Abstract
The immunosuppressive agent mycophenolate mofetil (MMF) has been approved for use in kidney transplant recipients and may thus be used concomitantly for the treatment of intercurrent herpesvirus infections with drugs such as acyclovir (ACV), ganciclovir (GCV), and penciclovir (PCV). We found that MMF and its parent compound mycophenolic acid (at concentrations that are attainable in plasma) strongly potentiate the antiherpesvirus (herpes simplex virus [HSV] type 1 [HSV-1], HSV-2, thymidine kinase-deficient [TK−] HSV-1, both wild-type and TK− varicella-zoster virus, and human cytomegalovirus) activities of ACV, PCV, and GCV (up to 350-fold increases in their activities). The mechanism of potentiation was found to reside in the depletion of endogenous dGTP pools, which favored the inhibitory effect of the triphosphate of ACV, GCV, or PCV on the viral DNA polymerase. The combination of topically applied 5% MMF with 0.1% ACV strongly protected against HSV-1-induced cutaneous lesions in hairless mice, whereas therapy with either compound used singly had no protective effect. Interestingly, the combination of topically applied 5% MMF with 5% ACV was also highly effective in protecting against TK− HSV-2-induced cutaneous lesions (that were refractory to ACV treatment) in athymic nude mice. Topical therapy with MMF was very well tolerated, and no signs of irritation were observed. When given perorally at 200 mg/kg of body weight/day, MMF potentiated to some extent the growth retardation induced by GCV in young NMRI mice. These observations may have clinical implications (i) for those transplant recipients who receive both MMF and either ACV, GCV, or PCV and (ii) for the treatment of ACV-resistant mucocutaneous HSV infections.
Mycophenolate mofetil (MMF), the morpholinoethyl ester of mycophenolic acid (MPA), is currently used as an immunosuppressant in kidney transplant recipients. After oral administration, MMF is hydrolyzed to MPA, the active immunosuppressive agent, which is a potent inhibitor of IMP dehydrogenase. Inhibition of this enzyme results in a depletion of the intracellular GTP and dGTP pools (19, 23). Acyclovir (ACV), ganciclovir (GCV), and penciclovir (PCV) are three acyclic purine nucleoside analogs with potent activities against different herpesviruses including herpes simplex virus (HSV) type 1 (HSV-1) and type 2 (HSV-2) and varicella-zoster virus (VZV). GCV is active against human cytomegalovirus (HCMV). These compounds are specifically phosphorylated to their monophosphate forms by virus-encoded kinases (HSV-1, HSV-2, or VZV-encoded thymidine kinase [TK] or the HCMV-encoded UL97 protein kinase with GCV-phosphorylating capacity) and are then further phosphorylated by cellular kinases to the triphosphate metabolites (8). These triphosphorylated metabolites may be expected to achieve a better inhibition of the viral DNA polymerase if the levels of the competing substrate dGTP are reduced. We reasoned that a depletion of the dGTP pools brought about by MPA may enhance the antiviral activities of these antiherpesvirus molecules. In addition, ACV and GCV can be phosphorylated by 5′-nucleotidase for which IMP is the phosphate donor (14, 15). Increased IMP pools may thus result in a more efficient phosphorylation of ACV or GCV by this enzyme.
Several important interactions with drugs and immunosuppressive agents have been reported (17). The effect of antiherpesvirus therapy has also been studied in animals that are immunosuppressed with cyclosporin A (10, 11) or other immunosuppressive agents. However, the interaction between MMF and ACV, GCV, or PCV has never been the subject of a study. We have now demonstrated that MPA and MMF markedly potentiate the antiherpesvirus activity of ACV, GCV, and PCV against wild-type and TK-deficient (TK−) herpesvirus strains both in vitro and in animal models. This observation may have clinical implications because (i) transplant recipients under MMF therapy may show a better response to antiherpetic treatment for intercurrent herpesvirus infections, and (ii) topical use of the combination ACV plus MMF may have potential for the treatment of mucocutaneous infections with TK− HSV strains. Moreover, from a toxicological viewpoint it may be of interest to monitor the toxicity of GCV in patients under MMF treatment since the latter has the potential to increase the side effects of GCV.
MATERIALS AND METHODS
Cells and viruses.
HCMV (strain Davis) and VZV (strains OKA and YS-R) were obtained from the American Type Culture Collection. The origins of HSV-1 KOS, and HSV-2 G, and TK− HSV-1 B2006s have been described before (9). TK− HSV-2 (HS-44) is a plaque-purified TK− strain isolated from a patient refractory to ACV treatment (21). Human embryonic lung (HEL) cells and Vero cells were propagated in minimal essential medium (MEM) supplemented with 10% fetal calf serum (FCS), l-glutamine, and bicarbonate. The human T-cell line CEM was propagated in RPMI medium supplemented with 10% FCS, l-glutamine, and bicarbonate.
Compounds.
MPA was purchased from Sigma (St. Louis, Mo.). ACV was from Glaxo Wellcome, GCV was from Sarva-Syntex, and PCV was from Smith Kline Beecham. MMF was provided by Roche (Palo Alto, Calif.).
Antiviral and cell growth assays.
HEL or Vero cells were grown to confluency in microtiter trays and were inoculated with one of the different HSV strains at 100 times the 50% cell culture infective dose. Confluent cultures of HEL cells were inoculated with 100 PFU of HCMV or 20 PFU of VZV. Compounds, either alone or in combination, were added after a 2-h virus adsorption period. The virus-induced cytopathic effect (CPE) was recorded microscopically at 2 to 3 days postinfection for HSV and 7 days postinfection for HCMV. VZV-induced plaque formation was evaluated at 5 days postinfection. The 50% effective concentrations were derived from graphical plots.
