Abstract
Aging is associated with a loss of skeletal muscle regeneration. We hypothesized that differentially regulated vascular endothelial growth factor (VEGF)A with aging partially underlies this loss of regenerative capacity. To assess the role of VEGFA in muscle regeneration, young (12–14 weeks old) and old C57BL/6 mice (24–25 months old) were subjected to cryoinjury in tibialis anterior (TA) muscle to induce muscle regeneration. The average cross-sectional area (CSA) of regenerating myofibers was 33% smaller in old as compared to young (p<0.01) mice, which correlated with a 2-fold loss of muscle VEGFA protein levels (p=0.02). The capillary density in the TA was similar between the two groups. To assess effects of VEGF on muscle cells, siRNA was utilized to knockdown VEGF expression in C2C12 myoblasts and resulted in impaired differentiation in comparison to control groups. Young VEGFlo mice, with a 50% decrease in systemic VEGFA activity, exhibited a 2-fold reduction in the average regenerating fiber CSA following cryoinjury (p<0.01) in comparison to their littermate controls. ML228, a hypoxia signaling activator known to increase VEGFA levels, augmented muscle VEGFA levels 2-fold and increased average CSA of regenerating fibers in both old mice (25% increase, p<0.01) and VEGFlo (20% increase, p<0.01) mice, but not in young or the littermate WT controls. A marked fatty deposition in the regenerating area noted in the VEGFlo group, which was significantly reduced with ML228 supplementation. These results suggest that VEGFA may be a therapeutic target in age-related muscle loss.
Keywords: muscle regeneration, aging, sarcopenia, hypoxia signaling
Graphical Abstract.
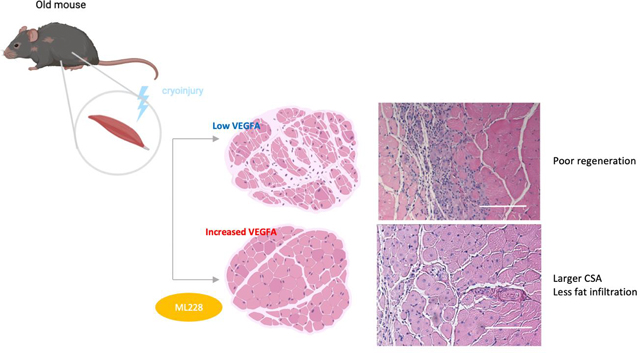
Aging is associated with a loss of skeletal muscle regeneration with a concurrent reduction in VEGFA. Low VEGF levels impairs myoblast differentiation in vitro, and does not seem to affect muscle stem cell (MuSC) activation / proliferation in vivo. Pharmacological restoration of VEGFA level with ML228 in skeletal muscle of old mice results in a significant improvement in muscle regeneration.
Introduction
Skeletal muscle possesses a remarkable ability to regenerate and repair itself following injuries. However, the regenerative potential of muscle declines with aging.1,2 Skeletal muscle stem cells (muSCs), which reside beneath the basement membrane on mature myofibers, are primarily responsible for skeletal muscle regeneration and efficient muscle repair following injury in adult.3,4 With normal aging, the myogenic potential of muSCs decreases and may potentially lead to aging-associated loss of skeletal muscle mass.5 The mechanisms underlying this age-related decline in muscle regenerative function remains largely undefined to this date; however, identifying the key pathways and effector molecules differentially regulated in aging may offer attractive targets for improving muscle regeneration and potentially for the treatment of sarcopenia, or aging-related muscle loss.6–8
With aging, both intrinsic changes in muSCs and extrinsic changes within the stem cell niche contribute to impaired muscle regeneration.9–11 Within skeletal muscle, satellite cells exist in the niche, or microenvironment near the basal lamina, and are influenced by a local milieu of growth factors released by interstitial cells, blood vessels, motor neurons, myofibers, as well as adjacent satellite cells.11 As such, differentially regulated factors in skeletal muscle with aging are potential targets to restore skeletal muscle regenerative potential. Vascular endothelial growth factor (VEGF)A, in particular, is known to promote skeletal muscle regeneration and VEGF signaling insufficiency occurs with aging across multiple organs, including skeletal muscle.12 Hypoxia signaling, which decreases substantially in skeletal muscle with aging, may underlie this loss of VEGFA.13 However, the role of VEGF signaling with regards to aging and muscle regeneration has not been previously characterized.
Multiple studies suggest VEGF is necessary for skeletal muscle homeostasis and muSC function. Skeletal muscle tissue is the most abundant producer of VEGFA in the body and its levels are higher in skeletal muscle than other VEGF isoforms. It has already been extensively studied in the skeletal muscle fibers in models of VEGFA knockout mice,14–18 as well as VEGFA overexpression.19–21 Collectively, these studies demonstrate the importance of VEGFA in skeletal muscle maintenance. Another study has demonstrated that the loss of VEGFA in muSCs limits their engraftment potential in dystrophic mice, and deletion or blockade of VEGF interaction with its decoy receptor Flt resulted in functional improvement in mdx dystrophic mice.22,23 Other studies have suggested that topical application of VEGF improves strength recovery and limits fibrosis following muscle injury.24 Separately, VEGF supplementation improved myofiber hypertrophy in vitro and increases the number of regenerating fibers in both normally perfused and ischemic muscle.25 Conversely, muscle hypertrophy in response to loading is limited with VEGF inhibition.26 Furthermore, VEGF deletion or pharmacological inhibition in muSCs have been shown to reduce their proximity to capillaries in vivo, which is essential for the maintenance of MuSC quiescence, which in turn dictates the regenerative capacity of muSCs.27 Taken together, these studies suggest that loss of VEGF may underlie some of the loss of myogenic potential in muSCs that occurs with aging. Given this, we hypothesized VEGF, a potent downstream signal well characterized in nascent vascular ingrowth and progenitor differentiation, is differentially regulated in aging 28,29 and may contribute to differences in regeneration seen in young and old muscle.
We found that skeletal muscle regeneration is significantly decreased in old mice, in corollary with decreased skeletal muscle VEGFA levels. In a myoblast cell line, knockdown of VEGFA diminished myofiber tube size in vitro. Next, using a genetically modified mouse model with systemically decreased levels of VEGFA,30 we found skeletal muscle regeneration was similarly decreased. Use of ML228, a hypoxia pathway activator that promotes VEGFA levels,21 reverses this loss of skeletal muscle myogenic potential in both old mice and mice with decreased VEGFA activity. Taken together, these experiments suggest that differential regulation of VEGFA, as occurs with aging, may impact loss of myogenic potential in skeletal muscle.
Methods
2.1. Animals
All animal procedures were approved by the Institutional Animal Care and Use Committee of Brigham and Women’s Hospital and were conducted within the guidelines of the US National Institutes of Health. Young C57BL/6 mice (12–14 weeks of age) were obtained from Jackson Laboratories. Old C57BL/6 (24–25 months of age) mice were obtained from the National Institute on Aging. VEGFlo mice (STOCK Vegfatm2.1Nagy/J) (12–14 weeks old) were procured from Jackson Laboratory (Stock No: 027315). In brief, these 129S1/SvImJ mice carry knock-in mutations of the Vegfa gene, which yields a gene product with reduced VEGFA activity (25–50% reduction).26 In experiments involving ML228 treatment, ML228 (TOCRIS #1357171-62-0, Cayman Chemical, Ann Arbor, MI, USA) was dissolved in DMSO (0.03 mg ML228 in 1 mL DMSO) and was injected intraperitoneally (0.1 mL) once daily for a total of 5 days.21 Cryoinjury was induced on the left legs of the animals immediately following the second injection of DMSO, as a vehicle control, or ML228 approximately 24 hours following the first injection.31 The animals were housed at the Brigham and Women’s Hospital Animal Care Facility and were given ad libitum access to food and water. An equal number of male and female mice were utilized for all experiments.
