Abstract
Objective
To investigate the associations between several anthropometric parameters and regulatory T cells (Tregs) and circulating cytokines in a population-based cohort.
Methods
Between 2018 and 2021, a total of 238 participants were examined up to three times within the scope of the MEGA study in Augsburg, Germany. Tregs were analyzed using flow cytometry and the serum concentrations of 52 cytokines were determined. Anthropometric parameters were measured, using also bioelectrical impedance analysis: body mass index (BMI), relative total body fat, relative visceral adipose tissue (rVAT), waist circumference (WC), waist-to-hip ratio (WHR) and body fat distribution. Associations were analyzed using linear mixed models with random intercept (Tregs) and conventional linear regression models (cytokines).
Results
WC and WHR were inversely associated with the general Treg subset. Four parameters (BMI, rVAT, WC, and WHR) were inversely associated with the conventional Treg population. Three cytokines showed a particularly strong association with several anthropometric parameters: the cutaneous T-cell attracting chemokine was inversely associated with anthropometric parameters, while hepatocyte growth factor and interleukine-18 showed positive associations.
Conclusions
Anthropometric measures are associated with Tregs and serum cytokine concentrations revealing new important interconnections between obesity and the adaptive immune system.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00011-023-01777-1.
Keywords: BMI, Body fat, Visceral fat, Cytokines, Flow cytometry, Regulatory T cells
Introduction
Within the last decades, overweight and obesity became one of the major health risks in western societies and worldwide [1, 2]. Many diseases are supposed to be causally associated with increased body mass index (BMI) and total body fat (TBF) in general, and visceral adipose tissue (VAT) in particular [3–6]. Since recently, it became more obvious, that the impact of obesity on the immune system explains a major part of the association between obesity and chronic disease [7]. At first, influences especially on the innate immune system (e.g., macrophages) were described in various studies, but more recent studies also reported connections with the adaptive immune system [7]. Still, the overall interplay between obesity and changes in the functionality of the immune system are far from being understood completely. To gain a deeper understanding of this relationship the present study investigates the associations between various anthropometric parameters and regulatory T cells (Tregs) in a population-based cohort with repeated measurements. In addition, serum cytokines were examined to inform the results for Tregs with data at the protein level.
Methods
Study population and data collection
This analysis is based on data from the MEGA study, a single center, population-based cohort study conducted between 2018 and 2021 in Augsburg, Germany. Participants between 25 and 65 years were recruited and examined up to 4 times within a time period of 9 months (baseline visit, follow-up visit after 1 and 6 months, final visit after 9 months). For this study, only patients without fever (body temperature ≤ 38.5 °C in the last 24 h) and without antibiotics or immunosuppressant use in the last 3 months were included. All examinations were performed in a 12 h overnight fasting state. All study participants gave written informed consent. Methods of data and biospecimen collection have been approved by the ethics committee of the Ludwig-Maximilians-Universität München, and the study was performed in accordance with the Declaration of Helsinki. The study was registered at the German Register of Clinical Studies (DRKS) with the project number DRKS00015784.
Data collection
Next to a variety of different examinations, every visit included the collection of venous blood samples and an extensive survey addressing comorbidities and existing diseases, risk factors, and mental health. Among other topics, the following information was obtained during the face-to-face interview: total daily alcohol consumption, smoking status (current smoker, never smoker, previous smoker), and education (no professional education, professional education, academic education).
At each study visit, anthropometric measurements were made (height, weight, waist circumference, hip circumference) and a body composition analysis via bioelectrical impedance analysis (BIA, SECA mBCA515 device) was conducted. For the present analysis, the following parameters were used as exposure variables: body mass index (BMI, kg/m2), relative total body fat (rTBF, in % of body weight), relative visceral adipose tissue (rVAT, in % of body weight), waist circumference (WC, in cm), waist-to-hip ratio (WHR) and body fat distribution (BFD; visceral body fat mass divided by total body fat mass).
All examination steps were carried out by trained study nurses in accordance with previously defined standard operating procedures.
