Abstract
Objectives:
Evaluate the association of frailty with the utilization of optimal guideline-directed medical therapy (GDMT) and outcomes in HFrEF.
Background:
The burden of frailty in HFrEF is high, and the patterns of GDMT use according to frailty status have not been studied previously.
Methods:
A post-hoc analysis of patients with HFrEF enrolled in the GUIDE-IT trial was conducted. Frailty was assessed using a frailty index (FI) using a 38-variable deficit model, and participants were categorized into three groups: class 1: (non-frail—FI:<0.21,), class 2 (intermediate frailty—FI:0.21–0.31,), and class 3 (high frailty—FI>0.31). Multivariate adjusted Cox models were used to study the association of frailty status with clinical outcomes. Utilization of optimal GDMT over time [beta-blockers, ACEi/ARB, and MRAs] across frailty strata was assessed using adjusted linear and logistic mixed effect models.
Results:
The study included 879 participants, of which 56.3% had high frailty burden (class 3 FI). A higher frailty burden was associated with a significantly higher risk of HF hospitalization or death in adjusted Cox models (HR [95% CI] high frailty vs. non-frail: 1.76[1.20–2.58]). On follow-up, participants with high frailty burden also had a significantly lower likelihood of achieving optimal GDMT (GDMT triple therapy use at study end: non-frail vs. high-frailty: 17.7% vs. 28.4%; P-interaction frailty class * time <0.001).
Conclusion:
Patients with HFrEF with a high burden of frailty have a significantly higher risk for adverse clinical outcomes and are less likely to be initiated and up-titrated on an optimal GDMT regimen.
Keywords: Frailty, Heart failure with reduced ejection fraction, mortality, guideline-directed medical therapy
INTRODUCTION
It is well-established that treatment with guideline-directed medical therapy (GDMT) titrated to the maximally tolerated or target dose improves clinical outcomes in patients with heart failure (HF) and reduced ejection fraction (HFrEF). Despite this, several studies have demonstrated that patients in contemporary clinical practice are often not treated with optimal GDMT regimens.(1–3) In the CHAMP-HF (Change the Management of Patients with Heart Failure) registry, only 1% of eligible HFrEF patients received target doses of all GDMT. It is essential to identify factors that influence the likelihood of meeting guideline-directed treatment goals.
Frailty—a state of reduced physiologic reserve and function across multiple systems that manifests as increased vulnerability to acute stressors —is common in patients with HF.(4) Frailty is usually assessed using validated models of physical function assessment such as the Fried Phenotype.(4) Frailty burden can also be evaluated using deficit accumulation model, in which individual health deficits are evaluated and summed to calculate a frailty index (FI).(5) The use of FI to assess frailty has facilitated the quantification of frailty burden in existing cohorts of patients with HF without the need for prospective physical function assessment.(6–8)
HF is intimately linked with frailty; patients with HF are predisposed to developing frailty, and frailty increases the risk of HF progression and adverse clinical outcomes.(9–14) Given that patients with coexisting frailty and HF identify a high-risk subset, optimization of GDMT is particularly important in this population. However, treatment patterns according to frailty status have not been studied previously. Frail patients may not receive adequate initiation or titration of GDMT due to the notion that they are more susceptible to adverse drug effects. Furthermore, difficulties with self-care and mobility may hinder the access of frail individuals to healthcare, causing delays in treatment modification. Therefore, in this post hoc analysis of the GUIDE-IT (Guiding Evidence-Based Therapy Using Biomarker Intensified Treatment in Heart Failure) trial,(15) we evaluated the association of baseline frailty status with the risk of adverse outcomes and optimization of GDMT among patients with HFrEF.
METHODS
Study Design and Participants:
The data for the present analysis was obtained from the National Institute of Health Biologic Specimen and Data Repository Coordinating Center. The design and primary results of the GUIDE-IT trial have been reported previously and detailed in the supplemental methods.(15,16) In brief, the trial enrolled patients with HFrEF and evaluated a strategy of augmented guideline-based therapy to suppress NT-proBNP concentrations to less than 1000 pg/ml, compared with usual care. The GUIDE-IT trial was approved by the institutional review board at each study site, and all participants provided written informed consent. As this is a posthoc analysis of the trial using de-identified data, it was considered exempt from an IRB review.
Frailty index:
Frailty, assessed using the FI, was the exposure of interest for this study. The FI was constructed based on the deficit accumulation model as previously described by Rockwood and colleagues.(17) The details of the FI variable selection and scoring are provided in the supplemental methods. Consistent with prior approaches to FI development in patients with HF,(6,8) we developed the FI using 38 parameters captured at baseline representing deficits across the following domains: comorbidities, anthropometric parameters, biomarkers, baseline functional status and quality-of-life parameters (supplementary table 1). Binary variables were assigned a score of 0 (absent) or 1 (present). Ordinal variables were coded by converting the number of possible ranks into equally spaced scores ranging from 0 to 1. Continuous variables were dichotomized as 0 (normal) or 1 (abnormal) based on established clinical thresholds, with 1 representing the most severe deficit. FI was calculated by dividing the total number of deficits present by the total number of deficits assessed. Supplementary figure 1 displays an example of how the FI was calculated for each patient. Consistent with prior approaches and conventional cutoffs of FI to identify frailty used previously in other investigators, the FI was categorized into three groups, according to the degree of frailty, Class-1 (non-frail: FI<0.21); Class-2 (intermediate frailty: FI 0.21–0.31) and Class-3 (high frailty: FI>0.31).(6,17,18)
Outcome of interest
Clinical outcomes:
The clinical endpoints of interest for the current study were: [1] the primary endpoint of the GUIDE-IT trial (time-to-first HF hospitalization or cardiovascular mortality); [2] all-cause mortality; and [3] heart failure hospitalization. A blinded clinical endpoint committee adjudicated all hospitalization and mortality events according to prespecified criteria as detailed previously.(15,16) Safety events of interest analyzed in this study were symptomatic hypotension, hyperkalemia (potassium > 6 meq/dl or requiring change in therapy), and worsening renal function (increase in Creatinine by 0.5 g/dl from the last visit or requiring change in therapy). The safety events were collected as per the trial protocol from randomization through the completion of the follow up period.
