Abstract
To evaluate a potential pharmacokinetic interaction of coadministration of fluconazole, and indinavir, a human immunodeficiency virus (HIV) protease inhibitor, 13 patients were enrolled in a multiple-dose, three-period, placebo-controlled, crossover study. Patients were randomly assigned to receive indinavir at 1,000 mg every 8 h for 7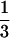 days (with fluconazole placebo), fluconazole at 400 mg once daily for 8 days (with indinavir placebo), and indinavir with fluconazole in combination. The pharmacokinetics of both drugs were measured on day 8 of each treatment period. The peak concentration in plasma (Cmax) and the time to reach Cmax were obtained by inspection, and the area under curve (AUC) was calculated for indinavir and fluconazole for each treatment period in which the respective drugs were administered. There was a marginally (P = 0.08) statistically significant decrease in the AUC from 0 to 8 h (AUC0–8) for indinavir when it was administered with fluconazole. However, the magnitudes of the decreases in Cmax and the concentration at 8 h postdosing (C8) were not as great as the decrease in AUC0–8. Although the 90% confidence interval for the geometric mean ratio was within the hypothesized limits, the clinical significance is not clear. Indinavir coadministration with fluconazole had no statistically (P > 0.5) or clinically significant effect on the Cmax and C8 of indinavir. Fluconazole coadministration with indinavir had no statistically or clinically significant effect on the pharmacokinetics of fluconazole. One patient was discontinued because of mild to moderate abdominal pain and diarrhea while on indinavir and fluconazole in combination. No serious adverse experience according to the results of laboratory tests was noted. Total bilirubin levels in serum were mildly increased in most patients treated with indinavir. This was not clinically significant and was not affected by the coadministration of fluconazole. Although the values of the pharmacokinetic parameters for indinavir decrease in the presence of fluconazole, indinavir and fluconazole can be administered concomitantly to HIV-infected patients without adjustment of the dose of either drug, and both drugs are generally well tolerated.
days (with fluconazole placebo), fluconazole at 400 mg once daily for 8 days (with indinavir placebo), and indinavir with fluconazole in combination. The pharmacokinetics of both drugs were measured on day 8 of each treatment period. The peak concentration in plasma (Cmax) and the time to reach Cmax were obtained by inspection, and the area under curve (AUC) was calculated for indinavir and fluconazole for each treatment period in which the respective drugs were administered. There was a marginally (P = 0.08) statistically significant decrease in the AUC from 0 to 8 h (AUC0–8) for indinavir when it was administered with fluconazole. However, the magnitudes of the decreases in Cmax and the concentration at 8 h postdosing (C8) were not as great as the decrease in AUC0–8. Although the 90% confidence interval for the geometric mean ratio was within the hypothesized limits, the clinical significance is not clear. Indinavir coadministration with fluconazole had no statistically (P > 0.5) or clinically significant effect on the Cmax and C8 of indinavir. Fluconazole coadministration with indinavir had no statistically or clinically significant effect on the pharmacokinetics of fluconazole. One patient was discontinued because of mild to moderate abdominal pain and diarrhea while on indinavir and fluconazole in combination. No serious adverse experience according to the results of laboratory tests was noted. Total bilirubin levels in serum were mildly increased in most patients treated with indinavir. This was not clinically significant and was not affected by the coadministration of fluconazole. Although the values of the pharmacokinetic parameters for indinavir decrease in the presence of fluconazole, indinavir and fluconazole can be administered concomitantly to HIV-infected patients without adjustment of the dose of either drug, and both drugs are generally well tolerated.
Human immunodeficiency virus (HIV) protease inhibitors are a new class of antiretroviral drugs with a high in vitro antiviral potency and a favorable toxicity profile. Recent clinical trials have demonstrated very promising results in terms of viral load, CD4-cell count, morbidity, and mortality, particularly in patients with advanced HIV infection.
Although these agents demonstrate antiretroviral effects exceeding those of the nucleoside analogs, as measured by CD4-cell count and plasma viremia levels, their long-term clinical benefit and their usefulness in patients with higher CD4-cell counts remain to be determined. Nevertheless, HIV protease inhibitors are increasingly used in the treatment of HIV-infected patients, particularly in those with advanced HIV disease and low CD4-cell counts. These patients are generally exposed to multiple drug interventions including other antiretroviral agents, drugs used as primary prophylaxis for and treatment of opportunistic infections, cancer chemotherapy, and symptomatic therapies.