Flow cytometry
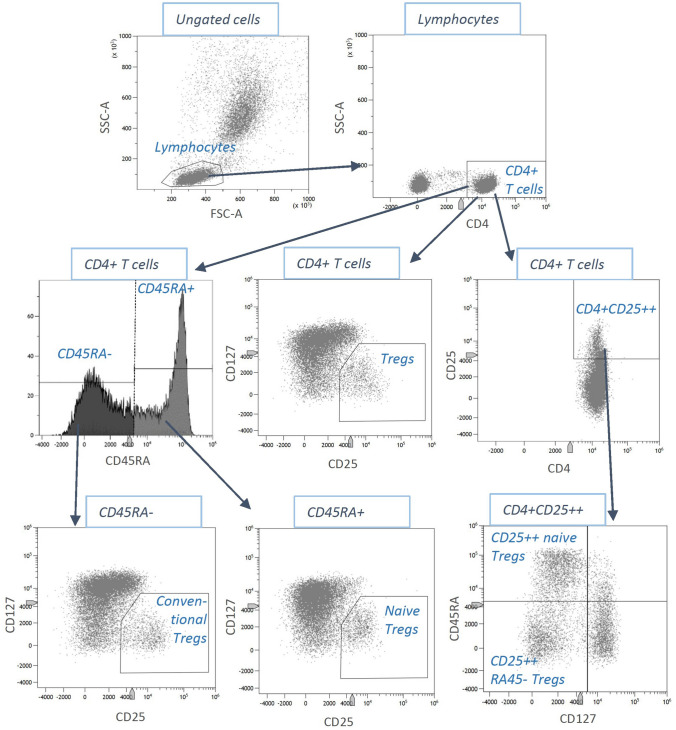
Venous EDTA blood samples were used for the flow cytometry measurements (Cytoflex LX flow cytometer, 6 lasers, Beckman Coulter GmbH, Krefeld, Germany). In a first step, erythrocytes were lysed by using VersaLyse Lysing Solution (Beckman Coulter) and subsequently immune cells were isolated in several washing steps. Leucocytes were treated with an FC receptor block, which avoids non-specific antibody binding. Then, antibody staining was performed with fluorescence-labelled liquid antibodies. The following antibodies were used for the analysis of Tregs: anti-CD4, anti-CD25, anti-CD127 and anti-CD45RA. The best concentration of antibodies was predetermined by titration. The antibody-coupled immune cells were fixed using IO-Test 3 Fixative Solution (Beckman Coulter). In a final step, the T cells were analyzed via flow cytometry. The gating was performed using Kaluza software (Beckman Coulter). Figure 1 displays the applied gating strategy. For each T cell subpopulation, the relative frequency of the respective cells (as a share of the total cell count of the parent gate) was estimated and used for the statistical analysis.
Fig. 1.
Gating strategy for the Treg panel. In the first step, T helper cells were identified by their expression of CD4. Then, different CD4 + T cell subsets were analyzed using the following antibodies: anti-CD25, anti-CD127, anti-CD45RA. The identified cell subsets were quantified by calculating their proportion of the parent gate cells
Cytokines measurement
Concentrations of 52 cytokines were measured in serum using the Bio-Plex Pro™ Human Cytokine Screening Panel, ICAM-1 set and VCAM-1 set, plus the Pro™ Human TH17 cytokine sCD40L set and IL31 set (all Bio-Rad Laboratories Inc., Hercules, California, USA). The assays were carried out according to the manufacturer’s specifications. In brief, 50 µl of diluted magnetic beads were placed in the wells and washed 2 times. Then the standard samples, blank and controls were pipetted into the respective well and incubated between 30 and 60 min on the shaker at 850 ± rpm at room temperature. This was followed by a threefold wash step, the addition of the diluted detection AB and another 30 min incubation (all incubation steps were carried out at room temperature). Subsequently and after another wash step, streptavidin PE was added and incubated for 10 min. Afterwards, the plate was washed 3 more times and re-suspended with 125 µl. For the measurements, Luminex xMAP technology instrument and Bioplex Manager Software was used. The measurements were performed in six batches. The technically related variations between the plates were corrected using intensity normalization (defined as x_norm = x − x̃_plate + x̃_overall, where x̃_plate and x̃_overall represent the plate-specific and overall medians).
For many measures the cytokine concentrations were below the limit of detection. For this analysis, we excluded all cytokines with missing values (in general due to concentrations below the limit of detection) in 25% or more of all measures. Consequently, linear regression models were calculated for 25 cytokines. For these cytokines, missing values due to concentrations below the limit of detection were set to the lower detection limit. 27 cytokines have not been used for the statistical analysis. In the supplementary material (Table S2) we give an overview of all cytokines measured and the number of missing values.
Sample size
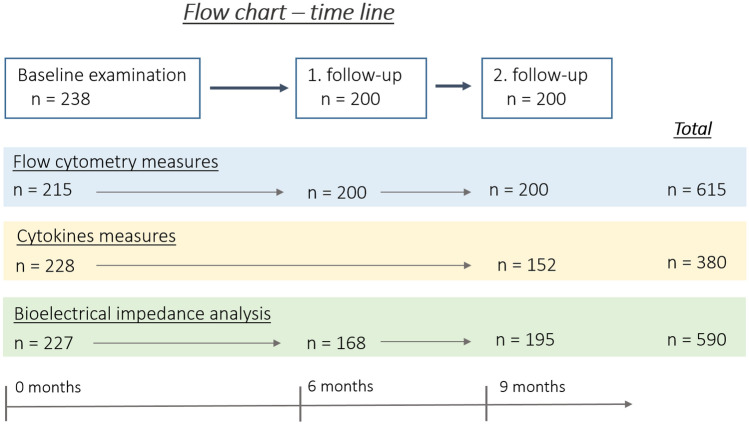
A total of 238 participants were examined at least once. The flow cytometry measures started with a short delay (after the start of the study), so baseline data for Tregs were available in 215 patients. For both follow-up visits (after 6-months and after 9-months), there was flow cytometry data available for 200 participants each. So overall, 615 measures could be included into the mixed model analyses.
Determination of cytokine concentrations was performed for baseline visits (n = 228) and 9-months follow up (n = 152) only. Consequently, a total of 380 measures was considered for the linear regression models. Due to restriction caused by the Covid-19 pandemics, the bioelectrical impedance analysis had to be temporarily removed from the examination. Consequently, 590 measurements of rTBF, rVAT and body fat distribution were available (baseline: 227, follow-up visits after 6-months 168, follow-up visits after 9-months: 195). Information on BMI, waist circumference and waist-to-hip ratio was available for every participant and for every examination visit. An overview of the sample sizes and the number of included measures is displayed in Fig. 2.
