Abstract
Objectives: Since 2018, we have routinely placed an Amplatzer vascular plug (AVP) in the proximal left subclavian artery (LSCA) to prevent embolic events during thoracic endovascular aortic repair with arch vessel debranching (d-TEVAR). Type II endoleaks of LSCA origin were observed in two patients (20%), and the coil-in-plug (CIP) method, i.e., microcatheter insertion through the plug and addition of coil embolization, which has been used since August 2019, was performed. This study aims to evaluate the effectiveness of the CIP method for LSCA embolization.
Methods: A total of 26 patients who underwent d-TEVAR for an aortic arch aneurysm between 2018 and 2022 were retrospectively reviewed. Ten patients who underwent d-TEVAR with a simple AVP placement (the control group) and 16 patients who underwent d-TEVAR with the CIP method (the CIP group) were compared.
Results: Two patients had type II endoleaks in the control group, whereas none had them in the CIP group. LSCA length was significantly shorter in patients with endoleaks than in those without endoleaks (24.5 vs. 50.3 mm; p<0.01). No perioperative deaths or cerebral infarctions occurred in either group.
Conclusions: AVP placement in the LSCA during d-TEVAR effectively prevented perioperative cerebral infarction. d-TEVAR with CIP was especially useful in patients with a short LSCA.
Keywords: aortic arch aneurysm, coil-in-plug, debranching thoracic endovascular aortic repair, left subclavian artery, stroke
Introduction
Thoracic endovascular aortic repair with arch vessel debranching (d-TEVAR) has been reported to have satisfactory results for aortic arch aneurysms,1–3) which has been indicated for the treatment of aortic arch aneurysms in some elderly patients with serious complications, such as chronic obstructive pulmonary disease (COPD) and cardiac dysfunction. Despite the advancements in equipment and technology, perioperative stroke remains an important complication after d-TEVAR. Previous studies have reported a stroke rate of approximately 3.8%–8%,4,5) i.e., indicating a higher number than simple TEVAR.5) Most cerebral infarctions during TEVAR are caused by flying atheroma during insertion and deployment of the stent graft. Therefore, balloon protection of the left subclavian artery (LSCA) has been reported to decrease cerebral infarction incidence.6,7) Occlusion of the LSCA without its reconstruction may also increase the risk of arm ischemia and ischemia of the vertebrobasilar artery system.8) In our hospital, all patients with landing zones 0, 1, and 2 have at least undergone an LSCA reconstruction.
Since 2018, our unit has been using an Amplatzer vascular plug (AVP) (St. Jude Medical, Plymouth, MN, USA), which is placed in the proximal LSCA before inserting the stent graft to prevent stroke. For type II endoleaks originating from the LSCA in several patients, we embolize the LSCA with AVP using the coil-in-plug (CIP) method to add coil embolization after a stent graft placement through a microcatheter placed beforehand via the AVP, a method our unit has been performing since August 2019. In addition, Kotoku et al. reported the usefulness of the CIP technique in internal iliac artery embolization.9) Therefore, this study aims to clarify the effectiveness of d-TEVAR using the CIP technique.
Materials and Methods
A total of 26 patients who underwent d-TEVAR for an aortic arch aneurysm between January 2018 and June 2022 were retrospectively reviewed. Those who underwent acute dissection were excluded. Ten patients who underwent d-TEVAR with simple AVP placement in the LSCA between January 2018 and July 2019 (the control group) and 16 patients who underwent d-TEVAR using the CIP technique after August 2019 (the CIP group) were compared. In addition to intraoperative differences, contrast-enhanced computed tomography (CT) was performed 3 months postoperatively to evaluate the presence of endoleaks. Thereafter, noncontrast CT was performed every 6 months, and if the aortic aneurysm was enlarged, contrast CT was performed again to reevaluate the presence of an endoleak. The length of the LSCA was defined as the distance from its origin to the vertebral artery bifurcation. The proximal aortic diameter was measured as the short diameter at the site where the proximal end of the stent graft was implanted. Preoperative cerebrovascular disease (CVD) was defined as a permanent focal or global neurologic dysfunction (sustained preoperatively) or a history of CVD. Ischemic heart disease was defined as a stenosis of at least 75% of one coronary artery. COPD was defined as a forced expiratory volume of <70% with the normal or daily routine use of bronchodilators. Chronic kidney disease was defined as a creatinine clearance of <50 ml/min or the need for hemodialysis. Patients with end-stage cancer, untreated carcinoma status, and a history of surgical or medical treatment for malignancy were defined as having malignancy. The diameter of the LSCA is measured 2 cm peripherally from the aortic bifurcation.
