Abstract
Background
Accurately assessing the risk of recurrence in patients with locally advanced rectal cancer (LARC) before treatment is important for the development of treatment strategies. The purpose of this study is to develop an MRI-based scoring system to predict the risk of recurrence in patients with LARC.
Methods
This was a multicenter observational study that enrolled participants who underwent neoadjuvant chemoradiotherapy. To evaluate the risk of recurrence in these patients, we developed the mrDEC scoring system and assessed inter-reader agreement. Additionally, we plotted Kaplan–Meier curves to compare the 3-year disease-free survival (DFS) and 5-year overall survival (OS) rates among patients with different mrDEC scores.
Results
A total of 1287 patients with LARC were included in this study. We observed substantial inter-reader agreement for mrDEC. Based on the mrDEC scores ranging from 0 to 3, the patients were categorized into four groups. The 3-year DFS rates for the groups were 91.0%, 79.5%, 65.5%, and 44.0% (P < 0.0001), respectively, and the 5-year OS rates were 92.9%, 87.1%, 74.8%, and 44.5%, respectively (P < 0.0001).
Conclusions
The mrDEC scoring system proved to be an effective tool for predicting the prognosis of patients with LARC and can assist clinicians in clinical decision-making.
Subject terms: Cancer imaging, Rectal cancer
Introduction
Locally advanced rectal cancer (LARC) is one of the most devastating cancers worldwide, with substantial recurrence rates despite standard treatment strategies. Local recurrence rates are estimated at 5–10%, while distant recurrence rates are estimated at 25–40% [1, 2]. Therefore, early identification of patients with a poor prognosis is crucial as it can inform treatment decisions and ensure that the benefits of the current treatment strategies are maximized. The search for the optimal neoadjuvant treatment strategy for LARC has been ongoing and has continued to evolve over time. However, these treatments tested in clinical trials may have unwanted side effects such as late neurotoxicity or a breached mesorectum [3, 4]. Therefore, a scoring system that differentiates between high-risk and low-risk recurrence in rectal cancer patients is critical for informed treatment decisions. Such a system enables clinical decision-makers to have more meaningful discussions with patients about the risks and benefits of treatment.
Magnetic resonance imaging (MRI) has become the most standardized approach for defining the locoregional clinical staging of rectal cancer and is a key factor in determining the success of selected treatment strategies [5–7]. Previous studies have confirmed the ability of MRI markers, including threatened extramural vascular invasion (EMVI) [8], tumor deposits (TDs) [9, 10], and circumferential resection margin (CRM) [11, 12], to stratify the risk of rectal cancer [13–15]. While the traditional neoadjuvant chemoradiotherapy (nCRT) strategy is commonly used in all patients with LARC (cT3-4/N0-2, or EMVI, or any T/N1-2, or lateral node positive, and M0) [5], the efficacy of neoadjuvant radiotherapy remains uncertain. In 2020, the National Institute for Health and Care Excellence (NICE) in the United Kingdom (UK) recommended preoperative radiotherapy for all patients with rectal cancer except those with radiologically staged T1–T2 or N0 tumors [16]. However, Lord et al. [17] argued that the criterion did not effectively identify the high-risk group, resulting in unwanted side effects from preoperative radiotherapy in many patients. Instead, MRI prognostic markers (EMVI, TDs, and CRM) are reliable high-risk markers for assessing the severity of rectal cancer as they can accurately predict pathological findings and substantially affect cancer outcomes [17]. Furthermore, the RAPIDO trial proposed a novel intensive neoadjuvant treatment strategy that identified high-risk MRI markers for metastasis, including T stage (T4a or T4b), EMVI, N stage (N2), involved CRM, or enlarged lateral lymph nodes [18]. In conclusion, there are no uniform MRI criteria to assist clinicians in making precise clinical decisions regarding different neoadjuvant treatment strategies.
Therefore, we aimed to develop a scoring system using MRI markers for rectal cancer to discriminate between patients at high risk of recurrence and those at low risk, thus assisting clinicians and patients in making informed decisions together.
Methods
Patients
This multicenter observational study involved Cohort 1 and Cohort 2 (a retrospective analysis of a previous prospective trial, No. NCT04271657) to assess the generalizability and prognostic power of the scoring system. Patients with LARC in Cohort 1 were retrospectively recruited from five hospitals in China, including Guangdong Provincial People’s Hospital (GDPH; Guangzhou, China), Shanxi Cancer Hospital (SXCH; Taiyuan, China), the Sixth Affiliated Hospital of Sun Yat-sen University (SYSU6; Guangzhou, China), Sun Yat-sen University Cancer Center (SYSUCC; Guangzhou, China), and Yunnan Cancer Hospital (YNCH; Kunming, China). The sample size was determined based on the number of cases in these areas during the study period.
Moreover, to assess the ability of this scoring system to predict the response to nCRT in clinical practice, a cohort study was conducted as a retrospective analysis of a prospective trial (No. NCT04271657; Cohort 2). The detailed prospectively recruiting procedure has been described in previously published literature [19]. The specific inclusion and exclusion criteria are shown in Supplementary Method 1, and the patient flow through the selection and recruitment process is given in Supplementary Fig. 1. All eligible patients received nCRT treatment (Supplementary Method 2). Patients in Cohort 1 subsequently underwent total mesorectal excision (TME) surgery, while the decision to perform TME surgery for patients in Cohort 2 was left to the discretion of the treating physician. The clinical information from patients was collected from the clinical records, and specific criteria of clinical information are in Supplementary Method 3.
This study complied with the principles of the Declaration of Helsinki and was approved by the ethics committee (retrospective study approval 2019ZSLYEC-169; NCT04271657 trial approval 2020ZSLYEC-009). This study was approved by all institutional review boards, and the need for informed consent was waived.
Definition of the follow-up and tumor regression grade (TRG)
The study evaluated two primary outcomes in Cohort 1: the 3-year disease-free survival (DFS) and 5-year overall survival (OS). In addition, the pathologists examined the histopathological TRG as a marker of nCRT response in Cohort 1 and Cohort 2. The TRG category used was based on the American Association of Cancer/College of American Pathologists (AJCC/CAP) system, with four categories ranging from complete response (TRG 0) to poor response (TRG 3) (Supplementary Method 4) [20]. We retrospectively collected the individual TRG category from the five participating centers. The TRG category data for the prospective trial were independently defined by two experienced gastroenterology pathologists who were blinded to the clinicopathological information of the patients. In cases where there was disagreement between the two pathologists, a third expert pathologist was responsible for making the final decision.