Indinavir is an orally bioavailable protease inhibitor that has shown a significant antiviral in vivo effect alone at the indicated dosage of 800 mg every 8 h (q8h) and in combination with zidovudine and lamivudine, with a 1- to 2-log reduction in the number of HIV type 1 (HIV-1) RNA copies in plasma and increases in the CD4-cell number of up to 80 to 140 after 48 weeks of treatment (5).
Fluconazole is the most widely used azole antifungal compound in HIV-infected patients. This implies that coadministration of indinavir and fluconazole could be a frequent situation in the clinical setting.
The major role of the P-450 isozyme CYP3A4 in the metabolism of indinavir suggests that coadministration with drugs such as fluconazole which inhibit P-450 enzymes might alter the pharmacokinetics of indinavir (1, 2, 7). The objectives of this study were to determine the effect of coadministration of fluconazole and indinavir on the pharmacokinetic profile of indinavir in plasma and to evaluate the safety and tolerability of coadministration. The effect of indinavir on the pharmacokinetic profile of fluconazole in plasma was also assessed.
MATERIALS AND METHODS
Patients.
HIV-positive patients of both sexes (provided that, for females, a serum pregnancy test was negative and barrier contraception was used) between the ages of 18 and 60 years, with a CD4-cell count of greater than 50 cells/mm3, and with no active AIDS-defining opportunistic infection were evaluated for the trial, provided that their weight was above 45.5 kg.
They were excluded in the case of a history of hepatic disease, a positive test for hepatitis B virus surface antigen or hepatitis C virus antibodies, or any elevation of serum aspartate aminotransferase, alanine aminotransferase, or bilirubin levels during the previous 3 months or a history of threefold or greater elevations in the levels of these components in the past. Other biological exclusion criteria included a serum creatinine level above 1.5 mg/dl, a granulocyte count below 1,000/mm3, or a hemoglobin level below 9.5 g/dl. No concomitant medication with the exception of acetaminophen (paracetamol) and prophylaxis with co-trimoxazole, aerosolized pentamidine, or topical antifungals agents was allowed. Daily intakes of greater than six 12-oz. portions of caffeine-containing beverages, more than two drinks of alcohol (wine, beer, or spirits), or more than 20 cigarettes were prohibited. This protocol was approved by the Ethical Review Committee of Centre Hospitalier Universitaire Saint-Pierre, and informed consent was obtained.
Study design and procedures.
The trial was designed as a multiple-dose, randomized, three-period, crossover study. Patients were randomly assigned to treatment sequences arranged according to a two-balanced 3-by-3 Latin Square design consisting of active indinavir with fluconazole placebo (treatment A), indinavir placebo with active fluconazole (treatment B), and active indinavir with active fluconazole (treatment C). Thus, data for six sequences of treatment were obtained: ABC, BCA, CAB, ACB, BAC, and CBA. Indinavir was administered at a dosage of 1,000 mg q8h for 7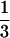 days on an empty stomach (2 h following or 1 h prior to a meal). At the time that the trial was designed, this was the highest dose shown to be well tolerated. Fluconazole was administered at a dosage of 400 mg once daily (q.d.) for 8 days. There was at least a 7-day washout period between each final dose of one treatment and the first dose of the subsequent treatment. Blood and urine for laboratory tests for drug safety were obtained prior to the administration of dose 1 and 4 h following the administration of the final dose of each treatment (day 8). Additionally, liver function tests (total and direct bilirubin, aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase levels) were determined on days 3 and 5 of each treatment.
days on an empty stomach (2 h following or 1 h prior to a meal). At the time that the trial was designed, this was the highest dose shown to be well tolerated. Fluconazole was administered at a dosage of 400 mg once daily (q.d.) for 8 days. There was at least a 7-day washout period between each final dose of one treatment and the first dose of the subsequent treatment. Blood and urine for laboratory tests for drug safety were obtained prior to the administration of dose 1 and 4 h following the administration of the final dose of each treatment (day 8). Additionally, liver function tests (total and direct bilirubin, aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase levels) were determined on days 3 and 5 of each treatment.
