ABSTRACT
Background
Endothelin A receptor antagonists (ETARA) slow chronic kidney disease (CKD) progression but their use is limited due to fluid retention and associated clinical risks. Sodium–glucose co-transporter 2 inhibitors (SGLT2i) cause osmotic diuresis and improve clinical outcomes in CKD and heart failure. We hypothesized that co-administration of the SGLT2i dapagliflozin with the ETARA zibotentan would mitigate the fluid retention risk using hematocrit (Hct) and bodyweight as proxies for fluid retention.
Methods
Experiments were performed in 4% salt fed WKY rats. First, we determined the effect of zibotentan (30, 100 or 300 mg/kg/day) on Hct and bodyweight. Second, we assessed the effect of zibotentan (30 or 100 mg/kg/day) alone or in combination with dapagliflozin (3 mg/kg/day) on Hct and bodyweight.
Results
Hct at Day 7 was lower in zibotentan versus vehicle groups [zibotentan 30 mg/kg/day, 43% (standard error 1); 100 mg/kg/day, 42% (1); and 300 mg/kg/day, 42% (1); vs vehicle, 46% (1); P < .05], while bodyweight was numerically higher in all zibotentan groups compared with vehicle. Combining zibotentan with dapagliflozin for 7 days prevented the change in Hct [zibotentan 100 mg/kg/day and dapagliflozin, 45% (1); vs vehicle 46% (1); P = .44] and prevented the zibotentan-driven increase in bodyweight (zibotentan 100 mg/kg/day + dapagliflozin 3 mg/kg/day = –3.65 g baseline corrected bodyweight change; P = .15).
Conclusions
Combining ETARA with SGLT2i prevents ETARA-induced fluid retention, supporting clinical studies to assess the efficacy and safety of combining zibotentan and dapagliflozin in individuals with CKD.
Keywords: bodyweight, dapagliflozin, fluid retention, hematocrit, zibotentan
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What is already known about this subject?
The development of endothelin receptor A antagonists (ETARA) in chronic kidney disease (CKD) has been limited by fluid retention and an associated increased risk for hospitalization for heart failure. Sodium–glucose co-transporter 2 inhibitors (SGLT2i) cause modest osmotic diuresis with proven outcome benefits in CKD and heart failure.
What this study adds?
Combining an SGLT2i with an ETARA may provide both superior efficacy and reduced risk of fluid retention. The present data indicate that the SGLT2i dapagliflozin mitigates the fluid-retaining effects of the ETAR antagonist zibotentan in a rodent model.
What impact this may have on practice or policy?
Dapagliflozin and zibotentan are currently under clinical investigation as a combination treatment for CKD (ZENITH-CKD; NCT04724837). The present preclinical data support further clinical investigation of combination treatment with zibotentan and dapagliflozin.
INTRODUCTION
In 2017, 698 million people were diagnosed with chronic kidney disease and, in 1.2 million people, chronic kidney disease (CKD) was attributed as the primary cause of death [1]. Endothelin 1 (ET-1), first described in the late 1980s [2], has been shown to modulate renal physiology and pathophysiology. ET-1 regulates kidney vascular tone and electrolyte and fluid homeostasis under normal physiological conditions [3] and is implicated in proinflammatory and fibrotic pathophysiological processes which are hallmarks of kidney disease [4–6]. ET-1 levels increase in CKD and are associated with increasing urinary albumin-to-creatinine ratio (UACR) levels and decreasing levels of glomerular filtration rate [3, 7, 8]. Accumulating evidence suggests that the pathological effects of ET-1, including proteinuria, vasoconstriction and inflammation, are driven primarily via its action on the endothelin A receptor (ETAR) [4, 5, 9].
Preclinical studies have demonstrated beneficial effects of ETA receptor antagonists (ETARA) in diabetic models of kidney disease by ameliorating established glomerular damage, inflammation and UACR [10–13]. Clinical studies have demonstrated that ETARA reduce albuminuria, slow kidney function loss over time and reduce the risk of kidney outcomes. However, clinical use of ETARA administration is limited due to sodium and fluid retention, leading to bodyweight increase, edema and potentially congestive heart failure, particularly in patients with reduced glomerular filtration rate in whom volume homeostasis is often impaired [14].
Sodium–glucose co-transporter 2 inhibitors (SGLT2i) block sodium and glucose reabsorption in the renal proximal tubule leading to glycosuria and osmotic diuresis [15–18]. These effects lead to reductions in HbA1c, total body water, weight and blood pressure. In large clinical trials, SGLT2i, including dapagliflozin, reduced the risk of heart failure and kidney disease progression [18, 19]. The modest osmotic diuretic effects of SGLT2i make them an attractive option to combine with ETARAs in order to mitigate the sodium and fluid retention induced by these agents. An exploratory post hoc analysis of a large clinical trial (Study Of Diabetic Nephropathy With Atrasentan (SONAR)) demonstrated that patients who initiated both the ETARA atrasentan and an SGLT2i experienced a greater reduction in albuminuria and a reduction in bodyweight whilst body weight increased in participants who initiated ETARA alone [20].
The aim of the current study was to address the hypothesis that combined initiation of SGLT2i with dapagliflozin and a selective ETARA zibotentan facilitates optimal fluid homeostasis. Consequently, we designed preclinical in vivo studies in Wistar rats fed a 4% salt diet. In the first experiment, increasing doses of zibotentan (30, 100 and 300 mg/kg) were administered once daily and the effects on hematocrit (Hct) and bodyweight were assessed as surrogate measures of fluid retention. In a subsequent study, zibotentan (30 and 100 mg/kg) was administered with and without dapagliflozin (3.0 mg/kg) to determine whether co-administration of dapagliflozin altered zibotentan-mediated effects on Hct and body weight.
