Abstract
An accurate estimation of net energy (NE) of wheat bran is essential for precision feeding of sows. However, the effects of inclusion level on NE of wheat bran have not been reported. Inclusion level was hypothesized to impact NE of wheat bran by regulating gut microbiota and partitioning of heat production. Therefore, twelve multiparous sows (Yorkshire × Landrace; 2 to 4 parity) were assigned to a replicated 3 × 6 Youden square with 3 successive periods and 6 diets in each square. The experiment included a corn-soybean meal diet (WB0) and five diets including 9.8% (WB10), 19.5% (WB20), 29.2% (WB30), 39.0% (WB40) and 48.7% wheat bran (WB50), respectively. Each period included 6 d of adaptation to diets followed by 6 d for heat production measurement using open-circuit respiration chambers. Compared with other groups, WB30, WB40, and WB50 enriched different fiber-degrading bacteria genera (P < 0.05). Apparent total tract digestibility of neutral detergent fiber and acid detergent fiber of wheat bran were greater in WB30 and WB40 (P < 0.05). Physical activity (standing and sitting) decreased as inclusion level increased (P = 0.04), which tended to decrease related heat production (P = 0.07). Thermic effect of feeding (TEF) was higher in WB50 than other treatments (P < 0.01). Metabolizable energy of wheat bran was similar among treatment groups (except for WB10). NE of wheat bran conformed to a quadratic regression equation with inclusion level (R2 = 0.99, P < 0.01) and peaked at an inclusion level of 35.3%. In conclusion, increasing inclusion level decreased energy expenditure of sows on physical activity and promoted growth of fiber-degrading bacteria, which improved energy utilization of fiber. Fermentation of wheat bran fiber by Prevotellaceae_UCG-003 and norank_f__Paludibacteraceae might increase TEF. Consequently, sows utilized energy in wheat bran most efficiently at an inclusion level of 35.3%.
Keywords: Gestating sow, Gut microbiota, Heat production, Inclusion level, Net energy, Wheat bran
1. Introduction
Excessive energy intake by gestating sows leads to lower feed intake in lactation and excessive fat deposition around the mammary gland and uterus, reducing the performance of sows (De et al., 2009; Woodworth et al., 2020). Fibrous diets are helpful to prevent sow obesity (high body condition score) and alleviate stress caused by restricted feeding in gestating sows (Ramonet et al., 1999; Sun et al., 2015).
Wheat bran is the primary by-product of flour milling and is rich in insoluble fibers such as insoluble arabinoxylan and cellulose (Jaworski et al., 2015). As a fibrous ingredient, wheat bran is used frequently in the diet of pigs to reduce cost of feed (Hassan et al., 2008). Compared with digestible energy (DE) and metabolizable energy (ME), net energy (NE) increases the accuracy for predicting available energy of ingredients by considering energy loss of heat increment, which was considered to be higher among animals fed a fibrous diet (Noblet et al., 1994; Ramonet et al., 2000). However, previous studies have focused on NE of wheat bran fed to growing pigs (Jaworski et al., 2016; Lyu et al., 2019). The digestibility of dietary fibers fed to growing pigs and adult sows was different (Dong et al., 2020; Niu et al., 2019). Previously, in our laboratory, we determined NE of wheat bran in gestating sows when wheat bran was included at 29.2% in the gestation diet (Wang et al., 2019). However, there is still a lack of research about the effect of inclusion level on NE content in wheat bran.
Inclusion level has a significant effect on the accuracy of determining available energy (Villamide, 1996). Increasing wheat bran inclusion impairs nutrient digestion and reduces available energy of swine (Huang et al., 2013). High dietary fiber concentration increases the thermic effect of feeding (TEF) by stimulating a rise in thermic effect of feeding long-term (TEFlt) (Ramonet et al., 2000). Research has been relatively consistent in finding that a higher dietary fiber level reduces energy expenditure related to physical activity (Rijnen et al., 2003; Serena et al., 2008). Recent studies have shown that the dietary concentration of wheat bran impacts microbial fermentation in growing pigs by shaping microbial communities, which ultimately influences energy metabolism (Iyayi and Adeola, 2015; Lyu et al., 2020). High dietary wheat bran content has been found to promote growth of fiber-degrading bacteria, which enhances utilization of fiber in wheat bran (Lyu et al., 2020).
We hypothesize that the inclusion level of wheat bran impacts the NE and nutrient digestibility of wheat bran through influencing certain bacterial communities and altering heat production (HP). Therefore, this study investigated how NE of wheat bran was influenced by dietary inclusion rate, gut microbiota and partitioning of heat production in gestating sows.
2. Materials and methods
2.1. Animal ethics statement
The experimental protocol was approved by the Institutional Animal Care and Use Committee of China Agricultural University (Beijing, China). Protocols were based on the National Research Council's Guide for the Care and Use of Laboratory Animals (AW11302202-1-1). The experiment was conducted at Fengning Swine Research Unit of China Agricultural University (Hebei Province, China). All experiments complied with the ARRIVE guidelines.
2.2. Animals, diets and experimental design
At d 30 to 37 of pregnancy, twelve multiparous sows (Yorkshire × Landrace; 2 to 4 parity) with initial body weight (BW) of 211 ± 6.1 kg were assigned to a replicated 3 × 6 Youden square with 3 successive periods and 6 diets. There were 6 sows and 6 open-circuit respiration chambers used in each Youden square resulting in 6 replicates for each treatment. Details about the repeated Youden square are shown in Table 1. The experiment included a corn-soybean basal diet (WB0) and five diets formulated to contain 9.8% (WB10), 19.5% (WB20), 29.2% (WB30), 39.0% (WB40), and 48.7% wheat bran (WB50), respectively. The chemical composition and nutrient content of the wheat bran used in this study and experiment diets are showed in Table 2, Table 3, respectively. Each period lasted for 12 d, including 6 d for adaptation and a subsequent 5 d for collection of feces and urine, determination of energy balance, HP measurements and an additional 1 d for measuring fasting heat production (FHP).
Table 1.
Experimental design of repeated Youden square.1
| Youden square_12 |
Youden square_23 |
||||||
|---|---|---|---|---|---|---|---|
| Animal | Period 1 | Period 3 | Period 5 | Animal | Period 2 | Period 4 | Period 6 |
| A | WB50 | WB40 | WB30 | G | WB0 | WB10 | WB20 |
| B | WB0 | WB50 | WB40 | H | WB10 | WB20 | WB30 |
| C | WB10 | WB0 | WB50 | I | WB20 | WB30 | WB40 |
| D | WB40 | WB30 | WB20 | J | WB50 | WB0 | WB10 |
| E | WB20 | WB10 | WB0 | K | WB30 | WB40 | WB50 |
| F | WB30 | WB20 | WB10 | L | WB40 | WB50 | WB0 |
Youden square_1 and Youden square_2 were conducted alternately between adaptation period and heat production measurement period. WB0, corn-soybean basal diet; WB10, diet including 9.8% wheat bran; WB20, diet including 19.5% wheat bran; WB30, diet including 29.2% wheat bran; WB40, diet including 39.0% wheat bran; WB50, diet including 48.7% wheat bran.
Youden squre_1 included 3 periods (Period 1, Period 3, and Period 5) of experiment, and six sows (A–F) were allotted to 6 dietary treatments.
Youden squre_2 included 3 periods (Period 2, Period 4, and Period 6) of experiment, and six sows (G–L) were allotted to 6 dietary treatments.
Table 2.
Analyzed chemical composition of wheat bran (%, as-fed basis).1
| Item | Wheat bran |
|---|---|
| Dry matter | 89.3 |
| Crude protein | 18.1 |
| Ether extract | 3.3 |
| Neutral detergent fiber | 34.3 |
| Acid detergent fiber | 10.0 |
| Insoluble dietary fiber | 32.0 |
| Soluble dietary fiber | 2.9 |
| Total dietary fiber | 34.9 |
| Starch | 29.3 |
| Ash | 4.8 |
| Ca | 0.2 |
| P | 0.8 |
| Gross energy, MJ/kg | 17.01 |
All data are the results of a chemical analysis conducted in duplicate.
Table 3.
Formulation and nutrient composition of the experimental diets (%, as-fed basis).1
| Item | Diet2 |
|||||
|---|---|---|---|---|---|---|
| WB0 | WB10 | WB20 | WB30 | WB40 | WB50 | |
| Corn | 81.6 | 73.4 | 65.3 | 57.1 | 48.9 | 40.8 |
| Soybean meal | 15.8 | 14.2 | 12.6 | 11.1 | 9.5 | 7.9 |
| Wheat bran | 0.0 | 9.8 | 19.5 | 29.2 | 39.0 | 48.7 |
| Dicalcium phosphate | 1.1 | 1.1 | 1.1 | 1.1 | 1.1 | 1.1 |
| Limestone | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 |
| Salt | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| Premix3 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Total | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Analyzed chemical composition | ||||||
| DM | 88.5 | 88.3 | 88.6 | 88.7 | 88.9 | 89.0 |
| Ash | 3.8 | 4.0 | 4.2 | 4.5 | 4.7 | 5.0 |
| CP | 14.5 | 14.3 | 14.9 | 15.2 | 15.5 | 15.7 |
| Ether extract | 1.3 | 1.7 | 2.3 | 1.7 | 2.2 | 2.4 |
| Starch | 54.5 | 52.0 | 49.5 | 48.0 | 47.2 | 41.8 |
| NDF | 8.6 | 11.3 | 13.5 | 18.3 | 19.7 | 21.7 |
| ADF | 2.9 | 3.6 | 4.2 | 5.1 | 6.0 | 6.6 |
| Insoluble dietary fiber | 9.2 | 11.2 | 13.3 | 16.4 | 17.8 | 19.1 |
| Soluble dietary fiber | 2.0 | 1.9 | 2.1 | 1.9 | 2.3 | 2.4 |
| Total dietary fiber | 11.2 | 13.1 | 15.4 | 18.4 | 20.0 | 21.5 |
| Gross energy, MJ/kg | 16.34 | 16.37 | 16.39 | 16.49 | 16.53 | 16.60 |
DM = dry matter; CP = crude protein; NDF = neutral detergent fiber; ADF = acid detergent fiber; SDF = soluble dietary fiber.
