ABSTRACT
Background
Hypoxia-inducible factor prolyl hydroxylase inhibitors such as vadadustat may provide an oral alternative to injectable erythropoiesis-stimulating agents for treating anemia in patients receiving peritoneal dialysis. In two randomized (1:1), global, phase 3, open-label, sponsor-blind, parallel-group, active-controlled noninferiority trials in patients with dialysis-dependent chronic kidney disease (INNO2VATE), vadadustat was noninferior to darbepoetin alfa with respect to cardiovascular safety and hematological efficacy. Vadadustat's effects in patients receiving only peritoneal dialysis is unclear.
Methods
We conducted a post hoc analysis of patients in the INNO2VATE trials receiving peritoneal dialysis at baseline. The prespecified primary safety endpoint was time to first major cardiovascular event (MACE; defined as all-cause mortality or nonfatal myocardial infarction or stroke). The primary efficacy endpoint was mean change in hemoglobin from baseline to the primary evaluation period (Weeks 24–36).
Results
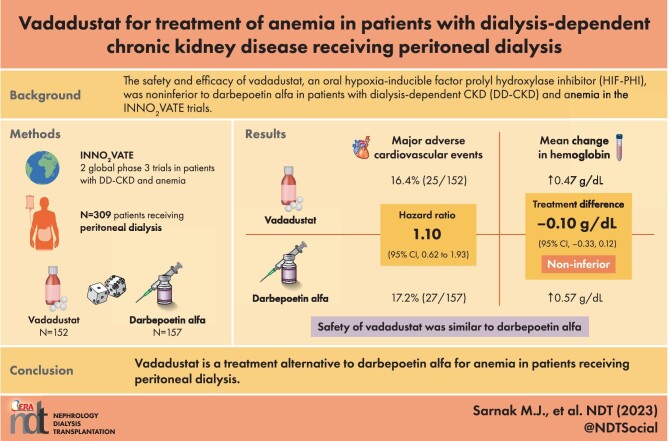
Of the 3923 patients randomized in the two INNO2VATE trials, 309 were receiving peritoneal dialysis (vadadustat, n = 152; darbepoetin alfa, n = 157) at baseline. Time to first MACE was similar in the vadadustat and darbepoetin alfa groups [hazard ratio 1.10; 95% confidence interval (CI) 0.62, 1.93]. In patients receiving peritoneal dialysis, the difference in mean change in hemoglobin concentrations was −0.10 g/dL (95% CI −0.33, 0.12) in the primary evaluation period. The incidence of treatment-emergent adverse events (TEAEs) was 88.2% versus 95.5%, and serious TEAEs was 52.6% versus 73.2% in the vadadustat and darbepoetin alfa groups, respectively.
Conclusions
In the subgroup of patients receiving peritoneal dialysis in the phase 3 INNO2VATE trials, safety and efficacy of vadadustat were similar to darbepoetin alfa.
Keywords: anemia, chronic kidney disease, hypoxia-inducible factor prolyl hydroxylase inhibitor, peritoneal dialysis, vadadustat
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What is already known about this subject?
In two global phase 3 trials in patients with dialysis-dependent chronic kidney disease who were or were not previously treated with erythropoiesis-stimulating agents (ESAs) (the INNO2VATE trials), vadadustat was noninferior to darbepoetin alfa with respect to cardiovascular safety and hematological efficacy.
Vadadustat's effect in the subset of patients in the INNO2VATE trials receiving only peritoneal dialysis is unclear.
What this study adds?
In the present post hoc subgroup analyses in patients receiving peritoneal dialysis, we show results similar to those observed in the INNO2VATE trials overall, with no new safety signals for vadadustat.
What impact this may have on practice or policy?
The potential advantages of an oral treatment for anemia are numerous for patients receiving home dialysis relative to those receiving in-center hemodialysis. Given its effectiveness and acceptable safety profile compared with darbepoetin alfa, vadadustat should be considered as a treatment alternative to ESAs in patients receiving peritoneal dialysis.
INTRODUCTION
Chronic kidney disease (CKD) is estimated to affect approximately 10% of the global population [1]. Among patients with CKD, anemia is a common complication that largely results from reduced erythropoietin production by the failing kidney [1–3]. The prevalence of CKD is increasing worldwide, with nearly 6 million patients anticipated to require kidney replacement therapy by 2030 [3]. Use of home dialysis modalities (peritoneal dialysis and home hemodialysis) is increasing because of greater convenience, flexibility, ability to maintain social distancing and cost-effectiveness compared with conventional in-center hemodialysis, while maintaining or potentially resulting in superior clinical outcomes and health-related quality of life [3]. In the USA, an Executive Order (the Advancing American Kidney Health initiative) signed in 2019 aims to have 80% of US patients with end-stage kidney disease receiving home dialysis or transplantation by 2025. As home dialysis includes both hemodialysis and peritoneal dialysis, this Executive Order will likely further increase the prevalence of peritoneal dialysis over the next several years [3].
It is to be expected that most patients receiving home dialysis will still require treatment for anemia [2]. Current treatments include iron supplements, erythropoiesis-stimulating agents (ESAs; recombinant human erythropoietin and its derivatives) and red blood cell (RBC) transfusions. ESAs can be administered intravenously during in-center or at-home hemodialysis sessions as a treatment for anemia. However, patients on home-based peritoneal dialysis therapies either need to self-administer ESAs subcutaneously, which can be difficult and uncomfortable for some patients, or make a trip to a healthcare facility for an injection, which can be time-consuming and challenging, especially for patients in rural areas, those unable to drive, or those with limited resources [4]. For those patients, oral agents may be preferable to the currently available injectable options. Vadadustat, an oral hypoxia-inducible factor prolyl hydroxylase inhibitor (HIF-PHI), can be stored at room temperature and could provide a benefit as it provides more convenient dosing for treatment of anemia in patients receiving peritoneal dialysis [3, 5].
Vadadustat stimulates endogenous erythropoietin and RBC production and is currently approved for the treatment of anemia in patients with CKD in Japan and patients with DD-CKD in the EU and Korea [6–10]. In two completed global phase 3 trials in patients with dialysis-dependent chronic kidney disease (DD-CKD; INNO2VATE trials), vadadustat met prespecified noninferiority criteria [upper confidence interval (CI) of 1.25] compared with darbepoetin alfa for the primary safety endpoint, time to first major adverse cardiovascular event (MACE; defined as all-cause mortality or nonfatal myocardial infarction or stroke) and the primary efficacy endpoint (correction/maintenance of hemoglobin concentrations) [7].
This analysis describes the safety and efficacy of vadadustat versus darbepoetin alfa in the subgroup of patients who received peritoneal dialysis in the INNO2VATE program.
