Abstract
Extracellular adenosine 5’-triphosphate (ATP) acts as an autocrine and paracrine agent, the actions of which on affected cells are mediated by P2 receptors (P2R), which include trans cell-membrane cationic channels (P2XRs), and G protein coupled receptors (P2YRs). The mammalian P2X receptors form homotrimeric or heterotrimeric cationic channels, each of which contains three ATP-binding sites. There are seven homotrimeric P2X receptors (P2X1-7) and three heteromeric (P2X2/P2X3, P2X4/P2X6, P2X1/P2X5). In the lungs and airways, ATP activates P2X3 and P2X2/3 receptors (P2X3R, P2X2/3R, respectively) localized on vagal sensory nerve terminals resulting in bronchoconstriction, and cough, and probably also localized release of pro-inflammatory neuropeptides via the axon reflex. Currently, several P2X3R and P2X2/3R antagonists are being developed as drug-candidates for the treatment of chronic cough. This report presents the receptor affinity data of a novel water-soluble small molecule, DT-0111, that acts as a selective P2X3R antagonist.
Keywords: Adenosine 5’-triphosphate, Bronchodilation, Vagus nerve, Cough, COPD, Asthma
Introduction
Adenosine 5’-triphosphate (ATP) is found in every cell of the human body where it plays a critical role as a source of energy for all cellular functions [1]. ATP is released from cells under physiologic and pathophysiologic conditions [2]. Extracellular ATP acts as an autocrine and paracrine agent, the effects of which on target cells are mediated by cell-surface receptors, P2R [2, 3]. These receptors are divided into two families: P2YR, which are seven trans-cell membrane domain G-protein coupled receptors (GPCR; metabotropic) [4], and P2XR, which are cationic channels (ionotropic) [5]. Eight P2YR and seven P2XR have been cloned heretofore. Several P2XR heterotrimers have also been identified including P2X2/3R, which manifests combined characteristics of P2X2R and P2X3R [6, 7].
Vagal sensory nerve endings in the airways and lungs express P2X3R and P2X2/3R [8]. In 1996, Pelleg and Hurt discovered that extracellular ATP triggers a pulmonary-pulmonary central vagal reflex by activating P2XR localized on vagal sensory nerve terminals in the canine lungs [9], which was later shown to cause bronchoconstriction [10]. Also in 1996, Pellegrino et al., showed that aerosolized ATP is a potent bronchoconstrictor in healthy human subjects and more so in asthmatic patients [11]. Based on these and subsequent studies using various in vitro and in vivo experimental models, it was proposed that extracellular ATP plays a major mechanistic role in chronic obstructive pulmonary disorders, what has been termed the “ATP Axis in Chronic Obstructive Pulmonary Diseases” [12]. Since then, voluminous data supporting this hypothesis have been published, and it is now well established that inflammatory processes in the lungs are associated with the release of ATP from different cell types causing bronchoconstriction and cough mediated by P2X3R and P2X2/3R in the airways, and which could also be pro-inflammatory due to localized release of neuropeptides via the axon reflex [13, 14].
In recent years, several molecules targeting P2X3R and/or P2X2/3R in the airways and lungs have been proposed as novel therapeutic agents for the treatment of chronic cough: Merck & Co.’s MK-7264 (Gefapixant) [15] (previously, AF-219) [16], and the structurally analogous AF-353 (both of which are allosteric inhibitors of P2X3 and P2X2/3 [17]), Beluss Health, Inc.’s BLU-5937 [18], Shionogi & Co., Ltd’s Sivopixant (S-600918) [19] and Bayer’s Eliapixant (BAY 1,817,080) [20] and Bay 1,902,607 [21].
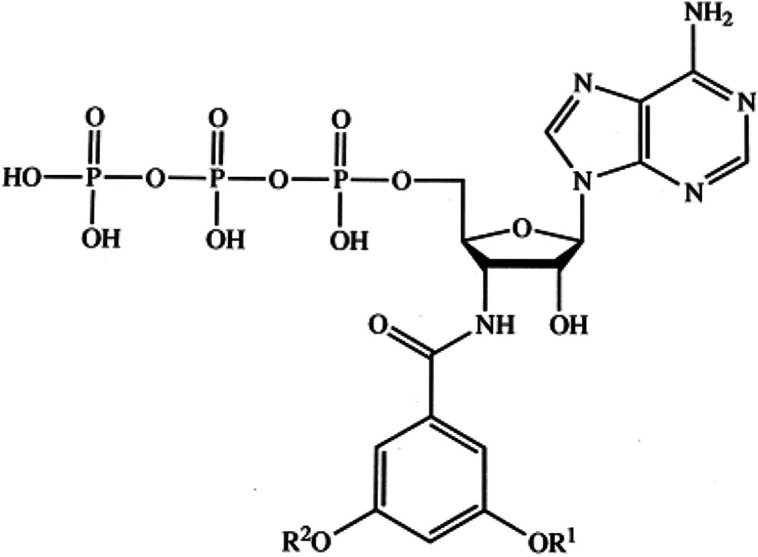
Here we report on the receptor selectivity of DT-0111 (Fig. 1) [22], a novel water-soluble small molecule that is being developed as an inhaled drug-candidate for the treatment of both chronic cough and chronic obstructive airway diseases such as COPD and asthma.
Fig. 1.
Molecular structure of DT-0111
Materials and methods
DT-0111
DT-0111 is a novel water-soluble small molecule, the synthesis pathway of which has been previously published (Fig. 1) [22]. Fresh batches were synthesized at Organix, Inc. (Woburn, Massachusetts, USA) for each of the studies outlined below.
Receptor selectivity
Calcium flux determination using Fluorescent Imaging Plate Reader (Gq Ca, FLIPR™), and TANGO™ binding assays at P2YR sites, α2B adrenergic receptor sites and the neurokinin-3 (NK3) receptor site were carried out through the National Institute of Mental Health's Psychoactive Drug Screening Program (Contract # HHSN-271–2018-00,023-C (NIMH PDSP)), which is Directed by Dr. Bryan L. Roth MD, at the University of North Carolina at Chapel Hill and Project Officer Jamie Driscoll at NIMH, Bethesda MD, USA. The materials and methods used at the PDSP’s lab have been previously published [23–25]. The DT-0111’s concetration range tested was 10−12 M – 10−6 M.
Funtional binding of DT-0111 at the rest of the GPCR (listed in Tabel 1) was quantified as follows:
The results are expressed as a percent of control specific binding:
and as a percent inhibition of control specific binding obtained in the presence of DT-0111:
The IC50 values (concentration causing a half-maximal inhibition of control specific binding) and Hill coefficients (nH) were determined by non-linear regression analysis of the competition curves generated with mean replicate values using Hill equation curve fitting:
where Y = specific binding, A = left asymptote of the curve, D = right asymptote of the curve, C = compound concentration, C50 = IC50, and nH = slope factor. This analysis was performed using software developed at Cerep (Hill software) and validated by comparison with data generated by the commercial software SigmaPlot® 4.0 for Windows® (© 1997 by SPSS Inc.).
