Abstract
Melanoma is a highly aggressive and life-threatening form of skin cancer that accounts for a significant proportion of cancer-related deaths worldwide. Although conventional cancer therapies, such as surgical excision, chemotherapy, and radiation, have been used to treat malignant melanoma, their efficacy is often limited due to the development of resistance and adverse side effects. Therefore, there is a growing interest in developing alternative treatment options for melanoma that are more effective and less toxic. Terpenes, a diverse group of naturally occurring compounds of plant origin, have emerged as potential anticancer agents due to their ability to inhibit tumor growth and induce apoptosis in cancer cells. In this review, the current understanding of the anticancer effects of terpenes (including, thymoquinone, β-elemene, carvacrol, limonene, α-pinene, β-caryophyllene, perillyl alcohol, taxol, betulinic acid, α-bisabolol, ursolic acid, linalool, lupeol, and artesunate) was summarized, with a special focus on their potential as therapeutic agents for malignant melanoma.
Keywords: Terpenes, Melanoma, Anticancer therapy
Introduction
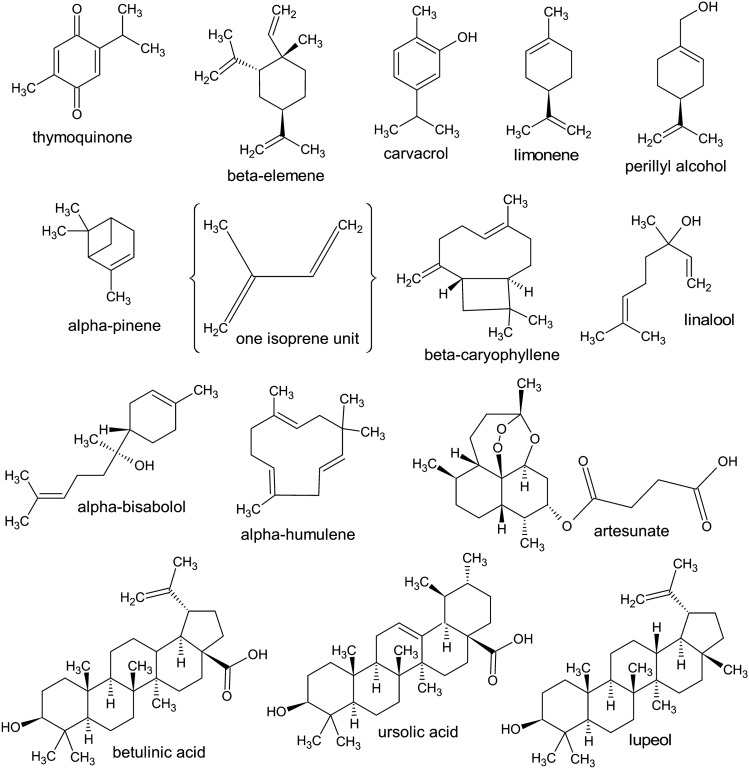
Terpenes (also known as isoprenoids) are a diverse large class of organic compounds found in plants, fungi, and some animals [1], characterized by a specific carbon skeleton composed of multiple isoprene units (Fig. 1), which can be arranged in a linear, branched, or cyclic manner [2].
Fig. 1.
Structural formulas of selected naturally occurring terpenes (ACD/ChemSketch vers. 2021.2.1 software)
Terpenes play important roles in the biosynthesis of plant secondary metabolites, such as essential oils and pigments, and are involved in various physiological processes, including growth and development, reproduction, and defense against biotic and abiotic stress [3, 4]. Terpenes are synthesized by plants and other organisms through the mevalonate pathway and are often found in essential oils, resins, and other plant-derived materials [5]. Terpenes are the subject of biochemical and molecular research due to their numerous biological activities, including, anticancer, anti-inflammatory, antimicrobial, and antiviral effects [6, 7]. Terpenes interact with specific biological targets, such as enzymes, receptors, ion channels, and can also modulate signaling pathways involved in various cellular processes, including apoptosis, proliferation, and cell differentiation [8].
One of the most notable biological activities of terpenes is their anti-inflammatory effect. Many terpenes modulate the immune system and reduce inflammation by inhibiting the activity of various enzymes and signaling pathways involved in the inflammatory response [9]. Some terpenes possess antioxidant activity, which can protect cells from damage caused by free radicals and oxidative stress [10].
Terpenes have also been reported to exhibit antimicrobial activity against a wide range of bacteria, fungi, and viruses [11]. This effect is thought to be due to the ability of some terpenes to disrupt the cell membrane of microorganisms, leading to their death.
The anticancer properties of terpenes have been widely studied in recent years [12]. Several preclinical studies have demonstrated the potential of terpenes as anticancer agents against various types of cancer, including melanoma [13]. In particular, the use of terpenes as adjuvant therapy in melanoma treatment has gained attention due to their ability to sensitize cancer cells to chemotherapeutic agents and reduce their toxicity [14]. This study aimed to present our expanding knowledge about the mechanisms of action of some terpenes involved in their anticancer effects on melanoma cells.
Melanoma is a type of skin cancer that originates in melanocytes, which are pigment-producing cells located in the basal layer of the epidermis [15]. It is the most aggressive form of skin cancer, with a high potential for metastasis and a poor prognosis if not detected and treated in its early stages [16]. Melanoma accounts for only 1% of all skin cancers, but it is responsible for the majority of skin cancer-related deaths [17].
Melanoma is caused by the accumulation of genetic mutations that disrupt the normal function of melanocytes and promote their uncontrolled proliferation [18]. Exposure to ultraviolet radiation from the sun or artificial sources, such as tanning beds, is the primary environmental risk factor for melanoma [19]. Other risk factors include fair skin, a history of sunburns, a family or personal history of melanoma, and certain genetic mutations [20].
The clinical presentation of melanoma varies depending on the location, stage, and subtype of the tumor. The most common presentation is a pigmented lesion on the skin that changes in size, shape, or color over time [21]. Other signs and symptoms may include itching, bleeding, or ulceration of the lesion, or the appearance of new moles or skin lesions [22].
Melanoma is a particularly challenging type of cancer to treat due to its propensity to metastasize, leading to poor survival rates for patients with advanced disease [23]. Treatment options for melanoma depend on the stage and location of the tumor, as well as the patient's overall health and preferences. Surgery is the primary treatment option for early-stage melanoma, while more advanced cases may require additional therapies, such as chemotherapy, radiation therapy, immunotherapy, or targeted therapy, especially, if melanoma metastases occur [24]. In recent years, there has been a growing interest in the use of natural products, such as terpenes, as adjuvant therapy for melanoma treatment, due to their potential to enhance the efficacy and reduce the toxicity of conventional pharmacotherapy [25, 26]. Additionally, some terpenes inhibit the growth of melanoma cells both in vitro and in vivo [27, 28].
One of the mechanisms by which terpenes may exert their anticancer effects is based on the ability of terpenes to modulate various signaling pathways involved in cell proliferation, apoptosis, and angiogenesis. Another potential mechanism by which terpenes may exhibit their anticancer effects is based on the induction of oxidative stress and DNA damage in cancer cells [29]. This effect can lead to the activation of the cell's apoptosis machinery, resulting in the death of cancer cells.