Fig. 2.
Flow chart and time line of the included participants and measures for the analysis of Tregs (flow cytometry) and cytokines
Statistical analysis
In general, categorical variables were compared using Chi-square tests and the results were presented as absolute frequencies with percentages. For normally-distributed continuous variables, Student’s t-tests were used. For continuous variables that were not normally-distributed we used nonparametric tests. The results were presented as mean and standard deviation (SD) or median and inter-quartiles range (IQR).
Mixed models with random intercept
For the majority of participants there was data on Treg subpopulations and body composition (BMI, body fat, visceral body fat) for three different time points available. An appropriate statistical method for this longitudinal structure of the data are random intercept models with identity link based on Maximum Likelihood estimation. We calculated such models to examine the associations between Treg subpopulations (outcomes) and parameters of body composition (exposures). For the Treg subpopulations, we used the relative proportion of these cells on the corresponding parent gate. We standardized the outcome as wells as the exposure variables to ensure comparability of the obtained effect sizes. In order to avoid excessive impact of single outliers, we removed observations with a deviation from the mean of greater than three standard deviations. According to literature review, all models were adjusted for the following potential confounder variables: sex, age, education, smoking status, alcohol consumption and hypothyroidism. Normal distribution of the regression residuals was checked graphically and considered to be fulfilled sufficiently.
Linear regression models
Data on cytokine concentrations was available for 2 time points (baseline, 9-months-follow-up). For that reason conventional linear regression models instead of mixed models were calculated. Only cytokines with missing values of less than 25% were analyzed. The models were adjusted for the same confounding variables as the mixed models for Tregs and additionally for the corresponding visit (baseline, follow-up). Just like for the mixed models, the exposure and outcome variables were standardized and outliers exceeding a deviation of more than 3 SD were removed from the analysis. Again, normal distribution of the regression residuals was checked graphically.
Within both, the mixed models and the linear regression models, the obtained p values were false discovery rate (FDR)-adjusted to control the effect of multiple testing (confidence intervals (CI) displayed in Figs. 3 and 4 and Table S2 were not FDR-adjusted). Results with p values of less than 0.05 were considered to be significant. For all models, the displayed effect estimates (β-coefficient and 95% CI) must be interpreted as the expected change in standardized outcome associated with one standard deviation increase in the exposure variable.
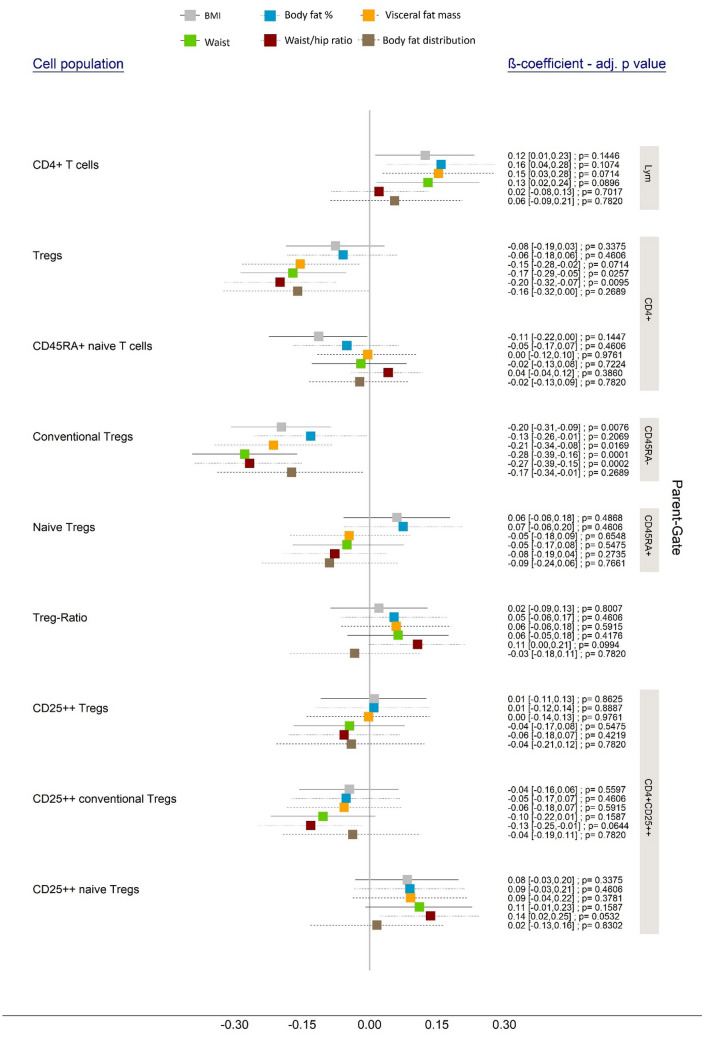
Fig. 3.
