Abstract
The activity of ampicillin-sulbactam against β-lactamase-producing Escherichia coli has been questioned. Therefore, in this study, the killing activity of ampicillin-sulbactam was investigated in an in vitro infection model which simulates human pharmacokinetics. One ampicillin-sensitive strain (E. coli ATCC 25922, ampicillin-sulbactam MIC = 4/2 μg/ml) and three ampicillin-resistant TEM-1-producing strains with various levels of ampicillin-sulbactam resistance (EC11, MIC = 4/2 μg/ml; TIM2, MIC = 12/6 μg/ml; and GB85, MIC > 128/64 μg/ml) were studied. The E. coli strains were exposed to ampicillin-sulbactam at a starting inoculum of 6 to 7 log10 CFU/ml. Ampicillin-sulbactam was infused over 30 min to simulate doses of 3 and 1.5 g every 6 h for 24 h. The 3-g ampicillin-sulbactam dose was bactericidal against E. coli ATCC 25922, EC11, and TIM2. The 1.5-g dose displayed bactericidal activity against ATCC 25922 and EC11 similar to that of the higher dose but failed to kill TIM2 due to inadequate time above the MIC and increased MICs over 24 h. GB85 was highly resistant and grew similarly to controls. Despite an MIC at 107 CFU/ml indicating resistance (20/10 μg/ml), TIM2 was killed by the 3-g dose of ampicillin-sulbactam. Current MIC breakpoints may not adequately portray the activity of ampicillin-sulbactam, considering both the activity in in vitro infection models and clinical data.
Ampicillin-sulbactam was introduced in 1986 as an effective alternative antibiotic for numerous infections, including those caused by bacteria producing β-lactamase. However, controversy has surrounded the in vitro determination of resistance to antibiotic–β-lactamase inhibitor combinations in the clinical laboratory (1, 7, 8). The disk diffusion and microdilution methods have used a fixed ratio of ampicillin to sulbactam. This approach has several potential problems: the inhibitor is not tested at concentrations that are achieved clinically, and the inhibition of β-lactamase activity may be sub- or supraphysiologic. Studies evaluating Escherichia coli susceptibility to ampicillin-sulbactam have shown a resistance rate of 20 to 80% (7, 12, 16). The mechanism of E. coli resistance to ampicillin consists primarily of the production of TEM-1 β-lactamase (17). Sulbactam is an effective inhibitor of TEM-1 and other β-lactamases, although it is less active than clavulanic acid (7). Therefore, the observed resistance could be a result of inadequate concentrations of sulbactam or difficulties with the susceptibility testing methodology.
To overcome these obstacles, ampicillin-sulbactam was investigated in an in vitro infection model simulating human pharmacokinetics against TEM-1-producing E. coli isolates for which the MICs vary.
MATERIALS AND METHODS
Bacterial strains.
Four E. coli strains were evaluated. E. coli EC11, TIM2, and GB85 (kindly supplied by Christine Sanders) produce different amounts of TEM-1 β-lactamase. E. coli ATCC 25922 served as a control strain since it does not produce a β-lactamase.
Antibiotics and medium.
Ampicillin, lot M00996-01, and sulbactam, lot Y013-39140, were provided by Pfizer, Inc., New York, N.Y. Appropriate antibiotic concentrations were produced immediately prior to experiments through dilution in distilled deionized water. Mueller-Hinton broth (Difco, Detroit, Mich.) supplemented with calcium (25 mg/liter) and magnesium (12.5 mg/liter) (SMHB) was used for all experiments and susceptibility testing.
Susceptibility testing.
MICs were determined by broth microdilution in SMHB according to National Committee for Clinical Laboratory Standards methods (14). MICs were also measured with an inoculum of 107 CFU/ml. Arithmetic antibiotic dilutions were used to obtain a more accurate MIC for TIM2.
In vitro model.