The inhibition constants (Ki) were calculated using the Cheng Prusoff equation:
where L = concentration of radioligand in the assay, and KD = affinity of the radioligand for the receptor. A Scatchard plot is used to determine the KD.
Binding data interpretation: Results showing an inhibition (or stimulation for assays run in basal conditions) higher than 50% are considered to represent significant effects of the test compounds. 50% is the most common cut-off value for further investigation (determination of IC50 or EC50 values from concentration–response curves). Results showing an inhibition (or stimulation) between 25 and 50% are indicative of weak to moderate effects.
Results showing an inhibition (or stimulation) lower than 25% are not considered significant and mostly attributable to variability of the signal around the control level.
Low to moderate negative values have no real meaning and are attributable to variability of the signal around the control level. High negative values (≥ 50%) that are sometimes obtained with high concentrations of test compounds are generally attributable to nonspecific effects of the test compounds in the assays. On rare occasion, they could suggest an allosteric effect of the test compound.
P2X3R VS. P2X2/3R selectivity
This study was performed at Axxam S.p.A. (Milan, Italy). CHO cell-line cells mitoClytin/pcDNA_P2X3R and mitoClytin/pcDNA_P2X2/3R were seeded 72 h before experiments (0.7 and 1 million cells per T225 flask, respectively). Just before experiments cells were washed twice with D-PBS w/o Ca2+/Mg2+ (Sigma: Euroclone, SpA, Milan, Italy) and detached from the flask with trypsin–EDTA (Merck Life Science S.r.l., Milan, Italy). Cells were then re-suspended in the suspension solution: 25 mL EX-CELL ACF CHO medium (Sigma); 0.625 mL HEPES (BioWhittaker); 0.25 mL of 100 × Penicillin/Streptomycin (Biowhittaker Europe Spr, Verviers, Belgium), 0.1 mL of Soybean Trypsin Inhibitor 10 mg/mL (Sigma) and placed on the automated electrophysiology platform QPatch 16X (Sophion, Copenhagen, Denmark). DT-0111 was solubilized as a 100 mM stock solution in 100% extracellular solution and stored at + 4 °C in aliquots. Compound solutions were prepared immediately before use on the QPatch 16X by dilution of the stock in extracellular solution. Standard whole-cell voltage clamp experiments were performed at room temperature using the multi-hole technology (10 holes for each of the 16 wells). Following the establishment of the whole-cell configuration, cells were held at -90 mV (gap-free protocol) and the human P2X3R and P2X2/3R currents were evoked by applying the agonist in the absence (control) or in the presence of five increasing concentrations of the compound under evaluation. The reference agonist α,β-Methylene ATP (Tocris, Bio-Techne, SRL, Milan, Italy) was used at 1 and 8 µM [26] to activate P2X3R and P2X2/3R, respectively. Compound has been tested according to the specific protocols of the QPatch Assays, in order to evaluate its blocking effect on the targets. The scheme of the QPatch application protocol is describe hereafter, where all the pipetting events (three agonist applications plus five increasing antagonist applications) are reported along the whole-cell period: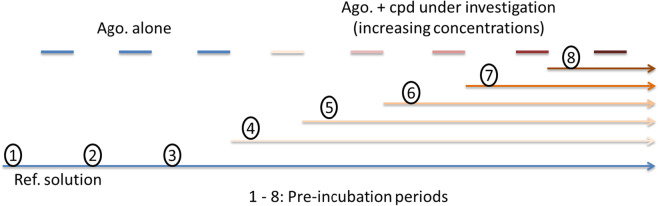
All the pre-incubation periods last at least three minutes.
Data were sampled at a rate of 2 kHz. The non-selective P2X3-P2X2/3 receptor antagonist [27] was used to block P2X3R and P2X2/3R.
The intracellular solution contained (mM) 135 CsF, 10 NaCl, 1 EGTA, 10 HEPES (pH 7.2 with CsOH); the extracellular solution contained (mM): 145 NaCl, 4 KCl, 0.5 MgCl2, 1 CaCl2, 10 HEPES, 10 Glucose (pH 7.4 with NaOH); the reference solution contained (mM) 145 NaCl, 4 KCl, 0.5 MgCl2, 1 CaCl2, 10 HEPES, 10 Glucose, 25 U/mL Hexokinase from Saccharomyces cerevisiae (Sigma) (pH 7.4 with NaOH). For data acquisition and analysis, Sophion and Excel software were used. The percentage of inhibition elicited by each concentration of the compound under investigation was calculated as: % of inhibition = 100—100 x (IP2XCP / IP2XCT); where IP2XCT and IP2XCP were the inward current elicited by the agonist in the absence (control) or in the presence of increasing concentrations of the compound under investigation, respectively. When the percentage of inhibition elicited by the highest concentration tested exceeded 50%, an IC50 value was determined using GraphPad Prism (v 8.2.1).
Results
DT-0111
Dt-0111 (Sodium 3'-N-(3,5-dimethoxybenzoyl)-3'-deoxy-β-D-adenosine 5'-triphosphate) is a white solid water-soluble molecule (Fig. 1). It was characterized by 1H NMR, 31P NMR, Mass spectometry data and purity was determined by HPLC and 31P NMR.
1H NMR (300 MHz, D2O) δ 8.51 (s, 1H), 8.21 (s, 1H), 6.98 (d, J = 2.2 Hz, 2H), 6.72 (t, J = 2.2 Hz, 1H), 6.20 (d, J = 4.1 Hz, 1H), 4.87 (m, 1H), 4.77 (m, 1H), 4.73 (m, 1H), 4.33 (m, 1H), 4.25 (m, 1H), 3.82 (s, 6H).
31P NMR (202 MHz, D2O) δ -6.39 (d, α-P), -10.36 (d, γ-P), -21.09 (t, β-P).