All data are the results of a chemical analysis conducted in duplicate.
WB0, corn-soybean basal diet; WB10, diet including 9.8% wheat bran; WB20, diet including 19.5% wheat bran; WB30, diet including 29.2% wheat bran; WB40, diet including 39.0% wheat bran; WB50, diet including 48.7% wheat bran.
Premix provided per kilogram of complete feed: 6,000 IU of vitamin A, 3,000 IU of vitamin D3, 20 IU of vitamin E, 1.8 mg of vitamin K3, 2.0 mg of vitamin B1, 6.0 mg of vitamin B2, 4.0 mg of vitamin B6, 3,000 mg of choline, 0.02 mg of vitamin B12, 26.0 mg of niacin, 18.0 mg of pantothenic acid, 3.2 mg of folic acid, 0.4 mg of biotin, 400 mg of Fe, 20 mg of Cu, 100 mg of Zn, 50 mg of Mn, 1.2 mg of I, 0.30 mg of Se, 8.0 g of Ca, 0.8 g of P, 5.6 g of sodium chloride, and 0.05% of lysine.
During the entire experiment, sows were housed individually in stainless-steel metabolism crates and had ad libitum access to water. Each metabolism crate (1.80 m × 0.65 m) was equipped with a feeder, nipple drinker, and slatted floor. During each period, sows were weighed at the beginning of collection period (d 7) and at the end of fasting period (d 13). Before d 6, sows kept in metabolism crates were housed in a room out of respiration chambers. On d 6, each sow was moved into a separate respiration chamber. HP measurement and total collection of feces and urine was conducted from d 7 to 11. On d 12, sows were fasted, and only urine was collected. According to Wang et al. (2019), FHP was calculated using gas exchange data from 22:00 (d 12) to 06:00 (d 13).
During every 12-d period, the sows had 1 d without feeding for FHP determination. Therefore, the feeding level was set as 593.5 kJ/(kg BW0.75 • d) rather than 544.0 kJ/(kg BW0.75 • d) based on the BW at the beginning of each period. Feeding level was equal to 1.3 times the maintenance requirement of gestating sows (NRC, 2012). Two meals with an equal quantity of feed were provided at 08:30 and 15:30. At 08:30 on d 7, about 10 g (1% of feed) ferric oxide was mixed into the diet, and the time between the first appearance of ferric oxide to its disappearance in feces was recorded. During the HP measurement period, at 08:30 the respiration chambers remained open for approximately half an hour for feeding sows, adding water into water storage, and collection of feces and urine. At 15:30, the respiration chambers remained open for approximately 5 min just for feeding sows and filling water storage. Data on gas concentration while the chambers were open was not used to calculate HP and the heat production measurement started again when the concentration of CO2 in the chamber was higher than 2,000 μL/L (Li et al., 2017).
Every chamber had a surveillance camera for observing and recording animal behavior. Behavioral analyses were based on video records collected on d 8 to 12. Behaviors of sows were partitioned into “lying frank”, “lying breast”, sitting, standing, and eating. According to Rijnen et al. (2003), “lying breast” was defined as a sow that lay on her legs with her head and spinal column upright, otherwise it would be considered as “lying frank”. Sitting was defined as a sow with backside on the floor and only forelegs upright. Standing was defined as a sow with all four legs upright and without eating. Eating was defined as a standing sow from the time it was given food until the food was completely consumed. Physical activity was defined as the total of sitting and standing without eating. The duration of the five behavioral characteristics was calculated within every 5-min interval during the gas collection period (Rijnen et al., 2003).
The volume of the six respiration chambers used in the present study was approximately 7.8 m3 and details were reported specifically by Zhang et al. (2014). Temperature and relative humidity in the chambers were maintained constantly at 20 ± 1 °C and 70% ± 5%, respectively. Air velocity was maintained at less than 0.2 m/s. Sows were exposed to light for 12 h daily (07:00 to 19:00). The devices for gas analysis and their operating parameters are described in detail by Wang et al. (2019).
2.3. Sample collection
All ingredients were collected prior to mixing of diets for dry matter (DM) analysis. Diets were also collected after preparation to determine chemical composition. Diet samples were collected daily before each feeding for calculating DM intake. Feed refusal and spillage for each sow were collected, weighed, and dried daily and separately to correct the DM intake during the HP measurement periods. During each HP measurement period, gas concentrations (CO2, O2 and CH4) were measured at 5-min intervals and total collection of feces and urine was conducted daily at 08:30. On d 12, fresh feces were collected for analysis of bacterial microbiota. On d 13 or d 1 of the next period, only urine was collected for calculating urinary nitrogen losses during fasting. Collected feces were stored immediately after collection at −20 °C and a subsample of fresh feces was placed in liquid nitrogen and stored at −80 °C for later microbial analysis. Urine was collected in plastic containers which contained 50 mL of 6 M HCl. After filtering with eight layers of gauze, filtered urine (1% of total) was put into a centrifuge tube (50 mL) and subsequently stored at −20 °C. At the end of each period, feces and urine were thawed, pooled, and a subsample was collected and stored at −20 °C. Fecal samples were oven-dried at 65 °C for 72 h and weighed after being exposed to room temperature for 12 h. Finally, all samples of the ingredients, diets, and feces were ground through a 1-mm screen and finely mixed for further analysis.
2.4. Sample analysis and calculation
Ingredients, diets, and fecal samples were analyzed for DM (method 930.15; AOAC, 2007), crude protein (CP, method 984.13; AOAC, 2007), and ash (Hortwitz and Latimer, 2007). Neutral detergent fiber (NDF), and acid detergent fiber (ADF) were determined according to the procedure of Van Soest et al. (1991). Samples of ingredients and diets were analyzed for ether extract according to the procedure of Thiex et al. (2003). The gross energy (GE) content of ingredients, diets, urine, and fecal samples was analyzed using an isoperibol calorimeter (Parr 6300 Calorimeter, Moline, IL USA) using benzoic acid as a standard. An enzymatic-colorimetric method was conducted to measure the starch content of ingredients and diets (Knudsen, 1997). Total dietary fiber, soluble dietary fiber, and insoluble dietary fiber were determined using the method described by Prosky et al. (1992). Concentration of amino acids in diets and ingredients was determined according to method 982.30 E (a, b, c; AOAC, 2007) using an AA analyzer (Hitachi L-8900; Hitachi Ltd., Tokyo, Japan) and high-performance liquid chromatography (Agilent 1200 Series; Agilent Technologies Inc., Santa Clara, CA).
Calculation of DM and GE intake is described in detail by Le Goff and Noblet (2001). Energy content of methane was assumed to be 39.54 kJ/L (Brouwer, 1965). The apparent total tract digestibility (ATTD) of energy and nutrients in diet was calculated as described by Noblet et al. (1994). The DE of diets was calculated as GE intake minus energy loss of feces. ME of diets was calculated by subtracting energy loss of urine and methane from DE. The difference method was used to calculate available energy of wheat bran (Noblet et al., 1994). The ATTD of nutrients in wheat bran was calculated according to Adeola (2001) as follows:
ATTDY = [ATTDWB − (100% − X%) × ATTDBD]/X%, in which ATTDY was the ATTD of nutrients in wheat bran (%), ATTDWB was the average ATTD of nutrients in diets including wheat bran (%), ATTDBD was the average ATTD of nutrients in the basal diet (WB0, %), and X% was the wheat bran inclusion rate in diets. Using ferric oxide (1% of feed) as a marker, gastrointestinal transit time was calculated as average of the time from ferric oxide first appeared in sows' feces to the time it disappeared (Wang et al., 2023).
The calculation and standardization for O2 consumption, and production of CH4 and CO2 is described by Wang et al. (2019). Total heat production (THP) was calculated according to Brouwer (1965): THP (kJ) = 16.18 × O2 (L) + 5.02 × CO2 (L) − 2.17 × CH4 (L) − 5.99 × urinary nitrogen excretion (g). Calculation of retention of dietary energy (RE) and partitioning of RE were conducted according to the equation described by Labussière et al. (2009).