Terpenes and cancer: preclinical studies
Preclinical studies have shown that terpenes can induce apoptosis, inhibit cell proliferation, and suppress tumor growth in animal models [30]. For example, β-elemene (a terpene found in plants such as Curcuma wenyujin) induces apoptosis in melanoma cells in vitro and inhibits tumor growth in melanoma-bearing mice in vivo [31]. Similarly, carvacrol (a monoterpenoid phenol found in oregano and thyme) inhibits the growth of melanoma cells in vitro and in vivo by inducing cell cycle arrest and apoptosis [32]. Limonene (a monocyclic monoterpene in citrus fruits) inhibits tumor growth and induces apoptosis in melanoma cells in both, in vitro and in vivo studies [33], while α-pinene (a terpene found in pine trees) induces apoptosis and inhibits cell proliferation in melanoma cells in vitro [34].
In addition to their direct anticancer effects, terpenes enhance the efficacy of conventional chemotherapy in preclinical models. For example, β-caryophyllene (a terpene found in many essential oils) sensitizes melanoma cells to doxorubicin by inhibiting the drug efflux pump responsible for drug resistance [35].
Preclinical in vitro studies have demonstrated that various terpenes, such as limonene, β-caryophyllene, and perillyl alcohol, can induce apoptosis, inhibit proliferation, and sensitize melanoma cells to conventional chemotherapy [12, 36]. Moreover, several terpenes have been found to exhibit anti-inflammatory effects, which can also contribute to their anticancer activity. For example, α-pinene inhibits the production of pro-inflammatory cytokines, such as TNF-α (Tumor Necrosis Factor α) and IL-6 (Interleukin 6), in melanoma cells [37]. In addition, β-elemene sensitizes melanoma cells to radiation by inhibiting the DNA damage repair pathway and inducing apoptosis [38]. Limonene enhances radiation-induced DNA damage and cell death in melanoma cells [39], and sensitizes vemurafenib-resistant melanoma cells to the drug by downregulating the expression of the drug efflux pump ABCB1 [40]. Similarly, perillyl alcohol overcomes resistance to BRAF and MEK inhibitors in melanoma cells by inducing apoptosis and inhibiting the MAPK signaling pathway [41].
Accumulating evidence indicates that terpenes exert their anticancer effects not only on melanoma, but also on various human cancers. For instance, taxol (paclitaxel—a diterpene) exerts its anticancer effect by binding to microtubules, stabilizing them, and inhibiting their depolymerization, leading to cell cycle arrest and apoptosis [42]. Thymoquinone (a monoterpenoid found in the seeds of Nigella sativa (black seed)), limonene, carvacrol, betulinic acid (a triterpenoid found in the bark of various trees) and α-bisabolol (a sesquiterpene alcohol found in chamomile) induce apoptosis, inhibit cell proliferation, and suppress angiogenesis in various human cancers [43–52].
Mechanisms of action of terpenes in cancer cells
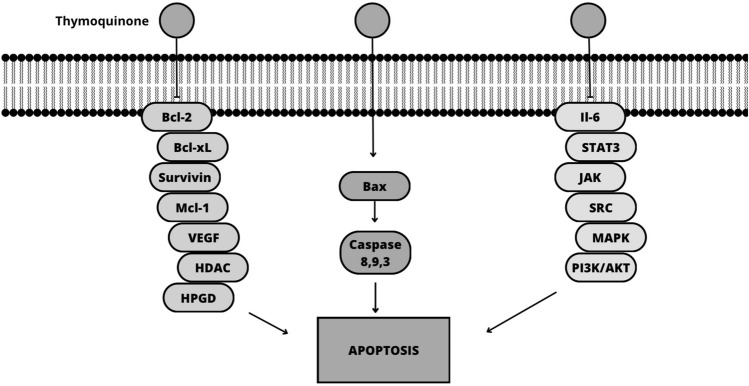
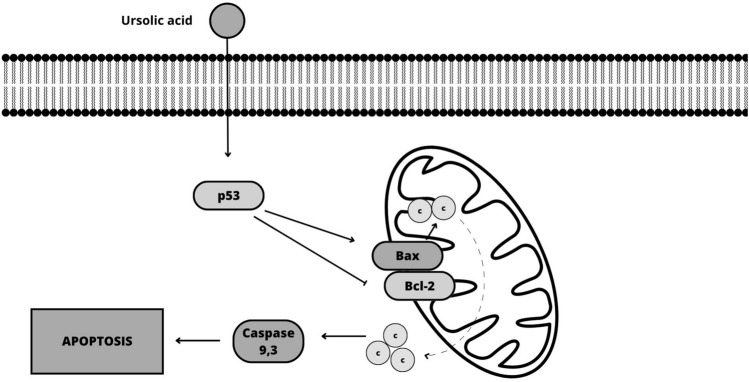
Accumulating evidence suggests that terpenes exert their effects through multiple molecular mechanisms, including regulation of apoptosis, induction of autophagy, inhibition of cellular signaling pathways, modulation of gene expression, inhibition of angiogenesis, and modulation of inflammation [53, 54]. More specifically, terpenes inhibit the expression of anti-apoptotic proteins, such as Bcl-2 and Bcl-xL, while upregulating pro-apoptotic proteins, such as Bax and caspases, which finally leads to the activation of the apoptotic pathway, promoting cell death [55–58]. Thymoquinone has shown effective results in treating melanoma (MDA-MB-435) by activating the intrinsic apoptosis pathway, while suppressing Akt phosphorylation, and increasing the Bax/Bcl-2 ratio (Fig. 2). This mechanism contributes to the inhibition of cancer cell growth. The presence of highly expressed caspase 3 is associated with the inhibitory effect observed. In addition, in silico target determination has indicated that thymoquinone induces DNA damage by specifically targeting histone deacetylase activity (HDAC) and human 15-hydroxyprostaglandin dehydrogenase (HPGD) [59, 60]. Betulinic acid activates the intrinsic apoptotic pathway and downregulates anti-apoptotic proteins [61]. α-Bisabolol activates the extrinsic apoptotic pathway [62]. The mechanism of action of ursolic acid in B16F-10 melanoma cells involves its inhibitory effect on cell growth by upregulating the expression of p53, Bax, and p21 proteins (Fig. 3). This upregulation leads to the activation of caspase 3-dependent apoptosis, which ultimately results in the programmed cell death of melanoma cells [63].
Fig. 2.
Schematic mechanisms involved in the anticancer effect of thymoquinone on melanoma cells (Canva for Windows)
Fig. 3.
Schematic mechanisms involved in the anticancer effect of ursolic acid on melanoma cells (Canva for Windows)
Terpenes can induce autophagy, a cellular process that helps degrade damaged proteins and organelles, leading to the inhibition of tumor growth [64].
Terpenes inhibit crucial signaling pathways involved in cancer cell proliferation and survival. One of these is the PI3K/Akt/mTOR pathway, which plays a significant role in cell proliferation and survival. Inhibition of this pathway by terpenes leads to apoptosis induction and inhibition of cell proliferation [47, 65]. Terpenes also inhibit the MAPK/ERK signaling pathway, which regulates proliferation, survival, and cell differentiation [66].