Bypass surgery procedure of d-TEVAR
The landing was zone 0 for 4 patients, and a Najuta stent graft (Kawasumi Laboratories, Inc., Tokyo, Japan)10,11) was used in all of them. One patient underwent bypass surgery using an 8-mm T-shaped expanded polytetrafluoroethylene (ePTFE) graft (W. L. Gore & Associates, Inc., Flagstaff, AZ, USA) from the right axillary artery to the left common carotid and LSCAs. Three patients underwent bypass surgery using an 8-mm ePTFE graft from the right to the left axillary artery.
The landing was zone 1 for three patients. Bypass surgery was performed using an 8-mm T-shaped ePTFE graft to the left common carotid artery and LSCA, with the right axillary artery as the inflow vessel.
The landing was zone 2 for 19 patients. Bypass surgery was performed using an 8-mm ePTFE graft to the LSCA, with the right axillary artery as the inflow vessel.
AVP placement method in the LSCA
After bypass surgery, 6-F 45-cm destination sheaths (Terumo Medical, Somerset, NJ, USA) were inserted through a puncture after a purse-string suture proximal to the left axillary artery anastomosis for the LSCA embolization.
The contrast was obtained from the sheath; AVP II was placed in the case of a sufficient distance from the LSCA bifurcation to the vertebral artery, and AVP I was placed in the case of a short distance. The plug was 30%–50% larger than the diameter of the embolized LSCA. After the plug placement, the stent graft was inserted and deployed via the femoral artery approach.
CIP method
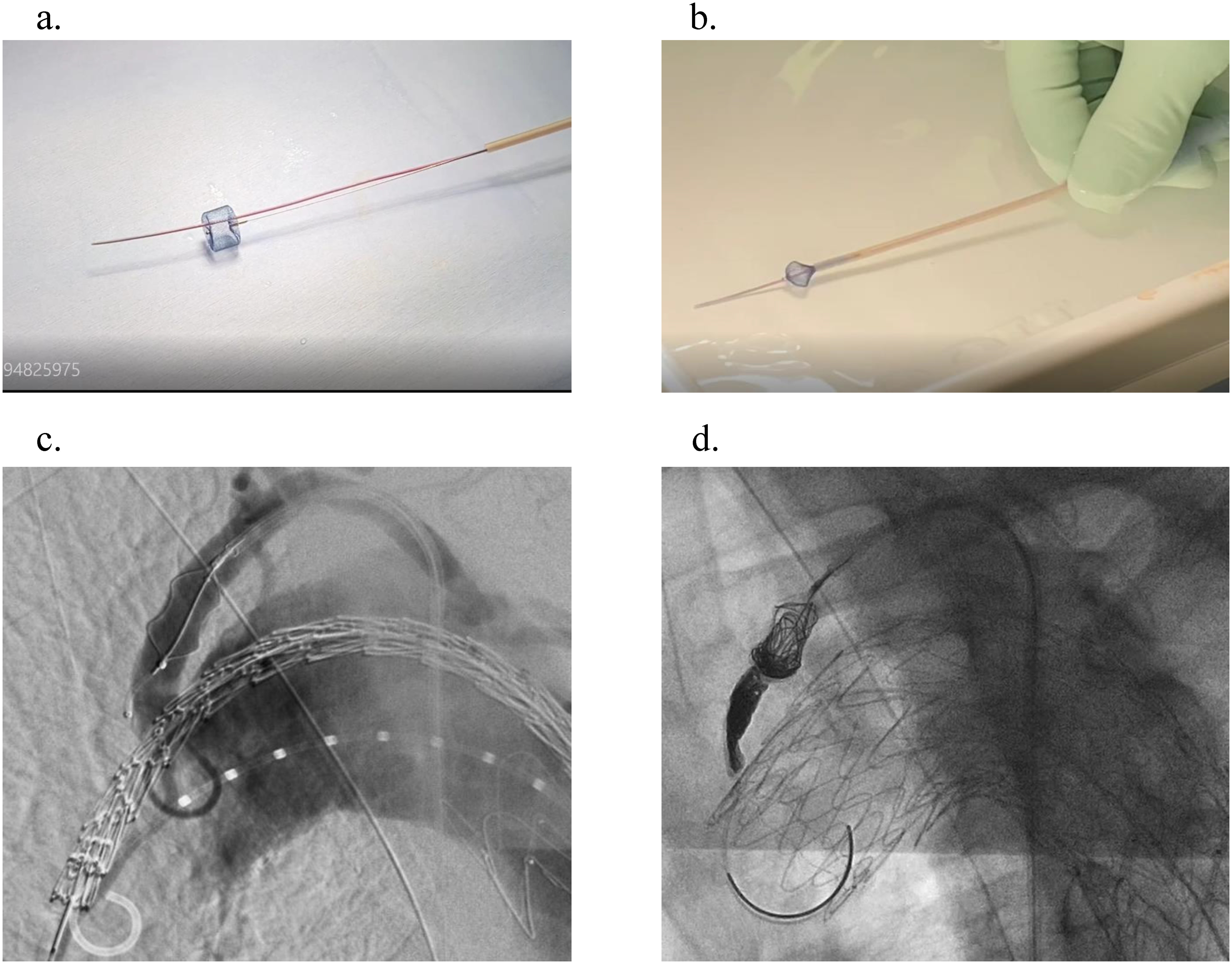