MS: (ESI−) [M-1] = 669.0
Receptor selectivity
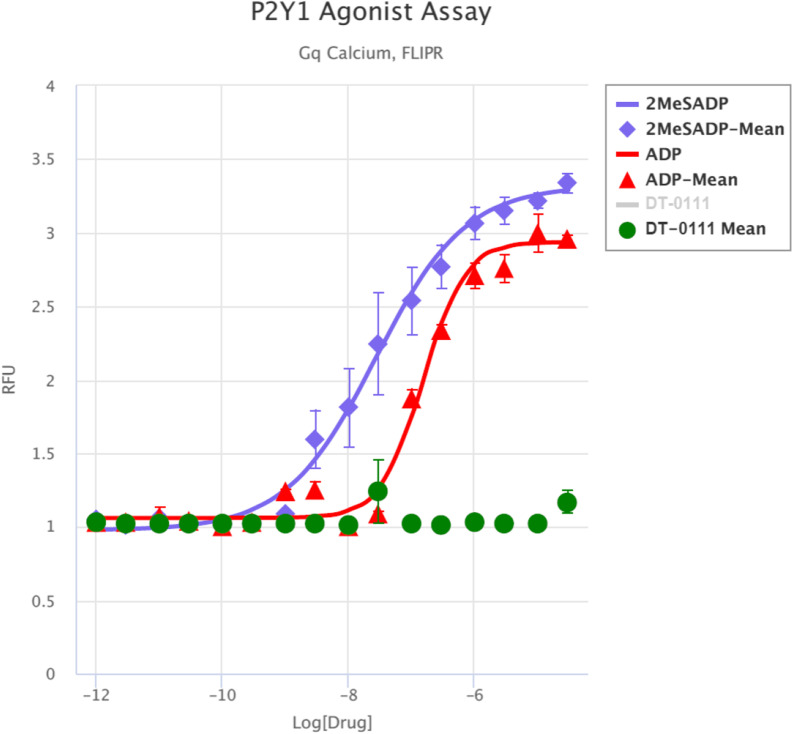
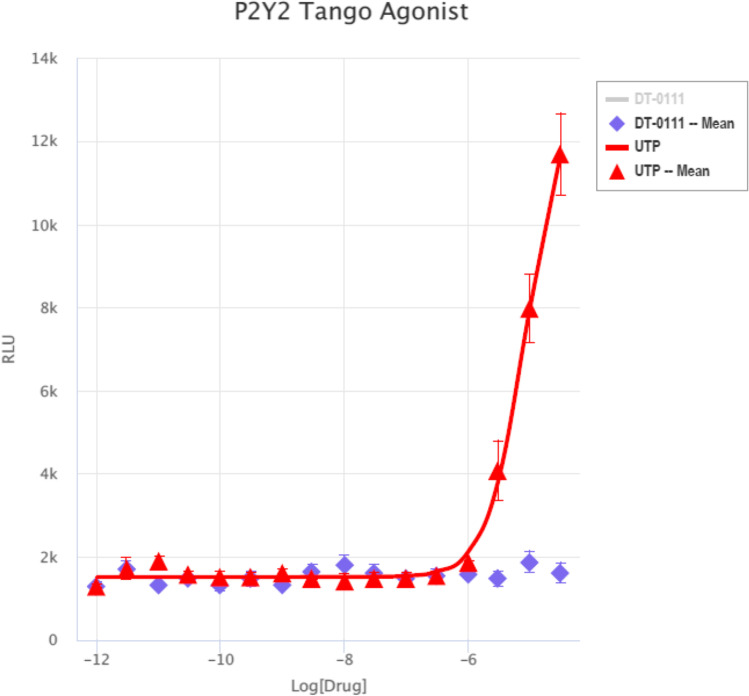
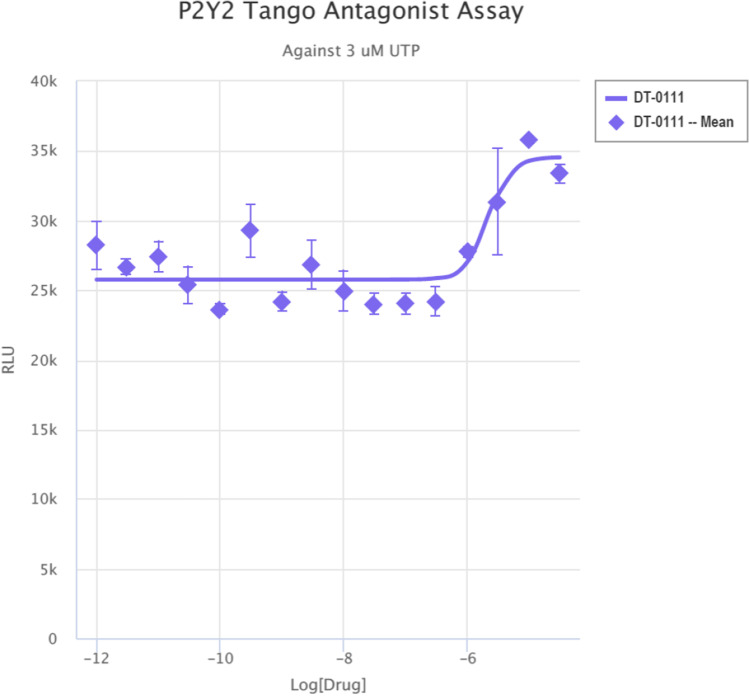
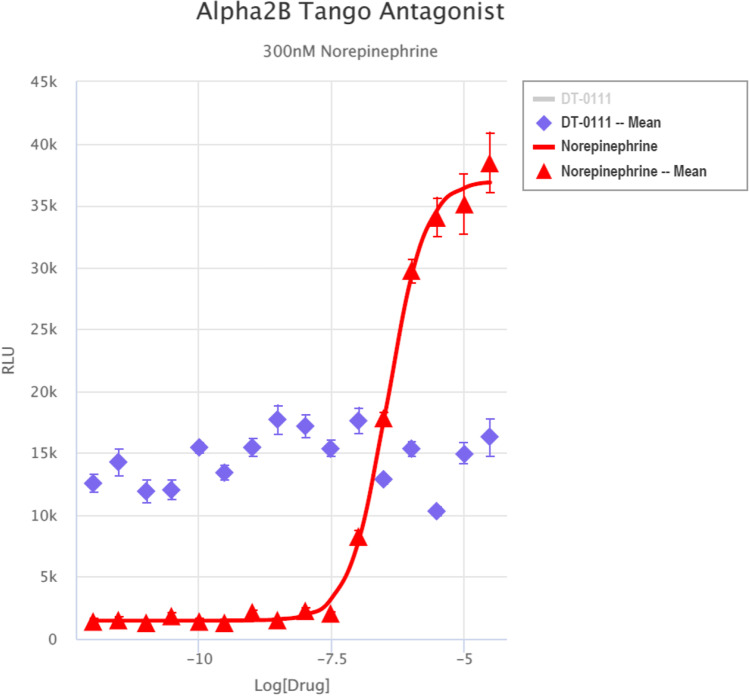
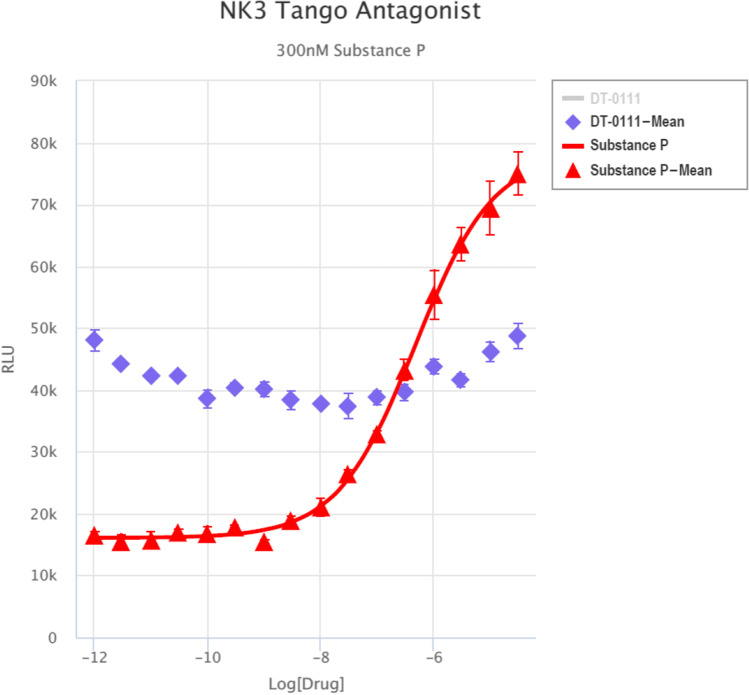
Gq Ca, FLIPR™ Assay: As shown in Fig. 2, DT-0111 did not induce any Ca2+ flux. TANGO™ assays: The potential agonist and antagonist activities of DT-0111 (at a concentration range of 10−12 M – 10−6 M) were determined at the following receptor sites: P2Y1R, P2Y2R, P2Y4R, P2Y6R, P2Y11R, P2Y12R, P2Y13R, and P2Y14R. In none of these receptor sites, DT-0111 acted as either agonist or antagonist; in fact, no dose response relationship was observed at any of the receptor sites tested. A typical example are agonist and antagonist data obtained at P2Y2R site, as shown in Figs. 3 and 4, respectively. In addition, DT-0111 did not act as an antagonist at the α2B adrenergic receptor and the neurokinin-3 (NK3R) receptor site, as shown in Figs. 5 and 6, respectively.
Fig. 2.
Dose response curves of DT-0111 and two agonists at P2Y1R site as determined by Ca.2+ influx. (SEM, n = 3–4; all figures)
Fig. 3.
Dose response curves of DT-0111 and UTP P2Y2R site
Fig. 4.
Dose response curve of DT-0111 at P2Y2R site
Fig. 5.
Dose responses curves of DT-0111 and nor-epinephrine (300 nM) at α2B adrenergic receptor
Fig. 6.
Dose response curves of DT-0111 and substance P (300 nM) at the neurokinin 3 (NK3) receptor site
Functional binding assays have shown that DT-0111 did not bind to a standard set of 28 GPCRs; and it did not bind to Ca2+, K+ and Na+ channels, three transporters and two nuclear receptors (Table 1).
Table 1.
List of 27 G protein coupled receptors (GPCR), four ionic channels, three transporters, and one enzyme to which DT-0111 had no significant affinity
| GPCR | A2aAdoR*, α1A & α2A, (adrenergic), β1 & β2 (adrenergic), BZD, CB1, CB2, CCK1, CCK2, D1, D2S, ETA, GR, H1, H2, M1-3, NMDA, N neuronal α4β2, δ (DOP), Kappa OP, µ (MOP), 5-HT1A,2A,1B2B,3, V1 |
| Enzyme | MAO-A |
