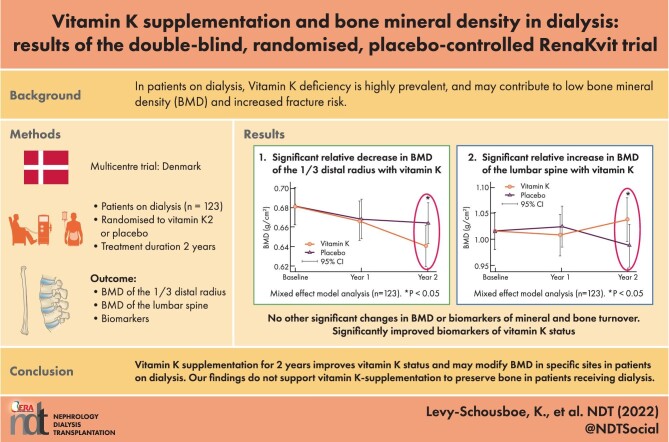
ABSTRACT
Background
Vitamin K deficiency is highly prevalent in patients on dialysis and may contribute to their low bone mineral density (BMD) and increased risk of fracture. This study investigated the effect of menaquinone-7 (MK-7) supplementation on BMD in patients on chronic dialysis.
Methods
In a multicentre, double-blind, placebo-controlled intervention trial, 123 patients on chronic dialysis were randomised to a daily oral supplement of either MK-7 360 µg or placebo for 2 years. BMD of the distal radius (1/3, mid, ultradistal and total), femoral neck, lumbar spine (L1–L4) and whole body was assessed by dual-energy X-ray absorptiometry. Serum levels of vitamin K1 and MK-7 and plasma levels of total osteocalcin, dephosphorylated-uncarboxylated matrix Gla protein and protein induced by vitamin K absence II were measured to assess vitamin K status.
Results
After 2 years, an accelerated BMD loss of the 1/3 distal radius was found with MK-7 supplementation {mean difference of changes relative to placebo −0.023 g/cm2 [95% confidence interval (CI) −0.039 to −0.008]}, whereas the decrease in lumbar spine BMD seen in the placebo group was prevented [mean difference of changes between groups 0.050 g/cm2 (95% CI 0.015–0.085)]. No significant effects were observed at the remaining skeletal sites. Vitamin K status strongly improved in MK-7-supplemented participants.
Conclusion
Compared with placebo, an accelerated BMD loss of the 1/3 distal radius was found after 2 years of MK-7 supplementation, whereas a decline in lumbar spine BMD was prevented. As such, MK-7 supplementation might modify BMD site-specifically in patients on dialysis. In aggregate, our findings do not support MK-7 supplementation to preserve bone in patients on dialysis.
Keywords: bone mineral density, chronic kidney disease, end-stage kidney disease, menaquinone-7, mineral and bone disorder
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What is already known about this subject?
In patients with chronic kidney disease on dialysis treatment, low bone mineral density (BMD) is prevalent.
Low BMD is associated with an increased risk of bone fracture and mortality.
Vitamin K deficiency may contribute to low BMD.
What this study adds?
In a 2-year randomised, double-blind, placebo-controlled study of patients on chronic dialysis treatment, an accelerated BMD loss of the 1/3 distal radius but preserved BMD of the lumbar spine were seen with 2 years of MK-7 supplementation compared with the changes in BMD with placebo in an intention-to-treat analysis. BMD at the other sites measured was unaffected.
Vitamin K supplementation improved the vitamin K status compared with placebo.
What impact this may have on practice or policy?
The results of the present study indicate that MK-7 supplementation might modify BMD site-specifically in patients receiving dialysis treatment but, in aggregate, do not support MK-7 supplementation to preserve bone.
INTRODUCTION
As kidney function declines, disturbances in the mineral metabolism cause abnormalities in bone turnover, mineralization and bone loss, as well as increased vascular and other soft-tissue calcification, the so-called chronic kidney disease–mineral and bone disorder (CKD-MBD) [1]. In patients with end-stage kidney disease (ESKD) on dialysis, the risk of fractures is increased up to 4 times compared with the general population [2]. Fractures are associated with increased morbidity and all-cause mortality [3]. Compared with the general population, low bone mineral density (BMD) is more frequent [4] and predicts future fractures in patients on dialysis treatment [5].
BMD may be affected by vitamin K status. In observational studies, vitamin K levels were positively associated with BMD in the elderly general population [6] and in patients on dialysis [7]. Furthermore, reduced levels of vitamin K are associated with an increased risk of fracture in both the elderly general population [8] and in patients on dialysis [9, 10]. In addition, treatment with vitamin K antagonists increases fracture risk [10].
Patients treated with dialysis are very often vitamin K deficient, with highly elevated levels of vitamin K–dependent proteins. Vitamin K supplementation has been demonstrated to improve vitamin K status, as systemic levels of osteocalcin [OC; as undercarboxylated osteocalcin (ucOC)], matrix Gla protein [MGP; as dephosphorylated-uncarboxylated matrix Gla protein (dp-ucMGP)] and protein induced by vitamin K absence II (PIVKA-II) significantly decreased during supplementation [11, 12]. So far, the effect of vitamin K supplementation on BMD in patients on dialysis treatment has not been reported by any studies.
The aim of this randomised, double-blind, placebo-controlled, study was to investigate the effect of vitamin K supplementation on BMD in patients on dialysis treatment.
MATERIALS AND METHODS
Study design
The RenaKvit trial was a 2-year randomised, multicentre, double-blind, placebo-controlled study examining the effects of vitamin K supplementation as menaquinone-7 (MK-7) on bone and vascular calcification in patients on dialysis treatment. The study design and the effects on vascular calcification have been described previously [13].
The study was approved by the Scientific Ethics Committee for the Region of Zealand (SJ-511) and the Danish Data Protection Agency and was registered at ClinicalTrial.gov (NCT02976246). The supporting CONSORT checklist can be found in Supplementary Table 1S.
Study population and intervention
In brief, eligible participants were adult patients on chronic dialysis treatment not treated with vitamin K antagonists, recombinant parathyroid hormones, anti-osteoporotic drugs or vitamin K supplements. Participants were excluded during follow-up if they initiated vitamin K antagonist treatment, were <50% adherent to or had non-acceptable side effects from the study tablets or received a kidney transplant. Informed consent was obtained from all participants included in the study.
Participants were randomised to receive one daily tablet containing 360 µg MK-7 or a visually identical placebo tablet using concealed block randomisation with blocks of four (2:2). Each block consisted of patients treated with the same dialysis modality at the same dialysis centre. Double blinding was maintained until the last patient's last visit.
Kappa Bioscience AS, Oslo, Norway, produced the synthetic MK-7 (K2VITAL Delta), according to the Hazard Analysis Critical Control Point principles, and Orkla Care AS, Ishøj, Denmark, produced all tablets used in the study.
Adverse events (AEs) and serious AEs (SAEs) were monitored according to the procedure described in Supplementary Table 2S.
Outcomes
The primary outcome was changes in BMD of the 1/3 distal radius, since BMD of the 1/3 distal radius predicts fractures in patients on dialysis treatment [5, 14, 15]. Secondary outcomes were changes in BMD of the remaining distal radial sites (mid, ultradistal and total), lumbar spine (L1–L4), femoral neck and whole body, as well as changes in biochemical markers of vitamin K status and bone metabolism, changes in abdominal aortic calcification (AAC) scores and clinical outcomes (bone fracture, parathyroidectomy, thromboembolic event and death) during the study period.