Associations between Treg subsets and anthropometric parameters. The figure displays the results of linear mixed models with random intercept and adjusted for sex, age, education, smoking status, alcohol consumption and hypothyroidism. Both, the exposure variables and the outcome were standardized. The results are presented on the right side (estimated β-coefficients with 95% CI, and FDR adjusted p values) and are graphically illustrated by the forest plot. The specific cell populations are shown on the left side and the respective parent gate is represented by the gray rectangles on the right edge of the figure
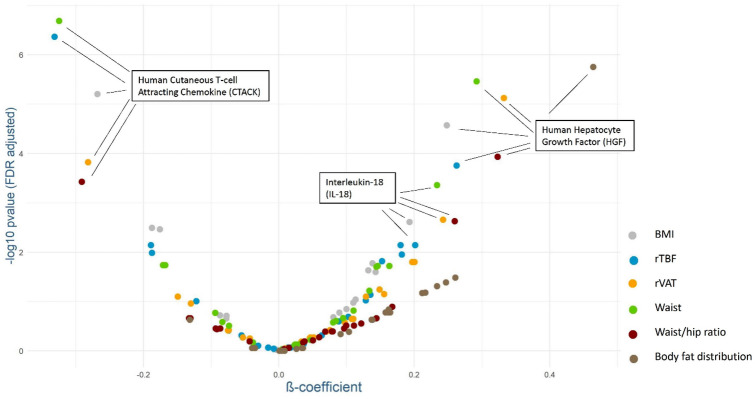
Fig. 4.
Associations between anthropometric parameters and serum cytokine concentrations. The Linear regression models were adjusted for sex, age, education, smoking status, alcohol consumption, hypothyroidism and corresponding visit (baseline, follow-up). Both, the exposure variables and the outcome were standardized. The figure displays the estimated β-coefficients (X-axis) and the − log10 transformed FDR-adjusted p values (Y-axis)
Since loss of follow-up was low and missing observations were very scarce, we performed a complete-case analysis.
All statistical analyses were performed using R version 4.2.1.
Results
Table 1 displays the baseline characteristics for the total sample and stratified for obesity (BMI ≤ 30 kg/m2, BMI > 30 kg/m2). Mean age of the cohort was 46.3 years (SD 11.8) and a majority of 68.9% were women. In total, 94 (39.5%) participants had a measured BMI of more than 30 kg/m2.
Table 1.
Baseline characteristics of the total sample and stratified by obesity (BMI ≤ 30 kg/m2, BMI > 30 kg/m2)
| Total sample (n = 238) | BMI ≤ 30 kg/m (n = 144) | BMI > 30 kg/m2 (n = 94) | p value | |
|---|---|---|---|---|
| Age (years) | 46.3 (11.8) | 45.9 (12) | 46.9 (11.6) | 0.5301 |
| Sex, female | 164 (68.9) | 108 (75) | 56 (59.6) | 0.0178 |
| Body weight (kg) | 83.8 (24.4) | 67.9 (10.8) | 108.1 (18.9) | < 0.001 |
| Body height (cm) | 170.8 (9.1) | 170.3 (8.5) | 171.6 (10) | 0.2724 |
| BMI (kg/m2) | 28.6 (7.6) | 23.3 (2.6) | 36.6 (5.2) | < 0.001 |
| Relative total body fat (%) | 34 (10.5) | 28.6 (8.1) | 42.6 (7.9) | < 0.001 |
| Relative visceral body fat (%) | 2.5 (1.8) | 1.5 (1.0) | 4 (1.7) | < 0.001 |
| Waist circumference (cm) | 93.1 (19.1) | 80.5 (9.6) | 112.4 (12.8) | < 0.001 |
| Waist-to-hip ratio | 0.9 (0.1) | 0.8 (0.1) | 0.9 (0.1) | < 0.001 |
| Body fat distribution | 7.2 (4.8) | 5.4 (3.7) | 10 (5) | < 0.001 |
| Hypertension | 63 (26.5) | 21 (14.6) | 42 (44.7) | < 0.001 |
| Diabetes mellitus | 10 (4.2) | 5 (3.5) | 5 (5.3) | 0.7698 |
| Total alcohol consumption (alcoholic beverages per day) | 0.15 (0.05–0.54) | 0.15 (0.12–0.54) | 0.15 (0.05–0.54) | 0.2447 |
| Physical activity | ||||
| Medium intensity | 0 (0–3) | 0 (0–3) | 0 (0–2.88) | 0.6213 |
| High intensity | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.425 |
| Smoking status | < 0.001 | |||
| Current smoker | 34 (14.3) | 13 (9.0) | 21 (22.3) | |
| Never smoker | 119 (50) | 87 (60.4) | 32 (34.0) | |
| Former smoker | 85 (35.7) | 44 (30.6) | 41 (43.6) | |
| Vegetarian diet | 0.2005 | |||
| No | 214 (89.9) | 128 (88.9) | 86 (91.5) | |
| Vegetarian | 12 (5) | 10 (6.9) | 2 (2.1) | |
| Vegan | 12 (5) | 6 (4.2) | 6 (6.4) | |
Mean (SD) or median (IQR) or n (%)
Anthropometric parameters and Tregs
Firstly, waist circumference (β-coefficient: − 0.17 [− 0.29, − 0.05], p value: 0.0257) and waist-to-hip ratio (β-coefficient: − 0.20 [− 0.32, − 0.07], p value: 0.0095) showed a significantly inverse associations with the Treg subset (as percentage of Tregs in CD4 + T cells) (Fig. 3). rVAT was significantly inversely associated with Tregs only before FDR-adjustment.
