Abstract
The therapeutic scenario of Human Epidermal Growth Factor Receptor 2 positive advanced breast cancer (ABC) has been recently enriched by a number of innovative agents, which are reshaping treatment sequence. While randomized trials have documented an advantage in terms of efficacy, for the newly available agents we lack effectiveness and tolerability evidence from the real-world setting. Similarly, the identification of predictive biomarkers might improve clinical decision. We herein describe the outline of a prospective/retrospective study which aims to explore the optimal sequence of treatment in HER2+, pertuzumab pre-treated ABC patients treated in II line with anti-HER2 agents in clinical practice. As part of the pre-clinical tasks envisioned by the STEP study, in vitro cell models of resistance were exploited to investigate molecular features associated with reduced efficacy of HER2 targeting agents at the transcript level. The aggressive behavior of resistant cell populations was measured by growth assessment in mouse models. This approach led to the identification of DARPP-32 and t-DARPP proteins as possible predictive biomarkers of efficacy of anti-HER2 agents. Biomarkers validation and the clinical goals will be reached through patients’ inclusion into two independent cohorts, i.e., the prospective and retrospective cohorts, whose setup is currently ongoing.
Keywords: HER2 positive metastatic breast cancer, Pertuzumab pretreated, Real-world evidence, PPP1R1B, DARPP-32 and t-DARPP
Introduction
The therapeutic landscape of HER2 positive (HER2+) advanced breast cancer (ABC) is dramatically evolving. The entrance of newly available anti-HER2 agents in clinical practice has significantly impacted patients’ outcome, and now imposes an extensive reconsideration of the pre-existing scenarios. In addition, results from randomized trials are not fully generalizable to patients’ populations from the real world setting [1,2].
In our prior work on the optimal sequence of treatment in HER2+ ABC patients, we exploited cell models of resistance. Cell populations resistant to trastuzumab+pertuzumab combination showed a marked reduction of HER2 expression on the cell membrane and its translocation to the nucleus, compared with trastuzumab-resistant and parental cells. This reduction resulted in lower efficacy of Trastuzumab emtansine (T-DM1), which may help interpret the significantly less favourable outcome of 2nd-Line-T-DM1 observed in 177 pertuzumab-pre-treated patients vs 194 pertuzumab-naïve patients [3].
HER2 downregulation may be part of an evolution process dictated by HER2 targeting agents. Adaptation may require multiple mechanisms including gene deregulations, fine tuning of signaling pathways activity, and/or changes in the interactions with tumor microenvironment [4,5]. Such phenotypic changes may include collateral sensitivities and/or overexpression of predictive biomarkers.
The STEP observational study is focused on the optimal sequence of treatment in HER2+, pertuzumab-pre-treated ABC pts. Both preclinical and clinical tasks are foreseen. We herein presented the outcome of the inherent preclinical research activities, which have led to the identification and characterization of DARPP-32 (dopamine- and cyclic-AMP-regulated phosphoprotein of molecular weight 32,000) and t-DARPP (truncated isoform of dopamine- and cAMP-regulated phosphoprotein) as potential predictors of efficacy of the anti-HER2 agents. We have also envisioned both a prospective (N=50) and a retrospective (N=50) patients’ cohort. The predictive role of DARPP-32/t-DARPP will be further investigated in the retrospective cohort, while the prospective cohort will generate real word evidence on the effectiveness/tolerability of the most innovative anti-HER2 agents and investigate the predictive/prognostic role of circulating biomarkers.
Materials and methods
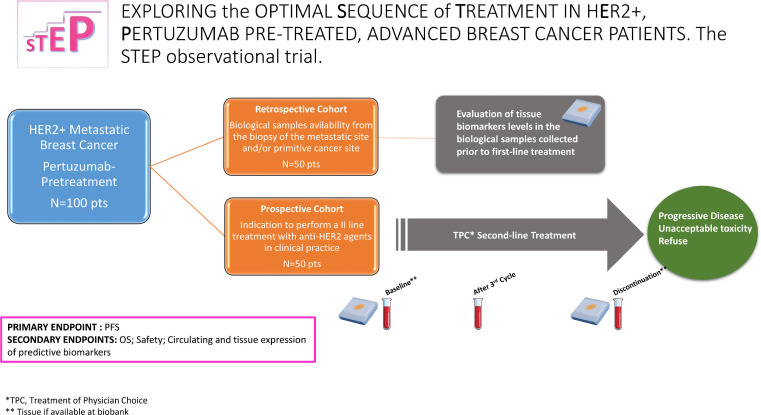
The STEP study is as a multicentric, observational study. The IRCCS Regina Elena Cancer Institute acts as coordinating center. The study protocol was approved by the Institutional Review Board of the coordinating and satellite centers (Register code: 1809/22). A flow diagram of the STEP is displayed in Fig. 1, while the methods applied to the clinical tasks are broadly summarized in the appendix 1.
