ABSTRACT
Introduction
We aimed to characterize the incidence and clinical presentation of membranous nephropathy (MN) after kidney transplantation (KT), and to assess allograft outcomes according to proteinuria rates and immunosuppression management.
Methods
Multicenter retrospective cohort study including patients from six Spanish centers who received a KT between 1991–2019. Demographic, clinical, and histological data were collected from recipients with biopsy-proven MN as primary kidney disease (n = 71) or MN diagnosed de novo after KT (n = 4).
Results
Up to 25.4% of patients with biopsy-proven MN as primary kidney disease recurred after a median time of 18.1 months posttransplant, without a clear impact on graft survival. Proteinuria at 3-months post-KT was a predictor for MN recurrence (rMN, HR 4.28; P = 0.008). Patients who lost their grafts had higher proteinuria during follow-up [1.0 (0.5–2.5) vs 0.3 (0.1–0.5) g/24 h], but only eGFR after recurrence treatment predicted poorer graft survival (eGFR < 30 ml/min: RR = 6.8). We did not observe an association between maintenance immunosuppression and recurrence diagnosis. Spontaneous remission after rMN was associated with a higher exposure to tacrolimus before recurrence (trough concentration/dose ratio: 2.86 vs 1.18; P = 0.028). Up to 94.4% of KT recipients received one or several treatments after recurrence onset: 22.2% rituximab, 38.9% increased corticosteroid dose, and 66.7% ACEi/ARBs. Only 21 patients had proper antiPLA2R immunological monitoring.
Conclusions
One-fourth of patients with biopsy-proven MN as primary kidney disease recurred after KT, without a clear impact on graft survival. Spontaneous remission after rMN was associated with a higher exposure to tacrolimus before recurrence.
Keywords: kidney transplantation, membranous nephropathy, proteinuria, recurrence, tacrolimus
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Membranous nephropathy (MN) is a leading cause of nephrotic syndrome in adults [1], This immune complex-mediated glomerular disease is histologically characterized by the accumulation of electron-dense subepithelial deposits consisting of immunoglobulin G, antigens, and complement components [2]. The immunological injury displays glomerular filtration barrier disruption and the subsequent loss of a large amount of proteinuria [3, 4]. In ∼80% of cases, MN has no underlying cause and is considered a primary disease. M-type phospholipase A2 receptor 1 (PLA2R, ∼70%–80% of primary cases) [5] and thrombospondin type 1 domain-containing 7A (THSD7A, ∼2%–5% of primary cases) [6] were the first target antigens described, confirming the disease's autoimmune nature in humans. Despite diagnostic advances and an improved treatment landscape, ∼10–30% of patients with primary MN will develop end-stage kidney disease and may be suitable candidates for kidney transplantation (KT) [7–9].
When occurring after KT, MN may present either as recurrent (rMN) or de novo disease (dnMN) [10–13]. Overall clinical recurrence rates range from 18% to 57% of primary MN cases, depending on surveillance biopsy policies [12, 14–20]. As in native kidneys, the pathophysiology of rMN generally relates to anti-PLA2R antibodies, which have shown a high positive predictive value for recurrence, especially at high pretransplant titers [16–18, 21]. Nonetheless, the posttransplant course of PLA2R-associated rMN may have some distinct characteristics compared with primary MN, including a lower likelihood of spontaneous remission, an earlier requirement for adjuvant immunosuppression to achieve remission or persistent histological activity in both treated and untreated patients [14, 22–24]. These features lead to consider rMN as a progressive disease if proteinuria persists over 1 g/day [25, 26]. Treatment with rituximab may be beneficial if proteinuria is >1 g/24 h [26], but management and outcomes in patients with subnephrotic proteinuria have been scarcely studied and need further assessment [23]. The role of maintenance immunosuppression and recurrence development and outcomes is at issue. One study demonstrated that no specific drug was associated with graft failure due to recurrence [27].
Analogously, the outcomes and posttransplant management of dnMN remain elusive due to its low incidence (0.7%–9.3%) and poorly understood pathophysiology [28, 29]. The diagnosis of dnMN is usually hampered by the absence of native kidney biopsy [30], making it difficult to differentiate from recurrence. Consequently, most cases remain unclassified and unstudied.
This multicenter study aimed to characterize the incidence and presentation form of MN after KT in a Spanish cohort and assess allograft outcomes according to proteinuria rates and therapeutical management.
MATERIALS AND METHODS
Study design and patients
Multicenter retrospective cohort study performed in six nephrology departments belonging to the Spanish Transplant Study Group (SENTRA) and the Spanish Glomerular Study Group (GLOSEN). Data from KT recipients aged >18 years old and transplanted between 1991 and 2019 with biopsy-proven MN as primary kidney disease or diagnosed de novo after transplantation, were collected. Patient identification was performed by reviewing histopathological charts and clinical histories. The median follow-up time since KT to graft loss or end of follow-up was 8.1 years (4.1–12.8 years), and the median follow-up time since MN diagnosis after KT (n = 22) was 1.3 years (IQR, 0.9–4.5 years). The study was approved by the Ethical Committee at the study coordinating center, Hospital del Mar, Barcelona, Spain, and conducted according to the guidelines as dictated by the Declaration of Helsinki. All data were recorded anonymously.
Data collection and definitions
Recorded baseline and follow-up data included: recipient characteristics and comorbidities, cause of renal failure, clinical presentation in native kidneys, time to kidney failure, and time on dialysis before transplantation, type of replacement therapy, type of donor, transplant-related factors such as HLA mismatch, cold ischemia time (CIT), immunosuppressive therapy and dose, and MN treatment after KT. Laboratory parameters analyzed using routine laboratory methods included serum creatinine, estimated glomerular filtration (eGFR) using the CKD-EPI equation [31], proteinuria, serum albumin, de novo HLA donor-specific antibodies (HLA-DSA), anti-PLA2R, and anti-THSD7A. Clinical events, such as delayed graft function (DGF), the development of rejection episodes and recurrence, nephrotic syndrome, nephritic syndrome, complete remission, partial remission, graft loss, and patient death, were recorded.
DGF was defined as the need for dialysis during the first week after KT followed by recovery of allograft function. Nephrotic syndrome was defined as proteinuria of >3.5 g/day plus serum albumin <3 g/dl. Complete remission (CR) was defined as proteinuria <0.3 g/day accompanied by normal serum albumin concentration and recovery of baseline kidney function. Partial remission (PR) was defined as urinary protein excretion between 0.3 and 3.5 g/day plus ≥50% reduction from its peak value at recurrence, along with normal albumin and stable renal function (maximum increase of serum creatinine <30% of baseline value). Spontaneous remission (SR) was defined as CR or PR in the absence of specific targeted immunosuppressive therapy.
Histopathologic data at the time of recurrence
Kidney biopsy specimens of patients with recurrent or de novo MN were examined in the pathology departments of participating hospitals. The following histopathological data were collected if available: percentage of sclerosed glomeruli, degree of interstitial fibrosis/tubular atrophy (IFTA), glomerulitis, peritubular capillaritis, tubulitis, interstitial inflammation, endarteritis, and C4d deposition in peritubular capillaries, transplant glomerulopathy, arteriosclerosis, mesangial expansion, PLA2R deposition, THSD7A deposition, IgG deposition and subtype, and Ehrenreich Churg category [32].