| Ionic channels | Ca2+ (L dihydropyridine), K+ hERG, Kv, Na+ |
| Transporters | Norepinephrine, Dopamine, 5-HT (5-hydroxytryptamine) |
*Abbreviations: Ado: Adenosine; AR: Androgen receptor; BZD: Benzodiazepine; CB: Cannabinoid; CCK: Cholecystokinin; D: Dopamine; ET: Endothelin; GR: glucocorticoid receptor; H: Histamine; HT: 5-hydroxytryptamine; M: Muscarinic; MAO-A: Monoamine oxidase A; MOP: mu opioid; NMDA: N-methyl-D-aspartate; N neuronal α4β2: α4β2 Nicotinic acetylcholine; OP: Opioid; R: Receptor; V: Vasopressin
P2X3R VS. P2X2/3R selectivity
Pharmacology of P2X3R and P2X2/3R channels recorded on the QPatch 16X
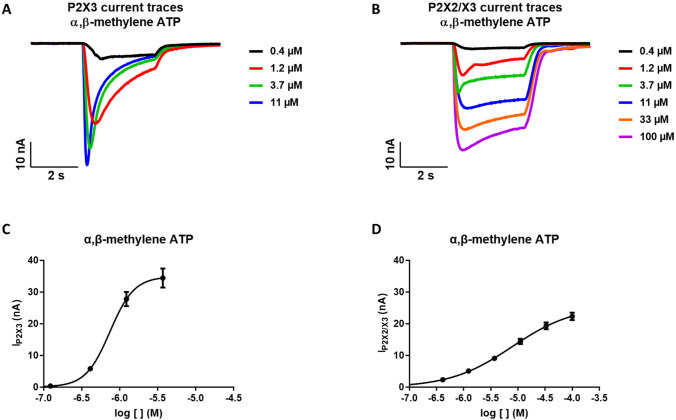
The two cell lines used for the compound profiling of DT-0111 were pharmacological characterized at QPatch 16X using α,β-methylene ATP as ligand, which is able to activate both the homomeric P2X3 and the heteromeric P2X2/3 channels.
Exposure to agonists (α,β-methylene ATP) led to typical transient P2X3 receptor currents characterized by a fast activation and a slower subsequent desensitization (Fig. 7). Concentration–response analyses were based on whole-cell current responses to four increasing agonist concentrations (Fig. 7a, 7c). The responses were highly reproducible. The peak current was identified in the cursor range between 2000 and 3000 ms. The current baseline was stable throughout the experiment period and no desensitization occurred after sequential agonist application (no tachyphylaxis). The EC50 value obtained was 0.74 μM, in agreement with the literature data (~ 1 μM, [28]). Three effects of increasing agonist concentrations were demonstrated: increased amplitudes of the whole-cell P2X3 receptor current; reduced time constants for activation and, in particular desensitization; increased degree of desensitization. The time to develop peak current was reduced with increasing agonist concentrations. In Fig. 5a the peak current was obtained at 967, 731, 510 and 379 ms after ligand application at 412 nM, 1.23 μM, 3.7 μM and 11.1 μM of α,β-methylene ATP, respectively.
Fig. 7.
