Abstract
A 64-year-old male patient who presented with symptoms indicative of hemolytic anemia was referred to our hospital. After obtaining the patient’s history, it was found that hemolysis occurred 14 years after he underwent ascending aortic replacement for acute type A aortic dissection. Enhanced computed tomography revealed an aortic pseudoaneurysm at the proximal anastomosis, which was thought to be the cause of hemolysis. Furthermore, aortic valve regurgitation and dilatation of the sinus of Valsalva were also found on a transthoracic echocardiogram. Therefore, the Bentall procedure was performed. During the surgery, aortic pseudoaneurysm formation and vascular graft stenosis were observed. The postoperative course was uneventful, and hemolysis diminished soon after the surgery.
Keywords: acute aortic dissection, aortic pseudoaneurysm, hemolytic anemia
Introduction
Operative survival for acute type A aortic dissection (ATAAD) has improved due to advancements in its diagnosis, surgical techniques, and perioperative management.1) However, several potential postsurgical complications, such as aortic pseudoaneurysms, still exist. Aortic pseudoaneurysms have been reported to occur in 5%–8% of cases.2) Anastomotic pseudoaneurysms can be caused by numerous factors, including the deterioration of sutures or grafts, arterial degeneration, and infection.3) Herein, we report our experience with a case complicated by hemolytic anemia due to external compression of the graft by an aortic pseudoaneurysm 14 years after emergency graft replacement for ATAAD.
Case Report
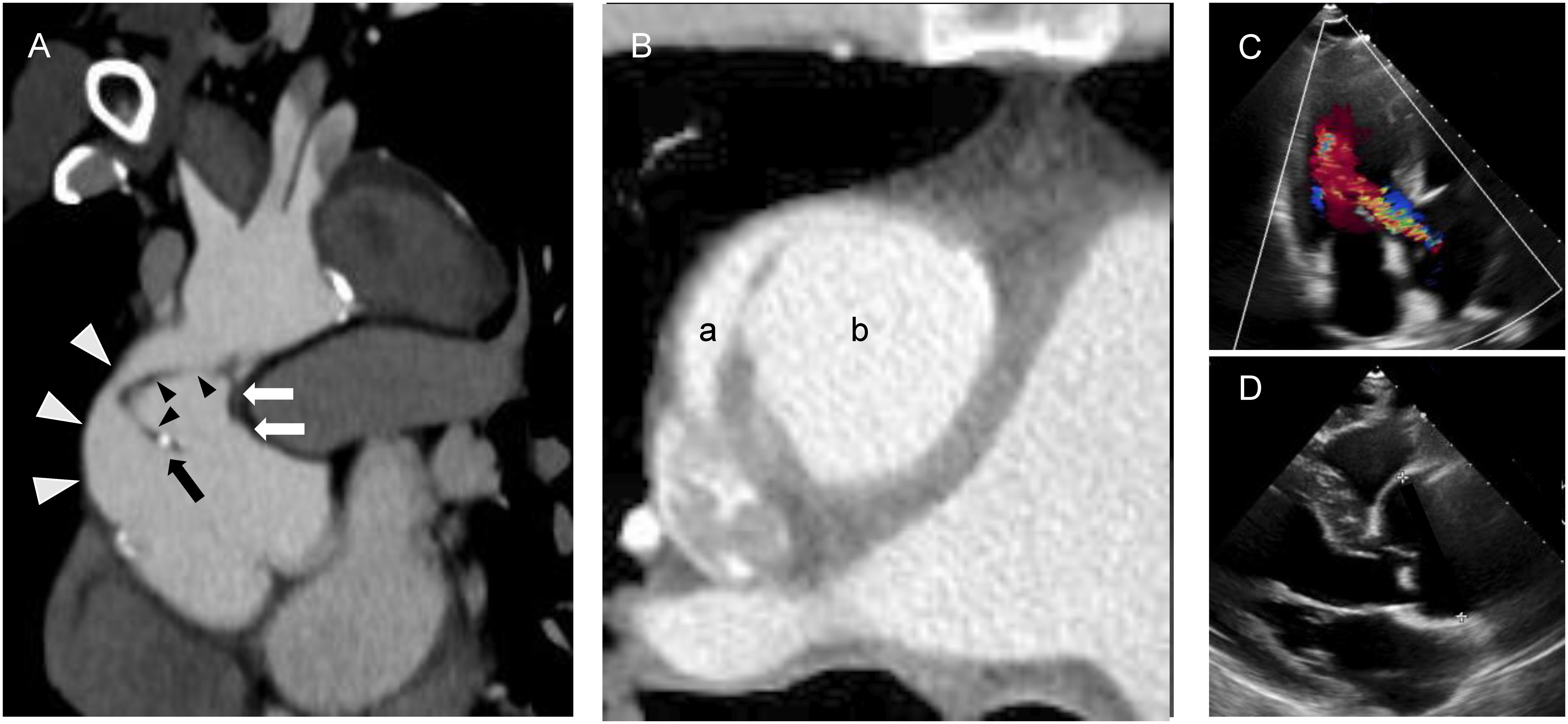
A 64-year-old male patient underwent emergency ascending aortic replacement for ATAAD at another hospital 14 years prior to the current presentation, during which his proximal and distal anastomotic sites were reinforced using internal and external felt strips and gelatin–resorcin–formalin (GRF) glue. The patient’s postoperative course was uneventful, and he showed no subjective symptoms during the follow up period. Fourteen years postoperatively, he noticed that he had dark-colored urine, yellowing of his skin, and difficulty breathing on exertion, which progressively worsened. He presented to the hospital with the abovementioned symptoms, and was found to be anemic, with laboratory results indicating hemolytic anemia and renal insufficiency. He was transferred to our hospital’s Department of Hematology for further evaluation