical structures and their innovative modes of action in the context of developing new drugs.4 The three main factors driving this trend away from synthetic contemporary pharmaceuticals and toward a wider usage of nutraceuticals are (1) cost effectiveness, (2) safety, and (3) accessibility.5
Phytoestrogens are phenolic plant compounds that are nonsteroidal and naturally occur and, by virtue of their size and molecular makeup, mirror the steroid estrogens produced by vertebrates. They fall into two categories: flavonoids and nonflavonoids. Isoflavones, coumestans, and prenylflavonoids are examples of flavonoids, whereas lignans are examples of nonflavonoids.6 Isoflavone phytoestrogens include genistein, daidzein (DDZ), glycitein, biochanin A, and formononetin. Humans primarily obtain isoflavones through their diets from soy and soy products, which are primarily composed of DDZ and genistein. They have estrogenic and/or antiestrogenic effects when ingested. Red clover’s methoxylated isoflavone formononetin is successfully transformed into DDZ in the human gastrointestinal tract; it serves as an indirect source of DDZ. Red clover extract based phytoestrogen dietary supplements are becoming more and more well-liked as an alternative therapy for the management of menopausal symptoms. Isoflavones are thought to be chemoprotective and can be used as an alternative therapy for a variety of hormonal illnesses, including menopausal symptoms, cardiovascular diseases, osteoporosis, and numerous cancer types, including breast and prostate cancers.7 DDZ (a phytoestrogen that belongs to the category of nonsteroidal estrogens), a nutraceutical ingredient so far, belongs to chemical class of isoflavones. It is a multitargeted herbal moiety that is used to address the challenges of a number of ailments, including osteoporosis, cancer, and inflammation, with antihemolytic, antioxidant, and anti-inflammatory effects. It has also protective properties against some illnesses like diseases of the cardiovascular system, diabetes, osteoporosis, and malignancy of the breast; these are connected by which estrogen control is regulated. Other biological functions not related to the ER (estrogen receptor) include safeguarding the skin and nerves, anti-inflammatory effect, anticancer function, and inhibition of oxidative damage. These positive outcomes are mostly attributable to immunological reaction modulation, oxygen free radical scavenging, proliferation inhibition, and other factors.8 DDZ has a chemical structure (as shown in Figure 1) similar to that of mammalian estrogens and acts in two directions by either replacing or influencing the ER complex, estrogen receptor, and the hormone estrogen. It can be found in foods produced from soy, such as textured soy protein, soy flour, and soy protein isolates, as well as tofu, tempeh, and miso. Additionally, in supplementary flours made from wheat, rice and maize are fortified with soy flour.9
Figure 1.

Daidzein chemical structure.
This review article is an attempt to bring DDZ to the forefront through the study of how its pharmacological properties have been used to treat a variety of ailments and how it has been incorporated into health supplements. The global market capitalization of DDZ reflects its importance as a multitargeted drug with numerous patents and clinical trials.
The enhancement of characteristics like quality, dependability, end-user requests, and applications has led to a significant segmentation of the DDZ market. The DDZ market research includes general success criteria, restrictions, and in-depth illumination of significant information on recent and upcoming examples that may affect development. In-depth analysis of current advancements, factors, and establishments is provided in the DDZ market research.
North America, Europe, the Middle East, Africa, Southeast Asia, and South America are among the major geographical areas that were examined. Figure 2 reveals that, according to the output capacity, utilization percentage, market base, supply and demand situation, profit income margin, and other factors, the top players in the DDZ market are projected to have a global presence.
Figure 2.

Market surveillance of daidzein by region. North America has the highest proportion (35%), followed by Asia Pacific, Europe, and the Middle East; the lowest contribution (5%) is by Africa. https://www.datalabforecast.com/industry-report/386257-daidzein-market/.
2. Sources of DDZ
Red clover (Trifolium pratense), soybean (Glycine max), lucerne (Medicago sativa), kudzu (Pueraria radix), and red lentils (Lens esculenta) and other legumes (Leguminosae) are among the plants that contain DDZ primarily in the form of glucosides (as depicted in Figure 3). The most plentiful sources of DDZ among them are thought to be soybeans and products made from soybeans.10 The amount of total isoflavones in soybean varies based on the variety of soybean, the geographical distribution, and the harvesting year. Owing to fermentation’s active increase in the concentration of aglycones, the quantity range of DDZ in the soured soybean type is larger than that in nonfermented soybeans.11
Figure 3.

Biological sources of DDZ which include red clover (Trifolium pratense), soybean (Glycine max), lucerne (Medicago sativa), kudzu (Pueraria radix), and red lentils (Lens esculenta).
It has been shown that several microorganisms, including Bacillus species, Rhizopus species, and Aspergillus species, participate in the fermentation process by a glycosylating isoflavone. Another well-known traditional Chinese medicine that is frequently taken as dietary supplements is kudzu root (Pueraria radix), which has been found to contain DDZ in both its glycoside and aglycone forms.12 Various plant species have varied amounts of daidzein, expressed in milligrams per kilogram of dry weight. The amount of daidzein in Glycine max, also referred to as soybeans, is relatively high at 37.6 mg/kg (Table 1). Garden peas, or Pisum sativum, have 0.4 mg/kg daidzein, which is a lesser concentration. Common beans, or Phaseolus vulgaris, have a moderate 23.2 mg/kg daidzein concentration. Lima beans (Phaseolus lunatus) and mung beans (Vigna radiata) have a lower daidzein concentration of 0.3 mg/kg. Adzuki beans, or Vigna angulariz, and fava beans, or Vicia faba, contain 4.6 and 5.0 mg/kg daidzein, respectively.13
Table 1. Contents of DDZ in Various Plants.
| species | daidzein (mg/kg dry wt) |
|---|---|
| Glycine max | 37.6 |
| Pisum sativum | 0.4 |
| Phaseolus vulgaris | 23.2 |
| Phaseolus lunatus | 0.3 |
| Vigna agnularis | 4.6 |
| Vigna radiata | 0.3 |
| Vicia faba | 5.0 |
3. Biosynthesis Pathway of DDZ
The biosynthesis pathway of DDZ is a seven-step enzyme mediated and irreversible chain of reactions as depicted in Figure 4. It starts with the precursor l-phenylalanine, which on going through nonoxidative deamination via the enzyme phenylalanine ammonia lyase, converted to cinnamic acid The next step is the conversion of cinnamic acid to p-coumarate, which is accomplished by the action of the enzyme cinnamic acid 4-hydrolase (C4H). The third step involves the transformation by the enzyme 4-coumarate:CoA ligase (p-coumaroyl-CoA; 4CL).
Figure 4.
A seven-step biosynthesis pathway of DDZ which starts in the presence of phenylalanine as it acts as a precursor. DDZ is formed from liquiritigenin in the presence of glucosyl transferase.
The formation of chalcone in the next step is aided by the enzyme chalcone synthase (CHS), which on reduction by chalcone reductase (CHR) generates isoliquiritigenin. The penultimate step is the isomerization of isoliquiritigenin to liquiritigenin with the help of the enzyme chalcone isomerase (CHI). The concluding step is the formation of DDZ, which involves the transfer of the glucosyl group and is accompanied by the enzyme glucosyl transferase (GT).14
4. Physicochemical Properties of DDZ
Daidzein with the molecular formula C15H10O4 has a melting point of 320 °C and boiling point of 512–513 °C at 760 mmHg. Moreover, its partition coefficient as reported by Vegas Software is 3.16. Additionally, its maximum absorption wavelength is 250 nm. A detailed description of its chemical and physical properties is given in Table 2 (https://health.ec.europa.eu/system/files/2022-10/sccs_o_263.pdf).
Table 2. Various Physicochemical Properties of Daidzein (European Commission for Safety Assessment)a.
| property | value |
|---|---|
| molecular formula | C15H10O4 |
| melting point | 315–323 °C |
| boiling point (760.00 mmHg) | 512.00–513.00 °C |
| flash point | 201 °C |
| UV/visible light absorption spectrum, λmax | 250 nm |
| organoleptic properties (color, odor, taste if relevant) | pale yellow or fine off-white solid or diluted alcohol prisms |
| partition coefficient (Log Po/w) | 3.16b |
| solubility | |
| pH 6 buffer | 18.76 ± 0.33 nmol/mL |
| pH 10.8 buffer | 1875 ± 292.01 nmol/mL |
| soybean oil | 10.88 ± 2.73 nmol/mL |
| molecular weight (daidzein) | 254.24 g/mol |
| empirical formula | C15H10O4 |
| CAS Number | 486-66-8 |
| EC Number | 207-635-4 |
Calculated by Vega Software.
5. Method of Analysis for DDZ
Daidzein has been analyzed using different analytical methods, such as high-performance liquid chromatography (HPLC), Raman spectroscopy, FTIR spectroscopy, and polarimetry.14 Daidzein and genistein analysis requires a more effective, precise, and easy procedure in the preparation of a soybean sample. Gradient elution systems have been utilized extensively in research; however, the isocratic method can improve this approach.15,16 Some of the methods are briefly described in Table 3.
Table 3. Analysis and Extraction of Daidzein by Various Analytical Techniques.
| method | details of the experiment | ref |
|---|---|---|
| capillary zone electrophoresis (CE) | Once the antioxidant butylated hydroxytoluene hydrolyzed the acid, the isoflavones from a coffee sample were extracted as well as purified using ether (BHT). An applied voltage of 25 kV, a buffer solution of 20 mmol/L Na2HPO4, a hydrodynamic injection lasting 3 s at 30 mbar, and UV detection at 254 nm were experimental parameters for the CE separation procedure. According to the findings, all three substances may be examined within 10 min with a linearity of 0.5–50 g/mL. | (17) |
| For DDZ, formononetin, and genistein, 0.134, 0.0642, and 0.0825 g/mL were the limits of detection, respectively. | ||
| HPLC | DDZ (15.2 min) and genistein, two isoflavonoids, were entirely separated by the HPLC technique (17.3 min). First, genistein and DDZ concentrations in the analyzed milk prepared from soy were calculated. Genistein (25.86 mg L–1 ± 0.66 SD) and DDZ (8.25 mg L–1 ± 1.13 SD) were found in commercial soy milk samples when isoflavones were tested. Genistein concentrations in soy milk were greater than DDZ concentrations. On the basis of the major isoflavone concentration of soy milk, the study’s findings can be used to estimate how much soy milk each individual can consume. | (18) |
| radioimmunoassay | Based on polyclonal antibodies against DDZ-4′-O-(carboxymethyl)ether-BSA, radioimmunoassay was used. The assay’s intra- and interassay coefficients of variation varied from 4.1 to 11.5% and from 5.6 to 21.7%, respectively, and it had a sensitivity of 0.4 pg/tube based on the sample’s concentration of DDZ and the technique (direct or extraction). Only 8% of the DDZ levels obtained by direct radioimmunoassay were achieved after the removal of human sera using diethyl ether. Mean and peak basal serum levels of free DDZ were recorded through this experiment. It implies that the initial DDZ unconjugated serial tests were made feasible by plasma as well as the initial phytoestrogen immunoassay in bodily fluids from humans. | (19) |
| extraction of supercritical fluids | An ideal environment for supercritical fluid extraction (SFE) was discovered. A solid–liquid extraction method using an aqueous methanol solution was tested at various temperatures, pressures, and cosolvent concentrations (methanol, ethanol, and acetonitrile). The extraction conditions were 50–70 °C, 176–380 bar, and a modifier of 0, 5, or 10 mol % cosolvents dissolved in water. The greatest concentrations of DDZ and genistein were discovered and extracted under the following conditions: static and dynamic extraction for 15 min at 60 °C, 380 bar of pressure, and 10% acetonitrile addition. It was found that, in comparison to supercritical extraction, the amounts of DDZ and genistein produced using solid–liquid extraction were higher by 86 and 63%, respectively. | (20) |
5.1. Impact of Physiochemical Properties on Formulation Development
As daidzein falls under Biopharmaceutics Classification System (BSC) class IV compounds, it is extremely clear that it has low solubility and penetrability, particularly through skin, which prevents the medicine from expressing its full therapeutic potential.21 To make the use of daidzein easier, scientists have developed many dosage forms, such as gels, complex formations, polymer formations, cocrystallization, etc. In a study by Qiu et al., it was discovered that daidzein preparations in solutions are more stable than those in solids.22 Since phenolic groups are naturally weakly acidic, they make it challenging to combine bases and salt at physiological pH.23 This can be avoided by forming hydrogen bonds with complementary groups, such as those found in daidzein, during the cocrystallization process, which will increase solubility. The solution to entering the skin and treating conditions that resemble melanoma is to use nanobased gels.24 Additionally, efforts have been made to create daidzein-loaded poly(lactide-co-glycolide) nanoparticles utilizing structural and crystal engineering to increase its solubility and stability,25 particularly when taken orally. Theophylline and cyclodextrin have also been used to make a number of complexes compatible with daidzein,21 improving solubility, but these methods restrict the use of daidzein to food and functional products as they require large amounts of reagents and are labor-intensive to carry out.25
5.2. Side Effects of Daidzein
The natural substance daidzein, which is present in some plants, especially soybeans, may cause a variety of adverse reactions, including bloating, colitis, constipation, diarrhea, sleeplessness, itching, nausea, skin rash, and changed thyroid hormone levels. Bloating, a sensation of fullness and discomfort in the abdomen, can occur in some people, while colitis, an inflammation of the colon that can result in diarrhea and abdominal pain, can happen to other people. In addition to diarrhea and constipation, irregular bowel movements might also happen. Another negative effect is insomnia, which is the inability to fall or stay asleep. Aside from skin rash and nausea, some people who consume daidzein may also feel itching and itchiness. Daidzein has also been linked to altered thyroid hormone levels.26
6. Pharmacodynamic and Pharmacokinetics (ADME) Properties of DDZ and Its Metabolic Characteristics
DDZ is mostly present in soy and many unfermented foods, where it can also be found in the forms of acetyl glycoside and aglycone. The glycoside conjugate27 of DDZ must first, by glucosidases in the small intestine, be converted into the aglycone form in order to be absorbed in the gut. Human gut bacteria either absorb or break down the aglycone form into a variety of metabolites, such as dihydrodaidzein, equol, and O-desmethylangolensin (O-DMA; a metabolite with no or little estrogenic activity). The structures of these metabolites are shown in Figure 5. Studies on DDZ distribution, bioavailability, absorption, and excretion, however, are still few; there are a number of procedures, including reduction, methylation, and demethylation. There are two processes: hydroxylation and C-ring cleavage that are involved in this intestinal biotransformation.28 DDZ is a physiologically inert component of plants that is present in the form of glycosides (also known as DDZ) and is unaltered during various food preparation processes.
Figure 5.

Three metabolites of DDZ. Equol is formed in the intestine, O-desmethylangolensin is formed in the colon, and an intermediate product, dihyrodaidzein, is formed in the intestine.