Inhibition of cell growth was evaluated by counting the cell cultures with a Coulter Counter. Briefly, Vero cells were seeded in microtiter trays at a density of 4,000 cells/well in MEM containing 20% FCS and were allowed to adhere to the plastic, after which different concentrations of the drugs in MEM containing 2% FCS were added. The cells were allowed to proliferate for 3 days, after which the percent inhibition of cell growth was determined. Inhibition of the growth of CEM cells was assessed in a similar fashion, except that 50,000 cells were added per well.
Analysis of drug combination effect.
The inhibitory effects of the drugs combined on the HCMV-induced CPE were examined with checkerboard combinations of various concentrations of the test compounds. The drug combination effect was analyzed by the isobologram method as described previously (1). In this analysis, the EC50 was used to calculate the fractional inhibitory concentration (FIC). When the minimum FIC index, which corresponds to the FICs of the compounds combined (e.g., FICX + FICy), is equal to 1.0, the combination is assumed to act in an additive fashion; when it is between 1.0 and 0.5, the combination should act subsynergistically, and when it is <0.5, it acts synergistically.
Virus yield assay.
Confluent cultures of Vero cells were infected with an input of HSV-1, HSV-2, or TK− HSV that caused 100% CPE at 3 days postinfection and were treated (or left untreated) with MPA or MMF. Cultures were harvested and frozen at 3 days postinfection. Upon freezing-thawing, cell debris was removed by centrifugation and serial dilutions were inoculated onto confluent Vero cell cultures. Virus titers were determined 2 to 3 days later.
Cell metabolism studies.
Confluent cultures of Vero cells grown in 25-cm2 culture flasks were either infected with HSV-1 or mock infected. After 1 day (when the CPE had reached about 40%) [8-3H]ACV (specific activity, 15 Ci/mmol) or [8-3H]GCV (specific activity, 18 Ci/mmol) was added at 5 or 1 μCi/4 ml, respectively. At 48 h postinfection, cultures were washed three times with cold phosphate-buffered saline and trypsinized. After centrifugation, the cell pellets were extracted with 70% ice-cold methanol and were left on ice for 10 min. After centrifugation at 10,000 rpm (Minifuge T; Hereaus), the supernatants were filtered and the metabolites were quantitated by high-pressure liquid chromatography analysis with a Partisil-sphere radial compression column (Pharmacia). Alternatively, intracellular nucleotide pools were determined in cells that had not been radiolabelled.
Intracutaneous HSV-1 or TK− HSV-2 infections in mice.
Hairless mice were inoculated intracutaneously at the lumbrosacral area (by scratching the skin with a scarifier) with HSV-1 KOS at 104 PFU per 0.05 ml per mouse. The mice were then treated for 5 days, starting at 2 h after the infection. Test compounds were applied topically twice a day at the indicated concentrations in dimethyl sulfoxide in a volume of 0.05 ml over an area of 1.5 cm2. Mice were monitored daily for the development of herpetic skin lesions and mortality.
Athymic nude (nu/nu) mice (Charles River Breeding, Sulzfeld, Germany) were infected in a similar fashion with TK− HSV-2 at 104 PFU/0.05 ml. Test compounds were applied topically, twice daily starting 2 h after infection for 23 consecutive days or in a second type of experiment for three periods of 5 consecutive days (with a 2-day break after each period). Lesions were scored daily (blind) on a scale of from 0 to 4 with increments of 0.5.
In vivo toxicity of the combination MMF plus GCV.
NMRI mice (weight, 13 g) were treated for 11 consecutive days with 200 mg of GCV (subcutaneous injection of a 200-μl volume in phosphate-buffered saline), per kg of body weight per day, 200 mg of MMF (0.5-ml gavage in a solution containing 0.9% benzyl alcohol, 0.4% polysorbate 80, 0.9% sodium chloride, and 0.5% sodium carboxymethyl cellulose) per kg per day, or the combination of 200 mg of GCV per kg per day (administered subcutaneously) and 200 mg of MMF per kg per day (administered orally). Body weight was measured daily.
RESULTS
In vitro potentiation of the anti-HSV, anti-VZV, and anti-HCMV activity of ACV, GCV, or PCV by MPA or MMF.
The effect of the combination of MPA with either ACV, GCV, or PCV was studied in Vero and HEL cells. Depending on the cell line used, marked differences in the antiviral activities of the molecules were observed; i.e., the EC50s were lower when the antiviral action was assayed in HEL cells than in Vero cells. Cell line-dependent variations in the antiviral activity of antiherpetic molecules has been reported previously (6). When MPA was used at concentrations of 0.25 to 10 μg/ml, which by themselves had little or no effect on the replication of HSV-1 and HSV-2 in HEL and Vero cells, MPA markedly increased the antiherpesvirus activities of ACV, GCV, and PCV (Table 1). For example, the EC50 of GCV for the inhibition of the HSV-1-induced CPE in Vero cells dropped when GCV was combined with MPA (at 1.0 μg/ml), from 1.0 to 0.028 μg/ml (30-fold). Similarly, and depending on the concentration of MPA used, the EC50s of ACV and PCV decreased by 20- to 100-fold following combination with MPA. A comparable or even a more pronounced enhancement of the antiviral potency was noted when ACV, GCV, or PCV was combined with MMF (Table 2). The combination ACV, GCV, or PCV with either MPA or MMF also had a marked synergistic effect on the replication of TK− HSV-1 (Tables 1 and 2). This was particularly striking for the combination of PCV with MMF or MPA; under these conditions, the EC50 of PCV fell from ≥100 μg/ml to 1 to 5 μg/ml and the EC50 of ACV dropped from 30 to 50 μg/ml to well below 1 μg/ml. Also, MPA markedly potentiated the antiviral effects of ACV and GCV against both wild-type and TK− VZV strains (Table 3).
TABLE 1.