MATERIALS AND METHODS
Trial design
We conducted two phase 3, global, open-label, sponsor-blind, parallel-group, active-controlled, noninferiority trials to compare the safety and efficacy of vadadustat with that of darbepoetin alfa. This current analysis was conducted with data from the subgroup of patients receiving peritoneal dialysis at baseline in the INNO2VATE trials [NCT02865850 incident DD-CKD (registration date: 15 August 2016) and NCT02892149 prevalent DD-CKD (registration date: 8 September 2016)]. The rationale, design, methods and primary results of the INNO2VATE trials have previously been described [7, 11].
The INNO2VATE trials were performed in compliance with the International Conference on Harmonisation, in accordance with Good Clinical Practice guidelines and applicable local regulatory requirements and laws, and in line with the principles of the Declaration of Helsinki. Institutional review board approval was obtained at each participating center. All patients provided written informed consent prior to enrollment.
Trial population
Of the 3923 patients randomized in the two INNO2VATE trials, 309 were receiving peritoneal dialysis (vadadustat, n = 152; darbepoetin alfa, n = 157) and were included in the presented analyses. Eligible patients (n = 309) were adults (aged ≥18 years) with DD-CKD treated with peritoneal dialysis and anemia with laboratory proxies of iron status of serum ferritin ≥100 ng/mL and transferrin saturation (TSAT) ≥20%, and who had not received an RBC transfusion within 8 weeks prior to randomization.
For the incident DD-CKD trial, patients were required to have initiated maintenance dialysis within 16 weeks prior to screening, with baseline hemoglobin concentrations of 8–11 g/dL and receipt of limited doses of ESAs (i.e. the receipt of more than two doses of a long-acting ESA (darbepoetin alfa or methoxy polyethylene glycol-epoetin beta) or no more than four doses of short-acting ESA (epoetin alfa, epoetin beta) within 8 weeks prior to screening). Patients were also excluded if they met the following criteria for ESA resistance within 8 weeks prior to screening: epoetin >7700 U/dose three times per week or >23 000 U per week, darbepoetin alfa >100 µg/week, or methoxy polyethylene glycol-epoetin beta >100 µg every other week or >200 µg every month. For the prevalent DD-CKD trial, patients were required to have received maintenance dialysis for at least 12 weeks prior to screening and to be currently receiving any form of ESA therapy, with baseline hemoglobin concentrations of 8–11 g/dL (US) or 9–12 g/dL (non-US). In both trials, patients were excluded if they had anemia considered secondary to causes other than CKD, uncontrolled hypertension or a recent cardiovascular event.
Trial procedures
In the two INNO2VATE trials overall, eligible patients were randomized 1:1 to receive vadadustat or darbepoetin alfa, stratified by geographic region (USA/Europe/other regions), New York Heart Association (NYHA) congestive heart failure class (0/I vs II/III) and hemoglobin concentration at entry (incident DD-CKD trial, <9.5 vs ≥9.5 g/dL; prevalent DD-CKD trial, <10 vs ≥10 g/dL). The proportion of patients receiving peritoneal dialysis at baseline in the two INNO2VATE trials was similar in both randomized groups (3.9% and 4.0% in the vadadustat and darbepoetin alfa groups, respectively). The trials had four defined periods: (i) a correction or conversion period (Weeks 0–23); (ii) a maintenance period (Weeks 24–52), including the primary (Weeks 24–36) and secondary (Weeks 40–52) evaluation periods; (iii) a long-term safety period [Week 53 to end of treatment (182 weeks)]; and (iv) a 4-week safety follow-up period after the end of treatment.
The vadadustat starting dose was 300 mg orally once daily (two 150-mg tablets), with doses of 150 mg, 300 mg, 450 mg and 600 mg available for adjustment to a maximum daily dose of 600 mg. In contrast to studies with other HIF-PHI agents, all patients randomized to vadadustat received the same starting dose (300 mg), irrespective of their prior dose of ESA. Darbepoetin alfa was administered subcutaneously by the staff at the site facility or by the patient at home according to the investigator's determination and local practice. The initial dose of darbepoetin alfa was based on dose prior to randomization for those patients already receiving darbepoetin alfa, or on the local product label for patients not receiving darbepoetin alfa prior to randomization but switching from a different ESA. Doses of vadadustat and darbepoetin alfa were titrated according to protocol-specified dose adjustment guidelines to maintain target hemoglobin concentrations (10–11 g/dL in the USA; 10–12 g/dL in all other countries). Iron supplementation was encouraged to maintain serum ferritin concentrations of ≥100 ng/mL or a transferrin saturation of ≥20%. During the first year of treatment, hemoglobin concentrations were measured and dose adjustments assessed every 2 weeks for Weeks 0–12 and every 4 weeks for Weeks 12–52. Modifications to trial treatment dose were made to maintain hemoglobin concentrations within the geography-specific target range. Dose adjustments were based on the investigator's clinical discretion, incorporating the protocol guidance and considering the patient's clinical condition, hemoglobin rate of rise, hemoglobin rate of decline, hemoglobin concentration and trial drug responsiveness.
Starting at 6 weeks, patients in both treatment groups could receive an ESA as rescue therapy if they experienced worsening symptoms of anemia, with a hemoglobin concentration <9.5 g/dL. If rescue therapy was required, trial drugs were temporarily discontinued while patients received an ESA. In the darbepoetin alfa group, an ESA rescue was defined post hoc if the dose was increased to at least double the previous darbepoetin alfa dose. In the event of an acute or severe loss of blood, an RBC transfusion was administered as clinically indicated. Trial drugs were continued during the transfusion period.
Endpoints
The prespecified primary safety endpoint, in which patients receiving peritoneal dialysis were a prespecified subgroup, was time to first MACE, as defined in the Introduction. A key secondary safety endpoint was first occurrence of “expanded MACE” (a MACE plus hospitalization for either heart failure or a thromboembolic event, excluding vascular access failure). The primary efficacy endpoint was mean change in hemoglobin concentration from baseline to the primary evaluation period (Weeks 24–36), and the key secondary endpoint was mean change in hemoglobin concentration from baseline to the secondary efficacy period (Weeks 40–52) in each trial. In addition, we assessed changes in iron indices, provision of RBC transfusions, hemoglobin excursions, and rate of rise, trial treatment modifications and incidence of treatment-emergent adverse events (TEAEs). Analyses for expanded MACE, iron-related parameters, RBC transfusions, hemoglobin excursions, and rate of rise, trial treatment modifications and TEAEs were not prespecified by dialysis modality.