The bioconversion of DDZ involves the gut bacteria in a significant way. Following ingestion, the small intestine’s lactase enzymes, certain strains of Bifidobacterium and Lactobacillus, hydrolyze the glycosidic bond of DDZ. This results in the synthesis of the bioactive aglycone moiety, DDZ. Following glycosylation, a number of bacterial varieties prevalent in the gut of human proceed to demethylate and reduce DDZ to produce its variations.29 According to certain reports, DDZ is hydrogenated into dihydrodaidzein (DHD) by the microorganisms Clostridium sp. TM-40 and Coprobacillus sp. MRG1. Using a two-step process and the reductive cleavage of a heterocyclic ring, DHD can be further metabolized to produce DMA and equol.30
Colonic bacteria further metabolize DDZ to create equol or O-desmethylangolensin through a process that produces the intermediate dihydrodaidzein. Equol is an estrogen that is not a steroid and that is only produced in the intestine by bacteria that are metabolizing the soy isoflavone DDZ. These metabolites can then move to the liver for further metabolization. Finally, bacterial enzymes in the colon deconjugate both the DDZ and biliary byproducts that are not absorbed before they are either reabsorbed or metabolized.31
The small intestine is the site where DDZ is absorbed, as evidenced by the emergence of a modest peak in plasma roughly 1 h after consumption.32 After 5–8 h, a bigger peak results from the colon’s absorption and recycling of the conjugates. It is interesting to note that most DDZ in plasma is found conjugated, with a very tiny amount in aglycone form.33 A clinical investigation revealed that eating DDZ in the glucoside form leads to higher bioavailability than doing so in the aglycone form, in contrast to earlier research that had revealed the opposite. The effect of additional isoflavones on their metabolic process may be responsible for these contentious outcomes. Despite these investigations, it seems that DDZ reaches the peak plasma concentration 7 h after it is ingested, which is thought to be related to the absorption process. A study revealed that practically all DDZ is quickly absorbed and digested because there is little excretion in stools or urine; however, up to 30% of DDZ intake can be retrieved in urine.34 During a 24 h incubation, it has been demonstrated that a mouse colon derived anaerobic bacterium (Mt1B8) converts roughly 80% of DDZ to equol. The upper small intestine uses passive diffusion to absorb the aglycone molecule from the digestive tract. When compared to persons who consume nonfermented soybean, people who eat fermented soy products may absorb isoflavones more readily. This may be because fermented foods have probiotic benefits that may increase the population of gut bacteria.35
When soy milk containing glucoside conjugates is ingested, DDZ absorption is significantly 2 h faster than when solid soy meals are consumed. Another study discovered that insoluble fiber, including inulin, may boost the absorption of DDZ in part by stimulating bacterial growth. Studying the possible advantages of DDZ, however, requires taking into account a crucial component of its metabolism. The correlation between plasma levels and poor concentration prevents the various tissues from really receiving it. In fact, how much isoflavones and their derivatives are present in human tissues is not always known and can vary greatly.31 For instance, equol levels in human glandular tissue range from 456 to 559 nmol/kg and those in breast adipose tissue range from 22 to 36 nmol/kg.36
Human serum proteins can be nonspecifically bound by DDZ. It has been found to be present in plasma in a very low concentration which accounts for only 12%. It has been proposed that only the unbound fraction, which is free to interact with the target receptor, may be capable of producing biological effects that are particular to the target.37 DDZ is also thought to be present in a variety of tissues, including the placenta, kidney, liver, muscle, and mammary gland. In the brain, within the first hour of dosing, a discernible concentration of DDZ was discovered. Finally, DDZ absorption, bioavailability, and metabolism may be influenced by a number of variables, including age, dietary habits, and the gut bacterial community.38
7. Pharmacological Action of DDZ in Different Diseases
Consuming isoflavones may boost one’s health and minimize the symptoms of menopause as well as the risk of a number of age-related illnesses, including heart disease, fragile bone condition, and cancers.39 Supplementing with DDZ has been shown to have significant impacts, to decrease insulin resistance and inflammation, and in changes in lipid profiles within plasma, dyslipidemia, and other issues linked to obesity.40,41
Anti-inflammatory, cardioprotective, neuroprotective, and antiaging actions are further noteworthy effects. The following describes the function of DDZ in the human body and its specific mode of action for various conditions in Table 4.
Table 4. Mechanisms of Action of Daidzein (DDZ) for Different Diseasesa.
| no. | pathology | mechanism of action | ref |
|---|---|---|---|
| 1. Cancer | |||
| 1.1 | breast cancer | I. DDZ regulated (replacement or interference) estrogen and estrogen receptor complex; at high concentration exhibited anticancer capacity. | (42) |
| II. DDZ induced mammary tumor cell invasion by TNF-α (anti-inflammatory effect). | |||
| II.1. In the NF-κB signaling pathway, daidzein therapy suppressed TNF-mediated NF-B and AP-1 in breast cancer cell line MDA-MB-231, followed by a reduction in uPA release which ultimately prevented the spread of the disease. | |||
| II.2. Daidzein stopped the Hedgehog (Hh) signaling pathway’s activation and expression of Gli1, which halts ER-negative cells from moving and invading human breast cancer cells from MCF10DCIS. | |||
| II.3. Via the Hh/Gli1 signaling pathway, daidzein decreased the function and expression of MMP-9 that was triggered by TNF. | |||
| III. DDZ has noninvasive effects by partially reducing the MMP expression. | |||
| IV. Blockage of the cell cycle between G1 and G2/M phases along with apoptosis resulted in antiproliferative actions. | |||
| V. Daidzein elevated the generation of intracellular reactive oxygen species (ROS; antioxidant action), which modifies the mitochondrial transmembrane potential that in turn helps the release of cytochrome c. The release of cytochrome c is aided by proapoptotic protein Bax and reduced antiapoptotic protein Bcl-2 expression. The function of caspase-9 and caspase-7 was turned on by these enzymes, thus leading to cell death. | |||
| 1.2 | prostate cancer | I. By causing cell cycle arrest at the G0/G1 phase and inhibiting angiogenesis by changing the expression of genes involved in the cyclin-dependent kinase-related pathway, three prostate cancer cell lines (LNCaP, DU 145, and PC-3) have antiproliferative characteristics. | (43) |
| II. Antiandrogen activity PART-1 (prostate androgen-regulated transcript-1) gene expression was reduced dose dependently by dihydrotestosterone (DHT). | |||
| III. A programmed apoptosis in tumor cells can be made to happen by stimulation of TRAIL (tumor necrosis factor-related apoptosis-inducing ligand). | |||
| IV. As a radio sensitizer, downregulating the expression of APE1/ref-1 limits tumorigenesis primarily modulating the functioning of NF-B and HIF-1, a type of AR-independent mechanism that promotes radiotherapy. | |||
| 1.3 | colon and colorectal cancer | I. An arrest in the cell cycle at the G0/G1 stage along with apoptosis caused by caspase-3 in a dependent manner resulted in tumor suppressing activities on LoVo cells. | (44) |
| II. In order to better understand how two natural flavonoids, chrysin and daidzein, affect the levels of amphiregulin (AREG), chemokine ligand (CXCL1), and matrix metalloproteinase-9 (MMP-9) in colorectal cancer caused by 1,2-dimethylhydrazine dihydrochloride (DMH), we looked at their effects. Results obtained included the following: | |||
| II.1. Cytochrome P450 2E1 specific antioxidant activity of high potency (CYP2E1) was shown. | |||
| II.2. Metabolic disturbances were reversed, and histopathological findings, such as uncontrolled growth of mucosal lining covering crypts with mild inflammation, disappearance of goblet cells, and abnormal epithelial cells, reverted to a state that is close to normal. | |||
| II.3. Lowered p-ERK/ERK and p-AKT/AKT protein expression was linked to anticancer action toward SW620 cells. | |||
| 1.4 | liver cancer | I. Hepatocarcinoma SK-HEP-1 cell lines decrease the rate of hepatocarcinoma cell development. | (45) |
| II. Cell death is directed by the Bcl-2 family through the mitochondrial channel. | |||
| 1.5 | skin cancer | 7,3′,4′-THIF (metabolite of daidzein) has a chemopreventive role in UVB induced nonmelanoma skin cancer. The metabolite binds to Cot and MKK4 directly to inhibit the activities of Cot and MKK4, which further markedly suppresses the expression of UVB-induced cyclooxygenase 2 (COX-2) ultimately, inhibiting the elongation and number and volume of tumors. | (46) |
| 1.6 | ovarian and thyroid cancers | On inducing apoptotic cell death, the molecule 7-(O)-carboxymethyl daidzein linked to N-t-Boc-hexylenediamine (cD-tboc) exhibits antithyroid cancer and antiepithelial ovarian cancer actions. | (47) |
| 1.7 | neuroblastoma and other cancers | DDZ prevents cell division, interrupts the cell growth at the G2/M period, and accelerates cell death. | (48) |
| 1.8 | choriocarcinoma | I. Treatment with daidzein prohibits choriocarcinoma cells from multiplying. Daidzein exposure leads to G1 phase cell cycle arrest. Daidzein works by inhibiting nuclear signaling of p-ERK1/2 and ERK1/2 phosphorylation. | (49) |
| II. Daidzein lessened the rate of increase and clone development of the choriocarcinoma cell lines JAR and JEG-3 in a dependent manner, both with respect to time and concentration, prompting the cell cycle to be frozen at the G1 phase in both cell types. C-myc, daidzein, and PCNA, all of which can speed up the advancement of the cell cycle, also decreased the expression of cyclin D1. | |||
| 1.9 | cervical cancer | I. Anticancer behavior of daidzein on HeLa cells was achieved by modulating the cell cycle distribution. In some cases, cancer cells were arrested at the G0/G1 phase, while in some cases cancer cells were arrested at the G2/M phase. | (50) |
| II. Daidzein has an additional growth-retarding action on HeLa cells through inducing apoptosis. A decrease in catalytic activity of a subunit of mRNA of HeLa cells was observed on treatment with daidzein. | |||
| 1.10 | bladder cancer | I. Treatment with daidzein was found to reduce the cell viability of all indicated cell lines (bladder carcinoma cells and normal urothelial cells (SV-HUC-1 cells). | (107) |
| II. Daidzein inhibits colony formation of bladder carcinoma cells. | |||
| III. RT112 cells displayed a decreased number of colonies with the increase of daidzein concentration, suggesting daidzein inhibited colony formation of RT112 cells. | |||
| IV. Daidzein induces G1/S phase arrest and apoptosis of RT112 cells. | |||
| V. Daidzein suppresses the FGFR3 signaling pathway in RT112 cells. | |||
| VI. Daidzein decreased the phosphorylation levels of FGFR3 that was activated by FGF1 addition. | |||
| 2. Cardiovascular Diseases | |||
| 2.1 | hypercholesteremia | I. DDZ regulates blood lipid metabolism. | (51) |
| II. The triglyceride (TG) level that is correlated to the ESR-RsaI genotype is markedly reduced. | |||
| III. Uric acid, a separate CVD risk factor, is downregulated. | |||
| 2.2 | atherosclerosis and CAD (platelet aggregation, monocytes and macrophage aggregation, and endothelial function) | I. The pretreatment was the inhibition of collagen-mediated aggregation as a function of dose. | (52) |
| II. Activation of γ interferon coupled with lipopolysaccharide in a macrophage cell (RAW-264-7) stopped the generation of nitric oxide and decreased the release of TNF-α in a dose-dependent manner. | |||
| III. TNF dose dependently curtailed the human umbilical vein endothelial cells’ ability to secrete monocyte chemoattractant protein-1. | |||
| IV. DDZ demonstrated an action similar to that of estrogen on caveolin-1 suppression and caused vasorelaxation dependent on the endothelium, thus enhancing nitric oxide bioavailability and relieving endothelial dysfunction. | |||
| V. It greatly improves the endothelium dependent NO and prostaglandin mechanism to monitor the function of response toward contraction and relaxation. Moreover, it stopped the oxidation of lipids (antioxidant effect). | |||
| VI. Daidzein can substantially lower the blood level of inflammatory factors (by promoting the anti-inflammatory action) in geriatric CHD patients. | |||
| 2.3 | hypertension | DDZ checks the vascular smooth muscle tone by maintaining an equilibrium between vasodilator and vasoconstrictor. Alters renal and humoral functions and thus eventually reduces blood pressure. Daidzein’s role in catecholamine synthesis and secretion also contributed to reduce the likelihood of CVD. | (53) |
| 2.4 | myocardial infarction | I. DDZ reduced overall myocardium damage caused by ischemia reperfusion in a rat model. For instance, improved cardiomyocyte loss of function and apoptosis control and resulted in a smaller infarct size. | (54) |
| II. NF-κB activation allowed it to control the expression of the inflammatory cytokine with its antioxidant activity. | |||
| III. Equol, a derivative of daidzein, is crucial for relieving arterial stiffness and exerting antiatherosclerotic actions. | |||
| 3. Osteoporosis | |||
| postmenopausal symptoms | I. DDZ has estrogen-like action. Osteoporosis can be prevented by the ability of equol released by osteoblasts to suppress the synthesis of osteoclast cells and its activation by favoring apoptosis triggered by TGF. The action of apoptosis is achieved by binding to ERα present in osteoclasts and further changing the expression of the FasL gene. | (55) | |
| II. DDZ inhibits bone reabsorption. | |||
| III. An increase in secretion of RANK-L ligand and osteoprotegerin (OPG) through ERβ channel helps in differentiation of osteoblast cells and is implicated in osteoclastogenesis also amplified ALP activity and mineralization. | |||
| IV. DDZ promotes apoptosis in osteoclast progenitor cells through the enzyme caspase-3. | |||
| V. In the ERβ pathway (ERS mechanism), secretion of OPG is reduced when the osteoclastogenesis inhibitory factor is expressed in osteoblast cells and also involved in regulation of osteoclast differentiation. | |||
| VI. DDZ stimulates secretion of calcitonin (bone resorption inhibition), suppresses osteoclast activity, and promotes the growth of osteoblast line cells. | |||
| 4. Diabetes | |||
| 4.1 | type 2 diabetes | I. Daidzein favored AMPK phosphorylation, thereby facilitating glucose uptake. A type 2 diabetic cell model, L6 myotubes, helps in getting the glucose transporter into the PM of muscle cells leading to a state of glucose homeostasis that is independent of insulin. | (56) |
| II. The antagonistic action of daidzein in type 2 diabetes was found to be linked with glucose stored in liver and also affected lipid reaction by making changes in enzyme action (C57BL/KsJ-db/db mice). | |||
| III. DDZ controls the metabolism of blood in the liver of db/db rat by decreasing the fraction of glucose-6-phosphatase (G6 Pase)/glucokinase (GK) and phosphoenolpyruvate carboxykinase (PEPCK). | |||
| IV. DDZ decreases the level of FFA present in blood in order to reduce β-oxidation with the help of the enzyme carnitine palmitoyl transferase (CPT-1), thereby improving hepatic lipid metabolism and subsequent glycemic control in type 2 rats suffering from diabetes. | |||
| 4.2 | type 1 diabetes (insulin dependent diabetes) | I. Daidzein administration prolongs the life of B cells of the pancreas and insulin output while having no impact on glucagon in nonobese diabetic (NOD) rats, a model for type 1 diabetes in humans. | (57) |
| II. DDZ enhances the function of enzymes like G6PD and malic, while on the other hand it mitigates the activity of β-oxidation of fatty acids and others protein and enzymes like PEPCK and G6Pase. | |||
| III. DDZ monitors the level of glucose and metabolism of lipid by activating the receptors and regulating the gene expression involved in the lipid and glucose reactions such as turning on the peroxisome proliferator-activated receptor (PPAR) and regulating the gene expression of PPAR-α and PPAR-γ. | |||
| IV. DDZ brings down postprandial spikes in blood glucose by prohibiting the enzymes glucosidase and amylase from digesting carbohydrates. | |||
| 4.3 | diabetic nephropathy | I. DDZ elevates the level of nitric oxide in kidney, blocking the RAAS pathway, and reduces the expression of caveloin in wistar mice model. | (58) |
| II. DDZ guards the kidney and its associated organs and system such as the renin–angiotensin mechanism. | |||
| III. Podocytes, endothelial cells, and mesangial cells in glomeruli are overexposed to reactive oxygen species as a result of prolonged hyperglycemia, which results in kidney damage. Anti-inflammatory, antioxidant, and antiapoptotic properties are all present in daidzein. | |||
| 5. Aging | |||
| aging | I. In the TGF-β (transforming growth factor)/smad signal pathway, daidzein enhances the deposition of collagen by promoting the synthesis of collagen through increasing the activity procollagen (type I) and helps in the degradation of collagen by limiting the levels of enzymes such as MP1 (matrix metalloproteinase 1) and MMP2. This regulates the extracellular matrix (ECM). | (59) | |
| II. Owing to its antioxidant potential, it is not affected by light as it clears reactive oxygen species from keratinocytes that has been exposed to UV light. | |||
