Abstract
Purpose of review
Renal osteodystrophy (ROD) is a complex disorder of bone metabolism that affects virtually all adults and children with chronic kidney disease (CKD). ROD is associated with adverse clinical outcomes including bone loss, mineralization and turnover abnormalities, skeletal deformities, fractures, cardiovascular events, and death. Despite current therapies, fracture incidence is 2- to 100-fold higher in adults and 2- to 3-fold higher in children with compared to without CKD. Limited knowledge of ROD pathogenesis, due to the lack of patient-derived large-scale multi-modal datasets, impedes development of therapeutics aimed at reducing morbidity and mortality of CKD patients. The purpose of the review is to define the much needed infrastructure for the advancement of RDO treatment.
Recent findings
Recently, we created a large-scale data and tissue biorepository integrating clinical, bone quality, transcriptomic and epigenomic data along with stored urine, blood and bone samples. This database will provide the underpinnings for future research endeavors leading to the elucidation and characterization of the pathogenesis of ROD in CKD patients with and without dialysis.
Summary
The availability of an open-access NIH-funded resource that shares bone-tissue based information obtained from patients with ROD with the broad scientific community represents a critical step in the process of discovering new information regarding unrecognized bone changes that have severe clinical complications. This will facilitate future high-impact hypothesis-driven research to redefine our understanding of ROD pathogenesis and pathophysiology and inform the development of disease modifying and prevention strategies
Keywords: chronic kidney disease, biorepository, bone disease, histomorphometry, bone transcriptomics
Introduction
More than one in ten Americans have chronic kidney disease (CKD)1. Renal osteodystrophy (ROD), a complex disorder of bone metabolism, affects virtually all adult and pediatric patients with CKD during their lifetimes2–6. ROD is associated with adverse clinical outcomes including bone loss 7, mineralization and turnover abnormalities5, skeletal deformities, fractures8–13, cardiovascular events14–16 and death17. Despite current therapies, in adults with CKD fracture incidence of the appendicular skeleton more than doubled from 1992 to 200918 and is 2- to 100-fold higher compared to age-, sex-matched adults without CKD. Similarly, despite treatment of CKD-MBD, > 50% of adults who developed CKD in childhood reported skeletal symptoms, with 20% reporting handicaps from their bone disease19 and fracture rates are 2- to 3-fold higher in children with compared to without CKD20. Dearth of knowledge regarding ROD pathogenesis impedes development of novel therapeutics aimed at reducing morbidity and mortality of CKD patients. This knowledge gap is primarily due to the lack of patient-derived large-scale multi-modal datasets that permit elucidation of ROD pathogenesis.
We have recently proposed to close this knowledge gap by creating the fundamental infrastructure to facilitate high-impact novel hypothesis-driven clinical and translational research in ROD. Indeed, a large-scale data and tissue biorepository integrating clinical, bone histomorphometric and quality, and transcriptomic data along with stored urine and blood will be created by a multi-disciplinary team of investigators with the use of state-of-the art methods in biomarkers, bone tissue-level materials science, cell biology, transcriptomics and systems biology. The resource creates capacity for research with different hypotheses and methodologies to work from a common empirical base; thus, mitigating key roadblocks that hinder major scientific discovery in ROD (e.g., cohort differences due to small sample sizes). We assume that establishment of this comprehensive resource will provide the underpinnings for high-impact future research endeavors leading to the elucidation and characterization of the pathogenesis of ROD in patients across the spectrum of kidney disease and the development of new therapies. Establishment of the proposed repository and analytical infrastructure will lay the foundation for ground-breaking discovery and the development of novel precision medicine approaches for treating ROD. The availability of an NIH-funded resource that freely shares bone-tissue based information obtained from patients with ROD with the broad scientific community represents a critical step in the process of discovering new information regarding unrecognized bone changes that have severe clinical complications.
ROD: something old
Chronic kidney disease (CKD) affects over 37 million individuals in the United States (US), and 752 million individuals worldwide (CDC.gov/kidneydisease)21. CKD grade 5 requiring dialysis or transplantation affects over 700,000 Americans. ROD, a systemic disorder of bone structure, metabolism, and cellular function leading to compromised bone strength is present in all these patients as well as in a sizeable fraction of the 37 million in the US with CKD grades 1–4. ROD severity worsens with declining kidney function and it persists even after correction of kidney function with transplantation in adults and children.