Statistics
Given the modest size of the subgroup of INNO2VATE patients receiving peritoneal dialysis, we would not expect the 95% CIs on the MACE endpoint to fall within the noninferiority margin (upper bound of the 95% CI of 1.25) specified for both INNO2VATE trials. Nevertheless, we present the hazard ratio (HR) and 95% CI here, derived from a proportional hazards (Cox) regression model stratified by region and baseline hemoglobin concentration and adjusted for the same, along with NYHA class, age, sex, self-reported race, and history of diabetes mellitus and cardiovascular disease. For the primary efficacy endpoint, a two-sided 95% CI with a lower bound noninferiority margin of –0.75 g/dL (for vadadustat minus darbepoetin alfa) was calculated to evaluate the difference between treatment groups. For efficacy-related data, analyses between groups were based on analyses of covariance, with the baseline values of laboratory tests as covariates, and central laboratory baseline hemoglobin group, geographic region, NYHA congestive heart failure class and treatment group as fixed effects. Results for total iron-binding capacity (TIBC), iron, TSAT, hepcidin and ferritin were compared with respect to mean change from baseline in the primary efficacy periods. Categorical data were tabulated by frequency count and proportions.
RESULTS
Baseline characteristics
Of the 3923 patients randomized in the two INNO2VATE trials, 309 (8%) were receiving peritoneal dialysis at baseline (vadadustat, n = 152; darbepoetin alfa, n = 157). The median duration of follow-up was 1.59 years (Q1, Q3: 1.08 to 2.14) and 1.60 years (Q1, Q3: 1.11 to 2.39) in vadadustat- and darbepoetin alfa–treated patients, respectively. Approximately half (52%) of these patients were male, with a mean age of 55 years (Table 1). Irrespective of treatment group, the mean age of patients undergoing peritoneal dialysis was lower than that of patients receiving hemodialysis at baseline (Table 1). Patients receiving peritoneal dialysis were less likely than patients receiving hemodialysis to have cardiovascular disease at baseline. However, in the peritoneal dialysis patient group, those in the vadadustat group were less likely to have cardiovascular disease at baseline than those in the darbepoetin alfa group. Peritoneal dialysis patients had lower levels of ferritin at baseline than did patients receiving hemodialysis, irrespective of trial treatment group.
Table 1:
Selected demographic baseline characteristics of patients receiving peritoneal dialysis and hemodialysis at baseline in the INNO2VATE trials (randomized population).
| Peritoneal dialysis | Hemodialysis | |||
|---|---|---|---|---|
| Vadadustat | Darbepoetin alfa | Vadadustat | Darbepoetin alfa | |
| Characteristic | (n = 152) | (n = 157) | (n = 1803) | (n = 1807) |
| Mean age, years (SD) | 54.8 (13.4) | 54.6 (13.9) | 58 (14.0) | 58.4 (13.9) |
| Sex, male, n (%) | 76 (50.0) | 86 (54.8) | 1019 (56.5) | 1031 (57.1) |
| Racial or ethnic group, n (%) | ||||
| White | 87 (57.2) | 86 (54.8) | 1175 (65.2) | 1153 (63.8) |
| Asian | 35 (23.0) | 37 (23.6) | 52 (2.9) | 70 (3.9) |
| Black or African American | 21 (13.8) | 24 (15.3) | 449 (24.9) | 454 (25.1) |
| Othera | 9 (5.9) | 10 (6.4) | 127 (7.0) | 130 (7.2) |
| Hispanic ethnic group, n (%) | ||||
| Hispanic/Latino | 52 (34.2) | 56 (35.7) | 699 (38.8) | 684 (37.9) |
| Not Hispanic/Latino | 96 (63.2) | 99 (63.1) | 1050 (58.2) | 1058 (58.6) |
| Region of enrollment, n (%) | ||||
| USA | 73 (48.0) | 90 (57.3) | 1112 (61.7) | 1097 (60.7) |
| Europe | 14 (9.2) | 5 (3.2) | 266 (14.8) | 292 (16.2) |
| Non-USA/Europe | 65 (42.8) | 62 (39.5) | 425 (23.6) | 418 (23.1) |
| Mean time since dialysis started, years (SD) | 2.8 (3.2) | 2.5 (3.0) | 3.7 (4.0) | 3.7 (4.0) |
| Disease history, n (%) | ||||
| Diabetes mellitus | 75 (49.3) | 87 (55.4) | 1000 (55.5) | 1007 (55.7) |
| Cardiovascular disease | 46 (30.3) | 56 (35.7) | 889 (49.3) | 948 (52.5) |
| NYHA CHF class, n (%) | ||||
| Class 0 (no CHF) or I | 141 (92.8) | 147 (93.6) | 1564 (86.7) | 1561 (86.4) |
| II or III | 11 (7.2) | 10 (6.4) | 239 (13.3) | 246 (13.6) |
| Mean BMI, kg/m2 (SD) | 28.0 (6.2) | 28.7 (8.1) | 28.5 (7.2) | 28.4 (7.0) |
| Mean hemoglobin concentration, g/dL (SD) | 10.1 (0.9) | 10.0 (0.9) | 10.2 (0.9) | 10.1 (0.9) |
| Iron-related parameters | ||||
| Hepcidin (ng/mL) | ||||
| Mean (SD) | 195.4 (145.6) | 189.5 (153.1) 139.3 | 186.7 (138.6) | 183.8 (133.3) |
| Median | 163.4 | 139.3 | 151.6 | 152.8 |
| Q1, Q3 | 80.3, 265.1 | 68.6, 264.3 | 85.5, 236.4 | 86.4, 234.8 |
| Ferritin (ng/mL) | ||||
| Mean (SD) | 585.9 (450.4) | 629.2 (507.5) | 831.8 (559.4) | 826.7 (534.4) |
| Median | 437.5 | 434.0 | 735.3 | 729.0 |
| Q1, Q3 | 256.8, 774.5 | 261.0, 895.5 | 404.0, 1135.0 | 418.5, 1121.0 |
| TIBC (µg/dL) | ||||
| Mean (SD) | 224.1 (36.2) | 222.5 (38.2) | 210.7 (36.6) | 212.2 (36.9) |
| Median | 222.8 | 218.0 | 206.5 | 210.0 |
| Q1, Q3 | 199.5, 247.3 | 195.0, 250.5 | 186.0, 231.0 | 187.0, 233.0 |
| Serum iron (µg/dL) | ||||
| Mean (SD) |
81.6 (28.6) |
87.1 (35.3) |
78.6 (29.1) |
78.2 (28.0) |
| Median | 76.0 | 77.0 | 73.0 | 72.0 |
| Q1, Q3 | 61.3, 97.8 | 65.5, 101.0 | 58.0, 92.0 | 59.0, 91.0 |
| TSAT (%) | ||||
| Mean (SD) | 36.9 (12.9) | 39.0 (13.9) | 37.5 (13.3) | 37.2 (13.1) |
| Median | 34.0 | 36.0 | 34.5 | 34.5 |
| Q1, Q3 | 28.0, 43.0 | 29.5, 43.5 | 28.0, 43.5 | 28.0, 43.0 |
Includes American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, multiple or not reported.