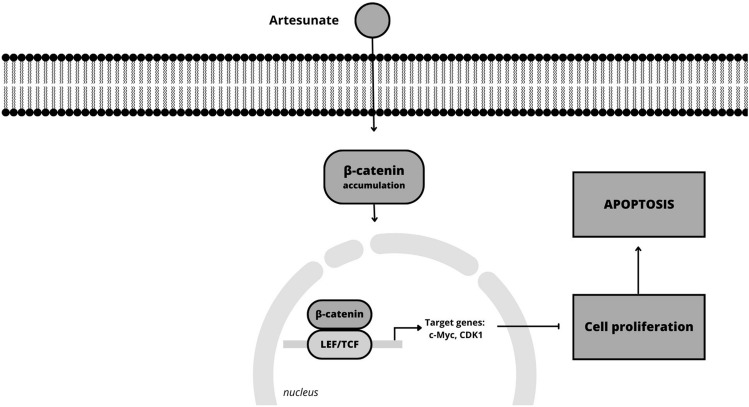
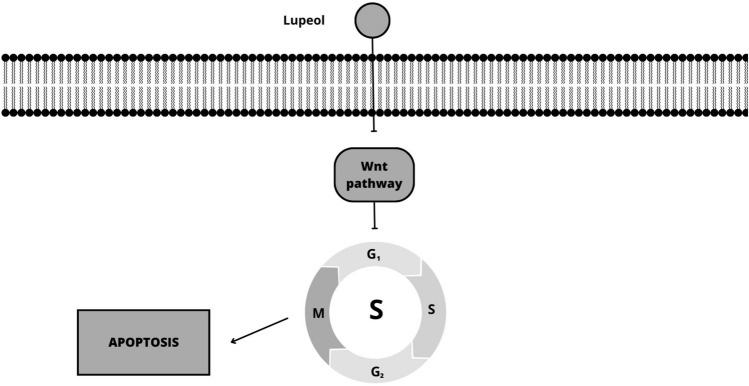
Terpenes inhibit cell cycle progression in melanoma by targeting different regulators. For instance, taxol stabilizes microtubules and blocks cell division, inhibiting cell cycle progression [67]. Carvacrol induces cell cycle arrest at the G0/G1 phase by downregulating cyclin D1 and CDK4/6 [68]. Artesunate exhibits its antitumor activity in uveal melanoma cells by inhibiting the accumulation of β-catenin and activating specific downstream genes, including c-Myc and CDK1 (Fig. 4), leading to the suppression of cancer cell growth and proliferation [69]. Lupeol demonstrated the ability to suppress the growth of melanoma cells (Mel-928, Mel-1241, Mel-1011) by interfering with the Wnt (Wingless signaling)/β-catenin pathway. It achieves this by effectively blocking the Wnt signaling pathway, a crucial pathway involved in cell proliferation and survival (Fig. 5). This inhibition of the Wnt/β-catenin pathway contributes to the antitumor effects of lupeol on melanoma cells [70].
Fig. 4.
Schematic mechanisms involved in the effect of artesunate on melanoma cells (Canva for Windows)
Fig. 5.
Schematic mechanisms involved in the effect of lupeol on melanoma cells (Canva for Windows)
Terpenes modulate the expression of several genes involved in the regulation of cell growth and survival, including the tumor suppressor gene p53 [71]. Terpenes exhibit anti-angiogenic effects by suppressing the expression of pro-angiogenic factors, such as VEGF (vascular endothelial growth factor) and bFGF (basic fibroblast growth factor). Inhibition of angiogenesis is critical for preventing tumor growth and metastasis [35, 66].
Terpenes inhibit angiogenesis in melanoma cells through various molecular pathways. For example, limonene downregulates VEGF and MMP-9 (matrix metallopeptidase 9) expression [72], but betulinic acid suppresses the expression of VEGF and MMP-2 (matrix metallopeptidase 2) [73], whereas thymoquinone downregulates HIF-1α (Hypoxia-inducible factor 1-alpha) and VEGF [74].
Terpenes can modulate chronic inflammation in melanoma cells by targeting different inflammatory pathways. For instance, α-bisabolol inhibits the expression of TNF-α and IL-1β [75]. Furthermore, terpenes such as β-elemene, perillyl alcohol, and limonene inhibit melanoma cell proliferation and induce apoptosis [76–78]. They can also inhibit melanoma cell migration and invasion by regulating the expression of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) [79–81]. Additionally, certain terpenes can enhance the antitumor activity of conventional chemotherapeutic agents, such as doxorubicin, cisplatin, and temozolomide, through various mechanisms [82–84].
Advantages and limitations of using terpenes as anticancer agents
Terpenes have gained increasing attention as potential anticancer agents due to their various pharmacological properties, including their ability to induce apoptosis, inhibit proliferation, sensitize cancer cells to chemotherapy drugs, and overcome resistance to targeted therapies. Furthermore, terpenes are widely distributed in plants and are easy to extract, making them a relatively inexpensive source of potential anticancer agents.
However, the use of terpenes as anticancer agents also has some limitations. One of the major limitations is their low bioavailability, which can limit their efficacy in vivo [85]. Terpenes are highly hydrophobic molecules, poorly soluble in water, and difficult to absorb and distribute in the body. Several strategies have been proposed to enhance the bioavailability of terpenes, such as encapsulation in liposomes or cyclodextrins [86, 87].
Another limitation of terpenes in anticancer therapy is their potential toxicity. Although terpenes are generally considered safe, some terpenes can exhibit cytotoxic effects on normal cells (i.e., eugenol at high concentrations) [88]. Therefore, it is important to carefully evaluate the safety and toxicity of terpenes before their clinical use.
Finally, the regulatory status of terpenes as drugs can also pose a challenge to their development as anticancer agents. Terpenes are classified as natural products and are subject to less stringent regulations than synthetic drugs. However, this can also limit their commercial potential due to the lack of intellectual property protection and the challenges in obtaining regulatory approval [89].
Clinical studies on the use of terpenes for melanoma treatment
Quite recently, some clinical studies have investigated the efficacy of terpenes in the treatment of melanoma. For instance, perillyl alcohol and limonene were studied in phase II of clinical trials, when evaluating their safety and efficacy in patients with advanced melanoma. Of note, both terpenes (perillyl alcohol and limonene) were well-tolerated, with no dose-limiting toxicities observed but, no objective responses were observed, with a median time to progression of 2 months [46, 90]. Similarly, a phase I clinical trial revealed that thymoquinone was well-tolerated, with no dose-limiting toxicities observed. However, no objective responses were observed, and the median time to progression was 2 months [91].
Terpenes may have potential as adjuvants to standard treatments, such as chemotherapy and immunotherapy.
Potential use of terpenes in combination with other anticancer therapies
Combination therapy, using two or more agents with different mechanisms of action, has become an important strategy in cancer treatment. In recent years, there has been increasing interest in using terpenes in combination with other anticancer therapies to enhance their efficacy and overcome drug resistance. For example, β-caryophyllene enhanced the antitumor activity of doxorubicin in melanoma cells [92]. Similarly, α-humulene enhanced the antitumor activity of cisplatin in melanoma cells [93]. Linalool potentiated the antitumor activity of temozolomide in melanoma cells [94].