Physical examinations were performed prior to the administration of the first dose (day 1) and between 0 and 8 h following the administration of the final dose for each treatment (day 8). Electrocardiograms were performed prior to the administration of the first dose (day 1) of the first treatment, as well as 1 h following the administration of the final dose of each treatment (day 8). Vital signs were measured at frequently scheduled times on the first and last days of dosing. Patients were asked to maintain a diary on a card to record the times of administration of all doses and to note any adverse experiences during each treatment. A poststudy evaluation for safety consisting of laboratory tests, physical examination, and electrocardiogram was performed 24 h following the final treatment.
Pharmacokinetics.
Blood was drawn for determination of the concentration of indinavir in plasma at 0, 0.5, 1, 1.5, 2, 3, 4, 6, and 8 h following the administration of the final dose of each treatment (day 8). Blood was drawn for determination of the concentration of fluconazole in plasma prior to the administration of the morning dose on days 3, 5, 6, 7, and 8 during each treatment as well as following the administration of the final dose of each treatment (day 8) at 1, 2, 4, 8, 12, 24, and 48 h postdosing. The blood drawing schedule was identical during each treatment to preserve the blinding of the sequence of treatments, but plasma was actually analyzed for indinavir and/or fluconazole levels only for the treatments when the active drug was administered. On day 8 urine was collected from 0 to 8 h for assay of indinavir levels. A blood sample as a blank for assays for fluconazole levels and a blood sample and a urine sample as blanks for assays for indinavir levels were obtained prior to administration of the first dose of the first treatment only.
The concentrations of indinavir in plasma were determined by the high-pressure liquid chromatography technique (10). We used a modified version of the high-pressure liquid chromatography assay reference procedure in which the assay limit of quantitation was 25 ng/ml (40.7 nM) instead of the originally described limit of 5 ng/ml (8 nM). The range of the linear standard curve was 25 to 5,000 ng/ml. The interday coefficient of variation was 6% for 75 ng/ml and 3.1% for 3,500 ng/ml. The concentrations of fluconazole in plasma were determined by solvent extraction followed by separation on a methyl silicone capillary column with electron capture detection. The limit of quantitation was 5.72 μg/ml. The range of the linear standard curve was 5.1 to 50 μg/ml. The interday coefficient of variation was 3.6% for 6.03 μg/ml and 6.8% for 55.28 μg/ml.
The peak concentration in plasma (Cmax) and the time to reach Cmax (Tmax) were obtained by inspection, and the areas under the curve (AUCs) were calculated for indinavir and fluconazole for each treatment in which the respective drugs were administered. AUCs for both indinavir and fluconazole were calculated over the interval from 0 to 8 h (AUC0–8) by the modified trapezoidal method by using stable piecewise cubic polynomials (11). For indinavir, the 0- to 8-h interval represents the final dosing interval. For fluconazole the 0- to 8-h interval represents the dosing interval of indinavir on day 8 because indinavir was not continued throughout the fluconazole dosing interval. The trough concentration was also assessed for both indinavir and fluconazole by using the concentration at 8 h postdosing (C8) for indinavir and the predose concentration (C0) for fluconazole (C0 was the last measurement of the trough concentration of fluconazole with indinavir coadministration). The actual times at which plasma was sampled were used to calculate the AUC. All concentrations below the limit of quantification were treated as zero for pharmacokinetic calculations.
Data analysis.
The a priori primary hypotheses to be tested were as follows. (i) The AUC0–8 and the C8 of indinavir after 1 week of coadministration with fluconazole would not be substantially altered compared to those observed after 1 week of coadministration with placebo; i.e., the 90% confidence interval about the geometric mean ratio (with fluconazole or with placebo) for both parameters would lie between 0.50 and 2.0 (hypothesis 1). (ii) Indinavir and fluconazole administered together at multiple doses for 1 week would be sufficiently safe and well tolerated to permit coadministration in subsequent studies (hypothesis 2).
The effect of fluconazole on indinavir was assessed by evaluating the relationship between the pharmacokinetics for indinavir given alone versus the pharmacokinetics for indinavir when indinavir and fluconazole were given in combination. The converse, the relationship between fluconazole given alone and in combination with indinavir, was also evaluated in an exploratory analysis. The primary pharmacokinetic parameters of interest for indinavir were AUC0–8 and C8. The AUC and the trough concentrations of indinavir and fluconazole were natural log transformed prior to analysis. An analysis of variance appropriate for a three-period two-balanced Latin Square crossover design with the factors subject, period, treatment, and carryover was used. The method of Tomasko et al. (9) was used to test for an interaction of treatment by pairs of periods.