Effect of MPA on the antiherpesvirus activity of GCV, ACV, or PCV in Vero and HEL cells
| Virus, cell | Antiviral agent | EC50 (μg/ml)a
|
||||||
|---|---|---|---|---|---|---|---|---|
| GCV alone | ACV alone | PCV alone | With MPA at 10 μg/ml | With MPA at 2.5 μg/ml | With MPA at 1.0 μg/ml | With MPA at 0.25 μg/ml | ||
| HSV-1, Vero | GCV | 1.06 ± 0.51 | 0.014 ± 0.005 | 0.017 ± 0.003 | 0.028 ± 0.008 | |||
| ACV | 5.32 ± 2.60 | 0.072 ± 0.012 | 0.100 ± 0.022 | 0.137 ± 0.002 | ||||
| PCV | 6.67 ± 2.22 | 0.45 ± 0.36 | 0.50 ± 0.33 | 0.60 ± 0.27 | 3.89 ± 3.18 | |||
| HSV-1, HEL | GCV | 0.0061 ± 0.0020 | 0.0005 ± 0.0004 | 0.0018 ± 0.0013 | 0.0012 ± 0.0008 | 0.0035 ± 0.0025 | ||
| ACV | 0.067 ± 0.017 | 0.0135 ± 0.0085 | 0.0125 ± 0.0025 | 0.0150 ± 0.007 | 0.0275 ± 0.0055 | |||
| PCV | 0.14 ± 0.08 | 0.0075 ± 0.0005 | 0.0053 ± 0.017 | 0.007 ± 0.000 | 0.048 ± 0.022 | |||
| HSV-2, Vero | GCV | 1.44 ± 0.68 | 0.047 ± 0.037 | 0.053 ± 0.046 | 0.045 ± 0.035 | 0.14 ± 0.11 | ||
| ACV | 2.6 ± 2.0 | 0.031 ± 0.0017 | 0.038 ± 0.030 | 0.12 ± 0.037 | 0.19 ± 0.14 | |||
| PCV | 5.3 ± 1.8 | 0.14 ± 0.07 | 0.67 ± 0.17 | 1.29 ± 0.87 | 2.9 ± 0.8 | |||
| HSV-2, HEL | GCV | 0.012 ± 0.006 | <0.0001 | ≤0.0001 | 0.0008 ± 0.0003 | 0.0064 ± 0.0057 | ||
| ACV | 0.050 ± 0.018 | 0.003 ± 0.001 | 0.003 ± 0.001 | 0.007 ± 0.003 | 0.015 ± 0.008 | |||
| PCV | 0.400 ± 0.111 | 0.009 ± 0.003 | 0.030 ± 0.032 | 0.029 ± 0.029 | 0.174 ± 0.028 | |||
| TK− HSV-1, Vero | GCV | 18.65 ± 3.70 | 0.25 ± 0.24 | 0.37 ± 0.33 | 0.61 ± 0.39 | 1.97 ± 0.26 | ||
| ACV | 56.50 ± 6.5 | 0.15 ± 0.14 | 0.27 ± 0.22 | 0.30 ± 0.20 | 1.29 ± 0.48 | |||
| PCV | >100 | 1.51 ± 1.41 | 2.61 ± 2.39 | 3.79 ± 3.28 | 6.00 ± 4.00 | |||
Data are mean values for at least three separate experiments. The EC50 of MPA alone is >10 μg/ml.
TABLE 2.
Effect of MMF on the antiherpesvirus activity of GCV, ACV, or PCV in Vero cells
| Virus | Antiviral agent | EC50 (μg/ml)a
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| GCV alone | ACV alone | PCV alone | With MMF at 50 μg/ml | With MMF at 25 μg/ml | With MMF at 10 μg/ml | With MMF at 2.5 μg/ml | With MMF at 1.0 μg/ml | ||
| HSV-1 | GCV | 0.53 ± 0.11 | 0.021 ± 0.011 | 0.014 ± 0.004 | 0.016 ± 0.006 | 0.019 ± 0.009 | 0.022 ± 0.000 | ||
| ACV | 2.45 ± 2.19 | 0.030 ± 0.020 | 0.030 ± 0.020 | 0.030 ± 0.020 | 0.056 ± 0.006 | 0.117 ± 0.033 | |||
| PCV | 6.2 ± 1.54 | 0.20 ± 0.07 | 0.24 ± 0.06 | 0.25 ± 0.07 | 0.37 ± 0.17 | 0.26 ± 0.11 | |||
| HSV-2 | GCV | 0.51 ± 0.38 | 0.002 ± 0.001 | 0.006 ± 0.003 | 0.010 ± 0.000 | 0.010 ± 0.002 | 0.076 ± 0.005 | ||
| ACV | 1.30 ± 0.71 | 0.010 ± 0.000 | 0.033 ± 0.016 | 0.055 ± 0.026 | 0.085 ± 0.015 | 0.201 ± 0.011 | |||
| PCV | 5.62 ± 1.55 | 0.008 ± 0.001 | 0.038 ± 0.053 | 0.040 ± 0.016 | 0.160 ± 0.010 | 1.18 ± 0.52 | |||
| TK− HSV-1 | GCV | 10.64 ± 3.73 | 0.46 ± 0.24 | 0.46 ± 0.24 | 0.43 ± 0.26 | 0.66 ± 0.33 | 0.79 ± 0.00 | ||
| ACV | 32.49 ± 4.74 | 0.13 ± 0.03 | 0.30 ± 0.20 | 0.36 ± 0.13 | 0.50 ± 0.00 | 0.75 ± 0.25 | |||
| PCV | ≥100 | 1.4 ± 0.7 | 1.8 ± 0.8 | 2.1 ± 1.4 | 3.5 ± 1.6 | 4.9 ± 1.1 | |||
Data are mean values for at least three separate experiments. The EC50 of MMF alone is >50 μg/ml.