THP was partitioned into FHP, heat production related to physical activity (AHP), thermic effect of feeding short-term (TEFst), and TEFlt as described by Labussière et al. (2015). The TEF was equal to the sum of TEFst and TEFlt. The heat production due to resting metabolism (RHP) was calculated by gas exchange data from 02:00 to 06:00 on 5 d that sows were fed in each period. TEFlt is the difference between RHP and FHP. RHP and FHP were both considered constant during feeding periods. Thus, the heat production of sows fits the following equation: ΔTHP = ΔAHP + ΔTEFst, where ΔTHP, ΔAHP, and ΔTEFst were, respectively, the differences of THP, AHP, and TEFst between two 5-min intervals. Heat production due to TEFst followed a gamma distribution (van Milgen et al., 1997) and increased sharply within 3 h after feeding, then slowly returned to a baseline level (Ramonet et al., 2000). Therefore, based on the premise that no gas data within 3 h after feeding had been used, we assumed there was a negligible contribution of TEFst to ΔTHP when the interval between two 5-min intervals was less than 15 min. Accordingly, ΔTHP depended almost entirely on ΔAHP. The coefficient of heat production on physical activity (AHPc) was predicted using the following model: ΔTHPijk = βi × ΔTAijk + Eijk, where ΔTHPijk = difference in THP of sow i between two 5-min intervals j and k (kJ); βi = AHPc of sowi (kJ/kg BW0.75 per min); ΔTAijk = physical activity time difference of sow i between two 5-min intervals j and k (min); Eijk = error term. In this study, at least 150 valid data points for each sow were used to fit an optimal model to obtain the regression coefficient of heat production on physical activity. AHP was calculated by multiplying the duration of the physical activity by BW0.75 and the coefficient β. The TEF was calculated by subtracting AHP and FHP from THP. TEFst was equal to TEF minus TEFlt.
2.5. Analysis of bacterial microbiota by 16S RNA sequencing
Bacterial DNA was extracted from fecal samples using a stool DNA Kit (Omega Bio-tek, Norcross, GA, USA). DNA concentration and purification were quantified using a NanoDrop 2000 UV-VIS spectrophotometer (Thermo Scientific, Wilmington, USA). The integrity of DNA was assessed by 1% agarose gel electrophoresis. The V3–V4 hypervariable regions of the bacteria 16S rRNA gene were amplified with primers 338F (5′-ACTCCTACGG GAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGG TWTCTAAT-3′) with a PCR analyzer (GeneAmp 9700, ABI, USA). The PCR products were extracted from a 2% agarose gel and then purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA) and quantified using a QuantiFluor-ST fluorometer (Promega, USA).
Pooled and purified amplicons in equimolar and paired-end reads were sequenced (2 × 300 bp) on an Illumina MiSeq platform according to standard protocols (Majorbio BioPharm Technology Co., Ltd., Shanghai, China). Raw fastq files were quality-filtered by Trimmomatic and merged by FLASH software. The remaining high-quality sequences were clustered into operational taxonomic units (OTU) at 97% similarity by UPARSE algorithm (http://www.drive5.com/usearch/manual/uparse_pipeline.html) and chimeric sequences were identified and removed by UCHIME algorithm (http://www.drive5.com/usearch/manual/uchime_algo.html). Each 16S rRNA gene sequence was taxonomically allocated on the basis of the silva (SSU128) 16S rRNA database by RDP Classifier (http://rdp.cme.msu.edu/) with a confidence threshold of 70%.
2.6. Statistical analysis
In the present study, each sow was considered as an experiment unit. All results of 6 dietary treatment groups were subjected to ANOVA where diets were treated as the fixed effect; period and animal were considered as the random effect using the GLM procedure of SAS (SAS Institute Inc., Cary, NC). When significant differences were observed, Tukey's test was used to adjust for multiple comparisons. However, differences in digestibility and energy contents of wheat bran among different inclusion levels were analyzed using Welch's t-test with “rstatix” package in R software. When significant differences were observed in these indices, Games-Howell's test was used to adjust for multiple comparisons. Orthogonal polynomial contrasts were used to test the linear and quadratic effects of wheat bran inclusion levels on all parameters.
A non-parametric factorial Kruskal–Wallis sum-rank test was used to determine the differential bacterial taxa then linear discriminant analysis (Threshold of 3.0) coupled with effect size (LEfSe) was used to identify key bacterial taxa from phylum to genus between different treatment groups. Alpha diversity of samples was calculated using Chao 1 and Shannon indices. The comparative analysis of alpha diversity between differential wheat bran inclusions was also conducted with the method Welch's t-test. Statistical significance was declared at P < 0.05 and tendency at 0.05 < P < 0.10.
3. Results
3.1. Chemical composition of diets
Table 2 shows the chemical composition of wheat bran used in this study. According to Table 3, dietary concentrations of DM, ash, CP, NDF, ADF, insoluble dietary fiber, total dietary fiber, and GE increased with increasing wheat bran inclusion except for WB10 that contained less DM and CP than the basal diet (Table 3). In contrast, dietary contents of starch decreased as dietary wheat bran increased. All diets had similar contents of ether extract and soluble dietary fiber.
3.2. The richness and biodiversity of bacterial communities
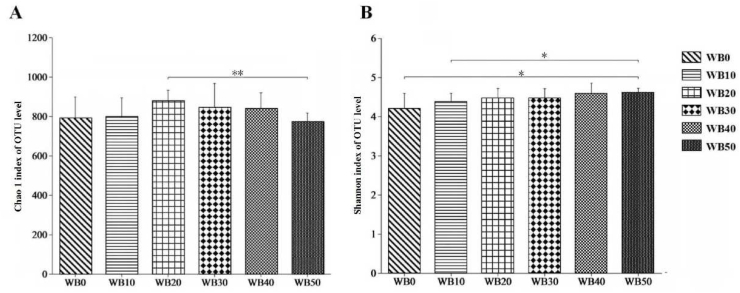
The indices of Chao 1 and Shannon at the OTU level were used to reflect bacterial richness and diversity (Fig. 1). Only a difference in Chao1 indices were observed for fecal bacterial communities of sows fed WB20 and WB50 (P < 0.05). The Shannon index of fecal bacterial communities in sows fed WB50 was higher than those fed WB0 and WB10 (P < 0.05).
Fig. 1.
Effects of wheat bran inclusion level on the richness and diversity of microbial communities in gestating sows. (A) Chao 1 index of bacterial community among treatments. (B) Shannon index of bacterial community among treatments. The results were analyzed by Welch's t-test and presented as mean values. n = 6, and a significant correlation is labeled by ∗P < 0.05, ∗∗P < 0.01. WB0, corn-soybean basal diet; WB10, diet including 9.8% wheat bran; WB20, diet including 19.5% wheat bran; WB30, diet including 29.2% wheat bran; WB40, diet including 39.0% wheat bran; WB50, diet including 48.7% wheat bran.
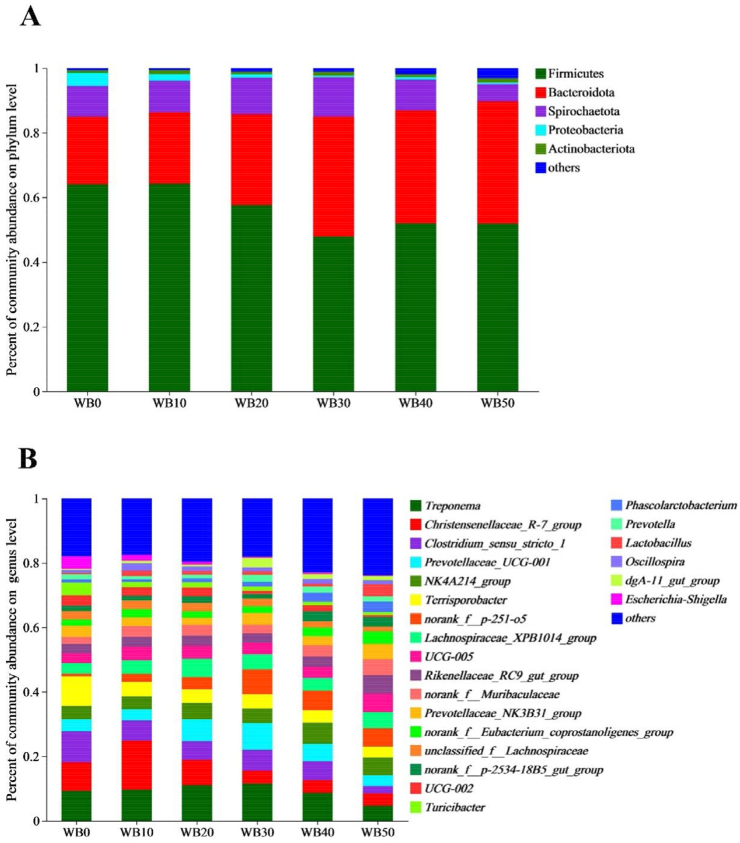
The most dominant phyla among bacterial communities were Firmicutes, Bacteroidetes, Spirochaetes, Proteobacteria, and Actinobacteriota (Fig. 2A). The combined phyla of Firmicutes and Bacteroidetes accounted for more than 85% of the total bacteria found in fecal samples of gestating sows. Sows fed WB30, WB40, and WB50 had a higher relative abundance of Bacteroidetes than sows fed WB0 and WB10 (P < 0.05). By contrast, Firmicutes was enriched in sows fed WB10 and WB20 compared with those fed WB30 and WB50 (P < 0.05). The predominant genera in Firmicutes consisted of Christensenellaceae_R-7_group, Clostridium_sensu_stricto_1, NK4A214_group, and Terrisporobacter (Fig. 2B). Prevotellaceae_UCG-001, norank_f__p-251-o5, and Rikenellaceae_RC9_gut_group were the predominant genera in Bacteroidetes (Fig. 2B).
