Abstract
Background and purpose
Health-related quality of life (HRQoL) is a key aspect of care for cancer survivors that can be improved by physical activity. Our aim was to explore the relationship between physical activity and time to deterioration (TTD) of the HRQoL in patients with lung adenocarcinoma (LUAD).
Methods
We conducted a hospital-based prospective study. The International Physical Activity Questionnaire long-form (IPAQ-L) was used to investigate the pre-treatment physical activity levels, and the EORTC Quality of Life Questionnaire version 3.0 (EORTC QLQ-C30) and EORTC Quality of Life Questionnaire-Lung Cancer (EORTC QLQ-LC13) were used to assess HRQoL at baseline and during follow-up. The QoLR package was used to calculate the HRQoL scores and determine TTD events (minimal clinically important difference=5 points). The effect of physical activity on the HRQoL was assessed using Cox regression analysis.
Results
For EORTC QLQ-C30, TTD events of physical functioning (PF) and dyspnea (DY) in functional scales and symptom scales were the most common during follow-up. Pre-treatment physical activity was found to significantly delay TTD of insomnia (HR=0.635, 95%CI: 0.437–0.922, P=0.017) and diarrhea (HR=0.475, 95%CI: 0.291–0.774, P=0.003). For EORTC QLQ-LC13 scales, deterioration of dyspnea (LC-DY) was the most common event. Physical activity was found to delay the TTD of dyspnea (HR=0.654, 95%CI: 0.474–0.903, P=0.010), sore mouth (HR=0.457, 95%CI: 0.244–0.856, P=0.015), and dysphagia (HR=0.315, 95%CI: 0.172–0.580, P<0.001).
Conclusions
Pre-treatment physical activity of LUAD patients may delay the TTD of multiple HRQoL indicators in EORTC QLQ-C30 and EORTC QLQ-LC13.
Implication for Cancer Survivors
Health-related quality of life (HRQoL) is a key aspect of care for cancer survivors (someone who is living with or beyond cancer), that can be improved by physical activity. Our aim was to explore the relationship between physical activity and time to deterioration (TTD) of the HRQoL in patients with lung adenocarcinoma (LUAD).
Supplementary Information
The online version contains supplementary material available at 10.1007/s11764-022-01259-z.
Keywords: Lung adenocarcinoma, Physical activity, Health-related quality of life, Time to deterioration
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide [1]. Lung adenocarcinoma (LUAD) accounts for approximately 40% of all primary lung tumors and is characterized by high mortality and metastasis rates [2]. Currently, surgery, adjuvant chemoradiotherapy, and immune checkpoint blockade therapy are the available curative options for lung cancer [3, 4], but the recurrence rate is still high (1-year recurrence rate of about 5%) [5]. In a recent report, the 5-year survival for advanced NSCLC was approximately 25% [6], an increase from the previous rate of 18% [7]. With an increase in the survival time, many lung cancer survivors experience health impairment [8]. Therefore, the improvement of the health-related symptoms of patients with LUAD is of much clinical relevance.
Health-related quality of life (HRQoL) is a broad multidimensional concept that includes perceptions of both physical and mental health [9]. It is a valuable index reflecting cancer survivorship outcomes [10]. Patients diagnosed with lung cancer were reported to experience a significant decline in psychosocial and physical function during and after treatment [11, 12]. Therefore, maintaining an adequate HRQoL is one of the goals of treatment for LUAD patients. Many factors can affect the HRQoL of patients with LUAD, and most attention is paid to modifiable behavioral factors [13]. Physical activity is a modifiable factor that is related to the prognosis of chronic diseases and cancer [14–16]. Previous studies have found that exercise prior to treatment or during rehabilitation can help improve outcomes of surgery, including cancer-related fatigue and dyspnea [17–19].
Physical activity is increasingly recognized as a valuable intervention as part of LUAD therapy. However, intermittent and missing HRQoL data in the follow-up period is a shortcoming of previous studies. The time to deterioration (TTD) model is a longitudinal time-event analysis used to assess post-treatment changes in the HRQoL of cancer patients over time, and it can address missing HRQoL data in long-term follow-up [20–22]. In this prospective study, we aimed to analyze the association between pre-treatment physical activity and the TTD in the HRQoL of LUAD survivors.
Materials and methods
Study patients
This was a hospital-based prospective study conducted in two hospitals in Fujian province (Thoracic Surgery and Respiratory Medicine of the First Affiliated Hospital of Fujian Medical University, Affiliated Union Hospital of Fujian Medical University). Patients recruited were newly diagnosed with primary LUAD, confirmed by fiber-optic bronchoscopy or histological examination, between May 2017 and November 2020. The inclusion criteria were (a) diagnosis of primary LUAD with pathological results and (b) patients able to answer the questionnaire clearly and autonomously sign an informed consent. The exclusion criteria were (a) patients diagnosed with benign lesions or secondary lung cancer; (b) patients lacking a pathological diagnosis; and (c) patients unable to answer the questionnaire. The study protocol was approved by the Ethics Committee of the Fujian Medical University, and written informed consent was obtained from all patients prior to their enrolment (Code: [2014] (98)).
Collection of baseline information
A structured questionnaire was designed for this study. Data was collected during face-to-face interviews with patients conducted by trained investigators. Data pertaining to the following variables were collected: general condition (age, sex, education level, height, and weight), history of smoking and alcohol consumption, physical activity, and baseline quality of life (QoL) scores. This data was collected at the time of admission to the hospital.
The International Physical Activity Questionnaire long form (IPAQ-L) was used to assess the level of physical activity [23, 24]. The IPAQ-L covers four domains (work or study, transportation, household duties, and sports leisure) and explores physical activity during the seven days immediately preceding the date of admission to the hospital. The number of days and the number of minutes in a day spent on physical activities were listed in the IPAQ-L. Data cleaning was undertaken to exclude any missing activity frequency or time data as well as any self-reported total time of physical activity of more than 960 min (the study assumed that each person had at least eight hours of sleep). Activity corresponding to time and weekly frequency was re-coded as 0 if the total time of physical activity was less than 10 min a day because at least 10 min of continuous physical activity could lead to good health outcomes). We then used the secondary truncation rule to calculate the level of physical activity. Firstly, if the daily duration of physical activity of a certain intensity exceeded 3 h, it was re-coded as 180 min. This principle allows for a maximum of 21 h (1260 min) per week of reported physical activity of each intensity level. Then, based on the rule of first truncation, the cumulative weekly hours of activities of the same intensity were added up and re-coded as 1260 min if the total time exceeded 1260 min. The physical activity level (MET-min/w) was calculated every week by the MET assigned to the physical activity (Supplement Table 1) multiplied by the weekly frequency (d/w) and the time spent each day (min/d). The sum of different intense physical activity levels was the total physical activity level. According to the IPAQ Working Group (Supplement Table 2), we re-coded the physical activity level (MET-min/w) into three gradient levels (low-level, moderate-level, and high-level).