MATERIALS AND METHODS
Animals
Experimental procedures were approved by the Local Ethics Review Committee on Animal Experiments (Göteborg region). Male, 8- to 9-week-old Wistar rats (Charles River, Germany) were housed in pairs in plastic cages, Makrolon type 3, in a temperature (20–22°C) and humidity (40%–60%) controlled facility with a 12 h light–dark cycle (lights on 06:00 am), and had free access to food (4% salt in chow diet #D20042901, Research Diets, USA) and tap water. The animal bedding consisted of aspen chips, gnaw blocks, shredded paper and a home for enrichment.
Experimental procedures
The study was divided into two experiments. In Experiment 1, the effect of zibotentan on Hct was determined when zibotentan was administered peroral (p.o.) daily for 14 days. Prior to administration of study drugs, the animals were randomized based on body weight into the following groups: (i) vehicle; (ii) Zibo 30 mg/kg; (iii) Zibo 100 mg/kg; and (iv) Zibo 300 mg/kg. Animals were weighed daily prior to dosing throughout the study. Blood samples (20 µL) for bioanalysis were taken at 4 and 24 h after the first dose via the tail vein from conscious animals and 24 h after the last dose via retroorbital route from anesthetized animals. Blood samples were collected via tail vein (100 µL) for Hct measurements on Days 7 and 14, 1–2 h after dosing (between 9:30 am and 12:00 pm). Twenty-four-hour urine was collected for the measurement of urinary glucose, urine volume and electrolytes between doses 9 and 10. Animals were terminated 24 h after the last dose.
In Experiment 2, the effect of zibotentan and dapagliflozin (alone or in combination) on Hct and body weight was determined. Seventy-two animals were randomized based on body weight and Hct prior to administration of study drugs into the following groups: (i) vehicle; (ii) zibotentan (Zibo) 30 mg/kg; (iii) Zibo 100 mg/kg; (iv) dapagliflozin (Dapa) 3 mg/kg; (v) Zibo 30 mg/kg + Dapa 3 mg/kg; and (vi) Zibo 100 mg/kg + Dapa 3 mg/kg. For practical reasons, the study was conducted in two consecutive slots with 36 animals (6/group) in each slot. Animals were weighed daily prior to dosing and throughout the study. Compounds were administered p.o. daily for 7 days. Blood samples for bioanalysis were collected at 4 and 24 h after the first dose and 24 h after the final dose for Hct analysis on Day 7 as described for Experiment 1. Twenty-four-hour urine was collected between the third and fourth dose. Twenty-four-hour food and water intake were measured between Day 2 and 3 and between Day 6 and 7. With two animals per cage, food and water intake per animal in a cage was assessed by dividing by two.
Experimenters were blinded to the treatment allocation of animals during termination, blood sampling and sample analyses.
On the day of termination, animals were anesthetized using 5% isoflurane, and blood was collected via the retroorbital route (in heparin-coated microvette, Sarsted) and centrifuged at 3500 r.p.m. at 4°C for 10 min. Plasma was collected and stored at –80°C degrees prior to analysis. Animals were then euthanized by removing the heart.
Compounds and dose selection
In Experiment 1, zibotentan (AZ11922817) was dosed orally at 30 mg/kg, 100 mg/kg or 300 mg/kg. Drugs were dissolved in saline [30% (w/w) triethylene glycol (TEG) 2% (w/w) EtOH 0.5% (w/w) hydroxypropyl methylcellulose (HPMC) 10 000 c.p.s. 0.1% (w/w) Tween 80 67.4% purified water]. The volume of injection was 10 mL/kg.
In Experiment 2, zibotentan was dosed orally at 30 and 100 mg/kg with or without 3 mg/kg dapagliflozin (AZ13219875). Drugs were dissolved in saline [30% (w/w) TEG 2% (w/w) EtOH 0.5% (w/w) HPMC 10 000 c.p.s. 0.1% (w/w) Tween 80 67.4% purified water]. The volume of injection was 10 mL/kg.
Dapagliflozin dose selection was performed according to human dose-equivalent estimation of 3 mg/kg in rat scaling to a 10 mg dose in human. Zibotentan dose selection was estimated from prior work with the ETARA sitaxentan where 30, 100 and 300 mg/kg reduced Hct in rats [21]. The corresponding doses of zibotentan, taking pharmacokinetics, plasma protein binding and ETA affinity differences into account were 30, 100 and 300 mg/kg with a free exposure in rat scaling respectively to a 250, 750 and 2500 mg dose in human.
Plasma and urine biochemistry
Plasma and urine albumin, creatinine, glucose, urea, potassium and sodium levels were analyzed using an ABX Pentra 400 instrument (Horiba Medical, Irvine, CA, USA) according to the manufacturer's protocol. Osmolality was calculated according to the formula: (2 × Na + K + urea + glucose)/creatinine.
Hematocrit and hemoglobin analysis
For Hct and hemoglobin (Hb), 100 µL of blood from tail vein was collected and measured using CG8 cartridges and analyzed with an iSTAT instrument (Abbott Point of Care Inc, Abbot Park, IL, USA). Estimated plasma volume was calculated according to the formula: 100 – Hct/Hb.