TABLE 3.
Effect of MPA on the anti-VZV (wild-type and TK− strains) activities of ACV and GCV
| Virus strain | Antiviral agent | EC50 (μg/ml)a
|
||||
|---|---|---|---|---|---|---|
| GCV alone | ACV alone | With MPA at 10 μg/ml | With MPA at 2.5 μg/ml | With MPA at 1.0 μg/ml | ||
| OKA (TK+) | GCV | 0.86 ± 0.89 | 0.09 ± 0.09 | 0.11 ± 0.12 | 0.11 ± 0.12 | |
| ACV | 0.45 ± 0.07 | 0.06 ± 0.04 | 0.05 ± 0.03 | 0.08 ± 0.02 | ||
| YS-R (TK−) | GCV | 27 ± 31 | 1.5 ± 1.1 | 1.6 ± 1.0 | 1.4 ± 1.4 | |
| ACV | 27 ± 20 | 0.8 ± 0.6 | 1.5 ± 0.7 | 0.55 ± 0.07 | ||
Data are mean values for two separate experiments. The EC50 of MPA alone is >10 μg/ml.
Although MPA and MMF had by themselves little or no effect on the development of a HSV-1-, HSV-2-, or TK− HSV-1-induced CPE, we determined the effects of these compounds on the yield of progeny virus. Both MPA and MMF caused a 2- to 10-fold reduction in virus yield when the compounds were added (at a concentration of 1 to 10 μg/ml [MPA] or 1 to 50 μg/ml [MMF]) to HSV-1-, HSV-2-, or TK− HSV-1-infected Vero cell cultures (data not shown).
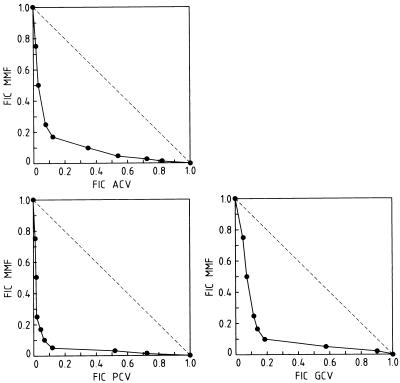
Interestingly, MPA and MMF as such exhibited some anti-HCMV activity (EC50s, ∼10 μg/ml). Furthermore, MPA and MMF with GCV, ACV, and PCV proved to have clearly synergistic activity against HCMV. The anti-HCMV activities of combinations of MMF with GCV, ACV, and PCV are depicted in Fig. 1. A marked synergistic activity was observed: the minimum FIC indices were 0.29, 0.29, and 0.17 for the combinations ACV and MMF, GCV and MMF, and PCV and MMF, respectively. For example, at an MMF concentration of 2.5 μg/ml (which alone had little or no effect on HCMV replication), the EC50 of ACV for the inhibition of HCMV replication decreased from 50 to 1 μg/ml and the EC50 of PCV decreased from >50 to 0.5 μg/ml.
FIG. 1.
Synergistic anti-HCMV activity of MMF with GCV, ACV, or PCV. Data are mean values for two to three separate experiments.
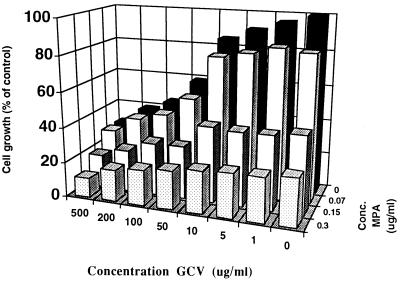
Effect of the combination ACV or GCV and MPA on the growth of CEM cells.
We evaluated the cytostatic action of the combination GCV with MPA on the growth of CEM cells. CEM cells were chosen because they are more susceptible than, for example, Vero cells to the cytostatic action of MPA. Under the conditions used no marked potentiation of the cytostatic effect of GCV by MPA was observed (Fig. 2).
FIG. 2.
Effect of the combination GCV plus MPA on the growth of CEM cells.
Metabolism of ACV and GCV in HSV-1-infected Vero cells in the absence or presence of MPA.
Since (i) ACV and GCV can be phosphorylated by cytoplasmic 5′-nucleotidase and (ii) IMP serves as the phosphate donor in this reaction, the expanded IMP pool in MPA-treated cells may facilitate the phosphorylation of ACV and GCV. We therefore studied the phosphorylation of ACV and GCV in HSV-1-infected Vero cells that were either incubated or not incubated with MPA at 10 μg/ml (Table 4). No increase in the phosphorylation of either ACV or GCV was observed in the MPA-treated cultures. Our findings indicate that the increased activities of combinations of ACV or GCV with MPA did not result from a higher velocity of the 5′-nucleotidase-catalyzed phosphorylation of ACV or GCV.
TABLE 4.
Effect of MPA on the phosphorylation of ACV or GCV in HSV-1-infected Vero cells
| Amt of MPA | Amt of metabolite (pmol/106 cells)a
|
|||||
|---|---|---|---|---|---|---|
| ACV-MP | GCV-MP | ACV-DP | GCV-DP | ACV-TP | GCV-TP | |
| None | 0.16 | 0.08 | 0.25 | 0.08 | 1.09 | 0.3 |
| 10 μg/ml | 0.12 | 0.08 | 0.18 | 0.07 | 0.87 | 0.2 |
Data from a representative experiment are shown. MP, monophosphate; DP, diphosphate; TP, triphosphate.
Depletion of dGTP pools.
The effect of MPA treatment on the intracellular nucleotide pools of either mock- or HSV-1-infected cells was studied. MPA (at 50 μg/ml) resulted in an 85% reduction in GTP pools in HSV-1-infected Vero cells (data not shown). The addition of exogenous guanosine reversed the potentiating effect of MPA on the anti-HSV-1 activities of ACV, PCV, and GCV (Table 5).