Immunotherapy, such as immune checkpoint inhibitors, has revolutionized the treatment of melanoma. However, not all patients respond to immunotherapy and there is a need to improve its efficacy. Terpenes have been shown to have immunomodulatory effects and may enhance the efficacy of immunotherapy. For example, β-caryophyllene enhanced the antitumor activity of anti-PD-1 immunotherapy in a mouse model of melanoma [95]. Similarly, β-elemene enhanced the antitumor activity of anti-PD-1 immunotherapy in a mouse model of melanoma by increasing T-cell infiltration and activation [96].
Radiation therapy is used in the treatment of melanoma, but its efficacy is limited by radiation resistance. Terpenes enhanced the radiosensitivity of cancer cells, potentially improving the efficacy of radiation therapy. For example, β-elemene enhanced the radiosensitivity of melanoma cells by inducing cell cycle arrest and apoptosis [97]. Similarly, thymoquinone enhanced the radiosensitivity of melanoma cells by inducing apoptosis and inhibiting DNA repair [98].
Targeted therapies, including BRAF and MEK inhibitors, have shown promise in the treatment of melanoma. However, resistance to these therapies is a major clinical problem. It has been shown that terpenes exert synergistic effects with targeted therapies, potentially overcoming resistance. For example, β-caryophyllene enhanced the antitumor activity of vemurafenib, a BRAF inhibitor, in melanoma cells [99]. Similarly, α-humulene enhanced the antitumor activity of trametinib, a MEK inhibitor, in melanoma cells [100].
Future directions for research on terpenes and melanoma treatment
There is growing interest in the potential use of terpenes for the treatment of melanoma, and future research in this area is likely to focus on several key areas. In in vivo melanoma models, terpenoids have shown the ability to increase the median overall survival time of animals with tumors, reduce tumor volume, decrease the expression of metastasis-associated chemokines and receptors, as well as lymph node metastasis, decrease the number and size of metastatic foci, alter the tumor microenvironment and the surrounding adipose tissue of lymph nodes and inhibit angiogenesis. Notably, plant-derived terpenoids generally exhibit lower-to-no toxicity towards non-cancerous cells or even enhance their photoprotection [57]. Further preclinical and clinical studies are needed to fully evaluate the safety and efficacy of terpenes in combination with other therapies for the treatment of melanoma. Although early studies have shown promising results, more extensive research is needed to establish the optimal doses, treatment regimens, and potential side effects of terpene-based therapies [98, 101–103]. One key advantage they possess over traditional chemotherapeutic agents is their lower cytotoxicity. Research conducted over the past eight years has revealed several effects of plant terpenoids on in vitro melanoma models. These include: demonstrating dose-dependent cytotoxicity, inducing apoptosis, necrosis, or autophagy, triggering the increased generation of reactive oxygen species, oxidative stress, and disruption of mitochondrial membrane potential, reducing oxygen consumption rate, extracellular acidification rate, oxidative phosphorylation, and the maximal respiratory capacity of the electron transport system, inducing endoplasmic reticulum stress, causing cell cycle arrest, inducing DNA damage, decreasing the expression and activity of proteins involved in melanogenesis, interfering with cell signaling pathways responsible for cell growth, proliferation, migration, adhesion, and invasion, reducing the expression of angiogenesis-related cytokines, inhibiting epithelial-mesenchymal transition, exhibiting radio- and photosensitization properties and displaying synergistic effects with other natural compounds or chemotherapeutics [57].
Despite the numbers of experiments, there is a need to explore the mechanisms underlying the effects of each terpene on melanoma cells. Further research in this area could provide valuable insights into the potential therapeutic applications of terpenes [98, 101–103]. There is a need to investigate the potential use of terpenes as adjuvant therapies in combination with immunotherapy. Terpenes may modulate immune responses and may therefore have the potential to enhance the effectiveness of immunotherapy for melanoma [104, 105]. Additionally, there is a need to explore the potential use of terpenes as chemopreventive agents for melanoma. Future research could investigate the potential use of these compounds for the prevention of melanoma [25, 106].
Conclusions
In recent years, there has been increasing interest in using terpenes in combination with other anticancer therapies to enhance their efficacy and overcome drug resistance. In combination with chemotherapy, β-caryophyllene, α-humulene, and linalool enhance the efficacy of chemotherapy agents in melanoma cells. Terpenes due to their immunomodulatory effects may enhance the efficacy of immunotherapy, especially, β-caryophyllene and β-elemene, which enhanced the antitumor activity of anti-PD-1 immunotherapy in mouse models of melanoma. In combination with radiation therapy, β-elemene, and thymoquinone may enhance the radiosensitivity of melanoma cells. Given the efficacy of terpenoids, future research must focus on conducting thorough preclinical evaluations of toxicity, bioavailability, pharmacodynamics, biomarkers, and comprehensive investigations into tumor suppression. Future studies are likely to focus on exploring the optimal use of terpenes in combination with other therapies, investigating the underlying mechanisms of their effects on melanoma cells, and exploring their potential use as adjuvant therapies or chemopreventive agents.
Abbreviations
- bFGF
Basic fibroblast growth factor
- BRAF
V-raf murine sarcoma viral oncogene homolog B1
- HDAC
Histone deacetylase activity
- HIF-1α
Hypoxia-inducible factor 1-alpha
- HPGD
15-Hydroxyprostaglandin dehydrogenase
- IL-1β
Interleukin 1 beta
- IL-6
Interleukin 6
- MAPK/ERK
Mitogen-activated protein kinase (MAPK)/Extracellular signal-Regulated Kinase (ERK)
- MEK
Mitogen-activated protein kinase kinase
- MMP-9
Matrix metallopeptidase 9
- PI3K/AKT/mTOR
Phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of the rapamycin (mTOR)
- TIMPs
Tissue inhibitors of metalloproteinases
- TNF-α
Tumor necrosis factor α
- VEGF
Vascular endothelial growth factor
- Wnt
Wingless signaling
Author contributions
Manuscript concept: PWŁ; Literature review and writing of the first draft: JB, JC; writing—review and editing: PWŁ, JJŁ; supervision: JJŁ. All authors have read and approved the final version of the manuscript.
Funding
Supported by a Grant (DS 474/2023) from Medical University of Lublin, Poland (JJŁ).