Analysis of blood creatinine by LC-MS
Ten µl of water was briefly mixed with 10 µL of blood or calibration standards in a 96-well plate. Calibration samples contained creatinine in 90% acetonitrile at concentrations ranging from 0.35 to 70 µM. One hundred microliters of internal standard solution (1 µM D3-creatinine in 90% acetonitrile and 10% water) was added to all samples and vortexed for 5 min. Separation was achieved in HILIC mode on a Waters BEH-Amide 5 cm × 2.1 mm column using 95% acetonitrile and 5% buffer (5 mM ammonium formate + 0.0625% formic acid) as A-phase and buffer (10 mM ammonium formate + 0.125% formic acid) as B-phase. Mass spectrometric detection was performed on a Waters Xevo TQ triple quadropol instrument.
Statistical analysis
The data for each feature measured was checked for a normal distribution and where appropriate a log transformation was applied to data with a non-normal distribution using R. Since the data were grouped by one factor zibotentan doses or combined therapy, a one-way ANOVA was used to analyze traits measured at a single time point (GraphPad version 8 or R statistics package emmeans) and a linear mixed effects model (nlme package) in R was used to analyze multiple measures data for bodyweight and provided estimated marginal means (EMM) for the difference in grams corrected for baseline bodyweight between an experimental group and vehicle. The differences between groups were tested using either a Dunnett test (emmeans package in R or GraphPad v8) when comparing treated groups to the vehicle group or a Tukey comparison (emmeans package in R or Graphpad v8) for specific pairwise comparisons of treated groups. Analysis of estimated plasma volume and calculated osmolality were performed using Brown–Forsythe and Welch ANOVA for multiple comparisons (GraphPad v9). For specific pairwise comparisons of treated groups. All model assumptions were checked using the model diagnostic plots in R statistics. Results are expressed as the mean ± standard error of the mean or mean ± standard deviation where described. Significance was set at P < .05.
RESULTS
Zibotentan causes fluid retention
On Day 7 of dosing, zibotentan (30, 100 and 300 mg/kg/day) significantly reduced Hct compared with vehicle treatment (Fig. 1). These effects were sustained at Day 14 in the two highest zibotentan doses (100 and 300 mg/kg/day) (Fig. 1). Body weight was numerically and consistently higher throughout the study in all three zibotentan groups (Fig. 2), with least square mean differences compared with vehicle of 5.4 g [standard error (SE) 2.6], 5.5 (2.6) and 2.2 (2.7) g for the zibotentan 30, 100 and 300 mg/kg/day dose groups, respectively. None of these differences reached statistical significance (all P-values >.13).
Figure 1:
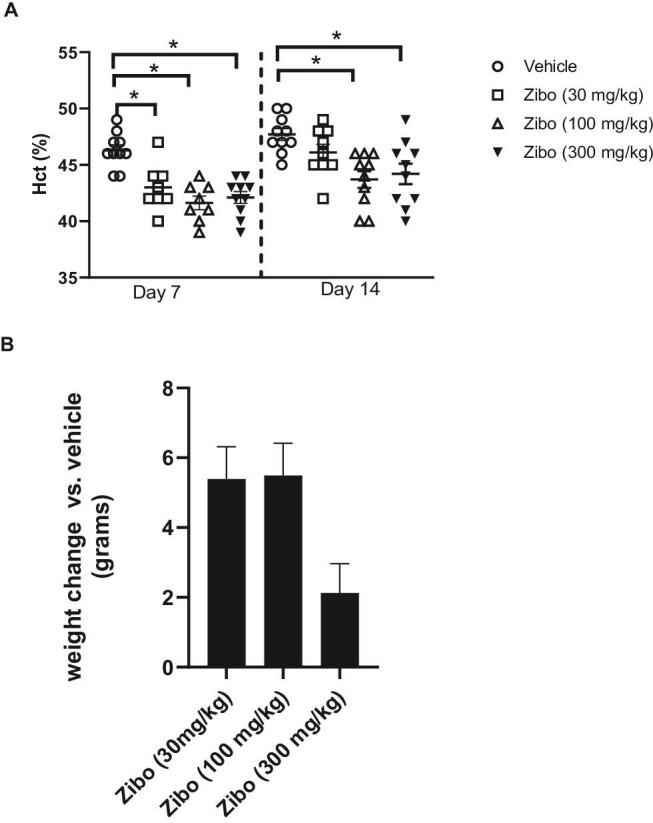
Zibotentan causes fluid retention. (A) Effect of 30, 100 and 300 mg/kg/day of zibotentan on Hct on Day 7 and Day 14 of administration. (B) Body weight presented as the difference in grams corrected for baseline body weight between groups. N = 8–10/group, *P < .05 vs vehicle. Data presented as mean ± SEM.
