Keywords: aging, core temperature, sweating
Abstract
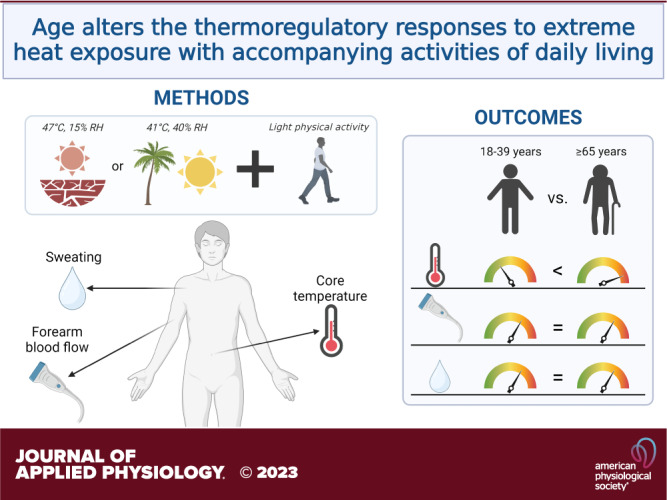
Older adults are at greater risk for heat-related morbidity and mortality, due in part to age-related reductions in heat dissipating capabilities. Previous studies investigating the impact of age on responses to heat stress used approaches that lack activities of daily living and therefore may not accurately depict the thermal/physiological strain that would occur during actual heatwaves. We sought to compare the responses of young (18–39 yr) and older (≥65 yr) adults exposed to two extreme heat simulations. Healthy young (n = 20) and older (n = 20) participants underwent two 3-h extreme heat exposures on different days: 1) DRY (47°C and 15% humidity) and 2) HUMID (41°C and 40% humidity). To mimic heat generation comparable with activities of daily living, participants performed 5-min bouts of light physical activity dispersed throughout the heat exposure. Measurements included core and skin temperatures, heart rate, blood pressure, local and whole body sweat rate, forearm blood flow, and perceptual responses. Δ core temperature (Young: 0.68 ± 0.27°C vs. Older: 1.37 ± 0.42°C; P < 0.001) and ending core temperature (Young: 37.81 ± 0.26°C vs. Older: 38.15 ± 0.43°C; P = 0.005) were greater in the older cohort during the DRY condition. Δ core temperature (Young: 0.58 ± 0.25°C vs. Older: 1.02 ± 0.32°C; P < 0.001), but not ending core temperature (Young: 37.67 ± 0.34°C vs. Older: 37.83 ± 0.35°C; P = 0.151), was higher in the older cohort during the HUMID condition. We demonstrated that older adults have diminished thermoregulatory responses to heat stress with accompanying activities of daily living. These findings corroborate previous reports and confirm epidemiological data showing that older adults are at a greater risk for hyperthermia.
NEW & NOTEWORTHY Using an experimental model of extreme heat exposure that incorporates brief periods of light physical activity to simulate activities of daily living, the extent of thermal strain reported herein more accurately represents what would occur during actual heatwave conditions. Despite matching metabolic heat generation and environmental conditions, we show that older adults have augmented core temperature responses, likely due to age-related reductions in heat dissipating mechanisms.
INTRODUCTION
Over the past ∼20 years, heatwaves have been the leading cause of death compared with all other environmental hazards (1, 2). The projected increase in the frequency, intensity, and duration of heatwaves in the coming decades heightens the concern over the impact of heatwaves on human health (3, 4). Humans have important regulatory mechanisms [e.g., cutaneous vasodilation and sweating (5)] directed to maintain body temperature within a relatively narrow range. However, should environmental and/or physiological conditions cause heat gain to exceed the capacity of these regulatory mechanisms for heat loss, body temperature will progressively increase (6, 7). In parallel with the consequences of climate change, in the United States, the percentage of individuals aged 65 yr or older is increasing (8). Thus, the number of older individuals exposed to progressively higher environmental temperatures, along with more frequent and intense heatwaves, will increase over the decades ahead.
Epidemiological studies indicate that older individuals are at a greater risk for heat-related morbidity and mortality (9–12), likely due (in part) to age-related reductions in heat-dissipating capabilities (13–17). For example, older adults have attenuated sweating and skin blood flow responses to heat stress, resulting in greater elevations in body temperature (13, 14, 16–19). However, previous studies investigating the impact of age on physiological responses to heat stress have used approaches that have not accounted for typical activities of daily living. Since activities of daily living will increase metabolic heat generation, previous work may underestimate the extent of thermal/physiological strain that would be observed during a real-world heatwave. Thus, we sought to compare the thermoregulatory responses of healthy young and older adults exposed to very hot and dry (DRY) and hot and humid (HUMID) extreme heat simulations inclusive of metabolic heat generation approximating activities of daily living (e.g., cooking, putting away groceries, cleaning, etc.; 20, 21). The environmental conditions were selected to represent the varied climates and types of heatwaves that occur across the United States. For example, the Los Angeles heatwave of 2018 had air temperatures upwards to 47°C but was dry (e.g., relative humidity <20%), whereas the 1995 Chicago heatwave had air temperatures reaching 41°C with high humidity (e.g., >40%). We hypothesized that, compared with young adults, older individuals would demonstrate greater increases in core temperature to the extreme heat exposures, resulting from attenuated sweating and skin blood flow responses.
METHODS
Participant Population
This study is part of a larger project investigating the integrative physiological responses to extreme heat exposures in young and older adults (Clinical Trials ID: NCT04538144). The study protocol and written informed consent were approved by the Institutional Review Boards at the University of Texas Southwestern Medical Center and Texas Health Presbyterian Hospital Dallas (STU-2019-1759), and the study was performed in accordance with the principles outlined in the Declaration of Helsinki. Healthy young (18–39 yr; n = 20, 10 male and 10 female) and older (≥65 yr; n = 20, 10 male and 10 female) participants were recruited from the greater Dallas-Fort Worth metropolitan area. We selected 65 yr of age as the lower cutoff for the older group given that around this age morbidity and mortality associated with heatwaves increase exponentially (12, 22–25). We did not control for the menstrual cycle phase for young females, given that fluctuations in female sex hormones that occur throughout the menstrual cycle do not alter thermoregulatory control (26–28). Exclusion criteria included: 1) known heart disease or other chronic medical conditions currently requiring regular medical therapy such as cancer, diabetes, uncontrolled hypertension, or uncontrolled hypercholesterolemia; 2) currently taking tricyclic antidepressants, loop diuretics, centrally acting calcium channel blockers, or beta-blockers; 3) abnormalities detected suggestive of provocable ischemia or undetected cardiac disease or resting left bundle branch block on screening electrocardiogram; 4) current smoker or regularly smoked within the past 3 years, 5) body mass index greater than or equal to 31 kg/m2; and 6) pregnant (confirmed in young females using a urine pregnancy test). All participants volunteered for a preliminary visit and two experimental visits. The experimental visits included 3-h extreme heat exposures performed on separate days (>6 days apart) in a randomized and counterbalanced order. Extreme heat simulations were carried out in a temperature and humidity-controlled environmental chamber (CANTROL, Ontario, Canada).
Preliminary Visit
During preliminary testing, participants completed a medical history form, physical activity questionnaire (IPAQ), and previous heat exposure questionnaire. The heat exposure questionnaire included questions regarding work and exercise to estimate the amount of time that individuals spent performing physical activity outdoors. In addition, we measured their height, mass, brachial artery blood pressure, heart rate and rhythm (via electrocardiogram), and body composition via dual-energy X-ray absorptiometry. A metabolic heat production assessment was also performed during the preliminary visit to estimate the workload to be used during the experimental visits. This test included a brief period of semirecumbent cycling (10–15 min) with collection and analysis of metabolic gasses using indirect calorimetry (PARVO Medics True-One Metabolic Measurement System, Parvo Medics, Salt Lake City, UT) to identify the workload that would elicit 3 METs.