Measurements
Dual-energy X-ray absorptiometry (DXA)
BMD of the distal half of the left radius [divided into the 1/3 (most proximal 20 mm), mid and ultradistal radius (most distal 15 mm)], lumbar spine (L1–L4), left femoral neck and whole body were assessed by DXA. DXA procedures are described in detail in Supplementary Material 4S.
Biochemical measurements
Serum vitamin K1 and MK-7 were analysed by mass spectrometry according to Boegh et al. [16] with modifications for MK-7 analysis as previously described [13]. Biochemical procedures are described in detail in Supplemental Material 4S.
Conventional X-ray of the lumbar spine
AAC scores were quantified as per Kauppila et al. [17] using a conventional laterally exposed X-ray of the lumbar spine (L1–L4). All X-rays were reviewed by the same experienced radiologist who was blinded to treatment allocation. The number of lumbar vertebral compression fractures (L1–L4), defined as a vertebral height reduction of at least 20% or 4 mm [18], was assessed at baseline and after 2 years of intervention.
Statistical methods
No data on changes in BMD over time of the 1/3 distal radius were available in patients on dialysis when the study was planned. The sample size calculation was therefore based on cross-sectional data in which a difference in BMD of the 1/3 distal radius of 0.26 g/cm2 in patients on dialysis with or without fractures and an overall standard deviation (SD) in BMD of the 1/3 distal radius of 0.46 g/cm2 was found [19]. To detect a minimal relevant change in the 1/3 distal radius BMD of 0.26 g/cm2 (5% significance level, 80% power) at least 2 × 49 patients were needed. With an expected annual dropout rate of 20% in our study population with a well-described high mortality, we aimed at including at least 2 × 70 patients in the study. Parametric and non-parametric statistics were applied on normally and non-normally distributed data, respectively. Categorical data were analysed with the Pearson's chi-squared or Fisher's exact test. Mixed effects linear regression models were used to estimate longitudinal treatment effects. With the applied methodology, missing values were handled and list exclusion avoided, meaning that analyses were intention-to-treat (ITT) analyses. Maximum likelihood procedures were used for estimation, thus, under the assumption of missing at random, unbiased effect sizes are estimated. The analyses were adjusted for baseline measures by constraining the baseline means of the two study groups to be equal, thereby considering participants as a random sample of the background prior to intervention, as suggested by Twisk et al. [20]. Examination of the underlying model assumptions was performed by visual inspection of QQ-plots of residuals as well as residuals versus predicted plots. If deviation from the assumption of variance homogeneity of the residuals was observed, the analyses were modified allowing for distinct error variances.
Due to severe heteroscedasticity in the MK-7 group, the variance of residuals at follow-up measurements was allowed to differ from residual variances at baseline. All analyses were repeated with adjustments for age and sex (partly adjusted) and adjustments for sex, age and baseline measures of 25-hydroxyvitamin D and dp-ucMGP (fully adjusted). The testing of secondary endpoints was not hierarchical. Mixed effects models were implemented in Stata version 16 (StataCorp, College Station, TX, USA) and other data processing and analyses using SPSS version 26 (IBM, Armonk, NY, USA). P-values <.05 were considered statistically significant.
RESULTS
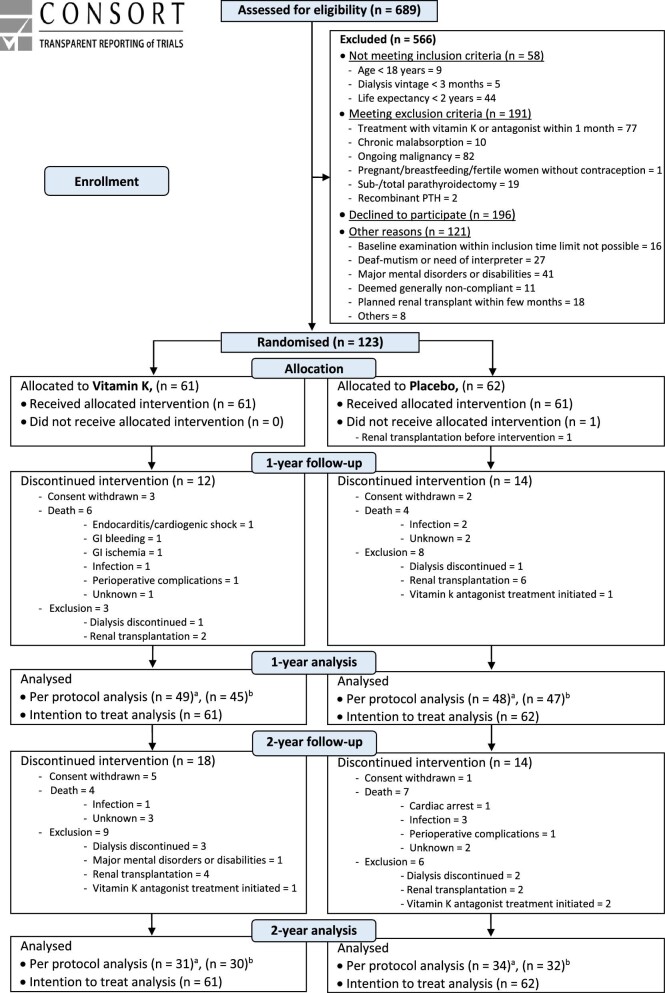
A total of 689 patients on dialysis were screened for eligibility (Fig. 1). Of those, 123 patients were enrolled and randomised to either MK-7 (n = 61) or placebo (n = 62). Twenty-six participants (21%) dropped out during year 1, leaving 97 participants (79%) available for the 1-year follow-up. An additional 32 participants dropped out during year 2, leaving 65 participants (53%) available for the 2-year follow-up (Fig. 1). All participants were Caucasian except one participant of Asian ancestry and one of Latino ancestry. Baseline characteristics are shown in Tables 1 and 2.
Figure 1:
CONSORT flow diagram. GI, gastro intestinal; PTH, parathyroid hormone. aNumber of overall completing participants. bNumber of participants completing the 1/3 distal radius DXA (primary outcome).
Table 1:
Baseline characteristics of participants.