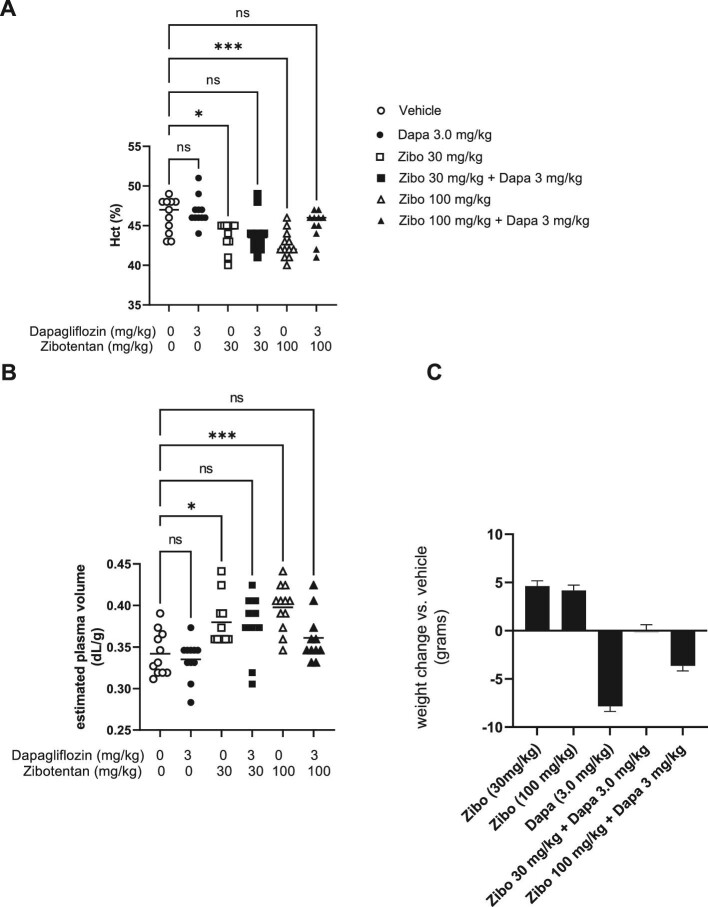
Figure 2:
Co-administration of zibotentan with dapagliflozin reverses zibotentan induced fluid retention. (A) Effect of zibotentan (30 and 100 mg/kg/day) with or without 3 mg/kg/day dapagliflozin and vehicle on Hct on Day 7 of dosing. (B) Effect of zibotentan (30 and 100 mg/kg/day) with or without 3 mg/kg/day dapagliflozin and vehicle on estimated plasma volumes on Day 7 of dosing. (C) Body weight presented as the difference in grams corrected for baseline body weight between groups. N = 8–12/group, *P < .05, *P < .01, *P < .001 vs vehicle. Data presented as mean ± SEM.
Dapagliflozin mitigates zibotentan-induced fluid retention
Co-administration of dapagliflozin (3 mg/kg/day) and zibotentan (30 or 100 mg/kg/day) was investigated in a second experiment. At Day 7, both zibotentan doses caused a significant reduction in Hct compared with the vehicle group (Fig. 2A). The decrease in Hct observed with zibotentan mono-treatment was ameliorated in the zibotentan–dapagliflozin combination groups. Plasma Hb levels were reduced by zibotentan (30 and 100 mg/kg) and this effect was mitigated by Day 7 of co-administration with dapagliflozin (Table 1). Consistently, estimated plasma volume was significantly increased by zibotentan (30 and 100 mg/kg) and mitigated by co-administration with dapagliflozin (Fig. 2B).
Table 1:
Effect of zibotentan, dapagliflozin and the combination on baseline corrected Hb.
| Zibo | Zibo | Dapa | Zibo (30 mg/kg) + | Zibo (100 mg/kg) + | ||
|---|---|---|---|---|---|---|
| Parameters | Vehicle | (30 mg/kg) | (100 mg/kg) | (3 mg/kg) | Dapa (3 mg/kg) | Dapa (3 mg/kg) |
| Basal Hb (g/L) | 159.4 ± 2.44 | 160.2 ± 1.62 | 158.5 ± 2.00 | 157.9 ± 2.34 | 159.3 ± 2.17 | 158.7 ± 1.04 |
| Day 3 Hb (g/L) | 159.1 ± 2.20 | 144.4 ± 2.32* | 147.5 ± 1.96* | 164.4 ± 1.42 | 151.3 ± 1.62* | 152.4 ± 1.02 |
| Day 7 Hb (g/L) | 157.5 ± 1.91 | 148.6 ± 1.86* | 144.8 ± 1.69* | 159.1 ± 1.88 | 149.7 ± 2.80 | 152.9 ± 1.99 |
Effect of 30 mg/kg and 100 mg/kg zibotentan with or without 3 mg/kg dapagliflozin, 3 mg/kg dapagliflozin alone and vehicle on Hb. N = 8–12/group, *P < .05 vs vehicle. Data analysis used one-way ANOVA, and Tukey's test for multiple comparisons. Data presented as mean ± SEM.
Twenty-four-hour urine volume and urea excretion were unchanged in zibotentan (30 and 100 mg/kg) mono-treatment groups compared with vehicle (Supplementary data, Table S1) between doses 3 and 4. Both 24-h urine volume and urea excretion significantly increased in all dapagliflozin-treated groups with or without co-administration of zibotentan. Urinary potassium excretion was unchanged across all groups. Urinary sodium excretion was not significantly different between groups but tended to be reduced by both zibotentan doses and increased by dapagliflozin with or without co-administration of zibotentan (Supplementary data,Table S1).
Both zibotentan 30 mg/kg and 100 mg/kg increased body weight from baseline compared with vehicle by 4.6 g (SE 1.8; P = .05) and 4.2 g (SE 1.8; P = .09) (Fig. 2C). Body weight significantly decreased in the dapagliflozin 3 mg/kg group compared with vehicle by 7.8 (SE 1.8) g (P < .001). Adding dapagliflozin 3 mg/kg to zibotentan 30 mg/kg or 100 mg/kg prevented the body weight increase observed in the zibotentan mono-treatment groups (Fig. 2C) with differences in body weight compared with vehicle of 0.1 (SE 1.8) and –3.7 (SE 1.8) g.