2.2. Muscle Cryoinjury and Assessment of Skeletal Muscle Regeneration
All mice were anesthetized using isoflurane. A skin incision was made on the lower leg and the tibialis anterior (TA) was exposed. A metal probe cooled in dry ice was placed on the skin for 10 seconds, creating a cryoinjury.31 Injured muscles were then allowed to recover for 5 or 10 days before harvest. Cryoinjury generates a reproducible, uniform injury in the muscle with a discrete border between uninjured and injured muscle, which remains distinct when evaluating the regenerative area. To quantify the area of regeneration, 10 photos per sample were taken spanning the entire regenerating area in cross section, and cross-sectional area (CSA) of 100 regenerating myofibers (identified by their centrally located nuclei) were measured using ImageJ. The CSA was then averaged to establish a mean CSA of regenerating fibers for each mouse. The CSA means were then used to compared between groups, as previously described.31,32
2.3. Histological Analysis
Muscles were fixed in 4% of formalin, washed in PBS and stored in 70% of ethanol for paraffin embedding. Hematoxylin and eosin (H&E) staining was used to evaluate the regenerating area. Newly formed myofibers were identified by their centrally located nuclei. All CSA measurements were completed in a blinded fashion and performed by two separate technicians. For immunostaining, skeletal muscle sections were blocked with Blocker BSA (#37520, ThermoFisher, Waltham, MA, USA), and stained with antibodies against CD31 (1:100, #ab7388, Abcam) or Pax7 (1:100, # PA5-68506, Thermo Fisher Scientific, USA) and Laminin (1:100, #ab11571, Abcam, Cambridge, UK). Fat infiltration was estimated as previously described using a commonly employed “pixel and area” classification steps on ImageJ (Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA).33 Briefly, the H&E cross-sectional images of injured muscles were converted into pixels and the background pixels were identified by their color. The area of the background pixels that presented fat droplets were identified by their eccentricity. The adjacency statistics were applied to the results to increase the accuracy of fat identification. This was performed on 10 non-overlapping, randomly selected images spanning the regenerating area per samples, and the average infiltration was estimated as a percentage of the total area of the tissue. Separately, C2C12 cells were stained with antibodies against myosin heavy chain (MF20) (1:100, #14-6503-82, ThermoFisher) and DAPI. Secondary antibodies used were (1:400, #A21470, ThermoFisher) and (1:400, #A11037, ThermoFisher), respectively. Bright field and fluorescent images were acquired using an Olympus BX53 microscope. 20X images were taken throughout the sectioned muscle. Within these high-powered fields, CD31 positive cells were quantified and normalized to the number of fibers within the field. Pax7-positive signals were counted per high-power-field, and the average numbers of signal per 10 images per sample were calculated. Fusion index of C2C12 cells after differentiation were calculated using the following formula:
2.4. Protein quantification
Whole muscle lysates were isolated from TA muscles using lysate buffer (Phosphosolutions, #SKU-100Lys) and subjected to immunoblotting and Elisa. For immunoblotting, proteins were separated on a 4%-12% of SDS-polyacrylamide gel with MOPS SDS Running Buffer (ThermoFisher) at 150 V. The gel was transferred onto an Immuno-Blot PVDF Membrane using the iBlot2 Dry Blotting System (ThermoFisher) for 7 minutes. The membranes were then blocked in blocking solution (Life Technologies, Carlsbad, CA) for 1 hour at room temperature then incubated with an antibody overnight at 4°C with constant shaking. The antibody utilized was VEGFA (Abcam ab46154). Following three 5-minutes washes in TBS-T buffer, the membranes were incubated with anti-rabbit IgG HRP-linked (Cell Signaling) secondary antibody. All the antibodies were diluted in blocking buffer. For immunodetection, the membranes were washed three times with TBS-T buffer, incubated with ECL solutions per manufacturer’s specifications (Amersham Biosciences, Little Chalfont, UK) and exposed to Hyperfilm ECL. The membranes were stripped and re-probed with an antibody recognizing GAPDH for normalization. Band intensities were determined using ImageJ software. Elisa was performed as per manufacturer’s instructions (Abcam ab209882), and the microplates were read at 450nm for OD. The concentration of VEGF protein in the samples were determined by interpolating the blank control subtracted absorbance values against the standard curve.
2.5. C2C12 siRNA treatment
C2C12 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS) and maintained at 37°C with 5% CO2 supply in a humidified incubator. At 75% confluency, cells were induced to undergo differentiation by adding DMEM supplemented with 2% horse serum for 5 days. Media was replaced every 24 hours during differentiation. For siRNA transfections, C2C12 cells were seeded in 6-well plates without coverslips and differentiated when 75% confluent. Cells were transfected every 24 hours from the day of adding the differentiation media referred to as Day 0 till Day 5 of differentiation. For each well, 150 uL of OPTI-MEM media (Life Technologies) was mixed with 3 μL of 10 uM siRNA (siControl or siVEGF from Santa Cruz Biotechnology, Dallas, TX, ISA) and 9 μL of transfection reagent Lipofectamine RNAiMAX (Life Technologies) in separate tubes and incubated for 5 minutes. After 5 minutes, 150 μL of the constituent solutions from each tube were combined and incubated for another 30 minutes at room temperature to generate 300 μL of transfection mix. A 250 μL of the transfection mix were added per well to have a final siRNA concentration of 11 nM per well. Cells were collected from Day 0 (undifferentiated and non-transfected control) to Day 5. Cells were harvested in 1 mL TRIzol for RNA extraction or fixed on coverslips with 4% PFA for immunofluorescence staining.
2.6. Quantitative RT-PCR
mRNA was extracted from C2C12 cells was extracted using RNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions. cDNA was prepared from mRNA using SuperScript III First-Strand kit (Invitrogen). Real-time quantitative PCR reactions were carried out in ABI StepOnePlus machine, using SYBR Green PCR mix (ThermoFisher). The temperature profile used was 95 degrees for 0 minutes, then, 40 cycles of 95 degrees C, 60 degrees C, 72 degrees C each for 30 seconds. All the gene expression levels were normalized to β-actin. Primers sequences are: VEGF-A and β-actin: F: (5′ CCTAAGGCCAACCGTGAAAA 3′) and R: (5′ AGCCATACAGGGACAGCACA 3′).
2.7. Statistical Analysis
Data are presented as the mean ± standard error. Statistical comparisons for normally distributed data were assessed for statistical significance using Student’s t-test. Results comparing more than two groups were assessed by one-way ANOVA with Tukey’s multiple comparison test (GraphPad Prism, GraphPad Software Inc, San Diego, CA, USA, RRID: SCR_002798). The investigators were blinded to the experimental group assignments for outcome assessment. Statistical significance was accepted at p<0.05.
Results:
Skeletal muscle regeneration and whole muscle VEGFA are reduced with aging.
To assess skeletal muscle regeneration, both young and old mice were subjected to cryoinjury of the TA. Old mice demonstrated markedly impaired regenerative response, as indicated by a significantly smaller myofiber CSA in comparison to that of young at both 5 (507±14 vs 386±10μm2 in young and old mice, respectively, p<0.001) and 10 days post injury (DPI) (1251±66 vs 833±18μm2 in young and old mice, respectively, p<0.001) (Figure 1A–C). The density of Pax7-positive satellite cells in the regenerating area was found to be significantly lower in old mice on DPI 10 compared to young mice (Figure D,E). Next, we evaluated the whole muscle VEGFA protein levels given its implicated role in skeletal muscle regeneration and our previous data demonstrating hypoxia signaling in skeletal muscle decreases with aging. VEGFA in the TA was found to be 1.5-fold lower in old as compared to young mice by immunoblotting at baseline (p=0.02) and 2-fold lower on DPI 5 (p=0.02) and 10 (p=0.02) (Figure 1F–I, Supplemental Figure 1A,B). The whole muscle VEGF levels were similar between the baseline and DPI10 timepoints in both young and old mice. (Supplemental Figure 1C). The differences in the VEGFA levels within skeletal muscle further prompted us to determine whether there were any effects on muscle angiogenesis, which depends on VEGF signaling. At the ages tested, the capillary-to-muscle-fiber ratio were similar between the two groups both at the baseline, (p=0.9) (Figure 1J,K), and on DPI 10 (p=0.42) (Supplementary Figure 2A,B).
Figure 1. Skeletal muscle regeneration decreases with aging and in correlation with loss of whole muscle VEGF.