Data availability
Not applicable.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tholl D. Terpene synthases and the regulation, diversity, and biological roles of terpene metabolism. Curr Opin Plant Biol. 2006;9(3):297–304. doi: 10.1016/j.pbi.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 2.Christianson DW. Structural biology and chemistry of the terpenoid cyclases. Chem Rev. 2017;117(17):11570–11648. doi: 10.1021/acs.chemrev.7b00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pichersky E, Raguso RA. Why do plants produce so many terpenoid compounds? New Phytol. 2018;220(3):692–702. doi: 10.1111/nph.14178. [DOI] [PubMed] [Google Scholar]
- 4.Gershenzon J, Dudareva N. The function of terpene natural products in the natural world. Nat Chem Biol. 2007;3(7):408–414. doi: 10.1038/nchembio.2007.5. [DOI] [PubMed] [Google Scholar]
- 5.Masyita A, Mustika Sari R, DwiAstuti A, Yasir B, RahmaRumata N, Emran TB, et al. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem X. 2022;13:100217. doi: 10.1016/j.fochx.2022.100217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baser KHC, Buchbauer G. Handbook of essential oils: science, technology, and applications. 2. Boca Raton: CRC Press; 2015. [Google Scholar]
- 7.Wink M. Modes of action of herbal medicines and plant secondary metabolites. Medicines. 2015;2(3):251–286. doi: 10.3390/medicines2030251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharifi-Rad J, Sureda A, Tenore GC, Daglia M, Sharifi-Rad M, Valussi M, et al. Biological activities of essential oils: from plant chemoecology to traditional healing systems. Molecules. 2017;22(1):70. doi: 10.3390/molecules22010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol. 2011;163(7):1344–1364. doi: 10.1111/j.1476-5381.2011.01238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross JA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- 11.Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils—a review. Food Chem Toxicol. 2008;46(2):446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 12.Kamran S, Sinniah A, Abdulghani MAM, Alshawsh MA. Therapeutic potential of certain terpenoids as anticancer agents: a scoping review. Cancers. 2022;14(5):1100. doi: 10.3390/cancers14051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arunasree KM. Anti-proliferative effects of carvacrol on a human metastatic breast cancer cell line, MDA-MB 231. Phytomedicine. 2010;17(8–9):581–588. doi: 10.1016/j.phymed.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Woo CC, Loo SY, Gee V, Yap CW, Sethi G, Kumar AP, et al. Anticancer activity of thymoquinone in breast cancer cells: possible involvement of PPAR-γ pathway. Biochem Pharmacol. 2011;82(5):464–475. doi: 10.1016/j.bcp.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 15.Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol. 2012;166(5):1069–1080. doi: 10.1111/j.1365-2133.2012.10830.x. [DOI] [PubMed] [Google Scholar]
- 16.Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, et al. Cancer statistics for the year 2020: an overview. Int J Cancer. 2021;149(4):778–789. doi: 10.1002/ijc.33588. [DOI] [PubMed] [Google Scholar]
- 17.Guy GP, Jr, Thomas CC, Thompson T, Watson M, Massetti GM, Richardson LC, et al. Vital signs: melanoma incidence and mortality trends and projections—United States, 1982–2030. MMWR Morb Mortal Wkly Rep. 2015;64(21):591–596. [PMC free article] [PubMed] [Google Scholar]
- 18.Shain AH, Bastian BC. From melanocytes to melanomas. Nat Rev Cancer. 2016;16(6):345–357. doi: 10.1038/nrc.2016.37. [DOI] [PubMed] [Google Scholar]
- 19.Bishop JN, Harland M, Bishop T. The genetics of melanoma. Br J Hosp Med. 2006;67(6):31–38. doi: 10.12968/hmed.2006.67.6.21288. [DOI] [PubMed] [Google Scholar]
- 20.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Cancer Society. Melanoma skin cancer. https://www.cancer.org/cancer/melanoma-skin-cancer/detection-diagnosis-staging/signs-and-symptoms.html. Accessed April 17, 2023.
- 22.Argenziano G, Ferrara G, Francione S, Di Nola K, Martino A, Zalaudek I. Dermoscopy-the ultimate tool for melanoma diagnosis. Semin Cutan Med Surg. 2009;28(3):142–148. doi: 10.1016/j.sder.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Tsao H, Atkins MB, Sober AJ. Management of cutaneous melanoma. N Engl J Med. 2004;351(10):998–1012. doi: 10.1056/NEJMra041245. [DOI] [PubMed] [Google Scholar]
- 24.American Cancer Society. Melanoma skin cancer: treatment options. https://www.cancer.org/cancer/melanoma-skin-cancer/treating/by-stage.html. Accessed 17 Apr, 2023.
- 25.Sharma SH, Thulasingam S, Nagarajan S. Terpenoids as anti-colon cancer agents—a comprehensive review on its mechanistic perspectives. Eur J Pharmacol. 2017;795:169–178. doi: 10.1016/j.ejphar.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Modzelewska A, Sur S, Kumar SK, Khan SR. Sesquiterpenes: natural products that decrease cancer growth. Curr Med Chem Anticancer Agents. 2005;5(5):477–499. doi: 10.2174/1568011054866973. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka T, Shnimizu M, Moriwaki H. Cancer chemoprevention by carotenoids. Molecules. 2012;17(3):3202–3242. doi: 10.3390/molecules17033202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raut PK, Lee HS, Joo SH, Chun KS. Thymoquinone induces oxidative stress-mediated apoptosis through downregulation of Jak2/STAT3 signaling pathway in human melanoma cells. Food Chem Toxicol. 2021;157:112604. doi: 10.1016/j.fct.2021.112604. [DOI] [PubMed] [Google Scholar]
- 29.Jiang M, Wu Z, Guo H, Liu L, Chen S. A review of terpenes from marine-derived fungi: 2015–2019. Mar Drugs. 2020;18(6):321. doi: 10.3390/md18060321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salehi B, Venditti A, Sharifi-Rad M, Kręgiel D, Sharifi-Rad J, Durazzo A, et al. The therapeutic potential of apigenin. Int J Mol Sci. 2019;20(6):1305. doi: 10.3390/ijms20061305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu J, Hu XJ, Jin B, Qu XJ, Hou KZ, Liu YP. β-Elemene induces apoptosis as well as protective autophagy in human non-small-cell lung cancer A549 cells. J Pharm Pharmacol. 2012;64(1):146–153. doi: 10.1111/j.2042-7158.2011.01371.x. [DOI] [PubMed] [Google Scholar]
