Abstract
Cardiovascular disease (CVD) is the leading cause of death worldwide. Despite medical advances, patients with CVD suffer from high morbidity and mortality rates, affecting their quality of life and death. Among CVD conditions, palliative care has been studied mostly in patients with heart failure, where palliative care interventions have been associated with improvements in patient-centered outcomes including quality of life, end-of-life care, and healthcare utilization. While palliative care is now incorporated into the AHA/ACC/HFSA guidelines for heart failure, the role of palliative care for non-heart failure CVD remains uncertain. Across all etiologies of CVD, palliative care can play an important role in all domains of CVD care from initial diagnosis to terminal care. In addition to general cardiovascular palliative care practices applicable to all areas, disease-specific palliative care needs may warrant individualized palliative care models. In this review, we discuss the role of cardiovascular palliative care for ischemic heart disease, valvular disease, arrhythmias, peripheral arterial disease, and adult congenital heart disease. While there are multiple barriers to cardiovascular palliative care, we recommend a framework for studying and developing cardiovascular palliative care models to improve patient-centered goal-concordant care for this underserved patient population.
Keywords: Palliative Care, Shared Decision Making, Goals of Care
Introduction
Cardiovascular disease (CVD), excluding hypertension, affects almost 10% of all adults.1 Despite advances in medical therapies, the morbidity and mortality rates remain high in this large patient population. As the leading cause of death worldwide, CVD accounts for more than 1900 deaths per day in the United States alone.2 With rising global CVD prevalence, there is an urgent need to address the quality of life and dying experiences for patients across multiple, specific CVD areas, many of which involve unique disease trajectories and patient experiences.
Palliative care is a multidisciplinary field that focuses on improving quality of life for patients with serious illness and their families by managing complex symptoms, using advanced communication skills to establish goals of care, and providing psychosocial and spiritual support.3 While hospice is a subset of palliative care that specifically provides multidisciplinary care to patients in the last months of life, palliative care addresses quality of life at any stage of chronic disease.3 As a specialized form of palliative care, cardiovascular palliative care has been most studied in patients with heart failure. Inpatient palliative care consults for heart failure have been associated with better quality of life and symptom burden.4 Longitudinal outpatient and home-based palliative care interventions have been associated with better anxiety, depression, symptom burden, functional class, satisfaction with care, and overall quality of life.5–9 Palliative care involvement has also been associated with increased quality of death measures for patients with heart failure, including increased documentation of wishes, advance care planning,4,10–13 and death at home.10,14,15 Finally, some studies of palliative care have shown reduced healthcare utilization for patients with heart failure, including hospital admissions,6,7,13,14,16 length of stay,7,11 and overall cost of care.17
Despite supportive evidence from multiple studies, palliative care application across the spectrum of CVD appears to lag compared with other disciplines such as oncology and nephrology. Palliative care is underutilized in heart failure with less frequent and later referrals than patients with cancer, despite similarly high mortality rates.18 Recognizing this unmet need, heart failure guidelines for the last 10 years have recommended consideration of specialty palliative care referral for stage D heart failure.19 Hospice referral in particular can be difficult, given the less clear prognostic trajectory in heart failure compared with cancer, and instead, expert consensus has shifted to focus on early integration of primary palliative care into heart failure management.20 In the 2022 update to the ACC/AHA/HFSA guidelines, primary palliative and supportive care is recommended for all heart failure patients, though practical implementation for this entire population would not be possible with current specialist availability and palliative training for CVD clinicians.21 The criteria for specialty palliative care referral is lacking, and a Delphi study attempted to create consensus for the role and timing of palliative care in heart failure management.22 These criteria prioritized patients with advanced, end-stage heart failure for specialty palliative care by including criteria such as severe complications and comorbidities, consideration of advanced therapies, limited life expectancy, severe symptom burden, frequent hospitalizations, and difficult decision-making.
Despite evidence of benefit and evolving guidelines for integrating palliative care into heart failure management, there is limited evidence for palliative care in other CVD entities, but similar signals suggest late or incomplete referral.23 The role and timing of palliative care for non-heart failure CVD also remains unclear and represents an unmet need for this large, high-risk patient population. To better understand cardiovascular palliative care across the spectrum of non-heart failure CVD, this review will highlight the current state of palliative care utilization, benefits specific to the CVD area, existing data on patient-centered outcomes, and recommendations for specialty palliative care integration into cardiovascular subspecialty care. Focusing on some of the unique needs of specific disease processes, this review will explore the role of palliative care for pain management in refractory ischemic heart disease, advance care planning in severe valvular disease, shared decision making in arrhythmias, end-of-life care in advanced peripheral arterial disease, and quality of life in adult congenital heart disease (Table 1). While discussing the benefits of integrated models of care with palliative care specialists embedded into cardiovascular teams, this review will also describe implementation challenges and provide recommendations for future directions.
Table 1:
Proposed Cardiovascular Palliative Care Interventions for Disease-Specific Challenges
| Type of CVD | Challenge | Palliative Care Intervention | Outcome |
|---|---|---|---|
| Ischemic heart disease | Refractory pain | Multidisciplinary angina program with integrated palliative care to address pain control, psychosocial support, mood symptoms | Holistic approach to pain management |
| Valvular heart disease | Complications after valve procedure | Advance care planning from initial diagnosis through post-procedural period | Valve preparedness plan to establish goal-concordant valve care |
| Arrhythmia | ICD shocks at end-of-life | Palliative care-facilitated shared decision-making regarding ICD implantation and subsequent ICD shock settings | Alignment of ICD settings with ongoing goals of care |
| Peripheral arterial disease | High rates of in hospital death | Assistance with end-of-life care including hospice referral when appropriate | Better quality of death in concordance with goals of care |
| Adult congenital heart disease | Quality of life with chronic illness at young age | Longitudinal goals of care discussions to prioritize quality of life at key milestones (pregnancy planning, career/financial difficulties, caregiving) | Psychosocial support throughout complex chronic illness in early to mid-adulthood |
Defining types of palliative care
Palliative care can be integrated into routine cardiovascular care through multiple models and across practice settings and is defined as primary or secondary depending on the expertise of the healthcare provider. Primary palliative care refers to the delivery of basic palliative care competencies required of most physicians and includes skills such as the ability to have goals of care discussions, basic symptom management, and conducting shared decision-making for medical care.24 In terms of cardiovascular palliative care, this often is provided by the patient’s primary cardiologist, primary care provider, or inpatient medical team. Secondary palliative care is provided by a palliative care specialist with additional training and resources to address more complex symptom, psychosocial, or care coordination needs.25 This is what is traditionally thought of as a palliative care consult or referral. With growing multidisciplinary care for complex CVD, an emerging model of care is embedded palliative care, where a palliative care specialist is integrated into the cardiovascular team in the outpatient or inpatient setting (Figure 1).
Figure 1:
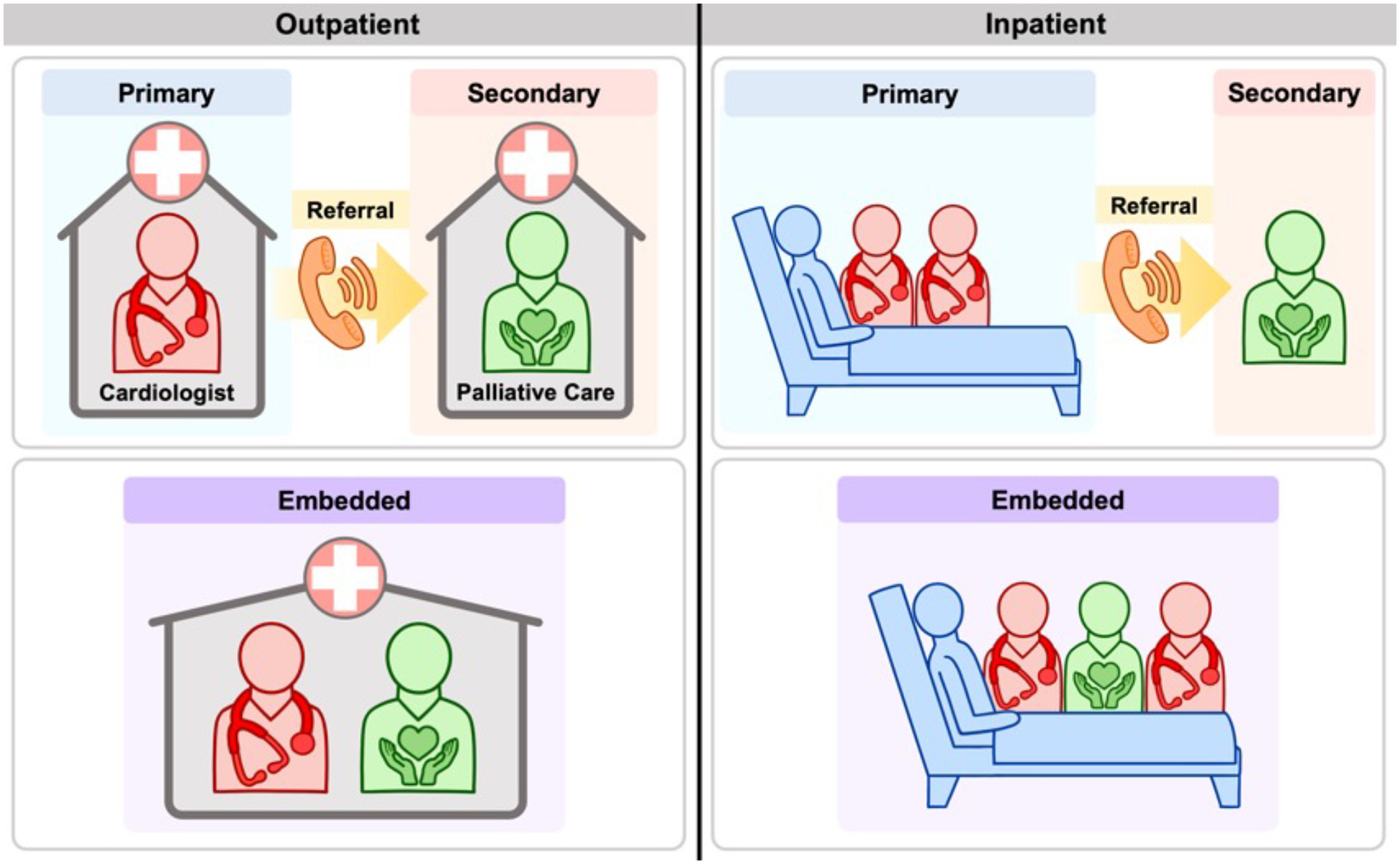
Models of Cardiovascular Palliative Care Across Settings
Patients with CVD have a high risk of suffering, significant morbidity and mortality, complicated and uncertain prognoses and face complex care decisions. This has led to an urgent need for both primary and secondary palliative care interventions spanning the disease state, from diagnosis to chronic illness management to critical and terminal care (Figure 2).26 Despite this urgent need for both, the majority of the literature on cardiovascular palliative care focuses on secondary palliative care.
