ABSTRACT
Background
This study aimed to determine the incidence and prevalence of immunoglobulin A nephropathy (IgAN) in Europe based on high-quality data from national registries.
Methods
IgAN incidences were obtained from a literature review of European studies of national kidney biopsy registry data in which IgAN diagnosis was biopsy-verified using contemporary techniques. Studies were eligible for the main analysis if published from 1990 to 2020. IgAN point prevalence was defined as the annual IgAN incidence multiplied by the estimated duration of disease. Incidence and prevalence estimates were made for three pooled populations: (i) patients of all ages; (ii) pediatric patients; and (iii) elderly patients.
Results
Across 10 European countries, the estimated annual IgAN incidence was 0.76 per 100 000 in patients of all ages. The corresponding pooled IgAN point prevalence was 2.53 per 10 000 (95% confidence interval: 2.51–2.55), ranging from 1.14 per 10 000 in Spain to 5.98 per 10 000 in Lithuania. Applied to 2021 population estimates, the number of expected prevalent IgAN cases was 47 027 across all 10 countries and ranged from 577 in Estonia to 16 645 in Italy. Among pediatric patients, IgAN incidence was 0.20 per 100 000 children and IgAN point prevalence was 0.12 per 10 000 children. Among elderly patients, IgAN incidence was 0.30 per 100 000 and IgAN point prevalence was 0.36 per 10 000.
Conclusions
Based on high-quality data from European national registries, IgAN point prevalence was estimated at 2.53 per 10 000 in patients of all ages. Prevalence was considerably lower in pediatric and elderly populations.
Keywords: glomerulonephritis, IgA, immunoglobulin A nephropathy, incidence, prevalence, registries
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What is already known about this subject?
Immunoglobulin A nephropathy (IgAN) is the most common form of primary glomerulonephritis worldwide. Although the incidence and prevalence of IgAN have been reported across various European nations, results vary according to different thresholds for performing kidney biopsy, different time periods covered, and the inclusion of single- versus multi-center studies. There is a need to determine the incidence and prevalence of IgAN in Europe using high-quality data from national registries.
What this study adds?
Based on high-quality data from European national registries, IgAN point prevalence was estimated at 2.53 per 10 000 in patients of all ages. Among pediatric patients the point prevalence was 0.12 per 10 000; among elderly patients the point prevalence was 0.36 per 10 000.
What impact this may have on practice or policy?
Monitoring IgAN incidence and prevalence at the population level is important to guide clinical decision-making and allocation of resources for this rare disease.
INTRODUCTION
Immunoglobulin A (IgA) nephropathy (IgAN) is the most common form of primary glomerulonephritis worldwide [1]. IgAN is defined by the presence of IgA-dominant or co-dominant immune deposits within kidney glomeruli [2]. It is typically characterized by increased levels of galactose-deficient (Gd) IgA1 in serum, and Gd-IgA1-containing immune complexes in the glomeruli [1, 3]. At present, IgAN diagnosis can only be confirmed by kidney biopsy [1].
The clinical presentation of IgAN is highly variable, and some patients may be asymptomatic with only minor urine abnormalities [4]. However, in many cases, IgAN is a progressive disease associated with proteinuria, hypertension, and kidney injury [4]. Approximately 30%–40% of patients will progress to kidney failure within 20–30 years of diagnosis, despite current treatment strategies [1].
Epidemiologic studies are essential to monitor IgAN progression at the population level, thereby guiding decision-making and allocation of resources. Although the incidence and prevalence of IgAN have been reported across various European nations, results vary according to different thresholds for performing kidney biopsy, different time periods covered, and the inclusion of single- or multi-center studies [5–7]. National biopsy registries provide the most comprehensive and accurate data for calculating IgAN incidence.
The aim of this study was to determine the incidence and prevalence of IgAN in Europe, based on high-quality data from national registries. First, incidence and prevalence were estimated in patients of all ages, based on the assumption that the pathogenetic mechanisms leading to IgAN are the same in adults and children [8]. Next, subanalyses were performed to specifically estimate IgAN incidence and prevalence in pediatric patients and in elderly patients.
MATERIALS AND METHODS
Study design
This was a descriptive epidemiologic study to determine the incidence and prevalence of IgAN in Europe, based on analyses of published studies identified via a literature review. Estimates were made for three populations: (i) adult and pediatric patients (i.e. patients of all ages); (ii) pediatric patients; and (iii) elderly patients.
Definitions and analyses
Incidence
For specific countries/regions, the annual incidence of biopsy-detected IgAN was taken directly from each publication, where reported. Where not reported, annual incidence was calculated as the annual number of IgAN cases divided by the reference population (i.e. the population at risk) at the midpoint of the study period. If a study reported several different study periods, data from the most recent period were used. The number of expected incident IgAN cases in each country was calculated by multiplying incidence by population size. Pooled incidence across all countries was calculated by summing the number of expected incident IgAN cases in each country, and dividing by the sum of the reference populations for each country.
Prevalence
IgAN point prevalence for each country/region was defined as the annual incidence of biopsy-detected IgAN multiplied by the estimated duration of disease [9]. The number of expected prevalent IgAN cases in each country was calculated by multiplying point prevalence by population size. Pooled prevalence across all countries was calculated by summing the number of expected prevalent IgAN cases in each country, and dividing by the sum of the reference populations for each country.
Duration of disease
For specific countries/regions, median duration of disease (years from IgAN diagnosis to death) was taken directly from each publication, where reported. Where not reported, median duration of disease was estimated from hazard rates, using survival analysis concepts in the absence of studies with follow-up periods sufficiently long enough to document median survival. Survival probability (S) can be determined from the following formula [10], where t = years of follow-up, and h = hazard rate:
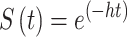 |
Survival probability can be used to calculate the hazard rate, where T0 represents a given time of follow-up:
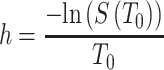 |
For an exponential distribution, the mean survival is 1/h and the median is ln(2)/h. Thus, from the hazard function, median survival time can be determined as follows:
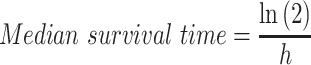 |
In certain cases, median duration of disease was calculated as median time from diagnosis to kidney replacement therapy (KRT) + median survival after initiation of KRT, using hazard rates to estimate the two components of the sum.