TABLE 5.
Effect of exogenously added guanosine on the potentiating effect of MPA on the anti-HSV-1 activity of ACV, GCV, or PCV in Vero cells
| Anti- viral agent | EC50 (μg/ml)a
|
||||
|---|---|---|---|---|---|
| GCV alone | ACV alone | PCV alone | With MPA at 1.0 μg/ml | With MPA at 1.0 μg/ml + guanosine at 25 μg/ml | |
| GCV | 0.49 ± 0.19 | 0.015 ± 0.007 | 0.64 ± 0.07 | ||
| ACV | 4.15 ± 4.0 | 0.09 ± 0.06 | 1.2 ± 0.2 | ||
| PCV | 2.9 ± 0.3 | 0.14 ± 0.0 | 3.15 ± 0.6 | ||
The EC50s for inhibition of HSV-1-induced CPE by MPA and guanosine were >25 μg/ml and >100 μg/ml, respectively.
MMF potentiates the anti-HSV-1 and anti-TK− HSV-2 activity of ACV in intracutaneously infected mice.
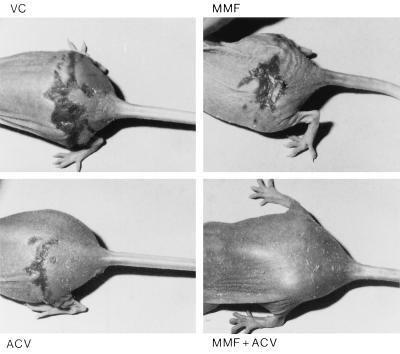
Hairless mice were inoculated intracutaneously on the back with HSV-1 KOS (Table 6). The animals were treated two times daily for a period of 5 consecutive days, starting 2 h after infection, with either placebo (dimethyl sulfoxide), a 0.1% ACV ointment (a concentration that does not cause protection), a 5% MMF ointment, or the combination of 0.1% ACV plus 5% MMF ointment. There was no effect on overall survival in the group receiving 0.1% ACV and the group receiving 5% MMF, although some minor delay in the mean day of death was observed. Those animals that received the combined treatment were almost completely protected against infection and the associated mortality. Also in this group, no signs of toxicity of MMF or local irritation from treatment with MMF were observed, and the infected area healed fast (Fig. 3).
TABLE 6.
Effect of topical treatment with ACV (0.1%) or MMF (5%), or both, on intracutaneous HSV-1 lesions and mortality in hairless mice
| Treatment | MDLAa | MDDa,b | No. of mice with lesions/total no. of mice | Mortality (no. of dead mice/total no. of mice) |
|---|---|---|---|---|
| Control | 5.2 ± 0.4 | 7.4 ± 0.7 | 9/9 | 9/9 |
| 5% MMF | 6.6 ± 2.0c | 9.7 ± 2.6d | 9/9c | 9/9c |
| 0.1% ACV | 5.5 ± 0.5c | 8.8 ± 1.5d | 8/9c | 8/9c |
| 5% MMF + 0.1% ACV | 7.0 | 14 | 1/9e | 1/9e |
Data are mean values for two independent experiments with a total of nine mice for each experimental condition.
MDD, mean day of death.
Not significant.
P < 0.05.
P < 0.001.
FIG. 3.
HSV-1-induced skin lesions in hairless mice treated topically with placebo (VC), 5% MMF, 0.1% ACV, or the combination 0.1% ACV plus 5% MMF.
The combination ACV plus MMF protects against a cutaneous ACV-refractory HSV-2 infection in nude mice.
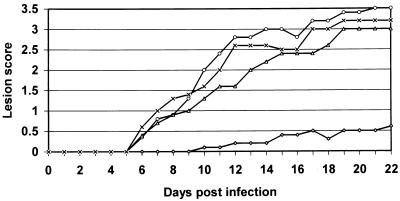
Athymic nu/nu mice were infected intracutaneously with TK− HSV-2 (Fig. 4). Animals received either placebo ointment, a 5% ACV ointment, a 5% MMF ointment, a 5% ACV plus 5% MMF ointment starting 2 h after infection twice daily for 23 consecutive days. Neither the 5% MMF ointment nor the 5% ACV ointment affected the appearance of the lesions (mean days of lesion appearance [MDLAs], 7.0 ± 0.0 [5% MMF], 6.8 ± 0.4 [5% ACV], and 6.6 ± 0.5 [placebo]) or the severity of the lesions (mean lesion scores [MLSs], 2.3 [5% MMF], 1.9 [5% ACV], and 2.2 [placebo]). However, in mice that were treated with the 5% ACV ointment plus 5% MMF ointment, lesions appeared significantly later compared to the time of appearance of lesions in mice treated with placebo (MDLA, 10.6 ± 2.8 [P < 0.05]) and remained very small (MLS, 0.25; P <1 × 10−7 for the difference in MLS between mice treated with ACV plus MMF versus mice treated with placebo alone and P < 5.0 × 10−6 for the difference in MLS between mice treated with ACV plus MMF versus mice treated with ACV alone). In a second experiment (data not shown), the infected mice (10 per condition) were treated for three periods of 5 consecutive days (with a 2-day weekend break after the first two treatment periods). Also under this condition, neither the 5% MMF ointment nor the 5% ACV ointment had an effect on the onset of the appearance of the lesions (MDLAs, 5.5 ± 0.8 [5% MMF] and 6.3 ± 1.9 [5% ACV] compared to the control [MDLA, 5.8 ± 1.7]) or the severity of the lesions (MLSs, 2.4 [5% MMF], 2.2 [5% ACV], and 2.7 [placebo]). In mice treated with the 5% ACV ointment plus 5% MMF ointment, lesions appeared significantly later (MDLA, 10 ± 2.6 [P < 0.005]) and were much smaller than those in the three other groups (MLS, 1.1 [P < 10−7 compared to the placebo group]).