All statistical tests were performed at the significance level of 0.05. P values which fall in the region of 0.05 to 0.1 are referred to as marginally statistically significant.
The 90% confidence interval for the mean natural log ratio of the pharmacokinetic parameters for combination treatment versus monotreatment was also calculated by using the mean square error from the analysis of variance. No clinically significant interaction for the primary hypothesis stated above would be concluded if the observed 90% confidence interval around the ratio of the geometric mean AUC0–8 and the concentration in plasma for the combination treatment versus monotreatment for indinavir fell within the equivalence interval (0.50 to 2.0).
Safety data were analyzed by tabulation of adverse events and inspection of the safety data for each treatment group.
Given a three-period, crossover study with 12 patients and a type I error of 0.05, there was a 99% probability that the observed 90% confidence interval for the ratio of geometric mean AUCs of indinavir (combination/indinavir alone) would fall within the equivalence interval (0.50 to 2.0) if the true ratio was 1.0.
There was 99% probability that the observed 90% confidence interval for the ratio of the geometric mean C8 of indinavir (combination/indinavir alone) would fall within the equivalence interval (0.50 to 2.0).
RESULTS
Patients.
Thirteen HIV-seropositive patients participated in the study. The demographic characteristics of the group are as follows. Of the 13 patients 11 were males, 2 were females, 11 were Caucasian, and 2 were black. The mean ± standard deviation (SD) age was 39 ± 10.4 years. The mean ± SD weight was 74.1 ± 10.7 kg. Two patients were discontinued from the study due to clinical adverse experiences. One patient was discontinued from the study while receiving both active drugs and was not replaced. One patient was discontinued from the study on day 2 and was replaced. Data for all 11 patients who completed the study are included in the pharmacokinetic analysis. Data for all 13 patients are included in the safety analysis.
Pharmacokinetics. (i) Effect of fluconazole on indinavir.
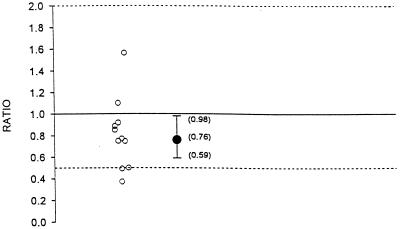
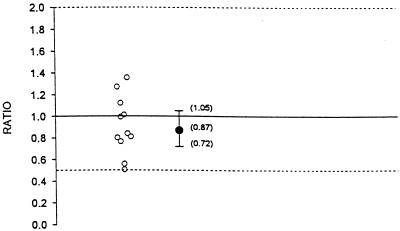
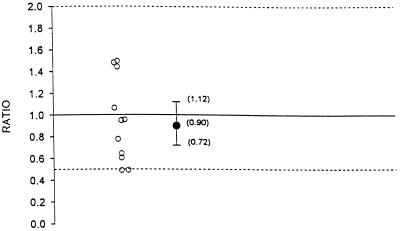
The geometric means, geometric mean ratios, and 90% confidence intervals for AUC0–8, Cmax, and C8 for indinavir administered alone and in combination with fluconazole are summarized in Table 1. Figures 1, 2, and 3 illustrate the individual AUC0–8, Cmax, and C8 ratios and geometric mean ratios with 90% confidence intervals for indinavir, respectively. Hypothesis 1 states that the AUC0–8 and C8 of indinavir after 1 week of coadministration with fluconazole would not be substantially altered compared to those observed after 1 week of coadministration with placebo (geometric mean ratio of values for combination therapy to those for monotherapy, no less than 0.50 and no more than 2.0). The geometric mean AUC0–8 values for indinavir administered alone and indinavir administered in combination with fluconazole were 39,142.3 and 29,831.4 nM · h, respectively (Table 1; Fig. 1). The geometric mean ratio of the values with combination therapy to the values with monotherapy was 0.76. This ratio was marginally statistically significant different from 1.0 (P = 0.08). The geometric mean AUC0–8s for indinavir did not differ by more than 41% between combination therapy and monotherapy. This is shown by the 90% confidence interval for the geometric mean ratio of 0.59 to 0.98. This was within the hypothesized interval of 0.50 to 2.0. The geometric mean Cmaxs of indinavir alone and in combination with fluconazole were 17,216.3 and 15,018.5 nM, respectively. The geometric mean ratio of the Cmax with combination therapy to the Cmax with monotherapy was 0.87. The 90% confidence interval for the geometric mean ratio is 0.72 to 1.05 (Table 1; Fig. 2). This was within the interval of 0.50 to 2.0. The geometric mean C8s of indinavir administered alone and indinavir administered in combination with fluconazole were 235.2 and 210.6 nM, respectively (Table 1; Fig. 3). The geometric mean ratio of the C8 with therapy combination to that with monotherapy was 0.90. The geometric mean C8 of indinavir did not differ by more than 28% between combination therapy and monotherapy. This is shown by the 90% confidence interval for the geometric mean ratio of 0.72 to 1.12. This was within the hypothesized interval of 0.50 to 2.0. The individual C8s of indinavir when it was coadministered with fluconazole ranged from 92.1 to 332.8 nM. The range is within the 95% confidence interval of 25 to 100 nM. Thus, there was a marginally statistically significant decrease in the AUC0–8 for indinavir when it was administered with fluconazole. Although the 90% confidence interval for the geometric mean ratio was within the hypothesized limits, the clinical significance is not clear. There was no statistical or clinical significant effect of coadministration of indinavir with fluconazole on Cmax or C8. In summary, there is no evidence of a clinically significant pharmacokinetic effect of the coadministration of indinavir and fluconazole on indinavir.