The accurate diagnosis of ROD type (low or high turnover, and mineralization characteristics) requires bone biopsy. In the absence of bone biopsy, circulating biomarkers of bone turnover can be used to diagnose ROD type with reasonable accuracy22, 23. Skeletal imaging with dual energy X-ray absorptiometry (DXA) or high resolution peripheral quantitative computed tomography (HR-pQCT) do not inform the diagnosis of the ROD type22; however, they provide valuable insight into abnormalities of bone quality and strength that may be impaired by ROD. Clinical management of ROD involves treating the biochemical abnormalities of CKD-MBD, including calcium, phosphorus, vitamin D and parathyroid hormone (PTH). In extreme cases, parathyroidectomy may be necessary.
Our current understanding of ROD pathogenesis, which can manifest as a range of disorders, including bone loss, hyperparathyroid bone disease, abnormal bone mineralization, and adynamic bone disease, has not changed in over 50 years24 and is based on the perception that elevations in PTH that occur with declining kidney function and active vitamin D deficiency results in ROD. The Turnover, Mineralization, Volume (TMV) classification system used to define ROD-type25 and to “…. greatly enhance communication, facilitate clinical decision-making, and promote the evolution of evidence-based clinical practice guidelines worldwide” reflects that notion26. However, our rudimentary understanding of ROD pathobiology does not reflect either state-of-the art science in skeletal biology or our current understanding of bone-cell signaling27–30. Lack of progress in improving ROD clinical outcomes despite strategies that consider the TMV classification18 support the need for treatments that are based on state-of-the art science in ROD pathobiology.
Fractures are the ultimate outcome of ROD progression. Fractures are a debilitating injury with prolonged rehabilitation, long-term pain, and increased costs. Only 50% of patients with a hip fracture regain their mobility and remain at their pre-fracture level of independence31. As the population ages, predictions about increasing numbers of fractures are staggering: from 1.7 million fractures to 6.3 million fractures between 1990 and 205032. Studies in adults and pediatric patients with CKD grades 3–48, 11, 20, 33, 34, on dialysis35, 36 and after transplantation10, 37 all demonstrate that patients with CKD have a 2- to 100-fold increase in fracture risk compared to age- and sex-matched individuals without CKD. A 40-year-old woman on dialysis has a 100-fold increased risk of having a hip fracture compared to a 40 year-old in the general population. Even at age 80, the risk in both men and women on dialysis is more than 2-fold greater in patients with, compared to age and sex-matched individuals without CKD10. Moreover, despite current CKD-MBD treatment guidelines, fracture incidence of the appendicular skeleton more than doubled from 1992 to 2009 in patients with CKD grade 5 requiring dialysis 18. Similar findings have been shown in adults with onset of CKD in childhood19. Indeed, although early treatment of vitamin D deficiency was estimated to result in >$19 million and 191 quality-adjusted life year (QALY)’s saved, there was minimal fracture incidence reduction, suggesting that better strategies are needed for their prevention38. The scope of the problem is staggering considering that mortality risk increases 100% in patients on dialysis who sustain a major fracture requiring hospitalization39, 40 and healthcare associated costs after fracture in patients with CKD exceeded $600 million in 201017.
ROD: something missing
Research in ROD is hindered by five key barriers: (1) The complex and heterogenous phenotypes that characterize ROD (i.e., bone loss associated with low, normal, high turnover coexisting with vascular calcifications); (2) The effects of co-morbidities that are concurrent with CKD and that also affect the skeleton (e.g, diabetes, inflammatory and genetic diseases); (3) The clinical and logistical difficulties associated with the bone biopsy process; (4) The skillset and competencies to perform the histologic, histomorphometric, bone quality and transcriptome analyses of the obtained samples; and (5) The lack of free-access to large-scale clinical datasets that span the CKD continuum and that are dedicated to CKD-relevant abnormalities in bone.