| All participants randomised (N = 123) | ||
|---|---|---|
| Characteristics | Vitamin K | Placebo |
| Participants, n | 61 | 62 |
| Males, n (%) | 45 (74) | 50 (81) |
| Age (years), mean ± SD | 65.2 ± 11.1 | 63.7 ± 12.0 |
| Height (cm), mean ± SD | 172.9 ± 9.6 | 174.0 ± 8.6 |
| Body mass index (kg/m2), mean ± SD | 26.3 ± 5.1 | 27.6 ± 5.6 |
| Smokers, n (%) | ||
| Current | 10 (17) | 11 (18) |
| Former | 31 (51) | 21 (34) |
| Comorbidity, n (%) | ||
| Previous fracturea | 3 (5) | 4 (6) |
| Cerebral strokeb | 8 (13) | 5 (8) |
| Peripheral arterial diseasec | 6 (10) | 5 (8) |
| Ischemic heart diseased | 7 (11) | 4 (6) |
| Heart failuree | 8 (13) | 8 (13) |
| Aetiology to ESKDf, n (%) | ||
| Polycystic kidney disease | 9 (15) | 7 (11) |
| Diabetic nephropathy | 7 (11) | 16 (26) |
| Glomerulonephritis | 10 (16) | 10 (16) |
| Vascular-hypertensive nephropathy | 12 (20) | 13 (21) |
| Post-renal nephropathy | 1 (2) | 0 (0) |
| Focal segmental glomerulosclerosis | 1 (2) | 0 (0) |
| Vasculitis | 1 (2) | 1 (2) |
| Chronic interstitial nephritis | 0 (0) | 1 (2) |
| Hereditary nephropathy | 0 (0) | 1 (2) |
| Other | 11 (18) | 6 (10) |
| Unknown | 9 (15) | 7 (11) |
| Dialysis modality, n (%) | ||
| Haemodialysis | 41 (67) | 38 (61) |
| Peritoneal dialysis | 20 (33) | 21 (34) |
| Hybrid dialysisg | 0 (0) | 3 (5) |
| Dialysis vintage (months), median (IQR) | 26.0 (9.3–46.0) | 17.0 (8.8–42.3) |
| Former kidney transplant, n (%) | 8 (13) | 12 (19) |
| Medication, n (%) | ||
| Vitamin D | ||
| Native | 40 (52) | 37 (48) |
| Activated | 40 (48) | 44 (52) |
| Phosphate binders | ||
| Calcium-containing | 28 (42) | 38 (58) |
| Non-calcium-containing | 39 (46) | 45 (54) |
| Calcimimetics (cinacalcet, etelcalcetid) | 11 (44) | 14 (56) |
| Biochemistry, median (IQR) | ||
| S-vitamin K1 (nmol/l) | 0.36 (0.21–0.66) | 0.46 (0.28–0.65) |
| S-MK-7 (nmol/l) | 0.40 (0.25–0.71) | 0.49 (0.30–0.76) |
| P-tOC (ng/ml) | 186.4 (112.4–349.9) | 154.1 (69.7–228.4) |
| P-dp-ucMGP (pmol/l)h | 1870 (1315–2651) | 1941 (1449–2629) |
| P-PIVKA-II (ng/ml)h | 36.1 (22.3–54.4) | 25.6 (21.1–42.1) |
| P-25-OH-vitamin D (D2 + D3) (nmol/l), mean ± SD | 74.2 ± 35.2 | 61.6 ± 28.7 |
| S-1,25(OH)2 vitamin D (pmol/l), mean ± SD | 54.4 ± 30.8 | 49.0 ± 25.7 |
| P-iPTH (pmol/l) | 28.5 (20.2–43.6) | 23.7 (12.3–43.6) |
| P-FGF-23 (ng/l) | 5 015 (1820–11 083) | 3463 (698–12 530) |
| P-ionized calcium (mmol/l), mean ± SD | 1.18 ± 0.09 | 1.19 ± 0.07 |
| P-phosphate (mmol/l), mean ± SD | 1.69 ± 0.41 | 1.59 ± 0.44 |
| P-magnesium (mmol/l), mean ± SD | 1.04 ± 0.17 | 1.00 ± 0.22 |
| P-BSAP (µg/l) | 10.7 (7.6–15.9) | 10.6 (5.9–15.6) |
| P-CTX-1 (ng/l), mean ± SD | 2086 ± 1228 | 1608 ± 965 |
| P-P1NP (µg/l) | 247.8 (135.6–423.1) | 205.4 (113.9–394.1) |
| P-albumin (g/l), mean ± SD | 34 ± 3.9 | 34 ± 4.4 |
B: blood; P: plasma; S: serum; FGF-23: fibroblast growth factor 23; BSAP: bone-specific alkaline phosphatase; CTX-1: type I collagen cross-linked C-telopeptide; P1NP, total procollagen type 1 N-terminal propeptide.
Previous fracture of the vertebra, upper- or forearm and/or upper or lower leg within the last 5 years and verified by X-ray.
bFormer sudden onset of focal neurological outcome >24 h duration, radiologically verified.
cFormer amputation, peripheral by-pass operation, Fontaine 3rd degree (resting pain) or 4th degree (ischaemic ulcer/gangrene), ankle blood pressure <50 or toe blood pressure <40 mmHg.
Former medically and biochemically verified acute myocardial infarction, percutaneous coronary intervention or coronary artery bypass graft.
eEjection fraction <40% assessed by echocardiography.
fAccording to medical records.
gHybrid dialysis treatment consisting of alternately haemo- and peritoneal dialysis.
hNormal reference range: dp-ucMGP 366–-646 pmol/l, PIVKA-II 8.4–131.0 ng/ml according to the manufacturer (Supplementary Material 4S).
Table 2:
Baseline bone characteristics of participants assessed by DXA.
| All participants randomised | ||
|---|---|---|
| Characteristics | Vitamin K | Placebo |
| Participants, n | 61 | 62 |
| 1/3 distal radius | ||
| BMD (g/cm2) | 0.669 ± 0.099 | 0.695 ± 0.110 |
| T-score | −2.08 ± 1.53 | −1.82 ± 1.59 |
| Z-score | −0.73 ± 1.61 | −0.58 ± 1.58 |
| Mid-distal radius | ||
| BMD (g/cm2) | 0.535 ± 0.096 | 0.560 ± 0.101 |
| T-score | −2.68 ± 1.66 | −2.25 ± 1.63 |
| Z-score | −1.59 ± 1.77 | −1.31 ± 1.59 |
| Ultradistal radius | ||
| BMD (g/cm2) | 0.375 ± 0.800 | 0.389 ± 0.097 |
| T-score | −2.34 ± 1.22 | −2.19 ± 1.50 |
| Z-score | −1.19 ± 1.37 | −1.12 ± 1.59 |
| Total distal radius | ||
| BMD (g/cm2) | 0.520 ± 0.089 | 0.543 ± 0.098 |
| T-score | −2.55 ± 1.45 | −2.30 ± 1.58 |
| Z-score | −1.31 ± 1.60 | −1.14 ± 1.62 |
| Lumbar spine (L1-L4) | ||
| BMD (g/cm2) | 1.003 ± 0.202 | 1.030 ± 0.195 |
| T-score | −0.63 ± 1.80 | −0.49 ± 1.73 |
| Z-score | 0.34 ± 1.96 | 0.44 ± 1.80 |
| Femoral neck | ||
| BMD (g/cm2) | 0.777 ± 0.145 | 0.797 ± 0.165 |
| T-score | −1.57 ± 0.94 | −1.41 ± 1.03 |
| Z-score | −0.88 ± 0.91 | −0.61 ± 1.05 |
| Whole body | ||
| BMD (g/cm2) | 1.049 ± 0.107 | 1.059 ± 0.123 |
| T-score | −1.43 ± 1.12 | −1.44 ± 1.36 |
| Z-score | −0.76 ± 1.02 | −0.76 ± 1.21 |
Data presented as mean ± SD unless stated otherwise.
DXA
DXA scans were performed in 92 of the 97 participants at year 1 and in 62 of the 65 participants completing the study at year 2 (Fig. 1). Causes of missing measurements are shown in Supplementary Table 5S. At baseline, the number of participants with a T-score below the osteoporosis defining threshold of −2.5 at any BMD site did not differ between the MK-7 and placebo groups (69% and 54%, respectively; P = .10).