Quality of life assessments
The European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire version 3.0 (EORTC QLQ-C30) and EORTC Quality of Life Questionnaire-Lung Cancer (EORTC QLQ-LC13) were used to assess the quality of life of patients at baseline and during follow-up. EORTC QLQ-C30 is a 30-item generic questionnaire that includes five functioning scales, three symptom scales, and a global health scale [25]. The EORTC QLQ-LC13 module comprises 13 questions for the assessment of lung cancer-associated symptoms, treatment-related side effects, and use of pain medication [26] (Supplement Table 3). For both the EORTC QLQ-C30 and QLQ-LC13, raw scores are transformed into scale scores ranging from 0 to 100. Higher scores reflect better HRQoL on the global health scale and functioning scales of QLQ-C30, while high scores are related to a high symptomatic level in symptom scales in QLQ-C30 and all scales in QLQ-LC13.
Follow-up
Survival time was defined as time from surgery (May 31, 2017–December 6, 2020) to death or the end of follow-up on September 11, 2021. All patients were followed up every 3–6 months in the first year, and annually thereafter.
Time to deterioration model
TTD was defined as the time from inclusion in the study to the first clinically meaningful deterioration compared to the baseline HRQoL scores in the respective HRQoL assessment tools [27]. The minimal clinically important difference refers to the smallest difference in HRQoL scores perceived as clinically important; it is an important indicator for judging the clinical relevance of the results [28]. In our study, TTD was defined as the time from the first observation with definitive deterioration with a > 5-point, and no subsequent observations with a <5-point decrease compared to baseline in the EORTC QLQ-C30 and EORTC QLQ-LC13 [29].
Statistical analysis
The QoLR package was used to calculate the HRQoL scores and determine the TTD events in EORTC QLQ-C30 and EORTC QLQ-LC13. Median and interquartile range were used to describe the HRQoL scores and TTD. And chi-squared test was performed to assess the differences in sociodemographic, clinical characteristics, and incidence rate of TTD events between patients with different levels of physical activity. Baseline HRQoL scores of three physical activity levels were compared using the Kruskal-Wallis test. Survival analysis was performed using the univariate and multiple Cox regression analysis after controlling for confounding factors; the results are shown as hazard ratios (HRs) with 95% confidence intervals (CIs). All statistical analyses were performed using R software (version 3.5.2) and Statistical Product and Service Solutions version 20.0 (SPSS 20.0).
Results
Sociodemographic, clinical characteristics, and HRQoL scores at baseline
A total of 440 participants completed the baseline questionnaire with a pathological diagnosis of primary LUAD. Among the 440 LUAD patients, 376 LUAD patients completed the first time EORTC QLQ-C30 and QLQ-LC13, 147 patients completed the second time, 80 patients completed the third time, 21 patients completed the fourth time, and three patients completed the fifth time. All patients included in our analysis (n=376) completed the baseline questionnaire and at least one follow-up EORTC QLQ-C30 and QLQ-LC13. Twenty-five patients died during the follow-up period and the median follow-up time was 25 months [19, 30]. Sixty-four patients dropped out during the follow-up (drop-out rate: 17.0%).
The sociodemographic and clinical characteristics of LUAD patients with different physical activity levels are shown in Table 1. There were significant differences between the three levels of physical activity with respect to the distribution of sex, education level, and history of smoking and alcohol consumption (P<0.05). However, there were no significant between-group differences with respect to age, body mass index (BMI), marital status, income, TNM stage, maximum tumor diameter, or therapeutic method. HRQoL scores are presented as a median and interquartile range in Table 2. Only QL scale scores showed significance differences between the three levels of physical activity.
Table 1.
Characteristics of study patients in demographics and clinical message at baseline
| Characteristic | n (%) | Levels of physical activity (n=376) | χ2 | P | ||
|---|---|---|---|---|---|---|
| Low-level N=44 n(%) | Moderate-level N=118 n(%) | High-level N=214 n(%) | ||||
| Age | 5.260 | 0.072 | ||||
| ≤60 | 190 (50.5) | 29 (65.9) | 54 (45.8) | 107 (50.0) | ||
| >60 | 186 (49.5) | 15 (34.1) | 64 (54.2) | 107 (50.0) | ||
| Gender | 30.837 | <0.001 | ||||
| Male | 162 (43.1) | 28 (63.6) | 68 (57.6) | 66 (30.8) | ||
| Female | 214 (56.9) | 16 (36.4) | 50 (42.4) | 148 (69.2) | ||
| BMI | 6.729 | 0.151 | ||||
| <18.5 | 22 (6.0) | 3 (6.8) | 9 (7.8) | 10 (4.9) | ||
| [18.5, 24) | 217 (59.6) | 24 (54.5) | 77 (67.0) | 116 (56.6) | ||
| ≥24 | 125 (34.3) | 17 (38.6) | 29 (25.2) | 79 (38.5) | ||
| Marital status | 2.851 | 0.240 | ||||
| Single (included divorced or widowed) | 34 (9.1) | 1 (2.3) | 12 (10.3) | 21 (9.9) | ||
| In a relationship | 338 (90.9) | 43 (97.7) | 104 (89.7) | 191 (90.1) | ||
| Family income per month | 0.731 | 0.694 | ||||
| ≤10000 | 208 (57.9) | 26 (61.9) | 61 (55.0) | 121 (58.7) | ||
| >10000 | 151 (42.1) | 16 (38.1) | 50 (45.0) | 85 (41.3) | ||
| Educational level | 8.587 | 0.014 | ||||
| Primary and below | 201 (54.0) | 19 (44.2) | 53 (45.7) | 129 (60.6) | ||
| Junior high school and above | 171 (46.0) | 24 (55.8) | 63 (54.3) | 84 (39.4) | ||
| Smoker | 10.895 | 0.004 | ||||
| No | 258 (68.6) | 24 (54.5) | 73 (61.9) | 161 (75.2) | ||
| Yes | 118 (31.4) | 20 (45.5) | 45 (38.1) | 53 (24.8) | ||
| Drinker | 9.818 | 0.007 | ||||
| No | 297 (79.8) | 30 (68.2) | 87 (74.4) | 180 (85.3) | ||
| Yes | 75 (20.2) | 14 (31.8) | 30 (25.6) | 31 (14.7) | ||
| TNM stage | 2.367 | 0.306 | ||||
| 0 and I | 167 (73.9) | 14 (60.9) | 45 (73.8) | 108 (76.1) | ||
| II and above | 59 (26.1) | 9 (39.1) | 16 (26.2) | 34 (23.9) | ||
| Maximum diameter of tumor | 0.144 | 0.930 | ||||
| ≤2.0 | 227 (63.9) | 23 (62.2) | 70 (63.1) | 134 (64.7) | ||
| >2.0 | 128 (36.1) | 14 (37.8) | 41 (36.9) | 73 (35.3) | ||
| Therapeutic method | 5.071 | 0.535 | ||||
| Untreated | 15 (4.0) | 2 (4.5) | 4 (3.4) | 9 (4.2) | ||
| Surgery alone | 275 (73.1) | 28 (63.6) | 84 (71.2) | 163 (76.2) | ||
| Chemotherapy/radiation alone | 17 (4.5) | 4 (9.1) | 6 (5.1) | 7 (3.3) | ||
| Treated with both chemotherapy/radiation and surgery | 69 (18.4) | 10 (22.7) | 24 (20.3) | 35 (16.4) | ||
Table 2.