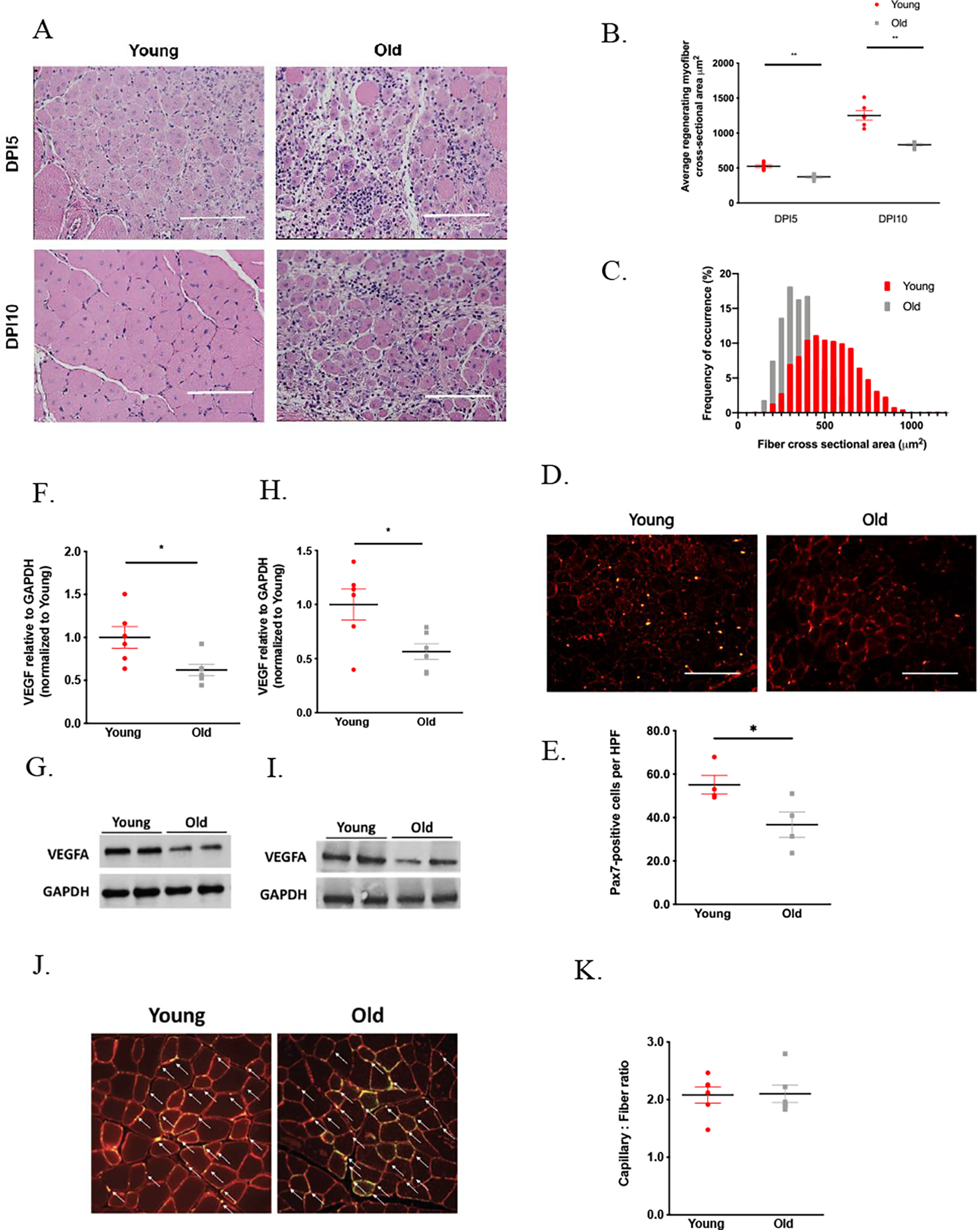
A. Young (12–14 weeks old) and Old (24–26 months old) were subjected to tibialis anterior cryoinjury and evaluated for size of regenerating fibers by histology. H&E staining of cross sections of muscles from young and old mice 5 and 10 days following cryoinjury. Scale bar = 100 μm. (A). Young mice exhibited significantly larger regenerating fiber size as well as fiber organization on both DPI 5 (525.5±21.45 vs 373.4±15.51 μm2 for young vs old, n=6 each, p<0.001) and 10 (1251.0±66.75 vs 833.3±44.82 μm2 for young vs old, n=6 each, p<0.001) (A-C). Whole muscle VEGFA levels are decreased by approximately 2-fold in skeletal muscle of old mice, both at baseline (1.00±0.13 vs 0.62±0.07 for young vs old, n=6 each, p=0.02) (F,G) and following skeletal muscle cryoinjury (1.00±0.14 vs 0.56±0.07 for young vs old, n=6 each, p=0.02) (H,I). The cross sections of injured muscles from young and old mice were stained for Pax7 (green) and laminin (red). Scale bar = 100 μm. (D). Fewer Pax7-positive satellite cells were found in the regenerating area in old mice on DPI 10 compared to young mice (55.1 ±4.3 vs 36.7±5.9 for young vs old, n=4 each, p=0.046) (D,E). The cross sections of uninjured muscles from VEGFlo and VEGF WT mice were stained for CD31 (green) and laminin (red). White arrows indicate the presence capillaries (J). Despite the decrease in whole muscle VEGFA, capillary density was similar between young and old mice at the ages tested (2.0 ±0.14 vs 2.1±0.15 for young vs old, n=6 each, p=0.923) (J,K).
VEGFA inhibition leads to poor skeletal muscle differentiation in vitro.
Next, to establish whether the loss of VEGFA is sufficient to limit differentiation in a myoblast cell line, C2C12 cells were subjected to small interfering (si)RNA to impair normal secretion of VEGFA. siRNA treatment of C2C12 cells resulted in 98±5% reduction of VEGFA expression in C2C12 cells, as compared to treatment with scrambled siRNA (n=4 per group, p<0.001). Treatment with VEGFA siRNA resulted in a 54% reduction (p<0.001) in muscle fiber diameter (Figure 2A,B), and 45% reduction (p=0.003) in fusion index (Figure 2C) in comparison to the control group treated with scrambled siRNA on day 5 differentiation. The total nuclei numbers were similar in both group on day 5 differentiation (p=0.3) (Figure 2D).
Figure 2. VEFGA inhibition limits skeletal muscle fiber hypertrophy in vitro.
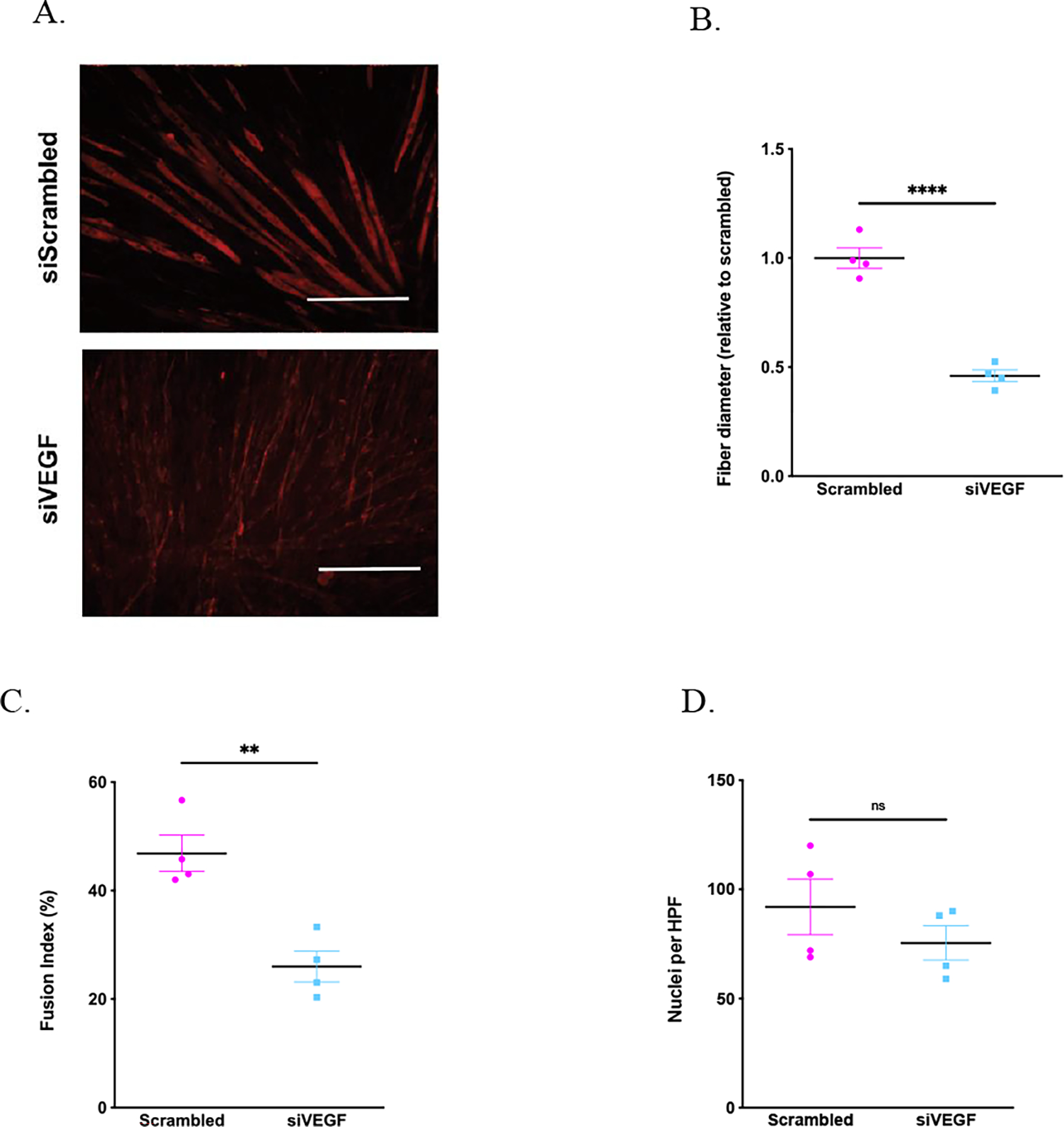
C2C12 cells were treated with either scrambled of VEGFA siRNA decreases VEGFA expression by in C2C12 cells. C2C12 cells were stained for MF20 on day 5 differentiation. Scale bar = 100 μm (A). VEGFA siRNA reduced muscle fiber diameter (1.00±0.05 vs 0.39±0.03 for scrambled vs VEGF siRNA, n=4 each, p<0.0001) (A,B) and fusion index (46.9±3.4 vs 26.0±2.8 % for scrambled vs VEGF siRNA, n=4 each, p=0.003) (C) in comparison to scrambled siRNA treatment. The total cell number remained similar in both groups on day 5 differentiation (92.0±12.7 vs 75.5±7.9 for scrambled vs VEGF siRNA, n=4 each, p=0.312) (D).