Figure 2:
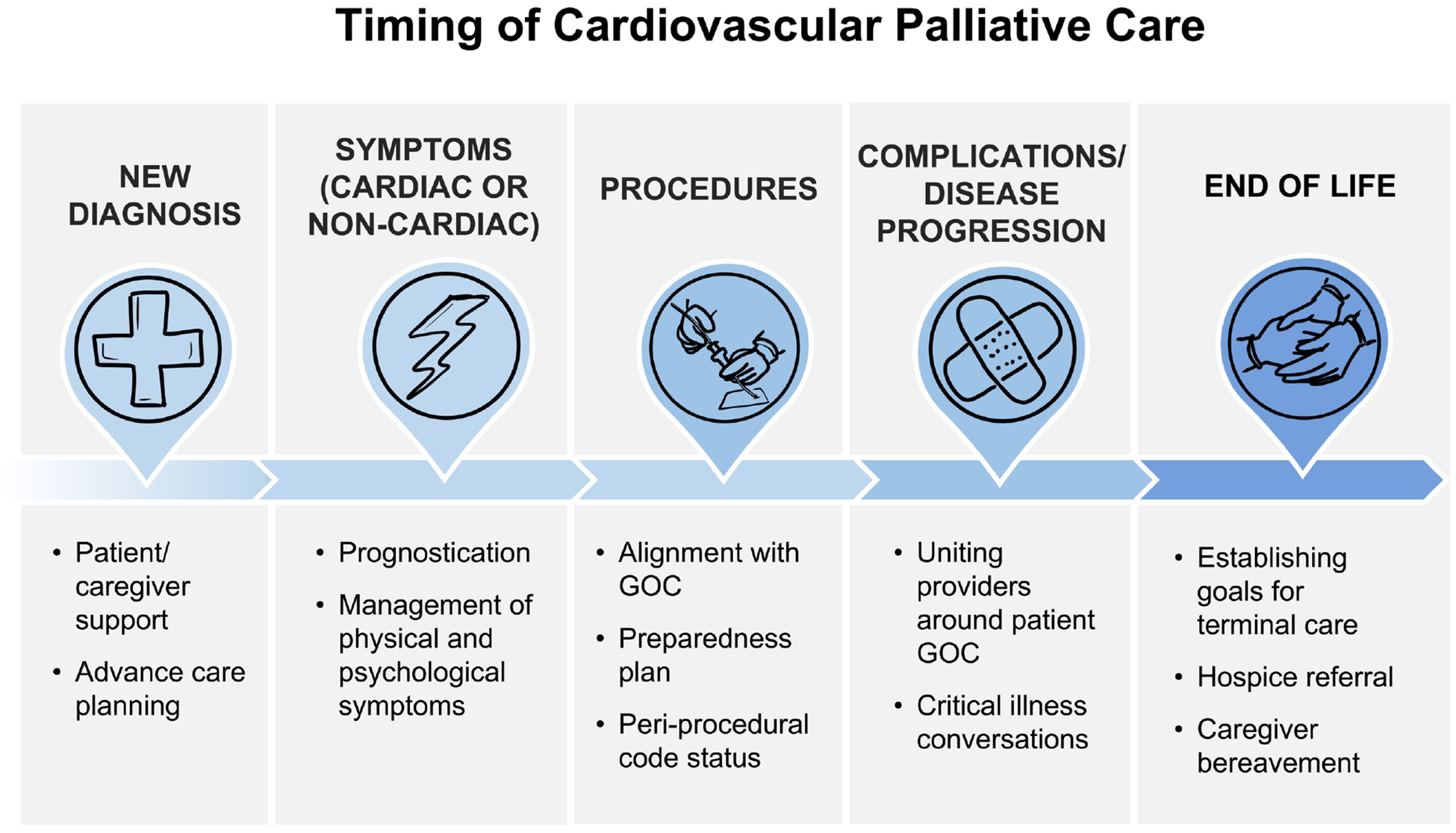
Timing of Palliative Care Throughout Cardiovascular Disease Course
Palliative care for pain management in refractory ischemic heart disease
Despite more than 7% of adults having ischemic heart disease, few patients are referred for palliative care.1 In a multicenter registry of CVD patients referred to palliative care, only 15% of referrals were for coronary artery disease compared with 70% for heart failure.27 Interestingly, the majority of those referrals were placed by general internal medicine practitioners with only 12% of palliative care referrals made by a cardiologist.
The low frequency of palliative care referrals may be attributed to the limited data on the efficacy of palliative care interventions for coronary artery disease. However, in addition to the general palliative care needs of CVD patients, patients with ischemic heart disease have a unique symptom burden. Unlike heart failure, where the chief concern for patients may be dyspnea or volume overload, patients with refractory ischemic disease suffer from significant pain with 2–24% of patients reporting daily to weekly angina.28 Refractory angina is defined as persistent longstanding pain despite antianginal therapy and revascularization.29 Regardless of the severity of coronary artery disease, patients with frequent angina have worse quality of life, often limiting their physical activity and social engagement to avoid intolerable symptoms.30 Among elderly patients with coronary heart disease, let alone refractory angina, geriatric syndromes such as multimorbidity, frailty, cognitive decline and delirium, disability, and sensory loss may limit optimal guideline-directed medical therapy.31
Recognizing the challenges of advanced ischemic disease, an AHA Scientific Statement on acute coronary syndrome (ACS) in older adults has advocated for incorporating goals of care conversations, evaluation of quality-of-life metrics, and formal study of palliative care interventions in ACS management.31 In addition to establishing goals of care, palliative care may have additional benefits specific for ischemic heart disease. For patients who have limited invasive options, there are multiple noninvasive targets for pain relief, including the neuropsychiatric experience of pain.32 Palliative care interventions can address pain beliefs and expectations, depression and anxiety, opioid and neurohormonal pain pathways, and self-management skills to augment pain relief from traditional antianginal therapy.32 While no large trials on palliative care for ischemic heart disease exist, small studies of noninvasive approaches to anginal pain provide evidence that multidisciplinary angina programs, including rehabilitation therapy and cognitive behavioral therapy, can have a meaningful impact on refractory angina.33 Studies have shown improvements in angina frequency and severity, treatment satisfaction, quality of life, anxiety and depression, physical function, and hospitalizations.34–38
The impact on important patient-centered outcomes and pain relief necessitates future study. As we can only improve what we measure, incorporation of quality-of-life screening tools, such as the short version of the Seattle Angina Questionnaire (SAQ), into routine outpatient care may be important to assess for life-limiting symptoms, track response to treatment, and guide individual and systemic strategies for angina management.39 Further investigation is particularly needed into the timing and role of palliative care-based interventions for this patient population. Incorporation of both primary and secondary palliative care into multidisciplinary angina programs, alongside physical rehabilitation, psychological support, and traditional cardiac care can significantly impact patients’ burden of pain symptoms.
Palliative care for advance care planning in severe valvular disease
Valvular heart disease comprises a significant portion of CVD worldwide with the greatest incidence of aortic stenosis, followed by mitral regurgitation and aortic regurgitation respectively.1 However, similar to ischemic heart disease, patients with valvular disease are uncommonly referred to palliative care, comprising only 4.2% of all such referrals in patients with CVD.13 Within valvular disease, the majority of the literature on palliative care is related to aortic stenosis with very limited data on other valvular lesions.
Aortic stenosis affects patients with high rates of morbidity and mortality, as demonstrated by the landmark PARTNER trial comparing transcatheter aortic valve replacement (TAVR) with the standard therapy (such as balloon valvuloplasty) in patients considered high risk for surgery.40 While this trial revolutionized our approach to aortic stenosis by reducing mortality versus standard therapy, the TAVR arm still had 43% mortality at 2 years compared with 68% in the control group. Further, once stratified by Society of Thoracic Surgeons (STS) risk score, that mortality benefit diminished for the highest risk patients, and among all TAVR recipients, 17% of those who survived to 2 years still had NYHA class III-IV symptoms.
With such significant risk of death and/or persistence of severe symptoms, the patient population with aortic stenosis is a natural target for palliative care intervention. Since 2005, frequency of palliative care referral for aortic stenosis has been increasing with significantly higher frequency for those undergoing TAVR compared with surgical replacement, likely reflecting the higher risk of the TAVR patient population.41 In a large study of patients from the NIS database, 8–13% of TAVR patients received a palliative care referral between 2011 and 2015. Markers of frailty or high surgical risk, including age over 80, electrolyte derangements, weight loss, and DNR code status, were all independently associated with higher frequency of palliative care referral.