| III. Selective activation of ERβ by ERβ pathway-S-equol results in increases in the levels of enzymes having antioxidant activity and possessing the ability to protect skin from dangerous radical species and low levels of Snail, which regulates translocation and proliferation of keratinocytes cells and ultimately increases the expression of both collagens (I and III). | |||
| IV. Photoprotective effects can be seen as a result of binding of daidzein to RAR and RARγ, thus improving their functioning. | |||
| V. Daidzein facilitates cutaneous HA (hyaluronic acid) formation leading to improved viscoelasticity of mice epidermis; it maintains hydration and inhibits elasticity loss. | |||
| 6. Oxidative Stress | |||
| oxidative stress | I. In direct action on liposomal membrane, upon binding to membrane and thus altering its fluid nature, it slowed oxidation of lipids by removing reactive species and restricts the movement of it. | (60) | |
| II. In indirect action, the activity of antioxidant enzymes like catalase, superoxide dismutase (SOD; CuZn- and Mn-SOD), and glutathione peroxidase (GPx) is enhanced by the action of daidzein. | |||
| III. In a study using rat hepatoma H4IIE cells, daidzein controlled the expression level of AOE to the highest level, i.e., 300 μmol/L. | |||
| IV. In the interest of minimizing oxidative stress, along with the mitigation of vascular dysfunction in streptozotocin- induced diabetic rats, daidzein boosted the inhibited action of SOD and downregulated the elevated concentration of MDA, a byproduct of lipid peroxidation. | |||
| V. It restored the concentrations of AE and AOE to the originals dose dependently. | |||
| VI. Through enhancing the activity and expression of catalase and SODs, the daidzein metabolites O-DMA and equol, 3-OH-daidzein, and 6-OH-daidzein, have greater antioxidant capacities. | |||
| VII. DDZ suppresses glutathione metabolism. | |||
| 7. Inflammation | |||
| inflammation | • By increasing the expression of TG2, which is necessary for efficient engulfment during efferocytosis, daidzein strengthened the capacity of macrophage cell RAW264.7 to engage in efferocytosis. Efferocytosis is finally made more effective by the elevated TG2, which also encouraged the phosphorylated Erk to trigger Rac1 as well as the downregulation of mitochondrial membrane potential. | (61) | |
| • In an attempt to control the transcriptional activation of a wide variety of intended genes, such as pro-inflammatory messengers like iNOS, COX-2, various cytokines, chemokines, and adhesion molecules, it is possible to suppress the expression of NF-B, a sort of transcription factor that is mainly associated with inflammation. | |||
| • On binding with PARP-1, daidzein not only suppressed the transcription of NF-B (sensitive inflammatory gene) and hindered the production of the chemokine Cxcl2 but was also involved in the reduction in the level. | |||
| 7.1 | periodontal inflammation | I. In RAW264.7 cells treated with Prevotella intermedia, it was observed that daidzein decreased levels of nitric oxide and IL-6 mRNA transcription as well as their synthesis. | (62) |
| II. The degeneration of IκB-α due to P. intermedia LPS was stopped by daidzein. | |||
| III. Suppression in translational activity of NF-κB can be achieved by monitoring the movement of nucleus and DNA-binding abilities toward the p50 subunit of NF-κB. | |||
| 7.2 | hepatic inflammation | I. Levels of p-ERK1/2, p-IB, and p-p65 can be reduced by the action of daidzein on damaged liver triggered by LPS. | (63) |
| II. By overexpressing Nrf2 and simultaneously suppressing Keap-1 activity, daidzein lowered the LPS induced reaction that produces reactive oxygen species and bumped up SOD activity by 88.4 ± 18.9%. | |||
| 7.3 | endometriosis | I. DDZ impeded the development of endometrium cells of human. | (64) |
| II. DDZ decreased the cytokines that cause inflammation and demonstrate ER-mediated action. | |||
| III. DDZ lessened the severity of lesions in a mouse model that resemble endometriosis. | |||
| 7.4 | rheumatoid arthritis | I. Daidzein lowered the value of p to below 0.0001, thus signifying the low activity of articular elastase, malondialdehyde, and TNF-α in mice suffering from induced rheumatoid arthritis. | (65) |
| II. Both low density and very low density lipoprotein cholesterol levels were decreased, but high density lipoprotein cholesterol levels rose by the action of daidzein. | |||
| III. Animals with arthritis treated with hesperidin and daidzein saw a decrease in free radical load as well as an increase in total antioxidant levels in their plasma. | |||
| 7.5 | pulmonary inflammation | I. Daidzein significantly inhibited LPS induced increases of macrophage and neutrophil infiltration of lung tissues, as well as markedly attenuated MPO activity. | (66) |
| II. Daidzein effectively reduced the inflammatory cytokine release and total protein in bronchoalveolar lavage fluids (BALF). | |||
| III. Daidzein significantly inhibited LPS induced toll-like receptor 4 (TLR4) and myeloid differentiation factor 88 (MyD88) protein upexpressions and NF-κB activation in lung tissues. | |||
| 7.6 | aortic aneurysm (vascular inflammation) | I. TNF, IL-1, and nuclear factor-B (NF-B) protein expression are inhibited, which has an anti-inflammatory impact. | (67) |
| II. In angiotensin II induced AAA mice, daizzein blocked inducible nitric oxide synthase (iNOS) protein expression and significantly reduced the gene expression of cyclooxygenase (COX) 2, and matrix metalloproteinase 2 (MMP 2). | |||
| III. Mitogen-activated protein kinase (MAPK) communication via p38 was prevented from being phosphorylated. | |||
| 7.7 | adipose inflammation (obesity related) | I. Daidzein upregulated PPAR to accelerate adipocyte proliferation and govern adipokine activity. | (68) |
| II. DDZ upregulated the expression of adiponectin and further decreased the expression of pro-inflammatory factor TNF-α and MCP-1, which plays an important role in suppressing macrophage infiltration in adipose tissue | |||
| III. Hyperplasia of adipocytes cells is minimized in patents with obesity associated with inflammation and having insulin resistance. | |||
| 7.8 | skin inflammation | I. Daidzein blocks macrophage intrusion into the epithelial membrane triggered by UVB. It whittles down the generation of reactive oxygen species (ROS) and also the expression of pro-inflammatory mediators such as iNOS and COX-2, also blocking the signaling route for mitogen-activated protein kinase (MAPK). | (69) |
| II. By lowering NF-B activation and the production of IL-6, TNF, and COX-2, daidzein inhibited TPA induced skin inflammation. | |||
| 8. Neurological Disorders | |||
| 8.1 | stroke | I. Although the mechanism is unclear, it is impactful in neuroprotective properties and functional resumption after hemorrhage. | (70) |
| II. A possible hypothesis follows: | |||
| II.1. Apoptosis of neuron cell is prevented by binding of daidzein to GPR30 and ERβ on the membrane via a caspase-dependent route. | |||
| II.2. Redevelopment of neuron is attained by blocking the MAG cAMP channel which in turn is triggered by promoting the formation of mRNA of arginase. | |||
| II.3. Activation of PPARγ by daidzein’s ability to control migration of nucleus leads to neuronal cell death and differentiation of cells of axon. | |||
| 8.2 | memory damage and drug induced amnesia | I. Male rats exposed to scopolamine suffered memory loss due to daidzein stimulated axon formation and extension. | (71) |
| II. Phosphorylation of PKCα attached to protein GAP-43 occurred upon activation of ERβ present on the membrane by daidzein. | |||
| III. DDZ promotes cell growth and slows down the process of gliosis by blocking the expression of genes in hippocampus such as caspase-3, GFAP, etc. | |||
| IV. DDZ assists in the formation of Ach by stimulating the enzyme for its generation via cholinergic signal and also helps to combat amnesia caused by scopolamine activator. | |||
| 8.3 | Alzheimer’s disease | I. Daidzein inhibited aggregation of Aβ. | (72) |
| II. Daidzein inhibited Aβ induced cytotoxicity (antioxidant and anti-inflammatory effect). | |||
| III. DDZ reduced the synthesis of pro-inflammatory mediators in response to stimulation via lipopolysaccharide. | |||
| IV. The viability of astrocytes caused by lipopolysaccharide due to underexpression of their mRNA prior to treatment was regained. | |||
| 8.4 | Parkinson’s disease | I. Uncertainty surrounds the neuroprotective benefits of equol against neurotoxins that cause toxicity in PD related models. | (73) |
| II. Neuroprotective effects against PD related neurotoxins including 6-hydroxydopamine (6-OHDA) and 1-methyl-4-phenylpyridinium (MPP+) induced cytotoxicity were evaluated in SH-SY5Y cells. The results include the following: | |||
| II.1. DDZ (10 μM) and equol (10 and 20 μM) showed cytoprotective effects by decreasing LPS-BV2-conditioned media induced cytotoxicity in SH-SY5Y cells. | |||
| II.2. DDZ protects neurons by decreasing the levels of 6-OHDA and cerebral toxicity stimulated by MPP+ in SH-SY5Y cells. | |||
| III. DDZ reduces the high oxidative stress by clearing out the reactive oxygen species generated due to Parkinson’s disease, thus displaying an antioxidant effect. | |||
| 8.5 | spinal cord ischemia/reperfusion injury (SCII) | The PI3K/Akt pathway is favorably stimulated by daidzein to prevent neuronal apoptosis and lessen injury in the case of a rat having SCII. | (74) |
| 8.6 | drug induced neurotoxicity | I. DDZ showed neuroprotective effects (regulation of various pathways). | (75) |
| II. DDZ showed anti-inflammatory action. | |||
| III. DDZ showed antioxidant effects. | |||
| IV. Daidzein attenuated the malondialdehyde amount, the formation of oxygen radicals, and the Ach level in medium and preserved mitochondrial membrane integrity in response to neurotoxic damage driven by chlorpyrifos in PC12 cells. Superoxide dismutase per-unit efficiency increased as a result of daidzein’s enhancement of the indigenous redox balance in PC12 cells. | |||
| 9. Muscular Disorders | |||
| muscular atrophy/degeneration | I. DDZ provided neuroprotection in neurogenerative diseases like Huntington’s and Parkinson’s associated with muscle dysfunction. | (76) | |
| II. DDZ regulated mitochondrial biogenesis in muscles (C2C12 murine muscle cell line) by increasing Tfam (mitochondrial transcription factor A) promoter activity through SIRT1-associated pathway. | |||
| III. The Tfam gene along with mitochondrial gene expression like COX1 and Cytb are both stimulated, and so is the amount of mitochondria. | |||
| IV. Cisplatin (DDP) induced muscle atrophy, inhibited the Glut4/AMPK/FoxO pathway, downregulated the expression of atrogin1 and MuRF1, and inhibited skeletal muscle protein degradation. DDZ could inhibit the Glut4/AMPK/FoxO pathway to reduce myotube atrophy (in DDP treated C2C12 myotubes). | |||
| 10. Other Conditions | |||
| 10.1 | postmenopausal symptoms (osteoporosis, CVS complications, sleep disturbances, genital atrophy, hot flashes, senile vaginitis, depression, etc.) | I. Estrogen-like actions include decreasing the rate of resorption of bone by inhibiting osteoclasts and atherosclerosis by increasing plasma HDL and decreasing plasma LDL levels. | (77) |
| II. DDZ showed neuroprotective effects. | |||
| III. DDZ showed cardioprotective effects. | |||
| IV. DDZ showed cytoprotective effects. | |||
| 10.2 | pulmonary fibrosis | I. The proteinase activated receptor 2 and TGF expression in pulmonary fibrosis induced by bleomycin were reduced by daidzein. | (78) |
| II. DDZ controls the high secretions of mucin releasing from respiratory epithelium cells that would have caused breathing problems. | |||
| 10.3 | renal poisoning | Daidzein was found to accelerate cadmium excretion in OVX mice to prevent the harm that a buildup of heavy metals would cause to renal function. | (79) |
| 10.4 | behavioral disturbances (anxiety, depression) | I. Anxiety was found to be reduced, locomotor activity increased, and aggressive and sexual behavior decreased in male Balb/cJ mice after daidzein therapy; the mechanism used may be connected to ER. | (80) |
| II. The social behavior of the female offspring was impacted by the mother’s daidzein intake. Daidzein showed no effect on anxiety, but it decreased the expression of ER in the brain, which caused behavioral masculinization in mature female mice. | |||
| 10.5 | thrombosis | Daidzein suppressed platelet aggregation brought on by ADP and collagen that were injected intraperitoneally in mice. | (81) |
| 10.6 | allergy | Both the DNP-BSA induced release of hexosaminidase and the rat PCA (passive cutaneous anaphylaxis) response were significantly reduced by daidzein. | (82) |
| 10.7 | female-specific anorectic effect | Daidzein modifies the expression of neuropeptides linked to appetite in the hypothalamus, and by delaying gastric emptying, it causes the anorectic effect. | (83) |
| 10.8 | myocardial fibrosis | I. TGF-1 and TGF RI levels and daidzein’s benign action on cardiac fibroblasts were progressively and dose dependently reduced by daidzein. | (84) |
| II. Daidzein blocks TGF-β1 induced migration and proliferation of cardiac fibroblasts. | |||
| III. The TGF-1/SMAD signaling pathway produced by TGF-1 in cardiac fibroblasts was blocked by daidzein. Daidzein also reduced the in vivo myocardial fibrosis and dysfunction brought on by MI. | |||
| 10.9 | acute pancreatitis (AP) | I. O-Desmethylangolensin (O-DMA) and equol are created from daidzein and the glycoside derivative. These metabolites have greater antioxidant capacities compared to daidzein and might thus be beneficial to human health. | (85) |
| II. Daidzein therapy increased the preventive role on the pancreas dose dependently against l-arginine by lowering these inflammatory markers substantially (P < 0.001) when compared to normal control. | |||
| III. Daidzein therapy boosting the antioxidants significantly increased antioxidant levels and may be considered comparable to the standard control group. | |||
The pharmacological action of DDZ has been shown in controlling the diseases and ultimately leading to their treatment.
7.1. Anticancer Activity of DDZ
7.1.1. Breast Cancer
Soy’s effect on the development of breast cancer has been extensively studied. According to a meta-analysis, isoflavone supplementation may be beneficial for those with ER-negative breast cancer and may be linked to a lower incidence of the disease.86 DDZ supplementation is often linked with reduction in relapse of breast cancer in postmenopausal women. A lesser expression of HER2/neu and proliferating cell nuclear antigen (PCNA) in tumors has also been linked to soy consumption (DDZ), which is directly linked to a more proliferative, malignant tumor phenotype.
DDZ is essential for controlling breast cancer cells encroaching brought on by tumor necrosis factor (TNF). The molecular foundation of this has been explained by two different signaling routes, one of which is the signaling network of nuclear factor kappa B (NF-κB). DDZ treatment decreased breast cancer cells releasing uPA after TNF-induced NF-B and AP-1 blocked the MDA-MB-231 breast cancer cell line, which prevented the spread of the disease. DDZ blocked the Hh/Gli1 signaling pathway, which prevented TNF from inducing MMP-9 activity and expression. DDZ also inhibits the proliferation of breast cancer cells by causing cell cycle arrest in the G1 and G2/M phases as well as the activation of death.87
DDZ increased the production of intracellular reactive oxygen species (ROS), which altered the transmembrane potential of the mitochondria and caused the release of cytochrome c. The discharge of cytochrome c was boosted because of the upregulation of the proapoptotic protein Bax and the antiapoptotic protein Bcl-2. These substances eventually caused cell death by increasing caspase-9 and caspase-7 activities.88
7.1.2. Prostate Cancer
DDZ described antiproliferative properties in three prostate cancer cell lines (LNCaP, DU 145, and PC-3), triggering G0/G1 phase cell cycle halt and inhibiting blood vessel formation via altering the transcription of genes and its expression in the route linked to cyclin-dependent kinase.89 A few of these genes are involved in the expression of angiogenesis genes and the pathway for sensing DNA damage; when these pathways are inhibited, the growth factors EGF and IGF are decreased, which slows the growth of tumors. Prostate cancer growth is androgen-dependent in LNCaP and prostate cancer cells. A newly discovered gene called prostate androgen-regulated transcript-1 (PART-1) responds to androgens and may be used as a biomarker for prostate cancer. Dihydrotestosterone (DHT)-induced PART-1 expression was dose dependently decreased by DDZ, indicating a potential antiandrogenic effect of DDZ. The relationship between the expansion of prostate tumors and the suppression of PART-1 expression has been the subject of several in vivo studies. DDZ has the ability to induce tumor necrosis factor related apoptosis-inducing ligand (TRAIL) mediated apoptotic death exclusively in tumor cells.90
The endogenous anticancer drug TRAIL causes LNCaP cells’ mitochondrial membrane potential to be disrupted, which encourages apoptosis. In general, DDZ indicates contributing to both the prevention and treatment of prostate cancer.91
7.1.3. DDZ Role in Other Types of Cancer
At different doses, DDZ exhibited a biphasic activity in human colon cancer cells, which is helpful in the treatment of colon cancer. Cell cycle capture in the G0/G1 phase and caspase-3-dependent apoptosis had little effect on differentiation but had tumor-suppressive effects in LoVo cells. DDZ was found to have no effect on healthy human hepatocytes, according to research using liver cancer SK-HEP-1 cells as a cell model. However, liver cancer cell proliferation was found to be inhibited by DDZs. The ability of DDZ to trigger apoptosis was associated with regulation of the Bcl-2 family via the mitochondrial pathway. In several types of murine and human neuroblastoma cell lines, DDZ still exhibits anticancer properties by inhibiting cell growth, stopping the cell cycle in the G2/M phase, and inducing cell death.