Systemic reduction of VEGFA impairs skeletal muscle regeneration.
To determine whether loss of VEGFA activity within skeletal muscle, as occurs with aging, is sufficient to impair muscle regeneration, we utilized genetically modified mice with a marked loss of VEGFA activity. Heterozygote mice carry a mutation within exon 8 which is partially replaced by the lacZ gene leading to downstream aberrant splicing events. These mutant transcripts are detectable by RT-PCR and precipitate a reduction in protein activity of 25–50%.30 All VEGFlo mice and littermate controls were 20 weeks old at the time of experimentation. In comparison to littermate controls, the levels of the normal VEGFA in skeletal muscle of VEGFlo mice are decreased by 61% compared to that of the littermate controls (p=0.01) at baseline and 56% following injury (p<0.001) (Figure 3A–D). Baseline CSA of uninjured muscle fibers were similar between the two groups (Supplemental Figure 4). Of note, the absolute regenerating fiber size is smaller overall in these 129S1/SvImJ mice as compared to the aging experiments, which utilized C57BL/6 mice.
Figure 3. Skeletal muscle regeneration decreases in a transgenic mouse model with reduced VEGFA activity.
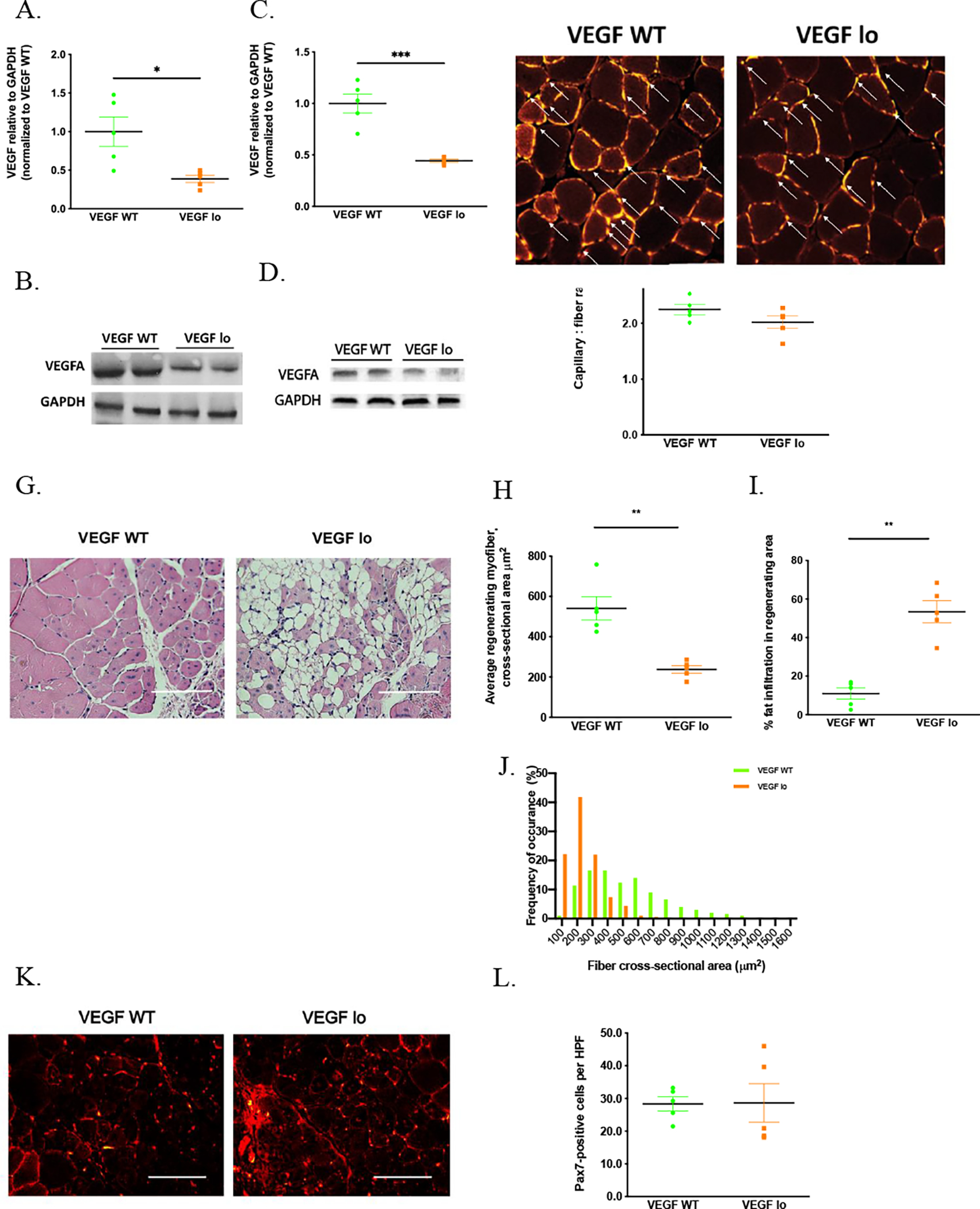
To evaluate whether loss of VEGFA impairs myogenic potential, VEGF WT and VEGFlo mice were utilized. Lower protein levels of VEGFA in VEGFlo mice were confirmed by immunoblotting in uninjured (1.00±0.19vs 0.39±0.05 for VEGF WT vs VEGFlo, n=5 each, p=0.01) (A,B) and injured muscles 10 days following cryoinjury (1.00±0.09vs 0.44±0.02 for VEGF WT vs VEGFlo, n=5 each, p<0.001) (C,D). H&E staining of the cross sections of muscle from the VEGFlo and control mice on DPI 10. Scale bar = 100 μm. (G). Following cryoinjury, the average CSA of regenerating fibers in the VEGFlo mice was significantly reduced on DPI 10 (541.2±5.2 vs 237.9±18.8μm2 for VEGF WT vs VEGFlo, n=5 each, p=0.001) (H,J), in comparison to the littermate controls. Significantly increased fatty deposition was observed after muscle injury in VEGFlo but not littermate control mice (11.0±2.9vs 53.36±5.8 % for VEGF WT vs VEGFlo, n=5 each, p<0.001) (G, I). The cross-sections of uninjured muscles from VEGFlo and VEGF WT mice were stained for CD31 (green) and laminin (red). White arrows indicate capillaries. (E). The capillary density of uninjured muscle was similar between both VEGF WT and VEGFlo mice in uninjured muscles (2.2±0.09vs 2.0±0.11 for VEGF WT vs VEGFlo, n=5 each, p=0.14) (E,F). The cross sections of injured muscles from VEGF WT and VEGFlo mice were stained for Pax7 (green) and laminin (red). Scale bar = 100 m (K). Similar numbers of Pax7-positive satellite cells were found in the regenerating area in VEGFlo and control mice on DPI 10 (D,E).