Outcomes
The main outcomes were: the development of biopsy-proven MN after KT; disease remission after recurrence or de novo disease; death-censored graft failure, defined as eGFR <15 ml/min per 1.73 m2 or the need for maintenance dialysis, and all-cause mortality.
Statistical analysis
Continuous data are presented as mean ± standard deviation (SD) or median and interquartile range (IQR). Categorical data are expressed as counts (%) according to their distribution. Comparison of continuous variables was performed by t-test or ANOVA for parametric data and Mann–Whitney U-test or Kruskal–Wallis test for nonparametric distributions. Nominal variables were compared using the chi-squared test or Fisher exact test, where appropriate. The predictors of recurrence and allograft failure were determined using Cox regression analysis. Predictors were selected as informed by prior literature and clinical practice. Those variables with P < 0.05 in the univariate Cox model were included in the multivariate analysis. Recurrence cumulative incidence and survival analyses were plotted using the Kaplan–Meier survival curves, applying the log-rank test and competing risks. Statistical significance was considered at P < 0.05. We used Stata/BE (v.17.0, StataCorp LLC, USA) for statistical analysis and GraphPad Prism software (v.9.3.1; GraphPad Software, San Diego, CA, USA) for graphical presentation.
RESULTS
Cohort demographics
Seventy-five patients fulfilled the inclusion criteria and were enrolled in the study (Fig. S1, see online supplementary material for a color version of this figure). Of them, 71 patients had biopsy-proven MN as primary kidney disease and 18 (25.4%) recurred after KT, within a median time of 18.1 (12.0–61.7) months (Fig. 1). Additionally, four MN cases were diagnosed de novo or unclassified after KT.
Figure 1:
Cumulative incidence of MN recurrence after KT.
Table 1 displays the demographic and clinical characteristics of patients with MN in their native kidneys according to the recurrence of the disease. No significant differences were observed with regard to age at diagnosis of the primary disease, clinical presentation form, or time to kidney failure. Five patients from the nonrecurrent group had a prior KT, one of which developed a recurrence. The recipient and donor's characteristics were similar. Moreover, most patients received a maintenance immunosuppression regimen based on corticosteroids, calcineurin inhibitors, and mycophenolate mofetil. The percentage of early steroid withdrawal only reached 8.5% of KT recipients. We found no differences in DGF or rejection episodes between groups.
Table 1:
Demographic and clinicopathological characteristics of patients with MN as primary kidney disease according to recurrence.
| Overall cohort (n = 71) | Recurrence (n = 18) | No recurrence (n = 53) | P value | |
|---|---|---|---|---|
| Native kidney disease | ||||
| Age at diagnosis, years, mean ± SD | 42.2 ± 15.9 | 46.7 ± 14.5 | 40.6 ± 16.2 | 0.163 |
| Female sex, n (%) | 19 (26.8) | 3 (16.6) | 16 (30.2) | 0.362 |
| Clinical presentation, an (%) | ||||
| Isolated nonnephrotic proteinuria | 1 (1.8) | 0 (0) | 5 (12.8) | 0.662 |
| Nephrotic proteinuria | 8 (14.3) | 3 (17.7) | 5 (12.8) | |
| Nephrotic syndrome | 43 (86.8) | 14 (82.4) | 29 (74.4) | |
| Nephritic syndrome | 4 (7.1) | 0 (0) | 4 (10.3) | |
| Considered primary MN at diagnosis, n (%) | 61 (93.9) | 14 (93.3) | 47 (94) | 1 |
| Creatinine at diagnosis, mg/dl, mean ± SD | 1.8 ± 1.2 | 1.7 ± 1.7 | 1.8 ± 0.9 | 0.736 |
| Proteinuria at diagnosis, g/24 h, median (IQR) | 6.4 [4.3–8.5] | 7 [4–10.6] | 6.1 [4.5–8.5] | 0.581 |
| Immunosuppression treatment | ||||
| None, n (%) | 4 (6.5) | 0 (0) | 4 (8.9) | 0.568 |
| Corticosteroids, n (%) | 19 (31.7) | 3 (18.8) | 16 (36.4) | 0.228 |
| Tacrolimus, n (%) | 28 (46.7) | 9 (52.9) | 19 (44.2) | 0.578 |
| Cyclophosphamide, n (%) | 12 (20) | 3 (17.7) | 9 (20.9) | 1 |
| Rituximab, n (%) | 6 (10) | 3 (17.7) | 3 (7.0) | 0.338 |
| Other, n (%) | 15 (25) | 3 (17.7) | 12 (27.9) | 0.520 |
| Time to kidney failure, months, median (IQR) | 70.1 (46–115.4) | 54.6 (34.1–99.2) | 72.4 (48–119.9) | 0.146 |
| Type of RRT, n (%) | ||||
| Hemodialysis | 42 (60) | 12 (66.7) | 30 (57.7) | 0.771 |
| Peritoneal dialysis | 21 (30) | 4 (22.2) | 17 (32.7) | |
| None (pre-emptive transplant) | 7 (10 | 2 (11.1) | 5 (9.6) | |
| Time on RRT, months, median (IQR) | 21.5 (7.8–43.4) | 9 (7.5–37.8) | 21.9 (9–43.4) | 0.441 |
| Recipient characteristics | ||||
| Age at transplantation, years, mean ± SD | 52.3 ± 13.9 | 54.8 ± 13.8 | 51.4 ± 13.9 | 0.374 |
| Hypertension, n (%) | 57 (80.3) | 17 (94.4) | 40 (75.5) | 0.098 |
| Diabetes mellitus, n (%) | 10 (14) | 3 (16.7) | 7 (13.2) | 0.706 |
| Cardiovascular disease, n (%) | 7 (9.9) | 3 (16.7) | 4 (7.6) | 0.359 |
| History of cancer, n (%) | 3 (4.2) | 1 (5.6) | 2 (3.8) | 1 |
| HCV, n (%) | 1 (1.4) | 0 (0) | 1 (1.9) | 1 |
| HBV, n (%) | 1 (1.4) | 1 (5.6) | 0 (0) | 0.254 |
| HIV, n (%) | 1 (1.4) | 0 (0) | 1 (1.9) | 1 |
| Prior kidney transplants, n (%) | 5 (7.3) | 0 (0) | 5 (9.8) | 0.316 |
| MN recurrence in prior kidney transplants, n (%) (n = 5) | 1 (20) | 0 (0) | 1 (20.0) | - |
| Kidney transplantation | ||||
| Donor age, years, mean ± SD | 53.2 ± 14.5 | 54.2 ± 13.5 | 52.9 ± 15 | 0.725 |
| Type of donor, n (%) | ||||
| Living donor | 10 (14.3) | 3 (16.7) | 7 (13.5) | 1 |