and treatment of hemolytic anemia. The laboratory results were as follows: white blood cell count, 8,400/mm3; red blood cell count, 2.05×106/mm3; platelet count, 121,000/mm3; hemoglobin, 6.8 g/dL; reticulocyte count, 9.0%; lactate dehydrogenase, 3,324 U/L; total bilirubin, 2.6 mg/dL; indirect bilirubin, 2.1 mg/dL; blood urea nitrogen, 29 mg/dL; creatinine, 2.32 mg/dL; C-reactive protein, 0.34 mg/dL; Coombs, negative; and haptoglobin, <10 mg/dL. Peripheral blood smears revealed the presence of numerous schistocytes. Plain computed tomography (CT) revealed abnormal findings in the ascending aorta, where the aortic graft or felts might have dehisced. Moreover, the ascending aorta was surrounded by a pseudoaneurysm, and there was a chronic aortic dissection from the aortic arch to the abdominal aorta. Hence, the possibility of hemolysis resulting from immunologic abnormalities or infections was relatively low, with mechanical trauma thought to be its cause. After improving the patient’s renal function through hydration, an enhanced CT was performed, which suggested that the aortic graft was dehisced and the felt was at the proximal anastomotic site (Figs. 1A and 1B). It also revealed a chronic communicating aortic dissection from the arch of the aorta to the abdominal aorta, with no organ ischemia. Therefore, intravascular hemolysis was suspected to be due to the site of the aortic graft, and the patient was referred to the Department of Cardiovascular Surgery for further management. A transthoracic echocardiogram revealed the following: an ejection fraction of 44%; moderate aortic regurgitation; and annuloaortic ectasia (Figs. 1C and 1D). Due to the presence of an anastomotic pseudoaneurysm compressing the graft, annuloaortic ectasia, and moderate aortic regurgitation, the Bentall procedure was considered for the patient.
Fig. 1 Preoperative computed tomography and transthoracic echocardiogram findings. (A) Coronal image of the ascending aorta. The white and black arrowheads indicate intact and detached aortic grafts, respectively. The white arrows indicate the wall of the pseudoaneurysm, and the black arrow indicates the detached proximal anastomotic site. (B) A horizontal image of the ascending aorta. The true lumen of the aortic graft (a) is compressed by the pseudoaneurysm (b). (C) An apical three-chamber view showing moderate aortic regurgitation. (D) A parasternal long-axis view showing dilatation of the sinus of Valsalva.