Following cryoinjury, VEGFlo mice exhibited poor regeneration, with a 2-fold decrease in CSA of regenerating fibers on DPI 10 (p= 0.001) (Figure 3G,H,J). In addition to the impairment in skeletal muscle regeneration, substantial fat deposition within the regenerating area was noted in the VEGFlo mice as compared to littermate controls on DPI10 (Estimated 11±2 vs 53±5% of fat within the regenerating area, control and VEGFlo mice, respectively, p<0.001) (Figure 3I). Capillary-to-muscle-fiber ratio was evaluated by CD31 staining, and found to be similar in muscles at the baseline in both VEGF WT and VEGFlo mice (2.2±0.1 vs 2.0±0.1 capillaries/fiber, control and VEGFlo mice, respectively, p=0.14) (Figure 3E,F) and on DPI10 (0.6±0.02 vs 0.65±0.02 capillaries/fiber, control and VEGFlo mice, respectively. p=0.42) (Supplemental Figure 3A,B).
Pharmacological stimulation of VEGFA improves skeletal muscle regeneration in old and VEGFlo mice.
To determine whether restoration of VEGF can improve skeletal muscle regeneration in old mice, ML228, a HIF pathway activator known to increase VEGFA levels,29 or vehicle control (DMSO), was administered prior to and immediately following cryoinjury once daily, for 5 days total. ML228 is a small molecule activator of the hypoxia inducible factor-pathway, which induces HIF1-α nuclear translocation and stimulates VEGF production. Previous studies suggest that ML228 augments VEGF-A protein levels in both rodent25 and murine models.13 ML228 supplementation improved skeletal muscle regeneration in old mice by 44% as compared to age-matched controls treated with vehicle (p<0.001) (Figure 4A,B). Following ML228 treatment, VEGFA protein levels increased by 3 folds at baseline compared to the DMSO treated littermate controls, (p<0.001) and remained elevated by 2 folds on DPI10 (p<0.001) (Figure 4C–F). Next, we sought to determine whether augmentation of functional VEGFA improves skeletal muscle regeneration in VEGFlo mice. ML228 supplementation augmented the average regenerating fiber CSA in VEGFlo in comparison to the VEGFlo mice treated with DMSO vehicle (661±84 μm2 vs 341±10μM, respectively, p=0.02) (Figure 5A,B). This was accompanied by a significant reduction in intramuscular fat (37±2 vs 24±3% of fat within the regenerating area in VEGFlo mice treated with ML228 vs DMSO, respectively, p=0.02) (Figure 5C). Treatment of VEGF WT mice with ML228 did not improve regeneration in comparison to treatment with vehicle (Figure 5A,B). Similarly, ML228 supplementation elevated the functional VEGFA levels in VEGFlo mice by nearly 2 folds (p=0.04), but not in VEGF WT mice, in comparison to DMSO treated control groups (Figure 5D,E). Capillary densities were found to be similar across all the groups tested (Supplementary Figure 5).
Figure 4. ML228 supplementation increases skeletal muscle VEGF A levels and improves skeletal muscle regeneration in old mice.
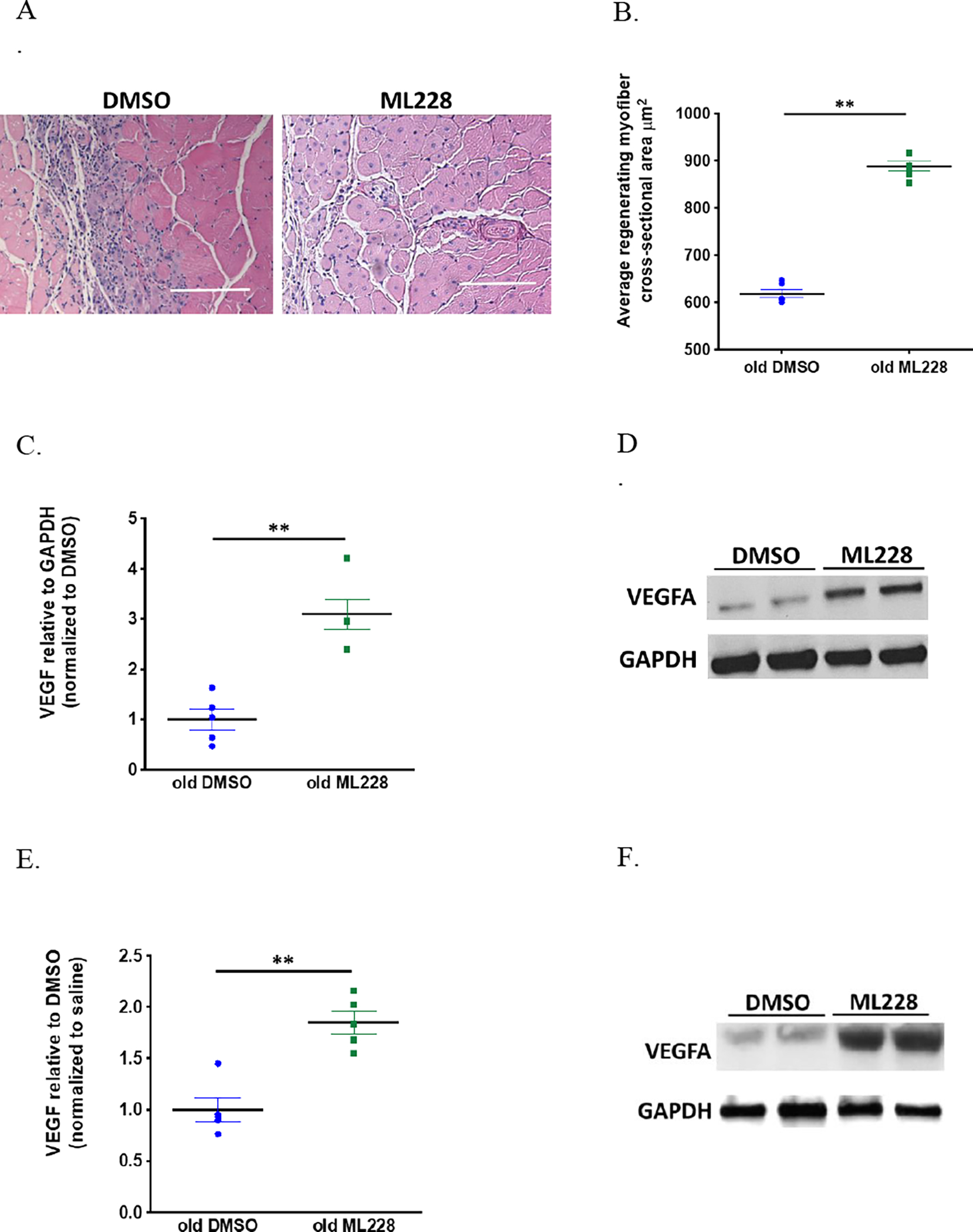
Old mice were treated with either ML228 or vehicle control for 5 days. H&E staining of the muscle cross sections from old mice treated with ML228 or DMSO 10 days following cryoinjury. Scale bar = 100 μm (A). Old mice treated with ML228 demonstrated a significant increase in regenerating fiber CSA versus old mice treated with vehicle control (617.9±8.3vs888.5±10.3 μm2 for old DMSO vs old ML228, n=6 each, p<0.0001) (A,B). Following ML228 treatment, VEGFA levels increased both at baseline (1.0±0.2vs888.5±10.3 for old DMSO vs old ML228, n=6 each, p<0.0001) (C,D) and 10 days following injury (1.0 ±0.1vs1.8±0.1 for old DMSO vs old ML228, n=6 each, p=0.0008) (E,F).
Figure 5. ML228 supplementation improves skeletal muscle regeneration in VEGFlo mice.