A, B: Representative traces of P2X3R-current and P2X2/3R-current evoked by increasing concentrations of the reference agonist α,β-methylene ATP. C: Fitting of the average (mean ± SE) of the dose response curves of α,β-methylene ATP for P2X3R (LogEC50 6.13 ± 0.05, n = 28). D: Fitting of the average (mean ± SE) of the dose response curves of α,β-methylene ATP for P2X2/3R (LogEC50 5.11 ± 0.11, n = 36)
The pharmacological profile of P2X2/3 receptor is unique and does not fit either homomeric P2X2 or P2X3 receptors alone [29]. Figure 6b described the whole-cell P2X2/X3 receptor current responses to six successive exposures to agonist. Since P2X2 homomeric receptors are insensitive to α,β-methylene ATP, and the kinetics of desensitization are slow rather than rapid, which would be indicative of P2X3 homomeric receptors, we can conclude that the majority to the receptors expressed in these cells are P2X2/3 heteromers. The peak current was identified in the cursor range between 2500 and 2800 ms, instead the sustained phase of the current was recorded in the range between 4850 and 4950 ms. The EC50 value obtained was 7.78 µm (Fig. 7d).
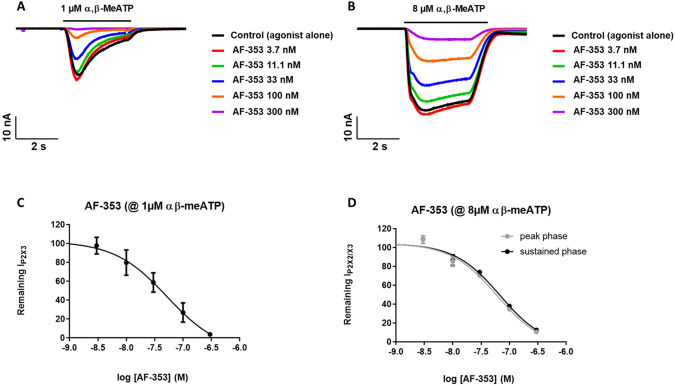
The effects of the selective, high affinity P2X3 and P2X2/3 receptors antagonist, AF-353, were investigated using five increasing concentrations of antagonist. A marked concentration-dependent reduction of the P2X3R mediated peak current amplitude was observed when stimulating the cells with 1 μM α,β-methylene ATP in the presence of AF-353 dose response experiments; five increasing concentrations) (Fig. 8). Figure 7a describes the effects of the antagonist AF-353 on whole-cell P2X3 receptor currents. The currents were first recorded in response to agonist alone, next the recordings were done in response to agonist in the presence of increasing concentrations of antagonist. Time constants for activation/desensitization were not significantly affected. The concentration–response relationship for the inhibitory effect of AF-353 on peak P2X3 receptor whole-cell currents is shown in Fig. 8c. The IC50 obtained (52.5 ± 0.43 nM, n = 7) compared well with values published in the literature (pIC50 range of 7.3—8.5, [27]).
Fig. 8.
A: Representative traces of P2X3R-current evoked by 1 µM α,β-methylene ATP in control (black line) and in the presence of increasing concentrations of reference compound AF353. B: Representative traces of P2X2/3R-current evoked by 1 µM α,β-methylene ATP in control (black line) and in the presence of increasing concentrations of reference compound AF353. C: Fitting of average (mean ± SE) of the dose response curves of AF353 on P2X3R peak currents (IC50 52.5 nM, n = 7). D: Fitting of average (mean ± SE) of the dose response curves of AF353 on both P2X2/3R-peak and sustained currents (IC50 59.9 and 68.6 nM, n = 10, respectively)
Currents mediated by P2X2/3R were induced by 8 μM α,β-methylene ATP, in order to investigate the pharmacological effects of reference antagonist AF-353. AF-353 was preincubated and then it was co-applied with the agonist (ligand-gated protocol). Five concentrations of AF-353 were applied to each well (“cumulative concentration response” approach), allowing full concentration response curves for each well to be constructed (Fig. 8b and 8d). This gave an IC50 value of 59.9 ± 0.76 and 68.6 ± 0.77 nM (n = 10), for the peak and the sustained phases of the current, respectively. The success rate for completed experiments was 90% and the cells were stable for over 35 min.
DT-0111 acts as a selective antagonist at P2X3R
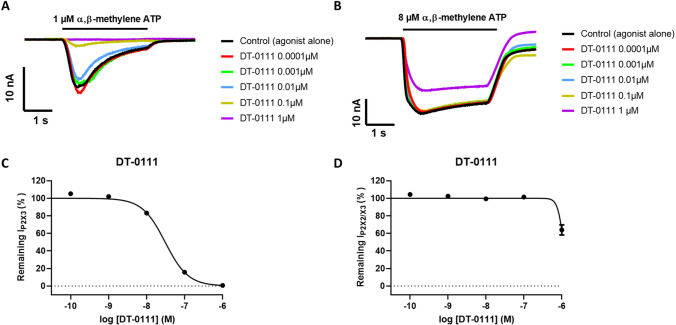
In order to study the pharmacology behavior of compound DT-0111, the molecule was preliminary tested on P2X3R at five increasing concentrations. As shown in Fig. 8a and 8c, DT-0111 showed a strong inhibition of peak current at the highest concentrations tested (1 and 0.1 µM), with a dose-dependent inhibition of P2X3R currents, elicited by 1 µM α,β-methylene ATP, and an IC50 value of 31.2 ± 2.15 nM (n = 6).
Compound DT-0111 was then tested on P2RX2/X3 at the same concentrations used for P2RX3, i.e. 0.0001; 0.001; 0.01; 0.1 and 1 µM. As shown in Fig. 9b and 9d, DT-0111 did not show a dose-dependent inhibition of P2X2/3R current, elicited by 8 µM α,β-methylene ATP, with a % of inhibition of 32% at 1 µM. Since the % of inhibition at the highest concentration tested (1 µM) was below 50% and no visible dose–response behavior could be recorded, it was not possible to calculate an IC50 value.
Fig. 9.
A: Representative traces of P2X3R-current evoked by 1 µM α,β-methylene ATP in control (black line) and in the presence of increasing concentrations of compound DT-0111. B: Representative traces of P2X2/3R-current evoked by 8 µM α,β-methylene ATP in control (black line) and in the presence of increasing concentrations of compound DT-0111. C: Fitting of average (mean ± SE) of the dose response curves of compound DT-0111 (IC50 31.2 ± 2.15 nM, n = 6). D: Fitting of average (mean ± SE) of the dose response curves of compound DT-0111 (n = 5)
Discussion
Currently, there is no effective drug available for the treatment of chronic cough. In addition, the hall mark treatment for COPD and asthma are long-acting muscarinic antagonists (LAMA) and long-acting beta-adrenergic agonists (LABA), both of which are bronchodilators, with or without the addition of cortico-steroids [30–33]. However, these therapies are not optimal and associated with serious side effects. Thus, chronic cough, COPD and asthma remain unmet clinical needs.