From January 2018 to July 2019, type II endoleaks were reported (2/10, 20%) in the group who underwent LSCA embolization by simply placing an AVP. Therefore, after August 2019, the CIP method was adopted using AVP I. Before implantation, the plug was deployed from the loader, and a 0.016-in guidewire (Asahi Meister; ASAHI INTECC CO., LTD., Aichi, Japan) was used to insert a 2.2-F microcatheter (Coiling Support; HI-LEX Corporation, Takarazuka, Japan) through the plug and stored in a loader (Fig. 1). Stowage was performed in a heparinized saline solution to prevent air from entering the plug, and the plug was placed before the stent graft was inserted. After the deployment of the stent graft, a microcatheter was placed through the plug and embolized between the stent graft and the plug and within the plug. AZUR CX18 (Terumo, Tokyo, Japan) and Penumbra (Penumbra Inc., Alameda, CA, USA) were used for embolization.
Fig. 1 Coil-in-plug method. (a) Before implantation, the Amplatzer vascular plug I was unfolded from the loader, and a 2.2-F microcatheter was inserted passing through the plug using a 0.016-in guidewire. (b) The plug was stored in the loader in a heparinized saline solution to prevent air from entering the plug. (c) The plug was placed in the left subclavian artery, and the stent graft was delivered to the aortic arch. (d) After deploying the stent graft, a microcatheter was placed through the plug and was embolized between the stent graft and the plug as well as inside the plug.
Endpoint analysis
The primary endpoints of the study were perioperative death, perioperative stroke, and a type II endoleak from the LSCA. Perioperative death was defined as intraoperative death and death up to 30 days postoperatively. Perioperative stroke was defined as a symptomatic cerebral infarction that occurred intraoperatively and up to 30 days postoperatively. Type II endoleak was assessed by performing intraoperative contrast and postoperative contrast-enhanced CT and based on the presence of an endoleak originating from the LSCA during the follow-up period.