For example, Maixnerova et al. reported a 10-year kidney survival of 77.1% in the Czech Republic [11], which was used to estimate the annual hazard rate as follows: h = –ln(0.771)/10 = 0.03. Subsequently, the hazard rate was used to estimate median kidney survival time (i.e. time from diagnosis to KRT): ln(2)/0.03 = 23 years. Median survival after initiation of KRT is 8 years in Europe [12], and thus total duration of disease in the Czech Republic was estimated as 23 + 8 = 31 years.
Sensitivity analyses
Two sensitivity analyses were performed to explore how Estimate 1 (patients of all ages) was affected by:
The addition of regional data to national data. Inclusion criteria were expanded to include regional studies, which may not be nationally representative.
Variation in median duration of disease. Duration of disease was adjusted by ±2 years, which was an arbitrary choice.
Data sources
Search strategy
Data to determine incidence and prevalence were obtained from a literature review with the aim of identifying publications of population-based studies of the epidemiology of IgAN in Europe. The same literature was also searched to identify publications of population-based studies on the long-term follow-up of IgAN (years from diagnosis to death) in Europe. The Medline (PubMed), ScienceDirect, and Cumulative Index to Nursing and Allied Health Literature databases were searched using the terms ‘IgA nephropathy’, ‘Berger's disease’, ‘glomerulonephritis’, ‘kidney biopsy registry’, ‘incidence’, ‘prevalence’, and ‘epidemiology’, and limited to studies of humans. Articles were eligible if they were published in the periods from 1 January 1990 to 1 October 2020 (Estimate 1: patients of all ages), 1 January 2000 to 1 May 2021 (Estimate 2: pediatric patients), and 1 January 2000 to 14 June 2021 (Estimate 3: elderly patients). Estimates 2 and 3 used shorter time periods than Estimate 1, since Estimate 1 revealed that all relevant studies were published post-2000. Relevant studies were also identified from three comprehensive reviews [5, 7, 13], and from the bibliographies of reviewed studies.
Study selection
To be eligible for use in estimating population-based rates, studies must be generalizable to a defined population, meaning that the study population is representative or inclusive of a region or country. Accordingly, the following inclusion criteria were applied: (i) IgAN incidence data were derived from a national (or regional, for sensitivity analyses) kidney biopsy registry with a clearly defined reference population {in terms of geographical location, age groups, and study period, such that denominator information [population at risk] could be determined}; (ii) IgAN diagnosis was assessed by kidney biopsy, and the study identified primary versus secondary glomerulonephritis (only primary was eligible): repeat biopsies and transplant biopsies were excluded to prevent double-counting of cases; and (iii) kidney biopsy methods indicated that contemporary diagnostic techniques were used (i.e. immunofluorescence, electron microscopy).
Studies were excluded for the following reasons: no IgAN-specific data; reference population was not specified; non-European study; not an observational study; small convenience sample; non-generalizable sample; or population-based incidence was not reported and could not be calculated.
Data extraction
To determine IgAN incidence, the following data were extracted from eligible articles, where available: country, registry, time frame, number of native kidney biopsies, reference population, and IgAN incidence. For the pediatric and elderly estimates, age group definitions were also extracted. To determine duration of disease, the following data were extracted from eligible articles, where available: country, population or registry, time frame, number of patients with biopsy-verified IgAN, median time from diagnosis to KRT, median survival after initiation of KRT, and median duration of disease.
Reference populations
Reference populations were extracted or estimated from the Eurostat data browser (https://ec.europa.eu/eurostat/databrowser/view/tps00001/default/table?lang = en) and the US Census International Database (https://www.census.gov/data-tools/demo/idb/#/country?YR_ANIM = 2021).
RESULTS
Estimate 1: IgAN incidence and prevalence in patients of all ages
Incidence
Of 5085 citations that were evaluated, 170 studies were reviewed in full. Nationally representative kidney biopsy registry data that met eligibility criteria were identified for 10 countries: Czech Republic, Denmark, Estonia, Italy, Lithuania, Norway, Poland, Scotland, Spain, and Sweden (Table 1). All studies considered combined adult and pediatric populations. Depending on the country, 578–14 607 kidney biopsies were considered for time periods ranging from 1 to 18 years.
Table 1:
IgAN annual incidence in European countries, based on national kidney biopsy registry data (Estimate 1: patients of all ages).
| Country | Registry | Time frame | Number of native kidney biopsies | Reference population (millions)a | Annual kidney biopsies per 100 000b | Annual IgAN incidence per 100 000 |
|---|---|---|---|---|---|---|
| Czech Republic [14] | Czech Registry of Renal Biopsies | 1994–2011 | 10 472 | 10.3 | 4.4–6.2 | 1.16 |
| Denmark [6, 15] | Danish Renal Biopsy Register | 1985–1997 | 2380 | 5.2 | 3.9 | 1.08 |
| Estonia [16] | Tartu University Hospital | 2001–2010 | 578 | 1.3 | 8.1 | 1.4 |
| Italy [17] | Italian Registry of Renal Biopsies | 1996–2000 | 14 607c | 57.6 | NA | 0.84d |
| Lithuania [18] | National Center of Pathology | 2007–2012 | 1643 | 3.2 | NA | 1.93d |
| Norway [19] | Norwegian Kidney Biopsy Registry | 1988–2004 | 633e | 4.3 | NA | 0.85d |
| Poland [20] | Polish Registry of Renal Biopsies | 2009–2014 | 9394f | 38.1 | 4.0 | 0.62d |
| Scotland [21] | Scottish Renal Biopsy Registry | 2017 | 651g | 5.4 | 12.0 | 1.22d |
| Spain [22] | Spanish Registry of Glomerulonephritis | 2014–2019 | 5174h | 46.5 | 4.8i | 0.34d |
| Sweden [23] | Four pathology departments (all kidney biopsies in Sweden) | 2002–2015 | 1746e | 9.4 | NA | 1.27d |
IgAN, immunoglobulin A nephropathy; NA, not available.
Taken from the publication or another source (see the ‘Reference populations’ subsection of the ‘Methods’).
Kidney biopsy rate and IgAN incidence are not strictly comparable as they reflect different study periods and time frames.