| Living related donor (n = 10) | 8 (80) | 2 (66.7) | 6 (85.7) | |
| DBD | 50 (71.4) | 13 (72.2) | 37 (71.2) | |
| DCD | 10 (14.3) | 2 (11.1) | 8 (15.4) | |
| Expanded criteria donor, n (%) | 24 (34.8) | 6 (33.3) | 18 (35.6) | 1 |
| KT decade | ||||
| 1991–2000 | 11 (15.5) | 3 (16.7) | 8 (15.1) | |
| 2001–2010 | 27 (38) | 7 (38.9) | 20 (37.4) | 0.048 |
| 2011–2020 | 33 (46.5) | 8 (44.4) | 25 (47.2) | |
| HLA-A/B/DR mismatch, mean ± SD | 3.9 ± 1.5 | 3.6 ± 1.8 | 4.1 ± 1.4 | 0.262 |
| CIT, hours, mean ± SD | 16 ± 7.8 | 17.1 ± 7.9 | 15.6 ± 7.8 | 0.528 |
| DGF, n (%) | 24 (35.3) | 6 (35.3) | 18 (35.3) | 1 |
| Baseline immunosuppression | ||||
| Induction therapy, n (%) | ||||
| None | 17 (24.3) | 6 (33.3) | 11 (21.2) | 0.246 |
| Antithymocyte globulin | 17 (24.3) | 2 (11.1) | 15 (28.9) | |
| ALG/ATGAM | 3 (4.3) | 0 (0) | 3 (5.8) | |
| Basiliximab | 29 (41.3) | 10 (55.6) | 19 (36.5) | |
| Daclizumab | 4 (5.7) | 0 (0) | 4 (7.7) | |
| Maintenance immunosuppression | ||||
| Corticosteroids, n (%) | 69 (98.6) | 18 (100) | 51 (98.1) | 1 |
| Early steroid withdrawal, n (%) | 10 (15.2) | 3 (16.7) | 7 (14.5) | 1 |
| Tacrolimus, n (%) | 64 (90.14) | 15 (83.3) | 49 (92.5) | 0.359 |
| Cyclosporine, n (%) | 7 (9.86) | 3 (16.7) | 4 (7.6) | 0.359 |
| Mycophenolate mofetil, n (%) | 68 (95.8) | 17 (94.4) | 71 (96.2) | 1 |
| De novo mTOR inhibitors, n (%)* | 2 (2.9) | 1 (5.9) | 1 (1.9) | 0.429 |
| Azathioprine, n (%) | 1 (1.45) | 0 (0) | 1 (1.9) | 1 |
| Kidney function and outcomes | ||||
| Mean eGFR during follow-up, mg/dl, mean ± SD | 46.6 ± 17.7 | 41.6 ± 14.5 | 48.3 ± 18.5 | 0.165 |
| Mean Proteinuria during follow-up, g/24 h, median (IQR) | 0.4 (0.2–0.7) | 1.2 (0.6–1.9) | 0.3 (0.1–0.4) | <0.001 |
| DGF, n (%) | 24 (35.3) | 6 (35.3) | 18 (35.3) | 1 |
| Acute rejection, n (%) | 15 (21.1) | 5 (27.8) | 10 (18.9) | 0.507 |
| Time to recurrence, months, median (IQR) | - | 18.1 (12–61.7) | - | |
| Follow-up time, years, median (IQR) | 8.5 (4.2–12.8) | 7.7 (4.4–11.4) | 8.8 (3.9–13.0) | 0.833 |
| Death-censored graft failure, n (%) | 14 (19.7) | 6 (33.3) | 8 (15.1) | 0.166 |
| Mortality, n (%) | 5 (7.0) | 0 (0) | 5 (9.4) | 0.320 |
Data available in 56 patients (78.9%): 1 (5.6%) in the group with recurrent disease versus 14 (26.4%) in the group without recurrence.
* 5 more patients were converted to mTORi during follow-up.
Clinical presentation, histology, and treatment
Table 2 shows the clinical presentation, histology, and treatment of rMN according to graft outcomes. KT recipients with and without allograft failure recurred within a median time of 17.8 and 24 months, respectively (n.s.). Only 16.7% of recurrences presented with nephrotic syndrome. Nonetheless, 83.4% of patients with graft loss developed nephrotic-range proteinuria compared to 33.4% of those with a nonnephrotic range (P = 0.098). Therefore, isolated nonnephrotic proteinuria was the predominant clinical presentation in KT recipients with recurrence and functioning graft at the end of follow-up. Median proteinuria rates reached 3.2 (1.4–15.6) vs. 2.2 (1.6–4.3) g/24 h in recipients with and without allograft failure (P = 0.030). Likewise, those with death-censored graft loss had worse creatinine values at recurrence (2.3 ± 1.1 vs. 1.7 ± 0.3; P = 0.014) that persisted after receiving recurrence treatment 2.4 ± 0.8 vs. 1.7 ± 0.3; P = 0.030).
Table 2:
Clinical presentation, histology, and treatment of MN recurrence according to death-censored graft loss.
| Overall cohort | Graft loss | No graft loss | ||
|---|---|---|---|---|
| (n = 18) | (n = 6) | (n = 12) | P value | |
| Clinical presentation | ||||
| Time to recurrence, months | 18.1 (12–61.7) | 17.8 (13.4–22.8) | 24 (11.4–84.9) | 0.512 |
| Clinical presentation, n (%) | ||||
| Isolated nonnephrotic proteinuria | 9 (50) | 1 (16.67) | 8 (66.7) | 0.098 |
| Isolated nephrotic proteinuria | 6 (33.3) | 4 (66.7) | 2 (16.7) | |
| Nephrotic syndrome | 3 (16.7) | 1 (16.7) | 2 (16.7) | |
| Serum albumin at recurrence, g/dl, mean ± SD | 3.5 ± 0.8 | 3.6 ± 0.7 | 3.6 ± 0.8 | 0.796 |
| eGFR at recurrence, per ml/min/1.73 m2, mean ± SD | 39.59 ± 11.87 | 33.45 ± 12.40 | 42.15 ± 11.18 | 0.177 |
| Proteinuria at recurrence, g/24, median (IQR) | 2.7 (1.6–4.6) | 3.2 (1.4–15.6) | 2.2 (1.6–4.3) | 0.030 |
| eGFR after treatment, per ml/min/1.73 m2, mean ± SD | 38.59 ± 12.85 | 29.86 ± 10.35 | 43.36 ± 11.83 | 0.033 |
| Proteinuria after treatment, g/24, median (IQR) | 0.6 (0.4–1) | 4.3 (0.6–10.5) | 0.5 (0.3–0.6) | 0.275 |
| Mean eGFR during follow-up, per ml/min/1.73 m2, mean ± SD | 41.6 ± 14.5 | 32.9 ± 13 | 46 ± 13.6 | 0.075 |
| Mean Proteinuria during follow-up, g/24 h, median (IQR) | 1.2 (0.6–1.9) | 3.2 (1.3–7.2) | 0.9 (0.5–1.4) | 0.039 |
| Kidney biopsy at recurrence | ||||
| Globally sclerotic glomeruli, %, median (IQR) (n = 16) | 2.56 (0–14.3) | 0 (0–7.1) | 3.85 (0–18.2) | 0.271 |
| IFTA, n (%) (n = 16) | ||||
| ≤20% | 13 (81.3) | 2 (50) | 11 (91.7) | 0.136 |
| 20–50% | 3 (18.8) | 2 (50) | 1 (8.3) | |
| >50% | 0 (0) | 0 (0) | 0 (0) | |
| Arterio- and arteriolosclerosis, n (%) (n = 16) | ||||
| ≤20% | 13 (81.3) | 2 (50) | 11 (91.7) | |
| 20–50% | 3 (18.8) | 2 (50) | 1 (8.3) | 0.136 |
| >50% | 0 (0) | 0 (0) | 0 (0) | |
| Transplant glomerulopathy, n (%) | 1 (5.9) | 0 (0) | 1 (8.3) | 1 |
| C4d deposition in peritubular capillaries, n (%) (n = 16) | 5 (31.25) | 3 (60) | 2 (18.18) | 0.245 |