ATP is released from multiple cell types under physiologic and pathophysiologic conditions [2], in the lungs, extracellular ATP stimulates both Aδ and C fibers’ nerve terminals [9, 13, 34], which play a critical mechanistic role in the cough reflex. In addition, the stimulations of these nerve terminals results in bronchoconstriction [10]. These observations constitute the rationale for the development of P2X3R and P2X2/3R antagonists as drug-candidate for the treatment of chronic cough and chronic obstructive pulmonary disorders including COPD and asthma. In recent years, P2X3R and P2X2/3R antagonists have been developed as drug-candidates for the treatment of pain and chronic cough [5, 35]. A more recently found antagonist, DT-0111, is being developed as a bronchodilator for the treatment of COPD and asthma as well as chronic cough. We have previously shown that aerosolized DT-0111 can prevent cough and bronchoconstriction induced by aerosolized ATP in free moving guinea-pigs [36]. Thus, DT-0111 can potentially become a novel drug for the treatment not only of chronic cough but also COPD and asthma. Furthermore, since ATP is released from cells under various pathophysiologic conditions in the lungs [14], DT-0111 could eventually be used for the treatment of other unmet clinical needs in this arena including ventilation induced lung injury [14].
P2X3Rs play an important role in the function of sensory neurons [37] in various tissues and organs. Of particular importance is their role in pain and taste sensations [38, 39]. Indeed, the first potent P2X3R-P2X2/3R antagonist, A-317491, was developed as a drug-candidate for the treatment of chronic pain [40]. The present results indicate that DT-0111 is a selective P2X3R antagonist, i.e., it effectively blocked P2X3R but did not act as an antagonist at P2X2/3R site.
A broad interest from academic and pharmaceutical scientists has focused on the search for P2X3R and P2RX2/X3R ligands and has led to the discovery of multiple new selective antagonists. Some of them have been studied in clinical trials for the treatment of pathological conditions such as bladder disorders, gastrointestinal and chronic obstructive pulmonary diseases. Of the multiple new molecules that act as antagonists at P2X3R and P2X2/3R, which have been discovered in recent years, only two have been shown to selective for P2X3 vs. P2X2/3R. High selectivity of BLU-5937 for P2X3R vs. P2X2/3R, 1/1500 was determined in vitro using cloned human P2X3 and P2X2/3 channels stably expressed in mammalian HEK293 cells as well as in rat and guinea pig using nodose ganglion neurons expressing P2X2/3 receptors and dorsal root ganglion (DRG) neurons expressing P2X3 receptors [35]. In addition, another molecule, Sivopixant (S-600918) was also found to be a selective antagonist, i.e., P2X3 IC50 of 4.2 nM, and P2X2/3 IC50, of 1100 nM. [19]. The 2016 publication of the crystallographic structure of the human P2X3R subtype gave an improvement of published patents in 2017 [41]. Hence, a great number of small molecules with dual antagonist activity on P2X3-P2X2/3R, a favorable pharmacokinetic profile, and reasonable oral bioavailability was discovered. The most promising compounds are the phenoxy-diaminopyrimidines including Gefapixant (AF-219), and the imidazo-pyridines like BLU-5937, which are in phase III and phase II clinical trials, respectively, for refractory chronic cough. Another P2X3R antagonist, Eliapixant [20], which is both highly potent and selective for P2X3R vs. P2XR subtypes in vitro, including P2RX2/3R, targets disorders associated with hypersensitive nerve fibers.
From a physiological and a pharmaceutical points of view, the selectivity between P2RX3 and P2RX2/3 (very close related genes) is very important to reach a potent anti-tussive effect and no taste alteration. In this paper, the compound DT-0111 showed a potency in the double digit nanomolar range towards P2Rx3 vs P2RX2/X3, and it does not affect the P2RX2/X3 current up to 10 uM (max 50% of inhibition at this concentration). Interestingly, DT-0111 is structurally different from all other known P2X3R antagonists [35, 42, 43]; it is the only molecule that is a simple analog of ATP, in which the three serially bonded phosphate-groups are intact. This structure on the one hand, affords relatively small molecular dimensions and high-water solubility, on the other hand, it probably shortens its half-life due to degradation by ecto-phosphatases. We have not tested the affinity of DT-0111 to P2XRs other than P2X3R vs. P2X2/3R. Although the molecular structure of DT-0111 is similar to that of ATP, the facts that it did not affect any P2YRs and was highly selective for P2X3R vs. P2X2/3R, indicate that DT-0111 does not mimic ATP in its receptor-affinity profile and the it is highly unlikely the DT-0111 would act as either agonist or antagonist in other P2XRs.
Our previous claim that DT-0111 acts as an antagonist at P2X2/3R was not based on selectivity data [36]; we hypothesized at that time that the pulmonary-pulmonary central vagal reflex triggered by extracellular ATP is similar to the cardio-cardiac central vagal reflex that ATP triggers by activating P2X2/3R in the antero-posterior wall of the left ventricle [44]. Our current data indicate that although the two reflexes are similar the mediating receptors could be different.
DT-0111 did not activate or block P2YR, NK3R, and a large series of other GPCRs. Importantly, in early clinical trials with AF-219, a drug-candidate for the treatment of chronic cough, significant reduction in taste sensation was observed at a certain dose range [16]. This side effect was explained by antagonistic activity at the P2X2/3R sites [18, 38]. This is in agreement with previously published studies on extracellular ATP and P2X2R and P2X3R receptors in fungiform papillae mediating the taste sensation [45, 46]. Thus, it seems that selectivity for P2X3R vs. P2X2/3R constitutes an advantage in these clinical settings, which the present findings indicate that DT-0111 poses.
Dr. Pelleg
is the founder and CEO and CSO of Danmir Therapeutics, LLC, an emerging biopharmaceutical company focusing on the development of drugs based on the pharmacologic manipulations of purinergic P2 receptors’ signal transductions. He had served in academia as a professor of medicine, physiology and pharmacology, during which time he was a consulted to several biopharmaceuticals companies. He was a member of the team that put the first two adenosine drugs on the market (Adenocard®, and Adenoscan®). Following his academic career, Dr. Pelleg served as an executive and CSO of several private and public biopharmaceutical companies.

Authors’ contribution
AP conceptualized the project; AP and ES wrote the manuscript, all authors read it and approved its submission; AM, and ES and J-FR supervised the work at Organix, Inc., and Axxam S.p.a., respectively; all authors review the data and approved its current presentation.
Funding
This project was supported in part by a Biotechnology Research Grant from the Dept. of health of the Commonwealth of Pennsylvania.
Data availability
N/A.
Declarations
Competing interests
Drs. Pelleg and Mahadevan are the CEOs of Danmir Therapeutics, LLC and Organix, Inc. respectively, which together are developing DT-0111 as a drug candidate for the treatment of several pulmonary disorders. Drs. Sirtori and Rolland declare no conflict of interest.