Experimental Protocol
All experimental trials began between 8:00 and 10:00 AM to avoid diurnal variations. Before experimental trials, participants were instructed to eat a light breakfast (without caffeinated beverages) on the morning of testing. Alcoholic beverages and aerobic/resistance exercise were prohibited 24 h before testing. Upon arrival, participants provided a urine sample. A euhydrated state before each trial was confirmed by a urine specific gravity value ≤1.020 (Atago Inc., Bellevue, WA). If urine specific gravity was between 1.020 and 1.024, participants ingested 500 mL of water before testing. If their urine specific gravity was ≥1.025, the trial was rescheduled to a later date. Participants rested in a temperate environment (∼22°C and 40% humidity) for at least 1 h while baseline measurements (e.g., heart rate, blood pressure, forearm blood flow, etc.) were performed. Following baseline measurements, participants entered the environmental chamber set to one of the following two extreme heat simulations: 1) DRY (47°C and 15% humidity; reflective of the 2018 Los Angeles heatwave) and 2) HUMID (41°C and 40% humidity; reflective of the 1995 Chicago heatwave). The duration of each exposure was 3 h, which represents the approximate duration of peak environmental temperatures during a heatwave (29, 30). Before instrumentation, participants measured their nude body mass using a precision balance scale with ±10 g accuracy (Mettler Toledo, OH). After a brief period (∼5 min) of instrumentation, participants were seated in a semirecumbent position on a chair with a breathable fabric thereby reducing impediments for thermal exchange from the regions in contact with the chair. To reduce the influence of clothing on thermoregulatory responses, participants wore athletic shorts (males) or athletic shorts and a sports bra (females). To decrease the effect of dehydration, participants consumed 3 mL/kg body mass of tap temperature water (14°C–16°C) every hour during the extreme heat simulation. This level of fluid ingestion was based on pilot data and was selected to maintain hydration to a level that would not compromise thermoregulatory, perceptual, or cardiovascular responses during heat exposure (31–33). Tap water temperature was used to mimic the water available to individuals during an actual heatwave. Following the extreme heat simulation, participants were deinstrumented and provided a towel to wipe off excess sweat before they weighed themselves nude.
Metabolic Heat Production
Participants performed seven 5-min bouts of light physical activity (either cycling: n = 39 or walking: n = 1) dispersed throughout the 3-h extreme heat exposure (Fig. 1). The intensity of the activity was maintained at a working metabolic rate of ∼3 METs to mimic activities of daily living such as cooking, cleaning, carrying groceries, moving around the home, etc. (20). Metabolic heat generation was verified using indirect calorimetry (PARVO Medics True-One Metabolic Measurement System, Parvo Medics, Salt Lake City, UT), with metabolic gases being collected during the first two 5-min periods of exercise. That identified workload was used for all subsequent exercise bouts. The average rate of oxygen consumption, respiratory exchange ratio, and workload were used to determine metabolic heat production, which was calculated using standard formulae (34).
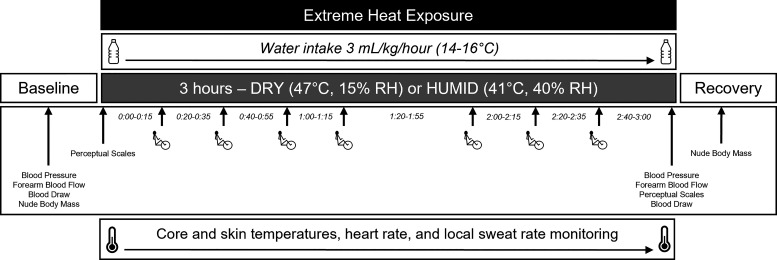
Figure 1.
Overview of experimental protocol. Healthy young (n = 20) and older (n = 20) participants were exposed to two 3-h extreme heat exposures on different days: 1) DRY (47°C and 15% humidity) and 2) HUMID (41°C and 40% humidity). To mimic heat generation comparable with activities of daily living, participants performed seven 5 min bouts of light physical activity (∼3 METs) dispersed throughout the heat exposure. Participants consumed 3 mL/kg body mass of tap temperature water (14°C–16°C) every hour during the heat exposures. Measurements included core and skin temperatures, heart rate, blood pressure, local and whole body sweat rate, forearm blood flow, plasma osmolality, change in plasma volume, and perceptual responses.
Instrumentation
Core temperature was measured via rectal temperature using a general-purpose thermocouple probe inserted 10 cm beyond the anal sphincter (Mon-a-therm, Mallinckrodt Medical, St. Louis, MO) (Young: n = 17, Older: n = 8) or a telemetric pill (e-Celsius performance pill, BodyCap, Caen, France) used as a rectal suppository (Young: n = 1, Older: n = 1). If participants did not consent to rectal temperature measurement, or if there were technical issues with the rectal thermocouple probe, core temperature was measured using an orally ingested telemetric pill (Young: n = 2, Older: n = 11). In these participants, the ingestible telemetric pill was taken no less than 1 h before beginning the extreme heat exposure. Importantly the time following ingestion of the telemetric pill (e.g., between 1 and 12 h) does not influence the validity of pill temperature as an index of core temperature (35). In addition, there is good agreeability among these methods to measure core temperature (36, 37), and for participants who had simultaneous measures of pill and rectal temperature in this study (n = 25) the intraclass correlation coefficient was 0.87. Mean skin temperature was obtained as the weighted average of local temperatures measured via the chest (22%), upper back (21%), lower back (19%), abdomen (14%), anterior thigh (13%), and calf (11%) (38).
Forearm blood flow, as a surrogate of changes in skin blood flow, was measured from brachial artery blood velocity and diameter values obtained by duplex ultrasonography (iE33/EPIQ 7, Philips Medical Systems, Andover, MA). A linear transducer (L12-3, Philips Medical Systems, Andover, MA), operating at an insonation angle of 60°, was placed proximal to the brachial artery bifurcation where the best spatial resolution of the artery could be obtained. Imaging was performed by the same sonographer throughout each heat exposure, and to aid in consistency between measurements sonographers used anatomical landmarks (e.g., ∼9 cm proximal to the medical epicondyle) to ensure similar probe placement. Blood velocity was measured using time-averaged mean velocity from pulsed-wave Doppler ultrasound. Offline two-dimensional (2-D) images of the brachial artery were used to measure vessel diameter at end-diastole. Brachial artery diameter and blood flow velocity were measured from recordings of five consecutive cardiac cycles at baseline and in the final 15 min of extreme heat exposure. Blood flow velocity and brachial artery diameter measurements were used to calculate forearm blood flow using a standard formula:
| (1) |
where v is velocity in m/s, and d is diameter in cm.
Heart rate was obtained from a six-lead electrocardiogram (GE Medical Systems, Madison, WI). Blood pressure was measured by automated auscultation of the brachial artery (Tango+, SunTech Medical, Morrisville, NC). Local sweat rate was measured on the upper back using a ventilated capsule technique where anhydrous compressed nitrogen was passed through the capsule at a flow rate of ∼300 mL/min. The water content of the nitrogen gas was then measured using capacitance hygrometers (Vaisala, Woburn, WA) and normalized for the area under the capsule.
Thermal perception was measured using an eight-point scale, with 0.5 increments ranging from 0.0 (“unbearably cold’’), 4 (“comfortable”), to 8.0 (‘‘unbearably hot’’). At the start and end of each extreme heat exposure, participants were given a modified environmental symptom questionnaire (39) to assess symptoms commonly associated with environmental stress such as “I feel lightheaded,” “I have a headache,” “I feel dizzy,” “I feel thirsty,” “I feel weak,” “I feel grumpy,” “It is hard to breathe,” “I have a muscle cramp,” “I feel tired,” “I feel nauseous,” “I feel hot,” “I have trouble concentrating,” “I feel ‘goose bumps’ or chills,” and “I can perform at my best,” on a scale of 1 (“not at all”) to 6 (“extreme”).