89.9% native and 10.1% transplant.
Calculated as (number of IgAN cases/study duration)/reference population at the midpoint of the study period.
Number of patients with biopsy-verified IgAN.
Including 951 re-biopsies.
Including 63 re-biopsies.
92% were first biopsies over the full study period (1994–2019).
For time frame 1994–1999 [24].
The estimated annual IgAN incidence ranged from 0.34 per 100 000 in Spain to 1.93 per 100 000 in Lithuania (Table 1), and the pooled incidence across all 10 countries was 0.76 per 100 000.
Duration of disease
Of 1086 citations that were evaluated, four studies provided population-based follow-up data relating to duration of disease in patients with IgAN in Europe (Table 2). Most excluded studies focused on specific subgroups of patients with IgAN (e.g. those with kidney transplant), which were not generalizable to all patients due to differing severity and/or stage of disease. The eligible studies represented Czech Republic, Norway, and Sweden; the VALIGA (Validation of the Oxford classification of IgAN) cohort, which includes 13 European countries, was also considered eligible. Depending on the study, 520–3622 patients with IgAN were followed, with a median follow-up of 4.7–13.6 years.
Table 2:
Population-based estimates of duration of disease (years from IgAN diagnosis to death) in European countries (Estimate 1: patients of all ages).
| Number of patients | Median time from | Median survival | Median duration | |||
|---|---|---|---|---|---|---|
| with biopsy-verified | diagnosis to KRT | after initiation of | of disease | |||
| Country | Population/registry | Time frame | IgAN | (years) | KRT (years) | (years) |
| Czech Republic [11] | Multiple Czech nephrology centers | 2000–2010 | 520 | 23a | 8a,b | 31c |
| Norway [19] | Norwegian Kidney Biopsy Registry | 1988–2004 | 633 | 31.1a | 9d | 40.1c |
| Sweden [23] | Four pathology departments (all kidney biopsies in Sweden) | 1974–2015 | 3622 | 32e | 10f | 42g |
| Varioush [25] | VALIGA cohort (55 centers in 13 countries) | 2011i | 1147 | 25.5a | 8a,b | 33.5c |
IgAN, immunoglobulin A nephropathy; KRT, kidney replacement therapy; VALIGA, Validation of the Oxford classification of IgAN.
Estimated from hazard rate.
Data to estimate survival time after initiation of KRT were not available for this country/region, and thus the value was estimated from overall European data obtained from the European Renal Association–European Dialysis and Transplant Association (ERA-EDTA) registry, which includes data from 53 national or regional registries within 37 European countries [12].
Calculated as median time from diagnosis to KRT + median survival after initiation of KRT.
Assumed to be intermediate between the European and Swedish values, as 5-year survival probability post-KRT is slightly higher in Norway (56%) than in Europe (52.4%) [12, 26].
Value read from Cox survival curve.
Calculated as median duration of disease − median time from diagnosis to KRT.
Calculated as median age at death − median age at IgAN diagnosis.
Croatia, Czech Republic, Estonia, Germany, Greece, Italy, The Netherlands, Poland, Portugal, Spain, Sweden, Turkey, UK.
Retrospective cohort assembled in 2011 [27].
Duration of disease estimates ranged from 31 years in the Czech Republic to 40–42 years in Norway and Sweden (Table 2).
Seven countries with incidence data in Table 1 had no corresponding duration of disease data in Table 2. To predict prevalence, a low, medium, or high duration of disease was assigned to these countries based on the life expectancy of the general population in that country [28–31]. Thus, the estimate from the Czech Republic (31 years) was applied to eastern European countries (Estonia, Lithuania, and Poland), which had lower than average general life expectancy. The mid-range estimate from the VALIGA cohort (33.5 years) was applied to the remaining countries (Denmark, Italy, Scotland, and Spain), based on average general life expectancy.
Prevalence
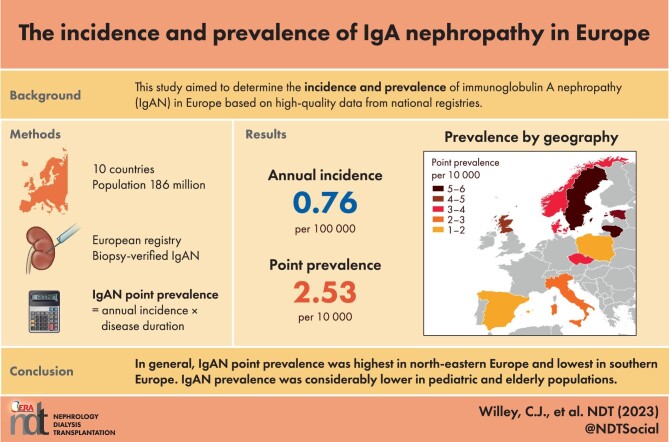
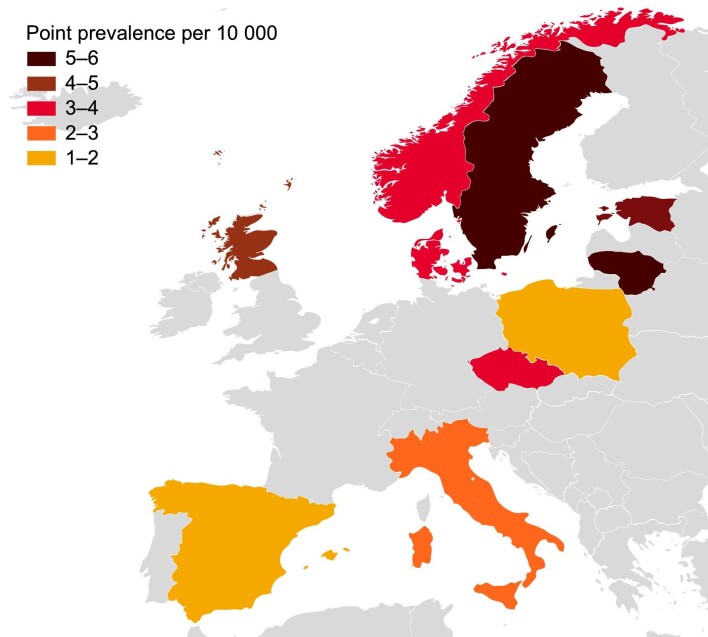
Across Europe, IgAN point prevalence was estimated at 2.53 per 10 000 {95% confidence interval [CI]: 2.51–2.55} based on national kidney biopsy registry data. The point prevalence ranged from 1.14 per 10 000 in Spain to 5.98 per 10 000 in Lithuania (Table 3). In general, point prevalence was lowest in southern Europe (Spain, Italy) and Poland, and highest in north-eastern Europe (Lithuania, Sweden, Estonia) (Fig. 1).