Fig. 2.
Effects of wheat bran inclusion level on fecal microbial community structure in gestating sows. (A) Microbial community bar plot at the phylum (A) and genus (B) level in gestating sows fed experimental diets. n = 6. WB0, corn-soybean basal diet; WB10, diet including 9.8% wheat bran; WB20, diet including 19.5% wheat bran; WB30, diet including 29.2% wheat bran; WB40, diet including 39.0% wheat bran; WB50, diet including 48.7% wheat bran.
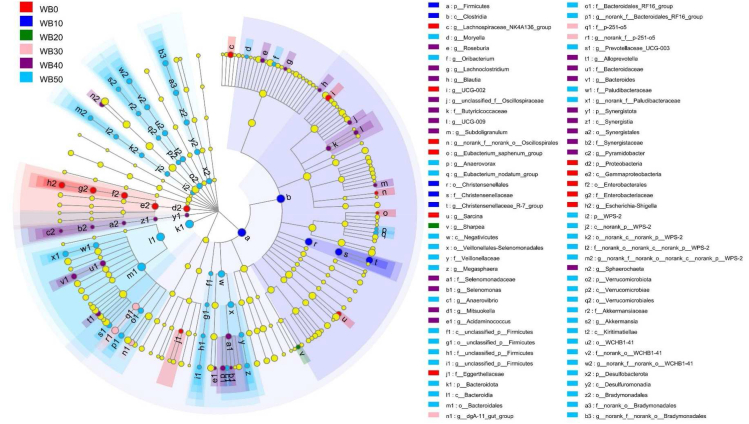
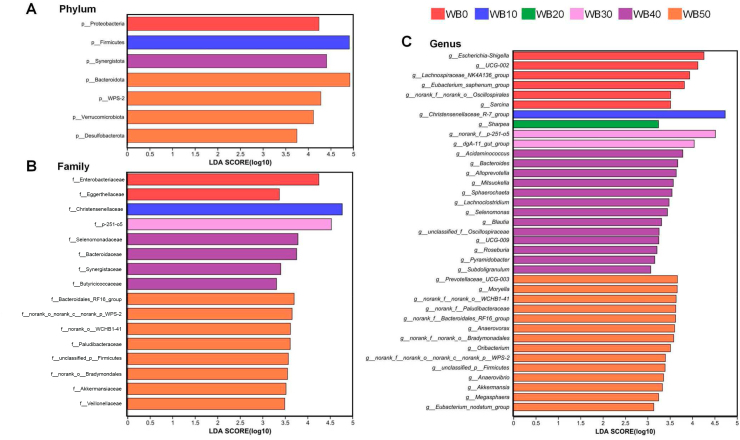
Cladograms of LEfSe analyzed by “all-against-all” showed all bacteria that were significantly enriched within each group from the phylum to the genus level (Fig. 3). Fig. 4 showed bacterial characteristics in each treatment group at phylum, family, and genus level, respectively. As shown in Fig. 3, Fig. 4, the bacteria varied according to dietary wheat bran inclusion rate. Compared with other treatments, the relative abundance of Eubacterium_saphenum_group was higher in sows fed WB0 and resulted in an increase in the population of phylum Proteobacteria. In addition, genera of Escherichia-Shigella, UCG-002, Lachnospiraceae_NK4A136_group, norank_f__norank_o__Oscillospirales, Sarcina and family of Eggerthellaceae were enriched in WB0. Christensenellaceae_R-7_group was enriched in WB10 and led to a higher relative abundance of phylum Firmicutes than other treatment groups. Sows fed WB20 were only enriched in genus Sharpea. Genera norank_f__p-251-o5 and dgA-11_gut_group that belongs to the phylum Bacteroidetes were enriched in sows fed WB30. Sows fed WB40 were enriched in 13 genera, in which 9 genera belonged to the phylum Firmicutes (Acidaminococcus, Lachnoclostridium, Blautia, Roseburia, unclassified_f__Oscillospiraceae, UCG-009, Subdoligranulum, Selenomonas, Mitsuokella), 2 genera belonged to phylum Bacteroidetes (Bacteroides and Alloprevotella), and 2 other genera (Pyramidobacter and Sphaerochaeta). A variety of bacteria were found to be enriched in sows fed WB50. Relative abundance of Prevotellaceae_UCG-003, norank_f__Bacteroidales_RF16_group, and norank_f__Paludibacteraceae of WB50 were higher than other treatments and resulted in a larger population of the phylum Bacteroidetes. Sows fed WB50 were enriched in Anaerovibrio and Megasphaera, which made relative abundance of class Negativicutes, that belongs to the phylum Firmicutes, larger. Additionally, these sows had a greater population of the genera Akkermansia, norank_f__norank_o__WCHB1-41, and norank_f__norank_o__Bradymonadales. The P-values of the above comparative analysis of bacteria composition among treatments from LEfSe were all less than 0.05.
Fig. 3.
Cladograms of linear discriminant analysis coupled with effect size from genus to phylum by all-against-all. n = 6, and light-yellow nodes represented no significant difference (P > 0.05) in bacteria among treatment groups. Threshold of linear discriminant analysis is 3.0. WB0, corn-soybean basal diet; WB10, diet including 9.8% wheat bran; WB20, diet including 19.5% wheat bran; WB30, diet including 29.2% wheat bran; WB40, diet including 39.0% wheat bran; WB50, diet including 48.7% wheat bran.
Fig. 4.
The linear discriminant analysis coupled with effect size (LEfSe) analysis of gut microbiota composition in sows provided different dietary treatments. Bacterial species differed among treatments at (A) phylum level, (B) family level, and (C) genus level, LEfSe analysis conducted by all-against-all, and n = 6. Threshold of linear discriminant analysis is 3.0. WB0, corn-soybean basal diet; WB10, diet including 9.8% wheat bran; WB20, diet including 19.5% wheat bran; WB30, diet including 29.2% wheat bran; WB40, diet including 39.0% wheat bran; WB50, diet including 48.7% wheat bran.
3.3. Dietary nutrient digestibility and available energy
As inclusion level of wheat bran increased from 0% to 48.7%, DM intake increased linearly (P < 0.01), but gastrointestinal transit time linearly and quadratically decreased from 104.7 to 57.5 h (P < 0.01; Table 4). Dietary ATTD of DM, GE, CP, NDF, and ADF linearly decreased as inclusion level of wheat bran increased (P < 0.01). As a consequence, dietary DE, ME, and NE decreased when dietary wheat bran increased (P < 0.01). There was little evidence for association between wheat bran inclusion level and energy utilization of diets.
Table 4.
The effect of inclusion level of wheat bran on nutrient digestibility and energy values in diets fed to gestating sows.
| Diet1 |
SEM | P-values |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Item | WB0 | WB10 | WB20 | WB30 | WB40 | WB50 | Diet | Linear | Quadratic | |
| No. of observations | 6 | 6 | 6 | 6 | 6 | 6 | ||||
| BW, kg | 206.8 | 217.0 | 202.7 | 214.4 | 207.7 | 217.4 | 4.80 | NS | NS | NS |
| DM intake, kg/d | 1.70 | 1.86 | 1.80 | 1.91 | 1.98 | 2.08 | 0.052 | <0.01 | <0.01 | NS |
| Gastrointestinal transit time2, h | 104.7a | 81.7b | 73.7bc | 63.6bc | 58.8c | 57.5c | 5.77 | <0.01 | <0.01 | <0.01 |
| Digestibility coefficients, % | ||||||||||
| DM | 92.0a | 89.2b | 87.1bc | 85.0cd | 82.8d | 79.9e | 1.44 | <0.01 | <0.01 | <0.01 |
| GE | 91.9a | 89.1b | 87.5b | 85.4c | 82.7d | 80.6e | 0.53 | <0.01 | <0.01 | <0.01 |
| CP | 90.6a | 89.8ab | 88.2bc | 87.3cd | 85.8de | 84.6e | 0.61 | <0.01 | <0.01 | <0.01 |
| NDF | 76.7a | 69.8ab | 63.2bc | 65.3bc | 60.6cd | 54.6d | 1.62 | <0.01 | <0.01 | 0.02 |
| ADF | 77.0a | 66.0b | 56.6c | 54.1c | 52.0c | 43.2d | 1.73 | <0.01 | <0.01 | <0.01 |
| Energy of diets, MJ/kg DM | ||||||||||
| DE | 16.96a | 16.52b | 16.17b | 15.89c | 15.39d | 15.03e | 0.098 | <0.01 | <0.01 | <0.01 |
| ME | 16.33a | 15.93b | 15.58b | 15.27c | 14.89cd | 14.55d | 0.115 | <0.01 | <0.01 | <0.01 |
| NE | 12.89a | 12.44b | 12.18bc | 12.14bc | 11.88c | 11.32d | 0.119 | <0.01 | <0.01 | 0.04 |
| Energy utilization, % | ||||||||||
| ME:DE ratio | 96.3 | 96.4 | 96.4 | 96.1 | 96.8 | 96.8 | 0.56 | NS | NS | NS |
| NE:ME ratio | 79.0 | 78.1 | 78.3 | 79.4 | 79.8 | 77.8 | 0.64 | NS | NS | NS |
BW = body weight; DM = dry matter; GE = gross energy; CP = crude protein; NDF = neutral detergent fiber; ADF = acid detergent fiber; DE = digestible energy; ME = metabolizable energy; NE = net energy.
a-e Means within a row without a common superscript differ significantly (P < 0.05). Values were means of six observations per treatment. The P-values more than 0.10 were presented with “NS”.