Secondly, four parameters were significantly and inversely associated with the conventional Treg population (defined as the proportion of CD25 + CD127− cells on all CD45RA− CD4 + T cells): BMI (β-coefficient: − 0.20 [− 0.31, − 0.09], p value: 0.0076), rVAT (β-coefficient: − 0.21 [− 0.34, − 0.08], p value: 0.0196), waist circumference (β-coefficient: − 0.28 [− 0.39, − 0.16], p value: 0.0001) and waist-to-hip ratio (β-coefficient: − 0.27 [− 0.39, − 0.15], p value: 0.0002) (Fig. 3). rTBF and body fat distribution showed significantly inverse associations before, but not after FDR-adjustment.
Anthropometric parameters and cytokines
Basically, three cytokines were particularly strongly associated with several anthropometric parameters (Fig. 4). On the one hand, the Human cutaneous T-cell Attracting Chemokine (CTACK) was significantly negatively associated with all parameters of body composition apart from body fat distribution. On the other hand, the human hepatocyte growth factor (HGF) showed a significant positive association with all six examined anthropometric parameters. Interleukine-18 (IL-18) was also inversely associated with significant FDR-adjusted p values for all parameters apart from body fat distribution.
In addition to these three cytokines, some more cytokines showed significant association (after FDR-adjustment) with some of the anthropometric parameters: Human vascular cell adhesion protein 1 [VCAM-1] was positively associated with BMI, rTBF, and waist circumference; Human Interleukin-4 [IL-4] was associated with BMI, rTBF, and waist circumference; Human Intercellular Adhesion Molecule-1 [Hu ICAM-1] was related to BMI, rTBF, and waist circumference; Human Interferon gamma-induced protein [IP-10] was associated with body fat distribution; Human Macrophage Inflammatory Protein-1 alpha [MIP-1a] was associated with BMI, rTBF, rVAT, waist circumference, and body fat distribution; Human Regulated upon activation, normal T cell expressed and secreted [RANTES] was related to body fat distribution; Human Eotaxin [Hu Eotaxin] was significantly associated with BMI, rTBF, and waist circumference. All results are presented in the supplementary.
Discussion
In this study, we found significant inverse associations between anthropometric traits and the frequency of Tregs and conventional Tregs. Moreover, three cytokines, CTACK, HGF and IL-18, were identified showing notably strong associations with anthropometric measurements.
Obesity and Tregs
First, it was noticeable, that for the general Treg population, we found significant association after FDR-adjustment only for the conventional measures waist circumference and waist-to-hip ratio. This comes somehow surprising, since both parameters can be measured easily and without advanced technology. Parameters like rTBF, rVAT or body fat distribution require elaborated measurements (bioelectrical impedance analysis) and therefore are suspected to provide more accurate information about body composition.
Tregs are a specific T cells with immunomodulatory properties, that play a crucial role in mitigation of inflammation and immune response [8]. For a variety of diseases (e.g. autoimmune diseases), Tregs are suspected to be involved in the pathophysiological processes [9]. On the other hand, recent research suggests, that obesity and changes in body composition affect the immune system und in this way are involved in the development of many diseases, but especially metabolic disorders [7, 10–12]. In this study, we show that several parameters of obesity are indeed inversely associated with the relative frequency of Tregs and conventional Tregs.
Few prior studies have investigated the connection between obesity/body composition and Tregs as well, and thereby reported differing results [7]. Some studies reported a (relative) decrease of Tregs in obesity-induced mice [13, 14] and in agreement with this the induction of Tregs improved insulin sensitivity [13]. Furthermore, another study indicated a body-mass dependent depletion of visceral adipose-tissue Tregs in mice and humans [15]. On the other hand, Travers et al. found an increased activation of adipose tissue Tregs in the presence of obesity, but no association for circulating blood Tregs in humans (n = 30) [16]. Similar results were reported by two further studies, showing an increased presence of Tregs in adipose tissue in individuals with obesity [17, 18]. The authors of a review article supposed that these cells accumulate in adipose tissue in order to attenuate obesity-related inflammation [19]. Additionally, van der Weerd et al. examined 13 morbidly obese subjects and 25 lean healthy controls and found that obesity was significantly associated with an increased number of peripheral blood Tregs [20], which is in contrast to the results of the present study.
Moreover, there are also studies indicating, that immunological changes in general and alterations in the Treg biology in particular affect metabolic processes and facilitate obesity. Beppu et al. for instance reported that Tregs promote obesity by Blimp-1-regulated IL-10 secretion, which suppresses adipocyte energy expenditure and thermogenesis [21].
Obesity and cytokines
In this study, we found a strong positive association between anthropometric measures and IL-18 and HGF. Both results are confirmed by a study from China, which also reported positive associations of IL-18 and HGF with BMI [22]. In the same study, both biomarkers were furthermore associated with an increased risk of cardiovascular diseases [22], which is confirmed by other publications as well [23–25].
IL-18 is a predominantly pro-inflammatory cytokine and supposedly involved in the pathogenesis of a variety of diseases [26]. Likewise, prior studies reported elevated IL-18 levels in individuals with obesity [27–31]. Moreover, it has been found that patients with obesity or type 2 diabetes mellitus are characterized by a IL-18 resistance after stimulation [29]. However, also anti-obesity effects of IL-18 have been described [32]. A recent study by Akimova et al. reported elevated IL-18 levels in overweight or obese lung transplant recipients and suggested an obesity-related Treg impairment induced by IL-18 [28].