The model consisted of separate central and peripheral compartments (13). The central compartment was a 450-ml glass chamber which allowed exposure of the peripheral compartment to simulated serum antibiotic concentrations. The peripheral compartment was a hollow 7-ml glass T tube containing the bacterial inoculum. Each end of the T tube was covered with an inert 0.2-μm-pore-size polysulfone membrane to both allow antibiotic penetration and hold the bacterial inoculum. A programmable peristaltic pump supplied fresh SMHB to the system at the rate at which antibiotic-containing broth was removed. This model clearance produced a logarithmic decline in antibiotic concentrations and a 1-h half-life. The entire apparatus was maintained in a water bath at 37°C. Model experiments were assessed over 24 h in duplicate on different days.
The bacterial inoculum was injected aseptically into the peripheral compartment 1 h prior to antibiotic infusion to allow exponential growth. Ampicillin-sulbactam was infused over 30 min into the central compartment at 0, 6, 12, and 18 h. Ampicillin-sulbactam regimens of 3 and 1.5 g every 6 h were simulated in the model. Desired peak concentrations of ampicillin for the 3- and 1.5-g doses were 100 and 50 μg/ml, respectively (5). Sulbactam peak concentrations were 50% of ampicillin concentrations.
Pharmacodynamic analysis.
Aliquots (0.1 ml) were removed for determination of bacterial counts at 0, 1, 2, 4, 6, 7, 8, 10, 12, 13, 14, 16, 18, 19, and 24 h. After suitable 10-fold dilutions with cold 0.9% sodium chloride, 20 μl was plated onto tryptic soy agar (TSA) in triplicate. The plates were incubated for 18 to 24 h, colonies were counted, and log10 CFU per milliliter were calculated.
Antibiotic carryover experiments were conducted to identify samples affected by residual ampicillin-sulbactam. Antibiotic samples were allowed to adsorb to TSA or placed in 10 ml of 0.9% sodium chloride and passed through a 0.45-μm-pore-size filter. Samples with known bacterial counts were then placed on TSA or filtered in the same way. Filters were then aseptically transferred to TSA plates and incubated overnight, and colonies were counted. Carryover was defined as a decrease in bacterial count to less than 80% of control growth for both methods. No filtered concentrations demonstrated antibiotic carryover. For samples with bacterial counts below the level of detection or dilutions affected by antibiotic carryover, 0.05-ml samples were filtered as described above. The overall limit of detection for this methodology was 200 CFU/ml. Killing curves were prepared by plotting the log10 CFU per milliliter versus time. The time required to achieve a 99.9% reduction in log10 CFU per milliliter was determined visually from the killing curves.
Resistance.
The development of resistance was monitored by determining MICs for isolates from the 6-, 12-, 18-, and 24-h time points. The samples were grown overnight and exposed to ampicillin-sulbactam (2:1) at 5 × 105 CFU/ml.
Pharmacokinetics analysis.
Samples were taken from the central compartment at 0.5, 1, 3, 6, 6.5, 9, and 12 h and from the peripheral compartment at 0.5, 3, 6, 9, and 12 h. Ampicillin and sulbactam half-lives were determined for the central compartment from the slope of the log concentration-versus-time curve. Ampicillin and sulbactam concentrations were determined by high-pressure liquid chromatography in the laboratory of Roger Bawdon as previously described (11). The coefficients of variation for ampicillin and sulbactam were less than 10%, and the assay was linear from 1 to 200 μg/ml. Time above the MIC (T>MIC) was calculated directly from the pharmacokinetic parameters and the MIC at 5 × 105 CFU/ml. The area under the curve from 0 to 6 h (AUC0–6) for the central compartment was determined by the trapezoidal-rule method.
Statistical analysis.
Analysis of variance was used to assess change in the log10 CFU per milliliter at 24 h with Tukey’s test for multiple comparisons. A P value of <0.05 was considered significant.
RESULTS
Susceptibility.