Baseline of patients QoL scores
| Levels of physical activity (n=376) | H | P | |||
|---|---|---|---|---|---|
| Low-level (M(P25,P75)) | Moderate-level (M(P25,P75)) | High-level (M(P25,P75)) | |||
| QLQ-C30 | |||||
| Global health status (QL) | 75.00 (66.67, 83.33) | 75.00 (66.67, 83.33) | 83.33 (66.67, 83.33) | 12.423 | 0.002 |
| Functional scales | |||||
| Physical functioning (PF) | 96.67 (81.67, 100.00) | 93.33 (86.67, 100.00) | 93.33 (86.67, 100.00) | 0.086 | 0.958 |
| Role functioning (RF) | 100.00 (66.67, 100.00) | 100.00 (100.00, 100.00) | 100.00 (100.00, 100.00) | 3.808 | 0.149 |
| Emotional functioning (EF) | 91.67 (83.33, 100.00) | 83.33 (75.00, 100.00) | 83.33 (75.00, 100.00) | 2.841 | 0.242 |
| Cognitive functioning (CF) | 100.00 (100.00, 100.00) | 100.00 (83.33, 100.00) | 100.00 (83.33, 100.00) | 0.724 | 0.696 |
| Social functioning (SF) | 83.33 (66.67, 100.00) | 66.67 (66.67, 100.00) | 66.67 (66.67, 100.00) | 0.792 | 0.673 |
| Symptom scales/items | |||||
| Fatigue (FA) | 11.11 (0.00, 22.22) | 11.11 (0.00, 33.33) | 11.11 (0.00, 33.33) | 0.311 | 0.856 |
| Nausea and vomiting (NV) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 2.163 | 0.339 |
| Pain (PA) | 8.33 (0.00, 33.33) | 0.00 (0.00, 16.67) | 0.00 (0.00, 16.67) | 1.920 | 0.383 |
| Dyspnea (DY) | 0.00 (0.00, 33.33) | 0.00 (0.00, 33.33) | 0.00 (0.00, 33.33) | 2.060 | 0.357 |
| Insomnia (SL) | 0.00 (0.00, 33.33) | 0.00 (0.00, 33.33) | 0.00 (0.00, 33.33) | 3.757 | 0.153 |
| Appetite loss (AP) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 33.33) | 2.082 | 0.353 |
| Constipation (CO) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.228 | 0.892 |
| Diarrhea (DI) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.281 | 0.869 |
| Financial difficulties (FI) | 0.00 (0.00, 33.33) | 0.00 (0.00, 33.33) | 0.00 (0.00, 33.33) | 3.310 | 0.191 |
| QLQ-LC13 | |||||
| Dyspnea (LC-DY) | 0.00 (0.00, 11.11) | 0.00 (0.00, 11.11) | 0.00 (0.00, 11.11) | 0.666 | 0.717 |
| Coughing (LC-CO) | 16.67 (0.00, 33.33) | 33.33 (0.00, 33.33) | 0.00 (0.00, 33.33) | 2.695 | 0.260 |
| Hemoptysis (LC-HA) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 1.262 | 0.532 |
| Sore mouth (LC-SM) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.651 | 0.722 |
| Dysphagia (LC-DS) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.717 | 0.699 |
| Peripheral neuropathy (LC-PN) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.457 | 0.796 |
| Alopecia (LC-HR) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 1.907 | 0.385 |
| Pain in chest (LC-PC) | 0.00 (0.00, 33.33) | 0.00 (0.00, 0.00) | 0.00 (0.00, 33.33) | 3.258 | 0.196 |
| Pain in aim or should (LC-PA) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 3.073 | 0.215 |
| Pain in other parts (LC-PO) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 2.167 | 0.338 |
Time to deterioration and HRQoL events
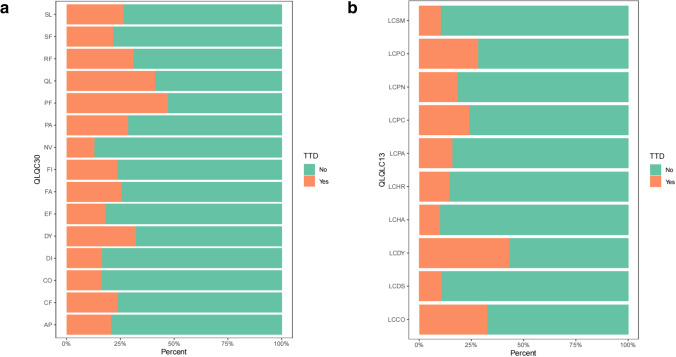
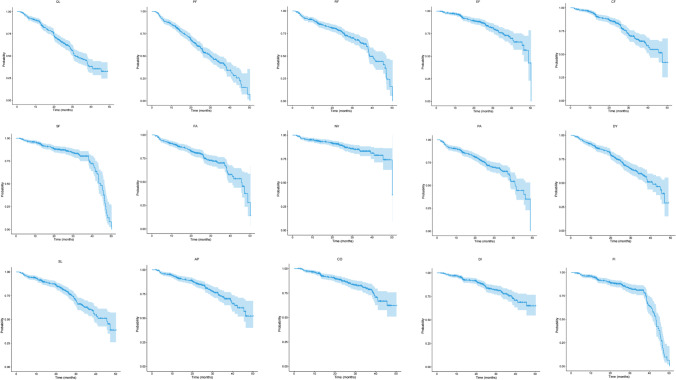
In the functioning scales of EORTC QLQ-C30, time to physical functioning (PF) deterioration event was the most common in our cohort during follow-up, while dyspnea (DY) was the most common in symptom scales of QLQ-C30 (Fig. 1a). The occurrence of TTD of dyspnea (LC-DY) events in EORTC QLQ-LC13 was the first, and coughing (LC-CO) was the second (Fig. 1b). TTD was calculated using the Kaplan-Meier method, HRQoL decreased over time. TTD in all scales of EORTC QLQ-C30 and LC13 are shown in Figs. 2 and 3.
Fig. 1.
The occurrence of TTD events in EORTC QLQ-C30 (a) and EORTC QLQ-LC13 (b)
Fig. 2.
The TTD of all EORTC QLQ-C30 scales
Fig. 3.