Optimal GDMT:
The criteria for optimal GDMT in the present analysis was consistent with prior reports from the GUIDE-IT trial and followed the guideline recommendations at the time of the GUIDE-IT trial.(3) Specifically, an optimal triple therapy GDMT regimen was defined as receiving beta-blockers and angiotensin converting enzyme (ACE) inhibitors at >=50% of the target doses and mineralocorticoid receptor antagonists (MRA) at any dose. An optimal triple therapy regimen for GDMT was defined as receiving any 2 of the 3 therapies at their optimal doses (beta-blockers or ACE -inhibitors at >=50% of the target doses or MRAs at any dose). Since the GUIDE-IT trial was conducted before the use of angiotensin receptor/neprilysin inhibitor was guideline-recommended and well before data supporting the use of sodium glucose co-transporter 2 inhibitors were published. Following outcomes were assessed to evaluated optimal GDMT utilization across frailty classes: [i] proportion of patients on triple therapy over time; [ii] proportion of patients on double therapy over time; and [iii] percent of the maximum recommended dose achieved for ACEi/ARBs and beta-blockers individually over time.
Statistical Analysis
Baseline characteristics were compared across the three frailty classes using the chi-squared test for categorical variables and the Kruskal Wallis test for continuous variables. The association between baseline frailty categories and clinical endpoints over time was evaluated using a Cox proportional hazards model with non-frail (class I) as the referent group with the following adjustments: model 1: adjusted for age, sex, race, treatment arm, and baseline body mass index (BMI); and model 2: variables in model 1 + baseline LVEF, HF etiology, baseline NT-proBNP, New York Heart Association (NYHA) class, and history of atrial fibrillation. The association between baseline frailty status and the likelihood of achieving optimal GDMT (triple therapy and double therapy as defined above) on follow-up was assessed using mixed-effects logistic models. Repeated measures of the optimal GDMT (yes vs. no) over time were modeled as the dependent outcome of interest. Participant ID was included as a random effect with adjustment for covariates in model 2. Similarly, for the individual components of GDMT, adjusted mixed-effects logistic models were also used to assess the association of frailty categories with the likelihood of initiation on MRA on follow-up. Finally, adjusted mixed-effect linear regression models were used to evaluate the association between baseline frailty status and percent of the maximum recommended dose achieved for ACEi/ARBs and beta-blockers. For this, separate models were constructed with the dose of ACEi/ARBs and beta-blockers on follow-up visits modeled as the dependent outcome with participant ID was included as a random effect. Models were adjusted for covariates detailed above in model 2. Multiplicative interaction terms were included (frailty category*time) to determine if the frailty status modified GMDT intensification over time. An exploratory time to event analysis using cumulative incidence curves was conducted to evaluate the association between the use of triple therapy and risk of primary endpoint among frail patients with high frailty burden at baseline. The analysis was performed using R software and a two-sided p-value of <0.05 was considered statistically significant.
RESULTS
Baseline characteristics
Of the 894 participants with HFrEF included in the GUIDE-IT trial, data for calculation of FI was available in 879 participants (mean age: 63 years, 31.5% women, median FI: 0.33). The FI was normally distributed (supplementary figure 2) with 18.5% (N = 163) participant considered non-frail, 25.1% (N =221) with intermediate frailty burden, and 56.3% (N = 495) with high frailty burden. Compared with non-frail participants, those with higher frailty burden were older, had higher BMI, higher NT-ProBNP levels, and higher burden of comorbidities such as diabetes, atrial fibrillation, kidney disease, stroke, and depression. There were no significant differences in race and sex distribution across the frailty classes (Table 1).
Table 1:
Baseline characteristics stratified by frailty index tertiles
| Variable | Non-Frail FI Class 1 (N=163) | Intermediate Frailty FI Class 2 (N=221) | High Frailty FI Class 3 (N=495) | P-value |
|---|---|---|---|---|
| Frailty Index | 0.163 [0.0278, 0.209] | 0.270 [0.210, 0.310] | 0.402 [0.310, 0.738] | <0.001 |
| Age, years | 58.0 [48.0, 68.0] | 63.0 [54.0, 72.0] | 64.0 [54.0, 72.0] | 0.001 |
| Female, % | 27.0 | 29.0 | 34.1 | 0.15 |
| White, % | 49.1 | 52.9 | 58.0 | 0.11 |
| BMI, kg/m 2 | 27.6 [24.3, 31.6] | 28.1 [24.7, 33.1] | 29.4 [24.8, 34.9] | 0.01 |
| NYHA Class I-II | 75.3 | 64.5 | 48.7 | <0.001 |
| Ejection Fraction | 20.0 [4.00, 40.0] | 22.5 [6.00, 40.0] | 25.0 [5.00, 40.0] | <0.001 |
| NT-Pro BNP | 2056 [103.0, 21293] | 2326 [49–61750] | 3004 [49–104280] | <0.001 |
| COPD, % | 6.1 | 18.1 | 28.3 | <0.001 |
| Ischemic HF, % | 31.6 | 31.2 | 56.6 | <0.001 |
| Diabetes, % | 24.5 | 41.6 | 43.4 | <0.001 |
| Atrial fib, % | 28.2 | 33.9 | 46.3 | <0.001 |
| PVD, % | 2.5 | 7.2 | 14.5 | <0.001 |
| Sleep Apnea, % | 8.0 | 18.1 | 29.7 | <0.001 |
| Kidney Disease, % | 14.1 | 32.6 | 46.3 | <0.001 |
| eGFR | 70 (55 – 86) | 61 (46 – 74) | 50 (36 – 72) | <0.001 |
| Stroke, % | 1.8 | 7.7 | 14.5 | <0.001 |
| Antidepressant, % | 6.7 | 8.1 | 21.6 | <0.001 |
| Biomarker arm, % | 54.6 | 52.0 | 47.1 | 0.18 |
| ACE or ARB, % | 36.2 | 30.8 | 29.5 | 0.275 |
| Beta blocker, % | 27.0 | 31.2 | 35.2 | 0.136 |
| Aldosterone antagonist, % | 60.1 | 47.1 | 48.1 | 0.02 |
| Double therapy, % | 38.7 | 30.8 | 31.7 | 0.201 |
| Triple therapy, % | 9.8 | 7.7 | 9.3 | 0.724 |
Data were presented as median (25th percentile – 75th percentile) for continuous and number (percentage) for categorical variables.
