Patients with chronic kidney disease (CKD) suffer from a high fracture burden, e.g. the hip fracture risk in hemodialysis patients is four to six times as high as that observed in age- and gender-matched controls, while vertebral fractures (VFs) show similar prevalence in CKD patients as the general population, varying between 18% and 55%, depending on the method of assessment [1, 2]. Fractures in CKD patients, moreover, are associated with higher morbidity and mortality [1]. The high fracture risk in CKD patients may be explained by an increased fall risk in combination with an impaired bone strength, which in turn reflects low bone density and/or quality. Bone quality integrates various characteristics including microarchitecture, bone turnover and bone mineralization. Both traditional and uremia-specific risk factors contribute to the impaired bone strength. These include hyperparathyroidism, inflammation and oxidative stress [3, 4]. Recent studies also point to vitamin K deficiency as a potential culprit of bone fragility in CKD. This renders vitamin K deficiency a potential therapeutic target.
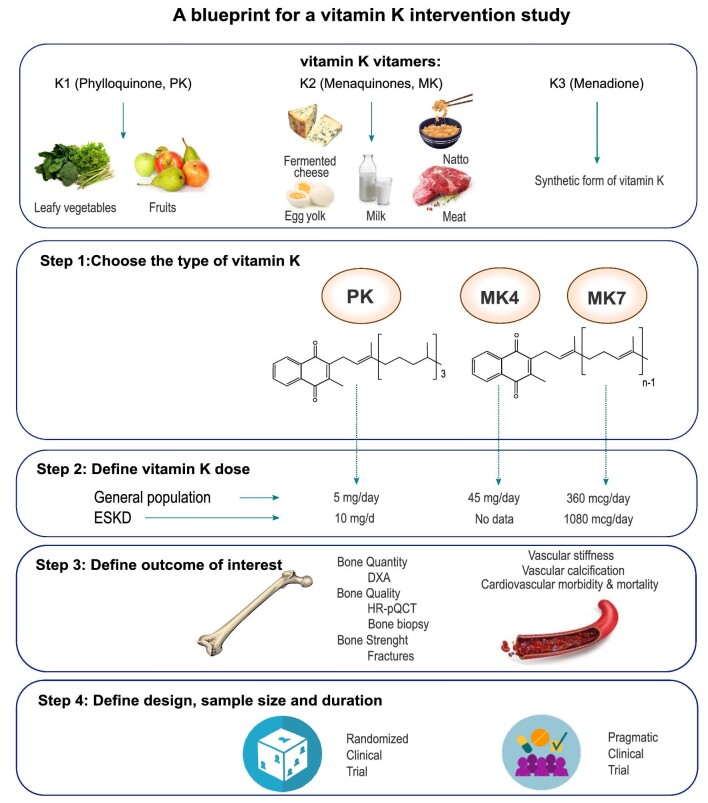
Vitamin K comprises a group of vitamins that share the presence of a 2-methyl-1,4-naphthoquinone ring, but differ in the (lipophilic) side chains at position 3. Vitamin K1 or phylloquinone (PK) is mainly found in green leafy vegetables (Fig. 1). All types of vitamin K2 or menaquinones, except menatetranone (MK4), are produced by the intestinal bacterial flora and can be found in fermented foods (Fig. 1). MK4 mainly originates from the conversion of PK through the removal or addition of a side chain in pancreatic, testicular or vascular tissue. Finally, vitamin K3 or menadione is a vitamin lacking side chains (Fig. 1). It is a synthetic analogue often added to foods, and is converted to MK4 in the liver [5].
Figure 1:
A blueprint for a vitamin K intervention study.
Vitamin K is a cofactor of γ-glutamyl carboxylase (GGCX), catalyzing the carboxylation of vitamin K–dependent proteins (VKDPs). So far, 17 VKDPs have been identified. These include coagulation factors but also proteins that are known to play an important role in skeletal and vascular (patho)biology, such as matrix Gla protein (MGP) and bone Gla Protein (BGP) [5, 6]. Furthermore, upon binding the steroid and xenobiotic receptor, MK4 may stimulate the expression of proteins such as matrilin-2 (Matn2), tsukushi (TsK), homeobox protein Msx2 and CD14, and as such influence bone remodeling and quality. Matn2 and Tsk are essential for the deposition of extracellular matrix and the formation of collagen, while Msx2 induces osteoblast differentiation and CD14 regulates osteoblastogenesis and osteoclastogenesis through the differentiation of B cells [5].