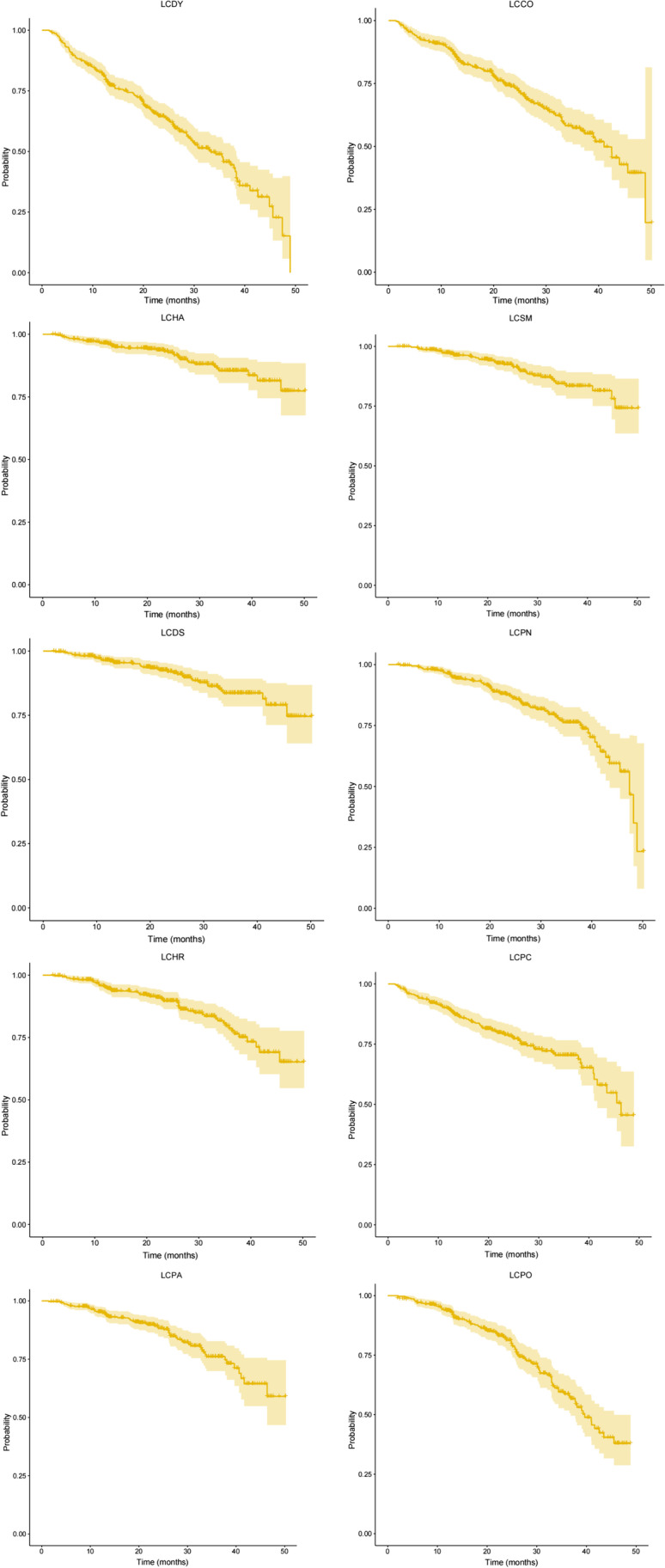
The TTD of all EORTC QLQ-LC13 scales
Association between TTD and physical activity
As shown in Table 3, the low-level physical activity group had a significantly higher proportion of patients with deterioration dyspnea (DY) events (P=0.024), insomnia (SL) events (P=0.036), and diarrhea (DI) events (P=0.033) in EORTC QLQ-C30. Deterioration of sore mouth (LC-SM) (P=0.003) was also higher in the low-level physical activity group. Three levels of physical activity were used as continuous variables to explore the association of physical activity with the HRQoL of LUAD patients. On univariate Cox regression analysis, a higher level of physical activity was associated with improved HRQoL of insomnia (SL) (HR=0.759, 95% CI: 0.586–0.982, P=0.036), diarrhea (DI) (HR=0.677, 95% CI: 0.489–0.938, P=0.019), dyspnea (LC-DY) (HR=0.798, 95% CI: 0.647–0.985, P=0.036), sore mouth (LC-SM) (HR=0.632, 95% CI: 0.425–0.940, P=0.029), and dysphagia (LC-DS) (HR=0.658, 95% CI: 0.443–0.978, P=0.038).
Table 3.
Comparison of time to deterioration in different level of physical activity and univariate cox regression analysis of physical activity level and time to deterioration event ≥ 5 points
| Time to deterioration event n(%) | χ2 | P | Time to deterioration M (P25, P75) | HR (95% CI) | P | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Low-level | Moderate-level | High-level | Low-level | Moderate-level | High-level | |||||
| QLQ-C30 | ||||||||||
| Global health status (QL) | 22 (50.0) | 40 (33.9) | 92 (43.0) | 4.285 | 0.117 | 21.70 (10.82, 33.18) | 20.80 (13.59, 27.77) | 22.14 (12.98, 29.13) | 1.035 (0.826-1.298) | 0.762 |
| Functional scales | ||||||||||
| Physical functioning (PF) | 23 (52.3) | 58 (49.2) | 96 (44.9) | 1.103 | 0.576 | 22.19 (10.99, 29.23) | 19.86 (12.61, 25.91) | 21.39 (12.53, 29.81) | 0.902 (0.736-1.105) | 0.319 |
| Role functioning (RF) | 15 (34.1) | 38 (32.2) | 63 (29.4) | 0.518 | 0.772 | 25.43 (18.79, 34.18) | 20.80 (13.22, 26.72) | 22.77 (12.90, 32.44) | 0.910 (0.706-1.173) | 0.467 |
| Emotional functioning (EF) | 11 (25.0) | 22 (18.6) | 35 (16.4) | 1.877 | 0.391 | 25.79 (19.37, 34.98) | 22.97 (14.13, 30.87) | 23.85 (13.42, 32.89) | 0.821 (0.594-1.134) | 0.232 |
| Cognitive functioning (CF) | 13 (29.5) | 29 (24.6) | 47 (22.0) | 1.240 | 0.538 | 25.43 (18.79, 34.34) | 22.90 (14.02, 29.24) | 23.85 (13.26, 31.96) | 0.877 (0.661-1.165) | 0.366 |
| Social functioning (SF) | 13 (29.5) | 29 (24.6) | 39 (18.2) | 3.704 | 0.157 | 26.20 (19.66, 34.98) | 22.75 (13.32, 29.96) | 23.84 (13.71, 32.89) | 0.819 (0.611-1.096) | 0.179 |
| Symptom scales/items | ||||||||||
| Fatigue (FA) | 15 (34.1) | 32 (27.1) | 49 (22.9) | 2.633 | 0.268 | 25.79 (19.37, 34.18) | 22.31 (13.12, 26.49) | 23.00 (13.03, 31.64) | 0.845 (0.645-1.107) | 0.221 |
| Nausea and vomiting (NV) | 9 (20.5) | 12 (10.2) | 27 (12.6) | 3.054 | 0.217 | 26.20 (19.79, 35.33) | 23.03 (14.13, 31.75) | 23.89 (13.36, 32.99) | 0.879 (0.598-1.294) | 0.514 |
| Pain (PA) | 15 (34.1) | 36 (30.5) | 56 (26.2) | 1.481 | 0.477 | 25.38 (18.79, 34.18) | 20.19 (13.17, 26.32) | 22.49 (12.53, 31.44) | 0.908 (0.702-1.174) | 0.462 |
| Dyspnea (DY) | 22 (50.0) | 37 (31.4) | 62 (29.0) | 7.448 | 0.024 | 25.79 (19.37, 34.18) | 22.75 (13.84, 29.24) | 23.08 (12.90, 31.84) | 0.827 (0.652-1.049) | 0.117 |
| Insomnia (SL) | 18 (40.9) | 33 (28.0) | 48 (22.4) | 6.662 | 0.036 | 25.43 (13.32, 33.72) | 22.55 (13.26, 29.24) | 23.36 (13.15, 32.18) | 0.759 (0.586-0.982) | 0.036 |
| Appetite loss (AP) | 15 (34.1) | 23 (19.5) | 39 (18.2) | 5.745 | 0.057 | 25.79 (19.37, 35.33) | 22.90 (13.84, 29.89) | 23.84 (13.32, 32.74) | 0.788 (0.588-1.057) | 0.111 |
| Constipation (CO) | 10 (22.7) | 22 (18.6) | 28 (13.1) | 3.456 | 0.178 | 25.79 (19.37, 34.80) | 22.97 (14.07, 31.75) | 23.95 (13.49, 33.48) | 0.751 (0.538-1.049) | 0.093 |