TABLE 1.
Pharmacokinetic parameters (geometric means) for indinavira
| Parameter | AUC0–8 (nM · h) | Cmax (nM) | C8 (nM) |
|---|---|---|---|
| Geometric mean without fluconazole | 39,142.3 | 17,216.3 | 235.2 |
| Geometric mean with fluconazole | 29,831.4 | 15,018.5 | 210.6 |
| Geometric mean ratio for combination therapy/monotherapy | 0.76 | 0.87 | 0.90 |
| 90% Confidence interval | 0.59–0.98 | 0.72–1.05 | 0.72–1.12 |
Results are based on data for 11 subjects.
FIG. 1.
Individual AUC0–8 ratios and geometric mean ratio with 90% confidence interval for indinavir (ratio = indinavir and fluconazole in combination/indinavir alone).
FIG. 2.
Individual Cmax ratios and geometric mean ratio with 90% confidence interval for indinavir (ratio = indinavir and fluconazole in combination/indinavir alone).
FIG. 3.
Individual C8 ratios and geometric mean ratio with 90% confidence interval for indinavir (ratio = indinavir and fluconazole in combination/indinavir alone).
(ii) Effect of indinavir on fluconazole.
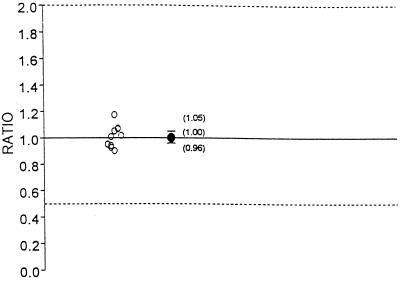
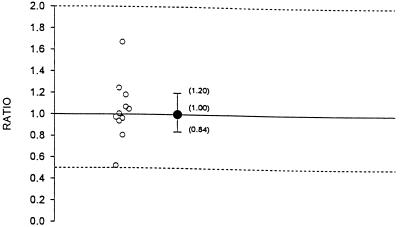
The pharmacokinetic parameters analyzed for fluconazole, which was administered at a dosage of 400 mg q.d. included AUC0–8 and C0 (or the trough concentration 24 h after dosing on day 7). There were two limitations to the study design. First, in order to evaluate the effect of indinavir on fluconazole over the 24-h dosing interval, all three indinavir doses on day 8 must have been administered; this was not required according to the protocol. Therefore, the AUC0–8 and C0 rather than AUC0–24 and C24 were calculated on day 8 for fluconazole. Second, fluconazole was administered for 8 days. However, trough concentrations indicated that the fluconazole concentrations did not reach steady state on day 8. The geometric mean AUC0–8 and C0 for fluconazole administered alone and in combination with indinavir are summarized in Table 2. The Cmax and Tmax for fluconazole were calculated but were not analyzed statistically. The Tmax values for fluconazole administered alone ranged from 0 to 24 h, and the Tmax values for fluconazole coadministered with indinavir ranged from 1 to 12 h. The arithmetic mean Cmax values for fluconazole administered alone and coadministered with indinavir were 20.79 and 20.17 μg/ml, respectively. The half-life of fluconazole is long, and thus concentrations in plasma are relatively constant throughout the fluconazole dosing interval on day 8. For a drug with this type of concentration profile in plasma (with no clear peak) after the administration of multiple doses, the parameters Tmax and Cmax are of limited value. The geometric mean AUC0–8 values for fluconazole administered alone and in combination with indinavir were 145.3 and 146.0 μg · h/ml, respectively. The geometric mean ratio of the AUC0–8 with combination therapy to that with monotherapy was 1.00 (Table 2; Fig. 4). The geometric mean AUC0–8 for fluconazole did not differ by more than 5% between combination therapy and monotherapy. This is shown by the 90% confidence interval for the geometric mean ratio of 0.96 to 1.05. This was within the interval of 0.50 to 2.0. The geometric mean C0s of fluconazole administered alone and in combination with indinavir were 13.5 