Statistical analysis
Continuous data are presented as the mean±standard deviation, whereas categorical variables are presented as frequencies and percentages. We compared continuous data using Student’s t-tests and categorical variables using χ2 or Fisher’s exact tests. Two-tailed p-values were used and p<0.05 was considered statistically significant. All data were analyzed using SAS software (version 16.1; SAS Institute, Inc., Cary, NC, USA).
Results
Baseline characteristics
Table 1 shows the patients’ characteristics. The mean ages were 78±10 and 79±6 years in the control and CIP groups, respectively, with a higher number of patients with COPD in the CIP group (p=0.05). Six patients (60%) in the control group and seven (44%) in the CIP group were taking oral antiplatelet agents. No patients were taking anticoagulants. The proximal landing zone was zone 0 for 4 patients in the CIP group and none in the control group; landing zone 1 for 2 patients in the CIP group and 1 patient in the control group; and landing zone 2 for 10 patients in the CIP group and 9 patients in the control group. The anatomy of the aorta and the LSCA and the devices used are shown in Table 2. No differences were observed in the proximal aortic diameter, the maximum short diameter of the arch aorta, the maximum short diameter of the aortic aneurysm, or the vessel diameter and length of the LSCA. The type of plug and stent graft used are shown in Table 3. The Najuta stent graft was used in all patients in landing zone 0. AVP I was used as the plug in all patients in the CIP group, whereas in the control group, AVP II and AVP I were used in 8 and 2 patients, respectively. The control group used plugs that were 60% oversized relative to the diameter of the LSCA whereas the CIP group used plugs that were 31% oversized. The control group had significantly larger plugs (p=0.01). The mean number of coils used in the CIP group was 4.2. The operative time was 203±60 and 243±62 min in the control and CIP groups, respectively, without significant differences (p=0.45).
Table 1 Patient characteristics.
| Control (n=10) | CIP (n=16) | p | |
|---|---|---|---|
| Median age (y) | 78±10 | 79±6 | 0.71 |
| Male | 6 (60%) | 14 (88%) | 0.1 |
| CVD | 1 (10%) | 2 (13%) | 0.84 |
| IHD | 2 (20%) | 4 (25%) | 0.85 |
| COPD | 1 (10%) | 9 (56%) | 0.05 |
| CKD | 3 (30%) | 10 (63%) | 0.1 |
| Malignancy | 2 (20%) | 1 (6%) | 0.66 |
| Emergency | 2 (20%) | 1 (6%) | 0.66 |
| History of type B dissection | 1 (10%) | 1 (6%) | 0.68 |
| Prior intervention on AAA | 1 (10%) | 1 (6%) | 0.68 |
| Prior median sternotomy | 1 (10%) | 1 (6%) | 0.68 |
| Prior TEVAR repair | 0 | 1 (6%) | 0.81 |
| Proximal Landing zone 0 | 0 | 4 | |
| Zone 1 | 1 | 2 | |
| Zone 2 | 9 | 10 |
CIP: coil-in-plug; CVD: cerebrovascular disease; IHD: ischemic heart disease; COPD: chronic obstructive pulmonary disease; CKD: chronic kidney disease; AAA: abdominal aortic aneurysm; TEVAR: thoracic endovascular aortic repair
Table 2 Morphology.
| Control (n=10) | CIP (n=16) | p | |
|---|---|---|---|
| Proximal aortic diameter (mm) | 30±5 | 32±2 | 0.19 |
| Diameter of the aorta arch (mm) | 57±7 | 56±9 | 0.76 |
| Maximal diameter of aneurysm (mm) | 58±6 | 57±8 | 0.74 |
| Diameter of the left subclavian artery (mm) | 9±1 | 9±2 | 0.6 |
| Length of the left subclavian artery (mm) | 45±10 | 51±9 | 0.14 |
CIP: coil-in-plug
Table 3 Plug and stent graft.