| Diarrhea (DI) | 13 (29.5) | 20 (16.9) | 29 (13.6) | 6.806 | 0.033 | 23.74 (18.37, 33.18) | 23.03 (14.07, 31.75) | 24.00 (13.71, 33.07) | 0.677 (0.489-0.938) | 0.019 |
| Financial difficulties (FI) | 12 (27.3) | 30 (25.4) | 47 (22.0) | 0.862 | 0.650 | 25.38 (18.79, 33.72) | 22.90 (13.64, 29.96) | 23.85 (13.61, 32.91) | 0.865 (0.645-1.161) | 0.334 |
| QLQ-LC13 | ||||||||||
| Dyspnea (LC-DY) | 25 (56.8) | 54 (45.8) | 83 (38.8) | 5.343 | 0.069 | 20.11 (9.24, 29.23) | 19.86 (12.58, 25.91) | 21.95 (12.22, 30.09) | 0.798 (0.647-0.985) | 0.036 |
| Coughing (LC-CO) | 15 (34.1) | 36 (30.5) | 71 (33.2) | 0.309 | 0.857 | 25.61 (19.37, 34.98) | 21.50 (13.27, 27.77) | 22.08 (12.53, 30.40) | 1.085 (0.842-1.400) | 0.528 |
| Hemoptysis (LC-HA) | 7 (15.9) | 11 (9.3) | 19 (8.9) | 2.085 | 0.353 | 26.20 (19.79, 35.63) | 23.18 (14.20, 33.19) | 24.72 (13.91, 34.05) | 0.807 (0.526-1.238) | 0.326 |
| Sore mouth (LC-SM) | 11 (25.0) | 10 (8.5) | 18 (8.4) | 11.470 | 0.003 | 26.50 (19.66, 35.33) | 23.18 (14.37, 33.19) | 24.72 (13.93, 34.07) | 0.632 (0.425-0.940) | 0.024 |
| Dysphagia (LC-DS) | 8 (18.2) | 15 (12.7) | 17 (7.9) | 4.802 | 0.091 | 26.20 (19.66, 34.98) | 22.90 (14.07, 30.87) | 24.82 (13.93, 33.98) | 0.658 (0.443-0.978) | 0.038 |
| Peripheral neuropathy (LC-PN) | 11 (25.0) | 21 (17.8) | 37 (17.3) | 1.483 | 0.476 | 26.20 (19.66, 34.98) | 22.90 (14.20, 32.78) | 24.57 (13.93, 33.48) | 0.836 (0.606-1.154) | 0.276 |
| Alopecia (LC-HR) | 9 (20.5) | 17 (14.4) | 28 (13.1) | 1.612 | 0.447 | 25.79 (19.37, 34.18) | 23.11 (14.20, 32.78) | 23.98 (13.36, 33.07) | 0.820 (0.571-1.177) | 0.282 |
| Pain in chest (LC-PC) | 13 (29.5) | 30 (25.4) | 48 (22.4) | 1.147 | 0.563 | 22.19 (15.14, 33.72) | 20.52 (13.12, 26.32) | 23.08 (13.07, 32.49) | 0.868 (0.658-1.146) | 0.318 |
| Pain in aim or should (LC-PA) | 9 (20.5) | 22 (18.6) | 29 (13.6) | 2.222 | 0.329 | 26.20 (19.66, 34.98) | 22.90 (14.07, 29.24) | 23.85 (13.51, 32.89) | 0.812 (0.582-1.134) | 0.221 |
| Pain in other parts (LC-PO) | 15 (34.1) | 34 (28.8) | 57 (26.6) | 1.035 | 0.596 | 25.61 (19.37, 35.33) | 20.31 (13.59, 28.63) | 23.44 (13.26, 32.44) | 0.931 (0.720-1.204) | 0.585 |
To minimize the influence of potential confounding factors, we adjusted for all baseline variables (including age, sex, education level, BMI, history of smoking, and alcohol consumption) and clinical variables (including stage, maximum tumor diameter, therapeutic method) in the multiple Cox regression analysis. The results obtained were similar to the univariate analysis. Physical activity was associated with reduced incidence of time to deterioration in insomnia (SL) (HR=0.635, 95%CI: 0.437–0.922, P=0.017), diarrhea (DI) (HR=0.475, 95%CI: 0.291–0.774, P=0.003), dyspnea (LC-DY) (HR=0.654, 95%CI: 0.474–0.903, P=0.010), sore mouth (LC-SM) (HR=0.457, 95%CI: 0.244–0.856, P=0.015), and dysphagia (LC-DS) (HR=0.315, 95%CI: 0.172–0.580, P<0.001) (Table 4).
Table 4.
Multivariate Cox analysis for time to deterioration event ≥ 5 points
| Items | HR (95%CI) | P |
|---|---|---|
| QLQ-C30 | ||
| Insomnia (SL) | 0.635 (0.437-0.922) | 0.017 |
| Diarrhea (DI) | 0.475 (0.291-0.774) | 0.003 |
| QLQ-LC-13 | ||
| Dyspnea (LC-DY) | 0.654 (0.474-0.903) | 0.010 |
| Sore mouth (LC-SM) | 0.457 (0.244-0.856) | 0.015 |
| Dysphagia (LC-DS) | 0.315 (0.172-0.580) | <0.001 |
Adjusted for all baseline variables (including age, gender, marital status, income, education, BMI, smoking and drinking) and clinical variables (including stage, maximum diameter of tumor, therapeutic method), boldface means P<0.05
Discussion
With the improvement in survival time of LUAD patients, HRQoL is increasingly being recognized as a key factor impinging on the prognosis of these patients. In this study, we constructed a TTD model for LUAD including EORTC QLQ-C30 and QLQ-LC13 in a prospective study. We identified that pre-treatment physical activity levels affected the TTD of insomnia, diarrhea, dyspnea, sore mouth, and dysphagia.