Distal radius BMD
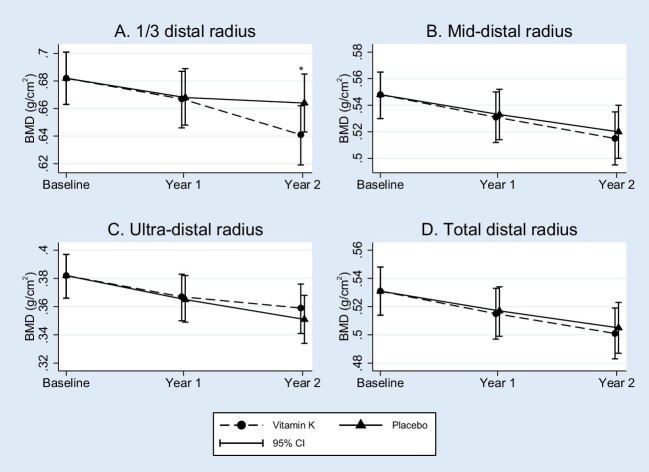
After 1 year, BMD of the 1/3 distal radius decreased significantly in both the group treated with MK-7 {−0.015 g/cm2 [95% confidence interval (CI) −0.025 to −0.005]} and placebo [−0.013 g/cm2 (95% CI −0.023 to −0.004)] with no between-group difference (P = .81) (Fig. 2A and Table 3). The absolute BMD levels were similar between groups (P = .13) (Supplementary Table 6S). After 2 years, BMD of the 1/3 distal radius was significantly decreased in both the MK-7 [−0.041 g/cm2 (95% CI −0.052 to −0.030)] and the placebo groups [−0.018 g/cm2 (−0.029 to −0.007)]. The decrease was significantly larger in the MK-7 group (P = .004) (Fig. 2A and Table 3) and the absolute level of BMD was significantly lower in the MK-7 group after 2 years (P = .03) (Supplementary Table 6S).
Figure 2:
Effects of 2-years of supplementation with vitamin K2 (MK-7, 360 μg daily) or placebo: Bone mineral density of the distal radius. Mixed effect model analysis of changes in bone mineral density of the distal radius. (A) The 1/3 distal radius. (B) Mid-distal radius. (C) Ultra-distal radius. (D) Total distal radius (n = 120baseline, 92year 1 and 62year 2). Variables are unadjusted and presented as mean and 95% confidence intervals (CI). Significant between-group differences *: P ≤ .005. BMD; bone mineral density.
Table 3:
Effects of 2 years of supplementation with vitamin K2 (MK-7, 360 µg/day) or placebo: Significant changes in bone mineral density.
| Unadjusted analyses | Unadjusted analyses | Partly adjusted analyses | Fully adjusted analyses | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean levels (95% CI) | Mean change in study groups from baseline (95% CI) | ||||||||||
| BMD (g/cm2) | Time | Vitamin K | Placebo | Vitamin K | Placebo | Differences of changes between study groups from baseline (95% CI) | P-value | Differences of changes between study groups from baseline (95% CI) | P-value | Differences of changes between study groups from baseline (95% CI) | P-value |
| 1/3 distal radius | Baseline | 0.682 (0.663–0.701) (n = 120) | |||||||||
| Year 1 | 0.667 (0.646–0.687) (n = 45) | 0.668 (0.648–0.689) (n = 47) | −0.015 (−0.025 to −0.005) | −0.013 (−0.023 to −0.004) | −0.002 (−0.015–0.012) | .81 | −0.002 (−0.015–0.012) | .79 | −0.001 (−0.015–0.012) | .86 | |
| Year 2 | 0.641 (0.619–0.662) (n = 30) | 0.664 (0.643–0.685) (n = 32) | −0.041 (−0.052 to −0.030) | −0.018 (−0.029 to −0.007) | −0.023 (−0.039 to −0.008) | .004 | −0.023 (−0.039 to −0.008) | .004 | −0.021 (−0.037 to −0.005) | .009 | |
| Lumbar spine (L1–L4) | Baseline | 1.016 (0.980–1.052) (n = 119) | |||||||||
| Year 1 | 1.008 (0.968–1.048) (n = 44) | 1.025 (0.985–1.064) (n = 48) | −0.008 (−0.029–0.014) | 0.009 (−0.012–0.029) | −0.017 (−0.046–0.013) | .27 | −0.017 (−0.046–0.013) | .26 | −0.019 (−0.049–0.011) | .23 | |
| Year 2 | 1.038 (0.996–1.080) (n = 29) | 0.988 (0.947–1.029) (n = 33) | 0.022 (−0.003–0.047) | −0.028 (−0.052–−0.004) | 0.050 (0.015–0.085) | .005 | 0.050 (0.015–0.084) | .005 | 0.050 (0.015–0.086) | .005 | |
| Femoral neck | Baseline | 0.786 (0.759–0.814) (n = 118) | |||||||||
| Year 1 | 0.766 (0.731–0.801) (n = 44) | 0.775 (0.741–0.810) (n = 47) | −0.021 (−0.048–0.007) | −0.011 (−0.038–0.016) | −0.010 (−0.047–0.028) | .61 | −0.008 (−0.046–0.029) | .66 | −0.004 (−0.042–0.034) | .84 | |
| Year 2 | 0.752 (0.712–0.791) (n = 28) | 0.717 (0.679–0.755) (n = 32) | −0.035 (−0.068 to −0.002) | −0.069 (−0.101–−0.038) | 0.035 (−0.010–0.079) | .13 | 0.037 (−0.008–0.081) | .12 | 0.043 (−0.003–0.088) | .07 | |
Mixed effects model analysis of changes in BMD. Partly adjusted for gender and age; fully adjusted for baseline vitamin D and dp-ucMGP levels.
After both 1 and 2 years of intervention, BMD of the mid, ultradistal and total distal radius decreased significantly in both the MK-7 and placebo groups, but without significant differences in mean changes or absolute BMD levels between groups (all P-values >.05) (Fig. 2B–D and Supplementary Tables 6S and 7S).
Adjustments for gender and age, and additionally baseline vitamin D and vitamin K levels, did not modify the results of MK-7 supplementation on BMD in a stepwise model of any of the regions of the distal radius (Table 3 and Supplementary Table 7S).
Lumbar spine, femoral neck and whole body BMD
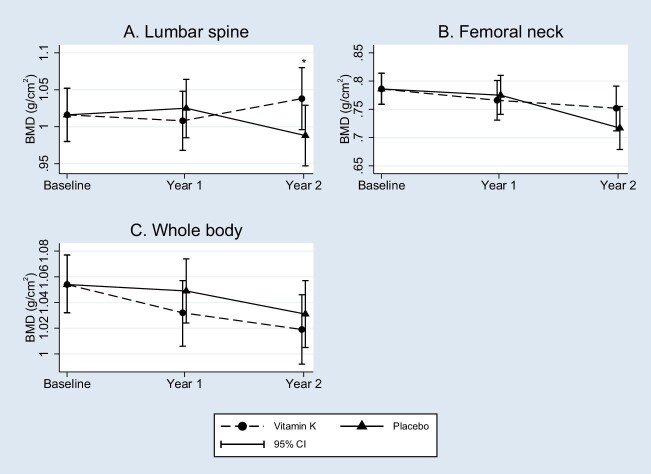
After 1 year, BMD of the lumbar spine did not change significantly in either the MK-7 or placebo groups and the mean changes from baseline or absolute BMD levels did not differ between study groups (P = .27 and .53, respectively) (Fig. 3A, Table 3 and Supplementary Table 6S). After 2 years, lumbar spine BMD was maintained with MK-7 but decreased significantly with placebo, and changes differed significantly between study groups (P = .005). The absolute BMD levels did not differ significantly between study groups at year 2 (P = .70) (Fig. 3A, Table 3 and Supplementary Table 6S).