| Control (n=10) | CIP (n=16) | |
|---|---|---|
| Plug | ||
| AVP I | 2 | 16 |
| AVP II | 8 | 0 |
| Stent-graft | ||
| Stent graft size (mm) | 37 (26–42) | 38 (34–44) |
| cTAG | 5 | 6 |
| Valiant | 2 | 4 |
| RELAY Plus | 2 | 1 |
| Najuta+cTAG | 0 | 3 |
| Najuta+Valiant | 0 | 1 |
| RELAY Plus+Valiant | 1 | 1 |
CIP: coil-in-plug; AVP: Amplatzer vascular plug cTAG (WL Gore & Associates, Inc., Newark, Del.), Valiant (Medtronic, Inc., Minneapolis, Minn.), RELAY Plus (Bolton Medical Inc., Sunrise, Fla.), Najuta (Kawasumi Laboratories, Inc., Tokyo, Japan)
Clinical outcomes
The mean observation period was 631±480 days. No perioperative death or perioperative cerebral infarction occurred in either group (Table 4). CT 3 months after TEVAR showed a type II endoleak of the LSCA origin in two patients (20%) in the control group: one with AVP I and the other with AVP II. In the control group, the length of the LSCA was significantly shorter in patients with endoleaks than in those without endoleaks (24.5 vs. 50.3 mm; p<0.01). Conversely, the diameter of the LSCA did not differ significantly between patients with endoleaks (7.8 mm) and those without endoleaks (9.3 mm). Patients with endoleaks used plugs that were 95% oversized for the LSCA vessel diameter, while patients without endoleaks used plugs that were 53% oversized. There was no significant difference. In one case of endoleak, the patient had a short subclavian artery with a stenosis in the middle, which caused the AVP II to protrude into the aorta. In this patient, an enlarged aortic aneurysm was observed on CT 6 months postoperatively; therefore, additional coil embolization was performed. In the same patient, the leak had spread to the greater curvature, which disappeared after additional embolization, and a reduction in aortic diameter could be observed on CT 6 months after embolization. Another patient is under observation without an enlarged aneurysm. No type II endoleak of LSCA origin was observed in the CIP group. Moreover, an aneurysmal enlargement due to distal stent graft-induced new entry was observed during the observation period in the CIP group; thus, an additional TEVAR was performed. One remote aortic-related death occurred in the CIP group. The patient had an enlarged thoracoabdominal aorta that ruptured. Due to an advanced age, the patient died without an indication for surgery.
Table 4 Clinical results.
| Control (n=10) | CIP (n=16) | |
|---|---|---|
| 30-day mortality | 0 | 0 |
| Late mortality | 0 | 1 |
| Stroke | 0 | 0 |
| Paraplegia | 0 | 0 |
| Type II EL | 2 | 0 |
| Reintervention | 1 | 1 |
CIP: coil-in-plug; EL: endoleak
Discussion
A favorable 30-day and hospital mortality rates of 3.2% and 4.8%, respectively, have been reported for median sternotomy in aortic arch aneurysms in Japan.12) Conversely, for extensive aortic aneurysms extending into the descending aorta, these rates increased to 7.5% and 11.2%, respectively. In Japan, the Japanese Circulation Society guidelines consider TEVAR with branch reconstruction for arch aneurysms unsuitable for open surgery.13) A relatively high incidence of perioperative stroke was observed in d-TEVAR,4,5) and the mortality rate of patients who develop postoperative stroke has also been reportedly high.14,15) However, solutions to prevent perioperative cerebral infarction during d-TEVAR have not been established.