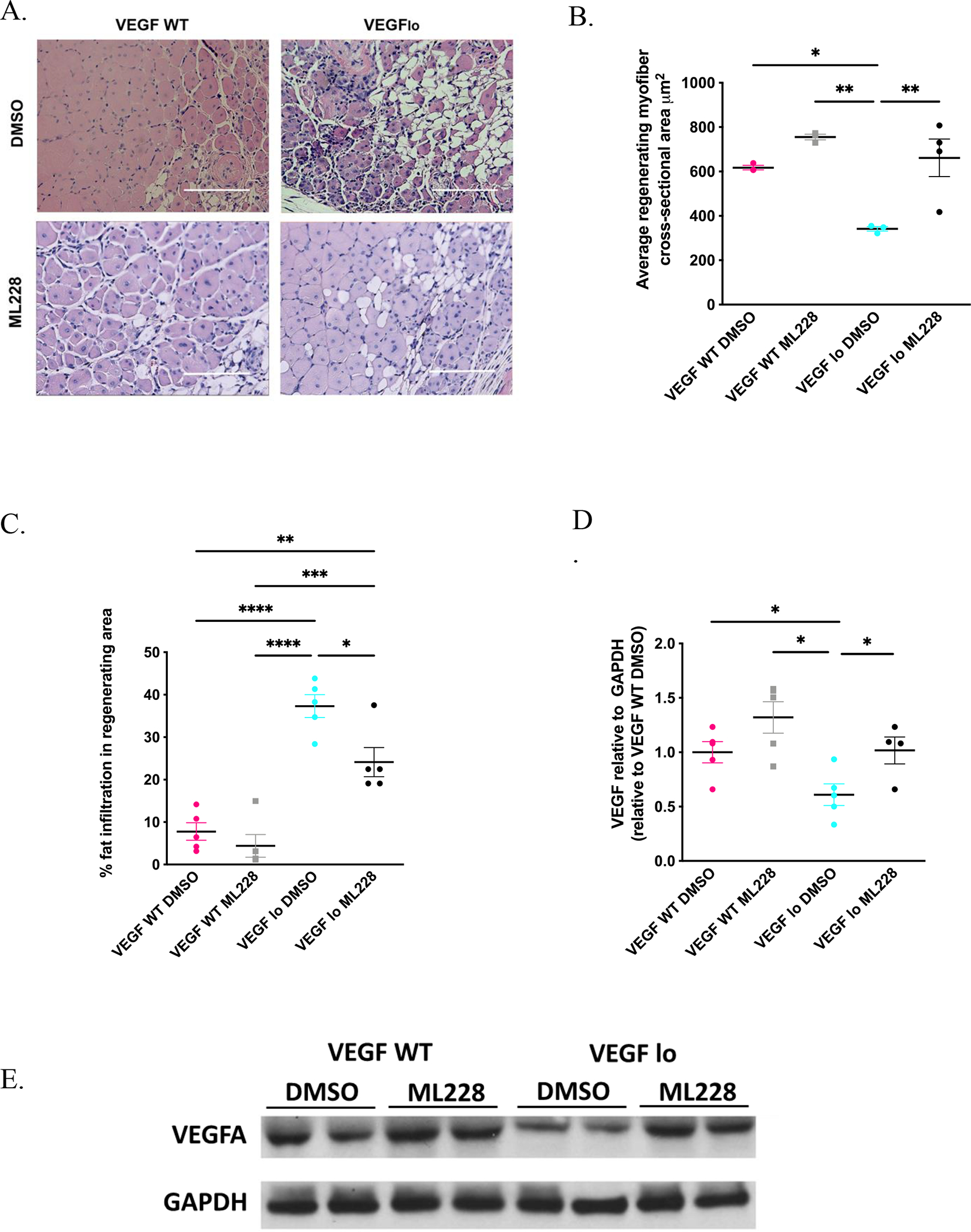
H&E staining of cross sections of injured muscles from VEGFlo and control mice 10 days after cryoinjury. Scale bar = 100 μm. (A). ML228 supplementation improved skeletal muscle regenerating fiber CSA in VEGFlo in comparison to VEGFlo mice treated with vehicle (341.5±10.4 vs 661.5±4.8 μm2 for VEGFlo with DMSO vs VEGFlo WT with ML228, n=4 each, p=0.01), but not in VEGF WT mice (617.4±10.3 vs 755.5±12.8 for μm2 VEGF WT with DMSO vs VEGF WT with ML228, n=4 each, p=0.37) (A,B). Fat percentage within the regenerating area also decreased following ML228 treatment in VEGFlo mice (37.3±2.7 vs 24.1±3.4 % for VEGFlo with DMSO vs VEGFlo WT with ML228, n=5 each, p=0.02) (A,C). ML228 supplementation also improved VEGFA levels in VEGFlo mice in comparison to vehicle (0.6±0.1 vs 1.0±0.1 % for VEGFlo with DMSO vs VEGFlo WT with ML228, n=4 each, p=0.04), but not in VEGF WT mice (1.0±0.1vs 1.3±0.1 for VEGF WT with DMSO vs VEGF WT with ML228, n=4 each, p=0.10) (D,E).
Discussion
Loss of skeletal muscle regeneration with aging may contribute to loss of muscle mass and function.35 Other studies have demonstrated that preservation of muscle SC function to replenish muscle fibers throughout aging may be sufficient to maintain muscle mass and limit the development of sarcopenia.36 Although the loss of muscle’s ability to regenerate with aging is likely multifactorial, changes in the intrinsic signaling in stem cells and the stem cell niche such as loss of growth factors, altered blood supply, and increased local inflammation are major determinants of skeletal muscle regenerative capacity.36
Previous studies demonstrated that hypoxia signaling is differentially regulated with aging21 and VEGFA, a key target of the hypoxia signaling pathway,37 may regulate skeletal muscle regeneration.25 We therefore hypothesized that the loss of VEGFA within skeletal muscle with aging may be responsible for the loss of regenerative potential with aging. Here, we report that VEGFA levels are significantly less in the skeletal muscle of old mice and that loss of VEGFA is correlated with poor skeletal muscle regeneration in both old mice and mice with systemically reduced VEGFA activity. The restoration of VEGFA, with the use of a pharmacological hypoxia pathway activator, ML228, improved skeletal muscle regeneration in both old and VEGFlo mice, suggesting that VEGFA may indeed have a role is skeletal muscle regeneration and its decline seen in aging. ML228 increased the whole muscle VEGFA levels, but it should be noted that ML228 also modulates hypoxia signaling in general. As such, we cannot definitely conclude that ML228 improves skeletal muscle regeneration in old and VEGFlo mice purely through a VEGFA-mediated pathway.
In order to determine the extravascular role of VEGFA on myofiber differentiation, we utilized a myoblast line and treated with siRNA to interfere with VEGFA expression during induced differentiation in differentiation media. VEGFA siRNA treatment resulted in a 2-fold reduction in fiber diameter and fusion index following differentiation in vitro without significantly affecting proliferation. These results suggest that production of VEGFA is required for normal differentiation of myoblasts into myofibers, and that differentiating myoblasts may act as a source of VEGFA that serves as a paracrine regulator of nearby satellite cells. Overall, this support previous studies demonstrating the ability of VEGFA to exert extra-angiogenic effects on myogenesis, including promotion and hypertrophy and fusion following injury.25 Similarly, others have observed that VEGFA supplementation promotes muscle function and myoglobin expression in ischemic skeletal muscle, independent of angiogenesis.38 In contrast to the reduction in the Pax7-positive satellite cell density observed in the regenerating area in old mice, VEGFlo mice exhibited a comparable satellite cell density in the regenerating area to that of the control mice. These results, together with the reduced regenerating myofiber CSA observed in VEGFlo mice, further suggest that the loss of VEGF affects muscle regeneration by hindering the differentiation and fusion of myofibers, rather than MuSC activation and proliferation.
It remains difficult, however, to determine the role of capillary density and vascular flow independently from the contribution of direct VEGFA effects. In a trauma model involving tibial fracture, muscle functional recovery was improved by VEGF supplementation,24 but was accompanied with significant new blood vessel formation in the limb. Within ischemic limbs, VEGFA supplementation promoted functional muscle recovery in conjunction with angiogenesis when delivered in a sustained release fashion.39 Interestingly, VEGF production is dramatically increased within mature myofibers and regenerating myotubes, suggesting VEGF, via the VEGFR2 receptor, may be involved in muscle recovery and function.40 Our study similarly suggests that VEGF promotes skeletal muscle regeneration, although the primary source of VEGF within skeletal muscle remains unclear. In addition, further studies are necessary to characterize the relative contributions of VEGF treatment on myogenesis and angiogenesis, and evaluate the effects of angiogenesis and blood flow on muscle regeneration. It should be noted, however, that capillary density within skeletal muscle was not different between the young and old mice at the ages tested, nor VEGFlo mice and littermate controls in uninjured and injured muscles. This suggests VEGF may influence myogenesis, independent from its angiogenic effects.”