Zibotentan does not alter dapagliflozin-driven glucosuria, food intake or water intake
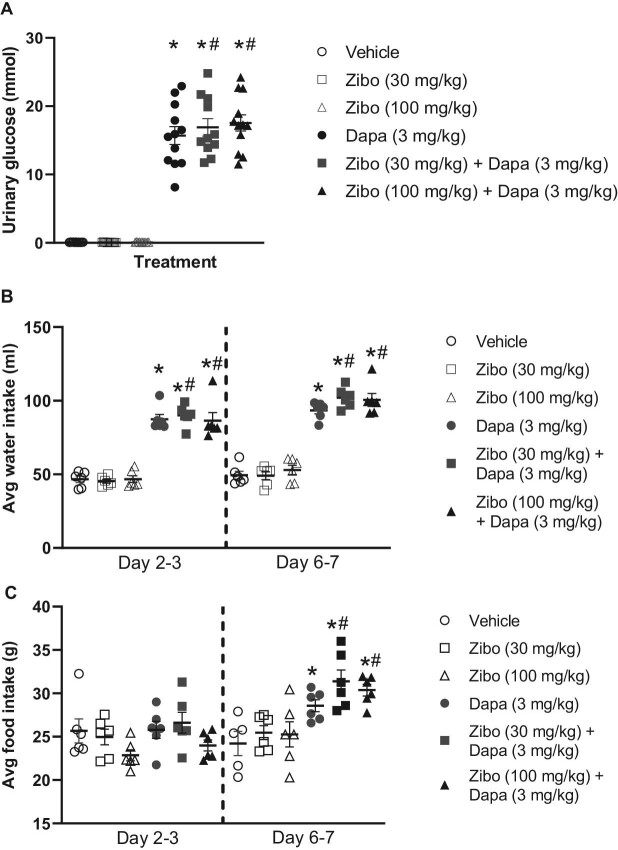
Dapagliflozin significantly increased 24-h urine glucose excretion vs vehicle treatment. There was no effect of zibotentan on urinary glucose (Fig. 3A). Furthermore, co-administration of dapagliflozin with zibotentan did not change dapagliflozin-mediated glycosuria. Dapagliflozin increased 24-h food intake and water intake compared with vehicle and zibotentan mono-treated groups (Fig. 3B and C). Dapagliflozin and zibotentan significantly increased water intake and food intake compared with vehicle, and to a similar level observed in animals administered dapagliflozin alone.
Figure 3:
Effect of zibotentan, dapagliflozin and the combination on 24-h urinary glucose excretion, food intake and water intake. (A) Effect of 30 mg/kg/day and 100 mg/kg/day zibotentan with or without 3 mg/kg/day dapagliflozin or vehicle on 24-h urinary glucose excretion. (B and C) Twenty-hour water intake and 24-h food intake respectively. For (B) and (C), each point represents mean food intake and water intake per animal with two animals/cage. N = 10–12/group, *P < .05 vs vehicle, #P < .05 vs zibotentan mono-treatment groups. Data presented as mean ± SEM.
DISCUSSION
ETARAs are in development for the treatment of CKD, however concern exists about the development of these agents in these patients due to the risk of fluid retention. Strategies that mitigate fluid retention may enable the use of ETARAs in the future. Because SGLT2i exert modest diuretic effects, this study tested the hypothesis that use of the EtARA zibotentan with the SGLT2i dapagliflozin mitigates ETARA-induced fluid retention. We demonstrated that zibotentan increases fluid retention in a dose-dependent fashion. Combining zibotentan with dapagliflozin, particularly zibotentan 30 mg/kg + dapagliflozin 3.0 mg/kg in the present study, completely prevented the change in Hct and body weight driven by zibotentan mono-treatment mediated fluid retention, suggesting that combination treatment may be an effective strategy to reduce fluid retention in the clinic.
This study measured changes in Hct and body weight as common and well established surrogates for fluid retention [22]. ETAR antagonism has been shown to reduce Hct and increase body weight acutely, an effect that is reversible upon treatment cessation and consistent with a hemodilution-driven effect as opposed to a loss of red blood cell mass or an increase in fat mass, respectively [21]. In patients with type 2 diabetes and CKD, changes in Hct and body weight during 6 weeks of treatment with the ETARA atrasentan were associated with a higher risk of edema and heart failure hospitalization supporting their use as early proxies of fluid retention during ETARA treatment. Although the lowest tested zibotentan dose (30 mg/kg) resulted in a decrease in Hct after 7 days in our study, the effect dissipated after 14 days. The lack of a sustained effect could be due to limitations in the sensitivity of Hct to detect subtle changes in fluid retention [23], or due to limitations of the employed experimental 4% salt induction model given compensatory physiological mechanisms of the kidney to achieve fluid homeostasis in the longer term [24]. By driving fluid retention through salt-loading there was a robust window to measure decreases in Hct and Hb pharmacologically, but salt-loading also reduced sensitivity to further increases in Hct or Hb levels. Thus, a clinically observed SGLT2i mechanism was not detectible in the salt-fed rats [25].
Body weight gain, another surrogate measure of fluid retention [26], increased during zibotentan treatment, although not as clearly as Hct in the present study. The highest dose of zibotentan, 300 mg/kg, resulted in a lower overall weight gain compared with other zibotentan doses which suggests a maximal pharmacological response was already achieved in the lower doses. Furthermore, since the estimated human-dose equivalent of rat zibotentan, 300 mg/kg, is 2500 mg or approximately 12 000% higher than the highest dose of zibotentan under clinical investigation, we conclude that further investigation of zibotantan 300 mg/kg is unlikely to be of therapeutic importance. Dapagliflozin acutely reduces body weight which is thought to reflect its diuretic effect, while during longer term treatment increased caloric loss may contribute to the body weight–lowering effects. That both the reduction in Hct and increase in body weight were abrogated when zibotentan was combined with dapagliflozin suggests that the observed effects are likely real and plausible.