MRI image acquisition and scoring system development
For each patient, axial high-resolution T2-weighted image (T2WI), contrast-enhanced T1-weighted image (CE-T1WI), and diffusion-weighted imaging (DWI) with two b values (0 and 800 s/mm2 or 1000 s/mm2) were acquired by 1.5 T or 3.0 T scanners at the participating hospitals. The acquisition parameters of MRI scans derived from specific-vendor devices are summarized in Supplementary Table 1. The raw data of MRI were exported from the PACS (picture archiving and communication systems) as DICOM files and subsequently evaluated by radiologists using RadiAnt DICOM Viewer (Version 5.0.1). The radiologists evaluated several imaging makers derived from pelvic MRI in rectal cancer, including the mrN and mrT stage, mrTDs, mrEMVI, and mrCRM. Diagnostic criteria for MRI markers of rectal cancer are detailed in Supplementary Table 2. MRI imaging markers were evaluated and measured by two radiologists with more than 5 years and less than 10 years experiences in interpreting abdominal imaging. In the event of disagreement, the final decision was made by an arbitration expert, a senior radiologist with over 10 years of experience.
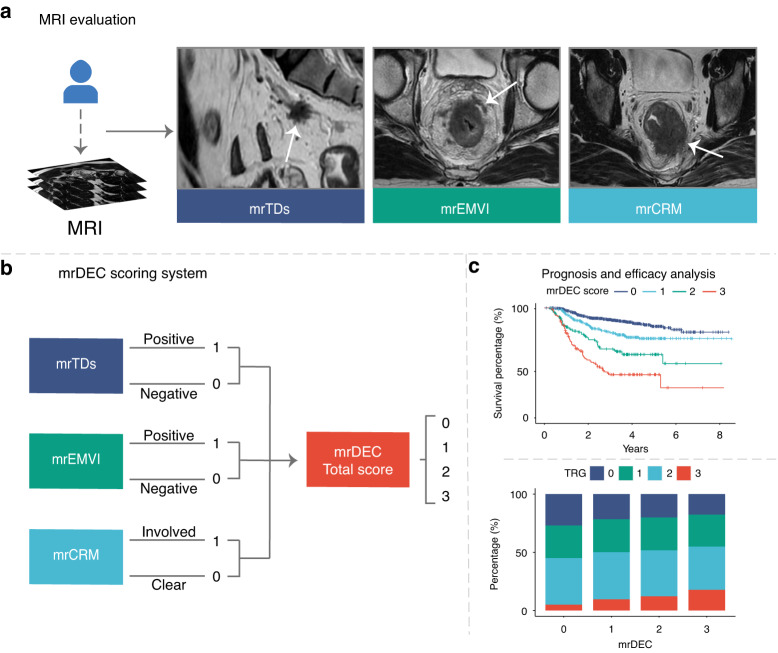
Subsequently, we attempted to establish a scoring system to complement the TNM staging system and evaluate the risk of recurrence in LARC patients. The system incorporated three MRI markers to achieve this objective, including mrCRM, mrEMVI, and mrTDs. Each marker was assigned a score of 1 for positive and 0 for negative, and then these scores were accumulated to create the mrDEC scoring system. The range of scores was from 0 (when all three MRI markers were negative) to 3 (when all three MRI markers were positive). With this scoring system, patients were categorized into four risk groups: low-risk (score = 0), intermediate-low-risk (score = 1), intermediate-high-risk (score = 2), and high-risk (score = 3). The entire workflow of our scoring system is presented in Fig. 1.
Fig. 1. Evaluation workstreams and construction of scoring system.
a Evaluation of rectal cancer magnetic resonance image. b The construction of the mrDEC scoring system. c Prognosis and efficacy analysis according to mrDEC score. mr magnetic resonance, TD tumor deposit, EMVI extramural vascular invasion, CRM circumferential resection margin, TRG tumor regression grade.
To evaluate the reproducibility of the MRI markers, we randomly selected 60 consecutive patients and had six radiologists independently assess the status of mrTDs, mrEMVI, and mrCRM. The radiologists were blinded to clinical and histopathological information, and were grouped according to their level of experience. The first group included two junior radiologists with between 3 and 5 years of training (WL, YL as reader 1 and 2), the second group consisted of two intermediate radiologists with between 5 and 10 years of training (MZ, QQ as reader 3 and 4), and the third group included two senior radiologists with over 10 years of training (LK, XY as reader 5 and 6). By categorizing the radiologists in this way, we aimed to assess the impact of experience on the reproducibility of the MRI markers.
Statistical analysis
Quantitative statistics are presented using mean ± standard deviation (SD). The Student’s test or Wilcoxon signed-rank test was used to compare continuous variables, while the χ2 test or Fisher’s exact test was used to compare categorical variables. Kaplan–Meier curves were plotted to compare 3-year DFS and 5-year OS rates for each patient group. All variables with P < 0.1 evaluated by univariate analysis were subjected to multivariate analysis. Forward step-wise selection was used in multivariate analysis to determine the independent variables. Cox multivariate analysis was used to estimate hazard ratios (HR) with 95% confidence intervals (CIs) to assess the prognostic value of each variable. Markers and system prognostic performance were quantified according to the concordance index (C-index). Two-sided P values at or below 0.05 were considered significant. R studio (version 3.1.0) was used for all statistical analyses.
The inter-reader agreement of MRI markers was calculated using Cohen’s kappa. Linear weighted kappa tests were used for mrTDs, mrEMVI, and mrCRM, and a squared weighted kappa test was used for mrDEC. The level of agreement was categorized as moderate agreement (kappa between 0.4 and 0.6), substantial agreement (kappa between 0.6 and 0.8), and excellent agreement (kappa >0.8) [21].
Results
Patients
Between September 2009 and June 2020, a total of 1287 patients from two cohorts were included in the study. Cohort 1 consisted of 1187 patients from five centers who underwent nCRT followed by radical surgery. The mean ages of these patients were 55.3 ± 9.9, 53.6 ± 10.6, 54.1 ± 11.5, 54.7 ± 11.6, and 56.1 ± 11.4 years, respectively (Table 1). In Cohort 2, we retrospectively analyzed 100 patients from the NCT04271657 trial, of whom 96 (96%) received radical resection, and 4 (4%) received local excision. The mean age of patients in Cohort 2 was 57.3 ± 12.1 years (Table 1). Table 1 summarizes the clinical characteristics and radiological information distribution across centers. Descriptive patient and tumor characteristics statistics depending on the MRI markers and mrDEC score are shown in Supplementary Tables 3, 4, 5, and 6.
Table 1.