and 13.5 μg/ml, respectively (Table 2; Fig. 5). The geometric mean ratio of the C0 with combination therapy to the C0 with monotherapy was 1.00. The geometric mean C0 of fluconazole did not differ by more than 20% between combination therapy and monotherapy. This is shown by the 90% confidence interval for the geometric mean ratio of 0.84 to 1.20. This was within the interval of 0.50 to 2.0. Collectively, the pharmacokinetic data indicate that the coadministration of fluconazole with indinavir had no statistically or clinically significant effect on the pharmacokinetics of fluconazole.
TABLE 2.
Pharmacokinetic parameters geometric means) for fluconazolea
| Parameter | AUC0–8 (μg · h/ml) | C0 (μg/ml) |
|---|---|---|
| Geometric mean without indinavir | 145.3 | 13.5 |
| Geometric mean with indinavir | 146.0 | 13.5 |
| Geometric mean for combination therapy/monotherapy | 1.00 | 1.00 |
| 90% Confidence interval | 0.96–1.05 | 0.84–1.20 |
Results are based on data for 11 subjects.
FIG. 4.
Individual AUC0–8 ratios and geometric mean ratio with 90% confidence interval for fluconazole (ratio = indinavir and fluconazole in combination/fluconazole alone).
FIG. 5.
Individual C0 ratios and geometric mean ratio with 90% confidence interval for fluconazole (ratio = indinavir and fluconazole in combination/fluconazole alone).
Safety.
Data for all 13 patients were included in the safety evaluation. Thirteen patients had clinical adverse experiences. The most common clinical adverse experiences were diarrhea, nausea, abdominal pain, acid regurgitation, headache, taste disturbances, hot flushes, and eye accommodation disorder during treatment with either indinavir alone, indinavir and fluconazole, or fluconazole alone. Eight patients had adverse experiences which were judged to be related to one of the study drugs. Among patients receiving the combination of indinavir and fluconazole, one patient had taste disturbance and one patient had taste loss, and another patient receiving indinavir alone reported taste loss. These were judged to be possibly related to study drugs. Four patients experienced nausea which was judged to be possibly drug related. One patient receiving indinavir alone and one patient receiving fluconazole alone had nausea. One patient receiving indinavir and fluconazole in combination had nausea. Two patients receiving indinavir and fluconazole in combination and while not receiving a study drug had nausea. All of these adverse experiences were mild or moderate.
Two patients were discontinued from the study due to clinical adverse experiences. One patient had a serious clinical adverse experience judged to be definitely not related to study drug. He received indinavir at 1,000 mg q8h plus fluconazole at 400 mg on day 1 while complaining of cough and sputum production. On day 2 he was hospitalized for a lung disorder and was discontinued from the study while off of drug. The other patient experienced nausea, taste disturbance, hot flushes, abdominal pain, and diarrhea while receiving both indinavir at 1,000 mg q8h and fluconazole at 400 mg q.d. He was discontinued on day 5 of the second treatment while receiving indinavir alone. These clinical adverse experiences were judged to be possibly related to study drug.