Plasma volume, as calculated from Hct and Hb, confirmed the effects of zibotentan and dapagliflozin on fluid retention. Urinary sodium excretion trended in the expected directions; the failure to achieve statistical significance was likely due, at least in part, to high variability, small sample size and intrinsic challenges in detecting relatively small differences in urinary sodium excretion between groups of rodents consuming a high salt diet.
SGLT2i and ETARAs have been shown to slow the progression of CKD and reduce the risk of kidney failure in well-conducted high-quality placebo-controlled randomized clinical outcome trials [14, 27]. These two drug classes are believed to confer nephroprotection via common and different mechanistic pathways. SGLT2i cause a reduction in glomerular hyperfiltration through the restoration of tubulo-glomerular feedback. This acute reduction in glomerular hyperfiltration is associated with stabilization of kidney function during prolonged treatment [28]. In addition, SGLT2i improve tubular oxygenation and reduce tubular hypoxic stress, and exert metabolic effects, including activation of hypoxia inducible factor-1 and sirtuin-1 mediated pathways, leading to improved mitochondrial and cellular function [29]. ETARAs reduce glomerular hyperfiltration, albeit moderately, but they also improve endothelin-1 mediated injury to the podocytes, endothelial glycocalyx, mesangium (extracellular matrix accumulation and proliferation) and tubulointerstitial (fibrosis) through direct actions on these cells and compartments [4, 30, 31]. Our finding that zibotentan co-administration with dapagliflozin attenuated fluid retention without any modification of dapagliflozin's primary mechanism on increasing glucosuria supports this hypothesis.
Two other experimental studies investigated the effects of dapagliflozin and atrasentan as mono-therapies and in combination. In contrast to the post hoc analysis from the SONAR trial, in the first experimental study neither dapagliflozin nor atrasentan, alone or in combination, reduced UACR or improved kidney function in db/db mice [32]. In another experimental study db/db mice were treated with empagliflozin, atrasentan or ramipril in combination of two or all three drugs. Triple therapy showed a modestly larger kidney protective effect than dual therapy but acute effects on sodium and fluid homeostasis were not assessed [33]. Acute effects on markers of fluid retention and kidney protection were, however, analyzed in a small post hoc analysis of the SONAR trial. The results of this analysis showed that initiation of combined treatment with SGLT2i and the ETARA atrasentan is associated with less fluid retention, as measured by changes in bodyweight and N-terminal pro B-type natriuretic peptide (NT-proBNP), compared with initiation of atrasentan alone. In addition, the combination resulted in a larger reduction in UACR and blood pressure compared with mono-therapy, suggesting that the combination of both drug classes may exert beneficial synergistic effects that augment UACR reduction while offsetting fluid retention. This beneficial effect has not been described to date with traditional diuretics and would require a head-to-head study to be fully addressed. The ongoing ZENITH study (Zibotentan and Dapagliflozin for the Treatment of CKD (ZENITH-CKD); NCT04724837) is a randomized, dose-finding, controlled clinical trial to provide more definitive evidence about the efficacy and safety of combined zibotentan–dapagliflozin treatment in patients with CKD.
The present study has limitations. Firstly, since our experimental study was designed to assess pharmacological aspects of combined treatment with zibotentan and dapagliflozin we did not assess whether combination treatment conferred additional kidney protection; this requires additional studies in experimental models of kidney disease. Secondly, the lack of NT-proBNP is another limitation of our study particularly since clinical studies have demonstrated that changes in NT-proBNP during ETARA treatment predict heart failure [34]. Thirdly, this study used significantly higher human-equivalent doses of zibotentan than under investigation in human zibotentan–dapagliflozin combination CKD trial (ZENITH-CKD; NCT04724837).
In conclusion, zibotentan treatment causes dose-dependent fluid retention. Co-administration with the SGLT2i dapagliflozin minimizes this effect. If proven in a prospective clinical trial, this would make the combination of both agents an attractive therapeutic option for clinical practice since both drug classes individually have already been shown to markedly reduce the risk of kidney failure.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank AstraZeneca Animal Science and Technology Vivarium Staff, Krister Bamberg for early data discussions, Anna Granqvist for practical discussions and Peter Konings for statistical review.
Contributor Information
Vandana Veenit, Bioscience Renal, Research and Early Development, Cardiovascular, Renal and Metabolism (CVRM), BioPharmaceuticals R&D, AstraZeneca, Gothenburg, Sweden.
Hiddo J L Heerspink, Department of Clinical Pharmacy and Pharmacology, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
Christine Ahlström, DMPK, Research and Early Development, Cardiovascular, Renal and Metabolism (CVRM), BioPharmaceuticals R&D, AstraZeneca, Gothenburg, Sweden.
Peter J Greasley, Early Clinical Development, Research and Early Development, Cardiovascular, Renal and Metabolism (CVRM), BioPharmaceuticals R&D, AstraZeneca, Gothenburg, Sweden.
Stanko Skritic, Innovation Strategies & External Liaison, Pharmaceutical Technologies & Development, AstraZeneca, Gothenburg, Sweden; Institute of, Medicine at Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; Institute of Medicine at Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden.