Interestingly, loss of VEGF resulted in dramatic fat deposition within the regenerating areas of muscle. Multiple studies have shown that Intermuscular adipose tissue (IMAT) increases with aging at both baseline and following injuries, although it was not as pronounced in the old mice in the present study.41,42 Interestingly, others have reported that upregulation of the hypoxia signaling pathway increases hepatic steatosis, mediated by both HIF-1α43and HIF-2α44. In skeletal muscle, HIF-1α improves glucose metabolism and insulin sensitivity,45 thereby potentially decreasing the risk of IMAT, but the role of hypoxia signaling and VEGF in IMAT remains unclear. While the source of adipocytes observed in VEGFlo mice is unknown, in the context of muscle regeneration, most adipogenesis occurs as a result of resident fibro-adipogenic progenitors (FAPs) expansion and differentiation. FAP expansion is an integral step in muscle repair, and plays an essential role in the regulation of myogenesis by influencing the myogenic behavior and the fate of SCs.46–48 FAP expansion occurs immediately following muscle injury, releasing cytokines and growth factors which in turn promote cell proliferation / survival but may block differentiation of myogenic cells. The subsequent reabsorption of FAPs is required to allow SC differentiation, fusion and formation of mature myofibers, and consequently, successful myogenesis.49 Myofibers in turn suppress the proliferation of FAPs, thus preventing further FAP mediated adipogenesis and fibrosis.50,51 Marked fat deposition observed in muscles containing lower levels of VEGFA may be a result of any one or the combination of abnormal FAP expansion, increased adipogenic differentiation or failure of FAP clearance. Loss of the inhibitory regulation on FAP-mediated adipogenesis due to failure in myofiber differentiation / maturation may offer a plausible mechanical explanation for the association between lower muscle VEGFA levels and increased fat deposition. Evidently, supplementation with ML228, a hypoxia signaling activator, resulted in reduced intramuscular fat deposition in the VEGFlo mice, with concurrent improvement in muscle regeneration. Whether this is a direct effect of lower levels of VEGF, or indirect effect through hypoxia signaling activation due to loss of negative feedback or poor vascularization of muscle remains to be determined, but that is beyond the scope of this study.
In addition to the limitations mentioned in the above section, further studies will be required to determine if poor myogenesis observed in the VEGFlo mice is due to the niche effects of lower whole muscle VEGF on SC or alterations in the intrinsic functions of SC themselves. In addition, the mechanism by which VEGFA improves skeletal muscle regeneration remains unclear. Previous studies suggest that VEGF can improve myoblast proliferation and survival following injury, suggesting that VEGF may promote a relative abundance of local muSC immediately following injury, thereby promoting improved regeneration.52 Separately, it may be possible that VEGF modulates key myogenic factor levels within skeletal muscle, such as Notch,53 which is required for muscle regeneration. Further research is necessary to elucidate mechanistically how restoration of VEGFA in muscle promotes regenerative capacity. Furthermore, VEGFA is abundantly produced within skeletal muscle, but we cannot rule out the contribution of other cell types of muscle VEGFA levels and how their expression of VEGFA may be differentially regulated in aging. In addition, a further study is required to evaluate any changes in the VEGF receptor expressions and their downstream effects to better characterize the relationships between age-related changes in VEGF signaling and regenerative response of skeletal muscles.
However, taken together, our data suggest that VEGF levels decline with aging and this may be partially responsible for a corollary loss of skeletal muscle regeneration. Pharmacological restoration of VEGF improves muscle regeneration, both in old mice and mice with a systemic reduction in VEGF levels and activity. These findings suggest that restoration of VEGF signaling within skeletal muscle may assist the maintenance of skeletal muscle mass and myogenic potential in aging.
Supplementary Material
Acknowledgments
Funding:
Y. Endo is supported by P30AG031679 from the Boston Pepper Center and NIA and I. Sinha is supported by K76AG059996 from NIA.
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Muñoz-Cánoves P, Neves J, Sousa-Victor P. Understanding muscle regenerative decline with aging: new approaches to bring back youthfulness to aged stem cells. FEBS J. 2020. Feb;287(3):406–416. [DOI] [PubMed] [Google Scholar]
- 2.Blau HM, Cosgrove BD, Ho AT. The central role of muscle stem cells in regenerative failure with aging. Nat Med. 2015. Aug;21(8):854–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang YX, Rudnicki MA. Satellite cells, the engines of muscle repair. Nat Rev Mol Cell Biol. 2011. Dec 21;13(2):127–33. [DOI] [PubMed] [Google Scholar]
- 4.Almada AE, Wagers AJ. Molecular circuitry of stem cell fate in skeletal muscle regeneration, ageing and disease. Nat Rev Mol Cell Biol. 2016. May;17(5):267–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brack AS, Muñoz-Cánoves P. The ins and outs of muscle stem cell aging. Skelet Muscle. 2016. Jan 18;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007. Aug 10;317(5839):807–10. [DOI] [PubMed] [Google Scholar]
- 7.Oh J, Sinha I, Tan KY, Rosner B, Dreyfuss JM, Gjata O, Tran P, Shoelson SE, Wagers AJ. Age-associated NF-κB signaling in myofibers alters the satellite cell niche and re-strains muscle stem cell function. Aging (Albany NY). 2016. Nov 14;8(11):2871–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005. Feb 17;433(7027):760–4. [DOI] [PubMed] [Google Scholar]
- 9.Feige P, Brun CE, Ritso M, Rudnicki MA. Orienting Muscle Stem Cells for Regeneration in Homeostasis, Aging, and Disease. Cell Stem Cell. 2018. Nov 1;23(5):653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Etienne J, Liu C, Skinner CM, Conboy MJ, Conboy IM. Skeletal muscle as an experimental model of choice to study tissue aging and rejuvenation. Skelet Muscle. 2020. Feb 7;10(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bentzinger CF, von Maltzahn J, Rudnicki MA. Extrinsic regulation of satellite cell specification. Stem Cell Res Ther. 2010. Aug 26;1(3):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grunewald M, Kumar S, Sharife H, Volinsky E, Gileles-Hillel A, Licht T, Permyakova A, Hinden L, Azar S, Friedmann Y, Kupetz P, Tzuberi R, Anisimov A, Alitalo K, Horwitz M, Leebhoff S, Khoma OZ, Hlushchuk R, Djonov V, Abramovitch R, Tam J, Keshet E. Counteracting age-related VEGF signaling insufficiency promotes healthy aging and extends life span. Science. 2021. Jul 30;373(6554):eabc8479. [DOI] [PubMed] [Google Scholar]
- 13.Endo Y, Baldino K, Li B, Zhang Y, Sakthivel D, MacArthur M, Panayi AC, Kip P, Spencer DJ, Jasuja R, Bagchi D, Bhasin S, Nuutila K, Neppl RL, Wagers AJ, Sinha I. Loss of ARNT in skeletal muscle limits muscle regeneration in aging. FASEB J. 2020. Dec;34(12):16086–16104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olfert IM, Howlett RA, Tang K, Dalton ND, Gu Y, Peterson KL, Wagner PD, Breen EC. 2009. Muscle-specific VEGF deficiency greatly reduces exercise endurance in mice. J Physiol 587:1755–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang K, Breen EC, Gerber H-P, Ferrara NM a, Wagner PD. 2004. Capillary regression in vascular endothelial growth factor-deficient skeletal muscle. Physiol Genomics 18:63–69. [DOI] [PubMed] [Google Scholar]
- 16.Wagner PD, Olfert IM, Tang K, Breen EC. 2006. Muscle-targeted deletion of VEGF and exercise capacity in mice. Respir Physiol Neurobiol 151:159–166. [DOI] [PubMed] [Google Scholar]
- 17.Delavar H, Nogueira L, Wagner PD, Hogan MC, Metzger D, Breen EC. Skeletal myofiber VEGF is essential for the exercise training response in adult mice. Am J Physiol - Regul Integr Comp Physiol. 2014. doi: 10.1152/ajpregu.00522.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knapp AE, Goldberg D, Delavar H, et al. Skeletal myofiber VEGF regulates contraction-induced perfusion and exercise capacity but not muscle capillarity in adult mice. Am J Physiol - Regul Integr Comp Physiol. 2016. doi: 10.1152/ajpregu.00533.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arsic N, Zacchigna S, Zentilin L, Ramirez-Correa G, Pattarini L, Salvi A, Sinagra G, Giacca M. 2004. Vascular endothelial growth factor stimulates skeletal muscle regeneration in vivo. Mol Ther 10:844–854. [DOI] [PubMed] [Google Scholar]