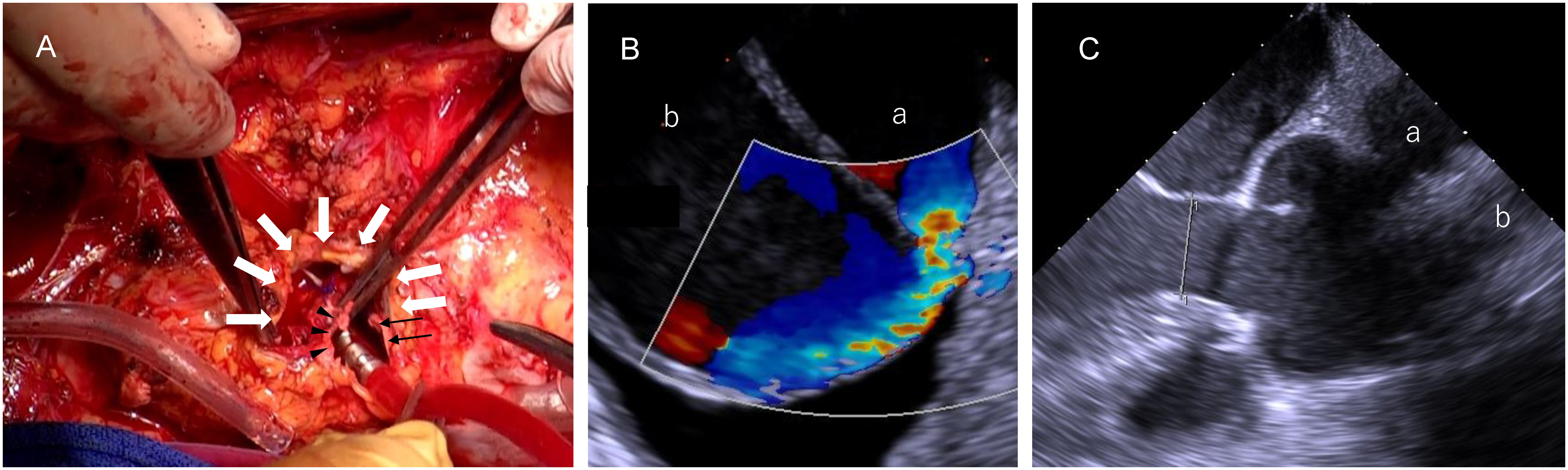
A redo median sternotomy was performed, and cardiopulmonary bypass was initiated through the femoral artery. Two-stage venous cannulas were inserted into the right atrium and the femoral vein. A pseudoaneurysm was observed under deep hypothermic circulatory arrest. After cutting it down, the proximal anastomotic site of the graft was found covered in the pseudoaneurysm. On cutting the graft, the proximal anastomosis was found to be dehisced by half a circle, and the aortic graft was compressed by the pseudoaneurysm (Fig. 2A). The distal anastomosis and the aortic graft were intact; therefore, we clamped the aortic graft and restarted cardiopulmonary bypass. Transesophageal echocardiography showed jet flow (Fig. 2B). The sinus of Valsalva and the aortic annulus were found to be dilated; therefore, the Bentall procedure was performed as planned (Fig. 2C). After the coronary buttons were separated from the sinus of Valsalva, we performed an aortic annulus anastomosis using the French cuff technique. The 30-mm J Graft SHIELD NEO (Japan Lifeline, Tokyo, Japan) and the 25-mm Inspiris Resilia aortic valve (Edwards Lifesciences, Irvine, CA, USA) were used for the procedure.4) The coronary buttons were re-implanted into the graft, and the old and new grafts were finally anastomosed.
Fig. 2 Intraoperative findings at the proximal anastomotic site and transesophageal echocardiogram findings. (A) The white arrows indicate the pseudoaneurysm wall, and the black arrows and arrowheads represent the intact and detached aortic grafts, respectively. (B) A mid-esophageal short-axis view of the ascending aorta shows blood flow from the aortic graft (a) to the pseudoaneurysm (b). (C) A mid-esophageal long-axis view showing dilatation of the sinus of Valsalva and the aortic annulus. (a) and (b) indicate the aortic graft and the pseudoaneurysm, respectively.
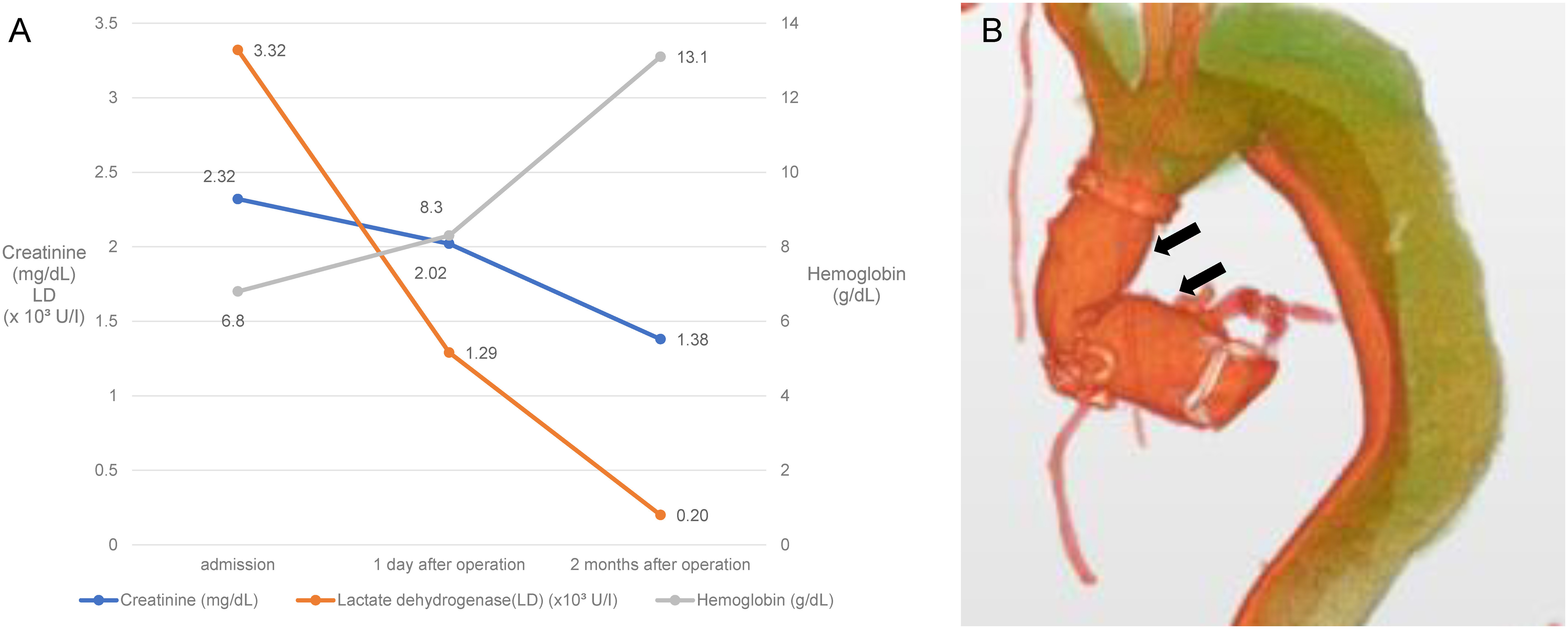