Natalie van Zuydam, Biostatistics Sweden, Data Science and Quantitative Biology, Discovery Sciences, BioPharmaceuticals R&D, AstraZeneca, Gothenburg, Sweden.
Donald E Kohan, Division of Nephrology, University of Utah Health, Salt Lake City, UT, USA.
Pernille B L Hansen, Bioscience Renal, Research and Early Development, Cardiovascular, Renal and Metabolism (CVRM), BioPharmaceuticals R&D, AstraZeneca, Gothenburg, Sweden.
Robert I Menzies, Bioscience Renal, Research and Early Development, Cardiovascular, Renal and Metabolism (CVRM), BioPharmaceuticals R&D, AstraZeneca, Gothenburg, Sweden.
CONFLICT OF INTEREST STATEMENT
H.J.L.H. and D.E.K. are consultants for AstraZeneca. V.V., C.A., P.J.G., S.S., N.v.Z., P.B.L.H. and R.I.M. are current or previous employees of AstraZeneca and may own stock or stock options.
AUTHORS’ CONTRIBUTIONS
P.B.L.H., P.J.G., C.A., V.V. and R.I.M. designed the experiments. H.J.L.H. and D.E.K. provided feedback on study plan and data produced. V.V. directed the rodent studies during the in-life phase and coordinated sample analysis. C.A. performed analysis confirming compound exposure. N.v.Z. performed the statistical analysis. V.V. and R.I.M. drafted the manuscript and all authors contributed to subsequent manuscript reviews. S.S. contributing to study/concept design in first line.
FUNDING
This study was funded by AstraZeneca. AstraZeneca develops and markets treatments for CKD, heart failure and metabolic diseases. Dapagliflozin is an approved product with defined benefits in CKD and heart failure, nondiabetic and diabetic indications. Zibotentan is an investigational medicinal product with no approved indication.
DATA AVAILABILITY STATEMENT
Data contained in this manuscript are submitted to US Patent US20220023295A1.
REFERENCES
- 1. GBD Chronic Kidney Disease Collaboration . Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet North Am Ed 2020;395:709–33. 10.1016/S0140-6736(20)30045-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yanagisawa M, Kurihara H, Kimura Set al. . A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988;332:411–5. 10.1038/332411a0 [DOI] [PubMed] [Google Scholar]
- 3. Kohan DE. Endothelins in the normal and diseased kidney. Am J Kidney Dis 1997;29:2–26. 10.1016/s0272-6386(97)90004-4 [DOI] [PubMed] [Google Scholar]
- 4. Dhaun N, Webb DJ. Endothelins in cardiovascular biology and therapeutics. Nat Rev Cardiol 2019;16:491–502. 10.1038/s41569-019-0176-3 [DOI] [PubMed] [Google Scholar]
- 5. Goddard J, Johnston NR, Hand MFet al. . Endothelin-A receptor antagonism reduces blood pressure and increases renal blood flow in hypertensive patients with chronic renal failure: a comparison of selective and combined endothelin receptor blockade. Circulation 2004;109:1186–93. 10.1161/01.CIR.0000118499.69469.51 [DOI] [PubMed] [Google Scholar]
- 6. Dhaun N, Yuzugulen J, Kimmitt RAet al. . Plasma pro-endothelin-1 peptide concentrations rise in chronic kidney disease and following selective endothelin A receptor antagonism. J Am Heart Assoc 2015;4:e001624. 10.1161/JAHA.114.001624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grenda R, Wuhl E, Litwin Met al. . Urinary excretion of endothelin-1 (ET-1), transforming growth factor- beta1 (TGF- beta1) and vascular endothelial growth factor (VEGF165) in paediatric chronic kidney diseases: results of the ESCAPE trial. Nephrol Dial Transplant 2007;22:3487–94. 10.1093/ndt/gfm300 [DOI] [PubMed] [Google Scholar]
- 8. Zeravica R, Cabarkapa V, Ilincic Bet al. . Plasma endothelin-1 level, measured glomerular filtration rate and effective renal plasma flow in diabetic nephropathy. Ren Fail 2015;37:681–6. 10.3109/0886022X.2015.1010990 [DOI] [PubMed] [Google Scholar]
- 9. Vachiery JL, Davenport A. The endothelin system in pulmonary and renal vasculopathy: les liaisons dangereuses. Eur Respir Rev 2009;18:260–71. 10.1183/09059180.00005709 [DOI] [PubMed] [Google Scholar]
- 10. Boffa JJ, Tharaux PL, Dussaule JCet al. . Regression of renal vascular fibrosis by endothelin receptor antagonism. Hypertension 2001;37:490–6. 10.1161/01.hyp.37.2.490 [DOI] [PubMed] [Google Scholar]
- 11. Sasser JM, Sullivan JC, Hobbs JLet al. . Endothelin A receptor blockade reduces diabetic renal injury via an anti-inflammatory mechanism. J Am Soc Nephrol 2007;18:143–54. 10.1681/ASN.2006030208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anguiano L, Riera M, Pascual Jet al. . Endothelin blockade in diabetic kidney disease. J Clin Med 2015;4:1171–92. 10.3390/jcm4061171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cahn A, Cernea S, Raz I. The SONAR study-is there a future for endothelin receptor antagonists in diabetic kidney disease? Ann Transl Med 2019;7:S330. 10.21037/atm.2019.09.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heerspink HJL, Parving HH, Andress DLet al. . Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial. Lancet North Am Ed 2019;393:1937–47. 10.1016/S0140-6736(19)30772-X [DOI] [PubMed] [Google Scholar]