Although DDZ has no effect on COX-2 expression, its biotransformation makes it a possible chemopreventive agent for skin cancer.92
By increasing cell apoptosis, the compound DDZ conjugated to N-t-Boc-hexylenediamine (cD-tboc) possesses antibodies against both thyroid and epithelial ovarian carcinomas.
7.2. DDZ Role in Osteoporosis
In contrast to other isoflavones, DDZ is special in that it may be used to treat osteoporosis. DDZ can also impede the absorption of bone. A study about cultivation of osteoblasts from young female pigs’ long bones showed that DDZ at a modest dose (1 nM) accelerated mineralization, increased ALP activity, and promoted osteoblast growth via the ER route. Additionally, the presence of ER was shown by an increase in the synthesis RANK ligand osteoprotegerin (OPG) which is RANK-L and runx2/Cbfa1, all of which are implicated in osteoclastogenesis. As a result, DDZ is essential for osteoblast development and function. DDZ also prevented osteoclast development and activation primarily by triggering caspase-3 to cause osteoclast progenitor death.93
Numerous DDZ analogues were shown to have antiosteoporosis effects by encouraging ER for separate development of stromal stem cells obtained from adipose tissue (ASCs) and mesenchymal stem cells (BMSCs) from bone marrow. For instance, the methoxy-DDZ isoformonetin decreased bone loss by preventing osteoblasts from death.94
7.3. Antidiabetic Activity
One of the most bioactive soy phytoestrogen ingredients, DDZ, has antidiabetic properties. Experiments both in vivo and in vitro have shown that DDZ has antihyperglycemic activity. DDZ increased glucose uptake by encouraging AMPK activation to enhance in a type 2 diabetic cell model; L6 myotubes, muscle cells’ PM, were translocated by glucose transporter 4. (The DDZ effect in the metabolism of glucose is depicted in Figure 6.) This resulted in glucose homeostasis that was insulin independent. In in vivo studies using db/db and KK-Ay mice as animal models for type 2 diabetes,95 DDZ was seen to control elevated blood sugar levels to demonstrate its antihyperglycemic action. DDZ has been shown to protect against type 2 diabetes and has the potential to be developed into an effective therapeutic phytochemical for the treatment of diabetes.
Figure 6.
Proposed molecular basis for daidzein and protective effects against faulty glucose metabolism.
DDZ has been seen to control elevated glucose levels in blood to demonstrate its action against hyperglycemia.96
Type 2 diabetes and DDZ have also been connected by adjusting the related enzyme activity; cholesterol and glucose metabolism in the liver are regulated. DDZ also decreased the increase in postprandial blood sugar levels by preventing glucosidase and amylase from digesting carbohydrates.97
7.4. Anti-inflammatory Activity
Treatment with DDZ is often shown to reduce the activation of many mediators of inflammation associated with various diseases such as insulin resistance, type 2 diabetes, and heart related disease. DDZ enhanced PPAR and adiponectin gene expression and adipogenic differentiation while downregulating expression and secretion of the MCP-1 gene.98
Macrophage cells treated with palmitate and DDZ treatment resulted in a significant increase in PPAR transcriptional activity as well as a reduction in JNK phosphorylation, followed by a decrease in Ccl2 and IL6 mRNA levels. Additionally, DDZ supplementation in the coculture system of adipocytes and macrophages lowered pro-inflammatory cytokine gene expression while increasing adiponectin gene expression through increasing PPAR transcriptional activity.99 The adipocytes and macrophages cocultured in a system are shown in Figure 7.
Figure 7.
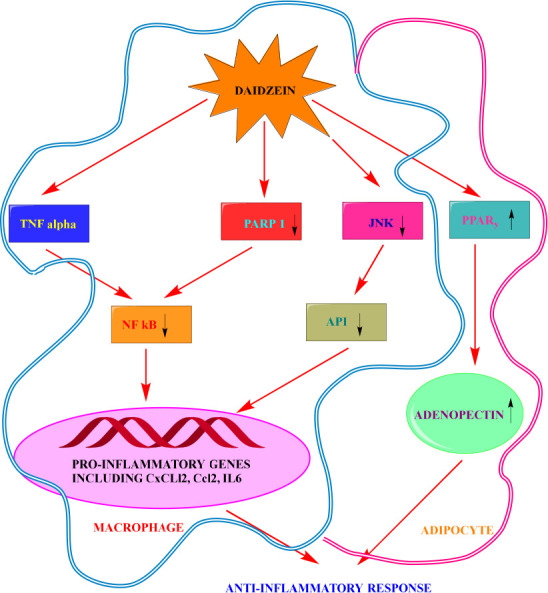
Anti-inflammatory activity of DDZ in coculture of adipocytes and macrophages. In adipocytes DDZ causes increased activity of PPARγ and adiponectin gene expression, while in macrophage it causes downregulation of NF κB, JNK, PARP 1 and AP-1, thus decreasing the activity of pro-inflammatory cell and resulting in anti-inflammatory activity.
DDZ’s anti-inflammatory properties have also been investigated using murine MLE-12 epithelial cells treated with TNFc. According to the findings, the administration of DDZ dramatically reduced TNF’s ability to promote NF-κB transcriptional activity, Cxcl2 expression and activity, and protein PARylation. Results from experiments with the NF-κB-Luc reporter plasmid and PARP1 expression plasmid revealed DDZ’s anti-inflammatory properties.100
7.5. Aging and Cognitive Activities
Soy supplementation (DDZ) in postmenopausal women shows positive results for muscle strength. Studies have shown that supplementation with isoflavones, specifically daidzein, help toward better cognitive health.9 The capacity of DDZ as a skin barrier, the capacity of DDZ in order to promote penetration through the skin, and the fact that DDZ exhibited nonionized forms, compared to ionized forms, cause higher skin layering.101 By use of the transdermal route, DDZ absorption was only mildly effective, but repeated transdermal administration of DDZ might increase its concentration in plasma despite the fact that aglycone combination and PEG400 can improve skin penetration.102
7.6. DDZ as an Antioxidant
DDZ’s antioxidant activity makes it good for animal health. DDZ reduced a result of lipid peroxidation, which is higher MDA levels, in streptozotocin-induced diabetic rats and boosted the suppressed action of SOD to reduce oxidative stress, including the avoidance of vascular damage. In a dose-dependent way, DDZ exerted its therapeutic effect by returning the regular AOE and AE concentrations.103 Compared to DDZ alone, certain of its metabolites, O-DMA with equol, for example, demonstrated higher antioxidant capacities in vitro. These compounds did this by enhancing catalase and SOD activity and expression. Two other DDZ metabolites, 3′-OH-DDZ and 6-OH-DDZ, were similarly more efficient than DDZ.104
7.7. Role of DDZ in Cardiovascular Diseases
DDZ is a potential therapy option for illnesses. It works by primarily controlling the metabolism of lipids in the blood, reducing high blood pressure readings, enhancing the capacity for antioxidants, and attenuating the dysfunction of endothelial cells. Use of DDZ for 6 months dramatically reduced triglyceride levels (TG).105
Glucose and other lipids were unaffected, although the concentration that is connected to the ESR-RsaI genotype was impacted. Another effect of DDZ was to downregulate an independent CVD risk factor. Additionally, DDZ and its metabolite equol were discovered to significantly reduce hypertension through regulating vascular modulating humoral systems, renal function, and the balance between vasodilators and vasoconstrictors to control smooth muscle tone function, which decreases blood pressure as a result. But DDZ’s ability to lower blood pressure has only been proven in animal models thus far, and human clinical trials are still required to confirm it.38,106
8. Clinical Trial Studies
DDZ has been studied clinically in different countries for various ailments such as hypertension, postmenopausal symptoms, type 2 diabetes, prostate cancer, etc. Some of the trials are approved or completed while others are pending. The investigation of the impact of phytoestrogens on serum lipids in postmenopausal women is one such study in Serbia. In the United States, a phase 3 trial of soy isoflavones with menopausal hot flashes is completed. In China, a trial was conducted and completed on a sample size of 400 that attempted to know the effects of DDZ on serum lipid profiles in hypercholesterolemia patients with different equol metabolism phenotypes. In the neurological disorder segment, research at the University of Wisconsin—Madison in the United States was successful in determining the potential benefits DDZ imparts for therapy of Alzheimer’s disease. A combinatorial examination was performed and concluded in New Zealand to unleash the effect of soy isoflavones with different green kiwifruit on hormonal levels, bone turnover, and fecal microflora in menopausal women. An analysis to study the impact of soy isoflavone on endothelial function and its ability to lower oxidative stress was carried out and finished in Indonesia for a sample size of 200. Various documented clinical trials to date have been listed in Table 5.
Table 5. Global Clinical Trial Studies on Daidzein Based Products to Access Their Effects in Various Conditions.
| no. | clinical trial no. | research topic | current status (phase) | state or condition | sample size | sponsor | location/country |
|---|---|---|---|---|---|---|---|
| 1 | NCT01270737 (U.S.) | whole soy and daidzein supplementation in a double-blind randomized controlled study to lower blood pressure in prehypertensive postmenopausal chinese women | completed (phase NA) | hypertension | 270 | Chinese University of Hong Kong | Center of Research and Promotion of Women’s Health, Hong Kong, China |
| 2 | NCT00179556 (U.S.) | menopausal hot flashes with soy isoflavones | completed (phase 3) | menopausal symptoms | 210 | 1. Beth Israel Deaconess Medical Center | Boston, MA |
| 2. Nichimo | Tokyo, Japan | ||||||
| 3 | NCT01556737 (U.S.) | impact of two distinct isoflavone supplements on gene expression of postmenopausal women | completed (phase NA) | postmenopausal | 72 | Wageningen University | Wageningen, Gelderland, Netherlands |
| 4 | NCT00951912 (U.S.) | studying how soy isoflavones affect postmenopausal Chinese women with impaired glucose regulation, metabolism of lipids and glucose | completed (phase NA) | type 2 diabetes mellitus | 165 | 1. Sun Yat-sen University | First Affiliated Hospital, Sun Yat-sen University; Guangzhou, Guangdong, China |
| 2. Nutrition Society of China | |||||||
| 3. International Danone Institute | |||||||
| 4. Department of Health of Guangdong Province | |||||||
| 5 | IRCT20100414003706N35 (Iran) | effect of a Menohelp Barij capsule on early menopause signs and symptoms: randomized placebo-controlled study | completed (phase 3) | menopausal and other perimenopausal disorders | 100 | Barij Essence Pharmaceutical Co., Kashan, Iran | Tabriz Public Health Centers, Tabriz, East Azarbaijan |
| 6 | EudraCT2020-000228-20 (Europe) | randomized controlled study of probiotic dietary intervention in polycystic ovary syndrome | ongoing (phase 1) | polycystic ovary syndrome (PCOS) | 180 | Medical University of Graz, Graz, Austria | Austria |
| 7 | UMIN000017190 (Japan) | plasma kinetics of the isoflavones daidzein and genistein in healthy females following consumption of fermented soymilk products | completed (phase NA) | healthy women (18–55 years) (menstrually active) | 10 | 1. Kagawa Nutrition University | Japan |
| 2. Yakult Honsha Co., Ltd. | |||||||
| 8 | UMIN000019450 (Japan) | effects of daily use of the isoflavone-rich soybean “Yukipirika” on the improvement of bone metabolism and menopausal symptoms examined in a random, double-blind, placebo-controlled, parallel group comparison study | completed (phase NA) | healthy adults and menopausal women | 20 | 1. Hokkaido Information University | Japan |
| 2. Northern Advancement Center for Science & Technology | |||||||
| 9 | ChiCTR2100043116 (China) | clinical study on application of PRF/phytoestrogen daidzein complex method in postmenopausal women with osteoporosis after tooth extraction | recruiting (not started) | alveolar bone loss | 90 | 1. Stomatology Department, Inner Mongolia Medical University Affiliated Hospital, Inner Mongolia Autonomous Area, China | Mongolia Medical University Affiliated Hospital, China |
| 2. Inner Mongolia Medical University Affiliated Hospital, China | |||||||
| 10 | ChiCTR2000034019 (China) | single-arm exploratory trail of combination of abiraterone with daidzein in the treatment of castration resistant prostate cancer | recruiting (not started) | prostate cancer | 30 | Shanghai Tongji Hospital, Shanghai, China | Shanghai Tongji Hospital, Shanghai, China |
| 11 | ChiCTR-TRC-11001222 (China) | preclinical trial of daidzein supplement for postmenopausal osteoporosis | completed (phase NA) | postmenopausal osteopenia or osteoporosis | 80 | 1. Third Military Medical University, Chongqing, China, | Third Military Medical University, Chongqing, China |
| 2. National Natural Science Fund of China, Beijing, China | |||||||
| 12 | ChiCTR-TRC-10001048 (China) | effects of daidzein on serum lipid profile in hypercholesterolemia patients with different equol metabolism phenotypes | completed (phase NA) | hypercholesterolemia | 400 | 1. Third Military Medical University, Chongqing, China | Third Military Medical University, Chongqing, China |
| 2. National Natural Science Fundation of China | |||||||
| 13 | NCT00661856 | impact of soy isoflavones on physical performance indicators and bone mineral density | completed (phase NA) | bone diseases, bone metabolism | 203 | 1. Creighton University | Creighton University Medical Center, Omaha, NE |
| 2. Solae, LLC | |||||||
| 14 | NCT00924339 | evaluation of urinary isoflavone excretion as compliance indicators in a soy food intervention trial (SOY FIT) for treatment of children and adolescents with familial hypercholesterolemia to confirm the beneficial impact of a soy-substituted diet | completed but not verified (phase NA) | familial hypercholesterolemia | 30 | Medical University of Vienna | Pediatrics Division, Division of Nutrition and Metabolism, Medical University of Vienna, Vienna, Austria |
| 15 | NCT04871750 | prospective, randomized controlled study on the effects of dietary soy protein on facial wrinkles | not started; completion June 2023 (phase NA) | photoaging | 80 | 1. Integrative Skin Science and Research | Sacramento, CA |
| 2. United Soybean Board | |||||||
| 16 | NCT00491595 | soy isoflavones: phase 1 multiple-dose clinical investigation in healthy postmenopausal women | phase 1 completed | drug toxicity | 36 | National Cancer Institute (NCI) at University of North Carolina | Chapel Hill, NC |
| 17 | NCT05073523 | randomized controlled study to evaluate dose–response associations for certain foods’ dietary biomarkers | ongoing; completion May 2022 (phase NA) | healthy | recruiting | Chalmers University of Technology | Department of Food & Nutrition and Sport Science Recruitment, University of Gothenburg, Gothenburg, Sweden |
| 18 | NCT01497977 | impact of phytoestrogens on serum lipids in postmenopausal women | completed (phase 4) | low serum lipid levels | 74 | American Medical Academy, Serbia | Ultramedica Clinic, American Medical Academy, Belgrade, Serbia |
| 19 | NCT01463436 | study of soy isoflavone 100 mg/day in postmenopausal women to elaborate effect of soy isoflavone in endothelial function and to reduce oxidative stress | completed (phase 3) | cardiovascular disease, osteoporosis | 200 | Trisakti University | Trisakti University, Jakarta, Indonesia |
| 20 | NCT01048606 | synergistic effect of exercise and phytoestrogens on postmenopausal women risk factors for CVD | completed (phase 4) | overweight | 45 | Canadian Institutes of Health Research (CIHR), Université de Sherbrooke | Centre of Research on Aging, CSSS-IUGS, Sherbrooke, Canada |
| 21 | NCT00669656 | men with biochemical recurrence of prostate cancer after initial local treatment: phase 2 study of combined herbal therapy | completed (phase 2) | prostate cancer | 43 | University of Southern California | Norris Comprehensive Cancer Center, USC, Los Angeles, CA |
| 22 | NCT00205179 | Alzheimer’s disease: potential benefit of isoflavones | completed (phase 2) | Alzheimer’s disease | 72 | 1. University of Wisconsin—Madison | University of Wisconsin—Madison, Madison, WI |
| 2. National Institute on Aging (NIA) |
9. Patent Repository on DDZ
The pharmacological benefits and other useful characteristics of DDZ have captivated scientists across the globe to conduct exhaustive research and studies, and in doing so they were successful in establishing numerous intellectual property rights in the shape of patents for several ailments like osteoporosis, skin treatment, etc. Table 6 outlines the information about some of the patents with their application numbers and invention details in various countries, with China having the most patents on DDZ.