For valvular heart disease, and aortic stenosis in particular, an important role of both primary and secondary palliative care is guiding goals of care discussions and shared decision-making regarding valvular interventions. In a survey of patients over age 75 considering TAVR, only 7% reported desiring the procedure to prolong their lifespan, whereas the remaining 93% reported their main priority was to maintain independence, be able to do a specific activity, or relieve symptoms.42 Thus, for many patients, TAVR serves as a palliative procedure that should be discussed in the context of a patient’s overall goals of care. Ideally, primary palliative care should be delivered at first point of contact from the cardiology team, incorporating patients’ goals of care into the treatment plan. For more complex shared decision-making, secondary palliative care can assist with incorporating TAVR as part of a patient’s symptom management, addressing the consequences of complications, and managing residual and non-CVD related symptoms after the procedure.
Further, incorporation of palliative care into valvular care promotes goal-concordance throughout the patient’s treatment. In a study that interviewed TAVR program coordinators in Washington and California, researchers asked about program policies regarding code status for DNR patients.43 They discovered there is no uniform approach to DNR code status; one program declines DNR patients, four programs have no policy, six programs maintain DNR status during the procedure as they consider it palliative, and 39 programs temporarily rescind the DNR status. Among those that rescind the DNR order, time frames on reinstituting the order range from 48 hours to 30 days after the procedure. Of note, the study did not examine how many patients required advanced cardiac life support (ACLS) while their code status was rescinded. However, the highly variable approaches to code status across just two states suggests a deeper and more widespread need for palliative care and goals of care discussions prior to valvular heart disease interventions.
Given the high risk of morbidity and mortality in an often elderly patient population, it has been proposed that specialty palliative care should be embedded into the valvular heart disease team from the initial diagnosis similar to the approach to LVAD in patients with advanced heart failure.44 As described by Steiner et al., embedded palliative care can assist with promoting goal-concordant care throughout the process, from advance care planning to developing a valve preparedness plan prior to intervention, establishing goals of care should complications or complex symptoms arise.44 Additionally, palliative care can assist with symptom management and hospice referral for those patients who are not likely to benefit from valve replacement or those who suffer a significant complication, such as devastating stroke. The data are limited on the impact of palliative care referral on important patient outcomes, which should prompt further study on the role of palliative care for aortic stenosis and other valvular heart disease.
Palliative care for shared decision-making in arrhythmias
In the United States alone, more than 100,000 implantable cardioverter defibrillators (ICDs) are implanted annually for primary prevention of ventricular arrhythmias and sudden cardiac death, and more than 30% of implantations are in patients over the age of 75.45 However, in this elderly patient population, the 1-year mortality rate after ICD implantation can be as high as 15–25%.46 While ICDs improve mortality from sudden cardiac death, ICD shocks can inflict significant suffering upon patients. Particularly among elderly patients with a high mortality risk, palliative care may be particularly influential for assuring goal-concordant care during ICD implantation, replacement, or deactivation. However, retrospective studies of deceased patients with ICDs have found that only 9–14% received a palliative care consult prior to death.47,48
Shared decision-making surrounding ICD implantation is important, as it has significant implications on patient quality of life and mental health. The experience of an ICD shock in the preceding 30 days is associated with worse perceived health, physical, emotional, and social functioning.49 Further, long-term effects can include increased risk of anxiety, depression, and post-traumatic stress disorder, particularly among those with more than 5 recurrent shocks.50,51 In one study of patients with prior ICD shocks, 23% of patients dreaded a future shock, and 5% of patients reported they wished they did not have an ICD.52
Presence of an ICD at end-of-life further influences patients’ quality of death with prior studies suggesting that shocks in the last 24 hours of life may be relatively common.53,54 Even among patients enrolled in hospice or with a DNR code status, more than 35% have been found to receive a shock within the last 30 days of life.55 Family members of deceased patients with ICDs report that shocks caused pain, fear, stress, and sadness for both patient and family members at end-of-life, and in one study, they rated their relative’s quality of death higher when their ICD was deactivated.56
From a technical perspective, ICD deactivation may include turning off anti-tachycardia pacing (ATP) therapy, shocks, or both; additional programming changes might include restricting the number of ICD shocks or changing the detection parameters for treatment. (We address pacing therapy separately below.) In the authors’ view, ICD deactivation discussions generally focus on the importance to the patient of prolonged longevity versus quality of life, and whether further treatment by the ICD fits those goals. Attempting to tailor device programming to narrowly treat arrhythmias only painlessly may not be realistic in most settings. Practically speaking, in most circumstances leaving ATP active while deactivating ICD shocks alone would not be recommended, as ATP can accelerate ventricular tachycardia and potentially worsen symptoms or render potentially self-limited or treatable arrhythmias fatal.
Assistance with shared decision-making regarding ICD implantation, replacement, and deactivation can be a target for palliative care intervention to improve goal-concordant care. In multiple studies interviewing ICD patients, the vast majority report never considering changing their ICD settings at end-of-life or discussing their ICD settings with a clinician.57,58 Ideally, patients should be counselled on their options for ICD deactivation at the time of ICD implantation and at subsequent changes in their clinical status by their primary cardiologist and/or electrophysiologist. For more complex goals of care needs, secondary palliative care can assist with communication around ICD settings in chronic illness. Though the studies are small, involvement of specialty palliative care has been found to reduce ICD implantations for patients whose goals of care do not align with ICD shocks.59 For patients with existing ICDs, palliative care consultation has been found to increase ICD deactivations and improve concordance between code status and device settings.47,48
Palliative care consultants occasionally become involved with patients with permanent pacemakers (PPM) when questions arise regarding the role of pacemaker deactivation. Previous Heart Rhythm Society guidance outlines the ethical, legal, and practical aspects of device deactivation.60 In general, when patients are critically or terminally ill and transitioned to comfort measures, PPM or CRT deactivation would rarely be indicated for several reasons. For patients who are not pacemaker-dependent, device deactivation would not be expected to influence their clinical course. For those who are dependent on pacing, the underlying disease or dying process will eventually render pacing therapy ineffective as progressive metabolic disarray increases the pacing capture threshold. Moreover, PPM or CRT deactivation may have unpredictable effects on symptoms (in awake patients) that would not be concordant with a comfort-oriented strategy.61 In selected cases, however, palliative care consultation along with electrophysiology involvement may identify cases where pacemaker deactivation, or more commonly deferral of generator replacement (such as in patients with severe dementia) may be appropriate, which would be both legal and ethical under carefully defined circumstances.62 This is another opportunity where patient care may benefit from formal palliative care consultation.
Decisions surrounding cardiac devices and procedures involve a complex balance of patient goals of care, procedural risk, and short vs. long-term benefits. Ideally, this should involve a multidisciplinary approach, including a combination of primary and secondary palliative care, to help guide patients towards goal-concordant care and bolster proceduralists to decline procedures that they may feel obligated to offer. Once a patient has a device, palliative care consultants can ensure ICD settings are not overlooked in end-of-life care.
Palliative care for end-of-life care in advanced peripheral arterial disease
The lifetime risk of peripheral arterial disease (PAD), defined as an ankle-brachial index (ABI) <0.9 over an 80-year lifespan, has been estimated to be 19–30%.1 Patients with PAD have a 4-fold greater risk of subsequent myocardial infarction, 2-fold greater risk of stroke, and 2-fold greater risk of death than their peers.63 Further, patients with an ABI less than 0.4 have a similarly high mortality risk to patients with stage III ovarian cancer.63,64 However, even among patients with the most advanced PAD requiring limb amputation, one study found less than 3% of patients received a palliative care consultation prior to amputation.65 In this cohort of 111 patients with chronic limb threatening ischemia, Kwong et al. found 22% of patients died within 1 year of amputation, and the median time from palliative care referral to death was only 9 days.65 This highlights that palliative care referral is currently underutilized and occurs very late in the disease course.
In addition to high morbidity and mortality, patients with advanced PAD have unique needs that lend themselves to a palliative care intervention. Patients with PAD suffer from chronic pain, limited mobility, social isolation, depression, anxiety, and the social stigma associated with chronic wounds and amputations.66 In one study of hospitalization among patients with new PAD, CVD, or stroke, higher frequency of depression, fatigue, and lack of social support predicted increased risk of hospitalization for only the patients with PAD.67 This highlights the distinctive psychological and social impacts of PAD that place patients at increased risk of healthcare utilization compared with other forms of CVD.
Studies of palliative care interventions for advanced PAD are mostly small, observational, and retrospective analyses. However, palliative care intervention has been associated with caregiver satisfaction with end-of-life care. In one study of patients undergoing high risk surgery, including vascular surgery, palliative care involvement was associated with better family-reported ratings of overall care, support, and end-of-life communication.68 In addition to family members feeling more supported, palliative care referral for PAD has been associated with better quality of death metrics, including higher frequency of hospice referral and death at home.69
Use of healthcare resources becomes particularly important at end-of-life as frequent hospitalization and/or invasive procedures may not always align with patient goals of care. While patient preferences surrounding end-of-life care vary widely, most patients state they wish to die at home.70 In one large study of deceased Canadian patients with PAD or diabetes, patients who underwent amputation spent more time in the last months of life in the hospital and were more likely to die there than patients without amputation.71 Further, patients with amputation were less likely to receive palliative care referral, but those who did were significantly more likely to die at home. Patients with PAD are at high risk for recurrent hospitalization, especially at end-of-life, and multiple studies have shown palliative care intervention reduces healthcare utilization.71,72 By incorporating palliative care into PAD management, palliative care specialists can assist with promoting goal-concordant end-of-life care, reducing hospital deaths, and facilitating referrals to hospice when appropriate.