BMI, body mass index; CHF, congestive heart failure; SD, standard deviation.
MACE in patients with chronic kidney disease and anemia undergoing peritoneal dialysis
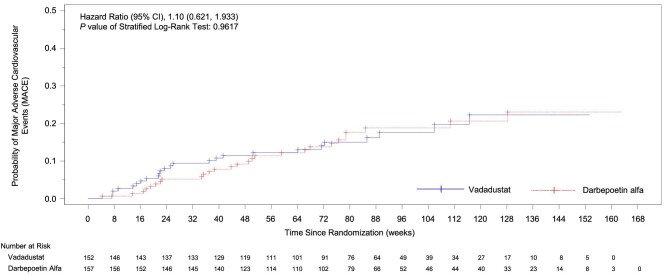
A first MACE occurred in 25 of 152 patients (16.4%) in the vadadustat group and 27 of 157 patients (17.2%) in the darbepoetin alfa group. The time to first MACE was similar in both vadadustat and darbepoetin alfa treatment groups (HR 1.10; 95% CI 0.62, 1.93; Fig. 1). MACE event rates per 100 person-years were 12.0 and 11.8 in the vadadustat and darbepoetin alfa treatment groups, respectively. Results for each component of MACE were similar in the two treatment groups: death from any cause occurred in 15 (9.9%) patients in the vadadustat group and 17 (10.8%) patients in the darbepoetin alfa group; nonfatal myocardial infarction occurred in 6 (3.9%) and 6 (3.8%) patients, respectively; and nonfatal stroke in 4 (2.6%) and 4 (2.5%) patients, respectively.
Figure 1:
Kaplan–Meier curve of time to first MACE in patients receiving peritoneal dialysis (safety population).
Expanded MACE in patients with CKD and anemia undergoing peritoneal dialysis
Expanded MACE occurred in 27 of 152 patients (17.8%) in the vadadustat group and 31 of 157 patients (19.7%) in the darbepoetin alfa group, with similar times to event (HR 1.09; 95% CI 0.63, 1.86). Expanded MACE event rates per 100 person-years were 14.0 and 14.9 in the vadadustat and darbepoetin alfa treatment groups, respectively. Although few hospitalizations for heart failure events occurred, they were less frequent: 2 (1.3%) in the vadadustat group and 6 (3.8%) in the darbepoetin alfa group.
Efficacy in patients with CKD and anemia undergoing peritoneal dialysis
Among patients who were undergoing peritoneal dialysis at baseline, vadadustat was noninferior to darbepoetin alfa with respect to the correction and maintenance of hemoglobin concentrations. Mean hemoglobin increased from 10.1 and 10.0 g/dL at baseline to 10.6 and 10.5 g/dL at Week 52 in the vadadustat and darbepoetin alfa treatment groups, respectively. The least-squares (LS) mean (±SE) change in hemoglobin concentration from baseline during Weeks 24 to 36 was 0.47 ± 0.16 g/dL in the vadadustat group and 0.57 ± 0.17 g/dL in the darbepoetin alfa group. The LS mean (±SE) change in hemoglobin concentration from baseline during Weeks 40–52 was 0.43 ± 0.19 g/dL in the vadadustat group and 0.62 ± 0.19 g/dL in the darbepoetin alfa group. The LS mean difference in change in hemoglobin concentration from baseline was –0.10 g/dL (95% CI –0.33, 0.12) during the primary efficacy period and –0.19 g/dL (95% CI –0.43, 0.05) during the secondary efficacy period (Fig. 2).
Figure 2:
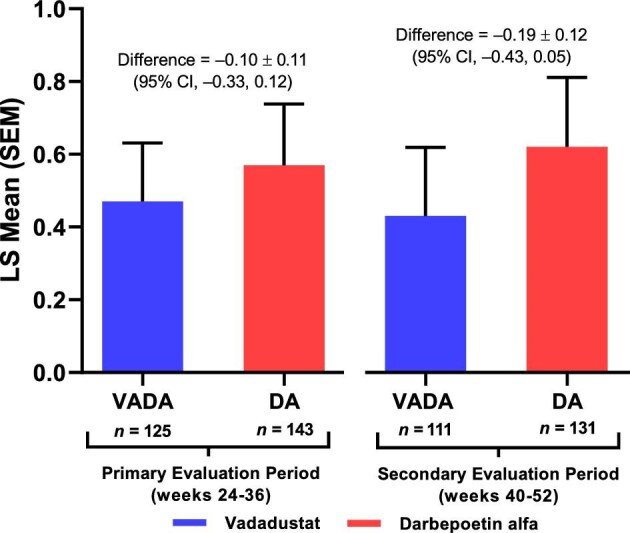
Mean change in hemoglobin from baseline during the primary and secondary evaluation periods in patients receiving peritoneal dialysis at baseline in the INNO2VATE trials (randomized population). DA, darbepoetin alfa; LS, least squares; VADA, vadadustat.
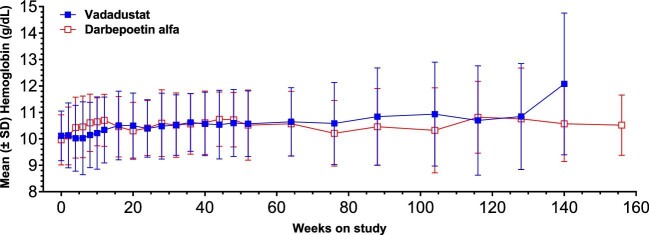
Mean hemoglobin concentration remained within geography-specific target ranges (10–11 g/dL in the USA; 10–12 g/dL in all other countries) throughout 156 weeks of treatment (Fig. 3). The proportion of patients who had mean hemoglobin concentrations within the geography-specific target ranges during the primary evaluation period (Weeks 24–36) was similar in the vadadustat (n = 84; 55.4%; 95% CI 51.97, 58.55) and darbepoetin alfa (n = 89; 56.4%; 95% CI 54.14, 58.60) groups [difference, % (95% CI) –0.0 (–0.1, 0.1); odds ratio (95% CI) 1.0 (0.6, 1.6)].