The MICs of ampicillin-sulbactam are listed in Table 1 for each isolate at 5 × 105 CFU/ml. The model also utilized an inoculum of 107 CFU/ml; therefore, MICs were evaluated for this inoculum as well. At 107 CFU/ml, the MICs for ATCC 25922 and TIM2 increased by 1 dilution. E. coli TIM2 was classified as having intermediate susceptibility at 5 × 105 CFU/ml and as resistant at 107 CFU/ml. E. coli GB85 was highly resistant to ampicillin-sulbactam.
TABLE 1.
Susceptibilities of E. coli strains to ampicillin-sulbactam
| E. coli strain | MIC, 2:1 (μg/ml), with an inoculum of:
|
|
|---|---|---|
| 5 × 105 CFU/ml | 1 × 107 CFU/ml | |
| ATCC 25922 | 4/2 | 8/4 |
| EC11 | 4/2 | 4/2 |
| TIM2 | 12/6 | 20/10 |
| GB85 | >128/64 | >128/64 |
Pharmacokinetics.
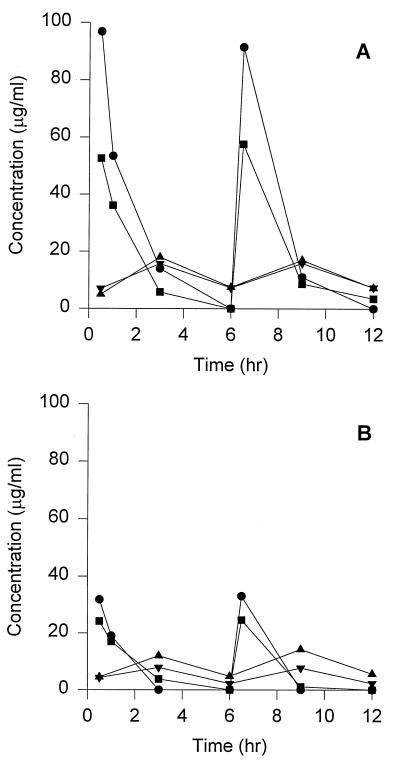
The pharmacokinetic profiles of ampicillin and sulbactam are shown in Fig. 1. Peak ampicillin and sulbactam concentrations for the simulated 3-g dose were 94.2 ± 3.8 μg/ml and 55.1 ± 3.5 μg/ml, respectively. The 1.5-g dose produced ampicillin and sulbactam peak concentrations of 32.4 ± 0.8 μg/ml and 24.4 ± 0.3 μg/ml, respectively. Trough concentrations for ampicillin and sulbactam were generally undetectable. The mean elimination half-life was 0.9 h in the central compartment. The AUC0–6s for the 3-g ampicillin and sulbactam doses were 150 and 86 μg · h/ml, respectively. The 1.5-g dose produced AUC0–6s of 52 μg · h/ml for ampicillin and 43 μg · h/ml for sulbactam. A limited number of samples were drawn from the peripheral compartment; therefore, comprehensive peak concentrations, AUCs, and elimination half-lives could not be calculated. Measured peripheral-compartment peak concentrations were above the MIC for ATCC 25922 and EC11; however, they were never above the MIC of 20/10 μg/ml for TIM2. Peak peripheral-compartment sulbactam concentrations were 15.7 and 7.9 μg/ml for the 3- and 1.5-g doses, respectively.
FIG. 1.
Ampicillin-sulbactam pharmacokinetic profiles for 3-g (A) and 1.5-g (B) doses. •, ampicillin in the central compartment; ▪, sulbactam in the central compartment; ▴, ampicillin in the peripheral compartment; ▾, sulbactam in the peripheral compartment.
Pharmacodynamics. (i) Ampicillin-sulbactam at a 3-g dose.