Similar to prior discussion, the complexity of symptom management and goals of care at end-of-life for patients with advanced PAD warrants further study of palliative care interventions for this population. The association between objective ABI cutoffs with morbidity and mortality indicates an opportunity to develop ABI-specific palliative care referral guidelines, allowing prioritization of those at highest risk for complications and amputation to preemptively discuss goals of care and end-of-life preferences. Integration of primary and secondary palliative care into a multidisciplinary approach to PAD is likely to improve quality of life and death for patients with complex medical and psychosocial needs.
Palliative care for quality of life in adult congenital heart disease
Adult congenital heart disease (ACHD) is a broad term with significant variability in the severity of cardiac malformations. It is estimated that more than 200,000 Americans are currently living with ACHD, excluding those with corrected defects, with 20% consisting of ventricular septal defects (VSDs), 20% of atrial septal defects (ASDs), 16% of patent ductus arteriosus (PDAs), and the remainder of more complex defects.1,73 While the associated outcomes are variable depending on the underlying anatomy, the 10-year incidence of advanced stage D heart failure is 11% in this population, with 3-fold greater odds for those with severe congenital defects.74
Though there are no major studies of palliative care intervention specifically for ACHD, the young age of this population warrants special considerations for palliative care. Interviews of patients with ACHD have shown that their illness has impacted their identity, created emotional distress, and influenced their adult decision-making with greater impacts on those with the most complex lesions.75 From the psychological impacts of the prolonged experience of chronic illness from early childhood to the financial stress of debilitating illness in early adulthood, patients with complex ACHD have specific challenges affecting their quality of life. Some ACHD experts have recommended involving specialist palliative care at age 18 to develop longitudinal goals of care, including making decisions about career, family planning, and end-of-life preferences.76
Among patients with ACHD who develop advanced heart failure, the one-year mortality rate has been found to be as high as 38% for a predominantly younger patient population than other causes of heart failure.74 When considering all congenital heart defects, less than 80% survive to age 68, and among those with complex lesions, less than 50% survive to middle age.74 Despite the significant mortality in this patient population, one study of deceased ACHD patients, with an average age of 37, found only 15% were referred to palliative care and 10% had documented end-of-life discussions during their terminal admission.77
In addition to needing general goals of care discussions at advanced stages of disease, complex ACHD patients throughout their disease course may have different concerns about quality of life as a younger age group compared with other forms of CVD. Among young palliative care patients in general, palliative care experts have recommended addressing their unique symptom burdens and goals of care when discussing quality of life and end-of-life care, including fertility, financial stability and career concerns, body image and sexual health, and caregiving.78 Studies of quality of life among patients with ACHD are small and limited with mixed results, though there has been some evidence of reduced quality of life, particularly in physical function domains.79 However, when studies focus on patients with complex ACHD, patients have significantly worse physical functioning, health perception, depression, and anxiety that could be addressed with palliative care intervention.80–82
There is very limited literature on the role and timing of palliative care intervention for complex ACHD. However, a survey of ACHD patients found the vast majority of patients were willing to discuss goals of care preferences and meet with a palliative care specialist.83 Despite this interest, as few as 1–13% report discussing advance care planning, life expectancy, and goals of care preferences.84–86 As a result, experts such as the ESC ACHD Working Group recommend discussing advance care planning at specific milestones, including disease progression, worsening prognosis, consideration of interventions, pre-pregnancy counselling, and changes in social system.76 Similarly, there is an AHA Scientific Statement that outlines the importance of early and longitudinal primary palliative care from the cardiovascular clinician through each phase of their care, from diagnosis to end of life.87 They advocate for routine quality of life assessments at follow-up visits, as well as clear referral guidelines for specialist palliative care. Like the other areas of CVD, further research is needed to assess palliative care impact on patients with complex ACHD.
Challenges and potential solutions to integration of palliative care in cardiovascular disease management
Integrated cardiovascular palliative care for patients with non-heart failure CVD has the potential for significant benefits for both patients and their caregivers. However, there are several barriers to widespread utilization of primary, secondary, and embedded palliative care (Figure 3). As an emerging field, cardiovascular palliative care does not yet have an established best model of care, and implementation may vary with the specific needs of the CVD etiology, patient population demographics, and health center priorities. The greatest barrier to widespread palliative care implementation is funding, as palliative care is not profitable compared to traditional CVD care. However, given the numerous benefits to patients, finding reimbursement models that prioritize patient-centered care is essential. While not exhaustive, we propose various approaches to improve primary, secondary, and embedded models of palliative CVD care.
Figure 3:
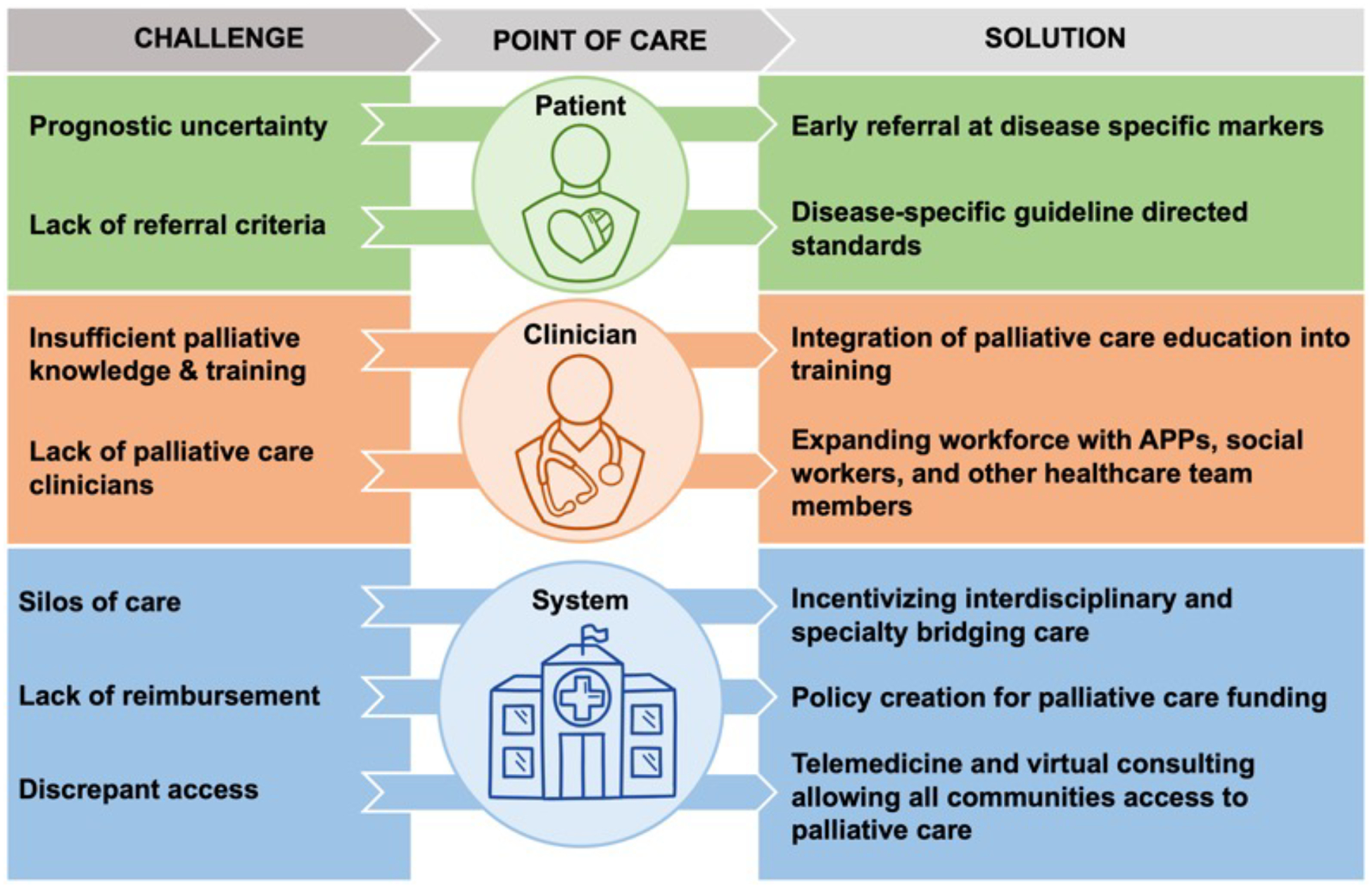
Challenges and Solutions to Implementation of Cardiovascular Palliative Care
APPs = advanced practice providers
For primary palliative care provided by cardiovascular practitioners, major challenges to implementation include inadequate clinician awareness, insufficient clinical time to discuss goals of care amid competing priorities, and inadequate communications training. Across a range of specialties, many clinicians lack a clear understanding of what palliative care entails. Many equate palliative care with hospice care, appropriate only for patients at end-of-life. Further, with the competing demands on short outpatient visits, in-depth goals of care conversations can be challenging. As such, providers often report time availability and lack of palliative care training as major barriers to advance care planning.88–90 In a national survey of cardiology fellows and faculty, less than 10% reported receiving required or elective training in palliative care during training.91
To improve the quality and frequency of primary palliative care, improved medical education and training in communications skills is needed. With the increasing complexity of cardiovascular care, incorporation of palliative care into cardiology training is not likely to occur without a nationwide initiative. The Accreditation Council of Graduate Medical Education (ACGME) should establish palliative cardiology competencies for general cardiology fellows, including communication skills, shared decision making, and goals of care discussions. More specialized competencies could be established for subspecialty training, such as management of ICDs and pacemakers at end-of-life for electrophysiology fellows. By establishing palliative cardiology principles as part of cardiology training, this would improve provider understanding of the goals of palliative care and provide a skillset to deliver effective primary palliative care.