HGF is a paracrine cellular growth factor that acts as a pleiotropic cytokine [33]. Next to the study from China mentioned above [22], several other studies have found positive associations between obesity and blood HGF levels as well [34–37]. In a study in mice, however, HGF was found to inhibit diet-induced obesity and to improve insulin resistance [38]. Thus, elevated HGF levels in individuals with obesity might primarily be a compensatory mechanism in reaction to (diet-induced) obesity.
In contrast to IL-18 and HGF, we found a strong inverse association of anthropometric parameters and CTACK, also known as CCL27 [39]. CTACK is a cytokine which is predominantly expressed in the skin and attracts memory T cells [40]. To the best of our knowledge, no prior studies have reported an association between CTACK and obesity so far. Future examinations are necessary to validate our findings.
Strengths and limitations
This study is characterized by some particular strengths. First, we examined a population-based cohort with a relatively high number of well-phenotyped individuals. For most participants we have one or two follow-up measurements with an overall low drop-out rate. The repeated measurements reduce the susceptibility to short-term immune cell fluctuations caused by infection or illness. Measurements were highly standardized and conducted by qualified and certified staff. The flow cytometry measurements were performed immediately after blood sampling.
Nevertheless, there are some limitations to consider. There is limited quantitative comparability of flow cytometry measurements across different laboratories (e.g. due to a different selection of staining antibodies, different gating strategies or deviating classification of specific subpopulations). In our study, we did not use FoxP3 antibodies for Treg classification, which recently became a commonly used marker for regulatory CD4 + T cells. Another limitation of this study is the missing investigation of Tregs in specific tissues like adipose tissue-resident Tregs. Since the overwhelming majority of our study sample consisted of a typical European population aged 25–65 years, the results might not be generalized to other ethnicities and age-groups. Finally, there might be some unmeasured confounding by disregarding relevant covariables and, as argued above, we cannot make reliable statements about the causality of the observed associations.
Conclusion
Anthropometric traits and changes in body composition are inversely associated with the relative frequency of Tregs and conventional Tregs. Moreover, the cytokines IL-18 and HGF showed strong positive associations with overweight and obesity. CTACK on the other hand showed a distinct negative association. These findings are essential for a comprehensive understanding of pathophysiological mechanisms underlying obesity and its secondary diseases.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all colleagues who were involved in the conduction of the study including examiners and study nurses, laboratory staff, secretaries, organization and documentation employees and student assistants. Our special thanks go to all study participants who enabled this study with their participation.
Author contributions
CM and JL conceived the study. TS performed the statistical analysis and drafted the manuscript. CM supervised data analysis. CM and JL were responsible for the acquisition of the data. DF contributed to the statistical analysis and the general evaluation strategy. All authors approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. The study was financed by institutional funds. The authors did not receive funding for this study.
Availability of data and materials
The datasets generated during and/or analyzed in the current study are not publicly available due to data protection aspects but are available in an anonymized form from the corresponding author on reasonable request.
Declarations
Conflict of interest
None declared.
Ethics approval and consent to participate
The study was registered at the DRKS (Deutsches Register Klinische Studien) under the number DRKS00015784 and was approved by the Ethics Committee of the Ludwig-Maximilians-Universität München (project number 18-637).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Muc Da Encarnacao M, NCD Risk Factor Collaboration Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet (London, England) 2017;390:2627–2642. doi: 10.1016/S0140-6736(17)32129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apovian CM. Obesity: definition, comorbidities, causes, and burden. Am J Manag Care. 2016;22:s176–s185. [PubMed] [Google Scholar]
- 3.Ortega FB, Lavie CJ, Blair SN. Obesity and cardiovascular disease. Circ Res. 2016;118:1752–1770. doi: 10.1161/CIRCRESAHA.115.306883. [DOI] [PubMed] [Google Scholar]
- 4.Pischon T, Nimptsch K. Obesity and risk of cancer: an introductory overview, recent results in cancer research. Fortschritte der krebsforschung. Progres Recherch Cancer. 2016;208:1–15. doi: 10.1007/978-3-319-42542-9_1. [DOI] [PubMed] [Google Scholar]