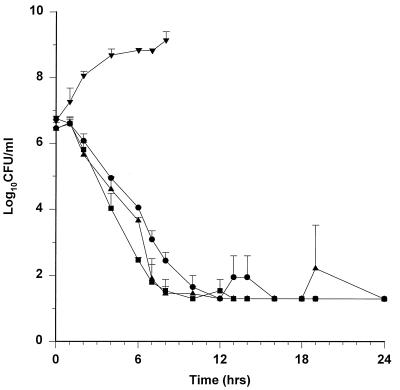
The killing curves for all isolates are shown in Fig. 2. Growth controls were similar for all strains (data not shown). Table 2 shows the bactericidal activities and pharmacodynamic parameters. Against strains ATCC 25922, EC11, and TIM2, the 3-g ampicillin-sulbactam dose required 4.6 to 6.5 h to decrease the bacterial count by 99.9%. E. coli GB85 grew unaffected by ampicillin-sulbactam. At 24 h, bacterial counts were either at or below the level of detection for ATCC 25922, EC11, and TIM2. The 24-h colony count was statistically higher for GB85 than for the other strains (P < 0.05). Ampicillin-sulbactam central-compartment concentrations were above the MIC for 3.2 to 4.6 h (53 to 77%) of the 6-h dosing interval for ATCC 25922, EC11, and TIM2. At no time were the concentrations in the central compartment above the MIC for GB85. MICs for isolates in the model did not show any changes during the 24-h experiment.
FIG. 2.
Killing curves for 3 g of ampicillin-sulbactam every 6 h. Values are means ± standard deviations. •, ATCC 25922; ▪, EC11; ▴, TIM2; ▾, GB85.
TABLE 2.
Bactericidal activities and pharmacodynamic parameters
| E. coli isolate | Dose (g) | Time for bacterial count to decrease 99.9% (h) | T>MIC (h) | Colony count at 24 h (log10 CFU/ml) |
|---|---|---|---|---|
| ATCC 25922 | 3 | 6.5 | 4.6 | 1.3 |
| 1.5 | 7.5 | 3.2 | 1.3 | |
| EC11 | 3 | 4.6 | 4.6 | 1.3 |
| 1.5 | 9.3 | 3.2 | 2.2a | |
| TIM2 | 3 | 6.1 | 3.2 | 1.3 |
| 1.5 | >24 | 1.6 | 9.1b | |
| GB85 | 3 | >24 | 0 | 9.2 |
| 1.5 | >24 | 0 | 9.2 |
P < 0.05, compared to all other values.
P < 0.05, compared to values for all strains except GB85 (3- and 1.5-g doses).
(ii) Ampicillin-sulbactam at a 1.5-g dose.
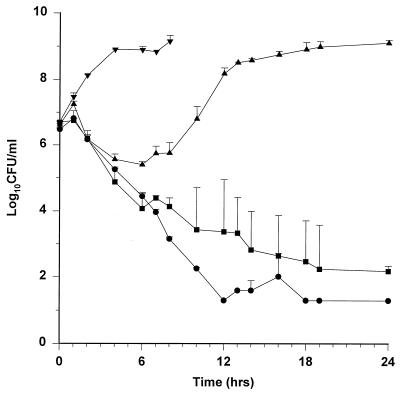
The killing curves for the 1.5-g dose are shown in Fig. 3. The lower dose of ampicillin-sulbactam was effective against ATCC 25922 and EC11. However, it had a minimal and short-lived inhibitory effect on the growth of TIM2. E. coli GB85 growth was similar to that of controls. The T>MICs were lower for all isolates than they were with the 3-g dose and were maintained for only 27% of the dosing interval for TIM2. The 24-h colony count for ATCC 25922 was statistically different from those for EC11, TIM2, and GB85 (P < 0.05). Colony counts at 24 h for TIM2 and GB85 were also statistically different from those for EC11 (P < 0.05). MICs for isolates from the TIM2 model experiments were increased at 12, 18, and 24 h (≥64/32 μg/ml).
FIG. 3.