| Ehreinreich Churg classification, n (%) (n = 16) | ||||
| Stage 1 | 10 (62.5) | 3 (50) | 7 (70) | 0.790 |
| Stage 2 | 4 (25) | 2 (33.3) | 2 (20) | |
| Stage 3 | 2 (12.5) | 1 (10) | 1 (16.7) | |
| PLA2R staining | ||||
| Negative | 2 (11.1) | 0 (0) | 2 (16.7) | 0.342 |
| Positive | 5 (27.8) | 3 (50) | 2 (16.7) | |
| Not performed | 11 (61.1) | 3 (50) | 8 (66.7) | |
| THSD7A staining | ||||
| Not performed | 18 (100) | 6 (100) | 12 (100) | 1 |
| IgG type | ||||
| IgG4 | 3 (16.7) | 1 (16.7) | 2 (16.7) | 1 |
| Not performed | 15 (83.3) | 5 (83.3) | 10 (83.3) | |
| Concomitant rejection, n (%) | ||||
| No | 16 (88.9) | 6 (100) | 10 (83.3) | 1 |
| ABMR | 1 (5.6) | 0 (0) | 1 (8.3) | |
| TCMR | 1 (5.6) | 0 (0) | 1 (8.3) | |
| Management of recurrence | ||||
| None | 1 (5.56) | 0 (0) | 1 (8.3) | 1 |
| ACEi/ARBsa, n (%) | 12 (66.7) | 3 (50) | 9 (75) | 0.344 |
| Steroidsa, n (%) | 7 (38.9) | 1 (16.7) | 6 (50) | 0.316 |
| Tacrolimus dose increasea, n (%) | 0 (0) | 0 (0) | 0 (0) | n.a. |
| Rituximaba, n (%) | 4 (22.2) | 3 (50) | 1 (8.3) | 0.083 |
| Outcomes | ||||
| CR, n (%) | 4 (23.5) | 0 (0) | 4 (36.4) | 0.237 |
| PRb, n (%) | 9 (50) | 3 (50) | 6 (50) | 1 |
| SR, n (%) | 5 (27.78) | 2 (33.3) | 3 (25) | 1 |
| Delta proteinuria 1 (proteinuria at 3 months post-KT vs. proteinuria at recurrence) | 2.2 (0.8–4.4) | 2.6 (1.1–14.4) | 1.9 (0.4–4.0) | 0.399 |
| Delta proteinuria 2 (proteinuria at recurrence vs. proteinuria after treatment) | 2.2 (1.1–3.5) | 2.1 (0.9–2.8) | 2.2 (1.1–3.6) | 0.546 |
Alone or in combination.
Includes patients without recurrence treatment or ACEi/ARBs alone.
Regarding histology features, no differences were found within the number of globally sclerotic glomeruli, IFTA scores, or other chronic and active lesions. Ehrenreich Churg's classification stage was similar between groups. Of note, a high percentage of cases (61%–100%) did not have PLA2R stain, THSD7A stain, or IgG subtype performed.
We observed no statistically significant differences in disease remission (complete, partial, and spontaneous) between groups. However, no recipients with CR lost their allograft during follow-up.
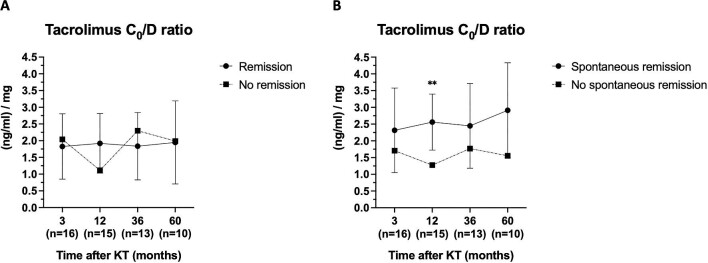
We next studied the possible effect of maintenance immunosuppression on recurrence development. Tacrolimus, mycophenolate, and corticosteroids levels and doses were similar between groups during the study period (Fig. S2, see online supplementary material for a color version of this figure). Likewise, disease remission was not associated with the mean levels of maintenance immunosuppression before recurrence. But, interestingly, patients with SR had higher trough concentration/dose (C0/D) ratio of tacrolimus before recurrence [2.86 (1.81–2.87) vs. 1.18 (0.96–1.48) (ng/ml)/mg, respectively; P = 0.028] (Fig. 2). Clinical and histological characteristics of patients with MN recurrence according to SR are displayed in Table S1 (see online supplementary material for a color version of this figure). We found no statistically significant differences in clinical presentation, proteinuria, or eGFR between groups. Most histological features were similar. However, patients with SR presented an increased percentage of globally sclerotic glomeruli (9.2% vs. 0%, P = 0.039).
Figure 2:
Tacrolimus C0/D ratio according to remission. (A) Complete or PR and (B) SR.
Concerning recurrence management, up to 94.4% of KT recipients received one or several treatments after recurrence onset: 22.2% rituximab, 38.9% increased corticosteroid dose, and 66.7% ACEi/ARBs. Of note, rituximab was only administered in patients with proteinuria ≥3 g/24 h (50% vs. 0%, respectively; P = 0.023; Table S2, see online supplementary material for a color version of this figure).
Kidney and patient outcomes
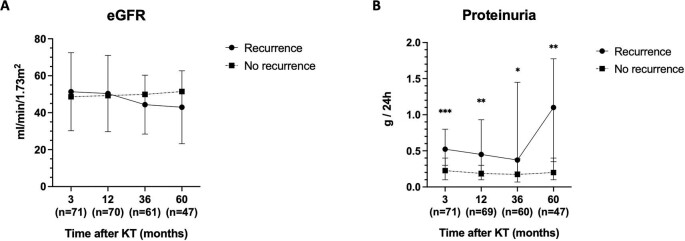
Average eGFR was plotted over time in patients with and without recurrence, indicating a similar eGFR in patients with rMN after 5 years of follow-up (Fig. 3A). Conversely, KT recipients with recurrence displayed greater proteinuria values at 3 months post-KT [0.5 (0.3–0.8) vs. 0.2 (0.1–0.4), P < 0.001] and in all the subsequent follow-up periods (Fig. 3B).
Figure 3:
Kidney function during the subsequent follow-up periods after KT (3, 12, 36, and 60 months) according to recurrence. (A) Average eGFR and (B) median proteinuria values.
Although graft losses were more numerous in the recurrence group (33.3% vs. 15.1%), no significant differences in death-censored graft survival were evident (log rank = 0.081; Fig. 4A). We next studied the cumulative incidence of death-censored graft failure according to recurrence after accounting for death as a competing event. Similar to Kaplan–Meier curve results, competing risk analysis showed no significant differences between groups (P = 0.059; Fig. S2, see online supplementary material for a color version of this figure). Of note, KT recipients with rMN lost their grafts due to recurrence (n = 5) and non-biopsied chronic injury (n = 1). In patients without recurrence, graft failure was attributed to urologic cause (n = 1), chronic rejection (n = 3), and non-biopsied chronic injury (n = 4). Regarding patient survival, Kaplan–Meier curves showed similar outcomes 5 years after transplantation in those recipients with and without recurrence (100% vs. 93.8%, respectively; log rank = 0.205; Fig. 4B).