When it comes to secondary palliative care referral, cardiovascular clinicians often delay referral due to prognostic uncertainty, beliefs that palliative care is designed for cancer patients, fear of taking away hope from patients for recovery, and unclear referral criteria.92–94 Though guidelines recommend primary palliative care for all patients with heart failure and secondary palliative care referral for those with stage D heart failure, there is no similar consensus for other forms of non-HF CVD, further complicating referrals.21 Given the limits of primary palliative care and prognostic uncertainty in CVD, some cardiovascular palliative care experts have argued that secondary palliative care should be involved at the time of diagnosis. However, this is complicated by the international shortage of specialist palliative care practitioners.95 To care for all patients with palliative care needs in the United States, there are fewer than 10,000 PC specialists currently practicing.95 Therefore, even among patients with CVD who are referred to palliative care, many may face lengthy wait times or have no access to a palliative care specialist.
Without dramatically increasing the number of palliative physicians, it is not feasible for our current system to refer all CVD patients to secondary palliative care. Therefore, we must find ways to prioritize the patients with the greatest need. One solution, as has been proposed for heart failure, would be to create consensus referral criteria in the guidelines for different etiologies of CVD. Some criteria would be universal, such as complex goals of care conversations, whereas others could be catered to the CVD area. As discussed above, referral criteria could include refractory angina despite maximally tolerated antianginal therapy, discussion of high-risk TAVR, consideration of ICD/CRT deactivation, specific ABI cutoffs, and severe complications of ACHD. In addition to guidelines for palliative care referral, metrics of monitoring ongoing palliative care needs can help determine when in the disease course a patient may benefit from secondary palliative care. There are ongoing studies to examine combining electronic medical records, patient, family, and medical team data to identify unmet palliative care needs.96 Additional research is needed to determine the appropriate timing and models of palliative care for CVD patients.
One potential solution to limited training and time for primary palliative care is to develop multidisciplinary CVD teams with embedded specialist palliative care practitioners. As outlined previously, embedded palliative care can assist in promoting holistic goal-concordant care that addresses complex pain management, advance care planning for valvular interventions, decision-making surrounding cardiac devices, and challenges to quality of life and death. Embedded specialists can have multiple different types of training, including physicians, nurse practitioners, physician assistants, and/or social workers to offset the burden on the palliative care physician workforce. Of note, this model is likely most relevant to large academic centers with multidisciplinary care, and embedded palliative care is only as effective as the collaborative nature of the team that equally values its palliative care members. Further, funding embedded palliative care models can be complicated in general, and in particular, by competing incentives for procedural volume. In these discussions, it is important to remember that incorporation of palliative care into the cardiovascular team helps ensure patient-centric decision making, promotes palliative procedures (e.g., TAVR), and improves appropriate patient selection to reduce adverse outcomes in those with significant frailty and conflicting goals of care (e.g., ICD placement). An additional challenge with embedded palliative care models is the growing silos of care as cardiology, and medicine, become more subspecialized. When a patient has multiple comorbidities (such as severe coronary artery disease and metastatic cancer), there can be a diffusion of responsibility for who should address goals of care.
Given cardiovascular palliative care’s infancy, there is no single approach to embedded palliative care, and different centers have different reimbursement models and priorities. However, to address the challenges of funding and silos of care, by including palliative specialists with a variety of training into a multidisciplinary model of care, embedded palliative care can leverage non-physician practitioners to improve access to palliative care, while also addressing the specific needs of a specific disease process (e.g., embedded palliative care in the TAVR team). Further, embedded palliative care teams can be adapted to different practice settings, integrating palliative care into the cardiac intensive care unit as well as the outpatient vascular disease clinic. In academic centers, specialized clinics or inpatient teams may have a dedicated palliative cardiology practitioner, whereas in smaller centers, there can be a preferred independent practitioner for all cardiology referrals, facilitating ease of access to palliative care. Finally, embedded palliative care specialists gain additional cardiovascular expertise by participating in multidisciplinary care for a specific type of CVD, as opposed to general palliative care practitioners who are not focused on one disease or its specific challenges.
Finally, it is important to recognize that the limited access to cardiovascular palliative care does not equally affect the CVD patient population. While most academic centers have specialty palliative care services, as few as 54% of public safety net hospitals have a palliative care team.97 This disparity applies to urban safety hospitals, as well as rural areas where heart failure patients are more likely to die in the hospital, on dialysis, and without hospice referral compared with patients in less remote areas.98 One potential solution to this would be to establish telemedicine palliative cardiology consult services, particularly for inpatient or ICU teams, to facilitate complex goals of care discussions remotely. In addition to socioeconomic disparities, there can also be unequal access to palliative care within the same hospital based upon patient factors. Palliative care studies have shown clinicians are less likely to offer palliative care referral when patients differed in culture, religion, or ethnicity.99 More data are clearly required to understand the best approach to cardiovascular palliative care, but it remains clear that support from specialist palliative care is both necessary and difficult to access.
Conclusions and future directions for cardiac palliative care
Cardiovascular palliative care is underutilized and often occurs at very advanced stages of cardiovascular disease. Many cardiology clinicians cite insufficient time or training to provide sufficient primary palliative care, while referral rates for secondary palliative care remain very low.13,88 Although palliative care referral for patients with heart failure has been incorporated into current guidelines and clinical practice, the role and timing of palliative care for non-heart failure CVD lacks consensus. Across all etiologies of CVD, palliative care can play an important role. In addition to end-of-life care, palliative care can augment cardiovascular care with advance care planning, shared decision-making, complex symptom management, caregiver support, challenging family dynamics, and psychological and spiritual support from the initial diagnosis (Figure 4).100
Figure 4:
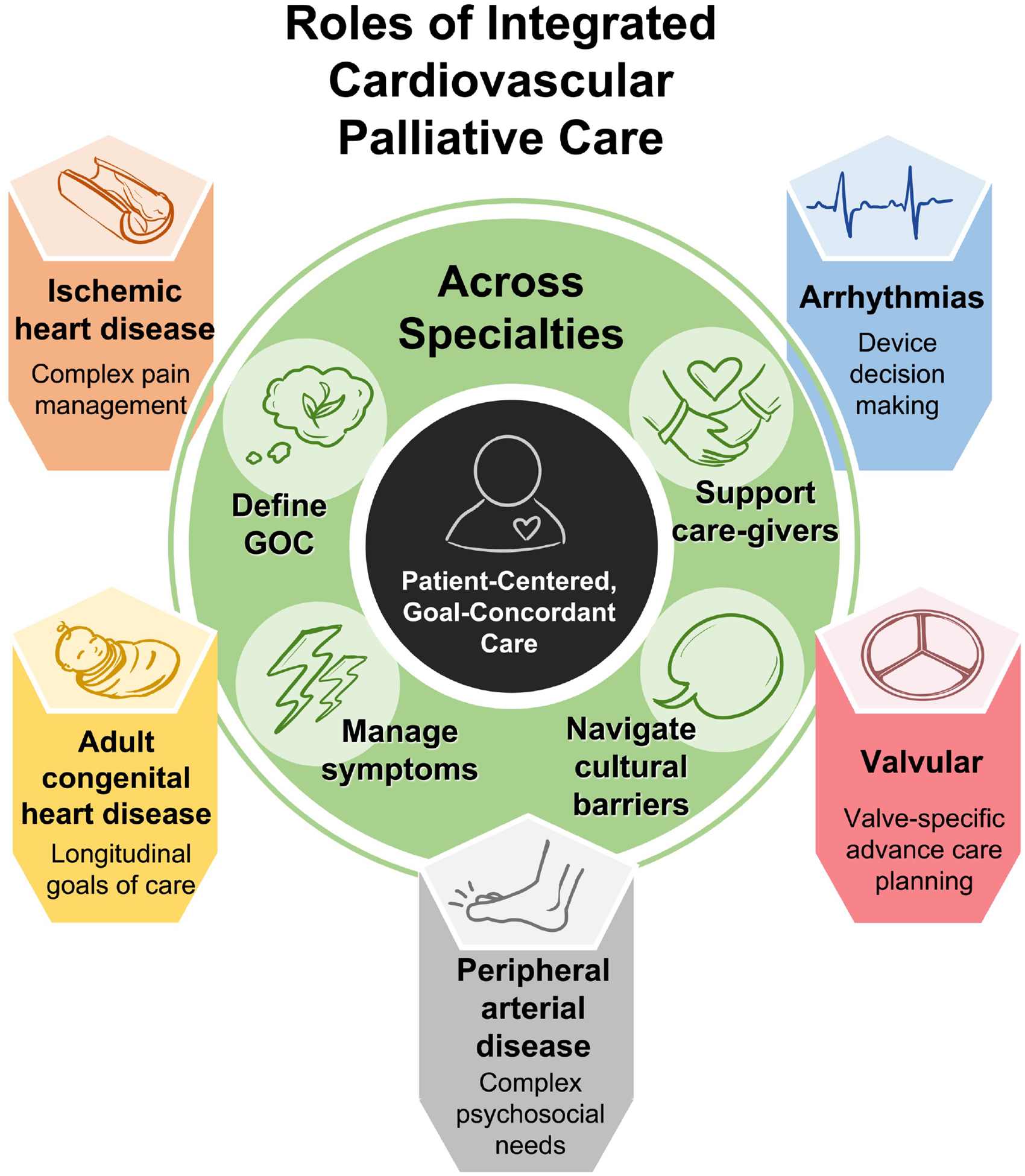
Roles of Integrated Cardiovascular Palliative Care Across Subspecialties
Apart from the general principles of cardiovascular palliative care that apply regardless of etiology, there are specific challenges for certain CVD diagnoses. As discussed above, palliative care for ischemic heart disease may focus more on pain relief, whereas palliative care for patients with valvular disease may focus on advance care planning and shared decision-making surrounding valvular procedures. Integration of palliative care into CVD care may require consideration of the unique needs of each patient population, in terms of both the timing of referral and nature of palliative care intervention. This diversity of needs necessitates a tailored approach incorporating primary palliative care from cardiology subspecialists in addition to secondary palliative care specialists with cardiac expertise.