Ethical approval
N/A.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nirody JA, Budin I, Rangamani P. ATP synthase: Evolution, energetics, and membrane interactions. J Gen Physiol. 2020;152(11):e201912475. doi: 10.1085/jgp.201912475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corriden R, Insel PA. Basal release of ATP: an autocrine-paracrine mechanism for cell regulation. Sci Signal. 2010;3(104):re1. doi: 10.1126/scisignal.3104re1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burnstock G. Purinergic signalling: from discovery to current developments. Exp Physiol. 2014;99(1):16–34. doi: 10.1113/expphysiol.2013.071951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobson KA, Delicado EG, Gachet C, Kennedy C, von Kugelgen I, Li B, Miras-Portugal MT, Novak I, Schoneberg T, Perez-Sen R, Thor D, Wu B, Yang Z, Muller CE. Update of P2Y receptor pharmacology: IUPHAR Review 27. Br J Pharmacol. 2020;177(11):2413–2433. doi: 10.1111/bph.15005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Illes P, Muller CE, Jacobson KA, Grutter T, Nicke A, Fountain SJ, Kennedy C, Schmalzing G, Jarvis MF, Stojilkovic SS, King BF, Di Virgilio F. Update of P2X receptor properties and their pharmacology: IUPHAR Review 30. Br J Pharmacol. 2021;178(3):489–514. doi: 10.1111/bph.15299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burnstock G. Purine and purinergic receptors. Brain Neurosci Adv. 2018;2:2398212818817494. doi: 10.1177/2398212818817494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobson KA, Muller CE. Medicinal chemistry of adenosine, P2Y and P2X receptors. Neuropharmacology. 2016;104:31–49. doi: 10.1016/j.neuropharm.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burnstock G, Brouns I, Adriaensen D, Timmermans JP. Purinergic signaling in the airways. Pharmacol Rev. 2012;64(4):834–868. doi: 10.1124/pr.111.005389. [DOI] [PubMed] [Google Scholar]
- 9.Pelleg A, Hurt CM. Mechanism of action of ATP on canine pulmonary vagal C fibre nerve terminals. J Physiol. 1996;490(Pt 1):265–275. doi: 10.1113/jphysiol.1996.sp021142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katchanov G, Xu J, Schulman ES, Pelleg A. ATP causes neurogenic bronchoconstriction in the dog. Drug Dev Res. 1998;45(3–4):342–349. doi: 10.1002/(SICI)1098-2299(199811/12)45:3/4<342::AID-DDR34>3.0.CO;2-P. [DOI] [Google Scholar]
- 11.Pellegrino R, Wilson O, Jenouri G, Rodarte JR. Lung mechanics during induced bronchoconstriction. J Appl Physiol (1985) 1996;81(2):964–75. doi: 10.1152/jappl.1996.81.2.964. [DOI] [PubMed] [Google Scholar]
- 12.Pelleg A, Schulman ES. Adenosine 5'-triphosphate axis in obstructive airway diseases. Am J Ther. 2002;9(5):454–464. doi: 10.1097/00045391-200209000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Pelleg A, Schulman ES, Barnes PJ. Extracellular Adenosine 5'-Triphosphate in Obstructive Airway Diseases. Chest. 2016;150(4):908–915. doi: 10.1016/j.chest.2016.06.045. [DOI] [PubMed] [Google Scholar]
- 14.Pelleg A. Extracellular adenosine 5'-triphosphate in pulmonary disorders. Biochem Pharmacol. 2021;187:114319. doi: 10.1016/j.bcp.2020.114319. [DOI] [PubMed] [Google Scholar]
- 15.Richards D, Gever JR, Ford AP, Fountain SJ. Action of MK-7264 (gefapixant) at human P2X3 and P2X2/3 receptors and in vivo efficacy in models of sensitisation. Br J Pharmacol. 2019;176(13):2279–2291. doi: 10.1111/bph.14677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdulqawi R, Dockry R, Holt K, Layton G, McCarthy BG, Ford AP, Smith JA. P2X3 receptor antagonist (AF-219) in refractory chronic cough: a randomised, double-blind, placebo-controlled phase 2 study. Lancet. 2015;385(9974):1198–1205. doi: 10.1016/S0140-6736(14)61255-1. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Wang Y, Cui WW, Huang Y, Yang Y, Liu Y, Zhao WS, Cheng XY, Sun WS, Cao P, Zhu MX, Wang R, Hattori M, Yu Y (2018) Druggable negative allosteric site of P2X3 receptors. Proc Natl Acad Sci U S A 115(19):4939-4944 [DOI] [PMC free article] [PubMed]
- 18.Garceau D, Chauret N. BLU-5937: A selective P2X3 antagonist with potent anti-tussive effect and no taste alteration. Pulm Pharmacol Ther. 2019;56:56–62. doi: 10.1016/j.pupt.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Kai H, Horiguchi T, Kameyma T, Onodera N, Itoh N, Fujii Y, Ichihashi Y, Hirai K, Shintani T, Nakamura K, Minami K, Kasai E, Yoneda S, Murakami Y, Ogawa H, Sekimoto R, Shinohara S, Yoshida O, Kurose N. Discovery of clinical candidate Sivopixant (S-600918): Lead optimization of dioxotriazine derivatives as selective P2X3 receptor antagonists. Bioorg Med Chem Lett. 2021;52:128384. doi: 10.1016/j.bmcl.2021.128384. [DOI] [PubMed] [Google Scholar]
- 20.Morice A, Smith JA, McGarvey L, Birring SS, Parker SM, Turner A, Hummel T, Gashaw I, Fels L, Klein S, Francke K, Friedrich C. Eliapixant (BAY 1817080), a P2X3 receptor antagonist, in refractory chronic cough: a randomised, placebo-controlled, crossover phase 2a study. Eur Respir J. 2021;58(5):2004240. doi: 10.1183/13993003.04240-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedrich C, Francke K, Birring SS, Van Den Berg JWK, Marsden P, McGarvey L, Turner A, Wielders P, Gashaw I, Klein S, Morice A (2020) Safety and efficacy of P2X3 antagonist BAY 1902607 in refractory chronic cough. Eur Respir J 56 [DOI] [PMC free article] [PubMed]
- 22.Pelleg A, Mahadaven A, Li J, Morency C ( 2022) US Patent No. US 11,440,935 B2, https://ppubs.uspto.gov/pubwebapp/