Table 3:
IgAN point prevalence and 2021 expected prevalent cases in European countries, based on national kidney biopsy registry data (Estimate 1: patients of all ages).
| Annual IgAN incidence | Median duration of | IgAN point prevalence | Expected prevalent | ||
|---|---|---|---|---|---|
| Country | per 100 000 | disease (years) | per 10 000a | Population in 2021b | IgAN cases in 2021c |
| Czech Republic | 1.16 | 31 | 3.60 | 10 494 836 | 3778 |
| Denmark | 1.08 | 33.5 | 3.62 | 5 840 045 | 2114 |
| Estonia | 1.4 | 31 | 4.34 | 1 330 068 | 577 |
| Italy | 0.84 | 33.5 | 2.81 | 59 236 213 | 16 645 |
| Lithuania | 1.93 | 31 | 5.98 | 2 795 680 | 1672 |
| Norway | 0.85 | 40.1 | 3.41 | 5 391 369 | 1838 |
| Poland | 0.62 | 31 | 1.91 | 37 840 001 | 7227 |
| Scotland | 1.22 | 33.5 | 4.07 | 5 500 000 | 2239 |
| Spain | 0.34 | 33.5 | 1.14 | 47 398 695 | 5403 |
| Sweden | 1.27 | 42 | 5.33 | 10 379 295 | 5532 |
| Pooled | – | – | 2.53 (95% CI: 2.51–2.55)d | 186 206 202 | 47 027 |
CI, confidence interval; IgAN, immunoglobulin A nephropathy.
Calculated as annual IgAN incidence × duration of disease.
See the ‘Reference populations’ subsection of the ‘Methods’ for sources.
Calculated as point prevalence × population.
Calculated as sum of expected prevalent cases/sum of population.
Figure 1:
Geographic variation in IgAN point prevalence across Europe, based on national kidney biopsy registry data for patients of all ages. See Table 1 for time frame of source data for each country.
When the point prevalences were applied to 2021 population estimates, the number of expected prevalent IgAN cases was 47 027 across all countries studied, and ranged from 577 in Estonia to 16 645 in Italy (Table 3).
Sensitivity analyses
Regional studies with incidence data were available for four additional countries: Germany, Northern Ireland, Romania, and Serbia. The addition of these countries (predominantly adult populations) to the 10 countries with national data increased the pooled IgAN point prevalence from 2.53 per 10 000 to 3.58 per 10 000 (95% CI: 3.56–3.60) (Supplemental Table S1).
Varying the median duration of disease by ±2 years resulted in estimates of point prevalence ranging from 2.48–2.79 per 10 000 (Supplemental Table S2).
Estimate 2: IgAN incidence and prevalence in pediatric patients
Incidence
Of 401 citations that were evaluated, 55 studies were reviewed in full. Nationally representative kidney biopsy registry data that met eligibility criteria were identified for six countries: Croatia, Czech Republic, Estonia, Lithuania, Poland, and Spain (Table 4). In addition, a single-center study from a region of Germany was included as a sensitivity analysis. The pediatric age range definition varied between countries, from 0–15 to 0–17 years (Table 4). Depending on the country, up to 1421 kidney biopsies were considered, for time periods ranging from 4 to 24 years.
Table 4:
IgAN annual incidence and 2021 expected incident cases in pediatric patients in European countries, based on national kidney biopsy registry data (and regional data for Germany) (Estimate 2: pediatric patients).
| Country | Registry | Time frame | Number of native kidney biopsies in pediatric patients | Pediatric age group definition | Annual IgAN incidence per 100 000 pediatric patients | Pediatric population in 2021a | Expected incident IgAN cases in pediatric patients in 2021b |
|---|---|---|---|---|---|---|---|
| Croatia [32] | Registry of Biopsy-Proven Renal Diseases | 1991–2004 | 565 | 0–17 | 0.51c | 716 172 | 4 |
| Czech Republic [14] | Czech Registry of Renal Biopsies | 1994–2011 | 1421 | 0–15 | 0.78c | 1 721 898 | 13 |
| Estonia [16] | Tartu University Hospital | 2001–2010 | 13d | 0–15 | 0.00 | 209 330 | 0 |
| Germany [33] | University Hospital Aachen | 1990–2013 | 23 | 0–15 | 0.19c | 11 037 903 | 21e |
| Lithuania [18] | National Center of Pathology | 2007–2012 | 114 | 0–16f | 0.79c | 467 450 | 4 |
| Poland | |||||||
| [20] | Polish Registry of Renal Biopsies | 2009–2014 | 384 | 0–17 | 0.18c | 6 706 811 | 12 |
| [34] | Polish pediatric IgAN registry | 2000–2015 | 140g | 0–17 | 0.16 | 11 | |
| Spain [35] | Spanish Registry of Glomerulonephritis | 2010–2013 | 238h | 0–16 | 0.03c | 7 913 453 | 2 |
| Pooled (incl. Germany) | – | – | – | – | 0.19i | 28 773 017 | 56j |
| Pooled (excl. Germany) | – | – | – | – | 0.20i | 17 735 114 | 35j |
IgAN, immunoglobulin A nephropathy.
See the ‘Reference populations’ subsection of the ‘Methods’ for sources.
Calculated as incidence × population.
Calculated as (number of IgAN cases/study duration)/reference population at the midpoint of the study period.
Number of pediatric patients with biopsy-confirmed primary glomerulopathy.
Germany data were from a single, internationally recognized teaching hospital.
No age range given in reference; definition assumed based on other studies.
Number of pediatric patients with biopsy-verified IgAN.
Number of pediatric patients with biopsy-verified IgAN over the full study period (1994–2013).
Calculated as sum of expected incident cases/sum of population.
The mean of the two Poland estimates was used (12 expected cases).