Fig. 1.
Flow diagram of the STEP observational trial.
Cell lines
The BT474 HER2+ breast cancer (BC) cell populations resistant to trastuzumab (T), pertuzumab (P), and trastuzumab+pertuzumab combination (TP) were obtained as reported elsewhere [1]. The cultures were maintained in RPMI medium supplemented with 10 % FBS, 1 % penicillin/streptomycin and 1 % glutamine (Invitrogen, Milan, Italy), and periodically tested for mycoplasma contamination.
Quantitative RT-PCR
Total RNA from parental and resistant cell lines was isolated by PureLink RNA Mini kit (Invitrogen) and reverse-transcribed using PrimeScript RT reagent kit (Takara), according to manufacturer's recommendations. Quantitative PCR (qPCR) was performed by SYBR Green on an ABI Prism 7500 apparatus (Applied Biosystems, Glasgow, UK) in 2 independent experiments in triplicate. The comparative threshold (∆Ct) method was used. Specific primer sequences were:
Darpp-32 FW: 5′-AGAGACACACGCGGAGAGGA-3′
Darpp-32 REV: 5′-TCTGCCTCTCCCGTCCTTCT-3′
t-Darpp FW: 5′-CTGCAGCCTTACAGAGACTGG-3′
t-Darpp REV: 5′-TGAGGCTCAGGGACCCAAAG-3′
GAPDH FW: 5′- TCCCTGAGCTGAACGGGAAG -3′
GAPDH REV: 5′- GGAGGAGTGGGTGTCGCTGT -3′
Antibodies and western blot analysis
Rabbit anti-DARPP32 (#2306) and mouse anti-HSP70 (N27F34) antibodies were purchased from Cell Signaling (Milan, IT) and Stressgen (Milan, IT), respectively. Peroxidase-conjugated secondary antibodies anti-IgGs were from Cappel and/or BioRad (Milan, IT).
To analyze t-DARPP and HSP70 proteins expression, the cells were lysed with RIPA buffer [50 mMTris (pH 8), 150 mMNaCl, 1 % Nonidet P40, 0.1 % deoxycholate, 0.1 % SDS, 1 mM PMSF, 5 mM Na3VO4, 50 mM protease inhibitors (SIGMA-Aldrich, Milan, IT)] for 30 min at 4°C. Total cell lysates were clarified by centrifugation at 14,000 rpm for 30 min at 4 °C. Aliquots of cell extracts containing equivalent amount of proteins were treated at 95 °C for 5 min, resolved by SDS-polyacrilamide gel electrophoresis 4-15 % (SDS-PAGE), and transferred to nitrocellulose. The horseradish peroxidase-conjugated goat anti-mouse or anti-rabbit were used as secondary antibodies. Signals were detected by LuminataTMClassico Western HRP substrate (Millipore). Identical amounts of total protein from three independent experiments were pooled and analyzed. Total loaded proteins were normalized by anti-HSP70.
RNA sequencing (RNA-seq) analysis
RNA-seq (whole-transcriptome sequencing) analysis was carried out in parental and resistant (T, TP) BT474 cell populations. A pertuzumab-resistant cell population (P) was also assessed as a further control population. Raw data processing from RNA-Seq experiments was performed by RNA-Seq Analysis Pipeline (RAP) webtool [6] using default parameters and hg19 as reference genome. Differentially expressed genes were identified with the cuffdiff module and genes with adjusted P-value <0.05 (Bonferroni correction) were considered significantly modulated between each condition. Plots were generated using the R statistical environment (v.4.2.3).