Blood Sampling and Analysis
Blood was taken from an arm vein after 30 min of supine rest at baseline and the end of the extreme heat simulations. We measured hematocrit (microcapillary technique) and hemoglobin (ABL90 Flex, Radiometer, Brønshøj, Denmark) to calculate changes in plasma volume using the Dill and Costill (40) equation. Blood samples were centrifuged to isolate plasma, and aliquots of plasma were sent to a nearby laboratory (Texas Health Presbyterian, Dallas TX) to determine plasma osmolality (Abbott Alinity, IL).
Calculations
We calculated mean body temperature as:
| (2) |
where Tcore and Tskin are core temperature and mean skin temperature, respectively.
We calculated mean arterial pressure (MAP) as:
| (3) |
where SBP and DBP are systolic and diastolic blood pressure, respectively.
We calculated forearm vascular conductance by dividing forearm blood flow by MAP. We calculated body mass loss as the difference in nude body weight (Mettler Toledo, OH) between pre- and postextreme heat exposure, and whole body sweat loss (WBSL) after correcting for fluid ingestion and urine output, which was collected and measured for volume. We calculated whole body sweat rate by dividing the whole body sweat loss by total heat exposure time.
Data Acquisition
Rectal temperature, skin temperature, local sweat rate, and heart rate were sampled at 250 Hz (Biopac MP150, Santa Barbara, CA) and converted into 1-min averages for data analysis. Core temperature measured via the telemetric pill (both ingested and as a suppository) was sampled every 30 s, and blood pressure was measured every 15 min during heat exposures.
Statistical Analyses
A power calculation was performed to detect a 0.5°C difference in the increase in core temperature between groups, with a standard deviation of that increase being 0.5°C. Based upon these estimates, and with an alpha of 0.05, 20 participants (10 participants per age group) would provide power of >0.80 to detect differences between age groups in the change in core temperature (power analyses from: NCSS PASS 2019). However, we enrolled 40 participants (20 participants per age group, with each age group comprising 10 males and 10 females) to account for possible attrition/missing data and to increase the capability to detect differences in other outcome variables that may have a greater degree of variability than core temperature. Due to technical difficulties, we were unable to obtain some measurements (e.g., skin temperature, local sweat rate, blood pressure, and limb blood flow) at either baseline or at the end of the extreme heat exposures in some participants. Similarly, we were unable to obtain metabolic heat production data in one young and one older participant. These missing data were excluded from final analyses, and the number of participants included for each variable is indicated in text, tables, and figures where appropriate. Before analyses, data were assessed to confirm that they met model assumptions (i.e., normality, equality of variance). Within each extreme heat simulation, we analyzed data using linear mixed effect models with main effects of time (within factor) and group (between factor; older vs. young) or unpaired t tests (two-tailed), as appropriate. Statistical analyses were performed in R studio (version 2022.07.1), and graphs were generated using GraphPad Prism 9.4 (GraphPad Software Inc., La Jolla, CA). Data in text, tables, and figures are presented as means ± standard deviations. Statistical significance was set a priori to P < 0.05.
RESULTS
Baseline Measurements
Participants’ baseline height, mass, body surface area, and lean mass were not different between the two cohorts (Table 1). However, there were differences in body mass index and body fat percentage (Table 1). Self-reported MET minutes of physical activity per week, quantified via the international physical activity questionnaire, were not different between the young (4,284 ± 5,049 MET min) and older (2,962 ± 3,133 MET min) cohorts (P = 0.348). In addition, the self-reported amount of time performing physical activity outdoors was not different between the young (4.4 ± 7.4 h/wk) and older (6.9 ± 8.2 h/wk) cohorts (P = 0.320). The medications reported upon screening are provided in Table 2.
Table 1.
Baseline participant characteristics
| Young | Older | P Value | |
|---|---|---|---|
| Males/Females | 10/10 | 10/10 | |
| Age, yr | 29 ± 5 | 70 ± 4 | |
| Height, cm | 171 ± 8 | 170 ± 8 | 0.707 |
| Body mass, kg | 68.8 ± 10.9 | 75.1 ± 11.3 | 0.083 |
| Body mass index, kg/m2 | 23.6 ± 2.8 | 26 ± 2.9 | 0.011 |
| Body surface area, m2 | 1.80 ± 0.17 | 1.86 ± 0.17 | 0.254 |
| Lean mass, kg | 49.4 ± 9.5 | 48.1 ± 10.3 | 0.682 |
| Body fat, % | 28.2 ± 8.2 | 36.3 ± 6.9 | 0.002 |
Values are presented as means ± standard deviation. Data were analyzed using unpaired t tests (two-tailed).
Table 2.
Medications reported at screening
| Young | Older | |
|---|---|---|
| 5-alpha reductase inhibitor | 0 | 1 |
| ACE inhibitors/ARBs | 0 | 7 |
| Alpha 1 blockers | 0 | 1 |
| Antidepressants | 1 | 3 |
| Antihistamines | 3 | 2 |
| Aspirin | 0 | 3 |
| Bisphosphonate derivatives | 0 | 2 |
| CNS stimulants | 2 | 0 |
| Diuretics | 0 | 3 |
| Birth control/estradiol | 2 | 1 |
| Gabapentin | 0 | 2 |
| Inhaled glucocorticoids | 0 | 4 |
| Thyroid drugs | 0 | 1 |
| Proton pump inhibitors | 0 | 2 |
| Semaglutide | 0 | 1 |
| Statins/fibric acid | 0 | 12 |
| Testosterone | 0 | 1 |
| Triptan | 1 | 0 |
| Vitamins | 5 | 7 |
Number of young (n = 20) and older (n = 20) participants using medication in each medication class. ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; CNS, central nervous system.
Environmental Conditions and Metabolic Heat Production
Average environmental conditions were 46.4 ± 0.4°C and 15.9 ± 2.3% relative humidity in the DRY simulations, and 40.8 ± 0.5°C and 40.3 ± 1.6% relative humidity during the HUMID simulations. Working metabolic rate (Young: 2.90 ± 0.22 METs vs. Older 2.84 ± 0.26 METs; P = 0.430) and metabolic heat production (Young: 2.98 ± 0.30 W/kg vs. Older 3.00 ± 0.36 W/kg; P = 0.882) were not different between groups.
Thermoregulatory and Cardiovascular Responses
DRY.