Figure 3:
Mean hemoglobin over time up to Week 156 in patients receiving peritoneal dialysis at baseline in the INNO2VATE trials (randomized population).
Effect on iron indices
There was an increase in TIBC in the vadadustat group from baseline to the primary evaluation period and a slight decrease in the darbepoetin alfa group for an LS mean difference of 34.0 μg/dL (range 26.1, 41.8). Serum iron increased slightly in the vadadustat group and decreased slightly in the darbepoetin alfa group, for an LS mean difference of 5.9 μg/dL (range–1.3, 13.1). TSAT decreased slightly in both groups, for an LS mean difference of −2.6% (range −5.7, 0.6) between treatment groups. There was a drop in hepcidin from baseline to the primary evaluation period in the vadadustat group, with −49.2 ng/mL versus −33.1 ng/mL in the darbepoetin alfa group, for an LS mean difference of −18.9 (range −45.0, 7.1). Similarly, there was a drop in ferritin in the vadadustat group (−21.2 ng/mL) but an increase in the darbepoetin alfa group (16.8 ng/mL), resulting in an LS mean difference of −36.5 ng/mL (range −113.3, 40.3) (Supplementary data, Table).
Red blood cell transfusions
Among patients undergoing peritoneal dialysis, the number receiving an RBC transfusion as rescue therapy during the primary evaluation period was low in both treatment groups [vadadustat group: 3 (2.4%); darbepoetin alfa group: 2 (1.4%)]. No patients received an RBC transfusion during the secondary evaluation period.
Hemoglobin-related safety endpoints
The proportion of patients with hemoglobin excursions >12.0–14.0 g/dL was similar in the vadadustat and darbepoetin alfa groups during the primary evaluation (Weeks 24–36) and secondary evaluation (Weeks 40–52) periods (Table 2). The proportion of patients with hemoglobin increases >1.0 within any 2-week interval and >2.0 g/dL within any 4-week interval or hemoglobin excursions <9 g/dL was also similar in the vadadustat and darbepoetin alfa groups during the primary and secondary evaluation periods.
Table 2:
Hemoglobin excursions and rate of rise during the primary and secondary evaluation periods in patients receiving peritoneal dialysis at baseline in the INNO2VATE trials (safety analysis set).
| Primary evaluation period (Weeks 24–36) | Secondary evaluation period (Weeks 40–52) | |||
|---|---|---|---|---|
| Vadadustat (n = 125) | Darbepoetin alfa (n = 143) | Vadadustat (n = 111) | Darbepoetin alfa (n = 131) | |
| Hb >12.0 g/dL, n (%) [95% CI] | 24 (19.2) [12.7, 27.2] | 29 (20.3) [14.0, 27.8] | 27 (24.3) [16.7, 33.4] | 28 (21.4) [14.7, 29.4] |
| Hb >13.0 g/dL, n (%) [95% CI] | 4 (3.2) [0.9, 8.0] | 7 (4.9) [2.0, 9.8] | 9 (8.1) [3.8, 14.8] | 8 (6.1) [2.7, 11.7] |
| Hb >14.0 g/dL, n (%) [95% CI] | 0 | 0 | 1 | 2 (1.5) [0.2, 5.4] |
| Hb increase >1.0 g/day within any 2-week interval, n (%) [95% CI] | 1 | 1 | 2 (1.8) [0.2, 6.4] | 2 (1.5) [0.2, 5.4] |
| Hb increase >2.0 g/dL within any 4-week interval, n (%) [95% CI] | 7 (5.6) [2.3, 11.2] | 10 (7.0) [3.4, 12.5] | 6 (5.4) [2.0, 11.4] | 3 (2.3) [0.5, 6.6] |
| Hb <9 g/dL, n (%) [95% CI] | 29 (23.2) [16.1, 31.6] | 28 (19.6) [13.4, 27.0] | 25 (22.5) [15.1, 31.4] | 28 (21.4) [14.7, 29.4] |
| Hb <8 g/dL, n (%) [95% CI] | 6 (4.8) [1.8, 10.2] | 10 (7.0) [3.4, 12.5] | 7 (6.3) [2.6, 12.6] | 7 (5.3) [2.2, 10.7] |
Hb increase of >2.0 g/dL within any 4-week period included increase >1 g/dL. Hb increase of >1 g/dL is not included in >2 g/dL increase.
Hb, hemoglobin.
Trial treatment modifications
During the first year of treatment, fewer mean dose modifications were required for patients receiving vadadustat than for those receiving darbepoetin alfa during the primary evaluation period from Weeks 24 to 36 (vadadustat: 1.3; darbepoetin alfa: 2.0) and secondary evaluation period from Weeks 40 to 52 (vadadustat: 1.2; darbepoetin alfa: 2.1) (Table 3). Also during the first year of treatment, a greater number of patients in the vadadustat group required dose interruptions than in the darbepoetin alfa group because of ESA rescue (Table 3). Of the 152 patients in the vadadustat group who started the trial on peritoneal dialysis, 19 (12.5%) switched to hemodialysis a median of 6.78 (Q1, Q3: 2.84 to 19.97) months into the trial. Of the 157 patients in the darbepoetin alfa group who started the trial on peritoneal dialysis, 23 (14.7%) switched to hemodialysis a median of 4.03 (Q1, Q3: 2.61 to 14.15) months into the trial.
Table 3:
Trial treatment modifications during the first year of treatment in patients receiving peritoneal dialysis at baseline in the INNO2VATE trials (randomized population).