WB0, corn-soybean basal diet; WB10, diet including 9.8% wheat bran; WB20, diet including 19.5% wheat bran; WB30, diet including 29.2% wheat bran; WB40, diet including 39.0% wheat bran; WB50, diet including 48.7% wheat bran.
Gastrointestinal transit time was calculated as average of the time ferric oxide first appeared in sows‘ feces and the time it disappeared.
3.4. Nitrogen balance and energy balance
Urinary nitrogen losses had no association with wheat bran inclusion level (Table 5). The higher inclusion level of wheat bran increased fecal nitrogen losses (P < 0.01). Total nitrogen losses increased linearly as dietary wheat bran increased (P = 0.04). However, no significant difference in nitrogen retention was observed among treatments. As dietary wheat bran inclusion increased, ME intake of sows increased linearly as dietary wheat bran inclusion increased (P = 0.03), but energy losses as CH4 decreased linearly as wheat bran inclusion increased (P < 0.01). There was no significant difference in THP among treatments. Dietary wheat bran level affected energy retained as fat and total retained energy (P < 0.05). In addition, total retained energy tended to increase as inclusion level of wheat bran increased (P = 0.09). When presented as a percentage of ME intake, there was no significant difference in total retained energy among treatments, but increasing inclusion level still tended to increase energy retained as fat (P = 0.08). Respiratory quotients were similar among treatments with an overall mean of 1.070.
Table 5.
Effect of wheat bran inclusion level on nitrogen balance and energy balance of gestating sows.
| Diet1 |
SEM |
P-values |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | WB0 | WB10 | WB20 | WB30 | WB40 | WB50 | Diet | Linear | Quadratic | ||
| No. of observations | 6 | 6 | 6 | 6 | 6 | 6 | |||||
| Nitrogen balance, g/d | |||||||||||
| Intake | 59.1 | 65.0 | 63.5 | 67.7 | 57.8 | 63.8 | 3.58 | NS | NS | NS | |
| Urinary losses | 32.0 | 31.6 | 31.5 | 31.1 | 32.0 | 31.6 | 2.57 | NS | NS | NS | |
| Fecal losses | 5.1e | 6.1de | 6.9cd | 8.2bc | 9.4ab | 11.1a | 0.43 | <0.01 | <0.01 | 0.03 | |
| Total losses | 37.1 | 37.7 | 38.4 | 39.3 | 41.4 | 42.7 | 2.59 | NS | 0.04 | NS | |
| Retention | 22.0 | 27.3 | 25.1 | 28.4 | 16.4 | 21.1 | 4.85 | NS | NS | NS | |
| Energy balance, MJ/d | |||||||||||
| DE intake | 35.08b | 36.49ab | 35.24b | 37.36ab | 36.74ab | 38.56a | 1.424 | <0.01 | NS | NS | |
| ME intake | 33.77b | 35.19ab | 33.95b | 35.91ab | 35.56ab | 37.31a | 1.475 | <0.01 | 0.03 | NS | |
| Energy losses in urine | 1.02 | 1.01 | 1.03 | 1.21 | 0.99 | 1.14 | 0.189 | NS | NS | NS | |
| Energy losses as CH4 | 0.28a | 0.29a | 0.26ab | 0.24ab | 0.20ab | 0.11b | 0.035 | <0.01 | <0.01 | <0.01 | |
| Total heat production | 25.84 | 27.15 | 25.99 | 26.66 | 26.08 | 27.82 | 0.635 | NS | NS | NS | |
| Retained energy, MJ/d | |||||||||||
| Total | 7.93b | 8.04b | 7.96b | 9.25ab | 9.48a | 9.49a | 0.766 | 0.03 | 0.09 | NS | |
| Retained as protein | 3.27 | 4.08 | 3.74 | 4.24 | 2.44 | 3.13 | 0.723 | NS | NS | NS | |
| Retained as fat | 4.66ab | 3.96b | 4.22ab | 5.01ab | 7.04a | 6.36ab | 1.106 | 0.04 | NS | NS | |
| Retained energy, % of ME intake | |||||||||||
| Total | 23.4 | 22.7 | 23.5 | 25.6 | 26.6 | 25.5 | 1.91 | NS | NS | NS | |
| Retained as protein | 9.5 | 11.6 | 11.1 | 11.8 | 6.8 | 8.4 | 2.00 | NS | NS | NS | |
| Retained as fat | 13.9 | 11.1 | 12.4 | 13.8 | 19.8 | 17.1 | 3.08 | 0.08 | NS | NS | |
| Respiratory quotient | 1.078 | 1.073 | 1.070 | 1.070 | 1.073 | 1.058 | 0.0107 | NS | NS | NS | |
DE = digestible energy; ME = metabolizable energy.
a-e Means within a row without a common superscript differ significantly (P < 0.05). Values were means of six observations per treatment. The P-values more than 0.10 were presented with “NS”.
WB0, corn-soybean basal diet; WB10, diet including 9.8% wheat bran; WB20, diet including 19.5% wheat bran; WB30, diet including 29.2% wheat bran; WB40, diet including 39.0% wheat bran; WB50, diet including 48.7% wheat bran.
3.5. Behavioral characteristics and heat production of physical activity
The time sows spent lying frank was different among treatment groups (P = 0.02) and tended to increase quadratically with increasing wheat bran inclusion (P = 0.06, Table 6). Total time spent resting tended to increase linearly as inclusion of wheat bran increased (P = 0.07). Sows spent more time eating as dietary wheat bran increased due to the increased DM intake (P < 0.01). In contrast, the time sows spent standing and physical activity linearly and quadratically decreased with increasing dietary wheat bran (P < 0.05). Therefore, AHP of sows trended to be lower in WB50 than those in WB10 (P = 0.07). AHPc was independent of dietary treatments and had an overall mean of 0.24 kJ/(kg BW0.75 • min).
Table 6.
Effect of wheat bran inclusion level on behavior and heat production related to physical activity of gestating sows.1
| Item | Diet2 |
SEM |
P-values |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| WB0 | WB10 | WB20 | WB30 | WB40 | WB50 | Diet | Linear | Quadratic | ||
| No. of observations | 6 | 6 | 6 | 6 | 6 | 6 | ||||
| Rest, % of 24 h | ||||||||||
| Lying frank | 78.80abc | 76.42c | 77.01bc | 77.62abc | 81.07ab | 81.65a | 1.319 | 0.02 | NS | 0.06 |
| Lying breast | 6.08 | 9.47 | 7.21 | 8.92 | 5.13 | 7.18 | 1.518 | NS | NS | NS |
| Total | 84.88ab | 85.89ab | 84.22b | 86.54ab | 86.19ab | 88.83a | 1.346 | 0.04 | 0.07 | NS |
| Eating, % of 24 h | 1.98b | 2.03b | 2.38 ab | 2.39ab | 2.52ab | 2.85a | 0.145 | <0.01 | <0.01 | 0.02 |
| Physical activity, % of 24 h | ||||||||||
| Sitting | 2.29 | 0.89 | 1.87 | 0.83 | 2.24 | 0.99 | 0.592 | NS | NS | NS |
| Standing | 10.84ab | 11.18a | 11.53a | 10.24ab | 9.04ab | 7.32b | 1.448 | 0.04 | 0.03 | <0.01 |
| Total | 13.13a | 12.07ab | 13.40a | 11.07ab | 11.28ab | 8.32b | 1.372 | 0.01 | 0.04 | 0.07 |
| AHP, MJ/d | 2.53 | 2.83 | 2.62 | 2.75 | 2.33 | 2.28 | 0.210 | 0.07 | NS | NS |
| AHPc, kJ/(kg BW0.75 • min) | 0.21 | 0.25 | 0.21 | 0.25 | 0.21 | 0.25 | 0.010 | NS | NS | NS |
AHP = heat production related to physical activity; AHPc = coefficient of heat production on physical activity; BW = body weight.
a-c Means within a row without a common superscript differ significantly (P < 0.05). Values were means of six observations per treatment. The P-values more than 0.10 were presented with “NS”.
Lying breast was defined as a sow laying on her legs with her head and spinal column upright, otherwise it would be considered as lying frank. Sitting was defined as a sow sitting with backside and forelegs kept upright. Standing was defined as a sow standing upright on four legs. Physical activity was defined as the total of sitting and standing without eating.
WB0, corn-soybean basal diet; WB10, diet including 9.8% wheat bran; WB20, diet including 19.5% wheat bran; WB30, diet including 29.2% wheat bran; WB40, diet including 39.0% wheat bran; WB50, diet including 48.7% wheat bran.