EORTC QLQ-C30 is widely used to assess HRQoL in the context of many cancers [31]. A previous study found a significant decrease in the EORTC QLQ-C30 score and a decrease in HRQoL in social, physical, and role functioning and in the dyspnea symptom score after therapy [32]. Similar results were found in our study, in that all scales in EORTC QLQ-C30 and LC-13 decreased over time. In functioning scales, TTD events of physical functioning were the most common, while role functioning was the second most common. TTD of dyspnea was also the first in symptom scales, in both QLQ-C30 and QLQ-LC13 (DY and LC-DY). These findings indicated that physical and role functioning and dyspnea symptoms warrant more clinical attention. Pre-surgery exercise has been shown to have substantially beneficial effects on lung cancer [33]. Our assessment of the association between TTD of HRQoL and physical activity also revealed that higher-level physical activity before treatment can significantly delay the TTD of insomnia (SL), diarrhea (DI), dyspnea (LC-DY), sore mouth (LC-SM), and dysphagia (LC-DS).
Insomnia is one of the most common sleep disorders, which seriously affects the daily life of patients [34]. Physical activity has been shown to reduce the incidence of insomnia, and insomnia is less prevalent in physically active individuals compared to individuals with a sedentary lifestyle [30, 35–37]. Increased daily physical activity of patients with cancer has been shown to improve sleep and alleviate insomnia [38, 39]. Physical inactivity has also been shown to be associated with gastrointestinal symptoms [40, 41]. A randomized controlled trial investigated the impact of exercise on the HRQoL of patients with prostate cancer and found that patients with exercise intervention had fewer diarrhea symptoms [42]. Another study on prostate cancer survivors also obtained the same results, with physical activity associated with an improvement in diarrhea [43]. Similar results were found in our study, suggesting that physical activity can significantly delay the deterioration of insomnia and diarrhea.
Dyspnea is one of the most common symptoms in patients with lung cancer. Increased physical activity over time has been shown to improve dyspnea after thoracic radiation therapy in patients with breast cancer, lung cancer, and lymphoma [44]. Another study conducted in Korea also observed an association of moderate to vigorous physical activity with fewer symptoms of dyspnea among breast and colorectal cancer survivors [45]. TTD of dyspnea (DY) in QLQ-C30 did not seem to be related to physical activity in our study; however, deterioration of dyspnea (LC-DY) measured by QLQ-LC13 was significantly delayed by physical activity. Sore mouth is a prominent symptom in cancer patients receiving chemotherapy [46, 47]. In patients with non-small cell lung cancer, chemotherapy with cisplatin or anlotinib was found to aggravate sore mouth [48, 49]. However, physical activity was found to delay the exacerbation of sore mouth in the current study, suggesting that physical activity may be applied as a non-pharmaceutical intervention to improve sore mouth in LUAD patients undergoing chemotherapy. Dysphagia is a persistent symptom in cancer patients after mediastinal radiation and chemotherapy [50] and has been shown to be associated with a worse prognosis and HRQoL in lung cancer patients [51, 52]. Methods to improve dysphagia are an important area of lung cancer therapy research. Higher levels of pre-treatment physical activity were found to significantly slow down the TTD of dysphagia in our prospective study. Exercise is an approach to improve muscular coordination and reduce pain in the masticatory muscles (53); dysphagia and sore mouth were associated with muscles and may also be reduced by exercise. The above results suggest that lifestyle interventions to improve physical activity may improve the HRQoL of patients with lung cancer.
To the best of our knowledge, this is the first prospective study to explore the relationship between HRQoL and physical activity based on a TTD model analysis. Our findings may provide a new perspective to improve the quality of life for patients with LUAD. Nevertheless, some limitations of our study should be acknowledged. Post-operative exercise also has a positive impact on the prognosis of many cancers; however, in this study, we did not assess the effect of post-treatment physical activity on the HRQoL. Second, 64 patients withdrew from our study probably due to disease progression or deterioration over a short time after therapy, or due to lack of follow-up. Thus, it is inevitable that there was some follow-up bias in our study causing biased exposure-outcome association estimates. Lastly, the IPAQ scale reflects the physical activity in the preceding seven days; however, physical activity levels 7 days prior to admission are likely to be affected by illness. The self-reported IPAQ scale also contained recall bias.
Conclusions
Pre-treatment physical activity is a modifiable factor that can delay the TTD of insomnia, diarrhea, dyspnea, sore mouth, and dysphagia, as assessed by EORTC QLQ-C30 and EORTC QLQ-LC13. Our report allowed us to generate the hypothesis that pre-treatment physical activity may help maintain a stable HRQoL in patients with LUAD.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
The authors thank all the medical staff who contributed to the maintenance of the medical record database. We greatly acknowledge all of the patients and their families for their cooperation with the questionnaire and support of our study.
Author contribution
Fei He designed the study. Jinman Zhuang performed the analysis. Fei He, Mengxin Lin, Jianbo Lin, Mingqiang Kang, and Zhijian Hu contributed to interpretation of the results. Jinman Zhuang, Yuhang Liu, Maolin Liu, Zishan Chen, and Shuyan Yang collected the data. Jinman Zhuang, Yuhang Liu, Xinying Xu, and Yuxin Cai drafted the manuscript. Fei He contributed to critical revision of the manuscript for important intellectual content. Fei He and Mengxin Lin approved the final version of the manuscript. All authors approved the submitted version.
Funding
This study was supported by Fujian Provincial Health Research Talents Training Programme Medical Innovation Project [grant number 2019-CX-33], Joint Funds for the innovation of science and Technology, Fujian province [grant number 2019Y9022] and Grant of Science and Technology of Fujian, China [grant number 2019L3006]
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mengxin Lin, Email: exlibralmx@163.com.
Fei He, Email: i.fei.he@fjmu.edu.cn.
References
- 1.Erratum: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2020;70(4):313. [DOI] [PubMed]
- 2.Siegel R, Miller K, Jemal A. Cancer statistics, 2019. CA: a cancer journal for clinicians. 2019;69(1):7-34. [DOI] [PubMed]
- 3.Antonia S, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. New Eng J Med. 2017;377(20):1919–29. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 4.Antonia S, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. New Eng J Med. 2018;379(24):2342–50. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 5.Nemesure B, Albano D, Bilfinger T. Lung cancer recurrence and mortality outcomes over a 10-year period using a multidisciplinary team approach. Cancer Epidemiol. 2020;68:101804. doi: 10.1016/j.canep.2020.101804. [DOI] [PubMed] [Google Scholar]
- 6.González M, Calvo V, Redondo I, Provencio M. Overall survival for early and locally advanced non-small-cell lung cancer from one institution: 2000–2017. Clini Transl Oncol: Off Publ Federation Span Oncol Soc National Cancer Inst Mexico. 2021;23(7):1325–33. doi: 10.1007/s12094-020-02521-5. [DOI] [PubMed] [Google Scholar]
- 7.Lin J, Cardarella S, Lydon C, Dahlberg S, Jackman D, Jänne P, et al. Five-year survival in egfr-mutant metastatic lung adenocarcinoma treated with EGFR-TKIs. J Thorac Oncol : Off Publ Int Assoc Study Lung Cancer. 2016;11(4):556–65. doi: 10.1016/j.jtho.2015.12.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colt H, Murgu S, Korst R, Slatore C, Unger M, Quadrelli S. Follow-up and surveillance of the patient with lung cancer after curative-intent therapy: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e437S–e54S. doi: 10.1378/chest.12-2365. [DOI] [PubMed] [Google Scholar]
- 9.Taylor VR. Measuring healthy days; population assessment of health-related quality of life. Centers for Disease Control & Prevention. 2015.