The distributions of demographic and tumor characteristics of rectal cancer patients in five centers and NCT04271657 trial.
| GDPH (n = 54) | SXCH (n = 185) | SYSU6 (n = 300) | SYSUCC (n = 477) | YNCH (n = 171) | Total (n = 1187) | Cohort 2 (n = 100) | |
|---|---|---|---|---|---|---|---|
| Age, years | |||||||
| Mean ± SD | 55.3 ± 9.9 | 53.6 ± 10.6 | 54.1 ± 11.5 | 54.7 ± 11.6 | 56.1 ± 11.4 | 54.6 ± 11.3 | 57.3 ± 12.1 |
| Sex | |||||||
| Male | 39 (72.2) | 110 (59.5) | 212 (70.7) | 314 (65.8) | 115 (67.3) | 790 (66.6) | 76 (76.0) |
| Female | 15 (27.8) | 75 (40.5) | 88 (29.3) | 163 (34.2) | 56 (32.7) | 397 (33.4) | 24 (24.0) |
| CEA levela | |||||||
| Normal | 45 (83.3) | 116 (62.7) | 178 (59.3) | 266 (55.8) | 91 (53.2) | 696 (58.6) | 0 (0.0) |
| Abnormal | 9 (16.7) | 69 (37.3) | 122 (40.7) | 211 (44.2) | 80 (46.8) | 491 (41.4) | 0 (0.0) |
| NA | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 100 (100) |
| Location | |||||||
| Low | 26 (48.1) | 83 (44.9) | 161 (53.7) | 221 (46.3) | 78 (45.6) | 569 (47.9) | 0 (0.0) |
| Middle | 27 (50.0) | 89 (48.1) | 130 (43.3) | 224 (47.0) | 84 (49.1) | 554 (46.7) | 0 (0.0) |
| High | 1 (1.9) | 13 (7.0) | 9 (3.0) | 32 (6.7) | 9 (5.3) | 64 (5.4) | 0 (0.0) |
| NA | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 100 (100) |
| cTNM stage | |||||||
| II | 3 (5.6) | 26 (14.1) | 52 (17.3) | 78 (16.4) | 34 (19.9) | 193 (16.3) | 0 (0.0) |
| III | 51 (94.4) | 159 (85.9) | 248 (82.7) | 399 (83.6) | 137 (80.1) | 994 (83.7) | 100 (100) |
| mrT stage | |||||||
| T1–T2 | 0 (0.0) | 0 (0.0) | 6 (2.0) | 2 (0.4) | 2 (1.2) | 10 (0.8) | 0 (0.0) |
| T3 | 47 (87.0) | 142 (76.8) | 246 (82.0) | 401 (84.1) | 141 (82.5) | 977 (82.3) | 79 (79.0) |
| T4 | 7 (13.0) | 43 (23.2) | 48 (16.0) | 74 (15.5) | 28 (16.4) | 200 (16.8) | 21 (21.0) |
| mrN stage | |||||||
| N0 | 14 (25.9) | 36 (19.5) | 80 (26.7) | 155 (32.5) | 63 (36.8) | 348 (29.3) | 23 (23.0) |
| N1 | 16 (29.6) | 73 (39.5) | 107 (35.7) | 188 (39.4) | 56 (32.7) | 440 (37.1) | 38 (38.0) |
| N2 | 24 (44.4) | 76 (41.1) | 113 (37.7) | 134 (28.1) | 52 (30.4) | 399 (33.6) | 39 (39.0) |
| mrTD status | |||||||
| Negative | 37 (68.5) | 128 (69.2) | 248 (82.7) | 421 (88.3) | 124 (72.5) | 958 (80.7) | 77 (77.0) |
| Positive | 17 (31.5) | 57 (30.8) | 52 (17.3) | 56 (11.7) | 47 (27.5) | 229 (19.3) | 23 (23.0) |
| mrEMVI status | |||||||
| Negative | 25 (46.3) | 121 (65.4) | 218 (72.7) | 417 (87.4) | 115 (67.2) | 896 (75.5) | 74 (74.0) |
| Positive | 29 (53.7) | 64 (34.6) | 82 (27.3) | 60 (12.6) | 56 (32.7) | 291 (24.5) | 26 (26.0) |
| mrCRM status | |||||||
| Clear | 26 (48.1) | 83 (44.9) | 210 (70.0) | 333 (69.8) | 108 (63.2) | 760 (64.0) | 50 (50.0) |
| Involved | 28 (51.9) | 102 (55.1) | 90 (30.0) | 144 (30.2) | 63 (36.8) | 427 (36.0) | 50 (50.0) |
| mrDEC score | |||||||
| 0 | 20 (37.0) | 61 (33.0) | 174 (58.0) | 303 (63.5) | 76 (44.4) | 634 (53.4) | 40 (40.0) |
| 1 | 8 (14.8) | 59 (31.9) | 59 (19.7) | 117 (24.5) | 45 (26.3) | 288 (24.3) | 34 (34.0) |
| 2 | 12 (22.2) | 31 (16.8) | 36 (12.0) | 28 (5.9) | 29 (17.0) | 136 (11.5) | 13 (13.0) |
| 3 | 14 (25.9) | 34 (18.4) | 31 (10.3) | 29 (6.1) | 21 (12.3) | 129 (10.9) | 13 (13.0) |
| TRG | |||||||
| 0 | 0 (0.0) | 31 (16.8) | 86 (28.7) | 112 (23.5) | 43 (25.1) | 272 (22.9) | 23 (23.0) |
| 1 | 1 (1.9) | 55 (29.7) | 98 (32.7) | 124 (26.0) | 40 (23.4) | 318 (26.8) | 32 (32.0) |
| 2 | 1 (1.9) | 55 (29.7) | 106 (35.3) | 239 (50.1) | 50 (29.2) | 451 (38.0) | 33 (33.0) |
| 3 | 0 (0.0) | 42 (22.7) | 10 (3.3) | 2 (0.4) | 38 (22.2) | 92 (7.8) | 12 (12.0) |
| NA | 52 (96.3) | 2 (1.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 54 (4.5) | 0 (0.0) |
CEA carcinoembryonic antigen, TNM tumor-node-metastasis, mr magnetic resonance, TD tumor deposit, EMVI extramural vascular invasion, CRM circumferential resection margin, TRG tumor regression grade, NA not applicable.
Unless otherwise indicated, data are number of patients, and data in parentheses are percentages.
aThe normal values for CEA level range from 0 to 5 ng/ml.
Inter-reader agreement for mrDEC score and MRI marker status
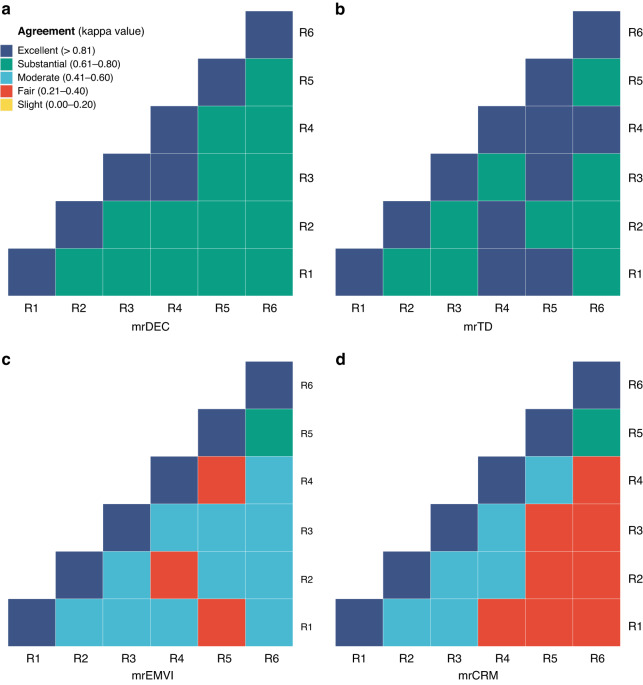
For senior radiologists, there was a substantial inter-reader agreement for mrTD, mrEMVI, mrCRM, and mrDEC (κ = 0.773, 0.651, 0.720, 0.774). Notably, mrTDs and mrDEC showed greater inter-reader agreement compared to mrEMVI and mrCRM. Moreover, the inter-reader agreement for mrDEC score was found to be substantially excellent among radiologists with the same level of experience (κ = 0.692, 0.815, 0.774) (Fig. 2 and Supplementary Table 7).