The pooled estimated annual IgAN incidence in pediatric patients was 0.20 per 100 000 children excluding Germany, and 0.19 per 100 000 children including Germany. The incidence ranged from 0.00 per 100 000 children in Estonia to 0.79 per 100 000 children in Lithuania (Table 4). Overall, 35 incident IgAN cases were expected in this pediatric population (excluding Germany) in 2021.
The Czech Republic study also determined the number of kidney biopsies (not specific to IgAN) in different pediatric age groups. Of 1421 biopsies, 16.3% were in patients aged 0–5 years, 30.7% were in patients aged 6–10 years, and 53.0% were in patients aged 11–15 years [14].
Prevalence
To calculate IgAN point prevalence in pediatric patients, duration of disease in childhood (years from IgAN diagnosis to age 18) was assumed to be approximately 6 years, based on a mean age at diagnosis of approximately 12 years (e.g. 12.7 years in the VALIGA pediatric cohort [36] and 11.4 years in a Polish pediatric study [34]).
Across Europe (excluding Germany), the corresponding IgAN point prevalence in pediatric patients was estimated at 0.12 per 10 000 children (i.e. annual incidence × duration of disease = 0.020 per 10 000 × 6).
Estimate 3: IgAN incidence and prevalence in elderly patients
Of 543 citations that were evaluated, 58 studies were reviewed in full. Nationally representative kidney biopsy registry data that met eligibility criteria were identified for five countries: Czech Republic, Estonia, Poland, Spain, and Sweden (Supplemental Table S3). A single-center study from Northern Ireland was also included because, despite being single center, it covered all kidney biopsies in the country. Finally, a single-center study from a region of Germany was included as a sensitivity analysis. The definition of elderly patients varied between studies, from ≥60 to >65 years (Supplemental Table S3). Depending on the country, up to 2000 kidney biopsies were considered, for time periods ranging from 6 to 42 years.
The pooled estimated annual IgAN incidence in elderly patients was 0.30 per 100 000 excluding Germany, and 0.70 per 100 000 including Germany. The incidence ranged from 0.08 per 100 000 in Estonia to 1.09 per 100 000 in Germany (Supplemental Table S3). Overall, 67 incident IgAN cases were expected in this elderly population (excluding Germany) in 2021.
A Spanish study presented IgAN cases in different elderly age groups: IgAN incidence was 0.26 per 100 000 in patients aged 65–80 years, and 0.09 per 100 000 in patients aged >80 years [22].
Prevalence
Life expectancy in elderly patients with IgAN was estimated at 75 years, based on a European life expectancy of 81 years (Eurostat), and the observation from a Swedish study that patients with IgAN died 6 years earlier than people without the disease [23]. Thus, duration of disease in elderly patients (years from IgAN diagnosis to death) was estimated at approximately 12 years, based on an age of diagnosis of 63 years in elderly patients [37].
Across Europe (excluding Germany), the corresponding IgAN point prevalence in elderly patients was estimated at 0.36 per 10 000 (i.e. annual incidence × duration of disease = 0.030 per 10 000 × 12).
DISCUSSION
The incidence and prevalence of IgAN in Europe in patients of all ages, in pediatric patients, and in elderly patients are summarized in Table 5.
Table 5:
Summary of annual IgAN incidence and point prevalence in European countries, based on national kidney biopsy registry data.
| Annual IgAN | IgAN point | |
|---|---|---|
| incidence per | prevalence per | |
| 100 000 | 10 000 | |
| Patients of all ages | 0.76 | 2.53 |
| Pediatric patients | 0.20 | 0.12 |
| Elderly patients | 0.30 | 0.36 |
In people of all ages, IgAN point prevalence in Europe was estimated at 2.53 per 10 000 based on national kidney biopsy registry data from 10 countries, with a reference population of 186 million. When applied to 2021 populations, the number of prevalent IgAN cases was estimated at 47 027 across the 10 countries studied.
The 95% CI for IgAN point prevalence was narrow (2.51–2.55), showing that the prevalence was relatively consistent across countries. This consistency may reflect that only high-quality, national, biopsy-detected data were included in the present analysis. Indeed, the range of annual IgAN incidences across countries in the present study (0.34–1.93 per 100 000) was smaller than in a previous review of IgAN incidence in adults (0.1–5.0 per 100 000 in European countries), which included local data, and which did not exclusively consider data from the most recent time frames [5]. Also of note, in the present study, higher biopsy rates (which ranged from 3.9 to 12.0 per 100 000) broadly corresponded with higher IgAN incidences (Table 1).
In children, the range of annual IgAN incidences across six European countries was up to 0.79 per 100 000 children (pooled incidence: 0.20 per 100 000), with a reference population of approximately 18 million children. In addition, an Italian study of biopsy data from 1992 to 1994 (missing the time frame cut-off for the present analysis) reported an IgAN incidence of 0.31 per 100 000 children aged 0–15 years [38]. In the present study, IgAN point prevalence was estimated at 0.12 per 10 000 children, indicating that IgAN is very rare in European children, and considerably less common in children than in adults (as previously reported [5]). It is possible that these incidence values are underestimates, due to the lack of school urinalysis screening programs in Europe, in contrast to certain countries in Asia, such as Japan and South Korea [39, 40]. Of note, a Spanish study suggested that IgAN incidence in pediatric patients declined over the period from 1994 to 2013 [35].
IgAN incidence was also lower in elderly adults (incidence across six countries: 0.08–0.68 per 100 000; pooled incidence: 0.30 per 100 000) than in patients of all ages, with a reference population of approximately 23 million. This corresponded to an IgAN point prevalence of 0.36 per 10 000. Data from one study in Spain suggest that IgAN incidence is lowest in the subset of elderly patients aged >80 years [22]. Prognosis is poor among elderly patients with IgAN, who often have advanced kidney disease at presentation [41].
In general, IgAN point prevalence was highest in north-eastern Europe and lowest in southern Europe. The exception was Poland, which had a low point prevalence among patients of all ages, potentially because the Polish study excluded some pediatric patients due to missing clinical and histopathologic data [20]. A similar pattern across Europe has been observed in genome-wide association studies (GWAS) on regional differences in genetic susceptibility to IgAN [42]. Genetic and environmental factors, together with differences in screening policies, kidney biopsy practices, and recording of kidney biopsy diagnosis, may explain the differences in IgAN incidence rates observed between geographic regions [43].