Killing curves for 1.5 g of ampicillin-sulbactam every 6 h. Values are means ± standard deviations. •, ATCC 25922; ▪, EC11; ▴, TIM2; ▾, GB85.
DISCUSSION
High rates of E. coli resistance to ampicillin-sulbactam have been reported; however, clinical resistance to ampicillin-sulbactam is infrequent (6–8). An in vitro infection model was employed to investigate ampicillin-sulbactam’s activity against E. coli producing TEM-1 β-lactamase.
The four E. coli strains were chosen to represent a range of TEM-1 β-lactamase production. E. coli ATCC 25922, a β-lactamase nonproducer, served as a control. E. coli EC11, TIM2, and GB85 produced various amounts of TEM-1 β-lactamase, as evidenced by the MICs for each strain. E. coli EC11 was ampicillin-sulbactam susceptible, with MICs similar to those for ATCC 25922. E. coli TIM2 had intermediate susceptibility at the inoculum of 5 × 105 CFU/ml. When tested at the inoculum of 107 CFU/ml, TIM2 was classified as resistant to ampicillin-sulbactam. Ampicillin-sulbactam was not expected to have activity against GB85, on the basis of its MIC and the achievable concentrations.
Ampicillin-sulbactam model pharmacokinetic parameters were similar to literature values except that the ampicillin peak concentration and AUC for the 1.5-g dose were lower than expected (5). The calculated T>MICs ranged from 0 to 77% of the dosing interval. For β-lactam antibiotics, the T>MIC is the best predictor of efficacy (9, 19). In this experiment, too, the T>MIC was correlated with efficacy. Failures were seen when T>MICs were 27% or less of the dosing interval. The minimum T>MIC necessary for efficacy is not well defined in the literature. However, T>MICs of less than 50% of the dosing interval are more frequently observed to result in poor efficacy (19). The pharmacodynamic parameter most associated with efficacy for β-lactamase inhibitors has not been well defined (8). However, sulbactam concentrations were above the 0.5 to 10 μg/ml necessary to restore ampicillin susceptibility (4, 15, 20). Sulbactam concentrations were similar to values reported for drug penetration into blister fluid (5).
Ampicillin-sulbactam administered in a 3-g dose every 6 h was effective against all E. coli strains except for the highly resistant GB85. The time required to reduce the viable counts by 99.9% was similar to that required for other β-lactams against gram-negative bacteria in this model (13). Minimal regrowth at the end of the dosing interval was observed for ATCC 25922 and TIM2. These findings were likely due to antibiotic concentrations falling below the MIC. In both instances, the next dose was capable of reducing the colony counts to the level of detection or below. The ampicillin-sulbactam 1.5-g dose was bactericidal against ATCC 25922 and EC11; however, it failed to kill TIM2. Overall, the time required for a 99.9% reduction in the viable counts was longer for the 1.5-g dose as a result of the shorter T>MIC. E. coli EC11 colony counts were statistically higher than those for ATCC 25922 at 24 h. However, this would not likely result in a clinical difference, since the bacterial count had already been reduced by 99.9% and subsequent doses would continue to contribute to efficacy. Against TIM2, the 1.5-g dose was insufficient to kill the organism or prevent the emergence of a resistant subpopulation. The lower-than-expected ampicillin peak concentrations may have contributed to the failure. However, the T>MIC would have increased to a maximum of only 2.3 h, or 38% of the dosing interval, which would be inadequate for maximum efficacy. In clinical situations with a large inoculum, a MIC of ≥16/8 to 32/16 μg/ml, and neutropenia, the 3-g dose would be preferred, based on these results.