Table 6. Brief Summary of Patents on Daidzein across the Globe.
| no. | inventor(s) | patent number | title of patent | details of invention |
|---|---|---|---|---|
| 1 | Helga Biergiesser, Thomas Doering, Stefan Gallinat, Ludger Kolbe, Franz Staeb, Kirsten Venzke | WO2002087517A3 | Use of isoflavonoids in skincare or cosmetic products to prevent or cure sensitive skin | The invention relates to the use of derivatives of isoflavones in cosmetic or dermatological preparations for the treatment and prophylaxis of the symptoms of inflammatory and/or itching skin conditions. |
| 2 | Barbara A. Bryan, Maryann C. Allred | EP0827698B2 (Europe) | Vegetable protein extract and material with added glucone isoflavones, as well as materials with high genistein and DDZ content and a method for making them | The current invention pertains to aglucone isoflavone enriched vegetable protein extract and protein material, as well as methods for generating such materials from aglucone isoflavone enriched protein material. |
| 3 | Susan M. Potter, Edna C. Henley, Doyle H. Waggle | TW486368B (Taiwan) | Pharmaceutical and food products that use DDZ material to lower LDL cholesterol levels and raise HDL cholesterol levels in the blood | This invention discusses a method for altering the concentration of cholesterol constituents in human blood. A DDZ material is administered to a human to increase the concentration of HDL cholesterol and to decrease the level of LDL cholesterol in the blood. |
| 4 | Tomomi Ueno, Adult Uchiyama, Shusui Suzuki | JP3864317B2 (Japan) | Composition containing equol-producing lactic acid bacteria | The present inventors have demonstrated the ability to assimilate DDZ glycoside, DDZ, or dihydrodaidzein and produce equol as a new bacterium (genus Lactococcus) that is essentially different from the previously isolated and identified microorganism. |
| 5 | Graham Edmund Kelly | NZ252051A (New Zealand) | Dietary supplement containing one of the following: genistein, DDZ, biochanin, or formononetin, which is a phytoestrogen | The present invention concerns a health supplement specifically enriched for isoflavones; their natural glycosides form in sufficient amounts to improve the health of a human. |
| 6 | Kenneth David Reginald Setchell, Sidney John Cole | US9408824B2 (United States) | S-Equol-containing substances, goods, and production processes | This invention discloses the composition for use in making commercial food and skin products comprising 5-equol or mixtures, including both a nonracemic mixture and a racemic mixture, of S-equol and R-equol. |
| 7 | Bert Vallee, Wing-Ming Keung | US20070270332A1 (United States) | Methods and assays useful in the treatment of alcohol dependence or alcohol abuse | A method for the treatment of alcohol abuse using DDZ and compounds analogous to DDZ is disclosed. Also disclosed is a method for screening compounds having antidipsotropic activity. |
| 8 | SuWeike, Li Jianjun | CN1321992C (China) | Method for extracting and separating isoflavone from kudzu | The present invention discloses a method for extracting and separating isoflavone from kudzu. |
| 9 | Lars Hoie | US20040234631A1 (United States) | Soy-based substances and their use to the treatment and/or prevention of a variety of illnesses | The invention concerns soy protein, phytoestrogens (DDZ and genistein), phospholipids, and dietary fibers and compositions thereof suitable for preventing, treating, and/or alleviating cardiovascular diseases. |
| 10 | Doyle H. Waggle, Susan M. Potter, Edna C. Henley | CA2306008C (Canada) | Composition and procedure for lowering the level of low-density lipoprotein cholesterol | The present invention is a composition comprising a plant sterol and a soy protein material and/or and isoflavone and their naturally occurring glycosides; a further method for preventing or minimizing the development of atherosclerosis in a human is also discussed. |
| 11 | Graham E. Kelly | US6340703B1 (United States) | Treatment or prevention of osteoporosis | The invention discusses a method for the treatment or prevention of menopausal symptoms wherein there is administered a therapeutically effective amount of the isoflavone DDZ. |
| 12 | Robin M. Bannister, John Brew, Gregory A. Stoloff | US10188668B2 (United States) | Cancer drug and uses | A pharmaceutical composition comprises a cancer therapeutic. In an embodiment, a cancer therapeutic is an isoflavone (DDZ, genistein, etc.). |
| 13 | Edmund Joseph Elder, Jr., Mark Joseph Sacchetti, Randall Joseph Tlachac, John L. Zenk | US10729674B2 (United States) | Nanoparticle isoflavone compositions and methods of making and using the same | The present invention is directed to formulations of genistein and DDZ and preparation methods. In particular, embodiments, the formulations described herein, include suspension formulations of nanoparticulate genistein and DDZ. |
| 14 | Grant E. Dubois, Indra Prakash | AU2007317458B2 (Australia) | High-potency sweetener formulations containing phytoestrogen and its sweetened derivatives | The present invention relates to different functional sweetener compositions and at least one functional ingredient, such as phytoestrogens (selected from the group consisting of genistein and DDZ). |
| 15 | Li Yaping | CN102060870 (China) | The composition of DDZ and phospholipids as well as its synthesis | The invention relates to a DDZ and phospholipid composite, and several preparations of a combination of DDZ and phospholipid are discussed. |
| 16 | Bhattacharya, Sushmita | WO2012004653 | A technique for NF-κB gene expression suppression | The present invention discloses a method of inhibition of the synthesis of NF-κB by inhibiting its gene expression using isoflavones DDZ and daidzein. Further, this invention provides that both natural and synthetic DDZ have the same biological activities. |
| 17 | Li Ying | CN102727482 (China) | DDZ-hydroxypropyl-beta-cyclodextrin clathrate and its preparation method | The invention discloses a DDZ–hydroxypropyl-β-cyclodextrin clathrate and its preparation method. The method discussed can substantially increase the solubility of DDZ; the prepared clathrate enables a high clathration rate. |
| 18 | Lei Hongtao | CN105669628 (China) | DDZ semi antigen and complete antigen and preparation method and application | The invention discloses a DDZ semiantigen, a complete antigen and a preparation method with its wide application. |
| 19 | Shimada, Yoshikazu | JP2010273647 (Japan) | Dihydrodaidzein-racemizing enzyme | The invention discloses an enzyme having the activity of racemizing dihydrodaidzein consisting of a specific amino acid sequence. A method for producing equol from DDZ is further discussed. |
| 20 | Bai Jun | EP2789604 (Europe) | DDZ derivative and pharmaceutically acceptable salt and preparation method thereof, and pharmaceutical composition containing the same | The invention discloses the DDZ hydrochloride derivative prepared from the DDZ derivative provided by the invention, especially 7-O-N,N-diethyl-amino acetyl DDZ hydrochloride, has good solubility and has a good effect in treating cardiovascular diseases. |
| 21 | So Hyun Lee | KR1020070014672 (Republic of Korea) | Skin external composition for treating and preventing hyperpigmentation, especially inhibiting phagocytosis of keratinocytes comprising DDZ or genistein | The skin external composition for inhibiting phagocytosis of keratinocytes comprises at least one selected from genistein and DDZ, which is an isoflavone produced from Leguminosae plants and shows estrogen effects; many formulations are available. |
| 22 | Yang Lizhi | CN104095822 (China) | DDZ containing tablet composition and preparation method thereof | The invention provides a specific prescription of DDZ-containing tablet and a preparation method. An added weak base enables DDZ to relatively well form a relatively stable, easily soluble salt. Thus, improvement of dissolution rate is facilitated, and absorption by the human body is facilitated. |
| 23 | Ren Lai | WO2018145364 | Use of 3,4,7-trihydroxyisoflavone or 3-methoxy daidzein in preparation of medicaments for inhibiting platelet aggregation and thrombosis | The invention reveals the use of 3,4,7-trihydroxyisoflavone or 3-methoxy-DDZ has an inhibitory effect on platelet aggregation. The use of said medicaments can reduce the risk of bleeding, is safe, and allows for broader clinical and medical uses. |
| 24 | Yi Chongqin | CN106880620 (China) | DDZ capsule and preparation method thereof | The invention is about the method of preparation of DDZ capsule having a dissolution rate or 80% or above by using DDZ and meglumine at different ratios. |
| 25 | Li Yaping | CN102258475 (China) | DDZ solid lipid nanoparticles and preparation method thereof | The invention outlines information about the preparation method of solid lipid nanoparticles with high oral bioavailability and of a hydrophobic medicine nature. |
| 26 | Wang Dongkai | CN101632650 (China) | Self-microemulsifying semisolid skeleton capsule of DDZ and preparation method thereof | The invention discloses a simple method of preparation of a self-microemulsifying semisolid skeleton capsule of DDZ which can be easily stored. The capsule absorbs water in the GI tract and automatically emulsifies to form a microemulsion. |
| 27 | John Casey | EP2365807 (Europe) | Oral composition comprising DDZ and an anthocyanidin | The invention is related to the composition of DDZ and anthocyanin in the range 1:1 to 1:100 for oral consumption which is free of soy protein and has an anti-inflammatory effect for skin inflammation. |
| 28 | Seung Bae Baek | KR102116416 (Republic of Korea) | Composition for preventing and treating cancer composed of 6′-sialyllactose and DDZ | The present invention relates to a composition for preventing or treating colorectal and gastric cancer composed of DDZ and 6′-sialyllactose in various combinations. |
| 29 | Ye Xiyun | CN102283787 (China) | Application of DDZ with function of promoting synthetic activity of collagen to cosmetics for removing and preventing crease. | The invention discloses application of DDZ as a cosmetic additive with a function of promoting the synthetic activity of collagen in cosmetics for removing and preventing creases. |
| 30 | Shen Qi | CN104784158 (China) | PLGA [poly(lactic-co-(glycolic) acid] electrospinning fiber loaded with DDZ NLCs (nanostructured lipid carrier) as well as preparation method | The invention discloses a PLGA (poly(lactic-co-(glycolic) acid) electrospinning fiber loaded with DDZ NLCs (nanostructure lipid carriers) as well as a preparation method having good foaming capacity, high encapsulation efficiency, easy to operate cand convenient to use. |
| 31 | Shen Qi | CN101204392 (China) | Self-emulsifying microemulsion DDZ oral liquid preparation composite and preparation method thereof | The invention discloses an oral preparation composition of a self-emulsified DDZ and a preparation method which can absorb water in the GI tract. The invention has the advantages of simple operation and easy preservation. |
| 32 | Liu Daicheng | CN110128386 (China) | Preparation method of DDZ pure product | The invention discloses a preparation method of a DDZ pure product. The method is simple in process and low in reagent consumption, the reagents are cheap and are easily available, and preparation cost is low. |
| 33 | Wang Yancai | CN102274202 (China) | DDZ nanocrystal immediate-release capsule and preparation process thereof | The invention relates to a prescription of a DDZ nanocrystal immediate-release capsule with good solubility and dissolution rate, good oral bioavailability, and a preparation process. |
| 34 | Graham Edmund Kelly | WO1998050026 | Treatment prevention of menopausal symptoms and osteoporosis | The invention describes the method for the treatment or prevention of menopausal symptoms or osteoporosis on administration of the isoflavone formononetin and DDZ (in the case of menopausal symptoms) optionally mixed sometimes with adjuvants, carriers, etc. |
| 35 | Anke Anton | CN-113896703-A (China) | Green preparation process of DDZ | The invention provides a green preparation process of DDZ, using etherate as a catalyst. The method has the advantages of, low cost, low risk, high yield, low reaction temperature, a nd mild and controllable reaction conditions. |
| 36 | Miao Shiwei, Li Minsheng | CN-113861152-A (China) | Preparation method of soybean isoflavone | The invention discloses a temperature sensitive preparation method of a soybean isoflavone, DDZ, with 90% product as isoflavone, thereby solving the problem of improving the content of soy isoflavones in industrialization. |
| 37 | Huang Kai, Chen Min, Liang Minglu, Shu Jiangcheng, Wu Yichen | CN-113813253-A (China) | Application of DDZ in preventing and treating heart related diseases | The invention discloses application of DDZ in preventing and treating disease caused by myocardial fibrosis and infarction of heart tissue and a preparation for inhibiting myocardial fibroblast inflammatory factor expression. |
| 38 | Gong Shenhai, Zeng Yunong, He Zhuo’En, Xiao Wei, Li Lei | CN-113730393-A (China) | Application of DDZ in preventing and treating acetaminophen induced acute liver injury | The invention relates to application of DDZ in preventing and treating acetaminophen induced acute liver injury. The invention has the beneficial effects that the DDZ relieves the symptoms of APAP induced acute liver injury. |
| 39 | Guo Cheng, Zhang Hong, Wan Lili, Zhang Jianping, Yang Quanjun | CN-113209076-A (China) | Application of DDZ in preparation of medicines for reducing toxicity of platinum medicines | The invention provides an application of DDZ or hydrate of DDZ (active ingredient) in preparing a medicament for reducing the toxicity of platinum drugs by downregulating skeletal muscle degradation related protein and for reducing the damage of platinum drugs to kidneys. |
| 40 | ZhiKe Hou, Jun Wei, Qi Kun, Li Meng, Yuan Hui, Li Yincheng | CN-113201137-B (China) | DDZ sol–gel surface molecularly imprinted polymer and preparation method thereof | The invention discloses a DDZ sol–gel surface molecularly imprinted polymer and a preparation method of its precursor. The method has the advantage of high adsorption selectivity on DDZ. |
| 41 | Wang Weichen, Zhang Wei, Yao Fawei, Ma Jianmin | CN-112812089-A (China) | Method for synthesizing DDZ | The invention discloses a method for synthesizing DDZ using boron trifluoride as a catalyst and acetophenone as a raw material. The method operates at low temperature, generates high yield, and maintains purity. |
| 42 | Miao Shiwei, Liu Xinmin | CN-213285743-U (China) | Novel DDZ molecular distillation extraction device | The invention relates to the technical field of soybean flavone molecular distillation extraction and discloses a novel soybean flavone molecular distillation extraction device. |
| 43 | Huang Zhiheng, Zhang Enze, Huang Zizheng, Liu Jiaqi, Yu Zijing, Zhang Sen, Duan Jin’Ao | CN-112111545-A (China) | 6′-O-Succinyl DDZ derived from biological method and application thereof in preparing neuroprotective drugs and health products | The invention discloses 6′-O-succinyl-DDZ from a biological method and its application preparing nerve protection medicaments and health care products wherein 6′-O-succinyl-DDZ is taken as an active ingredient and has the characteristic of a strong neuroprotective effect. |
| 44 | Chu Meijie | CN-111257382-A (China) | DDZ molecular imprinting electrochemical sensor and preparation method thereof | The invention discloses a DDZ molecular imprinting electrochemical sensor and two preparation methods for it using DDZ as the template molecule and dopamine hydrochloride; in the other one DDZ is used as the imprinted polymer of an O composite nanoporous material. |
| 45 | Li Ning, Hu Honglai, Deng Shangyong, Wang Qiangang | CN-111217787-A (China) | Method for purifying DDZ in radix Puerariae | The invention relates to a low cost, fast, and high yield method for purifying DDZ, in particular to a method for extracting and purifying a high-content and high-quality DDZ product from kudzu vine root. |
10. Commercial Products of DDZ
DDZ is present in significant quantities in a variety of dietary supplements, bodybuilding drinks, sports drinks, newborn formulas, etc. Therefore, there are different types of products by manufacturers that are available in the global market. Most of the products are solid like capsules and tablets. The quantity of DDZ varies in different product units with the most recommended daily dose of one to two capsules. The data of various commercial products listed in Table 7 have been collected from the Dietary Supplement Label Database (DSLD; https://dsld.od.nih.gov/).