The roles of palliative care for specific CVD etiologies discussed in this article are not exhaustive but represent some of the most common diagnoses with the best available data on palliative care integration. Future investigation and discussion should evaluate the role of palliative care for other subspecialties, such as cardio-oncology, cardio-obstetrics, critical care cardiology, stroke, and pulmonary hypertension to improve understanding of palliative care utilization and provide further data to make cardiovascular palliative care services more robust.
To improve incorporation of palliative care into CVD care based on current data highlighted in this review, we recommend the following: 1) dedicated trials of different palliative care interventions for specific CVD etiologies to establish evidence-based approaches, 2) primary palliative care training in general and subspecialty cardiology fellowships including communication education and palliative care principles for specific CVD populations, 3) clear consensus criteria for specialty palliative care referral within the guidelines, 4) embedded palliative care models, including non-physician palliative care specialists, in cardiac subspecialty programs, and 5) novel telemedicine palliative cardiology services to improve access for safety-net and rural centers. Through these broad aims, we would achieve a data-drive cardiovascular palliative care paradigm, incorporating both quality primary palliative care and guideline-directed secondary palliative care, to improve important patient-centered outcomes for patients with CVD.
Acknowledgements
The authors would like to thank Christine L. Chen, MD and Karim Saldahar, BS for creation of the article figures.
Conflict of Interest Disclosures
Sarah Godfrey – None
James Kirkpatrick – None
Daniel Kramer -- R01HL161697 grant
Melanie Sulistio – None
Non-standard Abbreviations and Acronyms
- ACGME
Accreditation Council of Graduate Medical Education
- ACS
Acute Coronary Syndrome
- ACHD
Adult Congenital Heart Disease
- ACLS
Advanced Cardiac Life Support
- APP
Advanced Practice Provider
- ABI
Ankle-Brachial Index
- ATP
Anti-Tachycardia Pacing
- ASD
Atrial Septal Defect
- CVD
Cardiovascular Disease
- ICD
Implantable Cardioverter-Defibrillator
- PDA
Patent Ductus Arteriosus
- PAD
Peripheral Arterial Disease
- PPM
Permanent Pacemaker
- SAQ
Seattle Angina Questionnaire
- STS
Society of Thoracic Surgeons
- TAVR
Transcatheter Aortic Valve Replacement
- VSD
Ventricular Septal Defect
References
- 1.Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, Baker-Smith CM, Beaton AZ, Boehme AK, Buxton AE, et al. Heart disease and stroke statistics-2023 update: A report from the American Heart Association. Circulation. 2023;147:e93–e621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention, National Center for Health Statistics. About Multiple Cause of Death, 1999–2020. CDC WONDER Online Database website. 2022; [Google Scholar]
- 3.Kelley AS, Morrison RS. Palliative care for the seriously ill. N Engl J Med. 2015;373:747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sidebottom AC, Jorgenson A, Richards H, Kirven J, Sillah A. Inpatient palliative care for patients with acute heart failure: outcomes from a randomized trial. J Palliat Med. 2015;18:134–142. [DOI] [PubMed] [Google Scholar]
- 5.Rogers JG, Patel CB, Mentz RJ, Granger BB, Steinhauser KE, Fiuzat M, Adams PA, Speck A, Johnson KS, Krishnamoorthy A, et al. Palliative Care in Heart Failure: The PAL-HF Randomized, Controlled Clinical Trial. J Am Coll Cardiol. 2017;70:331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong FKY, Ng AYM, Lee PH, Lam P-T, Ng JSC, Ng NHY, Sham MMK. Effects of a transitional palliative care model on patients with end-stage heart failure: a randomised controlled trial. Heart. 2016;102:1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brännström M, Boman K. Effects of person‐centred and integrated chronic heart failure and palliative home care. PREFER: a randomized controlled study. Eur J Heart Fail. 2014. [DOI] [PubMed] [Google Scholar]
- 8.Evangelista LS, Lombardo D, Malik S, Ballard-Hernandez J, Motie M, Liao S. Examining the effects of an outpatient palliative care consultation on symptom burden, depression, and quality of life in patients with symptomatic heart failure. J Card Fail. 2012;18:894–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ng AYM, Wong FKY. Effects of a Home-Based Palliative Heart Failure Program on Quality of Life, Symptom Burden, Satisfaction and Caregiver Burden: A Randomized Controlled Trial. J Pain Symptom Manage. 2018;55:1–11. [DOI] [PubMed] [Google Scholar]
- 10.Diop MS, Rudolph JL, Zimmerman KM, Richter MA, Skarf LM. Palliative Care Interventions for Patients with Heart Failure: A Systematic Review and Meta-Analysis. J Palliat Med. 2017;20:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dowd JE, Zimmerman P, Rowe C, Volz E, Patel L. Home Is Where the Heart Is: The Impact of Home-Based Palliative Care on Patients with Advanced Heart Failure. J Card Fail. 2020;26:S112. [Google Scholar]
- 12.O’Donnell AE, Schaefer KG, Stevenson LW, DeVoe K, Walsh K, Mehra MR, Desai AS. Social Worker–Aided Palliative Care Intervention in High-risk Patients With Heart Failure (SWAP-HF): A Pilot Randomized Clinical Trial. JAMA Cardiol. 2018;3:516–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maqsood MH, Khan MS, Warraich HJ. Association of Palliative Care Intervention With Health Care Use, Symptom Burden and Advance Care Planning in Adults With Heart Failure and Other Noncancer Chronic Illness. J Pain Symptom Manage. 2021;62:828–835. [DOI] [PubMed] [Google Scholar]
- 14.Pattenden JF, Mason AR, Lewin RJP. Collaborative palliative care for advanced heart failure: outcomes and costs from the “Better Together” pilot study. BMJ Support Palliat Care. 2013;3:69–76. [DOI] [PubMed] [Google Scholar]
- 15.Taylor GJ, Lee DM, Baicu CF, Zile MR. Palliative care for advanced heart failure in a department of veterans affairs regional hospice program: Patient selection, a treatment protocol, and clinical course. J Palliat Med. 2017;20:1068–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sahlollbey N, Lee CKS, Shirin A, Joseph P. The impact of palliative care on clinical and patient-centred outcomes in patients with advanced heart failure: a systematic review of randomized controlled trials. Eur J Heart Fail. 2020;22:2340–2346. [DOI] [PubMed] [Google Scholar]
- 17.Sahlen K-G, Boman K, Brännström M. A cost-effectiveness study of person-centered integrated heart failure and palliative home care: Based on a randomized controlled trial. Palliat Med. 2016;30:296–302. [DOI] [PubMed] [Google Scholar]
- 18.Liu AY, O’Riordan DL, Marks AK, Bischoff KE, Pantilat SZ. A Comparison of Hospitalized Patients With Heart Failure and Cancer Referred to Palliative Care. JAMA Netw Open. 2020;3:e200020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810–1852. [DOI] [PubMed] [Google Scholar]
- 20.Lemond L, Allen LA. Palliative care and hospice in advanced heart failure. Prog Cardiovasc Dis. 2011;54:168–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, et al. 2022 AHA/ACC/HFSA guideline for the Management of Heart Failure: Executive summary: A report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2022;145:e876–e894. [DOI] [PubMed] [Google Scholar]
- 22.Chang YK, Allen LA, McClung JA, Denvir MA, Philip J, Mori M, Perez-Cruz P, Cheng S-Y, Collins A, Hui D. Criteria for Referral of Patients With Advanced Heart Failure for Specialized Palliative Care. J Am Coll Cardiol. 2022;80:332–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kramer DB, Reynolds MR, Normand S-L, Parzynski CS, Spertus JA, Mor V, Mitchell SL. Hospice use following implantable cardioverter-defibrillator implantation in older patients: Results from the National Cardiovascular Data Registry. Circulation. 2016;133:2030–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munday D, Boyd K, Jeba J, Kimani K, Moine S, Grant L, Murray S. Defining primary palliative care for universal health coverage. Lancet. 2019;394:621–622. [DOI] [PubMed] [Google Scholar]
- 25.Quill TE, Abernethy AP. Generalist plus specialist palliative care--creating a more sustainable model. N Engl J Med. 2013;368:1173–1175. [DOI] [PubMed] [Google Scholar]
- 26.Kim JM, Godfrey S, O’Neill D, Sinha SS, Kochar A, Kapur NK, Katz JN, Warraich HJ. Integrating palliative care into the modern cardiac intensive care unit: a review. Eur Heart J Acute Cardiovasc Care. 2022;11:442–449. [DOI] [PubMed] [Google Scholar]
- 27.Warraich HJ, Wolf SP, Mentz RJ, Rogers JG, Samsa G, Kamal AH. Characteristics and trends among patients with cardiovascular disease referred to Palliative Care. JAMA Netw Open. 2019;2:e192375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davies A, Fox K, Galassi AR, Banai S, Ylä-Herttuala S, Lüscher TF. Management of refractory angina: an update. Eur Heart J. 2021;42:269–283. [DOI] [PubMed] [Google Scholar]