3.6. Components of heat production and net energy of diet
Intake of ME linearly and quadratically increased as inclusion level of wheat bran increased (P < 0.01; Table 7). The ME intake of sows was slightly higher than intended (593.5 kJ/kg BW0.75 per day) because of underestimation of ME content of wheat bran in this study. THP had an overall mean of 480.8 kJ/(kg BW0.75 • d), and no significant difference in THP among treatments was observed. Mean FHP of gestating sows in this study was 345.2 kJ/(kg BW0.75 • d) and was independent of dietary wheat bran inclusion. The average values of AHP, TEFlt and TEFst were 2.56, 1.73, and 3.21 MJ/d, respectively. There was no difference in TEFlt and TEFst among treatments. However, AHP of sows tended to be lower in WB50 than in WB10 (P = 0.07), and AHP:ME ratio of WB50 was lower than that of WB10 (P = 0.03). TEF increased significantly when dietary wheat bran reached 48.7% (P < 0.01). Besides, TEF:ME ratio of WB0 and WB30 was lower than that of WB50 (P < 0.01).
Table 7.
Effect of inclusion level on partitioning of heat production in gestating sows.
| Diets1 |
SEM |
P-values |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | WB0 | WB10 | WB20 | WB30 | WB40 | WB50 | Diet | Linear | Quadratic | |||
| No. of observations | 6 | 6 | 6 | 6 | 6 | 6 | ||||||
| ME intake, kJ/(kg BW0.75 d) | 619.4d | 622.9cd | 632.3bcd | 640.9abc | 649.9ab | 659.2a | 11.76 | <0.01 | <0.01 | <0.01 | ||
| HP, kJ/(kg BW0.75 d) | ||||||||||||
| THP | 474.2 | 480.9 | 484.4 | 476.7 | 476.9 | 491.9 | 12.06 | NS | NS | NS | ||
| RHP | 367.1 | 379.1 | 376.0 | 371.1 | 378.6 | 387.5 | 11.95 | NS | NS | NS | ||
| FHP | 344.0 | 344.5 | 346.3 | 345.2 | 345.5 | 345.6 | 11.92 | NS | NS | NS | ||
| Components of THP, MJ/d | ||||||||||||
| FHP | 18.75 | 19.45 | 18.58 | 19.31 | 18.88 | 19.57 | 0.663 | NS | NS | NS | ||
| AHP | 2.53 | 2.83 | 2.62 | 2.75 | 2.33 | 2.28 | 0.210 | 0.07 | NS | NS | ||
| TEF | ||||||||||||
| TEFlt | 1.26 | 1.92 | 1.59 | 1.44 | 1.82 | 2.36 | 0.301 | NS | NS | NS | ||
| TEFst | 3.30 | 2.96 | 3.20 | 3.15 | 3.05 | 3.61 | 0.301 | NS | NS | NS | ||
| Total | 4.57b | 4.88b | 4.78b | 4.60b | 4.87b | 5.96a | 0.195 | <0.01 | NS | NS | ||
| THP | 25.84 | 27.15 | 25.99 | 26.66 | 26.08 | 27.82 | 0.635 | NS | NS | NS | ||
| Heat increment, % of ME intake | ||||||||||||
| AHP:ME ratio | 7.5ab | 8.0a | 7.7ab | 7.7ab | 6.5ab | 6.2b | 0.61 | 0.03 | 0.06 | 0.03 | ||
| TEF:ME ratio | 13.5b | 13.9ab | 14.1ab | 12.8b | 13.7ab | 16.0a | 0.55 | <0.01 | NS | NS | ||
| Total | 21.0 | 21.9 | 21.8 | 20.5 | 20.2 | 22.2 | 0.64 | NS | NS | NS | ||
BW = body weight; ME = metabolizable energy; HP = heat production. THP = total heat production; RHP = heat production of resting metabolism; FHP = fasting heat production; AHP = heat production related to physical activity; TEF = thermic effect of feeding; TEFlt = thermic effect of feeding long-term; TEFst = thermic effect of feeding short-term.
a-d Means within a row without a common superscript differ significantly (P < 0.05). Values were means of six observations per treatment. The P-values more than 0.10 were presented with “NS”.
WB0, corn-soybean basal diet; WB10, diet including 9.8% wheat bran; WB20, diet including 19.5% wheat bran; WB30, diet including 29.2% wheat bran; WB40, diet including 39.0% wheat bran; WB50, diet including 48.7% wheat bran.
3.7. Nutrient digestibility and available energy of wheat bran
Inclusion levels had no significant effect on ATTD CP in wheat bran (Table 8). ATTD of DM changed quadratically as dietary wheat bran increased. ATTD of NDF and ADF in wheat bran were different among treatment groups (P < 0.05) and the two indices were relatively high in groups WB30 and WB40 compared with other treatments, respectively. As for energy available from wheat bran, only slight changes were observed for DE and ME among treatment groups except for WB10. NE changed quadratically as dietary wheat bran level increased (P < 0.01). A quadratic increase of NE:ME ratio as inclusion level increased was noted (P = 0.03).
Table 8.
Effect of inclusion level on digestibility of nutrient and energy values of wheat bran.
| Diets1 |
SEM |
P-values |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Item | WB10 | WB20 | WB30 | WB40 | WB50 | Diet | Linear | Quadratic | |
| No. of observations | 6 | 6 | 6 | 6 | 6 | ||||
| Digestibility coefficients, % | |||||||||
| CP | 77.0 | 72.2 | 73.7 | 74.4 | 73.9 | 2.05 | NS | NS | NS |
| DM | 61.0 | 67.7 | 68.6 | 69.3 | 68.1 | 2.53 | NS | NS | <0.05 |
| GE | 63.4 | 68.4 | 70.3 | 68.7 | 69.0 | 2.08 | NS | NS | NS |
| NDF | 60.5ab | 49.0b | 72.1a | 58.6ab | 50.2b | 2.84 | <0.01 | NS | NS |
| ADF | 34.0ab | 28.5b | 39.9ab | 44.5a | 34.9ab | 3.06 | 0.02 | NS | NS |
| Energy, MJ/kg DM | |||||||||
| DE | 12.09 | 13.04 | 13.39 | 13.09 | 13.15 | 0.381 | NS | NS | NS |
| ME | 11.93 | 12.63 | 12.80 | 12.79 | 12.80 | 0.529 | NS | NS | 0.05 |
| NE | 8.05 | 9.35 | 10.38 | 10.40 | 9.77 | 0.522 | NS | NS | <0.001 |
| Energy utilization, % | |||||||||
| ME:DE ratio | 98.7 | 96.9 | 95.7 | 97.7 | 97.4 | 2.94 | NS | NS | NS |
| NE:ME ratio | 67.4 | 74.0 | 81.0 | 81.3 | 76.3 | 4.14 | NS | NS | 0.03 |
CP = crude protein; DM = dry matter; GE = gross energy; NDF = neutral detergent fiber; ADF = acid detergent fiber; DE = digestible energy; ME = metabolizable energy; NE = net energy.
a-d Means within a row without a common superscript differ significantly (P < 0.05). Values were means of six observations per treatment. The P-values more than 0.10 were presented with “NS”.
WB10, diet including 9.8% wheat bran; WB20, diet including 19.5% wheat bran; WB30, diet including 29.2% wheat bran; WB40, diet including 39.0% wheat bran; WB50, diet including 48.7% wheat bran.
4. Discussion
4.1. Effects of inclusion level on fecal microbiota
Wheat bran has a typical non-starch polysaccharide composition of gramineous grain by-products that mainly consists of insoluble arabinoxylan and cellulose (Knudsen, 1997). The insoluble arabinoxylan and cellulose content of wheat bran is 19.4% and 6.4%, respectively (Jaworski et al., 2015). In general, Firmicutes and Bacteroidetes were the most dominant phyla in gestating sows (Zhuo et al., 2020). Consistent with previous studies (Ji et al., 2019; Shao et al., 2020), in this study, Firmicutes and Bacteroidetes were the most abundant phyla in all treatments regardless of the periods. Sows fed WB30, WB40, and WB50 diets exhibited lower relative abundance of Firmicutes compared with those fed WB0 and WB10. An opposite result was observed in the relative abundance of Bacteroidetes. Consistent with this result, high dietary insoluble fiber usually increases the relative abundance of Bacteroidetes in pigs (Chen et al., 2019; El Kaoutari et al., 2013). Bacteroidetes possesses special polysaccharide utilization loci and carbohydrase enzymes that cleave the linkages in complex polysaccharide structures (Sonnenburg et al., 2010). A variety of bacteria within phylum Bacteroidetes were enriched in sows fed WB40 and WB50 and many of them have the ability to degrade insoluble polysaccharides, such as Bacteroides, Alloprevotella, Prevotellaceae_UCG-003, and norank_f__Paludibacteraceae (Downes et al., 2013; Dyksma et al., 2020; Flint et al., 2008; MacFabe et al., 2007; Okeke and Lu, 2011). In addition, sows fed WB30 displayed higher relative abundance of norank_f__p-251-o5 and dgA-11_gut_group compared to the other treatments. Zhuo et al. (2020) found a positive correlation between dgA-11_gut_group and dietary fiber. Similarly, norank_f__p-251-o5 was one of the most dominant bacteria in horse at genus level, and low relative abundance of norank_f__p-251-o5 was related to impaired digestibility of fibrous feed (Li et al., 2022). It has been suggested that norank_f__p-251-o5 contributed to digestibility of fiber. By contrast, bacteria enriched in sows fed WB0, WB10, and WB20 did not belong to phylum Bacteroidetes, and the fiber-degrading function of these bacteria has not been reported. In addition, Christensenellaceae_R-7_group was enriched in sows fed WB10. Similarly, Yu et al. (2020) found that Christensenellaceae_R-7_group was enriched in sows fed a diet that included a moderate level of fiber supplementation (dietary crude fiber content of 9.15%) rather than a low or high dietary fiber level. Christensenellaceae_R-7_group has been shown to be negatively correlated with serum lipids (Waters and Ley, 2019).