- 32.Sampaio LA, Pina LTS, Serafini MR, Tavares DDS, Guimarães AG. Antitumor effects of carvacrol and thymol: a systematic review. Front Pharmacol. 2021;12:702487. doi: 10.3389/fphar.2021.702487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alipanah H, Farjam M, Zarenezhad E, Roozitalab G, Osanloo M. Chitosan nanoparticles containing limonene and limonene-rich essential oils: potential phytotherapy agents for the treatment of melanoma and breast cancers. BMC Complement Med Ther. 2021;21(1):1–10. doi: 10.1186/s12906-021-03362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsuo AL, Figueiredo CR, Arruda DC, Pereira FV, Scutti JA, Massaoka MH, et al. α-Pinene isolated from Schinus terebinthifolius Raddi (Anacardiaceae) induces apoptosis and confers antimetastatic protection in a melanoma model. Biochem Biophys Res Commun. 2011;411(2):449–454. doi: 10.1016/j.bbrc.2011.06.176. [DOI] [PubMed] [Google Scholar]
- 35.Park KR, Nam D, Yun HM, Lee SG, Jang HJ, Sethi G, et al. β-Caryophyllene oxide inhibits growth and induces apoptosis through the suppression of PI3K/AKT/mTOR/S6K1 pathways and ROS-mediated MAPKs activation. Cancer Lett. 2011;312(2):178–188. doi: 10.1016/j.canlet.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Di Sotto A, Mancinelli R, Gullì M, Eufemi M, Mammola CL, Mazzanti G, et al. Chemopreventive potential of Caryophyllane sesquiterpenes: an overview of preliminary evidence. Cancers. 2020;12(10):3034. doi: 10.3390/cancers12103034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang H, Woo J, Pae AN, Um MY, Cho NC, Park KD, et al. α-Pinene, a major constituent of pine tree oils, enhances non-rapid eye movement sleep in mice through GABAA-benzodiazepine receptors. Mol Pharmacol. 2016;90(5):530–539. doi: 10.1124/mol.116.105080. [DOI] [PubMed] [Google Scholar]
- 38.Qureshi MZ, Attar R, Romero MA, Sabitaliyevich UY, Nurmurzayevich SB, Ozturk O. Regulation of signaling pathways by β-elemene in cancer progression and metastasis. J Cell Biochem. 2019;120:12091–12100. doi: 10.1002/jcb.28624. [DOI] [PubMed] [Google Scholar]
- 39.Menichini F, Tundis R, Loizzo MR, Bonesi M, Provenzano E, de Cindio B, et al. In vitro photo-induced cytotoxic activity of Citrus bergamia and C. medica L. cv. Diamante peel essential oils and identified active coumarins. Pharm Biol. 2010;48(9):1059–1065. doi: 10.3109/13880200903486636. [DOI] [PubMed] [Google Scholar]
- 40.Abdel-Daim MM, Mahmoud OM, Al Badawi MH, Alghamdi J, Alkahtani S, Salem NA. Protective effects of Citrus limonia oil against cisplatin-induced nephrotoxicity. Environ Sci Pollut Res Int. 2020;27(33):41540–41550. doi: 10.1007/s11356-020-10066-x. [DOI] [PubMed] [Google Scholar]
- 41.Zhong J, Yan W, Wang C, Liu W, Lin X, Zou Z, et al. BRAF inhibitor resistance in melanoma: mechanisms and alternative therapeutic strategies. Curr Treat Options Oncol. 2022;23:1503–1521. doi: 10.1007/s11864-022-01006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4(4):253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 43.Banerjee S, Padhye S, Azmi A, Wang Z, Philip PA, Kucuk O, et al. Review on molecular and therapeutic potential of thymoquinone in cancer. Nutr Cancer. 2010;62(7):938–946. doi: 10.1080/01635581.2010.509832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gali-Muhtasib H, Diab-Assaf M, Boltze C, Al-Hmaira J, Hartig R, Roessner A, Schneider-Stock R. Thymoquinone extracted from black seed triggers apoptotic cell death in human colorectal cancer cells via a p53-dependent mechanism. Int J Oncol. 2004;25(4):857–866. [PubMed] [Google Scholar]
- 45.Zou Y, Zhou Z, Yin S, Huang C, Tang H, Yin Z. Targeting of gallbladder megalin receptors with DHA-conjugated limonene albumin nanoparticles. Nanoscale. 2022;14(16):6052–6065. doi: 10.1039/d1nr07767h. [DOI] [PubMed] [Google Scholar]
- 46.Yu X, Lin H, Wang Y, Lv W, Zhang S, Qian Y, et al. d-Limonene exhibits antitumor activity by inducing autophagy and apoptosis in lung cancer. Onco Targets Ther. 2018;11:1833–1847. doi: 10.2147/OTT.S155716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu F, Li X, Li X, et al. Carvacrol induces apoptosis and suppresses proliferation in human colorectal cancer cells. Onco Targets Ther. 2016;9:4557–4566. [Google Scholar]
- 48.Zhong Z, Wang B, Dai M, Sun Y, Sun Q, Yang G, et al. Carvacrol alleviates cerebral edema by modulating AQP4 expression after intracerebral hemorrhage in mice. Neurosci Lett. 2013;555:24–29. doi: 10.1016/j.neulet.2013.09.023. [DOI] [PubMed] [Google Scholar]
- 49.Fulda S, Kroemer G. Targeting mitochondrial apoptosis by betulinic acid in human cancers. Drug Discov Today. 2009;14(17–18):885–890. doi: 10.1016/j.drudis.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 50.Savova MS, Mihaylova LV, Tews D, Wabitsch M, Georgiev MI. Targeting PI3K/AKT signaling pathway in obesity. Biomed Pharmacother. 2023;159:114244. doi: 10.1016/j.biopha.2023.114244. [DOI] [PubMed] [Google Scholar]
- 51.Eddin LB, Jha NK, Goyal SN, Agrawal YO, Subramanya SB, Bastaki SMA, et al. Health benefits, pharmacological effects, molecular mechanisms, and therapeutic potential of α-bisabolol. Nutrients. 2022;14(7):1370. doi: 10.3390/nu14071370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen W, Hou J, Yin Y, Jang J, Zheng Z, Fan H, et al. alpha-Bisabolol induces dose- and time-dependent apoptosis in HepG2 cells via a Fas- and mitochondrial-related pathway, involves p53 and NFκB. Biochem Pharmacol. 2010;80(2):247–254. doi: 10.1016/j.bcp.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 53.Wang J, Wang D, Feng L, Li X, Gong Y, Wang Z, et al. Eremophilane-type and xanthanolide-type sesquiterpenes from the aerial parts of Xanthium sibiricum and their anti-inflammatory activities. Phytochemistry. 2023;208:113603. doi: 10.1016/j.phytochem.2023.113603. [DOI] [PubMed] [Google Scholar]
- 54.Magalhães DB, Castro I, Lopes-Rodrigues V, Pereira JM, Barros L, Ferreira ICFR, et al. Melissa officinalis L. ethanolic extract inhibits the growth of a lung cancer cell line by interfering with the cell cycle and inducing apoptosis. Food Funct. 2018;9(6):3134–3142. doi: 10.1039/c8fo00446c. [DOI] [PubMed] [Google Scholar]
- 55.Siraj MA, Islam MA, Al Fahad MA, Kheya HR, Xiao J, Simal-Gandara J. Cancer chemopreventive role of dietary terpenoids by modulating Keap1-Nrf2-ARE signaling system—a comprehensive update. Appl Sci. 2021;11(22):10806. doi: 10.3390/app112210806. [DOI] [Google Scholar]
- 56.Lee J-Y, Park H, Lim W, Song G. Therapeutic potential of α, β-thujone through metabolic reprogramming and caspase-dependent apoptosis in ovarian cancer cells. J Cell Physiol. 2021;236:1545–1558. doi: 10.1002/jcp.30086. [DOI] [PubMed] [Google Scholar]