- 10.Nekhlyudov L, Mollica M, Jacobsen P, Mayer D, Shulman L, Geiger A. Developing a quality of cancer survivorship care framework: implications for clinical care, research, and policy. Journal of the National Cancer Institute. 2019;111(11):1120–30. doi: 10.1093/jnci/djz089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gridelli C, Perrone F, Nelli F, Ramponi S, Marinis FD. Quality of life in lung cancer patients. Ann Oncol. 2001;12(suppl 3):S21–5. doi: 10.1093/annonc/12.suppl_3.S21. [DOI] [PubMed] [Google Scholar]
- 12.Li X, Qin K, Yuan C, Song S. The effect of enhancing quality of life in patients intervention for advanced lung cancer: protocol for a randomized clinical study. Medicine. 2020;99(51):e23682. doi: 10.1097/MD.0000000000023682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poghosyan H, Sheldon L, Leveille S, Cooley M. Health-related quality of life after surgical treatment in patients with non-small cell lung cancer: a systematic review. Lung cancer (Amsterdam, Netherlands). 2013;81(1):11–26. doi: 10.1016/j.lungcan.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 14.Park S, Lee I, Kim J, Park H, Lee J, Uhm K, et al. Factors associated with physical activity of breast cancer patients participating in exercise intervention. Support Care in cancer : Off J Multinatl Assoc Support Care Cancer. 2019;27(5):1747–54. doi: 10.1007/s00520-018-4427-3. [DOI] [PubMed] [Google Scholar]
- 15.Avancini A, Sartori G, Gkountakos A, Casali M, Trestini I, Tregnago D, et al. Physical activity and exercise in lung cancer care: will promises be fulfilled? The Oncologist. 2020;25(3):e555–e69. doi: 10.1634/theoncologist.2019-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koontz B, Levine E, McSherry F, Niedzwiecki D, Sutton L, Dale T, et al. Increasing physical activity in cancer survivors through a text-messaging exercise motivation program (ICanSTEP) Support Care Cancer: Off J Multinatl Assoc Support Care Cancer. 2021;29(12):7339–49. doi: 10.1007/s00520-021-06281-y. [DOI] [PubMed] [Google Scholar]
- 17.Edbrooke L, Granger C, Denehy L. Physical activity for people with lung cancer. Aust J Gen pract. 2020;49(4):175–81. doi: 10.31128/AJGP-09-19-5060. [DOI] [PubMed] [Google Scholar]
- 18.Witlox L, Hiensch A, Velthuis M, Steins Bisschop C, Los M, Erdkamp F, et al. Four-year effects of exercise on fatigue and physical activity in patients with cancer. BMC Med. 2018;16(1):86. doi: 10.1186/s12916-018-1075-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Liu X, Yin Y, Ma R, Yang Z, Cao H, et al. Effects of home-based exercise training for patients with lung cancer. Oncol Nurs For. 2019;46(4):E119–E34. doi: 10.1188/19.ONF.E119-E134. [DOI] [PubMed] [Google Scholar]
- 20.Hamidou Z, Dabakuyo T, Mercier M, Fraisse J, Causeret S, Tixier H, et al. Time to deterioration in quality of life score as a modality of longitudinal analysis in patients with breast cancer. Oncologist. 2011;16(10):1458–68. doi: 10.1634/theoncologist.2011-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonnetain F, Dahan L, Maillard E, Ychou M, Mitry E, Hammel P, et al. Time until definitive quality of life score deterioration as a means of longitudinal analysis for treatment trials in patients with metastatic pancreatic adenocarcinoma (Oxford, England : 1990) Eur J Cancer. 2010;46(15):2753–62. doi: 10.1016/j.ejca.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 22.Brusniak K, Feisst M, Sebesteny L, Hartkopf A, Graf J, Engler T, et al. Measuring the time to deterioration for health-related quality of life in patients with metastatic breast cancer using a web-based monitoring application: longitudinal cohort study. JMIR cancer. 2021;7(4):e25776. doi: 10.2196/25776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang Q, Wang Y, Lin F. Reliability and validity of Chinese version of 7-day physical activity recall in Chinese population. Chinese Journal of Rehabilitation Medicine. 2010.
- 24.Fan M, Lyu J, He P. [Chinese guidelines for data processing and analysis concerning the International Physical Activity Questionnaire]. Zhonghua liu xing bing xue za zhi = Zhonghua liuxingbingxue zazhi. 2014;35(8):961-4. [PubMed]
- 25.Nolte S, Liegl G, Petersen M, Aaronson N, Costantini A, Fayers P, et al. General population normative data for the EORTC QLQ-C30 health-related quality of life questionnaire based on 15,386 persons across 13 European countries, Canada and the Unites States (Oxford, England : 1990) Eur J cancer. 2019;107:153–63. doi: 10.1016/j.ejca.2018.11.024. [DOI] [PubMed] [Google Scholar]
- 26.Koller M, Hjermstad M, Tomaszewski K, Tomaszewska I, Hornslien K, Harle A, et al. An international study to revise the EORTC questionnaire for assessing quality of life in lung cancer patients. Ann Oncol: Off J Eur Soc Med Oncol. 2017;28(11):2874–81. doi: 10.1093/annonc/mdx453. [DOI] [PubMed] [Google Scholar]
- 27.Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol: Off J Am Soc Clin Oncol. 1998;16(1):139–44. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 28.Jaeschke R, Singer J, Guyatt G. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10(4):407–15. doi: 10.1016/0197-2456(89)90005-6. [DOI] [PubMed] [Google Scholar]
- 29.Hamidou Z, Dabakuyo T, Mercier M, Fraisse J, Causeret S, Tixier H, et al. Time to deterioration in quality of life score as a modality of longitudinal analysis in patients with breast cancer. Oncol. 2011;16(10):1458–68. doi: 10.1634/theoncologist.2011-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghrouz A, Noohu M, Manzar M, Bekele B, Pandi-Perumal S, Bahammam A. Short-term insomnia symptoms are associated with level and not type of physical activity in a sample of Indian college students. J Prev Med Hyg. 2021;62(2):E447–E54. doi: 10.15167/2421-4248/jpmh2021.62.2.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mercieca-Bebber R, Costa D, Norman R, Janda M, Smith D, Grimison P, et al. The EORTC quality of life questionnaire for cancer patients (QLQ-C30): Australian general population reference values. Med J Aust. 2019;210(11):499–506. doi: 10.5694/mja2.50207. [DOI] [PubMed] [Google Scholar]
- 32.Pompili C, Koller M, Velikova G, Franks K, Absolom K, Callister M, et al. EORTC QLQ-C30 summary score reliably detects changes in QoL three months after anatomic lung resection for Non-Small Cell Lung Cancer (NSCLC) Lung cancer (Amsterdam, Netherlands). 2018;123:149–54. doi: 10.1016/j.lungcan.2018.07.021. [DOI] [PubMed] [Google Scholar]