Abbreviation: SD, standard deviation; BMI, basal metabolic rate; NYHA, New York Heart Association; NT-Pro BNP, amino terminal pro-B type natriuretic peptide
At baseline, the proportion use of optimal GDMT (triple and double therapy regimens) was suboptimal and not significantly different across each frailty group. Among individual therapies, there were no significant differences in the proportional use of ACEi/ARBs and beta-blockers at their optimal guideline-recommended doses across the frailty groups. In contrast, the use of MRA at any dose was significantly higher in the non-frail vs. other groups (Table 1).
Association of Frailty Status at Baseline with Risk of Adverse Clinical Outcomes
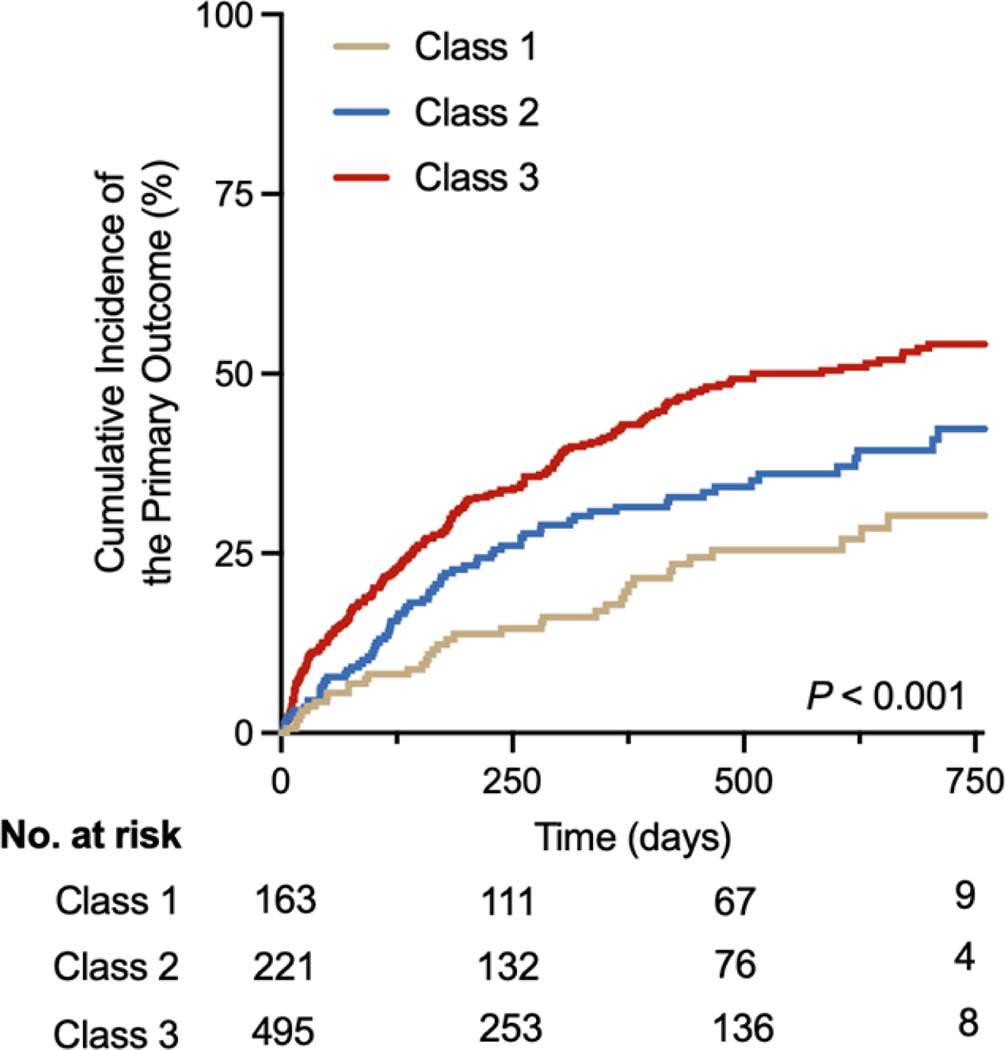
The cumulative incidence of primary composite endpoint increased across increasing frailty burden (non-frail category: 22.7% vs. intermediate frailty category: 33% vs. high frailty category: 43.2%, p log-rank < 0.001, Figure 1). In adjusted Cox models, compared with the non-frail participants, those in the intermediate and high frailty burden groups had a graded and statistically significant increase in the risk of primary composite endpoint after adjustment for demographic characteristics, treatment arm, and baseline body mass index (model 1, Table 2). The association between increasing frailty groups and greater risk of adverse outcome was attenuated modestly but remained statistically significant for the high frailty group (HR [95% CI): 1.76 [1.20 – 2.58], referent non-frail group) after additional adjustment for measures of HF severity (model 2, Table 2). For the secondary outcomes of interest, participants in the high frailty group also had a significantly higher risk of all-cause mortality [(HR [95% CI): 2.55 [1.25 – 5.20], referent non-frail group) and first HF hospitalization [(HR [95% CI): 1.61 [1.08 – 2.40], referent: non-frail group) in fully adjusted models (model 2, Table 2). The rate of safety events of interest (symptomatic hypotension, hyperkalemia, worsening renal function) during the trial period were low and comparable across the FI-based groups (supplementary table 2).
Figure 1: Association of Frailty status at baseline with the risk of heart failure hospitalization or cardiovascular mortality.