- 20.Bouchentouf M, Benabdallah BF, Bigey P, Yau TM, Scherman D, Tremblay JP. 2008. Vascular endothelial growth factor reduced hypoxia-induced death of human myoblasts and improved their engraftment in mouse muscles. Gene Ther 15:404–414. [DOI] [PubMed] [Google Scholar]
- 21.Yan H, Guo Y, Zhang P, Zu L, Dong X, Chen L, Tian J, Fan X, Wang N, Wu X, Gao W. 2005. Superior neovascularization and muscle regeneration in ischemic skeletal muscles following VEGF gene transfer by rAAV1 pseudotyped vectors. Biochem Biophys Res Commun 336:287–298. [DOI] [PubMed] [Google Scholar]
- 22.Deasy BM, Feduska JM, Payne TR, Li Y, Ambrosio F, Huard J. Effect of VEGF on the regenerative capacity of muscle stem cells in dystrophic skeletal muscle. Mol Ther. 2009. Oct;17(10):1788–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verma M, Shimizu-Motohashi Y, Asakura Y, et al. Inhibition of FLT1 ameliorates muscular dystrophy phenotype by increased vasculature in a mouse model of Duchenne muscular dystrophy. PLoS Genet. 2019. doi: 10.1371/journal.pgen.1008468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frey SP, Jansen H, Raschke MJ, Meffert RH, Ochman S. VEGF improves skeletal muscle regeneration after acute trauma and reconstruction of the limb in a rabbit model. Clin Orthop Relat Res. 2012. Dec;470(12):3607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arsic N, Zacchigna S, Zentilin L, Ramirez-Correa G, Pattarini L, Salvi A, Sinagra G, Giacca M. Vascular endothelial growth factor stimulates skeletal muscle regeneration in vivo. Mol Ther. 2004. Nov;10(5):844–54. [DOI] [PubMed] [Google Scholar]
- 26.Beckman SA, Chen WCW, Tang Y, et al. Beneficial effect of mechanical stimulation on the regenerative potential of muscle-derived stem cells is lost by inhibiting vascular endothelial growth factor. Arterioscler Thromb Vasc Biol. 2013. doi: 10.1161/ATVBAHA.112.301166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verma M, Asakura Y, Murakonda BSR, et al. Muscle Satellite Cell Cross-Talk with a Vascular Niche Maintains Quiescence via VEGF and Notch Signaling. Cell Stem Cell. 2018. doi: 10.1016/j.stem.2018.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wagatsuma A Effect of aging on expression of angiogenesis-related factors in mouse skeletal muscle. Exp Gerontol. 2006; 41:49–54. [DOI] [PubMed] [Google Scholar]
- 29.Ryan NA, Zwetsloot KA, Westerkamp LM, et al. Lower skeletal muscle capillarization and VEGF expression in aged vs. young men. J Appl Physiol. 2006; 100:178–185. [DOI] [PubMed] [Google Scholar]
- 30.Damert A, Miquerol L, Gertsenstein M, Risau W, Nagy A. Insufficient VEGFA activity in yolk sac endoderm compromises haematopoietic and endothelial differentiation. Development. 2002. Apr;129(8):1881–92. [DOI] [PubMed] [Google Scholar]
- 31.Endo Y, Karvar M, Sinha I. Muscle Cryoinjury and Quantification of Regenerating Myofibers in Mice. Bio Protoc. 2021. Jun 5;11(11):e4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oh J, Sinha I, Tan KY, Rosner B, Dreyfuss JM, Gjata O, Tran P, Shoelson SE, Wagers AJ. Age-associated NF-κB signaling in myofibers alters the satellite cell niche and re-strains muscle stem cell function. Aging (Albany NY). 2016. Nov 14;8(11):2871–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Homeyer A, Schenk A, Arlt J, Dahmen U, Dirsch O, Hahn HK. Fast and accurate identification of fat droplets in histological images. Comput Methods Programs Biomed. 2015. doi: 10.1016/j.cmpb.2015.05.009 [DOI] [PubMed] [Google Scholar]
- 34.Chen H, Li J, Liang S, Lin B, Peng Q, Zhao P, Cui J, Rao Y. Effect of hypoxia-inducible factor-1/vascular endothelial growth factor signaling pathway on spinal cord injury in rats. Exp Ther Med. 2017. Mar;13(3):861–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu W, Klose A, Forman S, Paris ND, Wei-LaPierre L, Cortés-Lopéz M, Tan A, Flaherty M, Miura P, Dirksen RT, Chakkalakal JV. Loss of adult skeletal muscle stem cells drives age-related neuromuscular junction degeneration. Elife. 2017. Jun 6;6:e26464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sousa-Victor P, García-Prat L, Muñoz-Cánoves P. Control of satellite cell function in muscle regeneration and its disruption in ageing. Nat Rev Mol Cell Biol. 2022. Mar;23(3):204–226. [DOI] [PubMed] [Google Scholar]
- 37.Darby IA, Hewitson TD. Hypoxia in tissue repair and fibrosis. Cell Tissue Res. 2016. Sep;365(3):553–62. [DOI] [PubMed] [Google Scholar]
- 38.van Weel V, Deckers MM, Grimbergen JM, van Leuven KJ, Lardenoye JH, Schlingemann RO, van Nieuw Amerongen GP, van Bockel JH, van Hinsbergh VW, Quax PH. Vascular endothelial growth factor overexpression in ischemic skeletal muscle enhances myoglobin expression in vivo. Circ Res. 2004. Jul 9;95(1):58–66. [DOI] [PubMed] [Google Scholar]
- 39.Borselli C, Storrie H, Benesch-Lee F, Shvartsman D, Cezar C, Lichtman JW, Vandenburgh HH, Mooney DJ. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proc Natl Acad Sci U S A. 2010. Feb 23;107(8):3287–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rissanen TT, Vajanto I, Hiltunen MO, Rutanen J, Kettunen MI, Niemi M, Leppänen P, Turunen MP, Markkanen JE, Arve K, Alhava E, Kauppinen RA, Ylä-Herttuala S. Expression of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 (KDR/Flk-1) in ischemic skeletal muscle and its regeneration. Am J Pathol. 2002. Apr;160(4):1393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waters DL. Intermuscular Adipose Tissue: A Brief Review of Etiology, Association With Physical Function and Weight Loss in Older Adults. Ann Geriatr Med Res. 2019. Mar;23(1):3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Correa-de-Araujo R, Addison O, Miljkovic I, Goodpaster BH, Bergman BC, Clark RV, Elena JW, Esser KA, Ferrucci L, Harris-Love MO, Kritchevsky SB, Lorbergs A, Shepherd JA, Shulman GI, Rosen CJ. Myosteatosis in the Context of Skeletal Muscle Function Deficit: An Interdisciplinary Workshop at the National Institute on Aging. Front Physiol. 2020. Aug 7;11:963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mesarwi OA, Moya EA, Zhen X, Gautane M, Zhao H, Wegbrans Giró P, Alshebli M, McCarley KE, Breen EC, Malhotra A. Hepatocyte HIF-1 and Intermittent Hypoxia Independently Impact Liver Fibrosis in Murine Nonalcoholic Fatty Liver Disease. Am J Respir Cell Mol Biol. 2021. Oct;65(4):390–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.hen J, Chen J, Fu H, Li Y, Wang L, Luo S, Lu H. Hypoxia exacerbates nonalcoholic fatty liver disease via the HIF-2α/PPARα pathway. Am J Physiol Endocrinol Metab. 2019. Oct 1;317(4):E710–E722. [DOI] [PubMed] [Google Scholar]
- 45.Görgens SW, Benninghoff T, Eckardt K, Springer C, Chadt A, Melior A, Wefers J, Cramer A, Jensen J, Birkeland KI, Drevon CA, Al-Hasani H, Eckel J. Hypoxia in Combination With Muscle Contraction Improves Insulin Action and Glucose Metabolism in Human Skeletal Muscle via the HIF-1α Pathway. Diabetes. 2017. Nov;66(11):2800–2807. [DOI] [PubMed] [Google Scholar]
- 46.Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010. Feb;12(2):153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heredia JE, Mukundan L, Chen FM, Mueller AA, Deo RC, Locksley RM, Rando TA, Chawla A. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell. 2013. Apr 11;153(2):376–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wosczyna MN, Konishi CT, Perez Carbajal EE, Wang TT, Walsh RA, Gan Q, Wagner MW, Rando TA. Mesenchymal Stromal Cells Are Required for Regeneration and Homeostatic Maintenance of Skeletal Muscle. Cell Rep. 2019. May 14;27(7):2029–2035.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lemos DR, Babaeijandaghi F, Low M, Chang CK, Lee ST, Fiore D, Zhang RH, Natarajan A, Nedospasov SA, Rossi FM. Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat Med. 2015. Jul;21(7):786–94. [DOI] [PubMed] [Google Scholar]
- 50.Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 2011. Sep;138(17):3625–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lukjanenko L, Karaz S, Stuelsatz P, Gurriaran-Rodriguez U, Michaud J, Dammone G, Sizzano F, Mashinchian O, Ancel S, Migliavacca E, Liot S, Jacot G, Metairon S, Raymon [Google Scholar]
- 52.Germani A, Di Carlo A, Mangoni A, Straino S, Giacinti C, Turrini P, Biglioli P, Capogrossi MC. Vascular endothelial growth factor modulates skeletal myoblast function. Am J Pathol. 2003. Oct;163(4):1417–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hellström M, Phng LK, Gerhardt H. VEGF and Notch signaling: the yin and yang of angiogenic sprouting. Cell Adh Migr. 2007. Jul-Sep;1(3):133–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