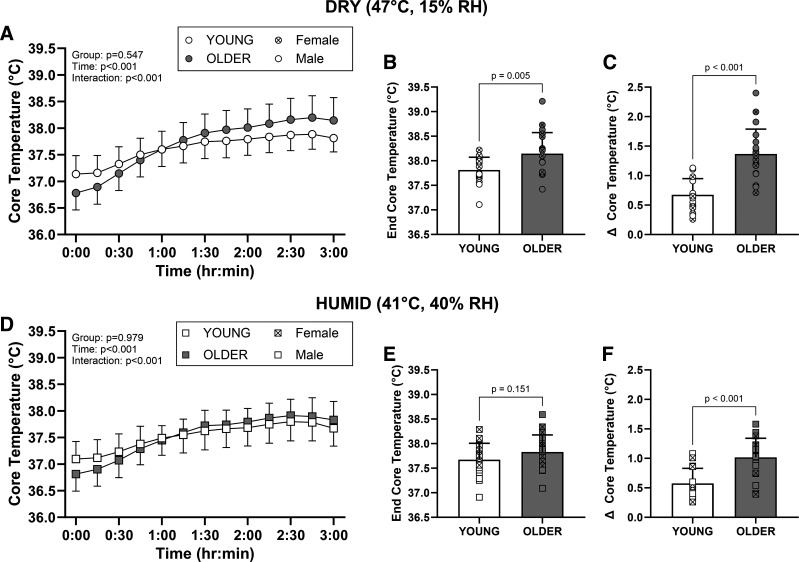
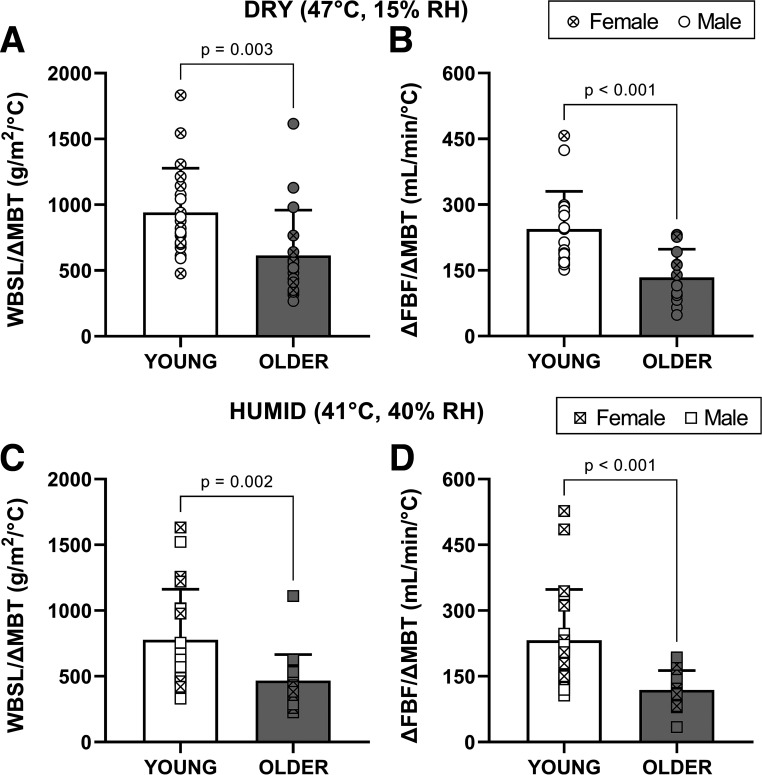
The thermoregulatory responses to the DRY condition are presented in Fig. 2 and Table 3. Δ core temperature (Young: 0.68 ± 0.27°C; Older: 1.37 ± 0.42°C; P < 0.001, d = 1.94) and ending core temperature (Young: 37.81 ± 0.26°C; Older: 38.15 ± 0.43°C; P = 0.005, d = 0.95) were greater in the older cohort following exposure to the DRY condition (Fig. 2). Whole body sweat rates (Young: 237 ± 40 g/m2/h vs. Older: 239 ± 46 g/m2/h; P = 0.885) and changes in forearm blood flow (interaction: P = 0.640; Table 3) were not different between groups. However, when indexed to changes in mean body temperature (i.e., divided by Δ mean body temperature), older adults had lower whole body sweat loss (P = 0.003, d = 0.96) and forearm blood flow responses (P < 0.001, d = 1.45; Fig. 3). Older adults had greater increases in heart rate (interaction: P = 0.039; Table 3), whereas there were similar decreases in mean arterial pressure in both groups (Table 3). Older adults had a higher plasma osmolality at baseline (P = 0.014; Table 4), however ending plasma osmolality (P = 0.209), body mass loss, and changes in plasma volume were not different between groups in the DRY condition (Table 4).
Figure 2.
Core temperature responses during the 3-h extreme heat exposures in young (n = 20) and older (n = 20) adults. Data are shown as means ± standard deviation with the circles/squares in B, C, E, and F representing individual data points. Older participants had greater increases in (Δ) core temperature and higher ending core temperatures in the DRY condition (B and C). Similarly, older participants had greater increases in (Δ) core temperature, but there was no difference in ending core temperature in the HUMID condition (E and F). Data in A and D were analyzed using linear mixed effects models with main effects of time (within: baseline and end of EHE) and group (between: young and older). Data in B, C, E, and F were analyzed using unpaired t tests (two-tailed). EHE, extreme heat exposure; RH, relative humidity.
Table 3.
Thermoregulatory, cardiovascular, and perceptual responses to 3-h extreme heat exposures
| Baseline | End of EHE | Δ | ANOVA | |
|---|---|---|---|---|
| DRY (47°C, 15% RH) | ||||
| Mean skin temperature, °C | Group: P = 0.113 Time: P < 0.001 Interaction: P = 0.553 | |||
| Young (n = 20) | 35.73 ± 0.63 | 37.57 ± 0.73 | 1.84 ± 0.77 | |
| Older (n = 17) | 35.29 ± 1.14 | 37.37 ± 0.85 | 2.08 ± 1.57 | |
| Mean body temperature, °C | Group: P = 0.317 Time: P < 0.001 Interaction: P < 0.001 | |||
| Young (n = 20) | 36.86 ± 0.29 | 37.76 ± 0.25 | 0.91 ± 0.26 | |
| Older (n = 17) | 36.46 ± 0.27 | 37.99 ± 0.41 | 1.53 ± 0.50 | |
| Local sweat rate, mL/min/cm2 | Group: P = 0.044 Time: P < 0.001 Interaction: P = 0.175 | |||
| Young (n = 20) | 0.20 ± 0.10 | 0.54 ± 0.13 | 0.34 ± 0.14 | |
| Older (n = 20) | 0.11 ± 0.08 | 0.51 ± 0.14 | 0.41 ± 0.15 | |
| Forearm blood flow, mL/min | Group: P = 0.907 Time: P < 0.001 Interaction: P = 0.640 | |||
| Young (n = 18) | 52 ± 26 | 268 ± 69 | 216 ± 57 | |
| Older (n = 18) | 56 ± 42 | 259 ± 99 | 203 ± 100 | |
| FVC, mL/mmHg/min | Group: P = 0.662 Time: P < 0.001 Interaction: P = 0.552 | |||
| Young (n = 18) | 0.6 ± 3.5 | 3.5 ± 1.1 | 2.7 ± 1.3 | |
| Older (n = 18) | 0.6 ± 0.5 | 3.3 ± 1.3 | 2.7 ± 1.3 | |
| Heart rate, beats/min | Group: P = 0.598 Time: P < 0.001 Interaction: P = 0.039 | |||
| Young (n = 20) | 64 ± 9 | 82 ± 9 | 18 ± 9 | |
| Older (n = 20) | 62 ± 10 | 87 ± 15 | 25 ± 11 | |
| Mean arterial pressure, mmHg | Group: P = 0.915 Time: P < 0.001 Interaction: P = 0.561 | |||
| Young (n = 20) | 86 ± 6 | 77 ± 7 | −9 ± 8 | |
| Older (n = 20) | 87 ± 7 | 76 ± 10 | −11 ± 11 | |
| Thermal sensation scale | Group: P = 0.833 Time: P < 0.001 Interaction: P = 0.106 | |||
| Young (n = 20) | 5 ± 1 | 6 ± 1 | 0.5 ± 1 | |
| Older (n = 20) | 5 ± 1 | 6 ± 1 | 1 ± 1 | |
| Environmental symptoms | Group: P = 0.736 Time: P < 0.001 Interaction: P = 0.558 | |||
| Young (n = 20) | 2 ± 2 | 8 ± 5 | 5 ± 5 | |
| Older (n = 20) | 3 ± 3 | 8 ± 5 | 4 ± 4 | |
| HUMID (41°C, 40% RH) | ||||
| Mean skin temperature, °C | Group: P < 0.001 Time: P < 0.001 Interaction: P = 0.119 | |||
| Young (n = 20) | 34.88 ± 0.93 | 36.65 ± 0.83 | 1.78 ± 1.26 | |
| Older (n = 19) | 33.69 ± 1.04 | 36.05 ± 0.58 | 2.36 ± 1.00 | |
| Mean body temperature, °C | Group: P = 0.018 Time: P < 0.001 Interaction: P < 0.001 | |||
| Young (n = 20) | 36.65 ± 0.39 | 37.47 ± 0.34 | 0.82 ± 0.30 | |
| Older (n = 19) | 36.19 ± 0.33 | 37.46 ± 0.21 | 1.27 ± 0.34 | |
| Local sweat rate, mL/min/cm2 | Group: P = 0.174 Time: P < 0.001 Interaction: P = 0.577 | |||
| Young (n = 18) | 0.18 ± 0.09 | 0.53 ± 0.19 | 0.35 ± 0.20 | |
| Older (n = 18) | 0.14 ± 0.06 | 0.47 ± 0.22 | 0.34 ± 0.21 | |
| Forearm blood flow, mL/min | Group: P = 0.123 Time: P < 0.001 Interaction: P = 0.279 | |||
| Young (n = 20) | 50 ± 19 | 227 ± 81 | 177 ± 82 | |