Figure 3:
Effects of 2-years of supplementation with vitamin K2 (MK-7, 360 μg daily) or placebo: Bone mineral density. Mixed effect model analysis of changes in bone mineral density. (A) Lumbar spine (n = 119baseline, 92year 1 and 62year 2). (B) Femoral neck (n = 118baseline, 91year 1 and 60year 2). (C) Whole body (n = 105baseline, 80year 1 and 55year 2). Variables are unadjusted and presented as mean and 95% confidence intervals (CI). Significant between group differences *: P ≤ 0.005. BMD, bone mineral density.
There were no significant differences between the study groups in BMD changes of the femoral neck or whole body (all P-values >.05), but after 2 years of MK-7 supplementation, femoral neck BMD was nearly maintained and changes between groups differed borderline significantly (P = .07) (Fig. 3B and C, Table 3 and Supplementary Tables 6S and 7S).
Adjustment for gender and age and baseline vitamin K and D levels did not significantly modify the results of MK-7 on BMD of the lumbar spine, femoral neck or whole body (Table 3 and Supplementary Table 7S).
Biochemical outcomes
Markers of vitamin K
At baseline, participants were highly vitamin K deficient, as demonstrated by the elevated levels of plasma dp-uc-MGP (P-dp-uc-MGP) and PIVKA-II.
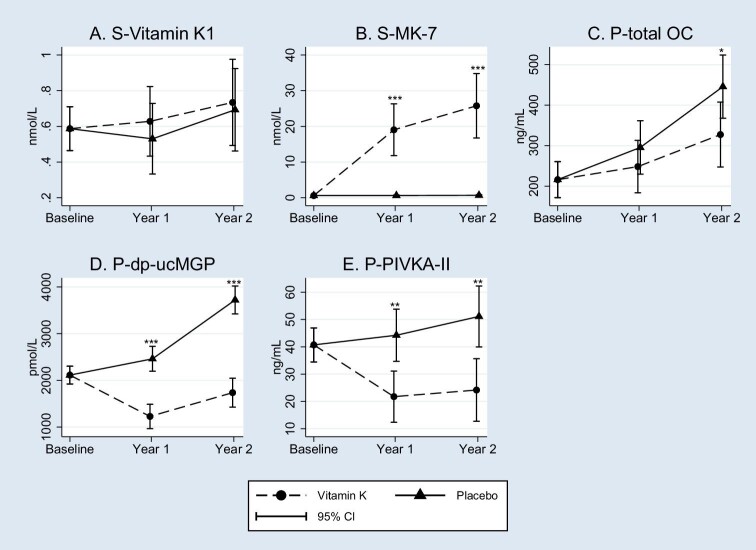
Serum MK-7 (S-MK-7) increased significantly in the MK-7 group but remained stable in the placebo group. The mean changes from baseline and the absolute MK-7 levels differed highly significantly between study groups (all P-values <.001) (Fig. 4 and Supplementary Table 8S).
Figure 4:
Effects of 2-year supplementation with vitamin K2 (MK-7, 360 μg daily) or placebo: Biochemical markers of vitamin K status. Mixed effect model analysis of changes in biochemical markers of vitamin K status. (A) vitamin K1 (n = 123baseline, 95year 1 and 65year 2). (B) MK-7 (n = 123baseline, 95year 1 and 65year 2). (C) total OC (n = 123baseline, 96year 1 and 60year 2). (D) dp-ucMGP (n = 123baseline, 96year 1 and 65year 2). (E) PIVKA-II (n = 122baseline, 96year 1 and 64year 2). Variables are unadjusted and presented as mean and 95% Confidence intervals (CI). Significant group differences (mean (95% CI)) at *P < .05, **P = 0.001, ***P < .001. dp-ucMGP, Dephosphorylated-uncarboxylated Matrix Gla Protein; MK-7, Menaquinone 7; P, Plasma; PIVKA-II, protein induced by vitamin K Absence-II; S, Serum.
Plasma total osteocalcin (tOC) levels were unchanged in the MK-7 group but increased significantly in the placebo group after 1 year. Over the 2-year study period, levels increased significantly in both study groups, yet to a larger extent with placebo (P = .03).
P-dp-ucMGP and PIVKA-II levels decreased significantly in the MK-7 group and increased significantly in the placebo group, with significant differences in changes and absolute levels between groups (all P-values <.001) (Fig. 4 and Supplementary Table 8S).
Markers of mineral and bone turnover
There were no significant differences between the study groups in the markers of mineral metabolism or bone turnover at any time (all P-values >.05) (Supplementary Table 9S).
Conventional X-ray of the lumbar spine
After 1 and 2 years, AAC scores increased in both study groups, but the changes from baseline (P = .51 and .41) and the absolute scores (P = .19 and .68) did not differ between groups (Supplementary Table 10S).
The number and extent of lumbar vertebral compression fractures did not differ between the study groups at baseline (P = .16) and remained unchanged after 2 years (Supplementary Table 11S).
Clinical outcomes
The number of participants who experienced bone fracture, parathyroidectomy, a thromboembolic event or death during the study did not differ between study groups (ITT analyses; all P-values >.05) (Supplementary Table 12S). Due to more events in one placebo-supplemented participant, the number of thromboembolic events differed between the study groups (P = .02).
Adherence and AEs
The mean adherence to study tablets was >90% after year 1 and 2 in both study groups. A total of 110 participants experienced 968 AEs during the trial and 481 SAEs occurred in 90 participants.
The number of participants who experienced an AE or SAE did not differ between groups (all P-values >.05) (Supplementary Tables 2S and 3S).
DISCUSSION
In the present randomised, double-blind, placebo-controlled trial, the effects on bone of daily supplementation with 360 µg vitamin K2 (MK-7) were compared with placebo in 123 patients receiving chronic dialysis treatment. In the ITT analysis, an accelerated BMD loss of the 1/3 distal radius was observed with MK-7 supplementation compared with placebo, whereas the decrease in lumbar spine BMD observed in the placebo-supplemented participants was prevented with MK-7. The RenaKvit trial is the first to investigate the effect of vitamin K supplementation on BMD in patients on dialysis treatment.
Surprisingly, we found the primary outcome, BMD of the 1/3 distal radius, to decrease ≈3% after 2 years of MK-7 supplementation compared with placebo, whereas BMD of the remaining distal radius sites was unaffected. However, these findings could not be confirmed in per protocol analysis. This may be because the participants able to complete the study represent only a subgroup of participants not randomly distributed between the groups. It may also be due to a type 1 statistical error in the ITT analysis. Four randomised studies, all in postmenopausal women with no kidney impairment, have examined the 2-year effect of vitamin K supplementation on distal radius BMD. One open-labelled study in 396 Japanese women examined the 1/3 distal radius and found a reduced BMD loss with supplements of vitamin K2 (MK-4) compared with no treatment [21]. Another open-label, non-placebo-controlled study found an increase in ultradistal radius BMD with vitamin K supplementation compared with vitamin D and calcium supplementation [22]. Yet, vitamin K supplementation did not affect the ultradistal and mid-distal radius BMD in two blinded and placebo-controlled studies [23, 24]. The present study is the first to find a worsening of BMD at any skeletal site during vitamin K supplementation compared with placebo. The difference in the 1/3 distal radius BMD between the study groups represents 20–25% of the SD, which translates into an ≈10% increased risk of fracture in patients with CKD according to Jamal et al. [15] and Prasad et al. [25].