Table 7. List of Dietary Supplement/Botanicals/Nutraceutical Products Available in the Global Marketa.
| no. | product | net content | recommended daily dose | amt of daidzein/serving unit | manufacturer/distributor | website |
|---|---|---|---|---|---|---|
| 1 | Apex Energetics Estrovite (K-5) | 90 vegetarian capsules | 1 capsule 3 times | 10 mg | Apex Energetics, U.S. | https://www.apexenergetics.com/Endocrine |
| 2 | Douglas Laboratories Isoflavone-250 with Genistein | 60 capsules | 1 capsule | NQ | Douglas Laboratories, U.S. | https://www.douglaslabs.com/ |
| 3 | GNC Natural Brand Soy Protein Natural Vanilla Flavor | 454.0 g | 1 scoop (32 g) with 1 cup (8 fl oz) of water, juice | 13.65 mg | General Nutrition Corp., U.S. | https://www.gnc.com/ |
| 4 | Health From the Sun Super FiProFLAX | 15.0 oz or 425.0 g | 2–4 tablespoons | 0.4 mg | Health From the Sun, U.S. | www.healthfromthesun.com |
| 5 | Healthy Choice Naturals Cholesterol Care | 60 tablets | 2 tablets | 40 mg | Healthy Choice Naturals, U.S. | https://shop.healthychoicenaturals.com/Default.asp |
| 6 | Higher Nature True Food Superpotency Soyabean | 90 tablets | 1 tablet | 3200 μg | Higher Nature Ltd., U.K. | https://www.highernature.com/ |
| 7 | Metagenics Selestro | 60 tablets | 1 tablet 1 or 2 times | NQ | Metagenics, U.S. | https://www.metagenics.com/testralin |
| 8 | Metagenics SpectraSoy | 90 tablets | 1 tablet 1 or 2 times | NQ | Metagenics, U.S. | https://kiwla.com/products/metagenics-spectrasoy |
| 9 | Metagenics Testralin | 60 tablets | 1 tablet | NQ | Metagenics, U.S. | https://www.metagenics.com/testralin |
| 10 | Metagenics Wellness Essentials Men’s Vitality | 30 packets | 1 packet | NQ | Nature’s Plus, U.S. | https://naturesplus.com/pages/our-values |
| 11 | Natures Plus Ultra Maximum Potency Isoflavone 100 | 60 tablets | 2 tablets | 35 mg | Nutri-West, U.S. | https://www.pureformulas.com/brands |
| 12 | Nutri-West Total Protect | 90 tablets | 1 tablet | NQ | Planetary Herbals, U.S. | https://www.planetaryherbals.com/ |
| 13 | Planetary Herbals Full Spectrum Soy 1000, 1000 mg | 240 tablets | 2 tablets twice daily | 13.4 mg | Planetary Herbals, U.S. | https://www.planetaryherbals.com/ |
| 14 | Planetary Herbals Full Spectrum Soy 1000, 1000 mg | 60 tablets | 2 tablets twice daily | 13.4 mg | Planetary Herbals, U.S. | https://www.planetaryherbals.com/ |
| 15 | Planetary Herbals Full Spectrum Soy 1000, 1000 mg | 120 tablets | 2 tablets twice daily | 13.4 mg | Planetary Herbals, U.S. | https://www.planetaryherbals.com/products/search/ |
| 16 | Planetary Herbals Soy Genistein Isoflavone Powder | 2.0 oz or 56.0 g | 1 teaspoon (2.5 g) twice daily | 19.5 mg | Planetary Herbals, U.S. | https://www.planetaryherbals.com/products/search/ |
| 17 | Planetary Herbals Soy Genistein Isoflavone Powder | 4.0 oz or 113.0 g | 1 teaspoon (2.5 g) twice daily | 19.5 mg | Planetary Herbals, U.S. | https://www.planetaryherbals.com/products/search/ |
| 18 | ProCaps Laboratories Bone & Body Factors | 60 packets, i.e., 240 capsules | 1 packet (4 capsules) | 30 mg | ProCaps Laboratories, U.S. | https://www.procapslabs.com/ |
| 19 | ProCaps Laboratories Women’s Wellness | 360 capsules | 1 or 2 capsules | 30 mg | ProCaps Laboratories, U.S. | https://www.procapslabs.com/ |
| 20 | ProCaps Ultimate Women’s Wellness | 360 capsules | 1 or 2 capsules | 30 mg | ProCaps Laboratories, U.S. | https://www.procapslabs.com/ |
| 21 | Solgar Iso Soy Natural Chocolate Caramel Flavor | 20.0 oz or 568.0 g | 28.4 g (≈1 level scoop) | NQ | Solgar Vitamin and Herb, U.S. | https://www.solgar.com/ |
| 22 | Solgar Iso Soy Natural Vanilla Bean Flavor | 20.0 oz or 568.0 g | 28.4 g (≈1 level scoop) | NQ | Solgar Vitamin and Herb, U.S. | https://www.solgar.com/ |
| 23 | Source Naturals Genistein Soy Complex, 1000 mg | 60 tablets | 2 tablets twice | 13.4 mg | Source Naturals, U.S. | https://www.sourcenaturals.com/ |
| 24 | Source Naturals Genistein Soy Complex, 1000 mg | 120 tablets | 2 tablets twice | 13.4 mg | Source Naturals, U.S. | https://www.sourcenaturals.com/ |
| 25 | Source Naturals Genistein Soy Complex, 1000 mg | 240 tablets | 2 tablets twice | 15.8 mg | Source Naturals, U.S. | https://www.sourcenaturals.com/ |
| 26 | Source Naturals Genistein Soy Supplement Powder | 100.0 g or 3.53 oz | 1 teaspoon (2.5 g) twice daily | 19 mg | Source Naturals, U.S. | https://www.sourcenaturals.com/ |
| 27 | Source Naturals Genistein Soy Supplement Powder | 200.0 g or 7.05 oz | 1 teaspoon (2.5 g) twice daily | 19 mg | Source Naturals, U.S. | https://www.sourcenaturals.com/ |
| 28 | Source Naturals Menopause Multiple | 30 tablets | 2–3 tablets | 6.7 mg | Source Naturals, U.S. | https://www.sourcenaturals.com/ |
| 29 | Source Naturals Menopause Multiple | 60 tablets | 2–3 tablets | 6.7 mg | Source Naturals, U.S. | https://www.sourcenaturals.com/ |
| 30 | Source Naturals Menopause Multiple | 120 tablets | 2–3 tablets | 17 mg | Source Naturals, U.S. | https://www.sourcenaturals.com/ |
| 31 | ProCaps Laboratories Bone & Body Factors | 240 capsules | 4 capsules | 30.0 mg | ProCaps Laboratories, U.S. | https://www.procapslabs.com/ |
| 32 | tnvitamins Soy Isoflavones, 650 mg | 180.0 capsules | 1 capsule | 650.0 mg | Total Nutrition, Inc., U.S. | https://tnvitamins.com/ |
These products are available in capsule and tablet form, and the majority of these supplement/botanicals/nutraceutical product are manufactured in the United States.
11. Conclusion
Recent years have seen a significant increase in the amount of research done on DDZ. In this review, the pharmacological characteristics of DDZ have been noted. Its outcomes include anticancer, anticardiovascular illnesses, antiosteoporosis, antidiabetes, anti-inflammation, antioxidant, antiaging, neuroprotective, and other effects. From many past years, traditional hormone replacement therapy has been utilized clinically to improve menopause symptoms; however, problems such as mammary cancer have restricted its use in therapeutic settings. It was established that DDZ has a structure identical to that of estrogen and is sensitive for the estrogen receptor. It is currently being utilized extensively for treatment of a variety of ailments, and there is optimism regarding the expansion of its therapeutic applications in the near future. The mechanisms of action are still not completely known, and its poor bioavailability considerably restricts the applications for which it can be used. Additionally, there have been reports of DDZ possibly causing certain unwanted side effects. Therefore, it is essential to further explain the mechanisms at play, in order to lessen the adverse effects associated with its usage.
Some biotransformation or synthetic analogues of DDZ, such as isoformonetin and 7,3′,4-THIF, may be more beneficial to health and/or less hazardous. Although certain bioactive analogues of DDZ with beneficial effects on human health have been discovered, many more possible metabolites remain unknown. Therefore, it is vital to discover new beneficial chemicals based on the DDZ structure. Furthermore, it is of the utmost importance to improve the absorption and bioavailability of DDZ. This may be accomplished either by modifying the drug in accordance with its physicochemical features and pharmacokinetic factors or by selecting the most appropriate route of administration. Therefore, additional research must be conducted on DDZ before it may be extensively advocated for its therapeutic usage.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia, for funding this research work through Project No. ISP22-2.
Author Contributions
Conceptualization, M.A.M., Z.I., and M.S.A.; methodology, M.U. and S.; investigation, M.U. and M.A.S.; resources, M.A.M., Z.I., and M.S.A.; data curation, S.M.K. and MA.; writing—original draft preparation, M.U. and S.; writing—review and editing, MU, H.A.M., A.N., and K.Z.; visualization, M.U. and M.A.H.; supervision, M.A.M., Z.I., and M.S.A. All authors have read and agreed to the final version of the manuscript.
The authors declare no competing financial interest.
References
- Shi Q.; Li L.; Huo C.; Zhang M.; Wang Y. Study on Natural Medicinal Chemistry and New Drug Development. Zhongcaoyao (Chin. Tradit. Herbal Drugs) 2010, 41 (10), 1583–1589. [Google Scholar]
- Singh S.; Singh D. B.; Singh S.; Shukla R.; Ramteke P. W.; Misra K. Exploring Medicinal Plant Legacy for Drug Discovery in Post-Genomic Era. Proceedings of the National Academy of Sciences, India Section B: Biological Sciences 2018 89:4 2019, 89 (4), 1141–1151. 10.1007/s40011-018-1013-x. [DOI] [Google Scholar]
- Ngo L. T.; Okogun J. I.; Folk W. R. 21st Century Natural Product Research and Drug Development and Traditional Medicines. Nat. Prod Rep 2013, 30 (4), 584–592. 10.1039/c3np20120a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H.; Ma Q.; Ye L.; Piao G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21 (5), 559. 10.3390/molecules21050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. C.; Doss R. B.; Lall R.; Srivastava A.; Sinha A. Nutraceuticals in Animal Health and Diseases. Nutraceuticals: Efficacy, Safety and Toxicity 2021, 1127–1141. 10.1016/B978-0-12-821038-3.00067-7. [DOI] [Google Scholar]
- Křížová L.; Dadáková K.; Kašparovská J.; Kašparovský T. Isoflavones. Molecules 2019, 24 (6), 1076. 10.3390/molecules24061076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aboushanab S. A.; Khedr S. M.; Gette I. F.; Danilova I. G.; Kolberg N. A.; Ravishankar G. A.; Ambati R. R.; Kovaleva E. G. Isoflavones Derived from Plant Raw Materials: Bioavailability, Anti-Cancer, Anti-Aging Potentials, and Microbiome Modulation. Crit. Rev. Food Sci. Nutr. 2023, 63, 261. 10.1080/10408398.2021.1946006. [DOI] [PubMed] [Google Scholar]
- Patisaul H. B. Endocrine Disruption by Dietary Phyto-Oestrogens: Impact on Dimorphic Sexual Systems and Behaviours. Proceedings of the Nutrition Society 2017, 76 (2), 130–144. 10.1017/S0029665116000677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshehri M. M.; Sharifi-Rad J.; Herrera-Bravo J.; Jara E. L.; Salazar L. A.; Kregiel D.; Uprety Y.; Akram M.; Iqbal M.; Martorell M.; Torrens-Mas M.; Pons D. G.; Daştan S. D.; Cruz-Martins N.; Ozdemir F. A.; Kumar M.; Cho W. C. Therapeutic Potential of Isoflavones with an Emphasis on Daidzein. Oxid. Med. Cell. Longevity 2021, 2021, 6331630. 10.1155/2021/6331630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Superfood and Functional Food: The Development of Superfoods and Their Roles as Medicine; Shiomi N., Waisundara V., Eds.; InTechOpen: 2017. 10.5772/65088. [DOI] [Google Scholar]
- Barnes S.; Kirk M.; Coward L. Isoflavones and Their Conjugates in Soy Foods: Extraction Conditions and Analysis by HPLC-Mass Spectrometry. J. Agric. Food Chem. 1994, 42 (11), 2466–2474. 10.1021/jf00047a019. [DOI] [Google Scholar]
- Shin D.; Jeong D. Korean Traditional Fermented Soybean Products: Jang. Journal of Ethnic Foods 2015, 2 (1), 2–7. 10.1016/j.jef.2015.02.002. [DOI] [Google Scholar]
- Kaufman P. B.; Duke J. A.; Brielmann H.; Boik J.; Hoyt J. E. A Comparative Survey of Leguminous Plants as Sources of the Isoflavones, Genistein and Daidzein: Implications for Human Nutrition and Health. Journal of alternative and complementary medicine 1997, 3 (1), 7–12. 10.1089/acm.1997.3.7. [DOI] [PubMed] [Google Scholar]
- Gupta O. P.; Nigam D.; Dahuja A.; Kumar S.; Vinutha T.; Sachdev A.; Praveen S. Regulation of Isoflavone Biosynthesis by MiRNAs in Two Contrasting Soybean Genotypes at Different Seed Developmental Stages. Front Plant Sci. 2017, 8, 567. 10.3389/fpls.2017.00567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulistyowati E.; Martono S.; Riyanto S.; Lukitaningsih E. Development and Validation for Free Aglycones Daidzein and Genistein in Soybeans (Glycine Max (L.) Merr.) Using RP HPLC Method. International Journal of Applied Pharmaceutics 2019, 11 (2), 138–142. 10.22159/ijap.2019v11i2.31222. [DOI] [Google Scholar]
- Mitani K.; Narimatsu S.; Kataoka H. Determination of Daidzein and Genistein in Soybean Foods by Automated On-Line in-Tube Solid-Phase Microextraction Coupled to High-Performance Liquid Chromatography. J. Chromatogr A 2003, 986 (2), 169–177. 10.1016/S0021-9673(02)02014-9. [DOI] [PubMed] [Google Scholar]
- Luan F.; Tang L. L.; Chen X. X.; Liu H. T. Simultaneous Determination of Daidzein, Genistein and Formononetin in Coffee by Capillary Zone Electrophoresis. Separations 2017, 4 (1), 1. 10.3390/separations4010001. [DOI] [Google Scholar]
- Golkhoo S.; Ahmadi A. R.; Hanachi P.; Barantalab F.; Vaziri M. Determination of Daidzein and Genistein in Soy Milk in Iran by Using HPLC Analysis Method. Pakistan Journal of Biological Sciences 2008, 11 (18), 2254–2258. 10.3923/pjbs.2008.2254.2258. [DOI] [PubMed] [Google Scholar]
- Lapčík O.; Hampl R.; Al-Maharik N.; Salakka A.; Wähälä K.; Adlercreutz H. A Novel Radioimmunoassay for Daidzein. Steroids 1997, 62 (3), 315–320. 10.1016/S0039-128X(96)00226-7. [DOI] [PubMed] [Google Scholar]
- Rostagno M. A.; Araújo J. M. A.; Sandi D. Supercritical Fluid Extraction of Isoflavones from Soybean Flour. Food Chem. 2002, 78 (1), 111–117. 10.1016/S0308-8146(02)00106-1. [DOI] [Google Scholar]
- Bhalla Y.; Chadha K.; Chadha R.; Karan M. Daidzein Cocrystals: An Opportunity to Improve Its Biopharmaceutical Parameters. Heliyon 2019, 5 (11), e02669 10.1016/j.heliyon.2019.e02669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu F.; Chen X.-Y.; Song B.; Zhong D.-F.; Liu C.-X. Influence of Dosage Forms on Pharmacokinetics of Daidzein and Its Main Metabolite Daidzein-7-O-Glucuronide in Rats 1. Acta Pharmacol. Sin. 2005, 26 (9), 1145–1152. 10.1111/j.1745-7254.2005.00187.x. [DOI] [PubMed] [Google Scholar]
- Uğur Kaplan A. B.; Öztürk N.; Çetin M.; Vural I.; Öznülüer Özer T. The Nanosuspension Formulations of Daidzein: Preparation and In Vitro Characterization. Turk J. Pharm. Sci. 2022, 19 (1), 84. 10.4274/tjps.galenos.2021.81905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugur Kaplan A. B.; Cetin M.; Orgul D.; Taghizadehghalehjoughi A.; Hacımuftuoglu A.; Hekimoglu S. Formulation and in Vitro Evaluation of Topical Nanoemulsion and Nanoemulsion-Based Gels Containing Daidzein. J. Drug Deliv Sci. Technol. 2019, 52, 189–203. 10.1016/j.jddst.2019.04.027. [DOI] [Google Scholar]
- Huang S.; Xue Q.; Xu J.; Ruan S.; Cai T. Simultaneously Improving the Physicochemical Properties, Dissolution Performance, and Bioavailability of Apigenin and Daidzein by Co-Crystallization With Theophylline. J. Pharm. Sci. 2019, 108 (9), 2982–2993. 10.1016/j.xphs.2019.04.017. [DOI] [PubMed] [Google Scholar]
- Daidzein (soy): Dosing, contraindications, side effects, and pill pictures. epocrates online. https://online.epocrates.com/drugs/alternative-medicines/820703/daidzein-soy/Adverse-Reactions (accessed 2023-06-19).