- 33.Himbert C, Klossner N, Coletta A, Barnes C, Wiskemann J, LaStayo P, et al. Exercise and lung cancer surgery: A systematic review of randomized-controlled trials. Crit Rev Oncol/hematol. 2020;156:103086. doi: 10.1016/j.critrevonc.2020.103086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med : JCSM : Off Publ Am Acad of Sleep Med. 2008;4(5):487–504. [PMC free article] [PubMed] [Google Scholar]
- 35.Janson C, Lindberg E, Gislason T, Elmasry A, Boman G. Insomnia in men-a 10-year prospective population based study. Sleep. 2001;24(4):425–30. doi: 10.1093/sleep/24.4.425. [DOI] [PubMed] [Google Scholar]
- 36.Morgan K. Daytime activity and risk factors for late-life insomnia. J Sleep Res. 2003;12(3):231–8. doi: 10.1046/j.1365-2869.2003.00355.x. [DOI] [PubMed] [Google Scholar]
- 37.Passos G, Poyares D, Santana M, Garbuio S, Tufik S, Mello M. Effect of acute physical exercise on patients with chronic primary insomnia. J Clin Sleep Med : JCSM : Off Publ Am Acad Sleep Med. 2010;6(3):270–5. [PMC free article] [PubMed] [Google Scholar]
- 38.Bernard P, Ivers H, Savard M, Savard J. Temporal relationships between sleep and physical activity among breast cancer patients with insomnia. Health Psychol : Off J Div Health Psychol Am Psychol Assoc. 2016;35(12):1307–15. doi: 10.1037/hea0000408. [DOI] [PubMed] [Google Scholar]
- 39.Armbruster S, Song J, Gatus L, Lu K, Basen-Engquist K. Endometrial cancer survivors' sleep patterns before and after a physical activity intervention: A retrospective cohort analysis. Gynecol Oncol. 2018;149(1):133–9. doi: 10.1016/j.ygyno.2018.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levy R, Linde J, Feld K, Crowell M, Jeffery R. The association of gastrointestinal symptoms with weight, diet, and exercise in weight-loss program participants. Clin Gastroenterol Hepatol : Off Clin Pract J Am Gastroenterol Assoc. 2005;3(10):992–6. doi: 10.1016/S1542-3565(05)00696-8. [DOI] [PubMed] [Google Scholar]
- 41.Ohlsson B, Manjer J. Physical inactivity during leisure time and irregular meals are associated with functional gastrointestinal complaints in middle-aged and elder subjects. Scand J Gastroenterol. 2016;51(11):1299–307. doi: 10.1080/00365521.2016.1209786. [DOI] [PubMed] [Google Scholar]
- 42.Mardani A, Pedram Razi S, Mazaheri R, Haghani S, Vaismoradi M. Effect of the exercise programme on the quality of life of prostate cancer survivors: a randomized controlled trial. Int J Nurs Pract. 2021;27(2):e12883. doi: 10.1111/ijn.12883. [DOI] [PubMed] [Google Scholar]
- 43.Gaskin C, Craike M, Mohebbi M, Salmon J, Courneya K, Broadbent S, et al. Associations of objectively measured moderate-to-vigorous physical activity and sedentary behavior with quality of life and psychological well-being in prostate cancer survivors. Cancer Causes Control : CCC. 2016;27(9):1093–103. doi: 10.1007/s10552-016-0787-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krishnan S, Narayan H, Freedman G, Plastaras J, Maity A, Demissei B, et al. Early Changes in Physical Activity and Quality of Life With Thoracic Radiation Therapy in Breast Cancer, Lung Cancer, and Lymphoma. Int J Radiat Oncol Biol Phys. 2021;109(4):946–52. doi: 10.1016/j.ijrobp.2020.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park J, Lee D, Kim S, Kim N, Jeon J. Moderate to vigorous physical activity participation associated with better quality of life among breast and colorectal cancer survivors in Korea. BMC Cancer. 2020;20(1):365. doi: 10.1186/s12885-020-06819-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown C, McGuire D, Peterson D, Beck S, Dudley W, Mooney K. The experience of a sore mouth and associated symptoms in patients with cancer receiving outpatient chemotherapy. Cancer Nurs. 2009;32(4):259–70. doi: 10.1097/NCC.0b013e3181a38fc3. [DOI] [PubMed] [Google Scholar]
- 47.Brown C, Beck S, Peterson D, McGuire D, Dudley W, Mooney K. Patterns of sore mouth in outpatients with cancer receiving chemotherapy. Support Care Cancer : Off J Multinatl Assoc Support Care Cancer. 2009;17(4):413–28. doi: 10.1007/s00520-008-0509-y. [DOI] [PubMed] [Google Scholar]
- 48.Si X, Zhang L, Wang H, Zhang X, Wang M, Han B, et al. Quality of life results from a randomized, double-blinded, placebo-controlled, multi-center phase III trial of anlotinib in patients with advanced non-small cell lung cancer. Lung cancer (Amsterdam, Netherlands) 2018;122:32–7. doi: 10.1016/j.lungcan.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 49.Morabito A, Piccirillo M, Maione P, Luciani A, Cavanna L, Bonanno L, et al. Effect on quality of life of cisplatin added to single-agent chemotherapy as first-line treatment for elderly patients with advanced non-small cell lung cancer: Joint analysis of MILES-3 and MILES-4 randomised phase 3 trials. Lung cancer (Amsterdam, Netherlands) 2019;133:62–8. doi: 10.1016/j.lungcan.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 50.Shields H, Li J, Pelletier S, Wang H, Freedman R, Mamon H, et al. Persistence of dysphagia and odynophagia after mediastinal radiation and chemotherapy in patients with lung cancer or lymphoma. Dis Esophagus : Off J Int Soc Dis Esophagus. 2017;30(2):1–8. doi: 10.1111/dote.12498. [DOI] [PubMed] [Google Scholar]
- 51.Marmor S, Cohen S, Fujioka N, Cho L, Bhargava A, Misono S. Dysphagia prevalence and associated survival differences in older patients with lung cancer: A SEER-Medicare population-based study. J Geriatr Oncol. 2020;11(7):1115–7. doi: 10.1016/j.jgo.2020.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brady G, Roe J, O' Brien M, Boaz A, Shaw C. An investigation of the prevalence of swallowing difficulties and impact on quality of life in patients with advanced lung cancer. Support Care Cancer : Off J Multinatl Assoc Support Care Cancer. 2018;26(2):515–9. doi: 10.1007/s00520-017-3858-6. [DOI] [PubMed] [Google Scholar]
- 53.Rashid A, Matthews N, Cowgill H. Physiotherapy in the management of disorders of the temporomandibular joint–perceived effectiveness and access to services: a national United Kingdom survey. Br J Oral & Maxillofac Surg. 2013;51(1):52–7. doi: 10.1016/j.bjoms.2012.03.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.