| Weeks 2–8 | Weeks 10–20 | Primary evaluation period (Weeks 24–36) | Secondary evaluation period (Weeks 40–52) | |||||
|---|---|---|---|---|---|---|---|---|
| VADA (n = 152) | DA (n = 157) | VADA (n = 152) | DA (n = 157) | VADA (n = 152) | DA (n = 157) | VADA (n = 152) | DA (n = 157) | |
| Patients with a dose modification, n (%) | 152 (100.0) | 157 (100.0) | 140 (92.1) | 152 (96.8) | 123 (80.9) | 141 (89.8) | 101 (66.4) | 128 (81.5) |
| Number of dose changes | ||||||||
| Mean (SD) | 1.4 (0.89) | 1.5 (1.18) | 1.4 (1.18) | 1.9 (1.32) | 1.3 (1.2) | 2.0 (1.6) | 1.2 (1.3) | 2.1 (1.8) |
| Median | 1.0 | 1.0 | 1.0 | 2.0 | 1.0 | 2.0 | 1.0 | 2.0 |
| Min, max | 0, 4 | 0, 6 | 0, 4 | 0, 7 | 0, 5 | 0, 7 | 0, 5 | 0, 10 |
| Reasons for dose modifications, n (%) | ||||||||
| Increased or decreased based on Hb assessment | 102 (67.1) | 96 (61.1) | 65 (46.4) | 98 (64.5) | 46 (37.4) | 89 (63.1) | 34 (33.7) | 83 (64.8) |
| Decreased due to AE | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Interrupted due to elevated Hb | 23 (15.1) | 47 (29.9) | 28 (20.0) | 31 (20.4) | 21 (17.1) | 42 (29.8) | 22 (21.8) | 31 (24.2) |
| Interrupted due to ESA rescuea | 13 (8.6) | 0 | 18 (12.9) | 0 | 12 (9.8) | 1 (0.7) | 14 (13.9) | 1 (0.8) |
| Interrupted due to AE | 8 (5.3) | 2 (1.3) | 9 (6.4) | 5 (3.3) | 8 (6.5) | 4 (2.8) | 2 (2.0) | 2 (1.6) |
| Restarted | 17 (11.2) | 23 (14.6) | 51 (36.4) | 52 (34.2) | 39 (31.7) | 39 (27.7) | 32 (31.7) | 36 (28.1) |
| Other | 2 (1.3) | 3 (1.9) | 2 (1.4) | 12 (7.9) | 5 (4.1) | 4 (2.8) | 1 (1.0) | 7 (5.5) |
During the first year of treatment, hemoglobin concentrations were measured and dose adjustments were assessed every 2 weeks for weeks 0–12 and every 4 weeks for Weeks 12–52. Modifications to trial treatment dose were made to maintain hemoglobin concentrations within the geography-specific target range.
Starting at Week 6, patients in both treatment groups could receive ESAs as rescue therapy if they had worsening symptoms of anemia and a hemoglobin concentration of <9.5 g/dL. In the darbepoetin alfa group, an ESA was defined post hoc as a rescue medication if the dose was at least double that of the previous dose of darbepoetin alfa.
AE, adverse event; DA, darbepoetin alfa; Hb, hemoglobin; SD, standard deviation; VADA, vadadustat.
TEAEs
As might be expected in a peritoneal dialysis population, and unlike the results of the overall dialysis-dependent population, peritonitis was the most common TEAE and serious adverse event, although it was more common in darbepoetin alfa–treated patients. The incidence of overall TEAEs was lower in the vadadustat group (88%) than in the darbepoetin alfa group (96%) (Table 4). The four most common TEAEs in both groups were peritonitis (vadadustat: n = 27, 18%; darbepoetin alfa: n = 43, 27%), hypertension (vadadustat: n = 22, 15%; darbepoetin alfa: n = 30, 19%), nasopharyngitis (vadadustat: n = 21, 14%; darbepoetin alfa: n = 20, 13%) and pneumonia (vadadustat: n = 18, 12%; darbepoetin alfa: n = 17, 11%). Hyperkalemia was reported in 9 (6%) patients randomized to vadadustat and 22 (14%) patients randomized to darbepoetin alfa.
Table 4:
Overall summary of TEAEs, and SAEs and TEAEs occurring in ≥10% of patients receiving peritoneal dialysis at baseline in the INNO2VATE trials (safety analysis set).
| Vadadustat (N = 152; exposure = 242.0 PY) | Darbepoetin alfa (N = 157; exposure = 262.2 PY) | |||
|---|---|---|---|---|
| Category | n (%) | Events (events per 100 PY) | n (%) | Events (events per 100 PY) |
| Overall summary of TEAEs | ||||
| Any TEAEs | 134 (88.2) | 1069 (441.7) | 150 (95.5) | 1464 (558.4) |
| Any drug-related TEAEs | 15 (9.9) | 25 (10.3) | 8 (5.1) | 9 (3.4) |
| Any severe TEAEs | 52 (34.2) | 148 (61.2) | 75 (47.8) | 218 (83.1) |
| Any serious TEAEs | 80 (52.6) | 263 (108.7) | 115 (73.2) | 347 (132.3) |
| Any serious TEAE resulting in hospitalization | 77 (50.7) | 235 (97.1) | 113 (72.0) | 328 (125.1) |
| Any drug-related serious TEAEs | 2 (1.3) | 2 (0.8) | 2 (1.3) | 2 (0.8) |
| Any TEAEs leading to trial treatment discontinuation | 6 (3.9) | 6 (2.5) | 5 (3.2) | 5 (1.9) |
| Any drug-related TEAEs leading to trial treatment discontinuation | 4 (2.6) | 4 (1.7) | 2 (1.3) | 2 (0.8) |
| Any TEAEs leading to death | 16 (10.5) | 16 (6.6) | 19 (12.1) | 19 (7.2) |
| All deaths | 16 (10.5) | 16 (6.6) | 20 (12.7) | 20 (7.6) |
| TEAEs occurring in ≥10% of patients | ||||
| Infections | 84 (55.3) | 235 (97.1) | 109 (69.4) | 309 (117.8) |
| Peritonitis | 27 (17.8) | 34 (14.0) | 43 (27.4) | 53 (20.2) |
| Nasopharyngitis | 21 (13.8) | 28 (11.6) | 20 (12.7) | 29 (11.1) |
| Pneumonia | 18 (11.8) | 22 (9.1) | 17 (10.8) | 18 (6.9) |
| Gastrointestinal disorders | 61 (40.1) | 126 (52.1) | 74 (47.1) | 164 (62.5) |
| Metabolism and nutrition disorders | 56 (36.8) | 94 (38.8) | 79 (50.3) | 172 (65.6) |
| Hyperkalemia | 9 (5.9) | 10 (4.1) | 22 (14.0) | 26 (9.9) |
| Vascular disorders | 47 (30.9) | 70 (28.9) | 58 (36.9) | 94 (35.9) |
| Hypertension | 22 (14.5) | 25 (10.3) | 30 (19.1) | 33 (12.6) |
| General disorders and administration-site conditions | 58 (38.2) | 74 (30.6) | 44 (28.0) | 77 (29.4) |
| Asthenia | 16 (10.5) | 21 (8.7) | 7 (4.5) | 11 (4.2) |
| Nervous system disorders | 43 (28.3) | 63 (26.0) | 41 (26.1) | 64 (24.4) |
| Injury, poisoning, and procedural complications | 38 (25.0) | 77 (31.8) | 45 (28.7) | 88 (33.6) |
| Musculoskeletal and connective tissue disorders | 36 (23.7) | 48 (19.8) | 47 (29.9) | 75 (28.6) |
| Respiratory, thoracic, and mediastinal disorders | 32 (21.1) | 50 (20.7) | 42 (26.8) | 57 (21.7) |
| Cardiac disorders | 29 (19.1) | 60 (24.8) | 40 (25.5) | 76 (29.0) |
| Skin and subcutaneous tissue disorders | 21 (13.8) | 22 (9.1) | 31 (19.7) | 41 (15.6) |
| Blood and lymphatic system disorders | 15 (9.9) | 22 (9.1) | 25 (15.9) | 37 (14.1) |
| Psychiatric disorders | 14 (9.2) | 14 (5.8) | 23 (14.6) | 29 (11.1) |
| Eye disorders | 15 (9.9) | 18 (7.4) | 16 (10.2) | 24 (9.2) |
PY, patient-years; SAEs, serious adverse events.