BMD of the lumbar spine decreased in the placebo group to a similar extent as reported in a previous 2-year observational study in patients on dialysis [26]. This decrease was prevented, as BMD was maintained in the MK-7-supplemented group. The observed difference in changes in lumbar spine BMD between the study groups, corresponding to an ≈5% BMD increase with MK-7 supplementation, translates into a 10% reduction in vertebral fractures according to observations in CKD [15, 25].
In postmenopausal women without kidney impairment, the influence of vitamin K on lumbar spine BMD varies. Whereas three neither blinded nor placebo-controlled Japanese randomised 2-year studies [22, 27, 28] and one Dutch randomised, blinded, placebo-controlled 3-year study [29] found similar protective effects on lumbar spine BMD, two 3-year interventional studies in Caucasian postmenopausal women found no effect of MK-7 or MK-4 on lumbar BMD [30, 31].
BMD of the femoral neck decreased in both study groups after 2 years, although to a lesser extent with MK-7 supplementation and with borderline significance between treatments groups. Four randomised, controlled trials in elderly Caucasian populations without kidney impairment investigated the 3-year effect of vitamin K supplementation on BMD of the femoral neck. Two studies observed increased BMD with supplements of MK-7 [29] and vitamin K1 [32], yet two other studies found no effect with supplements of lower dosages of MK-4 [31] or vitamin K1 [33]. No short-term studies have found any effect of vitamin K1, MK-4 or MK-7 on BMD [23, 24, 34–37].
In ESKD, low BMD predicts fractures [5, 38]. Osteoporosis is diagnosed by DXA of the lumbar spine and hip, as in the general population, although skeletal sites with a higher content of cortical bone, such as the 1/3 distal radius, hypothetically should be superior in discriminating fracture risk due to the effects of secondary hyperparathyroidism [14].
Similar to our findings, MK-7 supplementation had contrasting effects on trabecular and cortical bone as assessed by high-resolution peripheral quantitative computed tomography in a 3-year study of Caucasian postmenopausal women [30, 36]. This dual effect of MK-7 on bone metabolism appears to be similar to that of parathyroid hormone (PTH), which increases cortical resorption and trabecular bone formation simultaneously [39, 40]. In the present study, levels of intact PTH (iPTH) did not differ between study groups; still an interaction between MK-7 and iPTH cannot be excluded.
Vitamin D regulates the expression of OC and appropriate vitamin D status may therefore be of importance for the effects of vitamin K supplementation on bone [41]. In the present study, participants were vitamin D replete and adjustments for vitamin D status did not change the results.
In accordance with other studies [32, 35, 36], we found a significant lesser increase in tOC with MK-7 supplementation compared with placebo, which may reflect carboxylation of ucOC, which enables its binding to hydroxyapatite in the bone matrix, as seen in human in vitro studies [42]. The observed effect on tOC may reflect an improvement in bone mineral maturation and bone structure with MK-7 supplementation.
Even though markers of bone turnover may be affected by ESKD [43], MK-7 did not appear to influence bone turnover in terms of changes in bone turnover markers in the present study, which is in accordance with observations in the elderly general population [32, 35, 36].
In uraemia, this absence of convincing beneficial effects of MK-7 supplementation on bone might be explained by the profound disturbances in vitamin K lipoprotein distribution and metabolism as recently discussed by Kaesler et al. [44]. Accordingly, it should be noted that even though we demonstrated highly significant effects on functional vitamin K status, e.g. levels of dp-ucMGP, it still did not normalize after 2 years of vitamin K2 supplementation.
This study has strengths and limitations. Being a randomised, placebo-controlled, double-blind study, important biases were eliminated. Also, the high adherence to study supplementation demonstrated both by tablet counting and blood concentrations of MK-7 and PIVKA-II was an important strength.
According to our power calculation, we aimed at including 2 × 70 participants. Due to challenged recruitment, unfortunately we ended up with only 61 + 62 participants. Furthermore, the dropout rate in year 2 was higher than anticipated. For these reasons, only 30 + 32 patients contributed with 2-year primary outcome data, a number somewhat lower than required according to the power calculations (2 × 49). Nonetheless, we managed to detect significant changes in the primary outcome variable. It is possible that a larger sample size and a longer trial duration would have demonstrated more convincing differences in the response between treatment groups, and even other significant differences in outcomes as well.
Previous studies have reported worsening of BMD on the arteriovenous fistula arm [14]. The number of functioning fistulas was similar in the two study groups; unfortunately, neither the placement nor the number of closed fistulas was registered.
Lumbar spine BMD may be increased by osteoarthritis, compression fractures, severe aortic calcification, which is common in the dialysis population, and intestinal deposition of phosphate binders [45, 46]. In the present study, we cannot exclude that lumbar spine osteoarthritis may have been unevenly distributed in the two study groups. However, neither AAC scores nor vertebral fractures differed between the study groups. Although DXAs were performed with abdomens empty for dialysis fluids, intake of phosphate binders and leftover peritoneal dialysis fluids may have affected measurements of lumbar spine BMD. However, the number of participants treated with peritoneal dialysis and phosphate binders did not differ between study groups.
Finally, we cannot exclude a more pronounced effect if a higher dosage of MK-7 had been used, if vitamin K1 had been supplemented [44] or if only patients with a high risk of bone fracture such as female patients on dialysis with low BMD, a history of bone fracture or severe renal osteodystrophy were included.
In conclusion, 2 years of vitamin K (MK-7) supplementation may modify BMD site-specifically in patients receiving dialysis treatment, in addition to improvement in functional vitamin K status. Still, our findings do not support MK-7 supplementation to preserve bone in patients with ESKD receiving dialysis treatment.
Supplementary Material
ACKNOWLEDGEMENTS
The authors wish to express their gratitude to the lab technicians and staff at the Departments of Clinical Biochemistry and Immunology at Lillebælt Hospital, Vejle, and Zealand University Hospital, Denmark, and to the study nurses and lab technicians at the Department of Renal Medicine, Aarhus University Hospital, Department of Nephrology, Aalborg University Hospital, and the Department of Endocrinology at Zealand University Hospital, Aarhus University Hospital and Aalborg University Hospital. The authors are grateful to the nurses and the administrative staff of the involved dialysis departments. Finally, we would like to acknowledge the willingness of the many patients on dialysis who participated in this demanding study.
Contributor Information
Karin Levy-Schousboe, Department of Medicine, Zealand University Hospital, Roskilde, Denmark.
Peter Marckmann, Department of Medicine Sønderborg-Tønder, Hospital Sønderjylland, Sønderborg, Denmark.
Marie Frimodt-Møller, Steno Diabetes Center, Copenhagen, Denmark; Department of Nephrology, Herlev University Hospital, Copenhagen, Denmark.
Christian D Peters, Department of Renal Medicine, Aarhus University Hospital, Aarhus, Denmark.
Krista D Kjærgaard, Department of Renal Medicine, Aarhus University Hospital, Aarhus, Denmark.
Jens D Jensen, Department of Renal Medicine, Aarhus University Hospital, Aarhus, Denmark.
Charlotte Strandhave, Department of Nephrology, Aalborg University Hospital, Aalborg, Denmark.
Hanne Sandstrøm, Department of Radiology, Zealand University Hospital, Roskilde, Denmark.