Measuring vitamin K plasma levels directly is complex, partly due to lack of standardization and interference with triglycerides, which is especially relevant at low levels of vitamin K as encountered in CKD patients. The few clinical studies with the direct assay of vitamin levels are predominantly related to PK and less to MKs. Indeed, most studies evaluated vitamin K status indirectly, by quantifying undercarboxylated VKDPs. Using this approach, vitamin K deficiency is arbitrarily defined by high concentrations of dephosphorylated-uncarboxylated MGP (dp-ucMGP) [7].
Vitamin K deficiency is highly prevalent among CKD patients, due to dietary restrictions (limiting intake of PK-rich food items), microbiome alterations (limiting MK production), uremia per se (limiting recycling and cellular uptake) and drug interferences [5, 8]. The Vitamin K Italian (VIKI) observational study highlighted remarkable MK7 (35.4%), PK (23.5%) and MK4 (14.5%) deficiencies in hemodialysis patients [9–11].
Clinical cohort studies show an association between vitamin K deficiency and fractures in patients with CKD across stages of disease [6, 9]. In incident renal transplant recipients, above-mean dp-ucMGP levels were associated with a higher risk of incident fractures, even after adjusting for classic factors such as gender, age, previous fractures and femoral neck T-score [12]. In the VIKI observational study, vitamin K deficiency was associated with prevalent vertebral fractures. Importantly this association was found only for PK, thus corroborating data from the Hordaland Health Study [11, 13]. Taken together, observational data suggest that vitamin K deficiency may be associated with bone fragility, but that the association may not hold true for all types of vitamin K.
While biological plausibility and consistency of observational data suggest a causal relationship between vitamin K deficiency and bone fragility, the proof of the pudding remains in the eating. Yet, intervention studies with vitamin K are limited and unfortunately have yielded heterogeneous findings, probably reflecting case-mix and variability in type, dose and duration of the intervention, and end-point of interest. Vitamin K is currently commercially available in PK, MK4 and MK7 formulations. Intervention studies have demonstrated reduction in the incidence of bone fractures both with PK and MK4, while an improvement of bone mineral density (BMD) has only been seen with MK4 [5]. In a randomized, placebo-controlled, double-blind study of 440 postmenopausal women, Cheung et al. demonstrated that a daily intake of 5 mg of PK resulted in a reduced fracture rate, while it failed to attenuate the decline of BMD [14]. These findings suggest that vitamin K may improve bone quality rather than bone quantity [5, 15]. Of note, in Japan, MK4 (45 mg, once daily) is a well-recognized anti-osteoporosis drug [5].
Results from a recent vitamin K intervention study in dialysis patients by Levy-Schousboe et al. published in this issue of NDT, should be interpreted against this background [16]. The investigators conducted a randomized placebo-controlled (RCT), double-blind study to evaluate the impact of vitamin K supplementation on BMD in dialysis patients. Patients in the active study arm were treated with 360 μg of MK7 for 2 years. BMD results were mixed. Somewhat surprisingly, patients in the active study arm showed an accentuated BMD loss at the distal 1/3 radius site. No BMD differences were observed at other sites of the radius. MK supplementation reduced BMD loss at the lumbar spine but not at the femoral neck. VFs incidence and progression of vascular calcification, evaluated according to Kauppilla's method, did not differ between groups [16].
This study, being among the few RCT on vitamin K, should be applauded and warmly welcomed. However, power issues and peculiarities of the study design with regard to choice of (primary) endpoint and intervention call for a cautious interpretation of the results.