FIG. 4.
Effect of the combination MMF plus ACV on the development of skin lesions in athymic nude mice infected intracutaneously with TK− HSV-2. Animals were treated topically twice a day (starting 2 h after infection) for 23 consecutive days with placebo (×) (n = 5), 5% ACV (Δ) (n = 5), 5% MMF (○) (n = 5), and 5% MMF plus 5% ACV (◊) (n = 5).
Effect of combined systemic treatment with GCV and MMF.
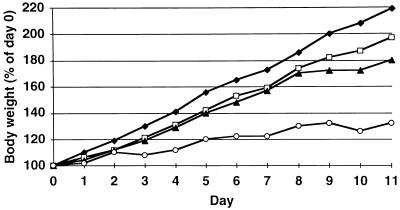
To study whether MMF can potentiate the in vivo toxicity of GCV, NMRI mice (weight, 13 g) were treated for 11 consecutive days with both GCV (given subcutaneously) at 200 mg/kg/day and MMF (given perorally) at 200 mg/kg/day (Fig. 5). MMF potentiated to some extent the inhibitory effect of GCV on the growth of these animals. However, after treatment was stopped, growth rapidly resumed (data not shown).
FIG. 5.
Effect of treatment with GCV (200 mg/kg/day; subcutaneously) (▴), MMF (200 mg/kg/day; perorally) (□), the combination MMF plus GCV (each at 200 mg/kg/day) (○), or carrier only (⧫) on the growth of young NMRI mice during treatment for 11 consecutive days.
DISCUSSION
We have found that the immunosuppressive agent MPA and its oral prodrug MMF markedly enhance the activities of ACV, GCV, and PCV against HSV-1, HSV-2, TK−, HSV, and VZV, while by themselves MPA and MMF do not substantially inhibit these viruses. Interestingly, MMF by itself has some inhibitory effect on the replication of HCMV. Again, when combined with GCV, ACV, or PCV, it strongly potentiates the activities of these compounds against HCMV. Of special interest is that the EC50s of the antiviral agents (especially ACV and PCV) for inhibition of replication of a TK− HSV-1 strain or HCMV (which can be considered a TK− virus) dropped from concentrations that are not attainable in plasma (>100 μg/ml for PCV and 30 to 50 μg/ml for ACV) to concentrations that can easily be reached in the plasma. For example, when combined with MPA, the EC50 of ACV for inhibition of HCMV dropped to values well below 1 μg/ml. The concentrations required for MPA to potentiate the antiviral activities of ACV, GCV, and PCV are not more than 1 μg/ml, that is, concentrations that can easily be reached in human plasma upon oral dosing with 1.5 to 3 g of MMF (7).
From a biochemical viewpoint the potentiating effect of MPA may result from two mechanisms. First, MPA might enhance the intracellular phosphorylation of ACV and GCV. Both ACV and GCV can be phosphorylated to the corresponding monophosphates by 5′-nucleotidase (15), for which IMP is an efficient phosphate donor. The relatively poor affinity of IMP for the enzyme (Km, 3 mM) would make the enzyme undersaturated under normal conditions, but because IMP pools may be expected to rise in MPA-treated cells, the enzyme may become progressively saturated, thus acquiring a higher velocity. However, we did not observe any substantial increase in the phosphorylation of ACV or GCV in MPA-treated HSV-1-infected cultures. Thus, an increase in 5′-nucleotidase-catalyzed phosphorylation of ACV or GCV may not be the mechanism by which MPA potentiates the antiviral activities of these antiviral agents. Ribavirin has been shown to potentiate the anti-HIV activity of 2′,3′-dideoxyinosine (ddI) and other purine 2′,3′-dideoxynucleosides (3, 5, 14). This potentiating effect has mainly been ascribed to the increased phosphorylation of ddI by 5′-nucleotidase in ribavirin-treated cells (4, 13).
Second, ACV, GCV, and PCV act, after their intracellular conversion to their triphosphates, as alternative substrate inhibitors of the viral DNA polymerase and compete with the natural substrate dGTP. Therefore, depletion of the endogenous dGTP pools may favor the inhibitory effects of the acyclic nucleoside triphosphates on the enzyme. Indeed, we found that in the HSV-1-infected cells, MPA causes a substantial decrease in the intracellular pools of GTP. Furthermore, we demonstrated that the potentiating effect of MPA on the antiviral activities of ACV, GCV, and PCV was reversed upon the addition of guanosine. Therefore, the synergistic action observed between MPA and the acyclic nucleoside analogs must be due to a depletion of the intracellular pools of the guanosine nucleotides. Potentiation of the antiviral activity of ACV, GCV, or PCV against TK− herpesviruses implies that at least traces of the monophosphates of ACV, GCV, and PCV (and, thus, also traces of their respective triphosphates) are formed in cells infected with these viruses. In cells with normal levels of intracellular dGTP the traces of the triphosphates of ACV, GCV, and PCV that are generated may not be sufficiently high to result in antiviral activity. However, in cells in which the intracellular dGTP pools are depleted (by MPA), the levels of the triphosphate forms of these drugs may be sufficient to result in inhibition of the viral DNA polymerase activity. Phosphorylation of trace amounts of ACV, GCV, and PCV may be accomplished by (i) the residual activity of the viral TK encoded by ACV-resistant strains (22), (ii) cellular thymidine kinase(s), and/or (iii) 5′-nucleotidase (although the last possibility can virtually be ruled out).