Several investigators have evaluated the efficacy of ampicillin-sulbactam against β-lactamase-producing strains of Enterobacteriaceae. Rice and colleagues evaluated ampicillin-sulbactam and cefoxitin against E. coli in a rat intra-abdominal-abscess model (16). Two strains, M6 and M44, produced TEM-1 β-lactamase, and the ampicillin-sulbactam MICs were 32/16 and 128/64 μg/ml, respectively. Ampicillin-sulbactam at a dosage of 500/250 mg/kg of body weight/day was administered by continuous infusion for 3 days. Cefoxitin was more effective in this model; however, ampicillin-sulbactam demonstrated acceptable activity against M6 despite the fact that it never reached concentrations above the MIC in serum. This may indicate that our current susceptibility testing method does not correlate directly with achievable concentrations in serum. Rice and colleagues concluded that ampicillin-sulbactam may be considered for the treatment of infections caused by moderately resistant β-lactamase (TEM)-producing isolates. Lister and Sanders investigated the efficacy of ampicillin-sulbactam in a murine bacteremia model (10). Both 3- and 1.5-g doses were simulated over only a 6-h period. Numerous E. coli strains producing TEM-1 β-lactamase were evaluated. Due to the higher clearance rates seen in this model, ampicillin-sulbactam was administered at the beginning of the experiment and 1 h later. This dosing method successfully simulated expected human peak concentrations, AUCs, and T>MICs. Ampicillin-sulbactam in both the 3- and 1.5-g doses was effective in preventing lethal septicemia from E. coli strains for which MICs were ≤32/16 μg/ml. Craig and Ebert studied 16 strains of Enterobacteriaceae in a neutropenic mouse thigh infection model (3). Pharmacokinetics comparable to those in humans were achieved through induction of reproducible nephrotoxicity. Mice were treated for 24 h with high and low ampicillin-sulbactam dosages given every 6 h. Enterobacteriaceae for which MICs were greater than 16/8 μg/ml were minimally killed; therefore, the in vivo MIC breakpoint appeared to be 16/8 μg/ml.
Direct comparisons between the present study and previous animal models are difficult. From this experiment and others, it appears that ampicillin-sulbactam has activity against E. coli for which MICs are 16/8 to 32/16 μg/ml. The findings of Lister and Sanders and of Craig et al. may be interpreted as conflicting; however, the use of a neutropenic host may have influenced the results. Both Rice et al. and Lister and Sanders observed that ampicillin-sulbactam maintained a low degree of activity even against E. coli for which MICs were 128/64 μg/ml. Lister and Sanders (10) hypothesized that the bactericidal activity of serum, subinhibitory antibiotic concentrations, and leukocyte phagocytosis may have contributed to these findings (9, 18). It is unknown how these factors may affect more susceptible bacteria.
Clinical data which evaluates ampicillin-sulbactam’s efficacy against resistant bacteria is limited. Castellano reviewed five lower respiratory tract infection studies and reported three patients with ampicillin-sulbactam-resistant pathogens (E. coli, Serratia marcescens, and Klebsiella pneumoniae) (2). The dosage was 3 g every 6 h for 5 to 10 days. Of these three patients, only the E. coli-infected patient was clinically and bacteriologically cured. Güneren evaluated a multicenter study of outpatient infections treated with 0.5/0.25 g of ampicillin-sulbactam given intramuscularly every 12 h (6). Treatment length ranged from 4 to 15 days depending on the type of infection. The sites of infections included the genitourinary tract; the ear, nose, and throat; the respiratory tract; and skin and soft tissue. Clinical efficacies (cure plus improvement) were similar regardless of ampicillin-sulbactam susceptibility. In fact, no clinical failures were seen in the 12 patients with ampicillin-sulbactam-resistant bacteria. Bacteriologic persistence occurred in 18 of 409 (4.4%), 5 of 43 (11.6%), and 1 of 12 (8.3%) patients infected with ampicillin-sulbactam-sensitive, intermediate, and resistant isolates, respectively. A greater rate of persistence in genitourinary tract infections was noted with E. coli. Possible explanations for this result may have been complicated patient presentation, low doses of ampicillin-sulbactam, and resistance mechanisms other than β-lactamase production. From this data, it is not clear that ampicillin-sulbactam resistance ultimately leads to clinical failure.