ROD: something new
The underlying causes of ROD are complex, and both genetic and environmental factors contribute to its development. The genetic determinants of ROD are complex and involve multiple genes and genetic polymorphisms. Several genes have been identified that are associated with ROD, including those involved in vitamin D metabolism, calcium sensing, and bone remodeling. Indeed, mutations in the VDR gene often lead to impaired bone mineralization. Mutations in the PTH1R gene can lead to abnormal responses to PTH, resulting in changes in bone mineral metabolism and the development of ROD.41 Several genetic polymorphisms have been identified that are associated with an increased risk of developing ROD.42, 43
In addition to the genetic factors, the other molecular mechanisms driving bone disorders in CKD are complex and not yet fully understood. However, Next-Generation Sequencing (NGS) tools have allowed researchers to identify key genes and pathways involved in bone metabolism and mineralization in CKD patients with ROD.44, 45
“Bulk” RNA sequencing, also known as RNA-seq, is a valuable technique in studying bone disorders as it offers a comprehensive understanding of biological constituents, helps to identify novel genes, and unravels the interconnected signaling pathways involved in disease mechanisms. However, despite its advantages, the major limitation of “bulk” RNA-seq is the lack of definition of the specific cellular subsets and determination of gene expression differences at the individual cell level, thereby constraining our comprehension of the biological and pathological processes of these diseases.
Recent advances in transcriptomics now permit single cell and single nuclei analyzes of bone cells 46–48. State-of-the art methods in single cell (sc) and single nuclei (sn) RNA sequencing of bone will lead to major advances in our understanding of ROD pathogenesis and inform the development of targeted disease modifying and curative strategies. In parallel, the identification of open chromatin regions and the profiling of chromatin accessibility in individual cells using sc assay for transposase-accessible chromatin sequencing (scATAC) seq allows the study of the epigenetic changes occurring in bone cells in animal and patients with ROD. These new tools can uncover cellular heterogeneity and specific subclusters, can infer the trajectories of various cell states, transitions, and differentiation, might aid in the creation of heterogeneous cellular signaling models through enrichment analyses and might uncover regulatory networks among clusters. Only by identifying the genes and pathways involved in ROD, can we develop targeted therapies that address the underlying causes of the disease, rather than just treating the symptoms or the end-organ effects of disease, such as hyperparathyroidism induced high turnover or increased cortical porosity.49 These novel methods are only now being applied to bone50 and more recently to ROD45, and only a few studies have investigated transcriptomic and epigenetic changes in ROD. Not only do we need further research using RNA-seq in large patient cohorts to fully understand the complex molecular mechanisms underlying ROD and to develop effective treatments but the fundamental infrastructure to develop patient-centered clinically relevant research is lacking, impeding the development of strategies that will improve the lives of patients living with CKD.
ROD: something needed
The establishment of the necessary infrastructure for conducting innovative research on the mechanisms of ROD. To address this gap, we proposed the creation of a large-scale data and tissue biorepository that will integrate clinical, bone histomorphometric and bone quality, transcriptomic and epigenetic data, along with stored urine and blood. This resource, the ROD-Precision Medicine Project (ROD-PMP), will enable high-impact hypothesis-driven clinical and translational research in ROD and lay the foundation for ground-breaking discoveries and the development of novel precision medicine approaches for treating ROD (Figure 1). The establishment of this comprehensive resource is poised to provide a common empirical base for research with different hypotheses and methodologies, thereby mitigating the key roadblocks that have hindered major scientific discovery in ROD. The repository and analytical infrastructure will facilitate the elucidation and characterization of the pathogenesis of ROD in CKD patients with and without dialysis and will represent a critical step in the process of discovering new information about bone changes that have severe clinical complications. The availability of this NIH-funded resource, which freely shares bone-tissue and bone-tissue-based information obtained from patients with ROD with the broader scientific community, has the potential to drive significant advances in the understanding and treatment of ROD in CKD patients.
Figure 1: Conceptualization of ROD Precision Medicine Program.
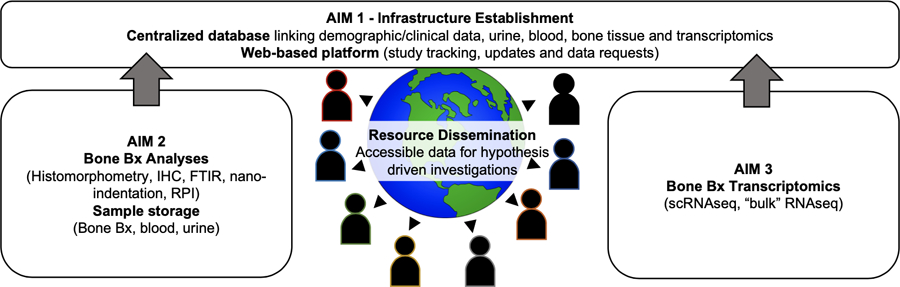
The proposal plans to create the fundamental infrastructure and build a centralized database integrating demographic and clinical bone histomorphometric and quality data, together with transcriptomic and stored urine and blood to facilitate high-impact novel hypothesis-driven clinical and translational research in ROD.