| Older (n = 17) | 43 ± 33 | 193 ± 58 | 150 ± 63 | |
| FVC, mL/mmHg/min | Group: P = 0.336 Time: P < 0.001 Interaction: P = 0.473 | |||
| Young (n = 20) | 0.6 ± 0.2 | 2.9 ± 1.2 | 2.4 ± 1.3 | |
| Older (n = 16) | 0.5 ± 0.4 | 2.6 ± 0.9 | 2.1 ± 1.0 | |
| Heart rate, beats/min | Group: P = 0.865 Time: P < 0.001 Interaction: P = 0.252 | |||
| Young (n = 20) | 65 ± 10 | 80 ± 10 | 15 ± 9 | |
| Older (n = 20) | 64 ± 10 | 82 ± 14 | 18 ± 8 | |
| Mean arterial pressure, mmHg | Group: P = 0.301 Time: P < 0.001 Interaction: P = 0.931 | |||
| Young (n = 20) | 86 ± 6 | 78 ± 5 | −8 ± 6 | |
| Older (n = 19) | 84 ± 8 | 76 ± 9 | −8 ± 9 | |
| Thermal sensation scale | Group: P = 0.819 Time: P = 0.339 Interaction: P = 0.960 | |||
| Young (n = 20) | 5 ± 1 | 5 ± 1 | 0 ± 1 | |
| Older (n = 20) | 5 ± 1 | 5 ± 2 | 0 ± 2 | |
| Environmental symptoms | Group: P = 0.752 Time: P < 0.001 Interaction: P = 0.925 | |||
| Young (n = 20) | 3 ± 3 | 6 ± 4 | 4 ± 4 | |
| Older (n = 20) | 2 ± 2 | 6 ± 5 | 4 ± 6 | |
Values are presented as means ± standard deviation. Baseline forearm blood flow was measured in a thermoneutral room before entering the environmental chamber, whereas mean skin temperature, thermal sensation, and environmental symptom questionnaire were measured after entering the environmental chamber following a brief period (∼5 min) of instrumentation. End measurements were taken just before exiting the environmental chamber. Data were analyzed using linear mixed effects models with main effects of time (within: baseline and end of EHE) and group (between: young and older). EHE, extreme heat exposure; FVC, forearm vascular conductance; RH, relative humidity.
Figure 3.
Whole body sweat loss (WBSL) and forearm blood flow (FBF) responses indexed to changes in mean body temperature in young and older adults. Data are shown as means ± standard deviation with the circles/squares representing individual data points. Whole body sweat loss and the changes in (Δ) forearm blood flow were lower in the older cohort when indexed to changes in (Δ) mean body temperature (MBT) during the DRY (A and B) and HUMID (C and D) heat exposures. Data were compared using unpaired t tests (two-tailed). RH, relative humidity.
Table 4.
Markers of hydration during 3-h extreme heat exposures
| Baseline | End of EHE | P Value | |
|---|---|---|---|
| DRY (47°C, 15% RH) | |||
| Plasma osmolality, mosmol/kgH2O | Group: P = 0.017 Time: P = 0.305 Interaction: P = 0.223 | ||
| Young (n = 20) | 286 ± 4 | 288 ± 4 | |
| Older (n = 17) | 290 ± 3* | 290 ± 4 | |
| Whole body sweat loss, L | P = 0.453 | ||
| Young (n = 20) | 1.42 ± 0.32 | ||
| Older (n = 20) | 1.50 ± 0.35 | ||
| Body mass loss, % | P = 0.585 | ||
| Young (n = 20) | −1.15 ± 0.37 | ||
| Older (n = 20) | −1.07 ± 0.47 | ||
| Plasma volume change, % | P = 0.400 | ||
| Young (n = 17) | −2.3 ± 7.8 | ||
| Older (n = 16) | −4.6 ± 7.2 | ||
| HUMID (41°C, 40% RH) | |||
| Plasma osmolality, mosmol/kgH2O | Group: P = 0.007 Time: P = 0.526 Interaction: P = 0.807 | ||
| Young (n = 20) | 285 ± 4 | 286 ± 3 | |
| Older (n = 18) | 288 ± 4* | 288 ± 4* | |
| Whole body sweat loss, L | P = 0.414 | ||
| Young (n = 20) | 0.97 ± 0.15 | ||
| Older (n = 20) | 1.03 ± 0.28 | ||
| Body mass loss, % | P = 0.789 | ||
| Young (n = 20) | −0.49 ± 0.25 | ||
| Older (n = 20) | −0.51 ± 0.35 | ||
| Plasma volume change | P = 0.400 | ||
| Young (n = 19) | 1.4 ± 5.2 | ||
| Older (n = 18) | −2.0 ± 5.9 | ||
Values are presented as means ± standard deviation. Data were analyzed using linear mixed effects models with main effects of time (within: baseline and end of EHE) and group (between: young and older) or unpaired t tests (two-tailed), as appropriate. EHE, extreme heat exposure; RH, relative humidity. *Difference between groups P < 0.05.
HUMID.
Following exposure to the HUMID condition, older participants had greater Δ core temperature (Young: 0.58 ± 0.25°C; Older: 1.02 ± 0.32°C; P < 0.001, d = 1.52), but there was no difference in ending core temperature (Young: 37.67 ± 0.34°C; Older: 37.83 ± 0.35°C; P = 0.151, d = 0.46; Fig. 2). Whole body sweat rates (Young: 161 ± 21 g/m2/h vs. Older: 164 ± 37 g/m2/h; P = 0.735) and forearm blood flow (interaction: P = 0.163; Table 3) were not different between groups. When indexed to changes in mean body temperature, older adults had lower sweating (P = 0.002, d = 1.01) and forearm blood flow responses (P < 0.001, d = 1.29; Fig. 3). There was no difference in the increase in heart rate between groups in the HUMID condition (interaction: P = 0.252), and groups had similar reductions in mean arterial pressure (Table 3). Older adults had a higher plasma osmolality at baseline (P = 0.022) and at the end of the heat exposure (P = 0.041), however, changes in plasma osmolality (interaction: P = 0.807), body mass loss, and changes in plasma volume were not different between groups in the HUMID condition (Table 4).