The patient’s urine color normalized immediately after the surgery, and his renal insufficiency and hemolytic anemia also improved (Fig. 3A). The postoperative course was uneventful, and no pseudoaneurysm was observed on the postoperative CT (Fig. 3B). The patient was discharged on postoperative day 15.
Fig. 3 Postoperative findings of laboratory results and three-dimensional computed tomography (CT). (A) Laboratory results showing the improvement of hemolytic anemia and renal dysfunction. (B) A three-dimensional image of an enhanced CT. The anastomotic sites are intact. There is no evidence of a pseudoaneurysm (black arrows), and both the new and old grafts show sufficient expansion.
Discussion
Hemolytic anemia secondary to aortic replacement is a relatively rare complication of the treatment of ATAAD. After encountering the 64-year-old male patient’s case, we performed a systematic review of the PubMed database and retrieved reports of only 32 similar cases between 1991 and 2022. Of these cases, Davison et al. evaluated 30 and found various causes for hemolytic anemia after surgery for ATAAD; these included aortic graft stenosis, graft kinking, external compression of the graft, and a folded elephant trunk appendage.5) Graft stenosis attributed to the inversion of the inner felt strip used for anastomosis was the most common, and only six cases were attributed to external compression, which was due to suture line dehiscence and the development of adjacent pseudoaneurysm in five cases and a constricting outer Dacron wrap aortoplasty in one5); the cause of suture line dehiscence in the five cases was not mentioned. In the present case, the patient’s dark-colored urine normalized immediately after the commencement of cardiopulmonary bypass; therefore, we suspected that the cause of hemolysis was present at the anastomotic site. Subsequently, external compression by an anastomotic pseudoaneurysm was found, and it was also considered the cause of hemolysis. Davison et al. reported that the mean onset of hemolysis following aortic dissection repair occurred 32.2 months (range, 0–180 months) after surgery.5) In comparison, hemolysis in our case took quite a while (14 years postoperatively) and was unique.