Aimed at advancing the understanding of ROD pathogenesis and developing targeted disease-modifying and curative strategies, the ROD-PMP aims to address the key barriers in ROD research and develop a bone-based infrastructure for clinical and translational research. The infrastructure includes a data and tissue biorepository, analytical infrastructure, and open-access data sharing. Designed to apply state-of-the-art methods in single-cell and single-nuclei RNA/ATAC sequencing of bone to ROD and link transcriptomic and epigenetic data to detailed clinical and bone histomorphometric and quality phenotypes, the proposed infrastructure will be available to the scientific community to conduct high-impact hypothesis-driven research on ROD.
Conclusion
The absence of large-scale datasets linking genomic architecture of bone cells to bone-tissue phenotypes, which correlate with bone strength, hinders the development of targeted strategies for preventing ROD-related morbidity and mortality. The proposed repository is not designed to test specific hypotheses but to create an evidence-base that future researchers can draw from and contribute to. Thus, the ROD-PMP offers promise to refine pathogenesis models, identify novel therapeutic targets and translate molecular findings into clinical applications for ROD. The establishment of this unique and critical resource will create new opportunities for scientific discoveries, and innovative and collaborative projects. We expect this resource will permit investigators to unlock knowledge gaps in the molecular pathogenesis of ROD, including circulating and local biomarkers of ROD stage, severity and subtype. These findings will ultimately translate into to the discovery of novel tailored bone targets for ROD diagnosis, monitoring, treatment and prevention.
KEY POINTS (3–5) (1 point is no more than 1 sentence).
A large comprehensive biorepository of urine, blood and bone tissue for phenotyping ROD from adults and children across the CKD continuum is lacking.
There is a lack of concomitant bone quality assessments of iliac crest bone biopsy samples by 2D and 3D histomorphometry, bone biomechanics by nanoindentation, bone protein assessment by immunohistochemistry, intrinsic bone material properties by reference point indentation and Fourier Transform Infrared Spectroscopy.
There is a need to determine the changes in osseous transcriptome of patients with ROD at tissue and cellular levels, and integrate these data into a framework that will facilitate future high-impact hypothesis-driven research to redefine our understanding of ROD pathogenesis and pathophysiology.
Financial support and sponsorship
This study was supported by R56DK127986 grant from National Institute of Health to TLN, IBS, HM and VD.
Footnotes
Conflict of interest
VD received research funding from Akebia and from Vifor Pharma and consulting honoraria from Keryx Biopharmaceuticals, Vifor Pharma, Luitpold and Amgen outside of submitted work. IBS has received honoraria from Akebia, Inozyme, Ultragenyx, Amgen, Abbvie outside of submitted work. TLN reports consultancy agreements with Pharmacosmos; receiving research funding from Amgen; and consulting honoraria from Amgen and Pharmacosmos outside of submitted work.