Participants with heart failure and reduced ejection fraction with higher frailty burden at baseline were more likely to experience the primary endpoint of heart failure hospitalization or cardiovascular mortality.
Table 2:
Association of baseline frailty class with risk of adverse clinical outcomes
| Event Rate | Model 1 | Model 2 | |||
|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | ||
| Primary outcome (HF hospitalization or death) | |||||
| Non-Frail (FI Class I) | 22.7% | Referent | |||
| Intermediate Frailty (FI Class II) | 33.0% | 1.60 (1.08–2.39) | 0.02 | 1.42 (0.94– 2.16) | 0.10 |
| High Frailty (FI Class III) | 43.2% | 2.31 (1.62–3.30) | <0.001 | 1.76 (1.20–2.58) | 0.004 |
| All-cause mortality | |||||
| Non-Frail (FI Class I) | 5.52% | Referent | |||
| Intermediate Frailty (FI Class II) | 12.7% | 2.09 (0.98–4.44) | 0.06 | 1.58 (0.73–3.40) | 0.24 |
| High Frailty (FI Class III) | 20.8% | 3.72 (1.87–7.40) | <0.001 | 2.55 (1.25– 5.20) | 0.01 |
| Heart failure hospitalization | |||||
| Non-Frail (FI Class I) | 21.5% | Referent | |||
| Intermediate Frailty (FI Class II) | 29.0% | 1.50 (0.99– 2.27) | 0.06 | 1.34 (0.87–2.08) | 0.18 |
| High Frailty (FI Class III) | 37.6% | 2.14 (1.48– 3.09) | <0.001 | 1.61 (1.08–2.40) | 0.02 |
Model 1: Adjusted for age, sex, race (white), treatment arm, baseline body mass index
Model 2: Model 1 + baseline ejection fraction + heart failure etiology+ baseline NT-proBNP + NYHA class + history of atrial fibrillation
Abbreviations: CI, confidence interval; HR, hazard ratio; HF, heart failure
Association of baseline frailty on optimization of guideline-directed medical therapy
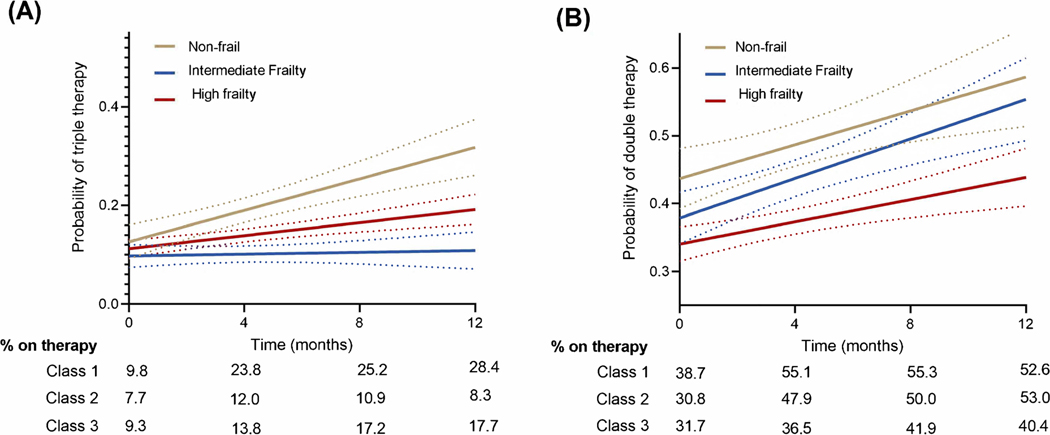
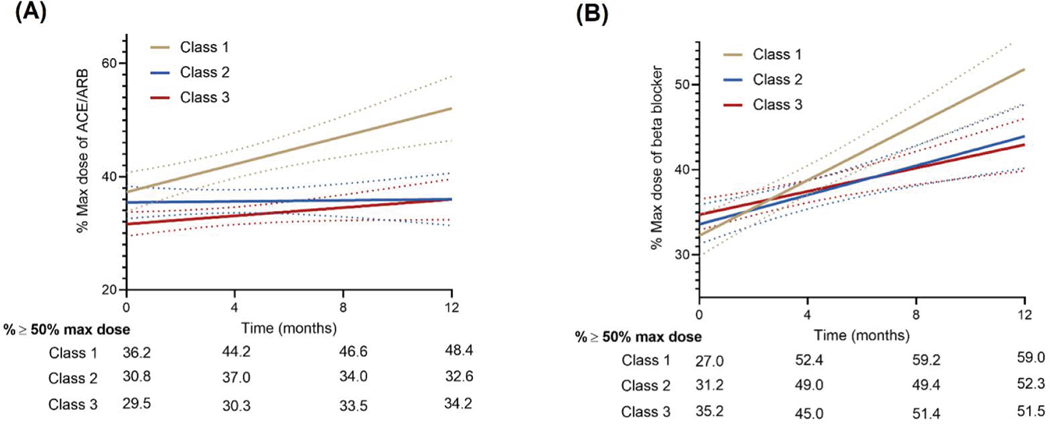
The proportion of participants on optimal GDMT (double therapy or triple therapy) increased over time. In adjusted mixed-effects models, the proportional use of optimal GDMT over time was modified by the baseline frailty burden (P-interaction FI class*time: <0.001 for optimal GDMT triple therapy and 0.007 for optimal GDMT double therapy, Figure 2, Table 3). Among non-frail participants, there was a significantly greater increase in the proportional use of optimal GDMT triple therapy (baseline to 12 months: 9.8% to 28.4%) and double therapy non-frail (baseline to 12 months: 38.7% to 52.6%) over time compared with participants in the high frailty group (Baseline to 12 months: GDMT Triple therapy from 9.3% to 17.7% and GDMT double therapy from 31.7% to 40.5%, Figure 2, Table 3). Among individual components of GDMT, a similar pattern of results was observed with a greater up titration in doses on ACEi/ARB and beta-blockers among non-frail and intermediate frail participants compared with those with high frailty burden (Figure 3, Table 3).