- Anupongsanugool E.; Teekachunhatean S.; Rojanasthien N.; Pongsatha S.; Sangdee C. Pharmacokinetics of Isoflavones, Daidzein and Genistein, after Ingestion of Soy Beverage Compared with Soy Extract Capsules in Postmenopausal Thai Women. BMC Clin. Pharmacol. 2005, 5 (1), 2. 10.1186/1472-6904-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murota K.; Nakamura Y.; Uehara M. Flavonoid Metabolism: The Interaction of Metabolites and Gut Microbiota. Biosci., Biotechnol., Biochem. 2018, 82 (4), 600–610. 10.1080/09168451.2018.1444467. [DOI] [PubMed] [Google Scholar]
- Otieno D. O.; Shah N. P. Endogenous Beta-Glucosidase and Beta-Galactosidase Activities from Selected Probiotic Micro-Organisms and Their Role in Isoflavone Biotransformation in Soymilk. J. Appl. Microbiol. 2007, 103 (4), 910–917. 10.1111/j.1365-2672.2007.03438.x. [DOI] [PubMed] [Google Scholar]
- Day A. J.; Dupont M. S.; Ridley S.; Rhodes M.; Rhodes M. J. c.; Morgan M. R. a.; Williamson G. Deglycosylation of Flavonoid and Isoflavonoid Glycosides by Human Small Intestine and Liver Beta-Glucosidase Activity. FEBS Lett. 1998, 436 (1), 71–75. 10.1016/S0014-5793(98)01101-6. [DOI] [PubMed] [Google Scholar]
- Alshehri M. M.; Sharifi-Rad J.; Herrera-Bravo J.; Jara E. L.; Salazar L. A.; Kregiel D.; Uprety Y.; Akram M.; Iqbal M.; Martorell M.; Torrens-Mas M.; Pons D. G.; Daştan S. D.; Cruz-Martins N.; Ozdemir F. A.; Kumar M.; Cho W. C. Therapeutic Potential of Isoflavones with an Emphasis on Daidzein. Oxid. Med. Cell Longevity 2021, 2021, 6331630. 10.1155/2021/6331630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espín J. C.; García-Conesa M. T.; Tomás-Barberán F. A. Nutraceuticals: Facts and Fiction. Phytochemistry 2007, 68 (22–24), 2986–3008. 10.1016/j.phytochem.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Piskula M. K. Soy Isoflavone Conjugation Differs in Fed and Food-Deprived Rats. J. Nutr. 2000, 130 (7), 1766–1771. 10.1093/jn/130.7.1766. [DOI] [PubMed] [Google Scholar]
- Setchell K. D. R.; Faughnan M. S.; Avades T.; Zimmer-Nechemias L.; Brown N. M.; Wolfe B. E.; Brashear W. T.; Desai P.; Oldfield M. F.; Botting N. P.; Cassidy A. Comparing the Pharmacokinetics of Daidzein and Genistein with the Use of 13C-Labeled Tracers in Premenopausal Women. Am. J. Clin. Nutr. 2003, 77 (2), 411–419. 10.1093/ajcn/77.2.411. [DOI] [PubMed] [Google Scholar]
- Raimondi S.; Roncaglia L.; de Lucia M.; Amaretti A.; Leonardi A.; Pagnoni U. M.; Rossi M. Bioconversion of Soy Isoflavones Daidzin and Daidzein by Bifidobacterium Strains. Appl. Microbiol. Biotechnol. 2009, 81 (5), 943–950. 10.1007/s00253-008-1719-4. [DOI] [PubMed] [Google Scholar]
- Maubach J.; Bracke M. E.; Heyerick A.; Depypere H. T.; Serreyn R. F.; Mareel M. M.; de Keukeleire D. Quantitation of Soy-Derived Phytoestrogens in Human Breast Tissue and Biological Fluids by High-Performance Liquid Chromatography. Journal of Chromatography B 2003, 784 (1), 137–144. 10.1016/S1570-0232(02)00789-4. [DOI] [PubMed] [Google Scholar]
- Prasain J. K.; Jones K.; Brissie N.; Moore R.; Wyss J. M.; Barnes S. Identification of Puerarin and Its Metabolites in Rats by Liquid Chromatography-Tandem Mass Spectrometry. J. Agric. Food Chem. 2004, 52 (12), 3708–3712. 10.1021/jf040037t. [DOI] [PubMed] [Google Scholar]
- Das D.; Sarkar S.; Bordoloi J.; Wann S. B.; Kalita J.; Manna P. Daidzein, Its Effects on Impaired Glucose and Lipid Metabolism and Vascular Inflammation Associated with Type 2 Diabetes. BioFactors 2018, 44 (5), 407–417. 10.1002/biof.1439. [DOI] [PubMed] [Google Scholar]
- Jooyandeh H. Soy Products as Healthy and Functional Foods. Middle-East J. Sci. Res. 2011, 7 (1), 71–80. [Google Scholar]
- Akhlaghi M. Non-Alcoholic Fatty Liver Disease: Beneficial Effects of Flavonoids. Phytotherapy Research 2016, 30 (10), 1559–1571. 10.1002/ptr.5667. [DOI] [PubMed] [Google Scholar]
- Kuryłowicz A. The Role of Isoflavones in Type 2 Diabetes Prevention and Treatment—A Narrative Review. Int. J. Mol. Sci. 2021, 22 (1), 218. 10.3390/ijms22010218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy K.; Samayoa C.; Krishnegowda N.; Tekmal R. R. Therapeutic Use of Estrogen Receptor β Agonists in Prevention and Treatment of Endocrine Therapy Resistant Breast Cancers: Observations From Preclinical Models. Prog. Mol. BiolTransl Sci. 2017, 151, 177–194. 10.1016/bs.pmbts.2017.08.002. [DOI] [PubMed] [Google Scholar]
- Izzo S.; Naponelli V.; Bettuzzi S. Flavonoids as Epigenetic Modulators for Prostate Cancer Prevention. Nutrients 2020, 12 (4), 1010. 10.3390/nu12041010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama A. A. A.; Allam R. M. Promising Targets of Chrysin and Daidzein in Colorectal Cancer: Amphiregulin, CXCL1, and MMP-9. Eur. J. Pharmacol. 2021, 892, 173763. 10.1016/j.ejphar.2020.173763. [DOI] [PubMed] [Google Scholar]
- Park H. J.; Jeon Y. K.; You D. H.; Nam M. J. Daidzein Causes Cytochrome C-Mediated Apoptosis via the Bcl-2 Family in Human Hepatic Cancer Cells. Food Chem. Toxicol. 2013, 60, 542–549. 10.1016/j.fct.2013.08.022. [DOI] [PubMed] [Google Scholar]
- Lee D. E.; Lee K. W.; Byun S.; Jung S. K.; Song N.; Lim S. H.; Heo Y. S.; Kim J. E.; Kang N. J.; Kim B. Y.; Bowden G. T.; Bode A. M.; Lee H. J.; Dong Z. 7,3′,4’-Trihydroxyisoflavone, a Metabolite of the Soy Isoflavone Daidzein, Suppresses Ultraviolet B-Induced Skin Cancer by Targeting Cot and MKK4. J. Biol. Chem. 2011, 286 (16), 14246–14256. 10.1074/jbc.M110.147348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K. K. L.; Siu M. K. Y.; Jiang Y.; Wang J.; Leung T. H. Y.; Ngan H. Y. S. Estrogen Receptor Modulators Genistein, Daidzein and ERB-041 Inhibit Cell Migration, Invasion, Proliferation and Sphere Formation via Modulation of FAK and PI3K/AKT Signaling in Ovarian Cancer. Cancer Cell Int. 2018, 18 (1), 65. 10.1186/s12935-018-0559-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S. L.; Park H. Y.; Vattem D. A.; Grammas P.; Ma H.; Seeram N. P. Equol, a Blood-Brain Barrier Permeable Gut Microbial Metabolite of Dietary Isoflavone Daidzein, Exhibits Neuroprotective Effects against Neurotoxins Induced Toxicity in Human Neuroblastoma SH-SY5Y Cells and Caenorhabditis Elegans. Plant Foods for Human Nutrition 2020 75:4 2020, 75 (4), 512–517. 10.1007/s11130-020-00840-0. [DOI] [PubMed] [Google Scholar]
- Zheng W.; Sun R.; Yang L.; Zeng X.; Xue Y.; An R. Daidzein Inhibits Choriocarcinoma Proliferation by Arresting Cell Cycle at G1 Phase through Suppressing ERK Pathway in Vitro and in Vivo. Oncol. Rep. 2017, 38 (4), 2518–2524. 10.3892/or.2017.5928. [DOI] [PubMed] [Google Scholar]
- Guo J. M.; Kang G. Z.; Xiao B. X.; Liu D. H.; Zhang S. Effect of Daidzein on Cell Growth, Cell Cycle, and Telomerase Activity of Human Cervical Cancer in Vitro. International Journal of Gynecologic Cancer 2004, 14 (5), 882–888. 10.1136/ijgc-00009577-200409000-00022. [DOI] [PubMed] [Google Scholar]
- He Y.; Wu X.; Cao Y.; Hou Y.; Chen H.; Wu L.; Lu L.; Zhu W.; Gu Y. Daidzein Exerts Anti-Tumor Activity against Bladder Cancer Cells via Inhibition of FGFR3 Pathway. Neoplasma 2016, 63 (04), 523–531. 10.4149/neo_2016_405. [DOI] [PubMed] [Google Scholar]
- Qin Y.; Shu F. R.; Zeng Y.; Meng X. G.; Wang B.; Diao L. P.; Wang L.; Wan J.; Zhu J. D.; Wang J.; Mi M. T. Daidzein Supplementation Decreases Serum Triglyceride and Uric Acid Concentrations in Hypercholesterolemic Adults with the Effect on Triglycerides Being Greater in Those with the GA Compared with the GG Genotype of ESR-β RsaI. J. Nutr. 2014, 144 (1), 49–54. 10.3945/jn.113.182725. [DOI] [PubMed] [Google Scholar]
- Liu P.; Zhao Y.-X.; Zhang Y. Clinical Observation of Daidzein Intervention on Serum Inflammatory Factors in Senile Patients with Coronary Heart Disease. Zhongguo Zhong Xi Yi Jie He Za Zhi 2006, 26 (1), 42–45. [PubMed] [Google Scholar]
- Yanagihara N.; Zhang H.; Toyohira Y.; Takahashi K.; Ueno S.; Tsutsui M.; Takahashi K. New Insights Into the Pharmacological Potential of Plant Flavonoids in the Catecholamine System. J. Pharmacol. Sci. 2014, 124 (2), 123–128. 10.1254/jphs.13R17CP. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Veliky C. v.; Birru R. L.; Barinas-Mitchell E.; Magnani J. W.; Sekikawa A. Potential Protective Effects of Equol (Soy Isoflavone Metabolite) on Coronary Heart Diseases—From Molecular Mechanisms to Studies in Humans. Nutrients 2021, 13 (11), 3739. 10.3390/nu13113739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illsley M.Osteogenic Properties of Novel Bioactive and Nanostructured Biomaterials for Mesenchymal Stromal Cell Differentiation. Ph.D. Thesis, University of Brighton, 2011. [Google Scholar]
- Cheong S. H.; Furuhashi K.; Ito K.; Nagaoka M.; Yonezawa T.; Miura Y.; Yagasaki K. Daidzein Promotes Glucose Uptake through Glucose Transporter 4 Translocation to Plasma Membrane in L6 Myocytes and Improves Glucose Homeostasis in Type 2 Diabetic Model Mice. J. NutrBiochem 2014, 25 (2), 136–143. 10.1016/j.jnutbio.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Choi M. S.; Jung U. J.; Yeo J.; Kim M. J.; Lee M. K. Genistein and Daidzein Prevent Diabetes Onset by Elevating Insulin Level and Altering Hepatic Gluconeogenic and Lipogenic Enzyme Activities in Non-Obese Diabetic (NOD) Mice. Diabetes Metab Res. Rev. 2008, 24 (1), 74–81. 10.1002/dmrr.780. [DOI] [PubMed] [Google Scholar]
- Kashihara N.; Haruna Y.; Kondeti V. K.; Kanwar Y. S. Oxidative Stress in Diabetic Nephropathy. Curr. Med. Chem. 2010, 17 (34), 4256–4269. 10.2174/092986710793348581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rungseevijitprapa W.; Yingngam B.; Chaiyasut C. Improvement of Biophysical Skin Parameters of Topically Applied Fermented Soybean Extract-Loaded Niosomes with No Systemic Toxicity in Ovariectomized Rats. Pharmaceutics 2021, 13 (7), 1068. 10.3390/pharmaceutics13071068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.; Ma X.; Lin Y.; Xiong Y.; Zheng C.; Hu Y.; Yu D.; Jiang Z. Dietary Supplementation with a High Dose of Daidzein Enhances the Antioxidant Capacity in Swine Muscle but Experts Pro-Oxidant Function in Liver and Fat Tissues. J. Anim Sci. Biotechnol. 2016, 7 (1), 43. 10.1186/s40104-016-0102-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. Y.; Pan L.; Ke Y. S.; Batnasan E.; Jin X. Q.; Liu Z. Y.; Ba X. Q. Daidzein Suppresses Pro-Inflammatory Chemokine Cxcl2 Transcription in TNF-α-Stimulated Murine Lung Epithelial Cells via Depressing PARP-1 Activity. Acta Pharmacol. Sin. 2014, 35 (4), 496–503. 10.1038/aps.2013.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi E. Y.; Jin J. Y.; Lee J. Y.; Choi J. I.; Choi I. S.; Kim S. J. Anti-Inflammatory Effects and the Underlying Mechanisms of Action of Daidzein in Murine Macrophages Stimulated with Prevotella Intermedia Lipopolysaccharide. J. Periodontal Res. 2012, 47 (2), 204–211. 10.1111/j.1600-0765.2011.01422.x. [DOI] [PubMed] [Google Scholar]
- Yu Z.; Yang L.; Deng S.; Liang M. Daidzein Ameliorates LPS-Induced Hepatocyte Injury by Inhibiting Inflammation and Oxidative Stress. Eur. J. Pharmacol. 2020, 885, 173399. 10.1016/j.ejphar.2020.173399. [DOI] [PubMed] [Google Scholar]
- Takaoka O.; Mori T.; Ito F.; Okimura H.; Kataoka H.; Tanaka Y.; Koshiba A.; Kusuki I.; Shigehiro S.; Amami T.; Kitawaki J. Daidzein-Rich Isoflavone Aglycones Inhibit Cell Growth and Inflammation in Endometriosis. J. Steroid Biochem Mol. Biol. 2018, 181, 125–132. 10.1016/j.jsbmb.2018.04.004. [DOI] [PubMed] [Google Scholar]