References
- 1.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA : the journal of the American Medical Association 2007;298(17):2038–47. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Spasovski GB, Bervoets AR, Behets GJ, et al. Spectrum of renal bone disease in end-stage renal failure patients not yet on dialysis. Nephrol Dial Transplant 2003;18(6):1159–66. doi: 10.1093/ndt/gfg116. [DOI] [PubMed] [Google Scholar]
- 3.Hamdy NA, Kanis JA, Beneton MN, et al. Effect of alfacalcidol on natural course of renal bone disease in mild to moderate renal failure. BMJ 1995;310(6976):358–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coen G, Mazzaferro S, Bonucci E, et al. Bone GLA protein in predialysis chronic renal failure. Effects of 1,25(OH)2D3 administration in a long-term follow-up. Kidney Int 1985;28(5):783–90. [DOI] [PubMed] [Google Scholar]
- 5.Malluche HH, Mawad HW, Monier-Faugere MC. Renal osteodystrophy in the first decade of the new millennium: analysis of 630 bone biopsies in black and white patients. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 2011;26(6):1368–76. doi: 10.1002/jbmr.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behets GJ, Spasovski G, Sterling LR, et al. Bone histomorphometry before and after long-term treatment with cinacalcet in dialysis patients with secondary hyperparathyroidism. Kidney Int 2015;87(4):846–56. doi: 10.1038/ki.2014.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nickolas TL, Stein EM, Dworakowski E, et al. Rapid cortical bone loss in patients with chronic kidney disease. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 2013;28(8):1811–20. doi: 10.1002/jbmr.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nickolas TL, McMahon DJ, Shane E. Relationship between Moderate to Severe Kidney Disease and Hip Fracture in the United States. J AmSocNephrol 2006;17(11):3223–32. [DOI] [PubMed] [Google Scholar]
- 9.Fried LF, Biggs ML, Shlipak MG, et al. Association of kidney function with incident hip fracture in older adults. J AmSocNephrol 2007;18(1):282–6. [DOI] [PubMed] [Google Scholar]
- 10.Ball AM, Gillen DL, Sherrard D, et al. Risk of Hip Fracture Among Dialysis and Renal Transplant Recipients. JAMA: The Journal of the American Medical Association 2002;288(23):3014–8. [DOI] [PubMed] [Google Scholar]
- 11.Dooley AC, Weiss NS, Kestenbaum B. Increased risk of hip fracture among men with CKD. Am J Kidney Dis 2008;51(1):38–44. [DOI] [PubMed] [Google Scholar]
- 12.Naylor KL, McArthur E, Leslie WD, et al. The three-year incidence of fracture in chronic kidney disease. Kidney Int 2014. doi: 10.1038/ki.2013.547. [DOI] [PubMed]
- 13.Isakova T, Craven TE, Scialla JJ, et al. Action to Control Cardiovascular Risk in Diabetes T. Change in estimated glomerular filtration rate and fracture risk in the Action to Control Cardiovascular Risk in Diabetes Trial. Bone 2015;78:23–7. doi: 10.1016/j.bone.2015.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351(13):1296–305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 15.London GM, Marchais SJ, Guerin AP, Metivier F. Arteriosclerosis, vascular calcifications and cardiovascular disease in uremia. Current opinion in nephrology and hypertension 2005;14(6):525–31. [DOI] [PubMed] [Google Scholar]
- 16.London GM, Marchais SJ, Guerin AP, et al. Association of bone activity, calcium load, aortic stiffness, and calcifications in ESRD. Journal of the American Society of Nephrology : JASN 2008;19(9):1827–35. doi: 10.1681/asn.2007050622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim SM, Long J, Montez-Rath M, et al. Hip Fracture in Patients with Non-Dialysis-Requiring Chronic Kidney Disease. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 2016. doi: 10.1002/jbmr.2862. [DOI] [PubMed]
- 18.Denburg M, Nickolas TL. Declining Hip Fracture Rates in Dialysis Patients: Is This Winning the War? Am J Kidney Dis 2018;71(2):154–6. doi: 10.1053/j.ajkd.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Groothoff JW, Offringa M, Van Eck-Smit BL, et al. Severe bone disease and low bone mineral density after juvenile renal failure. Kidney Int 2003;63(1):266–75. doi: 10.1046/j.1523-1755.2003.00727.x. [DOI] [PubMed] [Google Scholar]
- 20.Denburg MR, Kumar J, Jemielita T, et al. Fracture Burden and Risk Factors in Childhood CKD: Results from the CKiD Cohort Study. Journal of the American Society of Nephrology : JASN 2016;27(2):543–50. doi: 10.1681/ASN.2015020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bikbov B, Perico N, Remuzzi G, on behalf of the GBDGDEG. Disparities in Chronic Kidney Disease Prevalence among Males and Females in 195 Countries: Analysis of the Global Burden of Disease 2016 Study. Nephron 2018:1–6. doi: 10.1159/000489897. [DOI] [PubMed] [Google Scholar]