DISCUSSION
The purpose of this study was to compare the thermoregulatory responses of healthy young and older adults exposed to two distinct (DRY and HUMID) extreme heat simulations. To improve ecological relevance and to more accurately depict the extent of thermal stress during a real-world heatwave, we had participants perform brief bouts of light activity (3 METs) dispersed throughout the heat exposure to mimic heat generation comparable with activities of daily living (e.g., cooking, putting away groceries, or cleaning; 20). We found that older adults had greater core temperature responses to both extreme heat exposures (i.e., Δ and ending core temperature for the DRY trial, and Δ for the HUMID trial) compared with their young counterparts. These differences were likely a consequence of attenuated efferent/afferent thermoregulatory responses in older adults. For example, there were no differences in whole body sweat rate, local sweat rate, or forearm blood flow [an indicator of skin blood flow (41)] despite greater central drive (e.g., heightened thermal strain). Indeed, when sweating and forearm blood flow responses were indexed to changes in mean body temperature, older adults showed attenuated thermoregulatory responses. Notably, these age-related differences were observed despite the absence of dehydration in either group via prescribed water intake. These findings corroborate previous reports demonstrating that older adults have diminished thermoregulatory responses to heat stress (13, 14, 16–19), and confirm epidemiological data showing that older adults are at a greater risk for hyperthermia during heatwaves (9–12).
A novel aspect of our study was the incorporation of brief bouts of light activity to represent metabolic heat generation associated with activities of daily living that likely occur during a heatwave. Although a similar approach was used to examine responses in young adults (i.e., continuous low-intensity exercise throughout the 3-h exposure (42), we are the first to use intermittent metabolic heat generation associated with activities of daily living in older adults. Our approach resulted in greater thermal strain than what has been previously reported in the literature during similar heat exposures (i.e., both temperature and duration). That is, compared with our findings, previous studies with similar environmental conditions and duration in the absence of intermittent bouts of physical activity report lower increases in core temperature (i.e., <0.5°C), and smaller (<0.3°C) differences in core temperature responses between young and older adults (17, 43). In contrast, we report larger increases in core temperature (i.e., >0.5°C) in both groups and clear group-wide differences in thermal strain (i.e., ∼0.5°C–1°C difference in Δ core temperature between young and older participants). In fact, the present findings are similar to a prior report demonstrating the effect of age on thermal responses to heatwave-like conditions without the maintenance of euhydration, where the increase in core temperature in older individuals was ∼1.3°C after 3 h of exposure (19).
Given that individuals would likely have access to water during a heatwave, participants were provided with 3 mL/kg of tap temperature water every hour to offset some of the dehydration associated with sweat loss. Indeed, we noted only minimal changes in body weight loss and plasma osmolality post heat exposure (see Table 4), suggesting that participants were not dehydrated following that exposure. We propose that core temperature responses would likely be further magnified if water was restricted throughout the exposure. In addition, given an expected rate of dehydration in the absence of water administration, we speculate that increases in core temperature would likely be exacerbated without fluid replacement during longer heat exposure. This is a relevant concern for older adults who may be less likely to maintain hydration ad libitum due to attenuated thirst sensation, medication use, or cognitive/physical impairments (44, 45).
The reported differences in core temperature responses are likely due to established age-related alterations in heat dissipation. For example, data from experimental studies demonstrate that older adults have diminished sweating and skin blood flow responses to heat exposure (13–17). Attenuated sweating is likely to explain most of the difference between age groups in the present protocol, as sweating is the primary means for heat dissipation during heat exposure of the magnitude used in this protocol. Of note, we report no differences in whole body or local sweat rates between young and older adults, indicating similar levels of sweating. However, these similar sweating responses persisted despite greater thermoafferent inputs (i.e., greater mean body temperature) in the older adults, suggesting attenuated sweating for a given increase in body temperature. Similarly, there were no age-related differences in the change in forearm blood flow [an index of skin blood flow (41)] from baseline to the end of the heat exposure, but this also occurred when the older individuals had a greater increase in mean body temperature. As a heat dissipation mechanism, skin blood flow is dependent on the interplay of skin and environmental temperature gradients. With that in mind, increases in skin blood flow are still beneficial to minimize the environmental temperature to skin gradient and to improve the efficacy of evaporative cooling by returning cooled blood to the core. Nevertheless, our findings highlight that older adults may have impaired responses to thermoafferent signals, and/or impaired thermoeffector responses to the heat stimulus, resulting in similar increases in limb blood flow and sweating despite an approximately twofold greater increase in core temperature.
Experimental Considerations
We acknowledge that the duration of the used extreme heat exposure is shorter than actual heatwaves, often lasting multiple days (3). However, we chose a 3-h duration to replicate the duration of the peak periods of elevated daily temperatures during heatwaves (11, 43, 46–49). In addition, we simulated the environmental conditions of an outdoor heatwave, with access to shade, as there is a lack of large-scale data sets reporting indoor temperatures during heatwaves across a spectrum of housing types (e.g., a single dwelling home, high-density apartments, mobile/trailer homes, tents used by the homeless, etc.). Notably, in the absence of an air conditioner, in-home temperatures can resemble outside temperatures during extreme heat events (50–53). We recognize that individuals with certain health conditions are particularly susceptible to heat-induced morbidity and mortality (12, 49, 54), whereas our study used healthy older adults. With that in mind, the present work provides a foundation for subsequent highly focused studies of individuals with specific diseases exposed to similar levels of heat stress. Another limitation was that we indexed whole body sweat loss and changes in forearm blood flow to changes in mean body temperature. This method allowed us to better understand age differences in thermoregulation, though a key limitation to this approach is that it assumes a linear relation between these thermoregulatory responses and changes in body temperature. Clearly, both sweat rate and skin blood flow will at some point during heat exposure reach a plateau such that further increases in body temperature will result in no further increase in either measurement.
Perspectives
The frequency, intensity, and duration of extreme heat events are predicted to increase in the coming decades (3, 4). In parallel with climate changes, in the United Sates, the number of individuals aged 65 yr and older continues to rise (8), thus the number of older individuals exposed to progressively higher environmental temperatures will increase over the decades ahead. Our findings demonstrate that older adults, having attenuated efferent/afferent thermoregulatory responses, are at an increased risk for hyperthermia during actual heatwaves. Although the core temperature responses reported in this study are lower than what are typically associated with heat illness [e.g., heat stroke (56)], it is important to consider that higher core temperatures may be observed during actual heatwaves that last multiple days, or in diseased individuals who are particularly susceptible to heat-induced morbidity and mortality (12, 49, 54). Further, hyperthermia is not the only cause of morbidity and mortality during heatwaves. In fact, many of the excess hospitalizations and deaths during heatwaves are due to cardiovascular, renal, and/or respiratory causes (57). Thus, the impact of this elevated thermal stress on various physiological systems in older adults is of particular interest. In addition, the efficacy of various cooling strategies in groups most vulnerable to heat-related complications warrants future investigation. Although indoor air conditioning is the most effective strategy to prevent a heat-related injury and/or death, many individuals do not have access to air conditioners or the financial means to use them. Thus, special attention should be given to identifying non-air conditioning-dependent cooling modalities to attenuate excessive elevations in body temperature and associated physiological stress in older adults during heatwave conditions.
Conclusions
Using a model that incorporates brief periods of light physical activity to simulate activities of daily living, the extent of thermal strain reported herein more accurately represents what would occur during actual heatwave conditions. Importantly, despite matching metabolic heat generation and environmental conditions, our findings show that older adults have augmented increase in core temperature likely due to age-related reductions in heat dissipating mechanisms.
DATA AVAILABILITY
Data will be made available upon reasonable request.