Figure 2: Association of Frailty status at baseline with the probability of achieving optimal guideline directed medical therapy regimen (triple therapy or double therapy).
Non-frail participants (FI Class I) were significantly more likely to be started on the optimal GDMT triple therapy (A) and double therapy (B) over the study period compared with those with high frailty burden (FI Class III).
Table 3:
Association of baseline frailty status with optimization of guideline-directed medical therapies over 12 month follow up.
| Non-Frail (FI Class I) | Intermediate Frailty (FI Class II) | High Frailty (FI Class III) | P-int (Frailty class * time) | ||||
|---|---|---|---|---|---|---|---|
| Estimate (95% CI) | P-value | Estimate (95% CI) | P-value | Estimate (95% CI) | P-value | ||
| Optimal GDMT* (Triple therapy) | 1.23 (1.15, 1.32) | <0.001 | 1.01 (0.94, 1.08) | 0.78 | 1.07 (1.02, 1.13) | 0.002 | <0.001 |
| Optimal GDMT* (Double therapy) | 1.13 (1.06, 1.20) | <0.001 | 1.14 (1.08, 1.20) | <0.001 | 1.05 (1.02, 1.08) | 0.008 | 0.007 |
| ACE/ARB† | 1.17 (0.81, 1.53) | <0.001 | −0.02 (−0.35, 0.31) | 0.89 | −0.09 (−0.33, 0.15) | 0.49 | <0.001 |
| Beta Blockers† | 1.52 (122, 1.84) | <0.001 | 0.74 (0.49, 0.99) | <0.001 | 0.50 (0.34, 0.66) | <0.001 | <0.001 |
Adjusted mixed effect logistic regression models were constructed to assess the association of frailty categories with the likelihood of achieving optimal triple therapy GDMT, double therapy GDMT, and initiation on MRA as noted on repeated follow up visits over time.
Adjusted mixed effect linear regression models were constructed to assess the association of frailty categories with the percent of maximal dose of beta blocker and ACEi/ARB achieved as noted on repeated follow up visits over time.
All models included person ID as random effect; estimate is per 1-month. Models were adjusted for age, sex, race (white), treatment arm, baseline bmi, baseline ejection fraction, HF etiology, baseline NT-proBNP, NYHA class, Afib
Figure 3: (A) Proportion of patients on ≥50% target dose of ACEi/ARBs; (B) proportion of patients on ≥50% target dose of beta-blockers.
Non-frail participants (FI Class I) were significantly more likely to be uptitrated to achieve higher doses of evidence-based therapies as compared with those with high frailty burden (FI Class III)
In exploratory analysis, use of triple therapy at baseline was associated with lower risk of numerically lower incidence of primary composite events in participants with high frailty burden (Event rate: triple therapy – 30.3% vs. not on triple therapy – 44.5%; HR [95% CI] ref: not on triple therapy: 0.61 [0.35 – 1.05], P-value: 0.06, supplemental Figure 3).
DISCUSSION
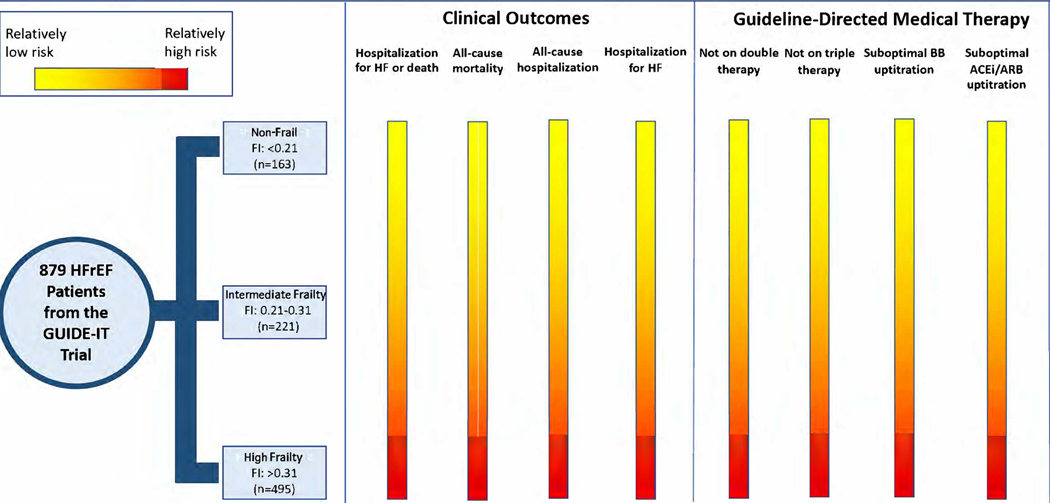
In this post hoc analysis of the GUIDE-IT trial, we report several key findings. First, among participants with HFrEF, higher baseline frailty was independently associated with a significantly higher risk of adverse clinical outcomes. Second, a higher frailty burden was also associated with a significantly less likelihood of up-titration/initiation of evidence-based pharmacotherapies for HFrEF and achieving optimal GDMT regimen by 12 months follow up. Our findings suggest that patients with HFrEF who have a higher burden of frailty are more likely to experience adverse outcomes and have underutilization of GDMT (central illustration).
Central Illustration: Impact of frailty on clinical outcomes and optimization of GDMT.
HFrEF patients with a higher burden of frailty are less likely to be initiated on GDMT and have dose escalation, despite being at a higher risk for adverse clinical outcomes.