A more recent review of the clinical and bacteriologic response to ampicillin-sulbactam has been completed by Jones and Dudley (8). Two hundred fifty-two patients with a defined clinical and bacteriologic response from an infection caused by members of the Enterobacteriaceae were reviewed. For the 185 patients who received the 3-g dose, there was no correlation between MIC and clinical or bacteriologic response. The percentages of patients with a clinical cure or improvement and bacteriologic eradication for MICs of ≤8/4, 16, 32, and ≥64 μg/ml were 85, 87.5, 80, and 81.6%, respectively. All eight patients with isolates for which the MIC was 16/8 μg/ml were either cured or improved, and seven had complete eradication of the baseline pathogen. Sixty-seven patients receiving the 1.5-g dose had greater percentages of bacteriologic persistence than those receiving the 3-g dose (13.4 versus 6.5%), and, of particular interest, the MICs for all of the persistent isolates in the 1.5-g group were ≤8/4 μg/ml. However, clinical responses for the doses were equivalent when isolates for which MICs were ≤16/8 μg/ml were evaluated (95 versus 93%). After considering this data and evaluating studies of in vitro susceptibility and interpretative error, Jones and Dudley recommended susceptible, intermediate, and resistant MIC breakpoints of ≤16/8, 32/16, and ≥64/32 μg/ml, respectively.
In conclusion, 3 g of ampicillin-sulbactam every 6 h was an effective regimen for all susceptible strains, including an E. coli strain for which the MIC was 20/10 μg/ml at the model inoculum of 107 CFU/ml. The lower dose of ampicillin-sulbactam was bactericidal against ATCC 25922 and EC11 but were not effective against TIM2 as a result of an inadequate T>MIC and the growth of a resistant subpopulation. A 3-g dose in this model and an ampicillin-sulbactam MIC of 16/8 μg/ml were predictive of efficacy; an MIC of 32/16 μg/ml would be somewhat less effective. Current MIC breakpoints may not adequately portray the activity of ampicillin-sulbactam, considering both the activity in in vitro infection models and clinical data.
ACKNOWLEDGMENT
This work was supported by a grant from Pfizer, Inc.
REFERENCES
- 1.Bradford P A, Sanders C C. Use of a predictor panel to evaluate susceptibility testing methods for ampicillin-sulbactam. Antimicrob Agents Chemother. 1993;37:251–259. doi: 10.1128/aac.37.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castellano, M. A. 1988. Sulbactam/ampicillin in the treatment of lower respiratory infections. Drugs 35(Suppl. 7):53–56. [DOI] [PubMed]
- 3.Craig W A, Ebert S. Abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. Activity of ampicillin/sulbactam with simulation of human pharmacokinetics in an animal model, abstr. A-61; p. 12. [Google Scholar]
- 4.English A R, Retsema J A, Girard A E, Lynch J E, Barth W E. CP-45,899, a beta-lactamase inhibitor that extends the antibacterial spectrum of beta-lactams: initial bacteriological characterization. Antimicrob Agents Chemother. 1978;14:414–419. doi: 10.1128/aac.14.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foulds, G. 1986. Pharmacokinetics of sulbactam/ampicillin in humans: a review. Rev. Infect. Dis. 8(Suppl. 5):S503–S511. [DOI] [PubMed]
- 6.Güneren, M. F. 1988. Clinical experience with intramuscular sulbactam/ampicillin in the outpatient treatment of various infections: a multicenter trial. Drugs 35(Suppl. 7):57–68. [DOI] [PubMed]
- 7.Itokazu G S, Danziger L H. Ampicillin-sulbactam and ticarcillin-clavulanic acid: a comparison of their in vitro activity and review of their clinical efficacy. Pharmacotherapy. 1991;11:382–414. [PubMed] [Google Scholar]