- 29.Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–477. [DOI] [PubMed] [Google Scholar]
- 30.Jespersen L, Abildstrøm SZ, Hvelplund A, Prescott E. Persistent angina: highly prevalent and associated with long-term anxiety, depression, low physical functioning, and quality of life in stable angina pectoris. Clin Res Cardiol. 2013;102:571–581. [DOI] [PubMed] [Google Scholar]
- 31.Damluji AA, Forman DE, Wang TY, Chikwe J, Kunadian V, Rich MW, Young BA, Page RL 2nd, DeVon HA, Alexander KP, et al. Management of acute coronary syndrome in the older adult population: A scientific statement from the American Heart Association. Circulation. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henry TD, Satran D, Jolicoeur EM. Treatment of refractory angina in patients not suitable for revascularization. Nat Rev Cardiol. 2014;11:78–95. [DOI] [PubMed] [Google Scholar]
- 33.Murphy I, Sivashankar A, Gadoud A. Refractory angina is a growing challenge for palliative medicine: a systematic review of non-invasive interventions. BMJ Support Palliat Care. 2020; [DOI] [PubMed] [Google Scholar]
- 34.Moore RK, Groves D, Bateson S, Barlow P, Hammond C, Leach AA, Chester MR. Health related quality of life of patients with refractory angina before and one year after enrolment onto a refractory angina program. Eur J Pain. 2005;9:305–310. [DOI] [PubMed] [Google Scholar]
- 35.Moore RKG, Groves DG, Bridson JD, Grayson AD, Wong H, Leach A, Lewin RJP, Chester MR. A brief cognitive-behavioral intervention reduces hospital admissions in refractory angina patients. J Pain Symptom Manage. 2007;33:310–316. [DOI] [PubMed] [Google Scholar]
- 36.Asbury EA, Webb CM, Probert H, Wright C, Barbir M, Fox K, Collins P. Cardiac rehabilitation to improve physical functioning in refractory angina: a pilot study. Cardiology. 2012;122:170–177. [DOI] [PubMed] [Google Scholar]
- 37.Tinson D Clinical and psychological outcomes of an angina management programme. Br J Cardiol. 2016;23:61–64. [Google Scholar]
- 38.Patel PA, Khan M, Thapar S. The short-and long-term impact of psychotherapy in patients with chronic, refractory angina. Br J Cardiol. 2016;23:57–60. [Google Scholar]
- 39.Spertus JA, Arnold SV. The evolution of patient-reported outcomes in clinical trials and management of patients with coronary artery disease: 20 years with the Seattle angina questionnaire. JAMA Cardiol. 2018;3:1035–1036. [DOI] [PubMed] [Google Scholar]
- 40.Makkar RR, Fontana GP, Jilaihawi H, Kapadia S, Pichard AD, Douglas PS, Thourani VH, Babaliaros VC, Webb JG, Herrmann HC, et al. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med. 2012;366:1696–1704. [DOI] [PubMed] [Google Scholar]
- 41.Ando T, Adegbala O, Uemura T, Akintoye E, Ashraf S, Briasoulis A, Takagi H, Afonso L. Incidence, Trends, and Predictors of Palliative Care Consultation After Aortic Valve Replacement in the United States. J Palliat Care. 2019;34:111–117. [DOI] [PubMed] [Google Scholar]
- 42.Coylewright M, Palmer R, O’Neill ES, Robb JF, Fried TR. Patient-defined goals for the treatment of severe aortic stenosis: a qualitative analysis. Health Expect. 2016;19:1036–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bernacki GM, Starks H, Krishnaswami A, Steiner JM, Allen MB, Batchelor WB, Yang E, Wyman J, Kirkpatrick JN. Peri-procedural code status for transcatheter aortic valve replacement: Absence of program policies and standard practices. J Am Geriatr Soc. 2022; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steiner JM, Cooper S, Kirkpatrick JN. Palliative care in end-stage valvular heart disease. Heart. 2017;103:1233–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Masoudi FM, Oetgen WJ. The NCDR ICD registry: A foundation for quality improvement. J Am Coll Cardiol. 2017;70:1673–1674. [DOI] [PubMed] [Google Scholar]
- 46.Fudim M, Carlisle MA, Devaraj S, Ajam T, Ambrosy AP, Pokorney SD, Al-Khatib SM, Kamalesh M. One-year mortality after implantable cardioverter-defibrillator placement within the Veterans Affairs Health System. Eur J Heart Fail. 2020;22:859–867. [DOI] [PubMed] [Google Scholar]
- 47.Stoevelaar R, Brinkman-Stoppelenburg A, van Driel AG, Theuns DA, Bhagwandien RE, van Bruchem-Visser RL, Lokker IE, van der Heide A, Rietjens JA. Trends in time in the management of the implantable cardioverter defibrillator in the last phase of life: a retrospective study of medical records. Eur J Cardiovasc Nurs. 2019;18:449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trussler A, Alexander B, Campbell D, Alhammad N, Enriquez A, Chacko S, Garrett T, Simpson C, Redfearn D, Abdollah H, Herx L, Baranchuk A. Deactivation of implantable cardioverter defibrillator in patients with terminal diagnoses. Am J Cardiol. 2019;124:1064–1068. [DOI] [PubMed] [Google Scholar]
- 49.Mark DB, Anstrom KJ, Sun JL, Clapp-Channing NE, Tsiatis AA, Davidson-Ray L, Lee KL, Bardy GH, Sudden Cardiac Death in Heart Failure Trial Investigators. Quality of life with defibrillator therapy or amiodarone in heart failure. N Engl J Med. 2008;359:999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sears SF, Rosman L, Sasaki S, Kondo Y, Sterns LD, Schloss EJ, Kurita T, Meijer A, Raijmakers J, Gerritse B, et al. Defibrillator shocks and their effect on objective and subjective patient outcomes: Results of the PainFree SST clinical trial. Heart Rhythm. 2018;15:734–740. [DOI] [PubMed] [Google Scholar]
- 51.Kajanová A, Eisenberger M, Řimnáčová Z. Comparison of health-related quality of life between patients with implantable cardioverter defibrillators and pacemaker recipients. In: Psychological, Emotional, Social and Cognitive Aspects of Implantable Cardiac Devices. Cham: Springer International Publishing; 2017. p. 67–84. [Google Scholar]
- 52.Ahmad M, Bloomstein L, Roelke M, Bernstein AD, Parsonnet V. Patients’ attitudes toward implanted defibrillator shocks. Pacing Clin Electrophysiol. 2000;23:934–938. [DOI] [PubMed] [Google Scholar]
- 53.Kinch Westerdahl A, Sjöblom J, Mattiasson A-C, Rosenqvist M, Frykman V. Implantable cardioverter-defibrillator therapy before death: high risk for painful shocks at end of life. Circulation. 2014;129:422–429. [DOI] [PubMed] [Google Scholar]
- 54.Mitchell LB, Pineda EA, Titus JL, Bartosch PM, Benditt DG. Sudden death in patients with implantable cardioverter defibrillators. J Am Coll Cardiol. 2002;39:1323–1328. [DOI] [PubMed] [Google Scholar]
- 55.Sherazi S, McNitt S, Aktas MK, Polonsky B, Shah AH, Moss AJ, Daubert JP, Zareba W. End-of-life care in patients with implantable cardioverter defibrillators: a MADIT-II substudy. Pacing Clin Electrophysiol. 2013;36:1273–1279. [DOI] [PubMed] [Google Scholar]
- 56.Stoevelaar R, Stoppelenburg A, van Bruchem-Visser RL, van Driel AG, Theuns DA, Lokker ME, Bhagwandien RE, van der Heide A, Rietjens JA. Advance care planning and end-of-life care in patients with an implantable cardioverter defibrillator: The perspective of relatives. Palliat Med. 2021;35:904–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kirkpatrick JN, Gottlieb M, Sehgal P, Patel R, Verdino RJ. Deactivation of implantable cardioverter defibrillators in terminal illness and end of life care. Am J Cardiol. 2012;109:91–94. [DOI] [PubMed] [Google Scholar]
- 58.Berlacher M, Abousaab C, Chen C, Suarez A, Garrett BE, Badia RR, Newcomer K, Lee S, Ayers C, Sulistio MS. ICD knowledge and attitudes at end of life in a diverse and vulnerable patient population. J Health Care Poor Underserved. 2022;33:1793–1808. [DOI] [PubMed] [Google Scholar]
- 59.Diop MS, Bowen GS, Jiang L, Wu W-C, Cornell PY, Gozalo P, Rudolph JL. Palliative Care Consultation Reduces Heart Failure Transitions: A Matched Analysis. J Am Heart Assoc. 2020;9:e013989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lampert R, Hayes DL, Annas GJ, Farley MA, Goldstein NE, Hamilton RM, Kay GN, Kramer DB, Mueller PS, Padeletti L, et al. HRS Expert Consensus Statement on the Management of Cardiovascular Implantable Electronic Devices (CIEDs) in patients nearing end of life or requesting withdrawal of therapy. Heart Rhythm. 2010;7:1008–1026. [DOI] [PubMed] [Google Scholar]
- 61.Kramer DB, Mitchell SL, Brock DW. Deactivation of pacemakers and implantable cardioverter-defibrillators. Prog Cardiovasc Dis. 2012;55:290–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zellner RA, Aulisio MP, Lewis WR. Should implantable cardioverter-defibrillators and permanent pacemakers in patients with terminal illness be deactivated? Circ. Arrhythm. Electrophysiol 2009;2:340–344. [DOI] [PubMed] [Google Scholar]
- 63.Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, Browner D. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381–386. [DOI] [PubMed] [Google Scholar]
- 64.Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, Gaudet MM, Jemal A, Siegel RL. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68:284–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kwong M, Curtis EE, Mell MW. Underutilization of Palliative Care for Patients with Advanced Peripheral Arterial Disease. Ann Vasc Surg. 2021;76:211–217. [DOI] [PubMed] [Google Scholar]