- 57.Kłos P, Chlubek D. Plant-derived terpenoids: a promising tool in the fight against melanoma. Cancers. 2022;14(3):502. doi: 10.3390/cancers14030502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shahidi F, Yeo JD. Insoluble-bound phenolics in food. Molecules. 2016;21(9):1216. doi: 10.3390/molecules21091216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ganji-Harsini S, Khazaei M, Rashidi Z, Ghanbari A. Thymoquinone could increase the efficacy of tamoxifen induced apoptosis in human breast cancer cells: an in vitro study. Cell J. 2016;18(2):245–254. doi: 10.22074/cellj.2016.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Attoub S, Sperandio O, Raza H, Arafat K, Al-Salam S, Al Sultan MA, Al Safi M, Takahashi T, Adem A. Thymoquinone as an anticancer agent: evidence from inhibition of cancer cells viability and invasion in vitro and tumor growth in vivo. Fundam Clin Pharmacol. 2013;27(5):557–569. doi: 10.1111/j.1472-8206.2012.01056.x. [DOI] [PubMed] [Google Scholar]
- 61.Liu W, Li S, Qu Z, Luo Y, Chen R, Wei S, Yang X, Wang Q. Betulinic acid induces autophagy-mediated apoptosis through suppression of the PI3K/AKT/mTOR signaling pathway and inhibits hepatocellular carcinoma. Am J Transl Res. 2019;11(11):6952–6964. [PMC free article] [PubMed] [Google Scholar]
- 62.Baumgartner J, Wilson C, Palmer B, Richter D, Banerjee A, McCarter M. Melanoma induces immunosuppression by up-regulating FOXP3(+) regulatory T cells. J Surg Res. 2007;141:72–77. doi: 10.1016/j.jss.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Manu KA, Kuttan G. Ursolic acid induces apoptosis by activating p53 and caspase-3 gene expressions and suppressing NF-κB mediated activation of bcl-2 in B16F–10 melanoma cells. Int Immunopharmacol. 2008;8(7):974–981. doi: 10.1016/j.intimp.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 64.Yang PM, Wu ZZ, Zhang YQ, Wung BS. Lycopene inhibits ICAM-1 expression and NF-κB activation by Nrf2-regulated cell redox state in human retinal pigment epithelial cells. Life Sci. 2016;155:94–101. doi: 10.1016/j.lfs.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 65.Xing X, Ma JH, Fu Y, Zhao H, Ye XX, Han Z, et al. Essential oil extracted from erythrina Corallodendron L. leaves inhibits the proliferation, migration, and invasion of breast cancer cells. Medicine. 2019;98(36):e17009. doi: 10.1097/MD.0000000000017009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yao W, Lin Z, Wang G, Li S, Chen B, Sui Y, et al. Delicaflavone induces apoptosis via mitochondrial pathway accompanying G2/M cycle arrest and inhibition of MAPK signaling cascades in cervical cancer HeLa cells. Phytomedicine. 2019;62:152973. doi: 10.1016/j.phymed.2019.152973. [DOI] [PubMed] [Google Scholar]
- 67.Stacchiotti A, Corsetti G. Natural compounds and autophagy: allies against neurodegeneration. Front Cell Dev Biol. 2020;8:555409. doi: 10.3389/fcell.2020.555409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Cássia da Silveira E Sá R, Lima TC, da Nóbrega FR, de Brito AEM, de Sousa DP. Analgesic-like activity of essential oil constituents: an update. Int J Mol Sci. 2017;18(12):2392. doi: 10.3390/ijms18122392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zheng L, Pan J. The anti-malarial drug artesunate blocks Wnt/β-catenin pathway and inhibits growth, migration and invasion of uveal melanoma cells. Curr Cancer Drug Targets. 2018;18(10):988–998. doi: 10.2174/1568009618666180425142653. [DOI] [PubMed] [Google Scholar]
- 70.Tarapore RS, Siddiqui IA, Saleem M, Adhami VM, Spiegelman VS, Mukhtar H. Specific targeting of Wnt/β-catenin signaling in human melanoma cells by a dietary triterpene lupeol. Carcinogenesis. 2010;31(10):1844–1853. doi: 10.1093/carcin/bgq169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim BY, Lee J, Park SJ, Bang OS, Kim NS. Gene expression profile of the A549 human non-small cell lung carcinoma cell line following treatment with the seeds of Descurainia sophia, a potential anticancer drug. Evid Based Complement Alternat Med. 2013;2013:584604. doi: 10.1155/2013/584604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pratheeshkumar P, Sreekala C, Zhang Z, Budhraja A, Ding S, Son YO, et al. Cancer prevention with promising natural products: mechanisms of action and molecular targets. Anticancer Agents Med Chem. 2012;12(10):1159–1184. doi: 10.2174/187152012803833035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gheorgheosu D, Duicu O, Dehelean C, Soica C, Muntean D. Betulinic acid as a potent and complex antitumor phytochemical: a minireview. Anticancer Agents Med Chem. 2014;14(7):936–945. doi: 10.2174/1871520614666140223192148. [DOI] [PubMed] [Google Scholar]
- 74.Tadros SA, Attia YM, Maurice NW, Fahim SA, Abdelwahed FM, Ibrahim S, et al. Thymoquinone suppresses angiogenesis in DEN-induced hepatocellular carcinoma by targeting miR-1-3p. Int J Mol Sci. 2022;23(24):15904. doi: 10.3390/ijms232415904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu S, Peng L, Sang H, Ping Li Q, Cheng S. Anticancer effects of α-Bisabolol in human non-small cell lung carcinoma cells are mediated via apoptosis induction, cell cycle arrest, inhibition of cell migration and invasion and upregulation of P13K/AKT signaling pathway. J BUON. 2018;23(5):1407–1412. [PubMed] [Google Scholar]
- 76.Ramadan MA, Shawkey AE, Rabeh MA, Abdellatif AO. Expression of P53, BAX, and BCL-2 in human malignant melanoma and squamous cell carcinoma cells after tea tree oil treatment in vitro. Cytotechnology. 2019;71(1):461–473. doi: 10.1007/s10616-018-0287-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Syed DN, Mukhtar H. Botanicals for the prevention and treatment of cutaneous melanoma. Pigment Cell Melanoma Res. 2011;24(4):688–702. doi: 10.1111/j.1755-148X.2011.00851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mukhtar YM, Adu-Frimpong M, Xu X, Yu J. Biochemical significance of limonene and its metabolites: future prospects for designing and developing highly potent anticancer drugs. Biosci Rep. 2018;38(6):BSR20181253. doi: 10.1042/BSR20181253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bai Z, Yao C, Zhu J, Xie Y, Ye XY, Bai R, et al. Anti-tumor drug discovery based on natural product β-elemene: anti-tumor mechanisms and structural modification. Molecules. 2021;26(6):1499. doi: 10.3390/molecules26061499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee YM, Kim GH, Park EJ, Oh TI, Lee S, Kan SY, et al. Thymoquinone selectively kills hypoxic renal cancer cells by suppressing HIF-1α-mediated glycolysis. Int J Mol Sci. 2019;20(5):1092. doi: 10.3390/ijms20051092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Machado TQ, da Fonseca ACC, Duarte ABS, Robbs BK, de Sousa DP. A narrative review of the antitumor activity of monoterpenes from essential oils: an update. Biomed Res Int. 2022;2022:6317201. doi: 10.1155/2022/6317201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mirzaei S, Gholami MH, Hashemi F, Zabolian A, Farahani MV, Hushmandi K, et al. Advances in understanding the role of P-gp in doxorubicin resistance: molecular pathways, therapeutic strategies, and prospects. Drug Discov Today. 2022;27(2):436–455. doi: 10.1016/j.drudis.2021.09.020. [DOI] [PubMed] [Google Scholar]