Melissano et al. reported that the incidence of cerebral infarction was 4.5% in patients with an open LSCA during a stent graft insertion, but not in patients with an occluded LSCA.16) Yoshitake et al. also reported that since the cerebellum is the main site of cerebral infarction during d-TEVAR surgery, cerebral embolization via the vertebrobasilar artery system is associated with problems.17) Occlusion of the LSCA during stent graft deployment has been reportedly effective in preventing cerebral infarction.6,13) Furthermore, coil devices are commonly used as an embolization material in the LSCA, but embolization using a plug has been reported to have a shorter procedure time and less risk of migration into the vertebral artery.18,19) Since August 2019, we have adopted LSCA embolization using a plug by adding coil embolization to the plug using the CIP method after observing two patients with a type II endoleak originating from the LSCA. The CIP method has been reported by Kotoku et al. to allow embolization with a shorter length and fewer coils.9) AVP I can be embolized with a shorter landing than AVP II; however, recanalization is considered more common.20) AVP II has a higher embolization efficacy, but its longer length may cause unscheduled branch embolization.21) Therefore, the CIP method overcomes the disadvantages of AVP I by inserting a microcatheter through the plug and adding embolization to the plug. Two of our patients may have had endoleaks because of the short length of the LSCA. In one patient, AVP I was used, whereas in another patient, AVP II was used. AVP I alone may not be sufficient for embolization. In the second patient, the placement of an excessively large AVP II in a short subclavian artery may have caused distortion, resulting in leakage. Both patients could have been treated without endoleaks if the CIP method had been used. If the LSCA is sufficiently long, treatment with AVP II alone may be possible without endoleaks. However, in cases where the LSCA is short, such as in our patients, we believe that the CIP method can provide reliable embolization with a short landing without crushing the vertebral artery. If a coil is added to the end of the plug, the coil may fall onto the vertebral artery if the subclavian artery is short. In this study, no cerebral infarction occurred, and AVP placement in the LSCA before stent graft insertion was useful in preventing cerebral infarction. After adopting the CIP method, no type II endoleak was observed in one patient, suggesting that this method is particularly useful for patients with a short LSCA.
Limitations
Our study has some limitations. This was a single-center, retrospective, observational study with a small sample size. More patients and observation periods are needed to support the usefulness of the CIP method in d-TEVAR. Furthermore, due to the higher number of type II endoleaks in the control group, the CIP method that we have utilized since August 2019 has been used. Therefore, the results of this study may be influenced by improvements in patient management and equipment.
Conclusions
AVP placement in the LSCA during d-TEVAR effectively prevented perioperative cerebral infarction. d-TEVAR with CIP was especially useful in patients with a short LSCA.
IRB Information
This study was approved by the ethics committees of the Kitasato University Hospital (approval number: B22-058).
Declaration of Conflict of Interest
The authors declare that there are no conflicts of interest.
Author Contributions
Study conception: KM
Data collection: SF, TM, HM
Analysis: FS
Investigation: MF, TK
Manuscript preparation: SF
Critical review and revision: all authors
Final approval of the article: all authors
Accountability for all aspects of the work: all authors
References
- 1).Patel HJ, Shillingford MS, Williams DM, et al. Survival benefit of endovascular descending thoracic aortic repair for the high risk patient. Ann Thorac Surg 2007; 83: 1628-34; discussion, 1633-4. [DOI] [PubMed] [Google Scholar]