The incidence of any serious TEAEs resulting in hospitalizations was lower in the vadadustat group than in the darbepoetin alfa group (vadadustat: n = 77, 50.7%; darbepoetin alfa: n = 113, 72%). The most common serious TEAEs (>5%) resulting in hospitalization were peritonitis (n = 17, 11.2%), pneumonia (n = 13, 8.6%) and sepsis (n = 8, 5.3%) in the vadadustat group, and peritonitis (n = 31, 19.7%), pneumonia (n = 9, 5.7%), sepsis (n = 9, 5.7%), acute myocardial infarction (n = 8, 5.1%) and hyperkalemia (n = 8, 5.1%) in the darbepoetin alfa group.
DISCUSSION
In the overall INNO2VATE trials of vadadustat versus darbepoetin alfa in patients with DD-CKD and anemia, vadadustat met prespecified noninferiority margins for safety and efficacy [7]. Here we present the results of a post hoc subgroup analysis in patients receiving peritoneal dialysis at baseline in the INNO2VATE trials. The efficacy of vadadustat in patients receiving peritoneal dialysis was similar to that observed in the INNO2VATE trials overall, with no new safety signals for vadadustat identified. Hemoglobin concentrations were within the target range for most patients in both the vadadustat and darbepoetin alfa groups.
Findings from this trial are in line with results from prior studies using HIF-PHIs for the treatment of anemia in patients with CKD undergoing peritoneal dialysis. Several trials in China and Japan have shown that oral HIF-PHIs were noninferior to ESAs for hemoglobin efficacy endpoints in patients receiving peritoneal dialysis [5, 12, 13]. In a recent international randomized phase 3 clinical trial, daprodustat was shown to be noninferior to ESA therapy with respect to both change in hemoglobin concentration from baseline and cardiovascular safety in patients with CKD undergoing hemodialysis and peritoneal dialysis [14]. The present report adds to the prior literature, extends previous observations in small national studies to a larger, global patient population, and further characterizes the safety and efficacy of HIF-PHIs as treatment for patients with CKD undergoing peritoneal dialysis.
The trial strengths include broad inclusion criteria (with respect to hemoglobin status, iron status, dialysis modality, ESA treatment status, RBC transfusion history), direct comparison with the current standard of care (the ESA darbepoetin alfa), and a relatively long follow-up period to observe potential side effects and MACE events. The limitations of this trial include potential bias in reporting of adverse events from open-label treatment. Moreover, dialysis type was not a stratification factor; thus, there was an imbalance in patients between regions that have differing hemoglobin treatment targets, which could have influenced the results. Additionally, the sample size and resulting number of events were relatively small compared with the overall INNO2VATE trial population; therefore, there was insufficient statistical power to evaluate differences in the MACE outcomes [7]. We provide point estimates and confidence limits for descriptive purposes.
In conclusion, safety and efficacy of vadadustat, shown to be noninferior to darbepoetin alfa in the INNO2VATE trials overall [7], were also similar in patients with DD-CKD who were receiving peritoneal dialysis. The potential advantages of an oral agent for the treatment of anemia are more numerous for patients receiving home dialysis (peritoneal or hemodialysis) than for those receiving in-center hemodialysis. Given its efficacy and acceptable safety profile compared with darbepoetin alfa, the present data highlight the suitability of vadadustat as a treatment alternative to ESAs in patients receiving home dialysis (peritoneal or home hemodialysis).
Supplementary Material
ACKNOWLEDGEMENTS
Medical writing assistance was provided by Syneos Health Medical Communications, LLC, and supported by Akebia Therapeutics, Inc. Data in the present manuscript were previously presented at Kidney Week 2021, the annual meeting for the American Society of Nephrology.
Contributor Information
Mark J Sarnak, Division of Nephrology, Tufts Medical Center, Tufts University School of Medicine, Boston, MA, USA.
Rajiv Agarwal, Indiana University School of Medicine, Indianapolis, IN, USA.
Neil Boudville, Medical School, University of Western Australia, Perth, Australia.
Pradip C P Chowdhury, Peritoneal Dialysis Center of America, Montebello, CA, USA.
Kai-Uwe Eckardt, Department of Nephrology and Medical Intensive Care, Charité – Universitätsmedizin Berlin, Berlin, Germany.
Carlos R Gonzalez, PI Health, Montebello, CA, USA.
Laura A Kooienga, Colorado Kidney Care, Denver, CO, USA.
Mark J Koury, Division of Hematology/Oncology, Vanderbilt University Medical Center, Nashville, TN, USA.
Kwabena A Ntoso, Pennsylvania Nephrology Associates, Philadelphia, PA, USA.
Wenli Luo, Akebia Therapeutics, Inc., Cambridge, MA, USA.
Patrick S Parfrey, Memorial University, St John's, NL, Canada.
Dennis L Vargo, Akebia Therapeutics, Inc., Cambridge, MA, USA.
Wolfgang C Winkelmayer, Baylor College of Medicine, Houston, TX, USA.
Zhiqun Zhang, Akebia Therapeutics, Inc., Cambridge, MA, USA.
Glenn M Chertow, Stanford University School of Medicine, Palo Alto, CA, USA.
FUNDING
These studies were funded by Akebia Therapeutics, Inc.
AUTHORS’ CONTRIBUTIONS
The INNO2VATE DD-CKD trials were conducted and funded by Akebia Therapeutics, Inc. The sponsor participated in the study design, data collection, analysis and interpretation, and approval of the manuscript. Analyses were conducted by the sponsor and all authors had access to and participated in the interpretation of the data. M.J.S. and G.M.C. wrote the first draft of the manuscript. All authors were involved in drafting and critical revision of the manuscript. All authors had access to study results, each contributed important intellectual content during manuscript drafting or revision, and all accept accountability for the overall work. Each author ensures that questions pertaining to the accuracy or integrity of any portion of the work will be appropriately investigated and resolved.