Fig. 2. Heat map of inter-reader agreement for mrDEC score and MRI markers.
The color-intensity in the heat map represents kappa value between each of the two readers. Inter-reader agreement was better for the mrDEC score and mrTD status (a, b) than for others (c, d). R1 and R2 means the junior radiologists (reader 1 and reader 2; WL, YL); R3 and R4 means the intermediate radiologists (reader 3 and reader 4; MZ, QQ); R5 and R6 means the senior radiologists (reader 5 and reader 6; LK, XY). R reader, mr magnetic resonance, TD tumor deposit, EMVI extramural vascular invasion, CRM circumferential resection margin.
Follow-up and TRG analysis
The median follow-up time was 3.58 years (Interquartile range [IQR], 2.28–4.68). The 3-year DFS in Cohort 1 was 79.6% (95% CI 77.2–82.1%), and the 5-year OS was 84.8% (95% CI 82.0–87.6%). The distribution of TRG 0-3 in Cohort 1 was as follows: 22.9% (n = 272), 26.8% (n = 318), 38.0% (n = 451), and 7.8% (n = 92) (Table 1). In Cohort 2, the respective proportions of TRG 0-3 were 23.0% (n = 23), 32.0% (n = 32), 33.0% (n = 33), and 12.0% (n = 12) (Table 1).
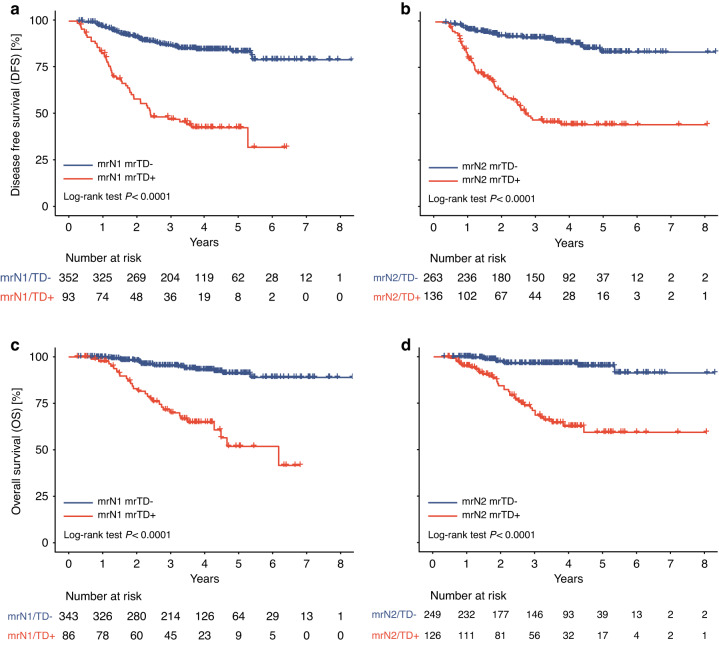
The prognostic performance of MRI markers as measured by the C-index is shown in Supplementary Table 8. Interestingly, the data in this table revealed that mrCRM has a significantly better prognostic performance than the mrT stage (C-index: 0.60 > 0.55). Furthermore, both mrTD and mrEMVI shown substantially better prognostic performance than the mrN stage (C-index: 0.67, 0.62 > 0.55). The results also indicated that the prognostic value of mrTD status remained significant among patients with the same mrN stage. In both mrN1 and mrN2 stage, mrTD-positive patients consistently exhibited significantly lower DFS and overall OS rates compared to mrTD-negative patients (both P < 0.0001; Fig. 3).
Fig. 3. The prognostic value of mrTD status in patients with the same mrN stage.
Kaplan–Meier survival curves for disease-free survival in patients with a mrN1 stage and b mrN2 stage according to mrTD status. Kaplan–Meier survival curves for overall survival in patients with c mrN1 stage and d mrN2 stage according to mrTD status. TD tumor deposit.
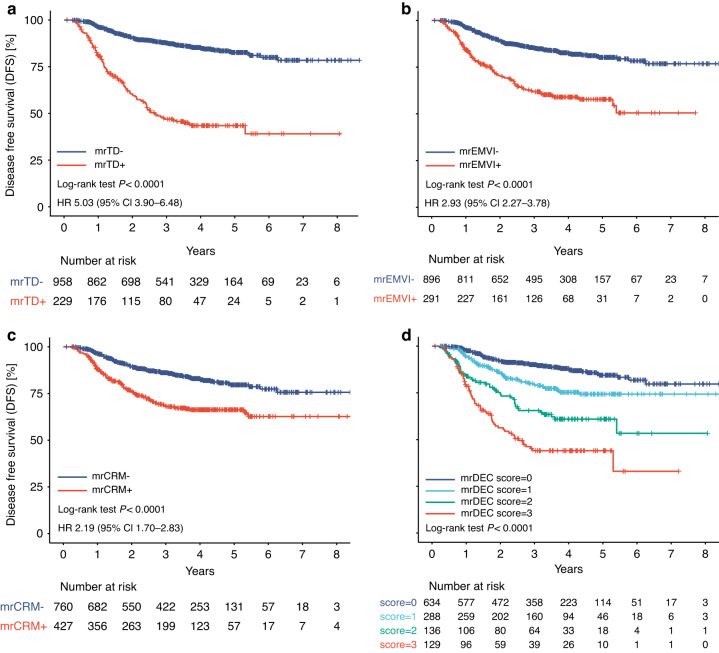
The mrDEC scoring system we developed included mrTDs, mrEMVI, and mrCRM. Increasing the mrDEC score translated into lower DFS and OS: the 3-year DFS for a total score of 0 to 3 were 91.0%, 79.5%, 65.5%, and 44.0%, while the 5-year OS were 92.9%, 87.1%, 74.8%, and 44.5%, respectively (Fig. 4 and Supplementary Fig. 2). Moreover, the mrDEC scoring system demonstrated a strong prognostic ability. That is, increasing mrDEC score was associated with decreased survival. For DFS, the HRs for score 1 to 3 were 1.92 (95% CI 1.36–2.70; P < 0.001), 3.68 (95% CI 2.54–5.34; P < 0.001), and 6.42 (95% CI 4.58–9.01; P < 0.001) (Table 2); for OS, the HRs for score 1 to 3 were 1.96 (95% CI 1.17–3.30; P = 0.011), 4.10 (95% CI 2.37–7.12; P < 0.001), and 9.65 (95% CI 6.01–15.5; P < 0.001) (Table 2). Moreover, the C-index indicated that the prognostic performance of the mrDEC scoring system was superior to individual MRI prognostic markers including mrTD, mrEMVI, and mrCRM (DFS: C-index: 0.69; OS: C-index: 0.72; Supplementary Table 8). Furthermore, we found that the mrDEC scoring system demonstrated a good prognostic ability even when stratified according to the TRG (Supplementary Figs. 3 and 4).