IgAN incidence can vary considerably from center to center within a single country [44]. In a sensitivity analysis, the addition of data from regional studies to data from national studies increased IgAN point prevalence from 2.53 per 10 000 to 3.58 per 10 000 among patients of all ages. Whereas national data were for adults and children, regional data were mostly from studies in adults; because IgAN incidence is greater in adults than in children, this may contribute to the increase in prevalence. Furthermore, data from a single, internationally recognized teaching hospital in Germany are likely to overestimate IgAN incidence, as this hospital attracts patients from other regions of Germany as well as from other European nations.
A diagnosis of IgAN was associated with shortened life expectancy, and most of the excess mortality risk occurred after kidney failure [23]. Median time to KRT differed between countries (23–32 years), whereas median survival after KRT was 8 years in the VALIGA cohort and 10 years in Sweden. The longest overall survival time was observed in Norway and Sweden, with differences between countries potentially related to differences in public healthcare (Norway and Sweden have universal and predominantly publicly financed healthcare systems) [31, 45].
In most cases, duration of disease was estimated by summing median time to KRT and survival time after KRT. This approach can be validated using the following data from Sweden (equivalent data were not available for other countries). In the Swedish study, median age at IgAN diagnosis was 35 years, and life expectancy was reduced by 6 years among patients with IgAN [23]. In the general Swedish population, individuals aged 35 years are expected to live for another 48 years (2019 data) [46]. Subtracting 6 from 48 gives an expected duration of disease for IgAN of 42 years, equal to that predicted in Table 2, thereby supporting this approach for estimating disease duration.
The present study is limited in that it only considered diagnosed patients, and diagnosis rates may vary between age groups. For example, incidence and prevalence might be underestimated in pediatric and elderly patients, for whom the threshold for performing a kidney biopsy in cases of urinary abnormalities may be higher. Urinalysis screening rates and thresholds to perform kidney biopsy may also vary between European regions. The incidence and prevalence of IgAN may be slightly underestimated in some countries because of missing data in kidney biopsy registries. Where reported, the percentage of non-diagnostic biopsies in national registries ranged from approximately 1% in Norway and Scotland to approximately 7% in Lithuania [18, 21, 26]. In one study, the percentage of non-diagnostic biopsies decreased over time [16], perhaps due to improved diagnostic technologies. To reduce this source of bias, the present analysis used the incidence rate reported for the most recent study cohort in each registry. Transplant biopsies were excluded to prevent double-counting of cases; however, there is a risk of missing a small number of patients who were diagnosed with IgAN after recurrence in the allograft. The inclusion of some patients with secondary IgAN cannot be ruled out, which may overestimate the incidence and prevalence of primary IgAN. Eligible studies were not available for all European regions, and the reference population of this study (186 million) represents approximately one-third of the overall European population excluding Russia, which may limit the generalizability of results to Europe as a whole. The duration of disease estimates could be influenced by treatment protocols across countries, and the proactiveness of treating physicians. Country-specific disease duration estimates were limited since IgAN is a slowly progressing disease [47] and few longitudinal studies had≥10 years of follow-up. However, a sensitivity analysis that varied duration of disease by ±2 years showed that prevalence was only minimally affected.
In conclusion, based on high-quality data from European national registries, IgAN point prevalence was estimated at 2.53 per 10 000 in patients of all ages. Among pediatric patients, IgAN point prevalence was 0.12 per 10 000, and, among elderly patients, IgAN point prevalence was 0.36 per 10 000. Monitoring IgAN incidence and prevalence at the population level is important to guide clinical decision-making and allocation of resources.
Supplementary Material
ACKNOWLEDGEMENTS
Writing support was provided by Chris Watling, PhD, assisted by his colleagues at Cambridge—a Prime Global Agency (Knutsford, UK), and funded by Otsuka Pharmaceutical Development & Commercialization Inc. (Princeton, NJ, USA).
Contributor Information
Cynthia J Willey, College of Pharmacy, University of Rhode Island, Kingston, RI, USA.
Rosanna Coppo, Fondazione Ricerca Molinette, Regina Margherita Hospital, Turin, Italy.
Franz Schaefer, Division of Pediatric Nephrology, Center for Pediatric and Adolescent Medicine, University of Heidelberg, Heidelberg, Germany.
Malgorzata Mizerska-Wasiak, Department of Pediatrics and Nephrology, Medical University of Warsaw, Warsaw, Poland.
Mohit Mathur, Visterra, Inc., Waltham, MA, USA.
Michaela J Schultz, Otsuka Pharmaceutical Development & Commercialization Inc., Princeton, NJ, USA.
FUNDING
This work was supported by Visterra, Inc. (Waltham, MA, USA), a member of the Otsuka family of companies. Authors affiliated with the sponsors were involved in the design of the study, the analysis and interpretation of data, the writing and reviewing of this article, and the decision to submit the article for publication.
AUTHORS’ CONTRIBUTIONS
C.J.W., M.M., and M.J.S. designed the study. C.J.W. acquired the data. C.J.W., R.C., F.S., M.M.W., M.M., and M.J.S. interpreted the data, participated in the drafting or the critical review of the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
DATA AVAILABILITY STATEMENT
The data underlying this article are available in the article and in its online supplementary material.
CONFLICT OF INTEREST STATEMENT
C.J.W. has served as an epidemiologic consultant to Otsuka/Visterra, Janssen Pharmaceuticals, and Healx. R.C. has been a consultant or advisory board member for Amgen, Argenx, Calliditas Therapeutics, Menarini, Novartis, Otsuka/Visterra, Purespring, Reata Pharmaceuticals, Stadapharm, and Travere Therapeutics. F.S. has received consultancy fees from Alexion, Otsuka, and Novartis. M.M.W. declares no conflict of interest. M.M. is a full-time employee of Visterra, Inc. M.J.S. was a full-time employee of Visterra, Inc. at the time of this work, and is currently a full-time employee of Otsuka Pharmaceutical Development & Commercialization Inc.