4.2. Effects of inclusion level on nutrient digestibility of diets
Many previous studies reported addition of wheat bran in the diet resulted in impaired nutrient digestibility (Lyu et al., 2019; Shi et al., 2021). Similarly, we found that addition of wheat bran in the diet decreased digestibility of most dietary nutrients. According to experiments conducted by Kim et al. (2007) and Jaworski et al. (2015), this phenomenon might be explained partly by significantly decreased gastrointestinal transit time, which shortens the digestion time of nutrients. Increasing insoluble fiber in the diet decreases the mean retention time in the small and large intestine of pigs (Wilfart et al., 2007). The increased DM intake causes a large volume of intestinal contents, which can exert a direct physical action on the intestine and thus stimulate gastrointestinal transit (Chasse et al., 2021). In addition, high chyme viscosity due to soluble fiber hinders contact between chyme and digestive enzymes (Gutierrez et al., 1994). In this study, shortened digestion time was associated with the level of dietary insoluble fiber, soluble fiber, and DM intake. Nevertheless, ATTD of ADF and NDF showed different responses to wheat bran inclusion level compared with other nutrients. In the present study, ATTD of dietary NDF and ADF decreased slightly (by 2.6% and 4.6%, respectively) as wheat bran inclusion level increased from 19.5% to 39.0%. Similarly, Huang et al. (2015) found ATTD of ADF and NDF of growing pigs fed diets with 9.65% wheat bran were lower than those fed a basal diet (corn-soybean meal), but they were similar to the diet containing wheat bran at 48.25% and the control group. As for adult sows, Shi et al. (2021) reported that the decrease in ATTD of NDF was less than one percentage point (58.92% vs. 59.43%), and ATTD of ADF increased (52.70% vs. 49.03%) in a diet containing 36.4% wheat bran compared with the control diet without wheat bran. Sows' adaptation to fibrous diets can partly explain this result. The gut microbiota undergo an adaptation process when exposed to fiber-rich diets, and the process is stimulated by increased fiber concentrations (Edwards, 1993). In the present study, a higher relative abundance of fiber-degrading genera belonging to phylum Bacteroides was observed in sows fed diets including more wheat bran. Increased fiber-degrading genera elevate the activities of enzymes such as cellulose decomposition (Castillo et al., 2007). In addition, a higher dietary fiber content promotes secretion of digestive juices and the adaptation process for the intestinal tract to absorb new products that come from degradation of fiber (Johnson, 1988; Zebrowska and Low, 1987). The significantly lower ATTD of dietary fiber in sows fed WB50 suggests these adaptations are limited and insufficient to compensate for the negative effects such as shortened time of digestion and increased difficulty in digestion of nutrients from high dietary wheat bran inclusion.
4.3. Effects of wheat bran inclusion level on nitrogen balance and energy balance
Fecal nitrogen losses of sows in this study increased linearly as dietary fiber increased. This finding was consistent with previous studies that demonstrated a positive relationship between dietary fiber and fecal nitrogen losses (Le Gall et al., 2009; Wang et al., 2019). The possible explanation was that wheat bran increased endogenous nitrogen losses due to excessive saliva, gastric and pancreatic secretions (Zebrowska and Low, 1987). Additionally, dietary fiber increases mechanical erosion of mucosa with desquamation of cells (Montagne et al., 2003). On the other hand, wheat bran resulted in increasing DM intake of sows, which decreased standard ileal digestibility for CP and amino acids (Moter and Stein, 2004). As for energy balance, ME intake increased as wheat bran level increased due to the underestimation of energy content of wheat bran. However, there was no significant difference in THP among treatment groups, and the average THP was 26.59 MJ/d or 480.8 kJ/(kg BW0.75 d), which was close to the values reported by Ramonet et al. (2000). Average FHP of gestating sows in this study was 344.9 kJ/(kg BW0.75 d) and was not affected by dietary wheat bran level. Similar results were observed in previous studies conducted by other researchers (Le Goff et al., 2002; Ramonet et al., 2000; Wang et al., 2019). Energy loss in CH4 decreased as wheat bran inclusion level increased. This result differs from studies that reported that high dietary fiber promoted production of CH4 (Jha and Berrocoso, 2015; Serena et al., 2008). However, some studies focused on wheat bran support our result. For example, Wang et al. (2019) reported a diet with 29.2% wheat bran reduced gestating sows' production of CH4 by 39% compared with a corn-soybean meal diet. Another study also observed reduced synthesis of CH4 as wheat bran inclusion increased (Jaworski et al., 2016). These inconsistent results may be attributed to differences in physical and chemical characteristics between wheat bran and other fibrous ingredients. Decreased transit time caused by the sulfates and fiber in wheat reduce the amount of time for microbial populations in the hindgut to ferment the fiber in wheat bran and may in part explain the reduced energy losses in CH4. Consistent with a previous study (Wang et al., 2019), even though ME intake changed, no significant differences in energy losses through urine and heat production were observed among treatment groups. Consequently, sows fed WB50 and WB40 displayed greater total retained energy than those fed diets with wheat bran inclusion rates lower than 19.5%. As wheat bran inclusion increased, energy retained as fat resulted in an increase in total energy retention.
4.4. Effects of wheat bran inclusion on behavior and heat production related to physical activity
Physical activity has always been an important part of animal energy expenditure. The behavior of sows in this study was observed by video records. The time spent resting tended to increase linearly as inclusion level of wheat bran increased. This observation was consistent with a previous study that observed less physical activity of pigs fed with a higher dietary fiber level (Serena et al., 2008). Sows in our study spent more than 85% of their day resting and about 13% of their time doing physical activity (sitting and standing). Similarly, Rijnen et al. (2003) reported sows spent 87.7% of their day resting and about 12.5% doing physical activity and eating. However, an inconsistent result was reported by Young et al. (2004) who found gestating sows fed one meal per day spent about 17% of their day sitting and standing. A possible reason is that the feeding pattern of one meal per day further frustrates the feeding motivation of sows (Holt et al., 2006). A linear and quadratic increase in time spent eating was observed in this study as inclusion level of wheat bran increased. Diets containing wheat bran had a lower ME content than a corn-soybean meal diet, therefore, sows needed a higher DM intake to satisfy their daily energy requirement. Increased DM intake helped to satisfy sows' feeding motivation. As a consequence, the AHP:ME ratio of sows decreased linearly and quadratically as dietary wheat bran increased. Decreased AHP is connected with a significantly decreased time that gestating sows spent on physical activity. Consistent with other studies (Rijnen et al., 2003; Young et al., 2004), sows produced 0.24 kJ/kg BW0.75 heat per minute of physical activity (sitting and standing) in this study.
4.5. Effects of inclusion level on thermic effect of feeding
The TEF was considered as energy losses related to the ingestion, digestion, absorption, and metabolism of feed. Consistent with a previous study (Le Goff et al., 2002), a significantly higher TEF was observed in sows fed WB50 compared with those fed WB0. Average TEF and average TEF:ME ratio were 4.94 MJ/d and 14%, respectively, which was consistent with Noblet et al. (1993) who used a method similar to the present study to evaluate AHP and TEF. Higher TEF is attributed to increased energy cost of digestion and excretion of undigested material caused by higher wheat bran inclusion (Le Goff et al., 2002). In studies of swine energy metabolism, TEF was further subdivided into TEFlt and TEFst. TEFlt is mainly derived from metabolic and fermentation processes, and TEFst is derived from processes such as ingestion, digestion, and absorption (Labussière et al., 2015; van Milgen et al., 1997). The average TEFlt and TEFst were 1.61 and 3.13 MJ/d, respectively, when inclusion level of wheat bran was within 0% to 39.0%. Therefore, increased TEF in sows fed WB50 can be mainly attributed to TEFlt (61%) rather than TEFst (39%). Similar results were found in the studies of Ramonet et al. (2000) and Le Goff et al. (2002), but they found TEFlt in sows fed a high-fiber diet was significantly higher than in those fed a low-fiber diet. These results suggested that dietary fiber promoted TEFlt of sows. It is widely known that fermentation of fiber is a process related to gut microbiota. Consequently, the fiber-degrading bacteria (Prevotellaceae_UCG-003 and norank_f__Paludibacteraceae) enriched in WB50 might contribute to an elevated TEFlt, which leads to an increase in TEF.