In vivo xenograft experiments
All animal procedures were compliant with the national and international directives (D.L. March 4th, 2014, no. 26; directive 2010/63/EU of the European Parliament and of the council; Guide for the Care and Use of Laboratory Animals, United States National Research Council 2011; Animal Research Guidelines Reporting of In Vivo Experiments (ARRIVE) guidelines and approved by the Italian Ministry of Health; authorization n. 743/2020-PR, released on July 28th 2020). The mice were maintained in high-efficiency, particulate air HEPA-filtered racks and were fed autoclaved laboratory rodent diet.
CB17-SCID (CB17/Icr-Prkdcscid/IcrIcoCrl, 6 week old) female mice (Charles River Laboratories, Calco, Italy) were injected subcutaneously with 5 × 106 of parental and resistant (T, TP) BT474 cells. Animals were supplemented with 200nM 17β-estradiol (E4389, Sigma-Aldrich) in drinking water.
Tumor volumes were measured in two dimensions using a caliper and calculated by the formula a × b2 /2, where “a” and “b” are the long and short sizes of the tumor, respectively. Each experimental group included six mice.
Results
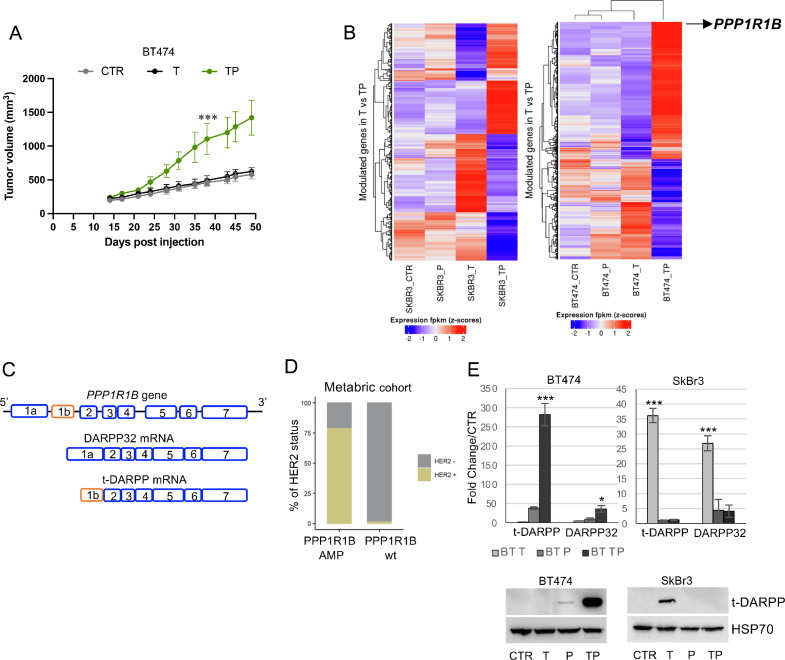
Mechanisms of resistance to HER2-targeted agents were explored in cell models of resistance to T, P, and their combination (TP) previously derived from BT474 and SkBr3 HER2+ BC cell lines and characterized in vitro [3]. We assessed the in vivo growth of BT474-derived T and TP cell populations following subcutaneous injection in immunocompromised mice. Unlike parental BT474-derived xenografts, T and TP-derived ones did not require estrogens supplementation for their growth (data available upon request). When compared with T and parental xenografts, the TP ones showed a significantly higher growth rate (Fig. 2A).
Fig. 2.
Pre-clinical models of resistance to anti-HER2 agents. A. Growth of tumors derived from subcutaneous implantation of CTR, T, and TP BT474 cells. B. RNA-Seq heatmap of the 1000 genes differentially expressed in TP as compared to T resistant cells (FC>1), showed in CTR, T, P, and TP cell populations (SkBr3 and BT474). C. Schematic representation of hyman PPP1R1B gene and exon organization of DARPP32 and its truncated isoform t-DARPP. D. Distribution of HER2 positivity among PPP1R1B amplified and wild type breast cancer cases from Metabric series. E. mRNA levels of t-DARPP and DARPP-32 in resistant BT474 and SkBr3 cells, represented as Fold Change as compared to parental cell population. F. Western Blot analysis revealing t-DARPP expression in resistant and parental BT474 and SkBr3 cell populations. The results are presented as mean +/- standard deviation of three independent experiments (*p < 0.05; **p < 0.001; ***p < 0.0001).