- Ahmad S.; Alam K.; Hossain M. M.; Fatima M.; Firdaus F.; Zafeer M. F.; Arif Z.; Ahmed M.; Nafees K. A. Anti-Arthritogenic and Cardioprotective Action of Hesperidin and Daidzein in Collagen-Induced Rheumatoid Arthritis. Mol. Cell. Biochem. 2016, 423 (1–2), 115–127. 10.1007/s11010-016-2830-y. [DOI] [PubMed] [Google Scholar]
- Feng G.; Sun B.; Li T. Z. Daidzein Attenuates Lipopolysaccharide-Induced Acute Lung Injury via Toll-like Receptor 4/NF-KappaB Pathway. Int. Immunopharmacol 2015, 26 (2), 392–400. 10.1016/j.intimp.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Liu Y. F.; Bai Y. Q.; Qi M. Daidzein Attenuates Abdominal Aortic Aneurysm through NF-ΚB, P38MAPK and TGF-Β1 Pathways. Mol. Med. Rep 2016, 14 (1), 955–962. 10.3892/mmr.2016.5304. [DOI] [PubMed] [Google Scholar]
- Fardoun M. M.; Maaliki D.; Halabi N.; Iratni R.; Bitto A.; Baydoun E.; Eid A. H. Flavonoids in Adipose Tissue Inflammation and Atherosclerosis: One Arrow, Two Targets. Clin Sci. 2020, 134 (12), 1403–1432. 10.1042/CS20200356. [DOI] [PubMed] [Google Scholar]
- Pan M. H.; Lai C. S.; Ho C. T. Anti-Inflammatory Activity of Natural Dietary Flavonoids. Food Funct 2010, 1 (1), 15–31. 10.1039/c0fo00103a. [DOI] [PubMed] [Google Scholar]
- Schreihofer D. A.; Redmond L. Soy Phytoestrogens Are Neuroprotective against Stroke-like Injury in Vitro. Neuroscience 2009, 158 (2), 602–609. 10.1016/j.neuroscience.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malar D. S.; Devi K. P. Dietary Polyphenols for Treatment of Alzheimer’s Disease - Future Research and Development. Current Pharm. Biotechnol. 2014, 15, 330–342. 10.2174/1389201015666140813122703. [DOI] [PubMed] [Google Scholar]
- Johnson S. L.; Park H. Y.; Vattem D. A.; Grammas P.; Ma H.; Seeram N. P. Equol, a Blood-Brain Barrier Permeable Gut Microbial Metabolite of Dietary Isoflavone Daidzein, Exhibits Neuroprotective Effects against Neurotoxins Induced Toxicity in Human Neuroblastoma SH-SY5Y Cells and Caenorhabditis Elegans. Plant Foods for Human Nutrition 2020 75:4 2020, 75 (4), 512–517. 10.1007/s11130-020-00840-0. [DOI] [PubMed] [Google Scholar]
- Goel R.; Chaudhary R. Effect of Daidzein on Parkinson Disease Induced by Reserpine in Rats. Brazilian Journal of Pharmaceutical Sciences 2020, 56, 1–7. 10.1590/s2175-97902019000318388. [DOI] [Google Scholar]
- Zhang F.; Ru N.; Shang Z. H.; Chen J. F.; Yan C.; Li Y.; Liang J. Daidzein Ameliorates Spinal Cord Ischemia/Reperfusion Injury-Induced Neurological Function Deficits in Sprague-Dawley Rats through PI3K/Akt Signaling Pathway. Exp. Ther. Med. 2017, 14 (5), 4878–4886. 10.3892/etm.2017.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y.; Xu J.; Xu M.; Shi S.; Xiong J. Genistein and Daidzein Reduced Chlorpyrifos Induced Damage in PC12 Cell. Oil Crop Science 2020, 5 (1), 1–5. 10.1016/j.ocsci.2020.03.008. [DOI] [Google Scholar]
- Yoshino M.; Naka A.; Sakamoto Y.; Shibasaki A.; Toh M.; Tsukamoto S.; Kondo K.; Iida K. Dietary Isoflavone Daidzein Promotes Tfam Expression That Increases Mitochondrial Biogenesis in C2C12 Muscle Cells. J. Nutr. Biochem. 2015, 26 (11), 1193–1199. 10.1016/j.jnutbio.2015.05.010. [DOI] [PubMed] [Google Scholar]
- Anderson J. J. B.; Anthony M. S.; Cline J. M.; Washburn S. A.; Garner S. C. Health Potential of Soy Isoflavones for Menopausal Women. Public Health Nutr 1999, 2 (4), 489–504. 10.1017/S1368980099000671. [DOI] [PubMed] [Google Scholar]
- Soumyakrishnan S.; Divya T.; Kalayarasan S.; Sriram N.; Sudhandiran G. Daidzein Exhibits Anti-Fibrotic Effect by Reducing the Expressions of Proteinase Activated Receptor 2 and TGFβ1/Smad Mediated Inflammation and Apoptosis in Bleomycin-Induced Experimental Pulmonary Fibrosis. Biochimie 2014, 103 (1), 23–36. 10.1016/j.biochi.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Om A. S.; Shim J. Y. Effect of Daidzein in Rats on Cadmium Excretion. Bull. Environ. Contam. Toxicol. 2007, 78 (6), 485–488. 10.1007/s00128-007-9098-6. [DOI] [PubMed] [Google Scholar]
- Yu C.; Tai F.; Zeng S.; Zhang X. Effects of Perinatal Daidzein Exposure on Subsequent Behavior and Central Estrogen Receptor α Expression in the Adult Male Mouse. Prog. NeuropsychopharmacolBiol. Psychiatry 2013, 43, 157–167. 10.1016/j.pnpbp.2012.12.015. [DOI] [PubMed] [Google Scholar]
- Vallance T. M.; Ravishankar D.; Albadawi D. A. I.; Osborn H. M. I.; Vaiyapuri S. Synthetic Flavonoids as Novel Modulators of Platelet Function and Thrombosis. Int. J. Mol. Sci. 2019, 20 (12), 3106. 10.3390/ijms20123106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S. H.; Park E. K.; Kim D. H. Passive Cutaneous Anaphylaxis-Inhibitory Activity of Flavanones from Citrus Unshiu and Poncirus Trifoliata. Planta Med. 2005, 71 (1), 24–27. 10.1055/s-2005-837746. [DOI] [PubMed] [Google Scholar]
- Fujitani M.; Mizushige T.; Adhikari S.; Bhattarai K.; Kishida T. Mechanism of Soy Isoflavone Daidzein-Induced Female-Specific Anorectic Effect. Metabolites 2022, 12 (3), 252. 10.3390/metabo12030252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu J.; Hu L.; Wu Y.; Chen L.; Huang K.; Wang Z.; Liang M. Daidzein Suppresses TGF-Β1-Induced Cardiac Fibroblast Activation via the TGF-Β1/SMAD2/3 Signaling Pathway. Eur. J. Pharmacol. 2022, 919, 174805. 10.1016/j.ejphar.2022.174805. [DOI] [PubMed] [Google Scholar]
- Farhana A.; Veeresh B.; Rao R. Protective Role of Diadzein in L-Arginine-Induced Acute Pancreatitis in Rats. YMER 2022, 21, 451. 10.37896/YMER21.06/44. [DOI] [Google Scholar]
- Shu X. O.; Zheng Y.; Cai H.; Gu K.; Chen Z.; Zheng W.; Lu W. Soy Food Intake and Breast Cancer Survival. JAMA 2009, 302 (22), 2437–2443. 10.1001/jama.2009.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valachovicova T.; Slivova V.; Bergman H.; Shuherk J.; Sliva D. Soy Isoflavones Suppress Invasiveness of Breast Cancer Cells by the Inhibition of NF-KappaB/AP-1-Dependent and -Independent Pathways. Int. J. Oncol. 2004, 25 (5), 1389–1395. 10.3892/ijo.25.5.1389. [DOI] [PubMed] [Google Scholar]
- Ma T.; Liang Y.; Li Y.; Song X.; Zhang N.; Li X.; Chen B.; Zhao W.; Wang L.; Yang Q. LncRNA LINP1 Confers Tamoxifen Resistance and Negatively Regulated by ER Signaling in Breast Cancer. Cell Signal 2020, 68, 109536. 10.1016/j.cellsig.2020.109536. [DOI] [PubMed] [Google Scholar]
- Rabiau N.; Kossaï M.; Braud M.; Chalabi N.; Satih S.; Bignon Y. J.; Bernard-Gallon D. J. Genistein and Daidzein Act on a Panel of Genes Implicated in Cell Cycle and Angiogenesis by Polymerase Chain Reaction Arrays in Human Prostate Cancer Cell Lines. Cancer Epidemiol 2010, 34 (2), 200–206. 10.1016/j.canep.2009.12.018. [DOI] [PubMed] [Google Scholar]
- Rabiau N.; Kossaï M.; Braud M.; Chalabi N.; Satih S.; Bignon Y. J.; Bernard-Gallon D. J. Genistein and Daidzein Act on a Panel of Genes Implicated in Cell Cycle and Angiogenesis by Polymerase Chain Reaction Arrays in Human Prostate Cancer Cell Lines. Cancer Epidemiol 2010, 34 (2), 200–206. 10.1016/j.canep.2009.12.018. [DOI] [PubMed] [Google Scholar]
- Szliszka E.; Czuba Z. P.; Mertas A.; Paradysz A.; Krol W. The Dietary Isoflavone Biochanin-A Sensitizes Prostate Cancer Cells to TRAIL-Induced Apoptosis. Urologic Oncology: Seminars and Original Investigations 2013, 31 (3), 331–342. 10.1016/j.urolonc.2011.01.019. [DOI] [PubMed] [Google Scholar]
- Sun M. Y.; Ye Y.; Xiao L.; Rahman K.; Xia W.; Zhang H. Daidzein: A Review of Pharmacological Effects. African Journal of Traditional, Complementary and Alternative Medicines 2016, 13 (3), 117–132. 10.4314/ajtcam.v13i3.15. [DOI] [Google Scholar]
- de Wilde A.; Lieberherr M.; Colin C.; Pointillart A. A Low Dose of Daidzein Acts as an ERβ-Selective Agonist in Trabecular Osteoblasts of Young Female Piglets. J. Cell Physiol 2004, 200 (2), 253–262. 10.1002/jcp.20008. [DOI] [PubMed] [Google Scholar]
- Horcajada M.-N.; Offord E. Naturally Plant-Derived Compounds: Role in Bone Anabolism. Curr. Mol. Pharmacol. 2012, 5, 205–218. 10.2174/1874467211205020205. [DOI] [PubMed] [Google Scholar]
- Cheong S. H.; Furuhashi K.; Ito K.; Nagaoka M.; Yonezawa T.; Miura Y.; Yagasaki K. Daidzein Promotes Glucose Uptake through Glucose Transporter 4 Translocation to Plasma Membrane in L6Myocytes and Improves Glucose Homeostasis in Type 2 Diabetic Model Mice. J. Nutr. Biochem. 2014, 25 (2), 136–143. 10.1016/j.jnutbio.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Park M. H.; Ju J. W.; Park M. J.; Han J. S. Daidzein Inhibits Carbohydrate Digestive Enzymes in Vitro and Alleviates Postprandial Hyperglycemia in Diabetic Mice. Eur. J. Pharmacol. 2013, 712 (1–3), 48–52. 10.1016/j.ejphar.2013.04.047. [DOI] [PubMed] [Google Scholar]
- Li H. Y.; Pan L.; Ke Y. S.; Batnasan E.; Jin X. Q.; Liu Z. Y.; Ba X. Q. Daidzein Suppresses Pro-Inflammatory Chemokine Cxcl2 Transcription in TNF-α-Stimulated Murine Lung Epithelial Cells via Depressing PARP-1 Activity. Acta Pharmacol Sin 2014, 35 (4), 496–503. 10.1038/aps.2013.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guru A.; Issac P. K.; Velayutham M.; Saraswathi N. T.; Arshad A.; Arockiaraj J. Molecular Mechanism of Down-Regulating Adipogenic Transcription Factors in 3T3-L1 Adipocyte Cells by Bioactive Anti-Adipogenic Compounds. Molecular Biology Reports 2020 48:1 2021, 48 (1), 743–761. 10.1007/s11033-020-06036-8. [DOI] [PubMed] [Google Scholar]
- Lin X.; Fang Y.; Jin X.; Zhang M.; Shi K. Modulating Repolarization of Tumor-Associated Macrophages with Targeted Therapeutic Nanoparticles as a Potential Strategy for Cancer Therapy. ACS Appl. Bio Mater. 2021, 4 (8), 5871–5896. 10.1021/acsabm.1c00461. [DOI] [PubMed] [Google Scholar]
- Shoelson S. E.; Lee J.; Goldfine A. B. Inflammation and Insulin Resistance. J. Clin Invest 2006, 116 (7), 1793–1801. 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugur Kaplan A. B.; Cetin M.; Orgul D.; Taghizadehghalehjoughi A.; Hacımuftuoglu A.; Hekimoglu S. Formulation and in Vitro Evaluation of Topical Nanoemulsion and Nanoemulsion-Based Gels Containing Daidzein. J. Drug Deliv Sci. Technol. 2019, 52, 189–203. 10.1016/j.jddst.2019.04.027. [DOI] [Google Scholar]
- Chadha G.; Sathigari S.; Parsons D. L.; Jayachandra Babu R. In Vitro Percutaneous Absorption of Genistein from Topical Gels through Human Skin. Drug Dev. Ind. Pharm. 2011, 37 (5), 498–505. 10.3109/03639045.2010.525238. [DOI] [PubMed] [Google Scholar]
- Khan A. Q.; Khan R.; Rehman M. U.; Lateef A.; Tahir M.; Ali F.; Sultana S. Soy Isoflavones (Daidzein & Genistein) Inhibit 12-O-Tetradecanoylphorbol-13-Acetate (TPA)-Induced Cutaneous Inflammation via Modulation of COX-2 and NF-ΚB in Swiss Albino Mice. Toxicology 2012, 302 (2–3), 266–274. 10.1016/j.tox.2012.08.008. [DOI] [PubMed] [Google Scholar]
- Mikulić M.; Krstonošić M. A.; Sazdanić D.; Cvejić J. Health Perspectives on Soy Isoflavones. Phytochemicals in Soybeans 2022, 1–44. 10.1201/9781003030294-1. [DOI] [Google Scholar]
- Qin Y.; Shu F. R.; Zeng Y.; Meng X. G.; Wang B.; Diao L. P.; Wang L.; Wan J.; Zhu J. D.; Wang J.; Mi M. T. Daidzein Supplementation Decreases Serum Triglyceride and Uric Acid Concentrations in Hypercholesterolemic Adults with the Effect on Triglycerides Being Greater in Those with the GA Compared with the GG Genotype of ESR-β RsaI. J. Nutr. 2014, 144 (1), 49–54. 10.3945/jn.113.182725. [DOI] [PubMed] [Google Scholar]
- Martin D.; Song J.; Mark C.; Eyster K. Understanding the Cardiovascular Actions of Soy Isoflavones: Potential Novel Targets for Antihypertensive Drug Development. Cardiovasc. Hematol. Disord.: Drug Targets 2008, 8, 297–312. 10.2174/187152908786786214. [DOI] [PubMed] [Google Scholar]