Fig. 4. Kaplan–Meier curves for disease-free survival (DFS).
Kaplan–Meier survival curves for disease-free survival according to a mrTD status, b mrEMVI status, c mrCRM status, and d mrDEC score. mr magnetic resonance, TD tumor deposit, EMVI extramural vascular invasion, CRM circumferential resection margin, HR hazard ratio.
Table 2.
Uni- and multivariate analyses for DFS and OS.
| Disease-free survival | Overall survival | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
| HR (95% CI) | P | HR (95% CI) | P | AHR (95% CI) | P | AHR (95% CI) | P | |
| Age | 1.01 (1.00–1.02) | 0.179 | 1.01 (0.99–1.02) | 0.417 | ||||
| Sex | ||||||||
| Male | Ref | Ref | ||||||
| Female | 0.84 (0.64–1.11) | 0.225 | 0.93 (0.64–1.37) | 0.724 | ||||
| CEA levela | ||||||||
| Normal | Ref | Ref | Ref | |||||
| Abnormal | 1.54 (1.20–1.99) | 0.001 | 1.33 (1.03–1.72) | 0.027 | 1.78 (1.24–2.55) | 0.002 | ||
| Location | ||||||||
| Low | Ref | Ref | Ref | |||||
| Middle | 0.78 (0.60–1.01) | 0.057 | 0.69 (0.53–0.89) | 0.005 | 0.81 (0.57–1.17) | 0.267 | 0.65 (0.45–0.94) | 0.022 |
| High | 0.67 (0.36–1.25) | 0.207 | 0.41 (0.22–0.77) | 0.005 | 0.22 (0.06–0.92) | 0.038 | 0.12 (0.03–0.47) | 0.003 |
| cTNM stage | ||||||||
| II | Ref | Ref | ||||||
| III | 1.19 (0.83–1.71) | 0.332 | 0.87 (0.55–1.36) | 0.530 | ||||
| mrT stage | ||||||||
| T1-T3 | Ref | Ref | ||||||
| T4 | 1.77 (1.33–2.38) | <0.001 | 2.36 (1.60–3.47) | <0.001 | ||||
| mrN stage | ||||||||
| N0 | Ref | Ref | ||||||
| N1 | 1.38 (0.99–1.93) | 0.056 | 1.56 (0.96–2.52) | 0.070 | ||||
| N2 | 1.61 (1.15-2.24) | 0.005 | 1.78 (1.09–2.90) | 0.021 | ||||
| mrTD status | ||||||||
| Negative | Ref | Ref | ||||||
| Positive | 5.03 (3.90–6.48) | <0.001 | 6.18 (4.31–8.85) | <0.001 | ||||
| mrEMVI status | ||||||||
| Negative | Ref | Ref | ||||||
| Positive | 2.93 (2.27–3.78) | <0.001 | 3.36 (2.35–4.82) | <0.001 | ||||
| mrCRM status | ||||||||
| Clear | Ref | Ref | ||||||
| Involved | 2.19 (1.70–2.83) | <0.001 | 3.16 (2.19–4.57) | <0.001 | ||||
| mrDEC score | ||||||||
| 0 | Ref | Ref | Ref | |||||
| 1 | 1.92 (1.36–2.70) | <0.001 | 1.98 (1.40–2.79) | <0.001 | 1.96 (1.17–3.30) | 0.011 | 2.08 (1.24–3.50) | 0.006 |
| 2 | 3.68 (2.54–5.34) | <0.001 | 3.82 (2.63–5.53) | <0.001 | 4.10 (2.37–7.12) | <0.001 | 4.54 (2.61–7.89) | <0.001 |
| 3 | 6.42 (4.58–9.01) | <0.001 | 6.88 (4.87–9.72) | <0.001 | 9.65 (6.01–15.5) | <0.001 | 11.7 (7.29–18.9) | <0.001 |
DFS disease-free survival, OS overall survival, HR hazard ratio, AHR adjusted hazard ratio, CI confidence interval, mr magnetic resonance, CEA carcinoembryonic antigen, TNM tumor-node-metastasis, TD tumor deposit, EMVI extramural vascular invasion, CRM circumferential resection margin.
aThe normal values for CEA level range from 0 to 5 ng/ml.
Note: P value was performed by χ2 test.
We also assessed the ability of MRI markers and mrDEC scoring system to predict the response to nCRT in both two cohorts. In Cohort 1, 17.1% of mrTDs positive patients with TRG results had TRG 0, compared with 25.6% of mrTDs negative patients with TRG 0 (P = 0.01; Supplementary Fig. 5A). Similarly, 19.1% of mrEMVI positive patients had TRG 0, compared with 25.5% of mrEMVI negative patients with TRG 0 (P = 0.04; Supplementary Fig. 5B). However, for mrCRM and mrDEC, patients whose TRG was 0 tended to be assessed with correspondingly lower marker scores, with borderline statistical significance (P = 0.05, 0.059; Supplementary Fig. 5C and D). Due to the small number of patients in Cohort 2, only a trend similar to Cohort 1 was observed for the proportion of TRG 0 was decreased with a correspondingly higher marker score (both P > 0.05; Supplementary Fig. 6).
Univariate and multivariate analysis
In the univariate analysis, it was found that all included factors, except age, sex, tumor location, and cTNM stage, were significantly associated with DFS. Furthermore, all factors were significantly associated with OS, except for age, sex, and cTNM stage (Table 2). The strongest predictor of both DFS and OS was mrTDs (DFS: HR 5.03 [95CI 3.90–6.48]; P < 0.001, OS: HR 6.18 [95CI 4.31–8.85]; P < 0.001; Table 2). In our study, mrTD, mrEMVI, and mrCRM were omitted from the multivariable model due to collinearity with the mrDEC score. For DFS, multivariate analysis revealed that abnormal CEA level (HR 1.33 [95% CI 1.03–1.72]; P = 0.027), higher tumor location (HR 0.41 [95% CI 0.22–0.77]; P = 0.005), and higher mrDEC score (HR 6.88 [95% CI 4.87–9.72]; P < 0.001) were predictive of a higher rate of recurrence (Table 2). For OS, multivariate analysis found that higher tumor location (HR 0.12 [95% CI 0.03–0.47], P = 0.003) and mrDEC score (HR 11.7 [95% CI 7.29–18.9], P < 0.001) were predictive of shorter OS (Table 2). Therefore, the pretreatment mrDEC score was considered one of the most critical prognostic factors for LARC in this study.