Figure 4:
Kaplan–Meier survival curves. (A) Death-censored graft survival according to recurrence. (B) Patient survival according to recurrence.
Demographic and clinical characteristics of patients according to death-censored graft loss are summarized in Table S3 (see online supplementary material for a color version of this figure) and Fig. 5A and B. Recipients who lost their grafts presented worse eGFR after one year of follow-up (P = 0.007, P = 0.013, and P = 0.005 at 1, 3, and 5 years post-KT, respectively) and increased proteinuria after the third year (P = 0.012 and P = 0.012 at 3 and 5 years post-KT, respectively). However, we found no significant differences concerning recipient and donor characteristics or immunosuppressive regimens.
Figure 5:
Kidney function during the subsequent follow-up periods after KT (3, 12, 36, and 60 months) according to kidney outcome. (A) Average eGFR and (B) median proteinuria values.
Risk factors for recurrence and graft loss
In univariable Cox regression analysis, rMN was associated with the recipient's age at transplantation, proteinuria at 3 months after KT, and KT decade (Table 3). After multivariable analysis, only proteinuria at three months after KT [HR 4.28 (95% CI: 1.47–12.48); P = 0.008] and recent KT decade [HR 3.03 (95% CI: 1.02–8.97); P = 0.046] remained associated with recurrence. Unfortunately, the presence and levels of autoantibodies could not be included in the analysis due to the limited number of immunological tests (both anti-PLA2R and anti-THSD7A) during follow-up (Table S4, see online supplementary material for a color version of this figure). When analyzing predictors of death-censored graft loss, the multivariate Cox regression model showed that eGFR after recurrence treatment [HR 0.85 (95% CI: 0.72–0.99), P = 0.037; eGFR <30 ml/min per 1.73 m2: RR = 6.2] was the only determinant of poorer graft survival (Table 4).
Table 3:
Cox regression analyses for determinants of MN recurrence after transplantation*.
| Variable | Univariate HR (95% CI) | P value | Multivariate HR [95% CI] | P value |
|---|---|---|---|---|
| Age at transplantation, per year | 1.06 (1.01–1.10) | 0.011 | 1.02 (0.98–1.07) | 0.310 |
| Sex | ||||
| Male | 1.00 (ref.) | |||
| Female | 1.68 (0.48–5.87) | 0.418 | NS | |
| Hypertension | ||||
| No | 1.00 (ref.) | |||
| Yes | 5.31 (0.70–40.43) | 0.107 | NS | |
| Time to kidney failure on native kidneys, per year | 0.98 (0.88–1.08) | 0.635 | NS | |
| Time on dialysis, per month | 1.00 (0.98–1.01) | 0.604 | NS | |
| KT decade | ||||
| 1991–2000 | 1.00 (ref.) | |||
| 2001–2010 | 6.20 (0.68–56.16) | 0.105 | ||
| 2011–2020 | 11.34 (1.29–99.95) | 0.029 | 3.03 (1.02–8.97) | 0.046 |
| Preemptive KT | ||||
| No | 1.00 (ref.) | |||
| Yes | 2.91 (0.81–10.40) | 0.100 | NS | |
| Type of donor | ||||
| DBD | 1.00 (ref.) | |||
| DCD | 0.95 (0.20–4.49) | 0.944 | ||
| Living donor | 1.51 (0.43–5.33) | 0.518 | ||
| Living related donor | 1.07 (0.24–4.78) | 0.925 | NS | |
| HLA-A/B/DR mismatch | ||||
| ≤3 | 1.00 (ref.) | |||
| >3 | 1.03 (0.38–2.77) | 0.949 | NS | |
| HLA-DRB1*03 | 0.47 (1.61–1.34) | 0.157 | NS | |
| CIT, per hour | 1.01 (0.94–1.08) | 0.758 | NS | |
| Induction therapy | ||||
| None | 1.00 (ref.) | |||
| Basiliximab/Daclizumab | 2.28 (0.74–7.01) | 0.151 | ||
| ATG/ALG/ATGAM | 0.48 (0.10–2.40) | 0.370 | NS | |
| Maintenance with CCT + CNI + AMF | ||||
| No | 1.00 (ref.) | |||
| Yes | 1.90 (0.23–16.01) | 0.555 | NS | |
| Steroid free/early steroid withdrawal | ||||
| No | 1.00 (ref.) | |||
| Yes | 0.41 (0.05–3.30) | 0.403 | NS | |
| DGF | ||||
| No | 1.00 (ref.) | |||
| Yes | 0.89 (0.32–2.41) | 0.818 | NS | |
| Rejection episode during follow-up | ||||
| No | 1.00 (ref.) | |||
| Yes | 1.73 (0.61–4.93) | 0.307 | NS | |
| eGFR 3 months after KT, per ml/min/1.73 m2 | 1.00 (0.97–1.02) | 0.743 | NS | |
| Proteinuria 3 months after KT, per g/24 h | 7.55 (2.49–22.86) | <0.001 | 4.28 (1.47–12.48) | 0.008 |
* Number of events: 18
Table 4:
Cox regression analyses for determinants of death-censored graft failure*.
| Variable | Univariate HR (95% CI) | P value | Multivariate HR [95% CI] | P value |
|---|---|---|---|---|
| Age at transplantation, per year | 1.05 (1–1.09) | 0.034 | 1.04 (0.96–1.13) | 0.339 |
| Sex | ||||
| Male | 1.00 (ref.) | |||
| Female | 2.05 (0.46–9.24) | 0.348 | NS | |
| Hypertension | ||||
| No | 1.00 (ref.) | |||
| Yes | 3.23 (0.42–24.80) | 0.259 | NS | |
| Diabetes | ||||
| No | 1.00 (ref.) | |||
| Yes | 0.89 (0.20–3.92) | 0.862 | NS | |
| Time on dialysis, per month | 1.00 (0.98–1.02) | 0.918 | NS | |
| Preemptive KT | ||||
| No | 1.00 (ref.) | |||
| Yes | 2.21 (0.49–10.09) | 0.305 | NS | |
| Type of donor | ||||
| DBD | 1.00 (ref.) | |||
| DCD | 1.56 (0.34–7.18) | 0.567 | NS | |
| Living donor | 1.28 (0.27–5.90) | 0.747 | NS | |
| HLA-A/B/DR mismatch | ||||
| ≤3 | 1.00 (ref.) | |||
| >3 | 2.39 (0.66–8.64) | 0.184 | NS | |
| CIT, per hour | 1.01 (0.94–1.09) | 0.741 | NS | |
| Induction therapy | ||||
| None | 1.00 (ref.) | |||
| Basiliximab/Daclizumab | 1.79 (0.43–7.43) | 0.421 | NS | |
| ATG/ALG/ATGAM | 2.87 (0.67–12.26) | 0.155 | NS | |
| Steroid free/early steroid withdrawal | ||||
| No | 1.00 (ref.) | |||
| Yes | 1.56 (0.35–6.99) | 0.561 | NS | |
| DGF | ||||
| No | 1.00 (ref.) | |||
| Yes | 1.19 (0.41–3.45) | 0.744 | NS | |
| Rejection episode during follow-up | ||||
| No | 1.00 (ref.) | |||
| Yes | 1.07 (0.30–3.83) | 0.922 | NS | |
| MN recurrence | ||||
| No | 1.00 (ref.) | |||
| Yes | 2.50 (0.86–7.24) | 0.092 | NS | |
| Recurrence clinical presentation | ||||
| Isolated nonnephrotic proteinuria | 1.00 (ref.) | |||
| Nephrotic proteinuria/Nephrotic syndrome | 7.51 (0.85–66.41) | 0.070 | NS | |
| eGFR 1 year after KT, per ml/min/1.73 m2 | 0.96 (0.92–0.99) | 0.010 | 1.04 (0.97–1.13) | 0.245 |
| eGFR at recurrence, per ml/min/1.73 m2 | 0.93 (0.86–1.00) | 0.085 | NS | |
| Proteinuria at recurrence, per g/dl | 1.07 (0.94–1.21) | 0.327 | NS | |
| eGFR after recurrence treatment, per ml/min/1.73 m2 | 0.90 (0.82–0.99) | 0.036 | 0.85 (0.72–0.99) | 0.037 |
| Proteinuria after recurrence treatment, per g/24 h | 1.07 (0.94–1.21) | 0.327 | NS |
* Number of events: 14
De novo or unclassified MN after transplantation
Table S5 (see online supplementary material for a color version of this figure) shows the clinical characteristics and management of four patients MN considered de novo or unclassified. All patients had a lacking native kidney biopsy and developed with a maximum of 15 months after KT. Proteinuria rates were very varied, and only one had concomitant ABMR. Despite all of them reaching partial or CR, only one patient had a functioning graft at the end of follow-up.