Like many RCTs, the study by Levy-Schousboe et al. suffered from recruitment difficulties and a higher than anticipated drop-out rate. As already highlighted, the outcome MK7 supplementation differed according to skeletal site under investigation: BMD loss at the distal 1/3 radius (primary endpoint) and BMD gain at the lumbar spine. While these different outcomes may have a biological explanation, as these sites refr to predominantly cortical and trabecular bone, respectively, one should also consider the possibility of analytical bias. Indeed, the dual-energy X-ray absorptiometry (DXA) BMD at the distal 1/3 radius has limitations as an outcome measure including a higher analytical variability and unknown bias by a functional arteriovenous fistula. These limitations confer a risk of type I statistical error. Furthermore, since skeletal health promoting effects of vitamin K may relate more to bone quality than quantity, other outcomes besides DXA BMD should be considered in future trials [5, 15].
MK7 was chosen to replenish vitamin K stores. While widespread availability and long half-life are truly assets, it should be acknowledged that epidemiological evidence linking MK7 to favorable bone outcomes is very limited, if not non-existant, especially for the synthetic form of MK7. More importantly, recent experimental and clinical data indicate that both metabolism and transport of MK7 is profoundly disturbed in CKD [17]. MK7 supplements thus seem not the best choice in CKD, or should be given at higher doses. Of note, while MK7 levels significantly increased in the active treatment arm, levels of dp-ucMGP, a marker of functional vitamin K deficiency, did not normalize. However, none of the trials that studied vitamin K supplementation in dialysis achieved a “normal” dp-ucMGP. These insights call for caution in extrapolating present results to other vitamin K treatment regimes, testing different vitamins, doses and/or follow-up time.
Designing the ideal study to assess (skeletal) efficacy of vitamin K supplementation is a real challenge (Fig. 1). Knowledge of the many pitfalls is evolving and will help in the pursuit of this challenge in the future. An RCT with a well-defined treatment regimen assessing hard endpoints would overall be considered the “nec plus ultra.” Such trials, however, are not always feasible, often deal with relatively short follow-up duration, and the participants in many cases are homogeneous and highly selected for safety considerations, and adherence to intervention is well-controlled. For several reasons, RCTs may both over- and underestimate treatment effects. As a result, the generalizability of the study findings to a broader range of subjects, and for chronic disease settings that require much longer follow-up, is often questionable. To address these issues and to evaluate the effectiveness of a treatment in real-world clinical practice, pragmatic trials are gaining popularity [18], also within the field of CKD–mineral and bone disorder [19].
In conclusion, while RCTs in the general population indicate that PK or MK4 confer bone protective, findings in CKD patients seem to be more puzzling. The latter may be related to the fact that the pathophysiology of bone disease in CKD is complex and that changing one risk factor does not necessarily translate into improved skeletal outcomes. Alternatively, heterogenous findings may be due to shortcomings in study design, in part related to incomplete knowledge of the metabolism of vitamin K in CKD. Importantly, not all types of vitamin K are equal. The present study, with negative findings, thus should not temper enthusiasm, but to the contrary, should help in designing the ideal trial answering the clinically highly relevant question of whether vitamin K supplementation prevents bone fractures and/or attenuates the progression of vascular calcification.
Contributor Information
Maria Fusaro, National Research Council (CNR) – Institute of Clinical Physiology (IFC), Pisa Via G. Moruzzi 1, Pisa, Italy; Department of Medicine, University of Padova, Padova, Italy.