- 22.Salam S, Gallagher O, Gossiel F, et al. Diagnostic Accuracy of Biomarkers and Imaging for Bone Turnover in Renal Osteodystrophy. J Am Soc Nephrol 2018;29(5):1557–65. doi: 10.1681/ASN.2017050584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jorgensen HS, Behets G, Viaene L, et al. Diagnostic Accuracy of Noninvasive Bone Turnover Markers in Renal Osteodystrophy. Am J Kidney Dis 2022;79(5):667–76 e1. doi: 10.1053/j.ajkd.2021.07.027. [DOI] [PubMed] [Google Scholar]
- 24.Slatopolsky E, Delmez J Renal Osteodystrophy. In: Coe FL, and Favus MJ, editor. Disorders of Bone and Mineral Metabolism New York: Raven Press; 1992. p. 905–34. [Google Scholar]
- 25.Malluche HH, Monier-Faugere MC. Renal osteodystrophy: what’s in a name? Presentation of a clinically useful new model to interpret bone histologic findings. Clinical nephrology 2006;65(4):235–42. doi: 10.5414/cnp65235. [DOI] [PubMed] [Google Scholar]
- 26.Moe S, Drueke T, Cunningham J, et al. Kidney Disease: Improving Global O. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2006;69(11):1945–53. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- 27. Araujo M, Bacelar Marques ID, Graciolli FG, et al. Comparison of serum levels with bone content and gene expression indicate a contradictory effect of kidney transplantation on sclerostin. Kidney Int 2019. doi: 10.1016/j.kint.2019.06.007. **This manuscript demonstrates that changes in circulating biomarkers after kidney transplantation do not reflect bone changes and suggest that treatment decisions post kidney transplantation cannot be based on measurements of serum biomarkers.
- 28.Pereira RC, Juppner H, Azucena-Serrano CE, et al. Patterns of FGF-23, DMP1, and MEPE expression in patients with chronic kidney disease. Bone 2009;45(6):1161–8. doi: S8756-3282(09)01819-5 [pii] 10.1016/j.bone.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fang Y, Ginsberg C, Seifert M, et al. CKD-induced wingless/integration1 inhibitors and phosphorus cause the CKD-mineral and bone disorder. Journal of the American Society of Nephrology : JASN 2014;25(8):1760–73. doi: 10.1681/ASN.2013080818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cejka D, Herberth J, Branscum AJ, et al. Sclerostin and Dickkopf-1 in renal osteodystrophy. Clinical journal of the American Society of Nephrology : CJASN 2011;6(4):877–82. doi: 10.2215/CJN.06550810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sernbo I, Johnell O. Consequences of a hip fracture: a prospective study over 1 year. Osteoporos Int 1993;3(3):148–53. doi: 10.1007/BF01623276. [DOI] [PubMed] [Google Scholar]
- 32.Johnell O The socioeconomic burden of fractures: today and in the 21st century. Am J Med 1997;103(2A):20S–5S; discussion 5S-6S. doi: 10.1016/s0002-9343(97)90023-1. [DOI] [PubMed] [Google Scholar]
- 33.Ensrud KE, Lui LY, Taylor BC, et al. Osteoporotic Fractures Research G. Renal function and risk of hip and vertebral fractures in older women. Archives of internal medicine 2007;167(2):133–9. doi: 10.1001/archinte.167.2.133. [DOI] [PubMed] [Google Scholar]
- 34.Nitsch D, Mylne A, Roderick PJ, et al. Chronic kidney disease and hip fracture-related mortality in older people in the UK. Nephrol Dial Transplant 2009;24(5):1539–44. doi: gfn678 [pii] 10.1093/ndt/gfn678. PubMed PMID: 19075194. [DOI] [PubMed] [Google Scholar]
- 35.Alem AM, Sherrard DJ, Gillen DL, et al. Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int 2000;58(1):396–9. doi: 10.1046/j.1523-1755.2000.00178.x. [DOI] [PubMed] [Google Scholar]
- 36.Maravic M, Ostertag A, Torres PU, Cohen-Solal M. Incidence and risk factors for hip fractures in dialysis patients. Osteoporos Int 2014;25(1):159–65. doi: 10.1007/s00198-013-2435-1. [DOI] [PubMed] [Google Scholar]
- 37.Naylor KL, Jamal SA, Zou G, et al. Fracture Incidence in Adult Kidney Transplant Recipients. Transplantation 2016;100(1):167–75. doi: 10.1097/TP.0000000000000808. [DOI] [PubMed] [Google Scholar]
- 38.Snyder S, Hollenbeak CS, Kalantar-Zadeh K, et al. Cost-Effectiveness and Estimated Health Benefits of Treating Patients with Vitamin D in Pre-Dialysis. Forum Health Econ Policy 2020. doi: 10.1515/fhep-2019-0020. [DOI] [PubMed]
- 39.Coco M, Rush H. Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. Am J Kidney Dis 2000;36(6):1115–21. doi: 10.1053/ajkd.2000.19812. [DOI] [PubMed] [Google Scholar]
- 40.Beaubrun AC, Kilpatrick RD, Freburger JK, et al. Temporal trends in fracture rates and postdischarge outcomes among hemodialysis patients. Journal of the American Society of Nephrology : JASN 2013;24(9):1461–9. doi: 10.1681/ASN.2012090916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Csukasi F, Bosakova M, Barta T, et al. Skeletal diseases caused by mutations in PTH1R show aberrant differentiation of skeletal progenitors due to dysregulation of DEPTOR. Frontiers in cell and developmental biology 2022;10:963389. doi: 10.3389/fcell.2022.963389. *This manuscript provides insight about the relationship between mutations in PTH1R, the parathyroid hormone (PTH)/PTH-related peptide (PTHrP) receptor, and DEPTOR, an inhibitor of the mechanistic target of rapamycin (mTOR) and establish DEPTOR as a key factor in skeletal progenitor differentiation.