- 66.Liles DR, Kallen MA, Petersen LA, Bush RL. Quality of life and peripheral arterial disease. J Surg Res. 2006;136:294–301. [DOI] [PubMed] [Google Scholar]
- 67.Honda Y, Mok Y, Mathews L, Hof JRV, Daumit G, Kucharska-Newton A, Selvin E, Mosley T, Coresh J, Matsushita K. Psychosocial factors and subsequent risk of hospitalizations with peripheral artery disease: The Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis. 2021;329:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yefimova M, Aslakson RA, Yang L, Garcia A, Boothroyd D, Gale RC, Giannitrapani K, Morris AM, Johanning JM, Shreve S, et al. Palliative care and end-of-life outcomes following high-risk surgery. JAMA Surg. 2020;155:138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morton C, Hayssen H, Kawaji Q, Kaufman M, Blitzer D, Uemura T, Kheirbek R, Nagarsheth K. Palliative Care Consultation is Associated with Decreased Rates of In-Hospital Mortality Among Patients Undergoing Major Amputation. Ann Vasc Surg. 2022; [DOI] [PubMed] [Google Scholar]
- 70.Gomes B, Calanzani N, Gysels M, Hall S, Higginson IJ. Heterogeneity and changes in preferences for dying at home: a systematic review. BMC Palliative Care. 2013;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.de Mestral C, Hsu AT, Talarico R, Lee DS, Hussain MA, Salata K, Al-Omran M, Tanuseputro P. End-of-life care following leg amputation in patients with peripheral artery disease or diabetes. Br J Surg. 2020;107:64–72. [DOI] [PubMed] [Google Scholar]
- 72.Wilson DG, Harris SK, Peck H, Hart K, Jung E, Azarbal AF, Mitchell EL, Landry GJ, Moneta GL. Patterns of care in hospitalized vascular surgery patients at end of life. JAMA Surg. 2017;152:183–190. [DOI] [PubMed] [Google Scholar]
- 73.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, et al. Heart disease and stroke statistics-2021 update: A report from the American Heart Association. Circulation. 2021;143:e254–e743. [DOI] [PubMed] [Google Scholar]
- 74.Egbe AC, Miranda WR, Jain CC, Bonnichsen CR, Anderson JH, Dearani JA, Warnes CA, Crestanello J, Connolly HM. Incidence and outcomes of advanced heart failure in adults with congenital heart disease. Circ Heart Fail. 2022;15:e009675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Steiner JM, Dhami A, Brown CE, Stout KK, Curtis JR, Engelberg RA, Kirkpatrick JN. It’s part of who I am: The impact of congenital heart disease on adult identity and life experience. Int J Cardiol Congenit Heart Dis. 2021;4:100146. [Google Scholar]
- 76.Schwerzmann M, Goossens E, Gallego P, Kovacs AH, Moons P, Swan L, Tobler D, de Stoutz N, Gabriel H, Greutmann M, et al. Recommendations for advance care planning in adults with congenital heart disease: a position paper from the ESC Working Group of Adult Congenital Heart Disease, the Association of Cardiovascular Nursing and Allied Professions (ACNAP), the European Association for Palliative Care (EAPC), and the International Society for Adult Congenital Heart Disease (ISACHD). Eur Heart J. 2020;41:4200–4210. [DOI] [PubMed] [Google Scholar]
- 77.Tobler D, Greutmann M, Colman JM, Greutmann-Yantiri M, Librach LS, Kovacs AH. End-of-life care in hospitalized adults with complex congenital heart disease: care delayed, care denied. Palliat Med. 2012;26:72–79. [DOI] [PubMed] [Google Scholar]
- 78.Clark JK, Fasciano K. Young adult palliative care: challenges and opportunities. Am J Hosp Palliat Care. 2015;32:101–111. [DOI] [PubMed] [Google Scholar]
- 79.Fteropoulli T, Stygall J, Cullen S, Deanfield J, Newman SP. Quality of life of adult congenital heart disease patients: a systematic review of the literature. Cardiol Young. 2013;23:473–485. [DOI] [PubMed] [Google Scholar]
- 80.Kamphuis M, Ottenkamp J, Vliegen HW, Vogels T, Zwinderman KH, Kamphuis RP, Verloove-Vanhorick SP. Health related quality of life and health status in adult survivors with previously operated complex congenital heart disease. Heart. 2002;87:356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kovacs AH, Saidi AS, Kuhl EA, Sears SF, Silversides C, Harrison JL, Ong L, Colman J, Oechslin E, Nolan RP. Depression and anxiety in adult congenital heart disease: predictors and prevalence. Int J Cardiol. 2009;137:158–164. [DOI] [PubMed] [Google Scholar]
- 82.Jackson JL, Leslie CE, Hondorp SN. Depressive and anxiety symptoms in adult congenital heart disease: Prevalence, health impact and treatment. Prog Cardiovasc Dis. 2018;61:294–299. [DOI] [PubMed] [Google Scholar]
- 83.Steiner JM, Stout K, Soine L, Kirkpatrick JN, Curtis JR. Perspectives on advance care planning and palliative care among adults with congenital heart disease. Congenit Heart Dis. 2019;14:403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tobler D, Greutmann M, Colman JM, Greutmann-Yantiri M, Librach LS, Kovacs AH. End-of-life in adults with congenital heart disease: a call for early communication. Int J Cardiol. 2012;155:383–387. [DOI] [PubMed] [Google Scholar]
- 85.Tobler D, Greutmann M, Colman JM, Greutmann-Yantiri M, Librach SL, Kovacs AH. Knowledge of and preference for advance care planning by adults with congenital heart disease. Am J Cardiol. 2012;109:1797–1800. [DOI] [PubMed] [Google Scholar]
- 86.Deng LX, Gleason LP, Khan AM, Drajpuch D, Fuller S, Goldberg LA, Mascio CE, Partington SL, Tobin L, Kim YY, et al. Advance care planning in adults with congenital heart disease: A patient priority. Int J Cardiol. 2017;231:105–109. [DOI] [PubMed] [Google Scholar]
- 87.Blume ED, Kirsch R, Cousino MK, Walter JK, Steiner JM, Miller TA, Machado D, Peyton C, Bacha E, Morell E, et al. Palliative care across the life span for children with heart disease: A scientific statement from the American Heart Association. Circ Cardiovasc Qual Outcomes. 2023;e000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fulmer T, Escobedo M, Berman A, Koren MJ, Hernández S, Hult A. Physicians’ views on advance care planning and end-of-life care conversations. J Am Geriatr Soc. 2018;66:1201–1205. [DOI] [PubMed] [Google Scholar]
- 89.Kavalieratos D, Mitchell EM, Carey TS, Dev S, Biddle AK, Reeve BB, Abernethy AP, Weinberger M. “Not the ‘grim reaper service’”: an assessment of provider knowledge, attitudes, and perceptions regarding palliative care referral barriers in heart failure. J Am Heart Assoc. 2014;3:e000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Singh GK, Davidson PM, Macdonald PS, Newton PJ. The perspectives of health care professionals on providing end of life care and palliative care for patients with chronic heart failure: An integrative review. Heart Lung Circ. 2019;28:539–552. [DOI] [PubMed] [Google Scholar]
- 91.Crousillat DR, Keeley BR, Buss MK, Zheng H, Polk DM, Schaefer KG. Palliative Care Education in Cardiology. J Am Coll Cardiol. 2018;71:1391–1394. [DOI] [PubMed] [Google Scholar]
- 92.Bonares MJ, Mah K, MacIver J, Hurlburt L, Kaya E, Rodin G, Ross H, Zimmermann C, Wentlandt K. Referral practices of cardiologists to specialist palliative care in Canada. CJC Open. 2021;3:460–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dunlay SM, Foxen JL, Cole T, Feely MA, Loth AR, Strand JJ, Wagner JA, Swetz KM, Redfield MM. A survey of clinician attitudes and self-reported practices regarding end-of-life care in heart failure. Palliat Med. 2015;29:260–267. [DOI] [PubMed] [Google Scholar]
- 94.Schallmo MK, Dudley-Brown S, Davidson PM. Healthcare providers’ perceived communication barriers to offering palliative care to patients with heart failure: An integrative review. J Cardiovasc Nurs. 2019;34:E9–E18. [DOI] [PubMed] [Google Scholar]
- 95.Kamal AH, Bull JH, Swetz KM, Wolf SP, Shanafelt TD, Myers ER. Future of the Palliative Care Workforce: Preview to an Impending Crisis. Am J Med. 2017;130:113–114. [DOI] [PubMed] [Google Scholar]
- 96.Cox CE, Olsen MK, Casarett D, Haines K, Al-Hegelan M, Bartz RR, Katz JN, Naglee C, Ashana D, Gilstrap D, et al. Operationalizing needs-focused palliative care for older adults in intensive care units: Design of and rationale for the PCplanner randomized clinical trial. Contemp Clin Trials. 2020;98:106163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Morrison RS, Augustin R, Souvanna P, Meier DE. America’s care of serious illness: a state-by-state report card on access to palliative care in our nation’s hospitals. J Palliat Med. 2011;14:1094–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hutchinson RN, Han PKJ, Lucas FL, Black A, Sawyer D, Fairfield K. Rural disparities in end-of-life care for patients with heart failure: Are they due to geography or socioeconomic disparity? J Rural Health. 2022;38:457–463. [DOI] [PubMed] [Google Scholar]
- 99.Smith CB, Brawley OW. Disparities in access to palliative care. In: Meeting the Needs of Older Adults with Serious Illness. New York, NY: Springer New York; 2014. p. 19–29. [Google Scholar]
- 100.Morrison RS, Meier DE. Palliative Care. N Engl J Med. 2004;350:2582–2590. [DOI] [PubMed] [Google Scholar]