REFERENCES
- 1. Lai KN, Tang SCW, Schena FPet al. IgA nephropathy. Nat Rev Dis Primers 2016;2:16001. 10.1038/nrdp.2016.1. [DOI] [PubMed] [Google Scholar]
- 2. Roberts ISD . Pathology of IgA nephropathy. Nat Rev Nephrol 2014;10:445–54. 10.1038/nrneph.2014.92. [DOI] [PubMed] [Google Scholar]
- 3. Kiryluk K, Novak J. The genetics and immunobiology of IgA nephropathy. J Clin Invest 2014;124:2325–32. 10.1172/JCI74475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coppo R. Clinical and histological risk factors for progression of IgA nephropathy: an update in children, young and adult patients. J Nephrol 2017;30:339–46. 10.1007/s40620-016-0360-z. [DOI] [PubMed] [Google Scholar]
- 5. McGrogan A, Franssen CFM, de Vries CS. The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol Dial Transplant 2011;26:414–30. 10.1093/ndt/gfq665. [DOI] [PubMed] [Google Scholar]
- 6. Fiorentino M, Bolignano D, Tesar Vet al. ; ERA-EDTA Immunonephrology Working Group. Renal biopsy in 2015 – from epidemiology to evidence-based indications. Am J Nephrol 2016;43:1–19. 10.1159/000444026. [DOI] [PubMed] [Google Scholar]
- 7. Schena FP, Nistor I. Epidemiology of IgA nephropathy: a global perspective. Semin Nephrol 2018;38:435–42. 10.1016/j.semnephrol.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 8. Coppo R, Robert T. IgA nephropathy in children and in adults: two separate entities or the same disease? J Nephrol 2020;33:1219–29. 10.1007/s40620-020-00725-0. [DOI] [PubMed] [Google Scholar]
- 9. Szklo M, Nieto FJ. Epidemiology: Beyond the Basics, 3rd edn. Jones & Bartlett Learning, LLC, Burlington, MA: 2014. [Google Scholar]
- 10. Kleinbaum DG, Klein M. Survival Analysis: A Self-Learning Text, 3rd edn. Springer, New York, NY: 2012. [Google Scholar]
- 11. Maixnerova D, Neprasova M, Skibova Jet al. IgA nephropathy in Czech patients – are we able reliably predict the outcome? Kidney Blood Press Res 2014;39:555–62. 10.1159/000368467. [DOI] [PubMed] [Google Scholar]
- 12. ERA-EDTA Registry . ERA-EDTA Registry Annual Report 2017. Amsterdam UMC, location AMC, Amsterdam, The Netherlands: 2019; https://era-edta-reg.org/files/annualreports/AnnRep2017.pdf (27 September 2021, date last accessed). [Google Scholar]
- 13. Woo KT, Chan CM, Lim Cet al. A global evolutionary trend of the frequency of primary glomerulonephritis over the past four decades. Kidney Dis (Basel) 2019;5:247–58. 10.1159/000500142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maixnerova D, Jancova E, Skibova Jet al. Nationwide biopsy survey of renal diseases in the Czech Republic during the years 1994–2011. J Nephrol 2015;28:39–49. 10.1007/s40620-014-0090-z. [DOI] [PubMed] [Google Scholar]
- 15. Heaf J, Løkkegaard H, Larsen S. The epidemiology and prognosis of glomerulonephritis in Denmark 1985–1997. Nephrol Dial Transplant 1999;14:1889–97. 10.1093/ndt/14.8.1889. [DOI] [PubMed] [Google Scholar]
- 16. Riispere Ž, Ots-Rosenberg M. Occurrence of kidney diseases and patterns of glomerular disease based on a 10-year kidney biopsy material: a retrospective single-centre analysis in Estonia. Scand J Urol Nephrol 2012;46:389–94. 10.3109/00365599.2012.693133. [DOI] [PubMed] [Google Scholar]
- 17. Gesualdo L, Di Palma AM, Morrone LFet al. ; Italian Immunopathology Group , Italian Society of Nephrology. The Italian experience of the national registry of renal biopsies. Kidney Int 2004;66:890–4. 10.1111/j.1523-1755.2004.00831.x. [DOI] [PubMed] [Google Scholar]
- 18. Brazdziute E, Miglinas M, Gruodyte Eet al. Nationwide renal biopsy data in Lithuania 1994–2012. Int Urol Nephrol 2015;47:655–62. 10.1007/s11255-015-0927-y. [DOI] [PubMed] [Google Scholar]
- 19. Knoop T, Vikse BE, Svarstad Eet al. Mortality in patients with IgA nephropathy. Am J Kidney Dis 2013;62:883–90. 10.1053/j.ajkd.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 20. Perkowska-Ptasinska A, Bartczak A, Wagrowska-Danilewicz Met al. ; Polish Society of Nephrology. Clinicopathologic correlations of renal pathology in the adult population of Poland. Nephrol Dial Transplant 2017;32:ii209–18. 10.1093/ndt/gfw365. [DOI] [PubMed] [Google Scholar]
- 21. The Scottish Renal Registry . Scottish Renal Biopsy Registry: Survey of Native Kidney Biopsies Performed in Scotland 2017. https://www.srr.scot.nhs.uk/Biopsy-Registry/Main.html (27 September 2021, date last accessed). [Google Scholar]
- 22. López-Gómez JM, Rivera F. Spanish registry of glomerulonephritis 2020 revisited: past, current data and new challenges. Nefrología (English Edition) 2020;40:371–83. 10.1016/j.nefroe.2020.08.004. [DOI] [PubMed] [Google Scholar]
- 23. Jarrick S, Lundberg S, Welander Aet al. Mortality in IgA nephropathy: a nationwide population-based cohort study. J Am Soc Nephrol 2019;30:866–76. 10.1681/ASN.2018101017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rivera F, López-Gómez JM, Pérez-García Ret al. ; Spanish Registry of Glomerulonephritis. Frequency of renal pathology in Spain 1994–1999. Nephrol Dial Transplant 2002;17:1594–602. 10.1093/ndt/17.9.1594. [DOI] [PubMed] [Google Scholar]
- 25. Coppo R, Troyanov S, Bellur Set al. ; VALIGA study of the ERA-EDTA Immunonephrology Working Group. Validation of the Oxford classification of IgA nephropathy in cohorts with different presentations and treatments. Kidney Int 2014;86:828–36. 10.1038/ki.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. The Norwegian Renal Registry . Annual Report 2018. November2019; https://www.nephro.no/nnr/AARSRAPPORT_NNR_2018_ToC.pdf (27 September 2021, date last accessed). [Google Scholar]