- 5.Withrow D, Alter DA. The economic burden of obesity worldwide: a systematic review of the direct costs of obesity. Obes Rev Off J Int Assoc Study Obes. 2011;12:131–141. doi: 10.1111/j.1467-789X.2009.00712.x. [DOI] [PubMed] [Google Scholar]
- 6.Mihalko WM, Bergin PF, Kelly FB, Canale ST. Obesity, orthopaedics, and outcomes. J Am Acad Orthop Surg. 2014;22:683–690. doi: 10.5435/JAAOS-22-11-683. [DOI] [PubMed] [Google Scholar]
- 7.McLaughlin T, Ackerman SE, Shen L, Engleman E. Role of innate and adaptive immunity in obesity-associated metabolic disease. J Clin Investig. 2017;127:5–13. doi: 10.1172/JCI88876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Josefowicz SZ, Lu L-F, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakaguchi S, Mikami N, Wing JB, Tanaka A, Ichiyama K, Ohkura N. Regulatory T cells and human disease. Annu Rev Immunol. 2020;38:541–566. doi: 10.1146/annurev-immunol-042718-041717. [DOI] [PubMed] [Google Scholar]
- 10.Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Investig. 2017;127:1–4. doi: 10.1172/JCI92035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersen CJ, Murphy KE, Fernandez ML. Impact of obesity and metabolic syndrome on immunity. Adv Nutr (Bethesda, Md.) 2016;7:66–75. doi: 10.3945/an.115.010207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Heredia FP, Gómez-Martínez S, Marcos A. Obesity, inflammation and the immune system. Proc Nutr Soc. 2012;71:332–338. doi: 10.1017/S0029665112000092. [DOI] [PubMed] [Google Scholar]
- 13.Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, Maezawa Y, Drucker DJ, Engleman E, Winer D, Dosch H-M. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deiuliis J, Shah Z, Shah N, Needleman B, Mikami D, Narula V, Perry K, Hazey J, Kampfrath T, Kollengode M, Sun Q, Satoskar AR, Lumeng C, Moffatt-Bruce S, Rajagopalan S. Visceral adipose inflammation in obesity is associated with critical alterations in tregulatory cell numbers. PLoS ONE. 2011;6:e16376. doi: 10.1371/journal.pone.0016376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Travers RL, Motta AC, Betts JA, Bouloumié A, Thompson D. The impact of adiposity on adipose tissue-resident lymphocyte activation in humans. Int J Obes. 2005;39(2015):762–769. doi: 10.1038/ijo.2014.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pereira S, Teixeira L, Aguilar E, Oliveira M, Savassi-Rocha A, Pelaez JN, Capettini L, Diniz MT, Ferreira A, Alvarez-Leite J. Modulation of adipose tissue inflammation by FOXP3+ Treg cells, IL-10, and TGF-β in metabolically healthy class III obese individuals. Nutrition (Burbank, Los Angeles County, Calif.) 2014;30:784–790. doi: 10.1016/j.nut.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 18.Zeyda M, Huber J, Prager G, Stulnig TM. Inflammation correlates with markers of T-cell subsets including regulatory T cells in adipose tissue from obese patients. Obesity (Silver Spring, Md.) 2011;19:743–748. doi: 10.1038/oby.2010.123. [DOI] [PubMed] [Google Scholar]
- 19.Zeng H, Chi H. The interplay between regulatory T cells and metabolism in immune regulation. Oncoimmunology. 2013;2:e26586. doi: 10.4161/onci.26586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Weerd K, Dik WA, Schrijver B, Schweitzer DH, Langerak AW, Drexhage HA, Kiewiet RM, van Aken MO, van Huisstede A, van Dongen JJM, van der Lelij A-J, Staal FJT, van Hagen PM. Morbidly obese human subjects have increased peripheral blood CD4+ T cells with skewing toward a Treg- and Th2-dominated phenotype. Diabetes. 2012;61:401–408. doi: 10.2337/db11-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beppu LY, Mooli RGR, Qu X, Marrero GJ, Finley CA, Fooks AN, Mullen ZP, Frias AB, Sipula I, Xie B, Helfrich KE, Watkins SC, Poholek AC, Ramakrishnan SK, Jurczak MJ, D'Cruz LM (2021) Tregs facilitate obesity and insulin resistance via a Blimp-1/IL-10 axis. JCI Insight 6 [DOI] [PMC free article] [PubMed]
- 22.Pang Y, Kartsonaki C, Lv J, Fairhurst-Hunter Z, Millwood IY, Yu C, Guo Y, Chen Y, Bian Z, Yang L, Chen J, Clarke R, Walters RG, Holmes MV, Li L, Chen Z. Associations of adiposity, circulating protein biomarkers, and risk of major vascular diseases. JAMA Cardiol. 2021;6:276–286. doi: 10.1001/jamacardio.2020.6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bahrami A, Sathyapalan T, Sahebkar A. The role of interleukin-18 in the development and progression of atherosclerosis. Curr Med Chem. 2021;28:1757–1774. doi: 10.2174/0929867327666200427095830. [DOI] [PubMed] [Google Scholar]