Next, we discuss the causes of anastomotic pseudoaneurysms. Infections can be considered one of the causes. Malvindi et al. reported that they found infections in one-third of the cases of anastomotic pseudoaneurysm.6) However, in our case, infection was not suspected due to the absence of fever and the non-elevation of the levels of inflammatory laboratory markers. Other than that, the deterioration of sutures or grafts can also be considered a potential cause of an anastomotic pseudoaneurysm. Hata et al. reported that postoperative aortic pseudoaneurysm at the site of anastomosis was observed in five of 56 patients after the use of the GRF glue, whereas no late complications were observed in patients treated without the GRF glue.7) The authors also reported that the mean interval between initial surgery and the detection of a pseudoaneurysm was 36.0 months (range, 13–76 months).7) Kunihara et al. explained the mechanism of the formation of an anastomotic pseudoaneurysm due to GRF glue. The glue consists of a GR solution (gelatin+resorcinol) and an F-activator that contains formaldehyde and glutaraldehyde. The GR solution cannot function as an adhesive until it is polymerized by the F-activator, which consists of cytotoxic formaldehyde. This cytotoxicity to the smooth muscles is thought to be the cause of anastomotic pseudoaneurysm.8) Notably, GRF glue was also used for our patient during the first surgery, which could have been a probable cause of dehiscence. This resulted in the formation of a pseudoaneurysm that gradually expanded and compressed the graft. Furthermore, a jet of blood may have hit the dehisced aortic graft or the site of stenosis, leading to hemolysis. However, the elaborating mechanism remains unclear. It is noteworthy that a prolonged duration of 14 years does not fall within the range reported by Hata et al.7) Also, this duration is the second longest of the previous 32 reports; thus, this case is unusual. The reason for such a long duration remains unclear, although we observed that the pseudoaneurysm expanded over time and subsequently compressed the graft. Intraoperative examination revealed a very tight pseudoaneurysm wall; accordingly, we assumed that it formed over a prolonged period of time.
Finally, we discuss the relationship between dehiscence at the anastomotic site and the time course. Strauch et al. reported that the suture line disruption incidence arising from anastomoses using the Teflon felt was 0.0052 per patient-year, and the mean interval between surgeries was 55.9 months (range, 4–180 months).9) This suggests that the possibility of dehiscence at the anastomotic site increases over time. Therefore, it is important to regularly follow up on the patients using imaging studies such as echocardiography or CT.
Conclusion
We describe a case of pseudoaneurysm at the proximal anastomosis that resulted in hemolytic anemia 14 years after emergency aortic replacement for ATAAD. GRF glue, which was used during the first surgery, was considered the probable cause of the pseudoaneurysm. Patients who have undergone aortic surgery should be followed up with blood tests and imaging studies over a prolonged period of time.
Acknowledgments
We would like to thank Editage for editing this manuscript in English.
Statement of Patient Consent
Written informed consent was obtained from the patient for the publication of this case report and accompanying images.
Disclosure Statement
The authors do not have any conflicts of interest to declare.
Author Contributions
Study conception: KI, YK
Data collection: KI, YK
Manuscript preparation: KI, YK
Critical review and revision: all authors
Final approval of the article: all authors
Accountability for all aspects of the work: all authors
References
- 1).Zhu Y, Lingala B, Baiocchi M, et al. Type A aortic dissection-experience over 5 decades: JACC historical breakthroughs in perspective. J Am Coll Cardiol 2020; 76: 1703-13. [DOI] [PubMed] [Google Scholar]
- 2).Luciani N, De Geest R, Lauria G, et al. Late reoperations after acute aortic dissection repair: single-center experience. Asian Cardiovasc Thorac Ann 2015; 23: 787-94. [DOI] [PubMed] [Google Scholar]
- 3).Izumiyama O, Yamashita A, Sugimoto S, et al. A case of anastomotic pseudoaneurysm at an anastomosis between two woven Dacron prostheses following aortic arch replacement. Jpn J Cardiovasc Surg 2000; 29: 191-4. (in Japanese) [Google Scholar]
- 4).Yan TD. Mini-Bentall procedure: the “French Cuff” technique. Ann Thorac Surg 2016; 101: 780-2. [DOI] [PubMed] [Google Scholar]
- 5).Davison MA, Norton DM, Popoff AM, et al. Hemolysis following wrap aortoplasty for Type A aortic dissection repair: case report and review of the literature. Vasc Med 2018; 23: 400-6. [DOI] [PubMed] [Google Scholar]
- 6).Malvindi PG, van Putte BP, Heijmen RH, et al. Reoperations for aortic false aneurysms after cardiac surgery. Ann Thorac Surg 2010; 90: 1437-43. [DOI] [PubMed] [Google Scholar]
- 7).Hata H, Takano H, Matsumiya G, et al. Late complications of gelatin-resorcin-formalin glue in the repair of acute type A aortic dissection. Ann Thorac Surg 2007; 83: 1621-6. [DOI] [PubMed] [Google Scholar]
- 8).Kunihara T, Iizuka K, Sasaki S, et al. Optimal proportions of gelatin–resorcin–formalin components in aortic surgery. Eur J Cardiothorac Surg 2009; 36: 962-6. [DOI] [PubMed] [Google Scholar]
- 9).Strauch JT, Spielvogel D, Lansman SL, et al. Long-term integrity of teflon felt-supported suture lines in aortic surgery. Ann Thorac Surg 2005; 79: 796-800. [DOI] [PubMed] [Google Scholar]