Frailty and HF commonly coexist; a recent study by Sze et al. demonstrated that in the older population, frailty is significantly more prevalent in patients with HF (30% to 52%) compared with patients without HF (2% to 15%), regardless of the tool used to assess frailty.(14) Other studies have also demonstrated a high burden of frailty in patients with HF.(8,19,20) The rate is even higher in patients who are hospitalized or have advanced HF.(21,22) In the present study, 56% of study participants had high frailty burden (FI > 0.31). The frailty burden in the present study was higher than prior studies of patients with chronic stable HFrEF that used a similar cumulative deficit-based FI. In the PARADIGM and ATMOSPHERE trial, high frailty burden (FI> 0.31) was noted in 27% of study participants.(6) The high frailty burden in the present study may be due to the greater disease severity of study participants than the PARADIGM and ATMOSPHERE trials. The >50% prevalence of high frailty burden in the present study is comparable to the frailty burden observed in studies of patients with acute HF hospitalization from the REHAB-HF trial and the FRAIL-HF cohort, which enrolled older patients and used the Fried phenotype for frailty assessment.(11,23)
Frailty and HF are intertwined; prefrailty and frailty burden in community-dwelling adults is associated with increased risk of HF.(24,25) Furthermore, HF is associated with a higher burden of frailty, and frailty predisposes to worse outcomes in patients with HF.(6–8,11,13,23) The inter-relationship between frailty and HF is driven by shared pathophysiological mechanisms, including high comorbidity burden, upregulation of inflammatory pathways, sarcopenia, and the global decline in functional capacity.(9) Several studies have also demonstrated that frailty is an independent predictor of poor prognosis in HF.(26) The current study reinforces these findings using a deficit accumulation model to measure frailty. We show that a higher burden of frailty in patients with HF is associated with an increased risk of adverse clinical outcomes independent of demographic characteristics, HF etiology, and severity of heart failure (assessed by LVEF, NYHA class, and NT-proBNP levels).
Besides the increased risk of adverse outcomes, we observed that a higher burden of frailty is associated with a lower likelihood of optimal GDMT implementation in patients with HFrEF. Despite advancement in available therapies for HFrEF, suboptimal implementation of GDMT continues to be a major challenge in the management of HFrEF. In an analysis of 3,518 participants in the CHAMP-HF registry, Greene and colleagues showed that only 1% of eligible HFrEF patients simultaneously received target doses of all three triple therapy medications.(1) In a multivariate-adjusted analysis, the authors found that lower medication utilization or dose was predicted by stigmata of frailty such as older age, lower blood pressure, higher NYHA class, renal insufficiency, and recent HF hospitalization.(1) A previous analysis of the GUIDE-IT trial also demonstrated similar findings. Only 15% of the 894 enrolled participants achieved optimal GDMT – defined as ≥50% of the target dose of beta-blockers or ACEi/ARBs and any dose of MRAs.(3) status at baseline significantly modifies GDMT up-titration over time in patients with HFrEF. Participants with a higher burden of frailty were less likely to receive optimal GDMT regimens. They were also more likely to be treated at lower doses of ACEis/ARBs and beta-blockers.
A possible explanation for the underutilization of GDMT in frail patients is the perception among physicians that these patients are prone to adverse effects or intolerance to medication. In a previous analysis of the GUIDE-IT trial, Fiuzat and colleagues demonstrated that one of the most common reasons cited by physicians for not up-titrating medications was that patients were “already at maximally tolerated therapy”.(3) Frailty is associated with higher rates of polypharmacy and an increased risk of adverse effects from drug interactions.(27) Clinical inertia with GDMT intensification may be related to the perceived risk of adverse events or intolerance to evidence-based therapies rather than actual occurrence. Clinical inertia may be exaggerated for frail patients deemed “clinically stable” despite having a significantly higher risk of adverse outcomes. In the present study, the rates of adverse events were low on follow-up across frailty groups. In the PARADIGM trial, despite greater discontinuation of the study drug in more frail patients, the treatment benefit of sacubitril/valsartan was consistent across frailty categories.(6) These findings support the notion that clinical benefits of optimal GDMT therapies are likely preserved in frail patients, and efforts should be made to continue GDMT at optimal tolerated doses in these patients. However, participants of the PARADIGM trial were less frail than those enrolled in the present study. It is noteworthy that older, frail patients have been underrepresented in the seminal clinical trials of HFrEF therapies. Thus, our understanding of the ideal combination therapies and dosing regimens in frail individuals is limited. Future HF trials should be designed with a ‘pragmatic’ approach where HFrEF patients across the spectrum of HF frailty burden are enrolled to better evaluate strategies for optimal GDMT titration in these patients.
Our study has important implications. It emphasizes the importance of assessing frailty in clinics to identify individuals at high risk of adverse clinical events and under-treatment with GDMT. Despite its well-established prognostic value, frailty assessment, using tools such as the Fried phenotype and FI, is not commonly performed in the routine patient care settings limiting its clinical utility. Deficit accumulation-based FI, which uses existing clinical information about patients, can be easily implemented in electronic medical records to estimate the frailty status of patients with HF in clinical settings. Other simple models of frailty screening tools, which are less time and resource intensive, have also been validated in patients with HF. It has been shown that a deficit-accumulation based FI has modest agreement with most other frailty tools, and comparable prognostic value.(28,29) Once identified, frail patients with HFrEF can be targeted with tailored approaches to optimize GDMT utilization in frail patients with HFrEF. The optimal GDMT regimen for HFrEF continues to evolve with the addition of new therapies such as sacubitril/valsartan and sodium glucose cotransporter 2 (SGLT2) inhibitors. Furthermore, increasing emphasis is being placed on first initiating all four key drug classes (beta-blockers, SGLT2 inhibitors, sacubitril/valsartan and MRAs) as soon as possible – followed by a focus on uptritration. This is particularly relevant as these tolerance to these therapies when used together is higher than when used alone.(30) Future studies are needed to determine if simultaneous initiation of multiple GDMT in patients would high frailty burden could be a feasible and safe strategy to improve their long-term utilization.
Recent studies have demonstrated improvement in frailty burden with interventions such as nutritional supplementation, multi-domain physical therapy, and exercise training.(31–35) Gorodeski and colleagues have proposed a ‘domain management approach’ targeting features seen in both HF and frailty, such as deficits in health, physical function, emotion, and cognition.(36) A comprehensive approach targeting frailty management with the implementation of such multidomain interventions may improve physical function and symptom burden and help in gradual optimization of GDMT with concurrent improvement in the frailty burden.