- 83.Castañeda AM, Meléndez CM, Uribe D, Pedroza-Díaz J. Synergistic effects of natural compounds and conventional chemotherapeutic agents: recent insights for the development of cancer treatment strategies. Heliyon. 2022;8(6):e09519. doi: 10.1016/j.heliyon.2022.e09519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Naeem A, Hu P, Yang M, Zhang J, Liu Y, Zhu W, et al. Natural products as anticancer agents: current status and future perspectives. Molecules. 2022;27(23):8367. doi: 10.3390/molecules27238367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Habtemariam S, Lentini G. Plant-derived anticancer agents: lessons from the pharmacology of geniposide and its aglycone, genipin. Biomedicines. 2018;6(2):39. doi: 10.3390/biomedicines6020039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang W, Zhao Y, Rayburn ER, Hill DL, Wang H, Zhang R. In vitro anti-cancer activity and structure-activity relationships of natural products isolated from fruits of Panax ginseng. Cancer Chemother Pharmacol. 2007;59(5):589–601. doi: 10.1007/s00280-006-0300-z. [DOI] [PubMed] [Google Scholar]
- 87.Rauf A, Imran M, Butt MS, Nadeem M, Peters DG, Mubarak MS. Resveratrol as an anti-cancer agent: a review. Crit Rev Food Sci Nutr. 2018;58(9):1428–1447. doi: 10.1080/10408398.2016.1263597. [DOI] [PubMed] [Google Scholar]
- 88.Dosoky NS, Setzer WN. Biological activities and safety of Citrus spp. Essential Oils Int J Mol Sci. 2018;19(7):1966. doi: 10.3390/ijms19071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 90.Liu G, Oettel K, Bailey H, Ummersen LV, Tutsch K, Staab MJ, et al. Phase II trial of perillyl alcohol (NSC 641066) administered daily in patients with metastatic androgen independent prostate cancer. Invest New Drugs. 2003;21(3):367–372. doi: 10.1023/a:1025437115182. [DOI] [PubMed] [Google Scholar]
- 91.Al-Amri A, Bamosa A. Phase I safety and clinical activity study of thymoquinone in patients with advanced refractory malignant disease. Shiraz E-Med J. 2009;10:107–111. [Google Scholar]
- 92.Dahham SS, Tabana Y, Asif M, Ahmed M, Babu D, Hassan LE, et al. β-Caryophyllene induces apoptosis and inhibits angiogenesis in colorectal cancer models. Int J Mol Sci. 2021;22(19):10550. doi: 10.3390/ijms221910550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Leite GM, Barbosa M, Lopes MJ, Delmondes G, Bezerra DS, Araújo IM, et al. Pharmacological and toxicological activities of α-humulene and its isomers: a systematic review. Trends Food Sci Technol. 2015;115:255–274. doi: 10.1016/j.tifs.2021.06.049. [DOI] [Google Scholar]
- 94.Danciu C, Soica C, Antal D, Alexa E, Pavel IZ, Ghiulai R, et al. Natural compounds in the chemoprevention of malignant melanoma. Anticancer Agents Med Chem. 2018;18(5):631–644. doi: 10.2174/1871520617666171121142522. [DOI] [PubMed] [Google Scholar]
- 95.Jung JI, Kim EJ, Kwon GT, Jung YJ, Park T, Kim Y, et al. β-Caryophyllene potently inhibits solid tumor growth and lymph node metastasis of B16F10 melanoma cells in high-fat diet-induced obese C57BL/6N mice. Carcinogenesis. 2015;36(9):1028–1039. doi: 10.1093/carcin/bgv076. [DOI] [PubMed] [Google Scholar]
- 96.Xie Q, Li F, Fang L, Liu W, Gu C. The antitumor efficacy of β-elemene by changing tumor inflammatory environment and tumor microenvironment. Biomed Res Int. 2020;2020:6892961. doi: 10.1155/2020/6892961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Balavandi Z, Neshasteh-Riz A, Koosha F, Eynali S, Hoormand M, Shahidi M. The use of ß-elemene to enhance radio sensitization of A375 human melanoma cells. Cell J. 2020;21(4):419–425. doi: 10.22074/cellj.2020.6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Al Bitar S, Ballout F, Monzer A, Kanso M, Saheb N, Mukherji D, et al. Thymoquinone radiosensitizes human colorectal cancer cells in 2D and 3D culture models. Cancers (Basel) 2022;14(6):1363. doi: 10.3390/cancers14061363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chang CT, Soo WN, Chen YH, Shyur LF. Essential oil of Mentha aquatica var. Kenting Water Mint suppresses two-stage skin carcinogenesis accelerated by BRAF inhibitor vemurafenib. Molecules. 2019;24(12):2344. doi: 10.3390/molecules24122344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tomko AM, Whynot EG, Ellis LD, Dupré DJ. Anti-cancer potential of cannabinoids, terpenes, and flavonoids present in cannabis. Cancers. 2020;12(7):1985. doi: 10.3390/cancers12071985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhai B, Zhang N, Han X, Li Q, Zhang M, Chen X, et al. Molecular targets of β-elemene, a herbal extract used in traditional Chinese medicine, and its potential role in cancer therapy: a review. Biomed Pharmacother. 2019;114:108812. doi: 10.1016/j.biopha.2019.108812. [DOI] [PubMed] [Google Scholar]
- 102.Gullì M, Percaccio E, Di Giacomo S, Di Sotto A. Novel insights into the immunomodulatory effects of caryophyllane sesquiterpenes: a systematic review of preclinical studies. Appl Sci. 2022;12:2292. doi: 10.3390/app12052292. [DOI] [Google Scholar]
- 103.Ambrož M, Šmatová M, Šadibolová M, Pospíšilová E, Hadravská P, Kašparová M, et al. Sesquiterpenes α-humulene and β-caryophyllene oxide enhance the efficacy of 5-fluorouracil and oxaliplatin in colon cancer cells. Acta Pharm. 2019;69(1):121–128. doi: 10.2478/acph-2019-0003. [DOI] [PubMed] [Google Scholar]
- 104.Pan P, Huang YW, Oshima K, Yearsley M, Zhang J, Arnold M, et al. The immunomodulatory potential of natural compounds in tumor-bearing mice and humans. Crit Rev Food Sci Nutr. 2019;59(6):992–1007. doi: 10.1080/10408398.2018.1537237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Takei M, Umeyama A, Lee J. The possible use of terpene compounds in DC immunotherapy against cancer. Recent Pat Endocr Metab Immune Drug Discovery. 2010;4(1):69–74. doi: 10.2174/187221410790226783. [DOI] [Google Scholar]
- 106.Hossain MS, Kader MA, Goh KW, Islam M, Khan MS, Harun-Ar Rashid M, et al. Herb and spices in colorectal cancer prevention and treatment: a narrative review. Front Pharmacol. 2022;13:865801. doi: 10.3389/fphar.2022.865801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.