Mette F Hitz, Department of Medicine, Zealand University Hospital, Køge, Denmark; Department of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark.
Bente Langdahl, Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark; Department of Clinical Medicine, Aarhus University, Aarhus, Denmark.
Peter Vestergaard, Department of Endocrinology, Aalborg University Hospital, Aalborg, Denmark; Steno Diabetes Center North Denmark, Aalborg University Hospital, Aalborg, Denmark; Department of Clinical Medicine, Aalborg University, Aalborg, Denmark.
Claus L Brasen, Department of Biochemistry and Immunology, Lillebælt Hospital, University Hospital of Southern Denmark, Denmark; Department of Regional Health Research, Faculty of Health Sciences, University of Southern Denmark, Odense, Denmark.
Anne Schmedes, Department of Biochemistry and Immunology, Lillebælt Hospital, University Hospital of Southern Denmark, Denmark.
Jonna S Madsen, Department of Biochemistry and Immunology, Lillebælt Hospital, University Hospital of Southern Denmark, Denmark; Department of Regional Health Research, Faculty of Health Sciences, University of Southern Denmark, Odense, Denmark.
Niklas R Jørgensen, Department of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark; Department of Clinical Biochemistry, Rigshospitalet, Copenhagen, Denmark.
Jens B Frøkjær, Department of Clinical Medicine, Aalborg University, Aalborg, Denmark; Department of Radiology, Aalborg University Hospital, Aalborg, Denmark.
Niels E Frandsen, Department of Medicine, Zealand University Hospital, Roskilde, Denmark.
Inge Petersen, Open Patient Data Explorative Network, Department of Clinical Research, University of Southern Denmark and Odense University Hospital, Odense, Denmark.
Ditte Hansen, Department of Nephrology, Herlev University Hospital, Copenhagen, Denmark; Department of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark.
FUNDING
The study was funded by the Danish Society of Nephrology, Danish Kidney Association, Region Zealand Research Foundation, Kappa Bioscience AS, Aase and Ejnar Danielsens Foundation, Helen and Ejnar Bjørnows Foundation, Beckett Foundation and Karen Elise Jensens Foundation. Kappa Bioscience AS and Orkla Care AS donated study tablets and DiaSorin donated the CLIA FGF-23 assay, but neither had further involvement in the study or any part in interpreting the data or acceptance of the results.
AUTHORS’ CONTRIBUTIONS
K.L.-S., D.H., M.F.-M., N.E.F. and P.M. designed the study. K.L.-S., C.D.P., K.D.K., C.S. and J.D.J. collected the clinical data. A.S., C.L.B., J.S.M. and N.R.J. were responsible for the specialized biochemical analyses. M.F.H., B.L. and P.V. conducted the DXA scans. H.S. and J.B.F. led and analysed the radiological investigations. K.L.-S. and I.P. did the statistical analyses and drafted the tables and figures. K.L.-S. and D.H. drafted the manuscript. All authors revised the article and approved the final version for publication.
DATA AVAILABILITY STATEMENT
Our data sharing statement is available as Supplementary Table 13S.
CONFLICT OF INTEREST STATEMENT
K.L.-S.’s salary was partially funded by Kappa Bioscience AS, the Danish Society of Nephrology and the Karen Elise Jensen Foundation during the RenaKvit trial. M.F.H. has received a research grant from Orkla Care AS. B.L. has received research grants from Amgen and honoraria for academic services from Amgen, UCB, Gilead, Astellas, AstraZeneca and Gedeon-Richter, unrelated to the RenaKvit trial. The remaining authors have nothing to declare.
REFERENCES
- 1. Moe S, Drüeke T, Cunningham Jet al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2006;69:1945–53. [DOI] [PubMed] [Google Scholar]
- 2. Hansen D, Olesen JB, Gislason GHet al. Risk of fracture in adults on renal replacement therapy: a Danish national cohort study. Nephrol Dial Transplant 2016;31:1654–62. [DOI] [PubMed] [Google Scholar]
- 3. Tentori F, McCullough K, Kilpatrick RDet al. High rates of death and hospitalization follow bone fracture among hemodialysis patients. Kidney Int 2014;85:166–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leonard MB, Wehrli FW, Ziolkowski SLet al. A multi-imaging modality study of bone density, bone structure and the muscle - bone unit in end-stage renal disease. Bone 2019;127:271–9. [DOI] [PubMed] [Google Scholar]
- 5. Iimori S, Mori Y, Akita Wet al. Diagnostic usefulness of bone mineral density and biochemical markers of bone turnover in predicting fracture in CKD stage 5D patients—a single-center cohort study. Nephrol Dial Transplant 2012;27:345–51. [DOI] [PubMed] [Google Scholar]
- 6. Booth SL, Broe KE, Peterson JWet al. Associations between vitamin K biochemical measures and bone mineral density in men and women. J Clin Endocrinol Metab 2004;89:4904–9. [DOI] [PubMed] [Google Scholar]
- 7. Evenepoel P, Claes K, Meijers Bet al. Poor vitamin K status is associated with low bone mineral density and increased fracture risk in end-stage renal disease. J Bone Miner Res 2019;34:262–9. [DOI] [PubMed] [Google Scholar]
- 8. Booth SL, Tucker KL, Chen Het al. Dietary vitamin K intakes are associated with hip fracture but not with bone mineral density in elderly men and women. Am J Clin Nutr 2000;71:1201–8. [DOI] [PubMed] [Google Scholar]
- 9. Kohlmeier M, Saupe J, Shearer MJet al. Bone health of adult hemodialysis patients is related to vitamin K status. Kidney Int 1997;51:1218–21. [DOI] [PubMed] [Google Scholar]
- 10. Fusaro M, Tripepi G, Noale Met al. Prevalence of vertebral fractures, vascular calcifications, and mortality in warfarin treated hemodialysis patients. Curr Vasc Pharmacol 2015;13:248–58. [DOI] [PubMed] [Google Scholar]
- 11. Westenfeld R, Krueger T, Schlieper Get al. Effect of vitamin K2 supplementation on functional vitamin K deficiency in hemodialysis patients: a randomized trial. Am J Kidney Dis 2012;59:186–95. [DOI] [PubMed] [Google Scholar]
- 12. Caluwé R, Vandecasteele S, Van Vlem Bet al. Vitamin K2 supplementation in haemodialysis patients: a randomized dose-finding study. Nephrol Dial Transplant 2014;29:1385–90. [DOI] [PubMed] [Google Scholar]
- 13. Levy-Schousboe K, Frimodt-Møller M, Hansen Det al. Vitamin K supplementation and arterial calcification in dialysis: results of the double-blind, randomized, placebo-controlled RenaKvit trial. Clin Kidney J 2021;14:2114–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Evenepoel P, Cunningham J, Ferrari Set al. European Consensus Statement on the diagnosis and management of osteoporosis in chronic kidney disease stages G4–G5D. Nephrol Dial Transplant 2021;36:42–59. [DOI] [PubMed] [Google Scholar]
- 15. Jamal SA, Hayden JA, Beyene J.. Low bone mineral density and fractures in long-term hemodialysis patients: a meta-analysis. Am J Kidney Dis 2007;49:674–81. [DOI] [PubMed] [Google Scholar]