Certain limitations should be considered when interpreting the results of this study. First, compared with HF registries, participants in the GUIDE-IT trial were relatively young. Thus, very old and frail patients were less represented.(15) Second, the use of sodium-glucose cotransporter 2 inhibitors and sacubitril/valsartan for HFrEF could not be assessed as these medications were approved for HFrEF during the trial. Finally, no adjustments for multiple comparisons were made in the analysis and our study findings are hypothesis generating.
In conclusion, our findings suggest that HFrEF patients with a higher burden of frailty are more likely to experience underutilization of GDMT, despite being at higher risk for adverse clinical outcomes.
Supplementary Material
CLINICAL PERSPECTIVES
Competency in patient care and procedural skills:
HFrEF patients with a higher burden of frailty are less likely to be initiated on guideline directed medical therapy and have dose escalation as per guideline targets despite being at an increased risk of adverse events.
Routine assessment of frailty in patients with heart failure and reduced ejection fraction may help tailor guideline directed medical therapies to high risk patients who are more likely to benefit from the same.
Translational Outlook:
Future research is needed to develop effective implementation strategies to promote utilization of guideline directed medical therapy among frail patients with heart failure and reduced ejection fraction
Funding/Support:
The GUIDE-IT study was funded by grants HL105448, HL105451 and HL105457 from the National Institutes of Health, and Roche Diagnostics provided support for NT-proBNP testing.
Disclosures:
Dr Greene received research support from the Duke University Department of Medicine Chair’s Research Award, American Heart Association, Amgen, AstraZeneca, Bristol Myers Squibb, Cytokinetics, Merck, Novartis, Pfizer, and Sanofi; has served on advisory boards for Amgen, AstraZeneca, Bristol Myers Squibb, Cytokinetics, and Sanofi; and serves as a consultant for Amgen, Bayer, Bristol Myers Squibb, Merck, and Vifor. Dr Fonarow is a consultant for Abbott, Amgen, AstraZeneca, Bayer, Janssen, Medtronic, Merck, and Novartis, and Associate Section Editor for the American Medical Association (JAMA) Cardiology. Dr Anker has received research support from Vifor International and Abbott Vascular and fees for consultancy and/or speaking from AstraZeneca, Bayer, Boehringer Ingelheim, Respicardia, Impulse Dynamics, Janssen, Novartis, Servier, and Vifor International. Dr Felker has received research grants from NHLBI, American Heart Association, Amgen, Bayer Merck, Cytokinetics, Myokardia; he has acted as a consultant to Novartis, Amgen, BMS, Cytokinetics, Medtronic, Cardionomic, Boehringer-Ingelheim, American Regent, Abbott, Astra-Zeneca, Reprieve, and Sequana, and has served on clinical endpoint committees/data safety monitoring boards for Amgen, Merck, Medtronic, EBR Systems, V-Wave, LivaNova, Siemens, and Rocket Pharma Dr. Januzzi has received grant support from Roche Diagnostics, Abbott Diagnostics, Singulex, Prevencio, Novartis, and Cleveland Heart Labs, consulting income from Roche Diagnostics, Abbott, Prevencio, and Critical Diagnostics, and participates in clinical endpoint committees/data safety monitoring boards for Siemens Diagnostics, Novartis, Bayer, AbbVie and Amgen. Dr. Butler has acted as a consultant to Abbott, Adrenomed, Amgen, Applied Therapeutics, Array, AstraZeneca, Bayer, Boehringer Ingelheim, CVRx, G3 Pharma, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, NovoNordisk, Relypsa, Sequana Medical, V-Wave Limited, and Vifor; and served on speaker’s bureaus for Novartis, Boehringer Ingelheim-Lilly, AstraZeneca, and Janssen. Dr Pandey is supported by the Texas Health Resources Clinical Scholars Program and has served on the advisory board of Roche Diagnostics.
ABBRIVIATIONS:
- GDMT
Guideline-directed medical therapy
- HFrEF
Heart failure with reduced ejection fraction
- CHAMP-HF
Change the Management of Patients with Heart Failure
- GUIDE-IT
Guiding Evidence-Based Therapy Using Biomarker Intensified Treatment
- FI
Frailty Index
- ACE inhibitors
Angiotensin converting enzyme (ACE) inhibitors
- MRA
Mineralocorticoid receptor antagonists
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Greene SJ, Butler J, Albert NM et al. Medical Therapy for Heart Failure With Reduced Ejection Fraction: The CHAMP-HF Registry. J Am Coll Cardiol 2018;72:351–366. [DOI] [PubMed] [Google Scholar]
- 2.Greene SJ, Fonarow GC, DeVore AD et al. Titration of Medical Therapy for Heart Failure With Reduced Ejection Fraction. J Am Coll Cardiol 2019;73:2365–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fiuzat M, Ezekowitz J, Alemayehu W et al. Assessment of Limitations to Optimization of Guideline-Directed Medical Therapy in Heart Failure From the GUIDE-IT Trial: A Secondary Analysis of a Randomized Clinical Trial. JAMA Cardiology 2020;5:757–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fried LP, Tangen CM, Walston J et al. Frailty in Older Adults: Evidence for a Phenotype. The Journals of Gerontology: Series A 2001;56:M146–M157. [DOI] [PubMed] [Google Scholar]
- 5.Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med 2011;27:17–26. [DOI] [PubMed] [Google Scholar]
- 6.Dewan P, Jackson A, Jhund PS et al. The prevalence and importance of frailty in heart failure with reduced ejection fraction - an analysis of PARADIGM-HF and ATMOSPHERE. Eur J Heart Fail 2020;22:2123–2133. [DOI] [PubMed] [Google Scholar]
- 7.Pandey A, Kitzman D, Reeves G. Frailty Is Intertwined With Heart Failure: Mechanisms, Prevalence, Prognosis, Assessment, and Management. JACC Heart Fail 2019;7:1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanders NA, Supiano MA, Lewis EF et al. The frailty syndrome and outcomes in the TOPCAT trial. Eur J Heart Fail 2018;20:1570–1577. [DOI] [PubMed] [Google Scholar]