- 8.Jones R N, Dudley M N. Microbiologic and pharmacodynamic principles applied to the antimicrobial susceptibility testing of ampicillin/sulbactam: analysis of the correlations between in vitro test results and clinical response. Diagn Microbiol Infect Dis. 1997;28:5–18. doi: 10.1016/s0732-8893(97)00013-8. [DOI] [PubMed] [Google Scholar]
- 9.Leggett J E, Fantin B, Ebert S, Totsuka K, Vogelman B, Calame W, Mattie H, Craig W A. Comparative antibiotic dose-effect relations at several dosing intervals in murine pneumonitis and thigh-infection models. J Infect Dis. 1989;159:281–292. doi: 10.1093/infdis/159.2.281. [DOI] [PubMed] [Google Scholar]
- 10.Lister P D, Sanders C C. Comparison of ampicillin-sulbactam regimens simulating 1.5- and 3.0-gram doses to humans in treatment of Escherichia coli bacteremia in mice. Antimicrob Agents Chemother. 1995;39:930–936. doi: 10.1128/aac.39.4.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maberry M C, Trimmer K J, Bawdon R E, Sobhi S, Dax J B, Gilstrap L C. Antibiotic concentration in maternal blood, cord blood and placental tissue in women with chorioamnionitis. Gynecol Obstet Invest. 1992;33:185–186. doi: 10.1159/000294878. [DOI] [PubMed] [Google Scholar]
- 12.Martinez O, Hernandez S, Cleary T. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Escherichia coli antibiotic susceptibility to ampicillin/sulbactam: evaluation of disk diffusion, MicroScan breakpoint panel, and E test methods, abstr. D-44; p. 68. [Google Scholar]
- 13.McGrath B J, Bailey E M, Lamp K C, Rybak M J. Pharmacodynamics of once-daily amikacin in various combinations with cefepime, aztreonam, and ceftazidime against Pseudomonas aeruginosa in an in vitro infection model. Antimicrob Agents Chemother. 1992;36:2741–2746. doi: 10.1128/aac.36.12.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 3rd ed. Approved standard M7-A3. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1993. [Google Scholar]
- 15.Pfaller M A, Barry A L, Fuchs P C, Gerlach E H, Hardy D J, McLaughlin J C. Comparison of fixed concentration and fixed ratio options for dilution susceptibility testing of gram-negative bacilli to ampicillin and ampicillin/sulbactam. Eur J Clin Microbiol Infect Dis. 1993;12:356–362. doi: 10.1007/BF01964434. [DOI] [PubMed] [Google Scholar]
- 16.Rice L B, Carias L L, Shlaes D M. Efficacy of ampicillin-sulbactam versus that of cefoxitin for treatment of Escherichia coli infections in a rat intra-abdominal abscess model. Antimicrob Agents Chemother. 1993;37:610–612. doi: 10.1128/aac.37.3.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanders C C, Sanders W E., Jr β-Lactam resistance in gram-negative bacteria: global trends and clinical impact. Clin Infect Dis. 1992;15:824–839. doi: 10.1093/clind/15.5.824. [DOI] [PubMed] [Google Scholar]
- 18.Tesh V L, Duncan R L, Jr, Morrison D C. The interaction of Escherichia coli with normal human serum: the kinetics of serum-mediated lipopolysaccharide release and its dissociation from bacterial killing. J Immunol. 1986;137:1329–1335. [PubMed] [Google Scholar]
- 19.Vogelman B, Gudmundsson S, Leggett J, Turnidge J, Ebert S, Craig W A. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in an animal model. J Infect Dis. 1988;158:831–847. doi: 10.1093/infdis/158.4.831. [DOI] [PubMed] [Google Scholar]
- 20.Wise R, Andrews J M, Bedford K A. Clavulanic acid and CP-45-899: a comparison of their in vitro activity in combination with penicillins. J Antimicrob Chemother. 1980;6:197–206. doi: 10.1093/jac/6.2.197. [DOI] [PubMed] [Google Scholar]