- 24.Bielinski SJ, Berardi C, Decker PA, Larson NB, Bell EJ, Pankow JS, Sale MM, Tang W, Hanson NQ, Wassel CL, de Andrade M, Budoff MJ, Polak JF, Sicotte H, Tsai MY. Hepatocyte growth factor demonstrates racial heterogeneity as a biomarker for coronary heart disease. Heart (British Cardiac Society) 2017;103:1185–1193. doi: 10.1136/heartjnl-2016-310450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bell EJ, Larson NB, Decker PA, Pankow JS, Tsai MY, Hanson NQ, Wassel CL, Longstreth WT, Bielinski SJ. Hepatocyte growth factor is positively associated with risk of stroke: the MESA (multi-ethnic study of atherosclerosis) Stroke. 2016;47:2689–2694. doi: 10.1161/STROKEAHA.116.014172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaplanski G. Interleukin-18: Biological properties and role in disease pathogenesis. Immunol Rev. 2018;281:138–153. doi: 10.1111/imr.12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nedeva I, Gateva A, Assyov Y, Karamfilova V, Hristova J, Yamanishi K, Kamenov Z, Okamura H. IL-18 serum levels in patients with obesity, prediabetes and newly diagnosed type 2 diabetes. Iran J Immunol IJI. 2022;19:193–200. doi: 10.22034/iji.2022.90095.1987. [DOI] [PubMed] [Google Scholar]
- 28.Akimova T, Zhang T, Christensen LM, Wang Z, Han R, Negorev D, Samanta A, Sasson IE, Gaddapara T, Jiao J, Wang L, Bhatti TR, Levine MH, Diamond JM, Beier UH, Simmons RA, Cantu E, Wilkes DS, Lederer DJ, Anderson M, Christie JD, Hancock WW. Obesity-related IL-18 impairs T-regulatory cell function and promotes lung ischemia-reperfusion injury. Am J Respir Crit Care Med. 2021;204:1060–1074. doi: 10.1164/rccm.202012-4306OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zilverschoon GRC, Tack CJ, Joosten LAB, Kullberg BJ, van der Meer JWM, Netea MG. Interleukin-18 resistance in patients with obesity and type 2 diabetes mellitus. Int J Obes. 2005;32(2008):1407–1414. doi: 10.1038/ijo.2008.109. [DOI] [PubMed] [Google Scholar]
- 30.Ahmad R, Al-Mass A, Al-Ghawas D, Shareif N, Zghoul N, Melhem M, Hasan A, Al-Ghimlas F, Dermime S, Behbehani K. Interaction of osteopontin with IL-18 in obese individuals: implications for insulin resistance. PLoS ONE. 2013;8:e63944. doi: 10.1371/journal.pone.0063944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Esposito K, Pontillo A, Ciotola M, Di Palo C, Grella E, Nicoletti G, Giugliano D. Weight loss reduces interleukin-18 levels in obese women. J Clin Endocrinol Metab. 2002;87:3864–3866. doi: 10.1210/jcem.87.8.8781. [DOI] [PubMed] [Google Scholar]
- 32.Murphy AJ, Kraakman MJ, Kammoun HL, Dragoljevic D, Lee MKS, Lawlor KE, Wentworth JM, Vasanthakumar A, Gerlic M, Whitehead LW, DiRago L, Cengia L, Lane RM, Metcalf D, Vince JE, Harrison LC, Kallies A, Kile BT, Croker BA, Febbraio MA, Masters SL. IL-18 production from the NLRP1 inflammasome prevents obesity and metabolic syndrome. Cell Metab. 2016;23:155–164. doi: 10.1016/j.cmet.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 33.Imamura R, Matsumoto K. Hepatocyte growth factor in physiology and infectious diseases. Cytokine. 2017;98:97–106. doi: 10.1016/j.cyto.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 34.Rehman J, Considine RV, Bovenkerk JE, Li J, Slavens CA, Jones RM, March KL. Obesity is associated with increased levels of circulating hepatocyte growth factor. J Am Coll Cardiol. 2003;41:1408–1413. doi: 10.1016/S0735-1097(03)00231-6. [DOI] [PubMed] [Google Scholar]
- 35.Jung C, Fritzenwanger M, Fischer N, Figulla HR. Hepatocyte growth factor is elevated in obese adolescents. J Pediatr Endocrinol Metabol JPEM. 2009;22:645–651. doi: 10.1515/JPEM.2009.22.7.645. [DOI] [PubMed] [Google Scholar]
- 36.Faber DR, van der Graaf Y, Westerink J, Kanhai DA, Monajemi H, Visseren FLJ. Hepatocyte growth factor and interferon-γ inducible protein-10 are related to visceral adiposity. Eur J Clin Invest. 2013;43:369–378. doi: 10.1111/eci.12054. [DOI] [PubMed] [Google Scholar]
- 37.Bell LN, Ward JL, Degawa-Yamauchi M, Bovenkerk JE, Jones R, Cacucci BM, Gupta CE, Sheridan C, Sheridan K, Shankar SS, Steinberg HO, March KL, Considine RV. Adipose tissue production of hepatocyte growth factor contributes to elevated serum HGF in obesity, American journal of physiology. Endocrinol Metab. 2006;291:E843–E848. doi: 10.1152/ajpendo.00174.2006. [DOI] [PubMed] [Google Scholar]
- 38.Muratsu J, Iwabayashi M, Sanada F, Taniyama Y, Otsu R, Rakugi H, Morishita R. Hepatocyte growth factor prevented high-fat diet-induced obesity and improved insulin resistance in mice. Sci Rep. 2017;7:130. doi: 10.1038/s41598-017-00199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Homey B, Wang W, Soto H, Buchanan ME, Wiesenborn A, Catron D, Müller A, McClanahan TK, Dieu-Nosjean MC, Orozco R, Ruzicka T, Lehmann P, Oldham E, Zlotnik A. Cutting edge: the orphan chemokine receptor G protein-coupled receptor-2 (GPR-2, CCR10) binds the skin-associated chemokine CCL27 (CTACK/ALP/ILC) J Immunol (Baltimore MD) 1950;164(2000):3465–3470. doi: 10.4049/jimmunol.164.7.3465. [DOI] [PubMed] [Google Scholar]
- 40.Morales J, Homey B, Vicari AP, Hudak S, Oldham E, Hedrick J, Orozco R, Copeland NG, Jenkins NA, McEvoy LM, Zlotnik A. CTACK, a skin-associated chemokine that preferentially attracts skin-homing memory T cells. Proc Natl Acad Sci USA. 1999;96:14470–14475. doi: 10.1073/pnas.96.25.14470. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed in the current study are not publicly available due to data protection aspects but are available in an anonymized form from the corresponding author on reasonable request.