- 9.Pandey A, Kitzman D, Reeves G. Frailty Is Intertwined With Heart Failure. JACC: Heart Failure 2019;7:1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vitale C, Uchmanowicz I. Frailty in patients with heart failure. European Heart Journal Supplements 2019;21:L12–L16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pandey A, Kitzman D, Whellan DJ et al. Frailty Among Older Decompensated Heart Failure Patients: Prevalence, Association With Patient-Centered Outcomes, and Efficient Detection Methods. JACC Heart Fail 2019;7:1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rich MW, Chyun DA, Skolnick AH et al. Knowledge Gaps in Cardiovascular Care of the Older Adult Population: A Scientific Statement From the American Heart Association, American College of Cardiology, and American Geriatrics Society. J Am Coll Cardiol 2016;67:2419–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shashikumar SA, Luke AA, Johnston KJ, Joynt Maddox KE. Assessment of HF Outcomes Using a Claims-Based Frailty Index. JACC Heart Fail 2020;8:481–488. [DOI] [PubMed] [Google Scholar]
- 14.Sze S, Pellicori P, Zhang J, Weston J, Clark AL. Identification of Frailty in Chronic Heart Failure. JACC: Heart Failure 2019;7:291–302. [DOI] [PubMed] [Google Scholar]
- 15.Felker GM, Anstrom KJ, Adams KF et al. Effect of Natriuretic Peptide–Guided Therapy on Hospitalization or Cardiovascular Mortality in High-Risk Patients With Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial. JAMA 2017;318:713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Felker GM, Ahmad T, Anstrom KJ et al. Rationale and design of the GUIDE-IT study: Guiding Evidence Based Therapy Using Biomarker Intensified Treatment in Heart Failure. JACC Heart Fail 2014;2:457–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatrics 2008;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blodgett J, Theou O, Kirkland S, Andreou P, Rockwood K. Frailty in NHANES: Comparing the frailty index and phenotype. Arch Gerontol Geriatr 2015;60:464–70. [DOI] [PubMed] [Google Scholar]
- 19.Denfeld QE, Winters-Stone K, Mudd JO, Gelow JM, Kurdi S, Lee CS. The prevalence of frailty in heart failure: A systematic review and meta-analysis. Int J Cardiol 2017;236:283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDonagh J, Martin L, Ferguson C et al. Frailty assessment instruments in heart failure: A systematic review. Eur J Cardiovasc Nurs 2018;17:23–35. [DOI] [PubMed] [Google Scholar]
- 21.Madan SA, Fida N, Barman P et al. Frailty Assessment in Advanced Heart Failure. J Card Fail 2016;22:840–4. [DOI] [PubMed] [Google Scholar]
- 22.Joyce E. Frailty in Advanced Heart Failure. Heart Fail Clin 2016;12:363–74. [DOI] [PubMed] [Google Scholar]
- 23.Vidan MT, Blaya-Novakova V, Sanchez E, Ortiz J, Serra-Rexach JA, Bueno H. Prevalence and prognostic impact of frailty and its components in non-dependent elderly patients with heart failure. Eur J Heart Fail 2016;18:869–75. [DOI] [PubMed] [Google Scholar]
- 24.Khan H, Kalogeropoulos AP, Georgiopoulou VV et al. Frailty and risk for heart failure in older adults: the health, aging, and body composition study. Am Heart J 2013;166:887–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Segar MW, Singh S, Goyal P et al. Prefrailty, impairment in physical function, and risk of incident heart failure among older adults. J Am Geriatr Soc 2021;69:2486–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang X, Lupón J, Vidán MT et al. Impact of Frailty on Mortality and Hospitalization in Chronic Heart Failure: A Systematic Review and Meta-Analysis. J Am Heart Assoc 2018;7:e008251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nwadiugwu MC. Frailty and the Risk of Polypharmacy in the Older Person: Enabling and Preventative Approaches. J Aging Res 2020;2020:6759521–6759521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oviedo-Briones M, Laso ÁR, Carnicero JA et al. A Comparison of Frailty Assessment Instruments in Different Clinical and Social Care Settings: The Frailtools Project. Journal of the American Medical Directors Association 2021;22:607.e7–607.e12. [DOI] [PubMed] [Google Scholar]
- 29.Op het Veld LPM, Beurskens AJHM, de Vet HCW et al. The ability of four frailty screening instruments to predict mortality, hospitalization and dependency in (instrumental) activities of daily living. European Journal of Ageing 2019;16:387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marti CN, Fonarow GC, Anker SD et al. Medication dosing for heart failure with reduced ejection fraction — opportunities and challenges. European Journal of Heart Failure 2019;21:286–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Travers J, Romero-Ortuno R, Bailey J, Cooney MT. Delaying and reversing frailty: a systematic review of primary care interventions. Br J Gen Pract 2019;69:e61–e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Behm L, Eklund K, Wilhelmson K et al. Health Promotion Can Postpone Frailty: Results from the RCT Elderly Persons in the Risk Zone. Public Health Nurs 2016;33:303–15. [DOI] [PubMed] [Google Scholar]
- 33.Bleijenberg N, Drubbel I, Schuurmans MJ et al. Effectiveness of a Proactive Primary Care Program on Preserving Daily Functioning of Older People: A Cluster Randomized Controlled Trial. J Am Geriatr Soc 2016;64:1779–88. [DOI] [PubMed] [Google Scholar]
- 34.Hildreth KL, Barry DW, Moreau KL et al. Effects of testosterone and progressive resistance exercise in healthy, highly functioning older men with low-normal testosterone levels. J Clin Endocrinol Metab 2013;98:1891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim CO, Lee KR. Preventive effect of protein-energy supplementation on the functional decline of frail older adults with low socioeconomic status: a community-based randomized controlled study. J Gerontol A Biol Sci Med Sci 2013;68:309–16. [DOI] [PubMed] [Google Scholar]
- 36.Gorodeski EZ, Goyal P, Hummel SL et al. Domain Management Approach to Heart Failure in the Geriatric Patient: Present and Future. J Am Coll Cardiol 2018;71:1921–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.