GRANTS
This research was supported by the National Institutes of Health (NIH) Grant R01AG069005 (to C.G.C.), American Heart Association (AHA) Grant 23POST1023065 (to Z.J.M.), NIH Grant F32HL154565 (to L.N.B.), AHA Grant 23CDA1037938 and NIH Grants F32HL154559 and K01HL160772 (to J.C.W.), and NIH Grant T32HL098040 (to W.C.A.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.N.B. and C.G.C. conceived and designed research; Z.J.M., J.F., W.C.A., L.N.B., J.C.W., C.P.J., and B.D.O. performed experiments; Z.J.M., J.F., W.C.A., L.N.B., and C.G.C. analyzed data; Z.J.M., J.F., W.C.A., L.N.B., J.C.W., and C.G.C. interpreted results of experiments; Z.J.M., J.F., W.C.A., and C.G.C. prepared figures; Z.J.M. and C.G.C. drafted manuscript; Z.J.M., J.F., W.C.A., L.N.B., J.C.W., C.P.J., and C.G.C. edited and revised manuscript; Z.J.M., J.F., W.C.A., L.N.B., J.C.W., C.P.J., B.D.O., and C.G.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the individuals who participated in this research. We also thank Mayah Benning, Elias Johnson, Courtney Kirby, and Frank Cimino for assistance with the study. Graphical abstract image was created with BioRender.com and published with permission.
REFERENCES
- 1. Coates L, Haynes K, O’Brien J, McAneney J, de Oliveira FD. Exploring 167 years of vulnerability: an examination of extreme heat events in Australia 1844–2010. Environ Sci Pol 42: 33–44, 2014. doi: 10.1016/j.envsci.2014.05.003. [DOI] [Google Scholar]
- 2. Luber G, McGeehin M. Climate change and extreme heat events. Am J Prev Med 35: 429–435, 2008. doi: 10.1016/j.amepre.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 3. Meehl GA, Tebaldi C. More intense, more frequent, and longer lasting heat waves in the 21st century. Science 305: 994–997, 2004. doi: 10.1126/science.1098704. [DOI] [PubMed] [Google Scholar]
- 4. Schär C, Vidale PL, Lüthi D, Frei C, Häberli C, Liniger MA, Appenzeller C. The role of increasing temperature variability in European summer heatwaves. Nature 427: 332–336, 2004. doi: 10.1038/nature02300. [DOI] [PubMed] [Google Scholar]
- 5. Gisolfi CV, Wenger CB. Temperature regulation during exercise: old concepts, new ideas. Exerc Sport Sci Rev 12: 339–372, 1984. [PubMed] [Google Scholar]
- 6. Bouchama A, Knochel JP. Heat stroke. N Engl J Med 346: 1978–1988, 2002. doi: 10.1056/NEJMra011089. [DOI] [PubMed] [Google Scholar]
- 7. Cramer MN, Gagnon D, Laitano O, Crandall CG. Human temperature regulation under heat stress in health, disease, and injury. Physiol Rev 102: 1907–1989, 2022. doi: 10.1152/physrev.00047.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.United States Census. 2017 National Population Projections. https://www.census.gov/data/tables/2017/demo/popproj/2017-summary-tables.html [2023 Feb].
- 9. Johnson H, Kovats RS, McGregor G, Stedman J, Gibbs M, Walton H, Cook L, Black E. The impact of the 2003 heat wave on mortality and hospital admissions in England. Health Stat Q 25: 6–11, 2005. [PubMed] [Google Scholar]
- 10. Knowlton K, Rotkin-Ellman M, King G, Margolis HG, Smith D, Solomon G, Trent R, English P. The 2006 California heat wave: impacts on hospitalizations and emergency department visits. Environ Health Perspect 117: 61–67, 2009. doi: 10.1289/ehp.11594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Linares C, Díaz J. Impact of high temperatures on hospital admissions: comparative analysis with previous studies about mortality (Madrid). Eur J Public Health 18: 317–322, 2008. doi: 10.1093/eurpub/ckm108. [DOI] [PubMed] [Google Scholar]
- 12. Semenza JC, McCullough JE, Flanders WD, Mcgeehin MA, Lumpkin JR. Excess hospital admissions during the July 1995 heat wave in Chicago. Am J Prev Med 16: 269–277, 1999. doi: 10.1016/s0749-3797(99)00025-2. [DOI] [PubMed] [Google Scholar]
- 13. Dufour A, Candas V. Ageing and thermal responses during passive heat exposure: sweating and sensory aspects. Eur J Appl Physiol 100: 19–26, 2007. doi: 10.1007/s00421-007-0396-9. [DOI] [PubMed] [Google Scholar]
- 14. Fennell WH, Moore RE. Responses of aged men to passive heating. J Physiol 231: 118P–119P, 1973. [PubMed] [Google Scholar]
- 15. Gagnon D, Romero SA, Cramer MN, Kouda K, Poh PYS, Ngo H, Jay O, Crandall CG. Age modulates physiological responses during fan use under extreme heat and humidity. Med Sci Sports Exerc 49: 2333–2342, 2017. doi: 10.1249/MSS.0000000000001348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Inoue Y, Nakao M, Araki T, Murakami H. Regional differences in the sweating responses of older and younger men. J Appl Physiol (1985) 71: 2453–2459, 1991. doi: 10.1152/jappl.1991.71.6.2453. [DOI] [PubMed] [Google Scholar]
- 17. Sagawa S, Shiraki K, Yousef MK, Miki K. Sweating and cardiovascular responses of aged men to heat exposure. J Gerontol 43: M1–M8, 1988. doi: 10.1093/geronj/43.1.m1. [DOI] [PubMed] [Google Scholar]
- 18. Crowe JP, Moore RE. Proceedings: physiological and behavioural responses of aged men to passive heating. J Physiol 236: 43P–45P, 1974. [PubMed] [Google Scholar]
- 19. Miescher E, Fortney SM. Responses to dehydration and rehydration during heat exposure in young and older men. Am J Physiol Regul Integr Comp Physiol 257: R1050–R1056, 1989. doi: 10.1152/ajpregu.1989.257.5.R1050. [DOI] [PubMed] [Google Scholar]
- 20. Ainsworth BE, Haskell WL, Leon AS, Jacobs DR Jr, Montoye HJ, Sallis JF, Paffenbarger RS Jr.. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc 25: 71–80, 1993. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 21. Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A. Physical Activity and Public Health. Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation 116: 1081–1093, 2007. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- 22. Berko J, Ingram DD, Saha S, Parker JD. Deaths attributed to heat, cold, and other weather events in the United States, 2006–2010. Natl Health Stat Report 76: 1–15, 2014. [PubMed] [Google Scholar]
- 23. Ellis FP, Nelson F, Pincus L. Mortality during heat waves in New York City July, 1972 and August and September, 1973. Environ Res 10: 1–13, 1975. doi: 10.1016/0013-9351(75)90069-9. [DOI] [PubMed] [Google Scholar]
- 24. Fouillet A, Rey G, Laurent F, Pavillon G, Bellec S, Guihenneuc-Jouyaux C, Clavel J, Jougla E, Hémon D. Excess mortality related to the August 2003 heat wave in France. Int Arch Occup Environ Health 80: 16–24, 2006. doi: 10.1007/s00420-006-0089-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kovats RS, Hajat S. Heat stress and public health: a critical review. Annu Rev Public Health 29: 41–55, 2008. doi: 10.1146/annurev.publhealth.29.020907.090843. [DOI] [PubMed] [Google Scholar]
- 26. Kolka MA, Stephenson LA. Control of sweating during the human menstrual cycle. Eur J Appl Physiol Occup Physiol 58: 890–895, 1989. doi: 10.1007/BF02332224. [DOI] [PubMed] [Google Scholar]