All 13 patients were included in the safety evaluation performed with data from laboratory tests. Three of 13 patients had adverse experiences according to the results of laboratory tests. One patient had pyuria on day 8 following the administration of fluconazole at 400 mg q.d. and on day 26 while off of either study drug. One patient had pyuria predosing on day 1 and had activated PT increased on day 25 following the administration of indinavir at 1,000 mg q8h and fluconazole at 400 q.d. The third patient had hyperkalemia on day 8 following the administration of indinavir at 1,000 mg q8h and fluconazole at 400 mg q.d. No patient had an adverse experience that was judged on the basis of laboratory test data to be related to study drug. No adverse experience was serious on the basis of laboratory test data. No patient was discontinued from the study due to an adverse experience on the basis of laboratory test data. Total bilirubin levels in serum were increased in some patients. The highest bilirubin level was 1.9 mg/dl. No clinically significant deviations in the evaluation of the data from tests for clinical safety such as physical examination, vital signs, or electrocardiogram were found.
DISCUSSION
Multiple drugs are commonly prescribed to HIV-infected patients, and the potential for drug interactions leading to either adverse events or reduced efficacy is great. It is thus particularly important to carefully evaluate potential interactions between drugs whose metabolic pathways are similar and which will commonly be administered concomitantly. This is the case for HIV protease inhibitors and azole derivatives, in particular, indinavir and fluconazole, whose coadministration could lead to increased plasma indinavir levels through inhibition of the CYP3A4 isozyme.
Several dosing regimens for indinavir have been investigated to date. At the time that this study was designed a progressive dose-response from 200 mg every 6 h (q6h) (0.8 g/day) to 600 mg q6h (2.4 g/day) had been demonstrated. In view of a potential risk of the development of resistance with dosages of less than 2.4 g/day, a study aimed at determining the maximum antiretroviral response possible with indinavir monotherapy was initiated. Three dosage regimens were investigated: 800 mg q8h (2.4 g/day), 1,000 mg q8h (3.0 g/day), and 800 mg q6h (3.2 g/day). At the time that the indinavir-fluconazole study started, the dosage of 1,000 mg q8h had been shown to be well tolerated and was selected for use in the trial. Following completion of the comparative trial with the three dosage regimens, the lower dosage (800 mg q8h) was selected for clinical development. Indinavir dosages above 2.4 g/day did not appear to exert a greater antiviral effect (8). The dosage of fluconazole chosen for the study, 400 mg q.d., is the highest dosage indicated for the treatment of fungal infections.
There was a decrease in the AUC0–8, C8, and Cmax for indinavir when it was coadministered with fluconazole. However, the magnitudes of the decreases in Cmax and C8 are not as great as the decrease observed for AUC0–8. Due to the nonlinear pharmacokinetics of indinavir, the AUC0–8 of indinavir when it is coadministered with fluconazole is anticipated to be reduced, but it is greater than the AUC0–8 seen with indinavir given at 600 mg q8h. The mechanism for the decrease in the AUC0–8 could be due to induction of P-450 metabolism by fluconazole. Fluconazole appears to induce some mammalian P-450 enzymes, and thus, in theory, fluconazole has the potential to increase as well as decrease the clearance of P-450-metabolized drugs (3).
However, the effects of fluconazole on the pharmacokinetics of other drugs (such as phenytoin, cyclosporine, and anticoagulants) appear to be mediated predominantly by P-450 inhibition, not P-450 induction. In this study, no increase in the AUC0–8 for indinavir consistent with P-450 inhibition was observed. In contrast, coadministration of indinavir with ketoconazole does result in an increase in plasma indinavir concentrations (6). This is consistent with the greater potency of ketoconazole versus that of fluconazole as a P-450 inhibitor (7). Another possibility for the apparent effect of fluconazole on indinavir pharmacokinetics is interference with absorption, but there is no known precedent for such an effect of fluconazole. Given that the effect of fluconazole on indinavir did not quite reach statistical significance, this result could well have been a chance occurrence. In any event, the clinical significance of this small decrease in the AUC0–8 for indinavir is unclear. However, this does not warrant a dose adjustment. The AUC0–8 and C0 values for fluconazole when it was and those for fluconazole when it was administered alone in combination with indinavir did not differ significantly. This suggests that indinavir, a potential CYP3A4 inhibitor, did not affect the metabolism of fluconazole. This is consistent with the minor role of hepatic metabolism in the clearance of fluconazole. The Tmax and Cmax values for fluconazole administered alone and coadministered with indinavir are of limited value. The half-life of fluconazole is long, and thus, concentrations in plasma are relatively constant, with no clear Cmax or Tmax on day 8 (4).