- 16. Boegh Andersen I, Lohman Brasen C, Skov Madsen Jet al. Quantitation of vitamin K1 in serum using online SPE-LC-MS/MS and the challenges of working with vitamin K. J Chromatogr B 2019;1117:41–8. [DOI] [PubMed] [Google Scholar]
- 17. Kauppila LI, Polak JF, Cupples LAet al. New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: a 25-year follow-up study. Atherosclerosis 1997;132:245–50. [DOI] [PubMed] [Google Scholar]
- 18. Eastell R, Cedel SL, Wahner HW. 3rd et al. Classification of vertebral fractures. J Bone Miner Res 1991;6:207–15. [DOI] [PubMed] [Google Scholar]
- 19. Ambrus C, Almasi C, Berta Ket al. Vitamin D insufficiency and bone fractures in patients on maintenance hemodialysis. Int Urol Nephrol 2011;43:475–82. [DOI] [PubMed] [Google Scholar]
- 20. Twisk J, Bosman L, Hoekstra Tet al. Different ways to estimate treatment effects in randomised controlled trials. Contemp Clin Trials Commun 2018;10:80–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ishida Y, Kawai S.. Comparative efficacy of hormone replacement therapy, etidronate, calcitonin, alfacalcidol, and vitamin K in postmenopausal women with osteoporosis: the Yamaguchi Osteoporosis Prevention Study. Am J Med 2004;117:549–55. [DOI] [PubMed] [Google Scholar]
- 22. Iwamoto J, Takeda T, Ichimura S.. Effect of menatetrenone on bone mineral density and incidence of vertebral fractures in postmenopausal women with osteoporosis: a comparison with the effect of etidronate. J Orthop Sci 2001;6:487–92. [DOI] [PubMed] [Google Scholar]
- 23. Cheung AM, Tile L, Lee Yet al. Vitamin K supplementation in postmenopausal women with osteopenia (ECKO trial): a randomized controlled trial. PLoS Med 2008;5:e196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bolton-Smith C, McMurdo ME, Paterson CRet al. Two-year randomized controlled trial of vitamin K1 (phylloquinone) and vitamin D3 plus calcium on the bone health of older women. J Bone Miner Res 2007;22:509–19. [DOI] [PubMed] [Google Scholar]
- 25. Prasad B, Ferguson T, Tangri Net al. Association of bone mineral density with fractures across the spectrum of chronic kidney disease: the Regina CKD-MBD Study. Can J Kidney Health Dis 2019;6:205435811987053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Malluche HH, Davenport DL, Cantor Tet al. Bone mineral density and serum biochemical predictors of bone loss in patients with CKD on dialysis. Clin J Am Soc Nephrol 2014;9:1254–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shiraki M, Shiraki Y, Aoki Cet al. Vitamin K2 (menatetrenone) effectively prevents fractures and sustains lumbar bone mineral density in osteoporosis. J Bone Miner Res 2000;15:515–21. [DOI] [PubMed] [Google Scholar]
- 28. Iwamoto J, Takeda T, Ichimura S.. Effect of combined administration of vitamin D3 and vitamin K2 on bone mineral density of the lumbar spine in postmenopausal women with osteoporosis. J Orthop Sci 2000;5:546–51. [DOI] [PubMed] [Google Scholar]
- 29. Knapen MH, Drummen NE, Smit Eet al. Three-year low-dose menaquinone-7 supplementation helps decrease bone loss in healthy postmenopausal women. Osteoporos Int 2013;24:2499–507. [DOI] [PubMed] [Google Scholar]
- 30. Rønn SH, Harsløf T, Oei Let al. The effect of vitamin MK-7 on bone mineral density and microarchitecture in postmenopausal women with osteopenia, a 3-year randomized, placebo-controlled clinical trial. Osteoporos Int 2021;32:185–91. [DOI] [PubMed] [Google Scholar]
- 31. Knapen MH, Schurgers LJ, Vermeer C.. Vitamin K2 supplementation improves hip bone geometry and bone strength indices in postmenopausal women. Osteoporos Int 2007;18:963–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Braam LA, Knapen MH, Geusens Pet al. Vitamin K1 supplementation retards bone loss in postmenopausal women between 50 and 60 years of age. Calcif Tissue Int 2003;73:21–6. [DOI] [PubMed] [Google Scholar]
- 33. Booth SL, Dallal G, Shea MKet al. Effect of vitamin K supplementation on bone loss in elderly men and women. J Clin Endocrinol Metab 2008;93:1217–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Binkley N, Harke J, Krueger Det al. Vitamin K treatment reduces undercarboxylated osteocalcin but does not alter bone turnover, density, or geometry in healthy postmenopausal North American women. J Bone Miner Res 2009;24:983–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Emaus N, Gjesdal CG, Almås Bet al. Vitamin K2 supplementation does not influence bone loss in early menopausal women: a randomised double-blind placebo-controlled trial. Osteoporos Int 2010;21:1731–40. [DOI] [PubMed] [Google Scholar]
- 36. Rønn SH, Harsløf T, Pedersen SBet al. Vitamin K2 (menaquinone-7) prevents age-related deterioration of trabecular bone microarchitecture at the tibia in postmenopausal women. Eur J Endocrinol 2016;175:541–9. [DOI] [PubMed] [Google Scholar]
- 37. Iwamoto I, Kosha S, Noguchi Set al. A longitudinal study of the effect of vitamin K2 on bone mineral density in postmenopausal women a comparative study with vitamin D3 and estrogen-progestin therapy. Maturitas 1999;31:161–4. [DOI] [PubMed] [Google Scholar]
- 38. Jamal S, Cheung AM, West Set al. Bone mineral density by DXA and HR pQCT can discriminate fracture status in men and women with stages 3 to 5 chronic kidney disease. Osteoporos Int 2012;23:2805–13. [DOI] [PubMed] [Google Scholar]
- 39. Silva BC, Bilezikian JP.. Parathyroid hormone: anabolic and catabolic actions on the skeleton. Curr Opin Pharmacol 2015;22:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yamaguchi M, Weitzmann MN.. Vitamin K2 stimulates osteoblastogenesis and suppresses osteoclastogenesis by suppressing NF-κB activation. Int J Mol Med 2011;27:3–14 [DOI] [PubMed] [Google Scholar]
- 41. Skjødt H, Gallagher JA, Beresford JNet al. Vitamin D metabolites regulate osteocalcin synthesis and proliferation of human bone cells in vitro. J Endocrinol 1985;105:391–6. [DOI] [PubMed] [Google Scholar]
- 42. Koshihara Y, Hoshi K.. Vitamin K2 enhances osteocalcin accumulation in the extracellular matrix of human osteoblasts in vitro. J Bone Miner Res 1997;12:431–8. [DOI] [PubMed] [Google Scholar]
- 43. Vervloet MG, Brandenburg VM.. Circulating markers of bone turnover. J Nephrol 2017;30:663–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kaesler N, Schreibing F, Speer Tet al. Altered vitamin K biodistribution and metabolism in experimental and human chronic kidney disease. Kidney Int 2022;101:338–48. [DOI] [PubMed] [Google Scholar]
- 45. Hochberg MC, Lethbridge-Cejku M, Tobin JD.. Bone mineral density and osteoarthritis: data from the Baltimore Longitudinal Study of Aging. Osteoarthritis Cartilage 2004;12(Suppl A):S45–8. [DOI] [PubMed] [Google Scholar]
- 46. Morgan SL, Prater GL.. Quality in dual-energy X-ray absorptiometry scans. Bone 2017;104:13–28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Our data sharing statement is available as Supplementary Table 13S.