DISCUSSION
Herein, we report the characteristics and outcomes of a multicenter observational cohort study, including patients with MN diagnosed after KT. Up to 25.4% of patients with biopsy-proven MN as primary kidney disease recurred after KT, without a clear impact on graft survival. Early-onset proteinuria could be a monitorable risk factor for recurrence. Although patients with graft loss had higher median proteinuria rates during follow-up, eGFR values after recurrence treatment were the only determinant of poorer graft survival. We did not observe an association between the doses and levels of maintenance immunosuppression and recurrence diagnosis. But, interestingly, patients with SR had higher C0/D ratio of tacrolimus before recurrence. To date, rituximab has been restricted to recurrences with proteinuria >3 g/24 h. The increase in cases observed in the last decade indicates an improvement in its diagnosis. However, the performance of pre- and post-KT immunological tests for monitoring rMN remains limited.
Reported rates of rMN (between 18% and 57% [12, 14–20]) are highly heterogeneous among transplant centers due to different rates of native kidney biopsies, surveillance biopsy policies, data collection period, and follow-up time after KT. The incidence of rMN in our cohort hovers in the lower range. Nonetheless, more than 50% of the cohort received a KT before 2010, when most centers’ surveillance biopsies were still not established. Indeed, our data showed a significant increase in recurrences in the last decade, indicating an improvement in its diagnosis, which could be a consequence of introducing protocol biopsy policies and ameliorating the diagnosis of native kidney disease.
Understandably, surveillance biopsies provide the earliest diagnosis of rMN and may be crucial for detecting a subclinical disease [10, 12, 14, 25, 33]. However, our data revealed that proteinuria rates were significantly higher from 3 months post-KT in those patients who were later diagnosed with rMN. Thus, early-onset proteinuria (>0.3 g/24 h) could be a monitorable predictor for recurrence, indicating the need for early protocol biopsy. This is noteworthy considering that isolated nonnephrotic-range proteinuria and insidious manifestations seem to be the predominant clinical presentation in ours and previous studies, in contrast to the primary disease [10, 12, 14, 17, 33]. Yet, the disease can progress quickly regardless of low amounts of proteinuria [12, 17, 34]. Careful monitoring of proteinuria in the posttransplant period is an advised standard procedure to detect recurrence early [12, 23]. But the evolution of proteinuria rates prior to recurrence has scarcely been analyzed [18]. Instead, Grupper et al. studied the higher recurrence risk in patients with higher proteinuria pretransplant [12].
Anti-PLA2R antibody concentration has also been associated with clinically significant rMN. The positive predictive value of pretransplant anti-PLA2R antibodies for disease recurrence is 83%. In the posttransplant period, high titers have been shown to correlate with a higher risk of recurrence and may associate with disease progression or resistance to treatment [16, 17, 34]. Lamentably, the performance of pre- and post-KT immunological assays monitoring early rMN in our cohort was limited. Therefore, there is still a long way toward improving recurrence monitoring for a prompt diagnosis and adequate treatment.
The impact of recurrence on graft survival is still controversial. Some authors have reported no significant difference in graft survival between recipients with and without rMN [15, 35]. In contrast, others have shown a progression to end-stage kidney disease or allograft loss between 45% and 65% of recipients with recurrence within 4 to 6 years from diagnosis [12, 20, 36, 37]. Pippias et al. evaluated death-censored graft survival in 708 patients with primary MN compared with patients with autosomal dominant polycystic kidney disease (n = 7 181) using the ERA-EDTA registry database. They described a detrimental effect of MN recurrence [38]. We found no significant differences in death-censored graft survival between groups in both Kaplan–Meier and competing risk analyses. Nonetheless, graft losses were more numerous in the recurrence group and up to 83.3% were due to recurrence.
As expected, our data showed that those patients who lost their grafts had higher protein rates during follow-up. But, more importantly, despite the frequent insidious clinical presentation in patients with recurrence, up to 83.4% of patients with graft loss developed nephrotic-range proteinuria with or without nephrotic syndrome. Additionally, no recipients with rMN who achieved CR lost their allograft. Recipients with diminished graft survival also had worse kidney function during follow-up. In fact, we observed that eGFR values after recurrence treatment were the only determinant of poorer graft survival. Similarly, others have identified proteinuria rates and kidney function as prognostic factors associated with poor graft outcomes in KT recipients with posttransplant glomerulonephritis and in primary MN [4, 39, 40].
Induction therapy and standard maintenance immunosuppression have been shown to induce immunologic remission in selected cases [10, 17]. Consequently, the question arises as to whether maintenance immunosuppression drugs used for treating primary MN (i.e. tacrolimus, steroids) might modulate the development and clinical course of rMN [16, 25]. In a Spanish study, one patient with negative PLA2R antibodies before KT had positive anti-PLA2R antibodies at the time of recurrence, presumably due to minimized immunosuppression [16]. Furthermore, in a French cohort, a reduced immunosuppressive regimen that did not include both induction therapy and combined treatment with a calcineurin inhibitor and mycophenolate was associated with a persistently positive anti-PLA2R activity and recurrence [34]. In contrast, Mulay et al. did not find any relation between maintenance immunosuppression drugs and the risk of graft loss due to a glomerulonephritis recurrence [27]. We did not observe an association between the doses and levels of maintenance immunosuppression and recurrence diagnosis. But, notably, patients with SR had higher C0/D ratio of tacrolimus before recurrence. SR has been associated with a lower incidence of death and end-stage kidney disease in patients with idiopathic MN and nephrotic syndrome [41]. Unfortunately, we could not assess this query due to the small number of recurrences. In fact, our data showed an increased percentage of globally sclerotic glomeruli in patients with SR, probably due to a sample size effect.
Regarding recurrence treatment, most studies agree that intensive treatment with immunosuppression therapy should be considered if significant proteinuria is present (>1 g/24 h) [17, 23, 25]. Particularly, rituximab has shown promising results with high rates of clinical remission [10, 14, 15, 33]. Yet, our data showed that, to date, rituximab had been restricted to recurrences with proteinuria >3 g/24 h. Considering that most patients from the recurrent group lost their graft due to recurrence, we should hereinafter contemplate rituximab use in earlier proteinuria stages, as recently suggested by the new clinical practice guidelines [26].