Discussion
In this study, we developed an mrDEC scoring system to help clinicians risk stratify patients with LARC before treatment and determine whether the intensive nCRT was necessary. The prognostic power of the scoring system for long-term survival in LARC was further validated in a multicenter study. The improved inter-reader agreement of the mrDEC scoring system compared to a single MRI marker also made it more practical for clinical use. Our study provides a reliable and accurate tool for the stratification of patients before treatment, enabling personalized clinical management with maximum benefits and minimum side effects.
During the development of our scoring system, we discovered that the mrDEC score, which was derived from cumulative summed scores of three acknowledged MRI marker (mrTDs, mrEMVI, and mrCRM), was more valid for the prognosis of rectal cancer than the individual factor (mrTDs) obtained from logistic regression analysis. So, our study did not use multivariate analysis for weight estimation, which differed from previous studies [22, 23]. The scoring system includes mrTDs, mrEMVI, and mrCRM, each assigned a score of 1 if positive and the total score ranging from 0 to 3. We validated this mrDEC scoring system in Cohort 1 and found that the 3-year DFS for a total score of 0 to 3 was 91.0%, 79.5%, 65.5%, and 44.0%, respectively. Patients with the highest score (total score = 3) had more than seven times the risk of DFS events than those with the lowest score (total score = 0) (9.0% vs. 56%). Moreover, high-risk patients (score = 3) had a worse prognosis than expected (44.0% vs. 79.6%), whereas low-risk patients (score = 0) had a better prognosis than expected (91.0% vs. 79.6%). Given the greater-than-average benefit from traditional nCRT, low-risk patients need no other intensive preoperative treatment therapies to avoid unwanted side effects. It’s worth noting that all patients in this study underwent neoadjuvant radiotherapy, so we could not assess its effect. However, Lord et al. [17] defined low-risk patients in their study, as we did, by the absence of any high-risk MRI markers. They found low-risk patients unsuitable for neoadjuvant radiotherapy due to their good prognosis. Conversely, high-risk patients with lower-than-average benefits from traditional nCRT may require additional preoperative therapies. The OS showed the same trend, with high-risk patients having a worse prognosis than expected (44.5% vs. 84.8%) and low-risk patients having a better prognosis than expected (92.9% vs. 84.8%). Intermediate-low-risk patients (score = 1) and intermediate-high-risk patients (score = 2) require further analysis to determine the needed therapy. Furthermore, in the same therapeutic context, implementing a more intensive surveillance program for high-risk patients is advisable. This approach facilitates the early detection of metastases and promises to improve the prognosis for these individuals. In addition, the inter-reader agreement of mrDEC scoring system was better than a single MRI marker (Fig. 2). In our study, the inter-reader agreement between intermediate and junior radiologists’ radiologists of mrEMVI and mrCRM was only at a moderate, which is consistent with previous research [24–27]. Unlike previous MRI standards, our scoring system has a broader coverage and is more concise and user-friendly [4, 18, 19, 28–30]. In summary, the mrDEC scoring system will help clinical decision-makers have risk-benefit discussions with patients about treatment options, facilitating clinical treatment decisions.
Our developed mrDEC system provides important complementary information to the TNM staging system. In accordance with the AJCC eighth edition criteria, TDs are classified as N1c staging, designated for patients without lymph node metastasis but with tumor deposits. However, the eighth edition TNM staging system completely disregards the impact of TD on patients when lymph node metastasis is present, rendering it highly unreasonable [9, 31]. Our study demonstrated that patients within the same mrN stage could be further stratified based on the status of mrTD, with mrTD-positive patients exhibiting significantly worse prognoses than their mrTD-negative counterparts (Fig. 4). Therefore, we recommend that the mrN stage and mrTD status be reported concurrently during diagnosis. And, we do not advocate for conflating mrEMVI and mrTD. Several studies have reported a significant association between mrTDs and mrEMVI [9]. It has even been suggested that TD and EMVI are continuations of the same process [32]. However, in our study, the univariate analysis indicated poor prognosis in patients with mrTDs was worse than those with mrEMVI. Furthermore, we found that compared to mrT stage, mrCRM status has a higher prognostic value for patients with rectal cancer, which is similar to previous studies [33, 34]. The mrDEC scoring system contains more comprehensive information compared to individual MRI markers, including the relationship between the tumor and surrounding tissues, extent of tumor development, vascular invasion, etc. The integration of these pieces of information contributes to a higher prognostic efficacy.
Our study provided evidence that the mrDEC scoring system had strong prognostic ability even when stratified by TRG (Supplementary Figs. 3 and 4). This finding is particularly noteworthy given that previous studies have identified the TRG system as an independent predictor of recurrence and survival in patients with LARC who undergo nCRT followed by TME [35–37]. Specifically, patients with TRG 0 exhibit a significantly better prognosis than those with higher TRG scores, indicating their suitability as candidates for organ-preserving strategies [38–40]. Interestingly, we observed a negative correlation between the mrDEC score and long-term prognosis in patients with TRG 0. These findings indicated that caution should be exercised when considering an organ-preserving strategy even in patients with high mrDEC scores who achieve TRG 0, as they have a greater risk of a poor prognosis. Furthermore, we found that the mrDEC scoring system could predict the response to nCRT. Patients with a score of 0 were more likely to achieve TRG 0 than those with the highest score (score = 3) (27.0% vs. 17.7%; Supplementary Fig. 5D). Higher scores indicated an increased risk of a poor response. These findings were confirmed in Cohort 2 (27.5% vs. 7.7%; Supplementary Fig. 6D). Consequently, the complementation of mrDEC score system to the existing clinicopathological prognosticators for LARC could provide additional prognostic value.
There are several limitations to acknowledge. Firstly, the study did not include patients who underwent surgery without nCRT, which limits the applicability of the results to this population. Secondly, the study did not investigate the impact of the mrDEC score on patient quality of life or functional outcomes, which are important considerations in the treatment of rectal cancer. Finally, the NCT04271657 trial is limited by a relatively small cohort size and a short follow-up period.
In conclusion, the mrDEC scoring system developed in this study is a reliable and accurate tool for risk stratifying patients with LARC and determining the need for intensive nCRT. The multicenter validation study demonstrated the prognostic ability of the mrDEC scoring system for patient outcomes, and its prognostic performance surpasses that of single MRI marker. The mrDEC scoring system has the potential to improve treatment decision-making and patient outcomes in clinical practice.
Supplementary information
Acknowledgements
This work was supported by the National Postdoctoral Program for Innovative Talents of China (No.BX20220359), Science Foundation of Yunnan Basic Research (202201AT070010), NSFC Incubation Project of Guangdong Provincial People’s Hospital (KY0120220037), National Science Foundation for Young Scientists of China (82202267), National Natural Science Foundation of China (No. 82001789), National Science Fund for Distinguished Young Scholars [81925023], Regional Innovation and Development Joint Fund of National Natural Science Foundation of China (No.U22A20345), and Guangdong Provincial Key Laboratory of Artificial Intelligence in Medical Image Analysis and Application [2022B1212010011].