- 2).Ferrero E, Ferri M, Viazzo A, et al. Is total debranching a safe procedure for extensive aortic-arch disease? A single experience of 27 cases. Eur J Cardiothorac Surg 2012; 41: 177-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Murashita T, Matsuda H, Domae K, et al. Less invasive surgical treatment for aortic arch aneurysms in high-risk patients: a comparative study of hybrid thoracic endovascular aortic repair and conventional total arch replacement. J Thorac Cardiovasc Surg 2012; 143: 1007-13. [DOI] [PubMed] [Google Scholar]
- 4).von Allmen RS, Gahl B, Powell JT. Incidence of stroke following thoracic endovascular aortic repair for descending aortic aneurysm: a systematic review of the literature with meta-analysis. Eur J Vasc Endovasc Surg 2017; 53: 176-84. [DOI] [PubMed] [Google Scholar]
- 5).Bellamkonda KS, Yousef S, Nassiri N, et al. Trends and outcomes of thoracic endovascular aortic repair with open concomitant cervical debranching. J Vasc Surg 2021; 73: 1205-12.e3. [DOI] [PubMed] [Google Scholar]
- 6).Seike Y, Matsuda H, Inoue Y, et al. Balloon protection of the left subclavian artery in debranching thoracic endovascular aortic repair. J Thorac Cardiovasc Surg 2019; 157: 1336-45.e1. [DOI] [PubMed] [Google Scholar]
- 7).Yoshitake A, Hachiya T, Okamoto K, et al. Postoperative stroke after debranching with thoracic endovascular aortic repair. Ann Vasc Surg 2016; 36: 132-8. [DOI] [PubMed] [Google Scholar]
- 8).Holt PJ, Johnson C, Hinchliffe RJ, et al. Outcome of the endovascular management of aortic arch aneurysm: implications for management of the left subclavian artery. J Vasc Surg 2010; 51: 1329-38. [DOI] [PubMed] [Google Scholar]
- 9).Kotoku A, Ogawa Y, Chiba K, et al. Clinical utility of coil in plug method (CIP) for internal iliac artery embolization during endovascular aortic aneurysm repair. Ann Vasc Dis 2020; 13: 269-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Kawaguchi S, Yokoi Y, Shimazaki T, et al. Thoracic endovascular aneurysm repair in Japan: experience with fenestrated stent grafts in the treatment of distal arch aneurysms. J Vasc Surg 2008; 48 Suppl: 24S-9S. [DOI] [PubMed] [Google Scholar]
- 11).Yokoi Y, Azuma T, Yamazaki K. Advantage of a precurved fenestrated endograft for aortic arch disease: simplified arch aneurysm treatment in Japan 2010 and 2011. J Thorac Cardiovasc Surg 2013; 145 Suppl: S103-9. [DOI] [PubMed] [Google Scholar]
- 12).Kuwano H, Amano J, Yokomise H. Thoracic and cardiovascular surgery in Japan during 2010: annual report by the Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2012; 60: 680-708. [DOI] [PubMed] [Google Scholar]
- 13).JCS/JSCVS/JATS/JSVS 2020. Guideline on diagnosis and treatment of aortic aneurysm and aortic dissection. https://www.j-circ.or.jp/cms/wp-content/uploads/2020/07/JCS2020_Ogino.pdf (accessed 2022-10-30)
- 14).Gutsche JT, Cheung AT, McGarvey ML, et al. Risk factors for perioperative stroke after thoracic endovascular aortic repair. Ann Thorac Surg 2007; 84: 1195-200; discussion, 1200. [DOI] [PubMed] [Google Scholar]
- 15).Mariscalco G, Piffaretti G, Tozzi M, et al. Predictive factors for cerebrovascular accidents after thoracic endovascular aortic repair. Ann Thorac Surg 2009; 88: 1877-81. [DOI] [PubMed] [Google Scholar]
- 16).Melissano G, Tshomba Y, Bertoglio L, et al. Analysis of stroke after TEVAR involving the aortic arch. Eur J Vasc Endovasc Surg 2012; 43: 269-75. [DOI] [PubMed] [Google Scholar]
- 17).Yoshitake A, Hachiya T, Okamoto K, et al. Postoperative stroke after debranching with thoracic endovascular aortic repair. Ann Vasc Surg 2016; 36: 132-8. [DOI] [PubMed] [Google Scholar]
- 18).Meyer C, Probst C, Strunk H, et al. Second-generation Amplatzer vascular plug (AVP) for the treatment of subsequent subclavian backflow type II endoleak after TEVAR. Cardiovasc Intervent Radiol 2009; 32: 1264-7. [DOI] [PubMed] [Google Scholar]
- 19).Iida Y, Ito T, Hayashi S, et al. Repair of acute type B aortic dissection complicated by aortic rupture with debranching thoracic endovascular aortic repair and left subclavian artery occlusion using Amplatzer vascular plug II. Ann Vasc Dis 2015; 8: 252-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Tuite DJ, Kessel DO, Nicholson AA, et al. Initial clinical experience using the Amplatzer vascular plug. Cardiovasc Intervent Radiol 2007; 30: 650-4. [DOI] [PubMed] [Google Scholar]
- 21).Lopera JE. The Amplatzer vascular plug: review of evolution and current applications. Semin Intervent Radiol 2015; 32: 356-69. [DOI] [PMC free article] [PubMed] [Google Scholar]