- 42.Herlin M, McGuigan FE, Luthman H, Åkesson K. Polymorphisms in inflammation associated genes ALOX15 and IL-6 are associated with bone properties in young women and fracture in elderly. Bone 2015;79:105–9. doi: 10.1016/j.bone.2015.05.035. [DOI] [PubMed] [Google Scholar]
- 43.Huang JV, Schooling CM. Inflammation and bone mineral density: A Mendelian randomization study. Scientific reports 2017;7(1):8666. doi: 10.1038/s41598-017-09080-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Agoro R, Nookaew I, Noonan ML, et al. Single cell cortical bone transcriptomics define novel osteolineage gene sets altered in chronic kidney disease. Frontiers in endocrinology 2023;14:1063083. doi: 10.3389/fendo.2023.1063083. **This manuscript was the first to experimentally assess distinct populations of osteoblasts/osteocytes at the single cell level in a mouse model of CKD, and showed that ROD is a bone pathology that occurs earlier than previously recognized.
- 45. Martinez-Calle M, Courbon G, Hunt-Tobey B, et al. Transcription factor HNF4α2 promotes osteogenesis and prevents bone abnormalities in mice with renal osteodystrophy. The Journal of clinical investigation 2023. doi: 10.1172/jci159928. **This study, established hepatocyte nuclear factor 4α2 (HNF4α2), as the major osteoblastic Hnf4α isoform that regulates osteogenesis, cell metabolism, and cell death, and is implicated in the development of ROD.
- 46. Greenblatt MB, Ono N, Ayturk UM, et al. The Unmixing Problem: A Guide to Applying Single-Cell RNA Sequencing to Bone. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 2019;34(7):1207–19. doi: 10.1002/jbmr.3802. **This manuscript described the application of single-cell sequencing approaches to skeletal tissues provided an overview of the steps involved with a single-cell sequencing analysis of bone.
- 47.Ayturk U RNA-seq in Skeletal Biology. Curr Osteoporos Rep 2019;17(4):178–85. doi: 10.1007/s11914-019-00517-x. [DOI] [PubMed] [Google Scholar]
- 48. Martin A, David V. Transcriptomics: a Solution for Renal Osteodystrophy? Curr Osteoporos Rep 2020;18(3):254–61. doi: 10.1007/s11914-020-00583-6. *This review summarized the progress and limitations in the study and treatment of ROD, and emphasized the need of transcriptomics approaches for the study of ROD.
- 49.Parfitt AM. A structural approach to renal bone disease. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 1998;13(8):1213–20. doi: 10.1359/jbmr.1998.13.8.1213. [DOI] [PubMed] [Google Scholar]
- 50. Xie Z, Yu W, Ye G, et al. Single-cell RNA sequencing analysis of human bone-marrow-derived mesenchymal stem cells and functional subpopulation identification. Experimental & molecular medicine 2022;54(4):483–92. doi: 10.1038/s12276-022-00749-5. *This manuscript provided new perspectives into the use of bone marrow precursors at the single-cell level, for clinical applications.