Author contributions
Study design: MNZ, LLF and ZK. Performed the research and collected data: MNZ, LLF, YFC, ZHL, CMX and XBW. Analyzed the data: MNZ, CLK, XYY, QQ, WRL and YTL. Manuscript drafting: MNZ and KZ. Provided discussion, critical feedback and manuscript editing: ZYL, CMX, KZ and XBW.
Funding
Funding sources can be found in “Acknowledgements” section.
Data availability
All data supporting the findings in this study are presented in the manuscript and the supplementary information, and additional raw data can be made available by the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Minning Zhao, Lili Feng, Ke Zhao, Yanfen Cui, Zhenhui Li.
Contributor Information
Ke Zhao, Email: zhaoke@gdph.org.cn.
ChuanMiao Xie, Email: xiechm@sysucc.org.cn.
Xiangbo Wan, Email: wanxbo@mail.sysu.edu.cn.
Zaiyi Liu, Email: liuzaiyi@gdph.org.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-023-02384-x.
References
- 1.van Gijn W, Marijnen CAM, Nagtegaal ID, Kranenbarg EM-K, Putter H, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575–82. doi: 10.1016/S1470-2045(11)70097-3. [DOI] [PubMed] [Google Scholar]
- 2.Denost Q, Fleming CA, Burghgraef T, Celerier B, Geitenbeek R, Rullier E, et al. An international multicenter prospective study evaluating the long-term oncological impact of adjuvant chemotherapy in ypN+ rectal cancer. Ann Surg. 2023;277:299–304. [DOI] [PubMed]
- 3.Ozer L, Yildiz I, Bayoglu V, Bozkurt M, Esen E, Remzi FH, et al. Tailored total neoadjuvant therapy for locally advanced rectal cancer: one size may not fit for all! Colorectal Dis. 2021;23:1662–9. doi: 10.1111/codi.15669. [DOI] [PubMed] [Google Scholar]
- 4.Dijkstra EA, Nilsson PJ, Hospers GAP, Bahadoer RR, Meershoek-Klein Kranenbarg E, Roodvoets AGH, et al. Locoregional failure during and after short-course radiotherapy followed by chemotherapy and surgery compared to long-course chemoradiotherapy and surgery—a five-year follow-up of the RAPIDO trial. Ann Surg. 2023. 10.1097/SLA.0000000000005799. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 5.Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv22–40. doi: 10.1093/annonc/mdx224. [DOI] [PubMed] [Google Scholar]
- 6.Bates DDB, Homsi ME, Chang KJ, Lalwani N, Horvat N, Sheedy SP. MRI for rectal cancer: staging, mrCRM, EMVI, lymph node staging and post-treatment response. Clin Colorectal Cancer. 2022;21:10–8. doi: 10.1016/j.clcc.2021.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kreis ME, Ruppert R, Ptok H, Strassburg J, Brosi P, Lewin A, et al. Use of preoperative magnetic resonance imaging to select patients with rectal cancer for neoadjuvant chemoradiation-interim analysis of the German OCUM Trial ( NCT01325649) J Gastrointest Surg. 2016;20:25–32. doi: 10.1007/s11605-015-3011-0. [DOI] [PubMed] [Google Scholar]
- 8.Rouleau Fournier F, Motamedi M, Brown C, Phang T, Raval M, Hague C, et al. Oncologic outcomes associated with MRI-detected extramural venous invasion (mrEMVI) in rectal cancer: a systematic review and meta-analysis. Ann Surg. 2022;275:303–14. doi: 10.1097/SLA.0000000000004636. [DOI] [PubMed] [Google Scholar]
- 9.Lord AC, D’Souza N, Shaw A, Rokan Z, Moran B, Abulafi M, et al. MRI-diagnosed tumor deposits and EMVI status have superior prognostic accuracy to current clinical TNM staging in rectal cancer. Ann Surg. 2022;276:334–44.. doi: 10.1097/SLA.0000000000004499. [DOI] [PubMed] [Google Scholar]
- 10.Lord AC, Graham Martínez C, D’Souza N, Pucher PH, Brown G, Nagtegaal ID. The significance of tumour deposits in rectal cancer after neoadjuvant therapy: a systematic review and meta-analysis. Eur J Cancer. 2019;122:1–8. doi: 10.1016/j.ejca.2019.08.020. [DOI] [PubMed] [Google Scholar]
- 11.Beets-Tan RG, Beets GL, Vliegen RF, Kessels AG, Van Boven H, De, et al. Accuracy of magnetic resonance imaging in prediction of tumour-free resection margin in rectal cancer surgery. Lancet. 2001;357:497–504. doi: 10.1016/S0140-6736(00)04040-X. [DOI] [PubMed] [Google Scholar]
- 12.Fernandes MC, Gollub MJ, Brown G. The importance of MRI for rectal cancer evaluation. Surg Oncol. 2022;43:101739. doi: 10.1016/j.suronc.2022.101739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunter CJ, Garant A, Vuong T, Artho G, Lisbona R, Tekkis P, et al. Adverse features on rectal MRI identify a high-risk group that may benefit from more intensive preoperative staging and treatment. Ann Surg Oncol. 2012;19:1199–205. doi: 10.1245/s10434-011-2036-1. [DOI] [PubMed] [Google Scholar]
- 14.Taylor FG, Quirke P, Heald RJ, Moran BJ, Blomqvist L, Swift IR, et al. Preoperative magnetic resonance imaging assessment of circumferential resection margin predicts disease-free survival and local recurrence: 5-year follow-up results of the MERCURY study. J Clin Oncol. 2014;32:34–43. doi: 10.1200/JCO.2012.45.3258. [DOI] [PubMed] [Google Scholar]
- 15.Beets-Tan RGH, Lambregts DMJ, Maas M, Bipat S, Barbaro B, Curvo-Semedo L, et al. Magnetic resonance imaging for clinical management of rectal cancer: updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol. 2018;28:1465–75. doi: 10.1007/s00330-017-5026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.NICE. Colorectal cancer. Jan 29, 2020. https://www.nice.org.uk/guidance/ng151. Accessed April 27, 2022.