- 27. Lei T-H, Stannard SR, Perry BG, Schlader ZJ, Cotter JD, Mündel T. Influence of menstrual phase and arid vs. humid heat stress on autonomic and behavioural thermoregulation during exercise in trained but unacclimated women. J Physiol 595: 2823–2837, 2017. doi: 10.1113/JP273176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Notley SR, Dervis S, Poirier MP, Kenny GP. Menstrual cycle phase does not modulate whole body heat loss during exercise in hot, dry conditions. J Appl Physiol (1985) 126: 286–293, 2019. doi: 10.1152/japplphysiol.00735.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Beizaee A, Lomas KJ, Firth SK. National survey of summertime temperatures and overheating risk in English homes. Build Environ 65: 1–17, 2013. doi: 10.1016/j.buildenv.2013.03.011. [DOI] [Google Scholar]
- 30. Gustin M, McLeod RS, Lomas KJ. Forecasting indoor temperatures during heatwaves using time series models. Build Environ 143: 727–739, 2018. doi: 10.1016/j.buildenv.2018.07.045. [DOI] [Google Scholar]
- 31. Cheuvront SN, Carter R 3rd, Sawka MN. Fluid balance and endurance exercise performance. Curr Sports Med Rep 2: 202–208, 2003. doi: 10.1249/00149619-200308000-00006. [DOI] [PubMed] [Google Scholar]
- 32. Sawka MN. Physiological consequences of hypohydration: exercise performance and thermoregulation. Med Sci Sports Exerc 24: 657–670, 1992. [PubMed] [Google Scholar]
- 33. Sawka MN, Burke LM, Eichner ER, Maughan RJ, Montain SJ, Stachenfeld NS; American College of Sports Medicine. American College of Sports Medicine position stand. Exercise and fluid replacement. Med Sci Sports Exerc 39: 377–390, 2007. doi: 10.1249/mss.0b013e31802ca597. [DOI] [PubMed] [Google Scholar]
- 34. Cramer MN, Jay O. Partitional calorimetry. J Appl Physiol (1985) 126: 267–277, 2019. doi: 10.1152/japplphysiol.00191.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Notley SR, Meade RD, Kenny GP. Time following ingestion does not influence the validity of telemetry pill measurements of core temperature during exercise-heat stress: the journal temperature toolbox. Temperature (Austin) 8: 12–20, 2021. doi: 10.1080/23328940.2020.1801119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Byrne C, Lim CL. The ingestible telemetric body core temperature sensor: a review of validity and exercise applications. Br J Sports Med 41: 126–133, 2007. doi: 10.1136/bjsm.2006.026344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gosselin J, Béliveau J, Hamel M, Casa D, Hosokawa Y, Morais JA, Goulet EDB. Wireless measurement of rectal temperature during exercise: comparing an ingestible thermometric telemetric pill used as a suppository against a conventional rectal probe. J Therm Biol 83: 112–118, 2019. doi: 10.1016/j.jtherbio.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 38. Taylor WF, Johnson JM, Kosiba WA, Kwan CM. Cutaneous vascular responses to isometric handgrip exercise. J Appl Physiol (1985) 66: 1586–1592, 1989. doi: 10.1152/jappl.1989.66.4.1586. [DOI] [PubMed] [Google Scholar]
- 39. Sampson JB, Kobrick JL. The environmental symptoms questionnaire: revisions and new filed data. Aviat Space Environ Med 51: 872–877, 1980. [PubMed] [Google Scholar]
- 40. Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 37: 247–248, 1974. doi: 10.1152/jappl.1974.37.2.247. [DOI] [PubMed] [Google Scholar]
- 41. Brothers RM, Wingo JE, Hubing KA, Crandall CG. Methodological assessment of skin and limb blood flows in the human forearm during thermal and baroreceptor provocations. J Appl Physiol (1985) 109: 895–900, 2010. doi: 10.1152/japplphysiol.00319.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Graham C, Morris NB, Harwood AE, Jay O. Ad libitum water consumption off-sets the thermal and cardiovascular strain exacerbated by dehydration during a 3-h simulated heatwave. Eur J Appl Physiol 120: 391–399, 2020. doi: 10.1007/s00421-019-04283-7. [DOI] [PubMed] [Google Scholar]
- 43. Kenny GP, Poirier MP, Metsios GS, Boulay P, Dervis S, Friesen BJ, Malcolm J, Sigal RJ, Seely AJ, Flouris AD. Hyperthermia and cardiovascular strain during an extreme heat exposure in young vs. older adults. Temperature (Austin) 4: 79–88, 2017. doi: 10.1080/23328940.2016.1230171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Begg DP. Disturbances of thirst and fluid balance associated with aging. Physiol Behav 178: 28–34, 2017. doi: 10.1016/j.physbeh.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 45. Phillips PA, Johnston CI, Gray L. Disturbed fluid and electrolyte homoeostasis following dehydration in elderly people. Age Ageing 22: S26–S33, 1993. doi: 10.1093/ageing/22.suppl_1.s26. [DOI] [PubMed] [Google Scholar]
- 46. Basu R, Malig B. High ambient temperature and mortality in California: exploring the roles of age, disease, and mortality displacement. Environ Res 111: 1286–1292, 2011. doi: 10.1016/j.envres.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 47. Mastrangelo G, Fedeli U, Visentin C, Milan G, Fadda E, Spolaore P. Pattern and determinants of hospitalization during heat waves: an ecologic study. BMC Public Health 7: 200, 2007. doi: 10.1186/1471-2458-7-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Medina-Ramón M, Zanobetti A, Cavanagh DP, Schwartz J. Extreme temperatures and mortality: assessing effect modification by personal characteristics and specific cause of death in a multi-city case-only analysis. Environ Health Perspect 114: 1331–1336, 2006. doi: 10.1289/ehp.9074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Michelozzi P, Accetta G, De Sario M, D'Ippoliti D, Marino C, Baccini M, Biggeri A, Anderson HR, Katsouyanni K, Ballester F, Bisanti L, Cadum E, Forsberg B, Forastiere F, Goodman PG, Hojs A, Kirchmayer U, Medina S, Paldy A, Schindler C, Sunyer J, Perucci CA; PHEWE Collaborative Group. High temperature and hospitalizations for cardiovascular and respiratory causes in 12 European cities. Am J Respir Crit Care Med 179: 383–389, 2009. doi: 10.1164/rccm.200802-217OC. [DOI] [PubMed] [Google Scholar]
- 50.Centers for Disease Control and Prevention. Heat-related deaths–Philadelphia and United States, 1993-1994. MMWR Morb Mortal Wkly Rep 43: 453–455, 1994. [PubMed] [Google Scholar]
- 51.Centers for Disease Control and Prevention. Heat-related deaths–United States. MMWR Morb Mortal Wkly Rep 42: 558–560, 1993. [PubMed] [Google Scholar]
- 52.Centers for Disease Control and Prevention. Heat-related deaths–Dallas, Wichita, and Cooke counties, Texas, and United States, 1996. MMWR Morb Mortal Wkly Rep 46: 528–531, 1997. [PubMed] [Google Scholar]
- 53.Centers for Disease Control and Prevention. Heat-related illnesses and deaths–Missouri, 1998, and United States, 1979-1996. MMWR Morb Mortal Wkly Rep 48: 469–473, 1999. [PubMed] [Google Scholar]
- 54. Díaz J, García R, Velázquez de Castro F, Hernández E, López C, Otero A. Effects of extremely hot days on people older than 65 years in Seville (Spain) from 1986 to 1997. Int J Biometeorol 46: 145–149, 2002. doi: 10.1007/s00484-002-0129-z. [DOI] [PubMed] [Google Scholar]
- 56. Bouchama A, Abuyassin B, Lehe C, Laitano O, Jay O, O'Connor FG, Leon LR. Classic and exertional heatstroke. Nat Rev Dis Primers 8: 8, 2022. doi: 10.1038/s41572-021-00334-6. [DOI] [PubMed] [Google Scholar]
- 57. Bobb JF, Obermeyer Z, Wang Y, Dominici F. Cause-specific risk of hospital admission related to extreme heat in older adults. JAMA 312: 2659–2667, 2014. doi: 10.1001/jama.2014.15715. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request.