- 15. Dhillon S. Dapagliflozin: a review in type 2 diabetes. Drugs 2019;79:1135–46. 10.1007/s40265-019-01148-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Park SH, Choi YJ, Rhee EJet al. . Retrospective analysis of the efficacy of dapagliflozin in patients with type 2 diabetes in a primary clinic in Korea. Endocrinol Metab 2019;34:70–9. 10.3803/EnM.2019.34.1.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Park HS, Jung YJ, Lee DYet al. . Use of dapagliflozin in patients with advanced diabetic kidney disease. Kidney Res Clin Pract 2018;37:292–7. 10.23876/j.krcp.2018.37.3.292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heerspink HJL, Stefansson BV, Correa-Rotter Ret al. . Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436–46. 10.1056/NEJMoa2024816 [DOI] [PubMed] [Google Scholar]
- 19. McMurray JJV, Docherty KF, Jhund PS. Dapagliflozin in patients with heart failure and reduced ejection fraction. Reply. N Engl J Med 2020;382:972. 10.1056/NEJMc1917241 [DOI] [PubMed] [Google Scholar]
- 20. Heerspink HJL, Kohan DE, de Zeeuw D. New insights from SONAR indicate adding sodium glucose co-transporter 2 inhibitors to an endothelin receptor antagonist mitigates fluid retention and enhances albuminuria reduction. Kidney Int 2021;99:346–9. 10.1016/j.kint.2020.09.026 [DOI] [PubMed] [Google Scholar]
- 21. Vercauteren M, Trensz F, Pasquali Aet al. . Endothelin ET(A) receptor blockade, by activating ET(B) receptors, increases vascular permeability and induces exaggerated fluid retention. J Pharmacol Exp Ther 2017;361:322–33. 10.1124/jpet.116.234930 [DOI] [PubMed] [Google Scholar]
- 22. Gagnon DR, Zhang TJ, Brand FNet al. . Hematocrit and the risk of cardiovascular disease—the Framingham study: a 34-year follow-up. Am Heart J 1994;127:674–82. 10.1016/0002-8703(94)90679-3 [DOI] [PubMed] [Google Scholar]
- 23. Miller WL. Fluid volume overload and congestion in heart failure: time to reconsider pathophysiology and how volume is assessed. Circ Heart Fail 2016;9:e002922. 10.1161/CIRCHEARTFAILURE.115.002922 [DOI] [PubMed] [Google Scholar]
- 24. Guyton AC, Young DB, DeClue JWet al. . Fluid balance, renal function, and blood pressure. Clin Nephrol 1975;4:122–6. [PubMed] [Google Scholar]
- 25. Sano M, Goto S. Possible mechanism of hematocrit elevation by sodium glucose cotransporter 2 inhibitors and associated beneficial renal and cardiovascular effects. Circulation 2019;139:1985–7. 10.1161/CIRCULATIONAHA.118.038881 [DOI] [PubMed] [Google Scholar]
- 26. Hoekman J, Lambers Heerspink HJ, Viberti Get al. . Predictors of congestive heart failure after treatment with an endothelin receptor antagonist. Clin J Am Soc Nephrol 2014;9:490–8. 10.2215/CJN.07040713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Heerspink HJL, Langkilde AM, Wheeler DC. Dapagliflozin in patients with chronic kidney disease. Reply. N Engl J Med 2021;384:388–90. 10.1056/NEJMc2032809 [DOI] [PubMed] [Google Scholar]
- 28. Jongs N, Chertow GM, Greene Tet al. . Correlates and consequences of an acute change in eGFR in response to the SGLT2 inhibitor dapagliflozin in patients with CKD. J Am Soc Nephrol 2022;33:2094–107. 10.1681/ASN.2022030306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Packer M. Critical reanalysis of the mechanisms underlying the cardiorenal benefits of SGLT2 inhibitors and reaffirmation of the nutrient deprivation signaling/autophagy hypothesis. Circulation 2022;146:1383–405. 10.1161/CIRCULATIONAHA.122.061732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boels MG, Avramut MC, Koudijs Aet al. . Atrasentan reduces albuminuria by restoring the glomerular endothelial glycocalyx barrier in diabetic nephropathy. Diabetes 2016;65:2429–39. 10.2337/db15-1413 [DOI] [PubMed] [Google Scholar]
- 31. Kohan DE, Barton M. Endothelin and endothelin antagonists in chronic kidney disease. Kidney Int 2014;86:896–904. 10.1038/ki.2014.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stuart D, Peterson CS, Hu Cet al. . Lack of renoprotective effects of targeting the endothelin A receptor and (or) sodium glucose transporter 2 in a mouse model of Type 2 diabetic kidney disease. Can J Physiol Pharmacol 2022;100:763–71. 10.1139/cjpp-2022-0082 [DOI] [PubMed] [Google Scholar]
- 33. Vergara A, Jacobs-Cacha C, Llorens-Cebria Cet al. . Enhanced cardiorenal protective effects of combining SGLT2 inhibition, endothelin receptor antagonism and RAS blockade in type 2 diabetic mice. Int J Mol Sci 2022;23:12823. 10.3390/ijms232112823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smeijer JD, Koomen J, Kohan DEet al. Increase in BNP in response to endothelin-receptor antagonist atrasentan is associated with incident heart failure. JACC Heart Fail 2022;10:498–507. 10.1016/j.jchf.2022.03.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data contained in this manuscript are submitted to US Patent US20220023295A1.