- 17.Lord AC, Corr A, Chandramohan A, Hodges N, Pring E, Airo-Farulla C, et al. Assessment of the 2020 NICE criteria for preoperative radiotherapy in patients with rectal cancer treated by surgery alone in comparison with proven MRI prognostic factors: a retrospective cohort study. Lancet Oncol. 2022;23:793–801. doi: 10.1016/S1470-2045(22)00214-5. [DOI] [PubMed] [Google Scholar]
- 18.Bahadoer RR, Dijkstra EA, van Etten B, Marijnen CAM, Putter H, Kranenbarg EM-K, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:29–42. doi: 10.1016/S1470-2045(20)30555-6. [DOI] [PubMed] [Google Scholar]
- 19.Feng L, Liu Z, Li C, Li Z, Lou X, Shao L, et al. Development and validation of a radiopathomics model to predict pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer: a multicentre observational study. The Lancet Digital Health. 2022;4:e8–17. doi: 10.1016/S2589-7500(21)00215-6. [DOI] [PubMed] [Google Scholar]
- 20.Benson A, Venook A, Al-Hawary M, Azad N, Chen Y, Ciombor K, et al. Rectal cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20:1139–67. doi: 10.6004/jnccn.2022.0051. [DOI] [PubMed] [Google Scholar]
- 21.Chmura Kraemer H, Periyakoil VS, Noda A. Kappa coefficients in medical research. Stat Med. 2002;21:2109–29. doi: 10.1002/sim.1180. [DOI] [PubMed] [Google Scholar]
- 22.Xu T, Wang L, Wu S, Zhou F, Huang H. Utility of a simple scoring system in differentiating bacterial infections in cases of fever of unknown origin. Clin Infect Dis. 2020;71:S409–15. doi: 10.1093/cid/ciaa1520. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Gale RP, Zhang M, Huang X, Jiang Q. A predictive scoring system for therapy-failure in persons with chronic myeloid leukemia receiving initial imatinib therapy. Leukemia. 2022;36:1336–42. doi: 10.1038/s41375-022-01527-y. [DOI] [PubMed] [Google Scholar]
- 24.van den Broek JJ, van der Wolf FSW, Lahaye MJ, Heijnen LA, Meischl C, Heitbrink MA, et al. Accuracy of MRI in restaging locally advanced rectal cancer after preoperative chemoradiation. Dis Colon Rectum. 2017;60:274–83. doi: 10.1097/DCR.0000000000000743. [DOI] [PubMed] [Google Scholar]
- 25.Pooni A, Al-Sukhni E, Milot L, Fruitman M, Victor JC, Schmocker S, et al. Selection of patients with rectal cancer for preoperative chemoradiotherapy: are T category and nodal status all that matters? Dis Colon Rectum. 2019;62:447–53. doi: 10.1097/DCR.0000000000001229. [DOI] [PubMed] [Google Scholar]
- 26.Liu L, Yang L, Jin E, Wang Z, Yang Z. Effect of gadolinium contrast-enhanced T1-weighted magnetic resonance imaging for detecting extramural venous invasion in rectal cancer. Abdom Radiol. 2016;41:1736–43. doi: 10.1007/s00261-016-0740-9. [DOI] [PubMed] [Google Scholar]
- 27.Lee ES, Kim MJ, Park SC, Hur BY, Hyun JH, Chang HJ, et al. Magnetic resonance imaging-detected extramural venous invasion in rectal cancer before and after preoperative chemoradiotherapy: diagnostic performance and prognostic significance. Eur Radiol. 2018;28:496–505. doi: 10.1007/s00330-017-4978-6. [DOI] [PubMed] [Google Scholar]
- 28.Kennedy ED, Simunovic M, Jhaveri K, Kirsch R, Brierley J, Drolet S, et al. Safety and feasibility of using magnetic resonance imaging criteria to identify patients with “good prognosis” rectal cancer eligible for primary surgery: the phase 2 nonrandomized QuickSilver Clinical Trial. JAMA Oncol. 2019;5:961–6. doi: 10.1001/jamaoncol.2019.0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williamson JS, Jones HG, Davies M, Evans MD, Hatcher O, Beynon J, et al. Outcomes in locally advanced rectal cancer with highly selective preoperative chemoradiotherapy. Br J Surg. 2014;101:1290–8. doi: 10.1002/bjs.9570. [DOI] [PubMed] [Google Scholar]
- 30.Figueredo A, Zuraw L, Wong RKS, Agboola O, Rumble RB, Tandan V, et al. The use of preoperative radiotherapy in the management of patients with clinically resectable rectal cancer: a practice guideline. BMC Med. 2003;1:1. doi: 10.1186/1741-7015-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaap DP, Voogt ELK, Burger JWA, Cnossen JS, Creemers G-JM, van Lijnschoten I, et al. Prognostic implications of MRI-detected EMVI and tumor deposits and their response to neoadjuvant therapy in cT3 and cT4 rectal cancer. Int J Radiat Oncol Biol Phys. 2021;111:816–25. doi: 10.1016/j.ijrobp.2021.06.013. [DOI] [PubMed] [Google Scholar]
- 32.Lord AC, Moran B, Abulafi M, Rasheed S, Nagtegaal ID, Terlizzo M, et al. Can extranodal tumour deposits be diagnosed on MRI? Protocol for a multicentre clinical trial (the COMET trial) BMJ Open. 2020;10:e033395. doi: 10.1136/bmjopen-2019-033395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel UB, Brown G, Machado I, Santos-Cores J, Pericay C, Ballesteros E, et al. MRI assessment and outcomes in patients receiving neoadjuvant chemotherapy only for primary rectal cancer: long-term results from the GEMCAD 0801 trial. Ann Oncol. 2017;28:344–53. doi: 10.1093/annonc/mdw616. [DOI] [PubMed] [Google Scholar]
- 34.Glynne-Jones R, Mawdsley S, Pearce T, Buyse M. Alternative clinical end points in rectal cancer-are we getting closer? Ann Oncol. 2006;17:1239–48. doi: 10.1093/annonc/mdl173. [DOI] [PubMed] [Google Scholar]
- 35.Germani P, Di Candido F, Léonard D, Cuicchi D, Elmore U, Allaix ME, et al. Contemporary snapshot of tumor regression grade (TRG) distribution in locally advanced rectal cancer: a cross sectional multicentric experience. Updates Surg. 2021;73:1795–803. doi: 10.1007/s13304-021-01044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song C, Chung J-H, Kang S-B, Kim D-W, Oh H-K, Lee HS, et al. Impact of tumor regression grade as a major prognostic factor in locally advanced rectal cancer after neoadjuvant chemoradiotherapy: a proposal for a modified staging system. Cancers. 2018;10:319. doi: 10.3390/cancers10090319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li JY, Huang XZ, Gao P, Song YX, Chen XW, Lv XE, et al. Survival landscape of different tumor regression grades and pathologic complete response in rectal cancer after neoadjuvant therapy based on reconstructed individual patient data. BMC Cancer. 2021;21:1214. doi: 10.1186/s12885-021-08922-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rutten HJT, Burger JWA. Bringing organ preservation closer for selected patients with rectal cancer. Lancet Gastroenterol Hepatol. 2023;8:294–5. doi: 10.1016/S2468-1253(22)00438-1. [DOI] [PubMed] [Google Scholar]
- 39.Fleming C, Vendrely V, Rullier E, Denost Q. Organ preservation in rectal cancer: review of contemporary management. Br J Surg. 2022;109:695–703. doi: 10.1093/bjs/znac140. [DOI] [PubMed] [Google Scholar]
- 40.Verrijssen AE, Ketelaers SHJ, Rutten HJT, Theuws J, Burger JWA, Cnossen JS. Organ preservation in rectal cancer: an overview of the Dutch perspective and recent developments. Clin Oncol (R Coll Radiol) 2023;35:107–16. doi: 10.1016/j.clon.2022.09.055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings in this study are presented in the manuscript and the supplementary information, and additional raw data can be made available by the corresponding author upon reasonable request.