To identify candidate molecular predictors of cross-resistance among sequential HER2-targeting agents, we used RNAseq to profile resistant and parental BT474 and SkBr3 cell populations. The PPP1R1B gene ranked as the top differentially expressed gene in BT474 cells resistant to dual HER2 blockade and was associated with resistance to trastuzumab in SkBr3 (Fig. 2B).
The PPP1R1B gene encodes for DARPP-32 and its truncated isoform (t-DARPP). In this latter form, this protein lacks the first 36 amino acids at the N-terminus (Fig. 2C). Since PPP1R1B is located together with ERBB2 on the 17q12-q21 amplicon, co-amplification of these two genes is a likely event. Data from the of the Metabric dataset of BCs (N=2,509) found a ERBB2 gene amplification in about 80 % of the cases with PPP1R1B gene amplification, which per sè shows an overall 13 % frequency (Fig. 2D).
To validate the RNAseq results, we first measured the levels of t-DARPP and DARPP-32 mRNAs by RT- PCR in cell models. We found a marked induction of both t-DARPP (up to 280-fold) and DARPP-32 (up to 40-fold) in BT474 TP cells (Fig. 2E, leftmost upper panel). Both isoforms were upregulated in SkBr3 T cells (t-DARPP 37-fold, and DARPP-32 27-fold induction) compared with parental SkBr3 (Fig. 2E, rightmost upper panel). The t-DARPP protein was significantly overexpressed (Fig. 2E, lower panels). Although co-amplification of PPP1R1B and ERBB2 was a recurrent event in HER2+ BC, we observed different patterns in our models. Increased mRNA levels of DARPP isoforms were accompanied by reduced HER2 expression [3], suggesting that a transcription-level regulatory mechanism may be involved in the onset of resistance to HER2 targeting agents.
Discussion
In pre- and post-therapy tissue sections from two HER2+ ABC patients treated with TP combination, we observed that the mRNA levels of both DARPP-32 and t-DARPP underwent significant but opposite alterations. Although extremely preliminary, this observation may support the predictive potential of these two DARPP isoforms. To our knowledge, only one single study has reported on the predictive role of DARPP-32 expression in ABC patients treated with trastuzumab [7]. Although t-DARPP expression has been involved in resistance to trastuzumab in in vitro cell models [8], [9], [10], we lack data on patients’ outcomes. The analysis of biological samples from the STEP retrospective cohort will add to this topic. Thus far, the retrospective cohort includes 30 patients, while 9 patients have entered the prospective cohort out of 16 patients screened.
Conclusions
The impact of innovative anti-HER2 agents on the general patients’ population will change its characteristics, along with the outcome of the lines of treatment administered sequentially in the near future. As a consequence, treatment sequence must be reconsidered. The STEP study holds the potentials to integrate current evidence by adding high quality RWE to the current scenario and to unveil key biological mechanisms of resistance to the currently available anti-HER2 agents.
Funding
This research was supported by Funds Ricerca Corrente 2023 from Italian Ministry of Health and GR-2018-12367431/Italian Minister of Health.
CRediT authorship contribution statement
Giulia Bon: Data curation, Formal analysis, Methodology, Investigation, Writing – original draft. Eriseld Krasniqi: Writing – review & editing. Manuela Porru: Writing – review & editing. Lorenzo D'Ambrosio: Writing – review & editing. Stefano Scalera: Writing – review & editing. Marcello Maugeri-Saccà: Writing – review & editing. Francesca Sofia Di Lisa: Writing – review & editing. Lorena Filomeno: Writing – review & editing. Teresa Arcuri: Writing – review & editing. Andrea Botticelli: Writing – review & editing. Daniele Santini: Writing – review & editing. Maria Agnese Fabbri: Writing – review & editing. Giuliana D'Auria: Writing – review & editing. Claudio Pulito: Data curation, Investigation, Writing – review & editing. Giovanni Blandino: Supervision, Writing – review & editing. Caterina Marchiò: Writing – review & editing. Maddalena Barba: Investigation, Writing – original draft. Gennaro Ciliberto: Writing – review & editing. Patrizia Vici: Supervision, Writing – review & editing. Laura Pizzuti: Conceptualization, Methodology, Data curation, Investigation, Writing – original draft, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: FSDL: Ipsen and Novartis, outside the submitted work. AB: Pfizer, Roche, Lilly, Novartis, Msd, Seagen, Gilead and Daiichi Sankyo, outside the submitted work. DS: Amgen, Astellas, Astrazeneca, Bayer, BMS, Eisai, Ipsen, Janssen, Merck, MSD, Novartis, Pfizer, outside the submitted work. GDA: Novartis, Amgen, Eli Lilly, outside the submitted work. CM: Bayer, Roche, AstraZeneca and Daiichi-Sankyo, outside the submitted work. P.V.: Pfizer, Novartis, Eisai, Daiichi Sankyo and Eli Lilly, outside of the manuscript under consideration L.P.: Novartis and Pfizer, outside of the manuscript under consideration. No potential conflicts of interest were disclosed for other authors
Acknowledgments
We thank Consorzio Interuniversitario Nazionale per la Bio-Oncologia for administrative and technical support and Dr Federico Cappuzzo for making available personnel from his division.