- 23.Besnard J, Ruda GF, Setola V, Abecassis K, Rodriguiz RM, Huang XP, Norval S, Sassano MF, Shin AI, Webster LA, Simeons FR, Stojanovski L, Prat A, Seidah NG, Constam DB, Bickerton GR, Read KD, Wetsel WC, Gilbert IH, Roth BL, Hopkins AL. Automated design of ligands to polypharmacological profiles. Nature. 2012;492(7428):215–220. doi: 10.1038/nature11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kroeze WK, Sassano MF, Huang XP, Lansu K, McCorvy JD, Giguere PM, Sciaky N, Roth BL. PRESTO-Tango as an open-source resource for interrogation of the druggable human GPCRome. Nat Struct Mol Biol. 2015;22(5):362–369. doi: 10.1038/nsmb.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang XP, Mangano T, Hufeisen S, Setola V, Roth BL. Identification of human Ether-a-go-go related gene modulators by three screening platforms in an academic drug-discovery setting. Assay Drug Dev Technol. 2010;8(6):727–742. doi: 10.1089/adt.2010.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spelta V, Jiang LH, Surprenant A, North RA. Kinetics of antagonist actions at rat P2X2/3 heteromeric receptors. Br J Pharmacol. 2002;135(6):1524–1530. doi: 10.1038/sj.bjp.0704591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gever JR, Soto R, Henningsen RA, Martin RS, Hackos DH, Panicker S, Rubas W, Oglesby IB, Dillon MP, Milla ME, Burnstock G, Ford AP. AF-353, a novel, potent and orally bioavailable P2X3/P2X2/3 receptor antagonist. Br J Pharmacol. 2010;160(6):1387–1398. doi: 10.1111/j.1476-5381.2010.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82(4):1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 29.Liu M, King BF, Dunn PM, Rong W, Townsend-Nicholson A, Burnstock G. Coexpression of P2X(3) and P2X(2) receptor subunits in varying amounts generates heterogeneous populations of P2X receptors that evoke a spectrum of agonist responses comparable to that seen in sensory neurons. J Pharmacol Exp Ther. 2001;296(3):1043–1050. [PubMed] [Google Scholar]
- 30.Koarai A, Sugiura H, Yamada M, Ichikawa T, Fujino N, Kawayama T, Ichinose M. Treatment with LABA versus LAMA for stable COPD: a systematic review and meta-analysis. BMC Pulm Med. 2020;20(1):111. doi: 10.1186/s12890-020-1152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koarai A, Yamada M, Ichikawa T, Fujino N, Kawayama T, Sugiura H. Triple versus LAMA/LABA combination therapy for patients with COPD: a systematic review and meta-analysis. Respir Res. 2021;22(1):183. doi: 10.1186/s12931-021-01777-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vanfleteren L, Fabbri LM, Papi A, Petruzzelli S, Celli B. Triple therapy (ICS/LABA/LAMA) in COPD: time for a reappraisal. Int J Chron Obstruct Pulmon Dis. 2018;13:3971–3981. doi: 10.2147/COPD.S185975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim LHY, Saleh C, Whalen-Browne A, O'Byrne PM, Chu DK. Triple vs Dual Inhaler Therapy and Asthma Outcomes in Moderate to Severe Asthma: A Systematic Review and Meta-analysis. JAMA. 2021;325(24):2466–2479. doi: 10.1001/jama.2021.7872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Undem BJ, Pelleg A. A-317491 inhibits the activation of guinea-pig pulmonary vagal sensory nerve terminals by α, β-methylene-ATP. Clin Immunol. 2005;115(Suppl 1):S59–60. [Google Scholar]
- 35.Marucci G, Dal Ben D, Buccioni M, Marti Navia A, Spinaci A, Volpini R, Lambertucci C. Update on novel purinergic P2X3 and P2X2/3 receptor antagonists and their potential therapeutic applications. Expert Opin Ther Pat. 2019;29(12):943–963. doi: 10.1080/13543776.2019.1693542. [DOI] [PubMed] [Google Scholar]
- 36.Pelleg A, Xu F, Zhuang J, Undem B, Burnstock G. DT-0111: a novel drug-candidate for the treatment of COPD and chronic cough. Ther Adv Respir Dis. 2019;13:1753466619877960. doi: 10.1177/1753466619877960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;377(6548):428–431. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- 38.Vandenbeuch A, Larson ED, Anderson CB, Smith SA, Ford AP, Finger TE, Kinnamon SC. Postsynaptic P2X3-containing receptors in gustatory nerve fibres mediate responses to all taste qualities in mice. J Physiol. 2015;593(5):1113–1125. doi: 10.1113/jphysiol.2014.281014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krajewski JL. P2X3-Containing Receptors as Targets for the Treatment of Chronic Pain. Neurotherapeutics. 2020;17(3):826–838. doi: 10.1007/s13311-020-00934-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jarvis MF, Burgard EC, McGaraughty S, Honore P, Lynch K, Brennan TJ, Subieta A, Van Biesen T, Cartmell J, Bianchi B, Niforatos W, Kage K, Yu H, Mikusa J, Wismer CT, Zhu CZ, Chu K, Lee CH, Stewart AO, Polakowski J, Cox BF, Kowaluk E, Williams M, Sullivan J, Faltynek C (2002) A-317491, a novel potent and selective non-nucleotide antagonist of P2X3 and P2X2/3 receptors, reduces chronic inflammatory and neuropathic pain in the rat. Proc Natl AcadSci U S A 99(26):17179-17184 [DOI] [PMC free article] [PubMed]
- 41.Mansoor SE, Lu W, Oosterheert W, Shekhar M, Tajkhorshid E, Gouaux E. X-ray structures define human P2X(3) receptor gating cycle and antagonist action. Nature. 2016;538(7623):66–71. doi: 10.1038/nature19367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bolcskei H, Farkas B. P2X3 and P2X2/3 receptor antagonists. Pharm Pat Anal. 2014;3(1):53–64. doi: 10.4155/ppa.13.70. [DOI] [PubMed] [Google Scholar]
- 43.Dane C, Stokes L, Jorgensen WT. P2X receptor antagonists and their potential as therapeutics: a patent review (2010–2021) Expert Opin Ther Pat. 2022;32(7):769–790. doi: 10.1080/13543776.2022.2069010. [DOI] [PubMed] [Google Scholar]
- 44.Xu J, Kussmaul W, Kurnik PB, Al-Ahdav M, Pelleg A. Electrophysiological-anatomic correlates of ATP-triggered vagal reflex in the dog. V. Role of purinergic receptors. Am J Physiol Regul Integr Comp Physiol. 2005;288(3):R651–R655. doi: 10.1152/ajpregu.00553.2004. [DOI] [PubMed] [Google Scholar]
- 45.Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310(5753):1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- 46.Ishida Y, Ugawa S, Ueda T, Yamada T, Shibata Y, Hondoh A, Inoue K, Yu Y, Shimada S. P2X(2)- and P2X(3)-positive fibers in fungiform papillae originate from the chorda tympani but not the trigeminal nerve in rats and mice. J Comp Neurol. 2009;514(2):131–144. doi: 10.1002/cne.22000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
N/A.