DATA AVAILABILITY STATEMENT
Proposals for access to original data should be sent to medicalinfo@akebia.com. De-identified patient-level data will be available 12 months after US and EU approval to qualified researchers with an appropriate research proposal. The research proposal is subject to review by an independent review board with final approval by Akebia Therapeutics, Inc.
CONFLICT OF INTEREST STATEMENT
M.J.S. has received steering committee fees from Akebia Therapeutics, Inc., which were paid to Tufts Medical Center. R.A. reports personal fees and nonfinancial support from Bayer Healthcare Pharmaceuticals Inc., Akebia Therapeutics, Inc., Boehringer Ingelheim, Eli Lilly and Vifor Pharma; has received personal fees from Lexicon and Reata; is a member of data safety monitoring committees for Vertex and Chinook; is a member of steering committees for randomized trials for Akebia Therapeutics, Inc., Bayer and Relypsa; and is a member of adjudication committees for Bayer. He has served as associate editor of the American Journal of Nephrology and Nephrology Dialysis Transplantation, has been an author for UpToDate, and has received research grants from the NIH and the US Veterans Administration. N.B. reports receiving grants from Amgen plus consulting or advisory board fees from Otsuka, AstraZeneca, Vifor, GlaxoSmithKline, FMC and Baxter. P.C.P.C. has no disclosures to report. K.-U.E. has received grant support from Amgen and Fresenius; lecture fees from Astellas Pharma; grant support and lecture fees from AstraZeneca, Bayer and Genzyme; consulting fees from Boehringer Ingelheim; advisory board fees from Retrophin; and grant support, advisory board fees and lecture fees from Vifor Pharma. C.R.G. and L.A.K. have no disclosures to report. M.J.K. reports personal fees from Akebia Therapeutics, Inc., as a consultant and member of steering committees for phase 3 trials of vadadustat; from TG Therapeutics, Inc., as a member of a data safety and monitoring committee; and from GlaxoSmithKline as a consultant and member of its educators’ network. He is also a contributor to BMJ Best Practices. K.A.N. has no disclosures to report. W.L. is an employee of Akebia Therapeutics, Inc. P.S.P. has received personal fees from Akebia Therapeutics, Inc., during the conduct of the trial, and advisory board fees from Vifor Pharma. D.L.V. is an employee of Akebia Therapeutics, Inc. W.C.W. has received steering committee fees from Akebia Therapeutics, Inc.; advisory fees from AstraZeneca, Bayer, Janssen, Merck, Otsuka, Reata and Zydus; steering committee and data safety monitoring fees from Bayer and Merck; and an honorarium for an invited lecture by Pharmacosmos. He receives stipends for being an associate editor for JAMA. He has received research grants from several institutes within the NIH. Z.Z. is an employee of Akebia Therapeutics, Inc. G.M.C. serves on the board of directors for Satellite Healthcare. He has served as a steering committee cochair, member, or advisor with Akebia Therapeutics, Inc., Ardelyx, AstraZeneca, CloudCath, CSL Behring, Durect, Gilead, Miromatrix, Outset, Reata, Renibus, Sanifit, Unicycive and Vertex. He has served as chair or member of DSMB/DMCs with Bayer, Gilead, Mineralys, Palladio and ReCor. He has received research grants from NIAID and NIDDK.
REFERENCES
- 1. Global Burden of Disease Chronic Kidney Disease Collaboration . Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study. Lancet 2020;395:709–33. 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stauffer ME, Fan T.. Prevalence of anemia in chronic kidney disease in the United States. PLoS One 2014;9:e84943. 10.1371/journal.pone.0084943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rastogi A, Lerma EV.. Anemia management for home dialysis including the new US public policy initiative. Kidney Int Suppl (2011) 2021;11:59–69. 10.1016/j.kisu.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kidney Disease: Improving Global Outcomes (KDIGO) . KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl 2012;2:292–8. [Google Scholar]
- 5. Hou YP, Mao XY, Wang C.et al. Roxadustat treatment for anemia in peritoneal dialysis patients: a randomized controlled trial. J Formos Med Assoc 2022;121:529–38. 10.1016/j.jfma.2021.06.004. [DOI] [PubMed] [Google Scholar]
- 6. Chertow GM, Pergola PE, Farag YMK.et al. Vadadustat in patients with anemia and non-dialysis-dependent CKD. N Engl J Med 2021;384:1589–600. 10.1056/NEJMoa2035938. [DOI] [PubMed] [Google Scholar]
- 7. Eckardt KU, Agarwal R, Aswad A.et al. Safety and efficacy of vadadustat for anemia in patients undergoing dialysis. N Engl J Med 2021;384:1601–12. 10.1056/NEJMoa2025956. [DOI] [PubMed] [Google Scholar]
- 8. Markham A. Vadadustat: first approval. Drugs 2020;80:1365–71. 10.1007/s40265-020-01383-z. [DOI] [PubMed] [Google Scholar]
- 9. Nangaku M, Kondo K, Kokado Y.et al. Phase 3 randomized study comparing vadadustat with darbepoetin alfa for anemia in Japanese patients with nondialysis-dependent CKD. J Am Soc Nephrol 2021;32:1779–90. 10.1681/ASN.2020091311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nangaku M, Kondo K, Ueta K.et al. Efficacy and safety of vadadustat compared with darbepoetin alfa in Japanese anemic patients on hemodialysis: a phase 3, multicenter, randomized, double-blind study. Nephrol Dial Transplant 2021;36:1731–41. 10.1093/ndt/gfab055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eckardt KU, Agarwal R, Farag YM.et al. Global phase 3 programme of vadadustat for treatment of anaemia of chronic kidney disease: rationale, study design and baseline characteristics of dialysis-dependent patients in the INNO2VATE trials. Nephrol Dial Transplant 2021;36:2039–48. 10.1093/ndt/gfaa204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nangaku M, Kondo K, Takabe S.et al. Vadadustat for anemia in chronic kidney disease patients on peritoneal dialysis: a phase 3 open-label study in Japan. Ther Apher Dial 2021;25:642–53. 10.1111/1744-9987.13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kanai H, Nangaku M, Nagai R.et al. Efficacy and safety of daprodustat in Japanese peritoneal dialysis patients. Ther Apher Dial 2021;25:979–87. 10.1111/1744-9987.13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Singh AK, Carroll K, Perkovic V.et al. ASCEND-D Study Group. Daprodustat for the treatment of anemia in patients undergoing dialysis. N Engl J Med 2021;385:2325–35. 10.1056/NEJMoa2113379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Proposals for access to original data should be sent to medicalinfo@akebia.com. De-identified patient-level data will be available 12 months after US and EU approval to qualified researchers with an appropriate research proposal. The research proposal is subject to review by an independent review board with final approval by Akebia Therapeutics, Inc.