The observations that we made in vitro also held in vivo in mice with HSV infections. Topical treatment with the combination 5% MMF plus 0.1% ACV proved to be highly protective against intracutaneous HSV-1 infections in hairless mice, whereas treatment with 5% MMF or 0.1% ACV (a subactive concentration) alone caused virtually no protective effect. Of special interest is our observation that the combined use of MMF (5%) and ACV (5%) is highly effective in protecting against a cutaneous infection with a TK− clinical HSV-2 strain that proved to be refractory to therapy with a 5% ACV ointment. Foscarnet is the drug of choice for the treatment of ACV-resistant HSV or VZV strains (2). However, resistance to foscarnet associated with a lack of clinical response has also been reported. Topical cidofovir can also be recommended for the treatment of ACV-resistant cutaneous or muco-cutaneous HSV-1 or HSV-2 infections (2, 20). The data from the present study suggest that a cream of ACV containing MMF may possibly serve as an alternative for the topical treatment of ACV-refractory cutaneous or mucocutaneous HSV or VZV lesions, although patients should also receive systemic antiviral therapy to prevent dissemination of the infection. Topical use of MMF may be expected to be well tolerated since we observed no signs of irritation or toxicity when the 5% MMF gel was applied to the scarified mouse skin (even after 23 days of treatment).
Transplant recipients under MMF treatment, compared to those receiving azathioprine, have a slightly increased risk of acquiring HCMV viremia (12), most likely because of the profound immunosuppressive action of MMF. However, treatment with the combination MMF and GCV is likely to cause a more pronounced inhibitory effect on the replication of the virus than if GCV is used alone. Use of MMF may thus be a double-edged sword. On the one hand, it may precipitate the reactivation of opportunistic herpesviruses (in particular, HCMV) in transplant recipients. On the other hand, once the patient receives GCV therapy for this infection, the synergistic action between the two compounds could possibly compensate for the increased risk of HCMV reactivation. Although we did not observe any stimulation of the cytostatic effect of GCV on growing T lymphocytes (CEM cells) by MPA, MMF potentiated to some extent the growth retardation induced by GCV in young mice. Therefore, we suggest that the potentially increased adverse effects of GCV in patients receiving MMF for immunosuppression and GCV for the treatment of HCMV infections be carefully monitored.
Also, prophylactic use of ACV for the prevention of HCMV infections in transplant patients has received considerable attention (16, 18, 24). Since MMF potentiates in vitro the anti-HCMV activity of ACV, it would be of interest to assess whether MMF also enhances the anti-HCMV activity of ACV in this cohort.
In conclusion, MMF is a potent enhancer of the antiherpesvirus activity of the acyclic purine nucleoside analogs ACV, GCV, and PCV. This potentiating effect has been demonstrated in vitro for HSV-1, HSV-2, TK− HSV-1, VZV, and HCMV infections and in vivo for HSV-1 and TK− HSV-2 infections. It would be of interest to evaluate the combined use of MMF and the acyclic nucleoside analogs as therapy and/or prophylaxis for herpesvirus (i.e., HSV, HCMV, and VZV) infections following organ transplantation and to monitor carefully a possible increase in the adverse effects of the antiviral agents, particularly GCV. Furthermore, topical therapy with ACV and MMF combined could serve as an alternative for the treatment of ACV-resistant cutaneous and mucocutaneous HSV lesions.
ACKNOWLEDGMENTS
This research was supported by the “Fonds voor Geneeskundig Wetenschappelijk Onderzoek” (grant 3.0180.95) and Geconcerteerde Onderzoeksacties (Ministerie van Onderwijs, Vlaamse Gemeenschap) (project 95/5).
We thank Miette Stuyck and Willy Zeegers for excellent technical assistance and Christiane Callebaut, Inge Aerts, and Dominique Brabants for dedicated editorial help. We are indebted to M. Waer for critically reading the manuscript. J. Neyts is a postdoctoral research assistant from the “Fonds voor Wetenschappelijk Onderzoek-Vlaanderen.”
REFERENCES
- 1.Baba M, Pauwels R, Balzarini J, Herdewijn P, De Clercq E, Desmyter J. Ribavirin antagonizes inhibitory effects of pyrimidine 2′,3′-dideoxynucleosides but enhances inhibitory effects of purine 2′,3′-dideoxynucleosides on replication of human immunodeficiency virus in vitro. Antimicrob Agents Chemother. 1987;31:1613–1617. doi: 10.1128/aac.31.10.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balfour H H, Jr, Benson C, Braun J, Cassens B, Erice A, Friedman-Kien A, Klein T, Polsky B, Safrin S. Management of acyclovir-resistant herpes simplex and varicella-zoster virus infections. J Acquired Immune Defic Syndr. 1994;7:254–260. [PubMed] [Google Scholar]
- 3.Balzarini J, Lee C-K, Schols D, De Clercq E. 1-α-d-Ribofuranosyl-1,2,4-triazole-3-carboxamide (ribavirin) and 5-ethynyl-1-α-d-ribofuranosylimidazole-4-carboxamide (EICAR) markedly potentiate the inhibitory effect of 2′,3′-dideoxyinosine on human immunodeficiency virus in peripheral blood lymphocytes. Biochem Biophys Res Commun. 1991;178:563–569. doi: 10.1016/0006-291x(91)90145-w. [DOI] [PubMed] [Google Scholar]
- 4.Balzarini J, Lee C-K, Herdewijn P, De Clercq E. Mechanism of the potentiating effect of ribavirin on the activity of 2′,3′-dideoxyinosine against human immunodeficiency virus. J Biol Chem. 1991;266:21509–21514. [PubMed] [Google Scholar]
- 5.Balzarini J, Naesens L, Robins M, De Clercq E. Potentiating effect of ribavirin on the in vitro and in vivo antiretrovirus activities of 2′,3′-dideoxyinosine and 2′,3′-dideoxy-2,6-diaminopurine riboside. J Acquired Immune Defic Syndr. 1990;3:1140–1147. [PubMed] [Google Scholar]
- 6.Boyd, M. R., S. Safrin, and E. R. Kern. 1993. Penciclovir: a review of its spectrum of activity, selectivity, and cross-resistance pattern. Antiviri. Chem. Chemother. 4(Suppl. 1):3–11.