Our study has the inherent limitations of all retrospective observational studies, and the results may be interpreted with caution. Despite its multicenter study design, the number of events is probably not large enough to show significant results regarding risk factors for recurrence or graft loss. Moreover, many patients did not have high-resolution HLA analysis performed to assess genetic predisposition nor serological monitoring for anti-PLA2R (or anti-THSD7A) levels before or after KT. One-third of patients did not have a follow-up biopsy performed, which may have underdiagnosed some cases of recurrent disease. Last, recurrence management was done at the discretion of the treating physician. Thus, we could not evaluate the use of rituximab in patients with mild proteinuria. However, this is a large series that explores allograft outcomes in patients with MN after KT according to proteinuria rates and maintenance immunosuppression management.
CONCLUSIONS
Our data show that one-fourth of patients with biopsy-proven MN as primary kidney disease recurred after transplantation, without a clear impact on graft survival. Early-onset proteinuria could be a monitorable risk factor for recurrence. Still, creatinine value after recurrence treatment was the only determinant of poorer graft survival. As a high tacrolimus exposure before rMN was associated to SR, maintenance immunosuppression seeking higher mean tacrolimus levels may be beneficial.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to the members of the SENTRA and GLOSEN working groups for their participation in helping this study become a reality.
Contributor Information
Anna Buxeda, Department of Nephrology, Hospital del Mar, Barcelona, Spain; Hospital del Mar Medical Research Institute (IMIM), Barcelona, Spain.
Fernando Caravaca-Fontán, Instituto de Investigación Hospital 12 de Octubre (imas12), Madrid, Spain.
Luis Alberto Vigara, Department of Nephrology, Hospital Universitario Puerta del Mar, Cádiz, Spain.
José Luis Pérez-Canga, Department of Nephrology, Hospital Universitario Marqués de Valdecilla / IDIVAL, Santander, Spain.
Emma Calatayud, Department of Nephrology, Hospital Universitari Doctor Peset, Valencia, Spain.
Ana Coloma, Department of Nephrology, Hospital Universitari de Bellvitge, Hospitalet de Llobregat, Barcelona, Spain.
Auxiliadora Mazuecos, Department of Nephrology, Hospital Universitario Puerta del Mar, Cádiz, Spain.
Emilio Rodrigo, Department of Nephrology, Hospital Universitario Marqués de Valdecilla / IDIVAL, Santander, Spain.
Asunción Sancho, Department of Nephrology, Hospital Universitari Doctor Peset, Valencia, Spain.
Edoardo Melilli, Department of Nephrology, Hospital Universitari de Bellvitge, Hospitalet de Llobregat, Barcelona, Spain.
Manuel Praga, Instituto de Investigación Hospital 12 de Octubre (imas12), Madrid, Spain; Department of Nephrology, Hospital Universitario 12 de Octubre, Madrid, Spain.
María José Pérez-Sáez, Department of Nephrology, Hospital del Mar, Barcelona, Spain; Hospital del Mar Medical Research Institute (IMIM), Barcelona, Spain.
Julio Pascual, Hospital del Mar Medical Research Institute (IMIM), Barcelona, Spain; Department of Nephrology, Hospital Universitario 12 de Octubre, Madrid, Spain.
FUNDING
AB has support from a Rio Hortega contract (CM19/00004, ISCIII), a M-AES grant (MV20/00072, ISCIII), and a Spanish Society of Nephrology scholarship.
AUTHORS’ CONTRIBUTIONS
Conception and design: A.B., M.J.P.-S., M.P., and J.P. Data acquisition: A.B., F.C.-F., L.A.V., A.M., J.L.P., E.R., E.C., A.S., A.C., and E.M. Analysis and interpretation of data: A.B., M.J.P.-S., and J.P. Draft of the manuscript: A.B. and M.J.P.-S. Critical revision or mentoring: A.M., E.M., E.R., A.S., M.P., M.J.P.-S., and J.P. A.B. was the major contributor in writing the manuscript. All the authors revised and approved the final version of the manuscript.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
CONFLICT OF INTEREST STATEMENT
Nothing to disclose.
REFERENCES
- 1. Rivera F, López-Gómez JM, Pérez-García R. Clinicopathologic correlations of renal pathology in Spain. Kidney Int 2004;66:898–904. 10.1111/j.1523-1755.2004.00833.x. [DOI] [PubMed] [Google Scholar]
- 2. Ronco P, Debiec H. Pathophysiological advances in membranous nephropathy: time for a shift in patient's care. Lancet North Am Ed 2015;385:1983–92. 10.1016/S0140-6736(15)60731-0. [DOI] [PubMed] [Google Scholar]
- 3. Couser WG. Primary membranous nephropathy. Clin J Am Soc Nephrol 2017;12:983–97. 10.2215/CJN.11761116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ronco P, Beck L, Debiec Het al. Membranous nephropathy. Nat Rev Dis Primers 2021;7. 10.1038/s41572-021-00303-z. [DOI] [PubMed] [Google Scholar]
- 5. Beck LH Jr, Bonegio RGB, Lambeau Get al. M-type phospholipase a2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 2009;361:11–21. 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tomas NM, Beck LH, Meyer-Schwesinger Cet al. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med 2014;371:2277–87. 10.1056/NEJMoa1409354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Donadio JV, Torres VE, Velosa JAet al. Idiopathic membranous nephropathy: the natural history of untreated patients. Kidney Int 1988;33:708–15. 10.1038/ki.1988.56. [DOI] [PubMed] [Google Scholar]
- 8. Schieppati A, Mosconi L, Perna Aet al. Prognosis of untreated patients with idiopathic membranous nephropathy. N Engl J Med 1993;329:85–89. 10.1056/NEJM199307083290203. [DOI] [PubMed] [Google Scholar]
- 9. Hofstra JM, Wetzels JFM. Alkylating agents in membranous nephropathy: efficacy proven beyond doubt. Nephrol Dial Transplant 2010;25:1760–6. 10.1093/ndt/gfq017. [DOI] [PubMed] [Google Scholar]
- 10. Dabade TS, Grande JP, Norby SMet al. Recurrent idiopathic membranous nephropathy after kidney transplantation: a surveillance biopsy study. Am J Transplant 2008;8:1318–22. 10.1111/j.1600-6143.2008.02237.x. [DOI] [PubMed] [Google Scholar]
- 11. Debiec H, Hanoy M, Francois Aet al. Recurrent membranous nephropathy in an allograft caused by IgG3k targeting the PLA2 receptor. J Am Soc Nephrol 2012;23:1949–54. 10.1681/ASN.2012060577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grupper A, Cornell LD, Fervenza FCet al. Recurrent membranous nephropathy after kidney transplantation: treatment and long-term implications. Transplantation 2016;100:2710–6. 10.1097/TP.0000000000001056. [DOI] [PubMed] [Google Scholar]
- 13. Truong L, Gelfand J, D'Agati Vet al. De novo membranous glomerulonephropathy in renal allografts: a report of ten cases and review of the literature. Am J Kidney Dis 1989;14:131–44. 10.1016/S0272-6386(89)80189-1. [DOI] [PubMed] [Google Scholar]