- 27. Tesar V, Troyanov S, Bellur Set al. ; VALIGA study of the ERA-EDTA Immunonephrology Working Group. Corticosteroids in IgA nephropathy: a retrospective analysis from the VALIGA study. J Am Soc Nephrol 2015;26:2248–58. 10.1681/ASN.2014070697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Christensen K, Davidsen M, Juel Ket al. The divergent life-expectancy trends in Denmark and Sweden – and some potential explanations. In: National Research Council (US) Panel on Understanding Divergent Trends in Longevity in High-Income Countries; Crimmins EM, Preston SH, Cohen B, (eds). International Differences in Mortality at Older Ages: Dimensions and Sources. National Academies Press, Washington, DC, USA: 2010;14. https://www.ncbi.nlm.nih.gov/books/NBK62583/(27 September 2021, date last accessed). [PubMed] [Google Scholar]
- 29. World Health Organization Regional Office for Europe . Interim Second Report on Social Determinants of Health and the Health Divide in the WHO European Region. 2011; https://www.euro.who.int/_data/assets/pdf_file/0010/148375/id5E_2ndRepSocialDet-jh.pdf (27 September 2021, date last accessed). [Google Scholar]
- 30. Zarocostas J. Life expectancy varies across WHO Europe region by 16 years. BMJ 2011;343:d5667. 10.1136/bmj.d5667. [DOI] [PubMed] [Google Scholar]
- 31. Nordic Burden of Disease Collaborators . Life expectancy and disease burden in the Nordic countries: results from the Global Burden of Diseases, Injuries, and Risk Factors Study 2017. Lancet Public Health 2019;4:e658–69. 10.1016/S2468-2667(19)30224-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Batinić D, Šćukanec-Špoljar M, Milošević Det al. Kliničke i patohistološke karakteristike biopsijom dokazanih bubrežnih bolesti djece u Hrvatskoj [Clinical and histopathological characteristics of biopsy-proven renal diseases in Croatia]. Acta Med Croatica 2007;61:361–4. [PubMed] [Google Scholar]
- 33. Zink CM, Ernst S, Riehl Jet al. Trends of renal diseases in Germany: review of a regional renal biopsy database from 1990 to 2013. Clin Kidney J 2019;12:795–800. 10.1093/ckj/sfz023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mizerska-Wasiak M, Turczyn A, Such Aet al. IgA nephropathy in children: a multicenter study in Poland. Adv Exp Med Biol 2016;952:75–84. 10.1007/5584_2016_65. [DOI] [PubMed] [Google Scholar]
- 35. Gutiérrez E, Praga M, Rivera Fet al. ; All members of the Spanish Registry of Glomerulonephritis. Changes in the clinical presentation of immunoglobulin A nephropathy: data from the Spanish Registry of Glomerulonephritis. Nephrol Dial Transplant 2018;33:472–7. 10.1093/ndt/gfx058. [DOI] [PubMed] [Google Scholar]
- 36. Coppo R. Pediatric IgA nephropathy in Europe. Kidney Dis (Basel) 2019;5:182–8. 10.1159/000495751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. O'Shaughnessy MM, Hogan SL, Poulton CJet al. Temporal and demographic trends in glomerular disease epidemiology in the southeastern United States, 1986–2015. Clin J Am Soc Nephrol 2017;12:614–23. 10.2215/CJN.10871016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Coppo R, Gianoglio B, Porcellini MG, Maringhini S.. Frequency of renal diseases and clinical indications for renal biopsy in children (report of the Italian National Registry of Renal Biopsies in Children). Nephrol Dial Transplant 1998;13:293–7. 10.1093/oxfordjournals.ndt.a027821. [DOI] [PubMed] [Google Scholar]
- 39. Murakami M, Hayakawa M, Yanagihara T, Hukunaga Y.. Proteinuria screening for children. Kidney Int 2005;67:S23–7. 10.1111/j.1523-1755.2005.09406.x. [DOI] [PubMed] [Google Scholar]
- 40. Cho BS, Kim SD. School urinalysis screening in Korea. Nephrology (Carlton) 2007;12:S3–S7. 10.1111/j.1440-1797.2007.00873.x. [DOI] [PubMed] [Google Scholar]
- 41. Sevillano AM, Diaz M, Caravaca-Fontán Fet al. Spanish Group for the Study of Glomerular Diseases (GLOSEN). IgA nephropathy in elderly patients. Clin J Am Soc Nephrol 2019;14:1183–92. 10.2215/CJN.13251118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kiryluk K, Li Y, Sanna-Cherchi Set al. Geographic differences in genetic susceptibility to IgA nephropathy: GWAS replication study and geospatial risk analysis. PLoS Genet 2012;8:e1002765. 10.1371/journal.pgen.1002765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yeo SC, Goh SM, Barratt J. Is immunoglobulin A nephropathy different in different ethnic populations? Nephrology (Carlton) 2019;24:885–95. 10.1111/nep.13592. [DOI] [PubMed] [Google Scholar]
- 44. McQuarrie EP, Mackinnon B, Young Bet al. ; Scottish Renal Biopsy Registry. Centre variation in incidence, indication and diagnosis of adult native renal biopsy in Scotland. Nephrol Dial Transplant 2009;24:1524–8. 10.1093/ndt/gfn677. [DOI] [PubMed] [Google Scholar]
- 45. Ranabhat CL, Kim CB, Park MB, Acharaya S.. Multiple disparities in adult mortality in relation to social and health care perspective: results from different data sources. Global Health 2017;13:57. 10.1186/s12992-017-0283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. World Health Organization . Global Health Observatory Data Repository, Life Tables by Country – Sweden. December2020; https://apps.who.int/gho/data/?theme=main&vid=61600 (3 February 2022, date last accessed). [Google Scholar]
- 47. Glassock RJ. Mortality risk in IgA nephropathy. J Am Soc Nephrol 2019;30:720–2. 10.1681/ASN.2018121255. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.