- 7.Bullingham R, Monroe S, Nicholls A, Hale M. Pharmacokinetics and bioavailability of mycophenolate mofetil in healthy subjects after single-dose oral and intravenous administration. J Clin Pharmacol. 1996;36:315–324. doi: 10.1002/j.1552-4604.1996.tb04207.x. [DOI] [PubMed] [Google Scholar]
- 8.De Clercq E. Trends in the development of new antiviral agents for the chemotherapy of infections caused by herpesviruses and retroviruses. Rev Med Virol. 1995;5:149–164. [Google Scholar]
- 9.De Clercq E, Descamps J, Verhelst G, Walker R T, Jones A S, Torrence P F, Shugar D. Comparative efficacy of different antiherpes drugs against different strains of herpes simplex virus. J Infect Dis. 1980;141:563–574. doi: 10.1093/infdis/141.5.563. [DOI] [PubMed] [Google Scholar]
- 10.Field H J, Tewari D, Sutton D, Thackray A M. Comparison of efficacies of famciclovir and valaciclovir against herpes simplex virus type 1 in a murine immunosuppression model. Antimicrob Agents Chemother. 1995;39:1114–1119. doi: 10.1128/aac.39.5.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Field H J, Thackray A M. The effects of delayed-onset chemotherapy using famciclovir or valaciclovir in a murine immunosuppression model for HSV-1. Antivir Chem Chemother. 1995;6:210–216. [Google Scholar]
- 12.Fulton B, Markham A. Mycophenolate mofetil. A review of its pharmacodynamic and pharmacokinetic properties and clinical efficacy in renal transplantation. Drugs. 1996;51:278–298. doi: 10.2165/00003495-199651020-00007. [DOI] [PubMed] [Google Scholar]
- 13.Hartman N R, Ahluwalia G S, Cooney D A, Mitsuya H, Kageyama S, Fridland A, Broder S, Johns D G D A. Inhibitors of IMP dehydrogenase stimulate the phosphorylation of the anti-human immunodeficiency virus nucleosides 2′,3′-dideoxyadenosine and 2′,3′-dideoxyinosine. Mol Pharmacol. 1991;40:118–124. [PubMed] [Google Scholar]
- 14.Johns D G, Ahluwalia G S, Cooney D A, Mitsuya H, Driscoll J S. Enhanced stimulation by ribavirin of the 5′-phosphorylation and anti-human immunodeficiency virus activity of purine 2′-β-fluoro-2′,3′-dideoxynucleosides. Mol Pharmacol. 1993;44:519–523. [PubMed] [Google Scholar]
- 15.Keller P M, McKee S A, Fyfe J A. Cytoplasmic 5′-nucleotidase catalyzes acyclovir phosphorylation. J Biol Chem. 1985;260:8664–8667. [PubMed] [Google Scholar]
- 16.Kletzmayr J, Kotzmann H, Popow-Kraupp T, Kovarik J, Klauser R. Impact of high-dose oral acyclovir prophylaxis on cytomegalovirus (CMV) disease in CMV high-risk renal transplant recipients. J Am Soc Nephrol. 1996;7:325–330. doi: 10.1681/ASN.V72325. [DOI] [PubMed] [Google Scholar]
- 17.Lake, K. D., and D. M. Canafax. 1995. Important interactions of drugs with immunosuppressive agents used in transplant recipients. J. Antimicrob. Chemother. 36(Suppl. B):11–22. [DOI] [PubMed]
- 18.Patel R. Cytomegalovirus prophylaxis in solid organ transplant recipients. Transplantation. 1996;61:1279–1289. doi: 10.1097/00007890-199605150-00001. [DOI] [PubMed] [Google Scholar]
- 19.Ransom J T. Mechanism of action of mycophenolate mofetil. Ther Drug Monit. 1995;17:681–684. doi: 10.1097/00007691-199512000-00023. [DOI] [PubMed] [Google Scholar]
- 20.Safrin S, Elbeik T, Phan L, Robinson D, Rush J, Elbaggari A, Mills J. Correlation between response to acyclovir and foscarnet therapy and in vitro susceptibility result for isolates of herpes simplex virus from human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 1994;38:1246–1250. doi: 10.1128/aac.38.6.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snoeck R, Andrei G, De Clercq E, Gerard M, Clumeck N, Tricot G, Sadzot-Delvaux C. A new topical treatment for resistant herpes simplex infections. N Engl J Med. 1993;329:968–969. doi: 10.1056/NEJM199309233291317. [DOI] [PubMed] [Google Scholar]
- 22.Snoeck R, Andrei G, Gérard M, Silverman A, Hedderman A, Balzarini J, Sadzot-Delvaux C, Tricot G, Clumeck N, De Clercq E. Successful treatment of progressive mucocutaneous infection due to acyclovir-and foscarnet-resistant herpes simplex virus with (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine (HPMPC) Clin Infect Dis. 1994;18:570–578. doi: 10.1093/clinids/18.4.570. [DOI] [PubMed] [Google Scholar]
- 23.Suthanthiran M, Morris R E, Strom T B. Immunosuppressants: cellular and molecular mechanisms of action. Am J Kidney Dis. 1996;28:159–172. doi: 10.1016/s0272-6386(96)90297-8. [DOI] [PubMed] [Google Scholar]
- 24.Zaia J A. Prophylaxis and treatment of CMV infections in transplantation. Adv Exp Med Biol. 1996;394:117–134. doi: 10.1007/978-1-4757-9209-6_13. [DOI] [PubMed] [Google Scholar]