- 14. El-Zoghby ZM, Grande JP, Fraile MGet al. Recurrent idiopathic membranous nephropathy: early diagnosis by protocol biopsies and treatment with anti-CD20 monoclonal antibodies. Am J Transplant 2009;9:2800–7. 10.1111/j.1600-6143.2009.02851.x. [DOI] [PubMed] [Google Scholar]
- 15. Sprangers B, Lefkowitz GI, Cohen SDet al. Beneficial effect of rituximab in the treatment of recurrent idiopathic membranous nephropathy after kidney transplantation. Clin J Am Soc Nephrol 2010;5:790–7. 10.2215/CJN.04120609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Quintana LF, Blasco M, Seras Met al. Antiphospholipase A2 receptor antibody levels predict the risk of posttransplantation recurrence of membranous nephropathy. Transplantation 2015;99:1709–14. 10.1097/TP.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 17. Kattah A, Ayalon R, Beck LHet al. Anti-phospholipase A2 receptor antibodies in recurrent membranous nephropathy. Am J Transplant 2015;15:1349–59. 10.1111/ajt.13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gupta G, Fattah H, Ayalon Ret al. Pre-transplant phospholipase A2 receptor autoantibody concentration is associated with clinically significant recurrence of membranous nephropathy post-kidney transplantation. Clin Transplant 2016;30:461–9. 10.1111/ctr.12711. [DOI] [PubMed] [Google Scholar]
- 19. Cosio FG, Cattran DC. Recent advances in our understanding of recurrent primary glomerulonephritis after kidney transplantation. Kidney Int 2017;91:304–14. 10.1016/j.kint.2016.08.030. [DOI] [PubMed] [Google Scholar]
- 20. Allen PJ, Chadban SJ, Craig JCet al. Recurrent glomerulonephritis after kidney transplantation: risk factors and allograft outcomes. Kidney Int 2017;92:461–9. 10.1016/j.kint.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 21. De Vriese AS, Glassock RJ, Nath KAet al. A proposal for a serology-based approach to membranous nephropathy. J Am Soc Nephrol 2017;28:421–30. 10.1681/ASN.2016070776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gupta G, Raynaud M, Kumar Det al. Impact of belatacept conversion on kidney transplant function, histology, and gene expression - a single-center study. Transpl Int 2020;33:1458–71. 10.1111/tri.13718. [DOI] [PubMed] [Google Scholar]
- 23. Leon J, Pérez-Sáez MJ, Batal Iet al. Membranous nephropathy posttransplantation: an update of the pathophysiology and management. Transplantation 2019;103:1990–2002. 10.1097/TP.0000000000002758. [DOI] [PubMed] [Google Scholar]
- 24. Spinner ML, Bowman LJ, Horwedel TAet al. Single-dose rituximab for recurrent glomerulonephritis post-renal transplant. Am J Nephrol 2015;41:37–47. 10.1159/000371587. [DOI] [PubMed] [Google Scholar]
- 25. Passerini P, Malvica S, Tripodi Fet al. Membranous nephropathy (Mn) recurrence after renal transplantation. Front Immunol 2019;10:1–9. 10.3389/fimmu.2019.01326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. KDIGO Clinical Practice Guideline on Glomerular Diseases . 2020;(June). https://kdigo.org/wp-content/uploads/2017/02/KDIGO-GN-GL-Public-Review-Draft_1-June-2020.pdf [Google Scholar]
- 27. Mulay AV, Van Walraven C, Knoll GA. Impact of immunosuppressive medication on the risk of renal allograft failure due to recurrent glomerulonephritis. Am J Transplant 2009;9:804–11. 10.1111/j.1600-6143.2009.02554.x. [DOI] [PubMed] [Google Scholar]
- 28. Ponticelli C, Glassock RJ. De novo membranous nephropathy (MN) in kidney allografts. A peculiar form of alloimmune disease? Transpl Int 2012;25:1205–10. 10.1111/j.1432-2277.2012.01548.x. [DOI] [PubMed] [Google Scholar]
- 29. Honda K, Horita S, Toki Det al. De novo membranous nephropathy and antibody-mediated rejection in transplanted kidney. Clin Transplant 2011;25:191–200. 10.1111/j.1399-0012.2010.01213.x. [DOI] [PubMed] [Google Scholar]
- 30. Registro Español de Enfermos Renales (REER) . Available at: http://www.ont.es/infesp/Registros/MEMORIA%20REER%202020_PRELIMINAR.pdf. Published online2020; [Google Scholar]
- 31. Levey AS, Stevens LA, Schmid CHet al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. http://www.nserc-crsng.gc.ca/Promoter-Promotion/PromoScience-PromoScience/Eligibility-Admissibilite_eng.asp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ehrenreich T, Churg J. Pathology of membranous nephropathy. Pathol Annu 1968;3:145–86. [Google Scholar]
- 33. Rodriguez EF, Cosio FG, Nasr SHet al. The pathology and clinical features of early recurrent membranous glomerulonephritis. Am J Transplant 2012;12:1029–38. 10.1111/j.1600-6143.2011.03903.x. [DOI] [PubMed] [Google Scholar]
- 34. Seitz-Polski B, Payré C, Ambrosetti Det al. Prediction of membranous nephropathy recurrence after transplantation by monitoring of anti-PLA2R1 (M-type phospholipase A2 receptor) autoantibodies: a case series of 15 patients. Nephrol Dial Transplant 2014;29:2334–42. 10.1093/ndt/gfu252. [DOI] [PubMed] [Google Scholar]
- 35. Moroni G, Gallelli B, Quaglini Set al. Long-term outcome of renal transplantation in patients with idiopathic membranous glomerulonephritis (MN). Nephrol Dial Transplant 2010;25:3408–15. 10.1093/ndt/gfq223. [DOI] [PubMed] [Google Scholar]
- 36. Cosyns J, Couchoud C, Pouteil-Noble Cet al. Recurrence of membranous nephropathy after renal transplantation: probability, outcome and risk factors. Clin Nephrol 1998;50:144–53. [PubMed] [Google Scholar]
- 37. Josephson M, Spargo B, Hollandsworth. The recurrence of recurrent membranous glomerulopathy in a renal transplant recipient: case report and literature review. Am J Kidney Dis Published online1994;24–875. [DOI] [PubMed] [Google Scholar]
- 38. Pippias M, Stel VS, Aresté-Fosalba Net al. Long-term kidney transplant outcomes in primary glomerulonephritis: analysis from the ERA-EDTA registry. Transplantation 2016;100:1955–62. 10.1097/TP.0000000000000962. [DOI] [PubMed] [Google Scholar]
- 39. Requião-Moura LR, Moscoso-Solorzano GT, Franco MFet al. Prognostic factors associated with poor graft outcomes in renal recipients with post-transplant glomerulonephritis. Clin Transplant 2007;21:363–70. 10.1111/j.1399-0012.2007.00650.x. [DOI] [PubMed] [Google Scholar]
- 40. Reichert L, Koene RA, Wetzels JF. Prognostic factors in idiopathic membranous nephropathy. Am J Kidney Dis 1998;31:1–11. 10.1053/ajkd.1998.v31.pm9428445. [DOI] [PubMed] [Google Scholar]
- 41. Polanco N, Gutiérrez E, Covarsí Aet al. Spontaneous remission of nephrotic syndrome in idiopathic membranous nephropathy. J Am Soc Nephrol 2010;21:697–704. 10.1681/ASN.2009080861. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.