Footnotes
Optimal Sequence of Treatment in HER2+ Pertuzumab-pretreated advanced breast cancer patients.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.neo.2023.100937.
Appendix. Supplementary materials
References
- 1.Swain S.M., Kim S.B., Cortés J., Ro J., Semiglazov V., Campone M., Ciruelos E., Ferrero J.M., Schneeweiss A., Knott A., et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a multicenter, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14:461–471. doi: 10.1016/S1470-2045(13)70130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nader-Marta G., Martins-Branco D., de Azambuja E. How we treat patients with metastatic HER2-positive breast cancer. ESMO Open. 2022;7 doi: 10.1016/j.esmoop.2021.100343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bon G., Pizzuti L., Laquintana V., Loria R., Porru M., Marchiò C., Krasniqi E., Barba M., Maugeri-Saccà M., Gamucci T., et al. Loss of HER2 and decreased T-DM1 efficacy in HER2 positive advanced breast cancer treated with dual HER2 blockade: the SePHER Study. J. Clin. Exp. Cancer Res. 2020;39:279. doi: 10.1186/s13046-020-01797-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mullard A. Stemming the tide of drug resistance in cancer. Nat. Rev. Drug Discov. 2020;19:221–223. doi: 10.1038/d41573-020-00050-y. [DOI] [PubMed] [Google Scholar]
- 5.Brady S.W., McQuerry J.A., Qiao Y., Piccolo S.R., Shrestha G., Jenkins D.F., Layer R.M., Pedersen B.S., Miller R.H., Esch A., et al. Combating subclonal evolution of resistant cancer phenotypes. Nat. Commun. 2017;8:1231. doi: 10.1038/s41467-017-01174-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Antonio M., D'Onorio De Meo P., Pallocca M., D'Erchia A.M., Caloger R.A., Castrignanò T., Pesole G. RAP: RNA-Seq Analysis Pipeline, a new cloud-based NGS web application. BMC Genom. 2015;16:S3. doi: 10.1186/1471-2164-16-S6-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotecha S., Lebot M.N., Sukkarn B., Ball G., Moseley P.M., Chan S.Y., Green A.R., Rakha E., Ellis I.O., Martin S.G., et al. Dopamine and cAMP-regulated phosphoprotein 32 kDa (DARPP-32) and survival in breast cancer: a retrospective analysis of protein and mRNA expression. Sci. Rep. 2019;9:16987. doi: 10.1038/s41598-019-53529-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belkhiri A., Dar A.A., Peng D.F., Razvi M.H., Rinehart C., Arteaga C.L., El-Rifai W. Expression of t-DARPP mediates trastuzumab resistance in breast cancer cells. Clin. Cancer Res. 2008;14:4564–4571. doi: 10.1158/1078-0432.CCR-08-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lenz G., Hamilton A., Geng S., Hong T., Kalkum M., Momand J., Kane S.E., Huss J.M. t-darpp activates IGF-1R signaling to regulate glucose metabolism in trastuzumab-resistant breast cancer cells. Clin. Cancer Res. 2018;24:1216–1226. doi: 10.1158/1078-0432.CCR-17-0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong J., Katsha A., Lu P., Shyr Y., Belkhiri A., El-Rifai W. Regulation of ERBB2 receptor by t-Darpp mediates trastuzumab resistance in human esophageal adenocarcinoma. Cancer Res. 2012;72:4504–4514. doi: 10.1158/0008-5472.CAN-12-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.