A review of reports of adverse experiences and tabulated summaries of selected laboratory values indicate that indinavir and fluconazole administered alone or together were generally well tolerated. Two patients were discontinued from the study due to clinical adverse experiences. One patient had a serious clinical adverse experience, judged not to be drug related, following day 1 of treatment with indinavir plus fluconazole. He was hospitalized for a lung disorder. The other patient was discontinued from the study due to nausea, taste disturbance, hot flashes, abdominal pain, and diarrhea while receiving both indinavir and fluconazole. These clinical adverse experiences were judged to be possibly drug related. No patient had an adverse experience that was judged on the basis of data from laboratory tests to be related to one of the study drugs. No adverse experience was serious on the basis of data from laboratory tests. No patient was discontinued from the study due to an adverse experience on the basis of data from laboratory tests.
In summary, indinavir and fluconazole may be administered concomitantly to HIV-seropositive patients without adjustments of the dose of either drug. The two drugs given concurrently generally appear to be well tolerated.
REFERENCES
- 1.Balani S, Woolf E, Hoagland V, Sturgill M, Deutsch P, Yeh K, Lin J H. Disposition of indinavir, a potent HIV-1 protease inhibitor, after an oral dose in humans. Drug Metab Dispos. 1996;24:1389–1394. [PubMed] [Google Scholar]
- 2.Chiba M, Hensleigh M, Nishime J, Balani S, Lin J. Role of cytochrome P450 3A4 in human metabolism of MK-639, a potent human immunodeficiency virus protease inhibitor. Drug Metab Dispos. 1996;24:307–314. [PubMed] [Google Scholar]
- 3.Debruyne D, Ryckelynck J P. Clinical pharmacokinetics of fluconazole. Clin Pharmacokinet. 1993;24:10–27. doi: 10.2165/00003088-199324010-00002. [DOI] [PubMed] [Google Scholar]
- 4.Grant S M, Clissold S P. Fluconazole: a review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential in superficial and systemic mycoses. Drugs. 1990;39:877–916. doi: 10.2165/00003495-199039060-00006. [DOI] [PubMed] [Google Scholar]
- 5.Gulick R M, Mellors J, Havlir D, Eron J, Gonzales C, McMahon D, Richman D, Valentine F, Rooney J, Jonas L, Meiborn A, Emini E, Chodakewitz J. Abstracts of the XI International Conference on AIDS. 1996. Potent and sustained antiretroviral activity of indinavir (IDV) zidovudine (ZDV) and lamivudine (3TC), abstr. Th.B.931. [Google Scholar]
- 6.McCrea, J., E. Woolf, A. Sterrett, C. Matthews, P. Deutsch, K. Yeh, S. Waldman, and T. Bjornsson. 1996. Effects of ketoconazole and other P-450 inhibitors on the pharmacokinetics of indinavir. Pharm. Res. 13(Suppl.):S485.
- 7.Shaw J T B, Tarbit M, Troke P. Cytochrome P450 mediated sterol synthesis and metabolism: differences in sensitivity of fluconazole and other azoles. In: Fromtling R A, editor. Recent trends in the discovery, development and evaluation of antifungal agents. J. R. Barcelona, Spain: Prous Science Publishers, S.A.; 1987. pp. 125–139. [Google Scholar]
- 8.Steigbigel R, Berry P, Toppler H, Mellors J, Drusano G, Leavitt R, Hildebrand C, Jonas L, Nessly M, Deutsch P, Chodakewitz J. Abstracts of the XI International Conference on AIDS. 1996. Extended follow-up of patients in a study of indinavir at 800 mg q8h (2.4 G/D), 1000 mg q8h (3.0 G/D) and 800 mg q6h (3.2 G/D), abstr. Mo.B.412. [Google Scholar]
- 9.Tomasko L, et al. XVIIth International Biometric Conference. 1994. Comparison of two modeling procedures for the three-period, two treatment crossover pharmacokinetic drug interaction study. [Google Scholar]
- 10.Woolf E, Au T, Haddix H, Matuszewski B. Determination of L-735524, an human immunodeficiency virus protease inhibitor, in human plasma and urine via high-performance liquid chromatography with column switching. J Chromatogr A. 1995;692:45–52. doi: 10.1016/0021-9673(94)00608-c. [DOI] [PubMed] [Google Scholar]
- 11.Yeh K, Small R. Pharmacokinetic evaluation of stable piecewise cubic polynomials as numerical integration functions. J Pharmacokinet Biopharm. 1989;17:721–740. doi: 10.1007/BF01062126. [DOI] [PubMed] [Google Scholar]