Pieter Evenepoel, Department of Microbiology, Immunology and Transplantation, Nephrology and Renal Transplantation Research Group, KU Leuven, Leuven, Belgium; Department of Medicine, Division of Nephrology, University Hospitals Leuven, Leuven, Belgium.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Evenepoel P, Cunningham J, Ferrari Set al. European Consensus Statement on the diagnosis and management of osteoporosis in chronic kidney disease stages G4–G5D. Nephrol Dial Transplant 2021;36:42–59. 10.1093/ndt/gfaa192 [DOI] [PubMed] [Google Scholar]
- 2. Fusaro M, Tripepi G, Noale Met al. High prevalence of vertebral fractures assessed by quantitative morphometry in hemodialysis patients, strongly associated with vascular calcifications. Calcif Tissue Int 2013;93:39–47. 10.1007/s00223-013-9722-x. [DOI] [PubMed] [Google Scholar]
- 3. Cannata-Andía JB, Martín-Carro B, Martín-Vírgala Jet al. Chronic kidney disease–mineral and bone disorders: pathogenesis and management. Calcif Tissue Int 2021;108:410–22. 10.1007/s00223-020-00777-1. [DOI] [PubMed] [Google Scholar]
- 4. Jørgensen HSK, David K, Salam Set al. Traditional and non-traditional risk factors for osteoporosis in CKD. Calcif Tissue Int 2021;108:496–511. 10.1007/s00223-020-00786-0. [DOI] [PubMed] [Google Scholar]
- 5. Fusaro M, Cianciolo G, Brandi MLet al. Vitamin K and osteoporosis. Nutrients 2020;12:3625. 10.3390/nu12123625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fusaro M, Cianciolo G, Evenepoel Pet al. Vitamin K in CKD bone disorders. Calcif Tissue Int 2021;108:476–85. 10.1007/s00223-020-00792-2. [DOI] [PubMed] [Google Scholar]
- 7. Fusaro M, Cosmai L, Evenepoel Pet al. Vitamin K and kidney transplantation. Nutrients 2020;12:2717. 10.3390/nu12092717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fusaro M, Cozzolino M, Plebani Met al. Sevelamer use, vitamin K levels, vascular calcifications, and vertebral fractures in hemodialysis patients: results from the VIKI study. J Bone Miner Res 2021;36:500–9. 10.1002/jbmr.4214. [DOI] [PubMed] [Google Scholar]
- 9. Neogi T, Booth S, Zhang YQet al. Low vitamin K status is associated with osteoarthritis in the hand and knee. Arthritis Rheum 2006;54:1255–61. 10.1002/art.21735. [DOI] [PubMed] [Google Scholar]
- 10. Pilkey RM, Morton RA, Boffa MBet al. Subclinical vitamin K deficiency in hemodialysis patients. Am J Kidney Dis 2007;49:432–9. 10.1053/j.ajkd.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 11. Fusaro M, Noale M, Viola Vet al. Vitamin K, vertebral fractures, vascular calcifications, and mortality: Vitamin K Italian (VIKI) dialysis study. J Bone Miner Res 2012;27:2271–8. 10.1002/jbmr.1677. [DOI] [PubMed] [Google Scholar]
- 12. Evenepoel P, Claes K, Meijers Bet al. Poor vitamin K status is associated with low bone mineral density and increased fracture risk in end-stage renal disease. J Bone Miner Res 2019;34:262–9. 10.1002/jbmr.3608. [DOI] [PubMed] [Google Scholar]
- 13. Apalset EM, Gjesdal CG, Eide GEet al. Intake of vitamin K1 and K2 and risk of hip fractures: the Hordaland Health Study. Bone 2011;49:990–5. 10.1016/j.bone.2011.07.035. [DOI] [PubMed] [Google Scholar]
- 14. Cheung AM, Tile L, Lee Yet al. Vitamin K supplementation in postmenopausal women with osteopenia (ECKO Trial): a randomized controlled trial. PLoS Med 2008;5:e196. 10.1371/journal.pmed.0050196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rønn SH, Harsløf T, Pedersen SBet al. Vitamin K2 (menaquinone-7) prevents age-related deterioration of trabecular bone microarchitecture at the tibia in postmenopausal women. Eur J Endocrinol 2016;175:541–9. 10.1530/EJE-16-0498. [DOI] [PubMed] [Google Scholar]
- 16. Levy-Schousboe K, Marckmann P, Frimodt-Møller Met al. Vitamin K supplementation and bone mineral density in dialysis: results of the double-blind, randomised, placebo-controlled RenaKvit trial. Nephrol Dial Transplant 2022; 10.1093/ndt/gfac315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaesler N, Felix S, Speer Tet al. Altered vitamin K biodistribution and metabolism in experimental and human chronic kidney disease. Kidney Int 2022;101:338–48. 10.1016/j.kint.2021.10.029. [DOI] [PubMed] [Google Scholar]
- 18. Sacristán JA, Dilla T. Pragmatic trials revisited: applicability is about individualization. J Clin Epidemiol 2018;99:164–6. 10.1016/j.jclinepi.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 19. Edmonston DL, Isakova T, Dember LMet al. Design and rationale of HiLo: a pragmatic, randomized trial of phosphate management for patients receiving maintenance hemodialysis. Am J Kidney Dis 2021;77:920–30.e1. 10.1053/j.ajkd.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]