4.6. Effects of inclusion level on energy content and nutrient digestibility of wheat bran
ATTD of CP and GE in wheat bran were close among treatment groups, and their average values were 74.2% and 68.0%, respectively. These values were similar to those reported in previous work conducted with gestating sows (Dong et al., 2020; Le Gall et al., 2009; Wang et al., 2019). Consistent with previous studies (Huang et al., 2013; Le Gall et al., 2009), in the current research, ATTD of ADF and NDF peaked at an inclusion rate of wheat bran within 29.2% to 39.0%. The high inclusion level might promote the adaptation of sows to diets including wheat bran, and the digestibility of ADF and NDF in wheat bran was improved when inclusion level increased from 9.8% to 29.2%. These processes include increasing gut microbiota with fiber-degrading properties, elevating activities of fiber-degrading enzymes, and increasing secretion of digestive juices (Castillo et al., 2007; Edwards, 1993; Johnson, 1988). By contrast, as inclusion rate exceeded 39%, there was a negative relationship between ATTD of dietary fiber in wheat bran and dietary concentration of wheat bran. This observation suggests that gestating sows have a limited capacity to degrade fiber in wheat bran. As a consequence, ME of wheat bran tended to have a quadratic relationship with increased inclusion rate. Average DE and ME of wheat bran in this study were 12.95 MJ/kg DM and 12.59 MJ/kg DM, respectively, and within the range of values reported by Le Goff et al. (2002) and Wang et al. (2019). Consistent with the study by Le Goff et al. (2002), the average ME:DE ratio of wheat bran in this study was 97.3%. The average NE of wheat bran was 9.59 MJ/kg DM and within the range reported by Le Goff et al. (2002) and Wang et al. (2019). Taking into account the differences in dietary wheat bran and nutritional composition, the NE:ME ratio for wheat bran in this study was 76.0%, and was slightly lower than values (78.5% and 82.8%) reported by Wang et al. (2019) and Le Goff et al. (2002). Different from the similar DE and ME estimates of wheat bran among different treatment groups (except for WB10), NE of wheat ban changed quadratically with increased inclusion level. A similar trend was observed in NE:ME ratio. The quadratic regression equation between NE or NE:ME ratio and inclusion level of wheat bran showed that the inclusion level with the greatest NE and NE:ME ratio of wheat bran was 35.3% and 39.5%, respectively. Based on a similar ME of wheat bran among treatments, NE:ME ratio was determined mainly by AHP and TEF (Labussière et al., 2015). As shown in this study, wheat bran reduced the physical activity of sows and led to an increase in NE:ME ratio. In contrast, higher dietary fiber from wheat bran caused a higher TEF (Le Goff et al., 2002; Ramonet et al., 2000) and decreased NE:ME ratio. Conserved energy from the depressed AHP of gestating sows only partially compensated for increased TEF in the present experiment. ME of wheat bran was similar among groups (except for WB10) due to the sows' adaptation to wheat bran fiber, but effects of inclusion rate on AHP and TEF caused NE or NE:ME ratio of wheat bran to change quadratically as dietary wheat bran increased.
5. Conclusions
In conclusion, high dietary concentration of wheat bran promoted growth of fiber-degrading bacteria in sows, which improved energy utilization of fiber resulting in similar ME of wheat bran among diets. Fermentation by bacteria Prevotellaceae_UCG-003 and norank_f__Paludibacteraceae might stimulate an increase in TEF. Wheat bran decreased sows' energy expenditure for physical activity. Consequently, sows utilized energy most efficiently at a level of 35.3% of wheat bran in the diet.
Author contributions
Song Xu: Conceptualization, Visualization, Methodology, Investigation, Writing-original draft, and Formal analysis; Zirou Yu: Data curation, Investigation; Zhongliang Li: Data curation, Investigation; Zijie Wang: Validation; Chenyu Shi: Validation; Jian Li: Data curation, Resources; Fenglai Wang: Supervision, Writing-review & editing, Project administration; Hu Liu: Supervision, Writing-review & editing, Project administration.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
This work was supported by the National Key Research and Development Program of China, China (2021YFD1300202), Bureau of Animal husbandry of Ministry of Agriculture, PRC (16190294).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Adeola O. 2nd ed. CRC Press; Washington, DC: 2001. Digestion and balance techniques in pigs. [Google Scholar]
- AOAC . 18th ed. AOAC Int; Arlington, VA: 2007. Official methods of analysis. [Google Scholar]
- Brouwer E. Academic Press; London: 1965. Report of sub-committee on constants and factors. [Google Scholar]
- Castillo M., Martin-Orue S.M., Anguita M., Perez J.F., Gasa J. Adaptation of gut microbiota to corn physical structure and different types of dietary fibre. Livest Sci. 2007;109(1–3):149–152. [Google Scholar]
- Chasse E., Guay F., Knudsen K.E.B., Zijlstra R.T., Letourneau-Montminy M.P. Toward precise nutrient value of feed in growing pigs: effect of meal size, frequency and dietary fibre on nutrient utilisation. Animals. 2021;11(9):2076–2085. doi: 10.3390/ani11092598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Chen D., Tian G., Zheng P., Mao X., Yu J., et al. Soluble fiber and insoluble fiber regulate colonic microbiota and barrier function in a piglet model. BioMed Res Int. 2019;2019 doi: 10.1155/2019/7809171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De W., Ai-Rong Z., Yan L., Sheng-Yu X., Hai-Yan G., Yong Z. Effect of feeding allowance level on embryonic survival, IGF-1, insulin, GH, leptin and progesterone secretion in early pregnancy gilts. J Anim Physiol Anim Nutr. 2009;93(5):577–585. doi: 10.1111/j.1439-0396.2008.00844.x. [DOI] [PubMed] [Google Scholar]
- Dong W., Zhang G., Li Z., Liu L., Zhang S., Li D. Effects of different crude protein and dietary fiber Levels on the comparative energy and nutrient utilization in sows and growing pigs. Animals (Basel) 2020;10(3):495–509. doi: 10.3390/ani10030495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes J., Dewhirst F.E., Tanner A.C.R., Wade W.G. Description of Alloprevotella rava gen. nov., sp nov., isolated from the human oral cavity, and reclassification of Prevotella tannerae Moore et al. 1994 as Alloprevotella tannerae gen. nov., comb. nov. Int J Syst Evol Microbiol. 2013;63(4):1214–1218. doi: 10.1099/ijs.0.041376-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyksma S., Jansen L., Gallert C. Syntrophic acetate oxidation replaces acetoclastic methanogenesis during thermophilic digestion of biowaste. Microbiome. 2020;8(1):105–119. doi: 10.1186/s40168-020-00862-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards C. Interactions between nutrition and the intestinal microflora. Proc Nutr Soc. 1993;52(2):375–382. doi: 10.1079/pns19930073. [DOI] [PubMed] [Google Scholar]
- El Kaoutari A., Armougom F., Gordon J.I., Raoult D., Henrissat B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat Rev Microbiol. 2013;11(7):497–504. doi: 10.1038/nrmicro3050. [DOI] [PubMed] [Google Scholar]
- Flint H.J., Bayer E.A., Rincon M.T., Lamed R., White B.A. Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nat Rev Microbiol. 2008;6(2):121–131. doi: 10.1038/nrmicro1817. [DOI] [PubMed] [Google Scholar]
- Gutierrez J.P., Canon J., Rico M. Comparison of 2 models for estimation of variance-components in a sample of Spanish Holstein-Friesians. J Anim Breed Genet. 1994;111(2):169–174. doi: 10.1111/j.1439-0388.1994.tb00452.x. [DOI] [PubMed] [Google Scholar]
- Hassan E.G., Awad Alkareem A.M., Moniem I.A. Effect of fermentation and particle size of wheat bran on the antinutritional factors and bread quality. Pak J Nutr. 2008;7(4):521–526. [Google Scholar]
- Holt J.P., Johnston L.J., Baidoo S.K., Shurson G.C. Effects of a high-fiber diet and frequent feeding on behavior, reproductive performance, and nutrient digestibility in gestating sows. J Anim Sci. 2006;84(4):946–955. doi: 10.2527/2006.844946x. [DOI] [PubMed] [Google Scholar]
- Hortwitz W., Latimer G. 18th ed. AOAC Int; Gaithersburg: 2007. Official methods of analysis of AOAC international. [Google Scholar]
- Huang Q., Piao X.S., Liu L., Li D.F. Effects of inclusion level on nutrient digestibility and energy content of wheat middlings and soya bean meal for growing pigs. Arch Anim Nutr. 2013;67(5):356–367. doi: 10.1080/1745039X.2013.837233. [DOI] [PubMed] [Google Scholar]
- Huang Q., Su Y.B., Li D.E., Liu L., Huang C.F., Zhu Z.P., et al. Effects of inclusion levels of wheat bran and body weight on ileal and fecal digestibility in growing pigs. Asian-Australas J Anim Sci. 2015;28(6):847–854. doi: 10.5713/ajas.14.0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyayi E.A., Adeola O. Quantification of short-chain fatty acids and energy production from hindgut fermentation in cannulated pigs fed graded levels of wheat bran. J Anim Sci. 2015;93(10):4781–4787. doi: 10.2527/jas.2015-9081. [DOI] [PubMed] [Google Scholar]
- Jaworski N.W., Laerke H.N., Knudsen K.E.B., Stein H.H. Carbohydrate composition and in vitro digestibility of dry matter and nonstarch polysaccharides in corn, sorghum, and wheat and coproducts from these grains. J Anim Sci. 2015;93(3):1103–1113. doi: 10.2527/jas.2014-8147. [DOI] [PubMed] [Google Scholar]
- Jaworski N.W., Liu D.W., Li D.F., Stein H.H. Wheat bran reduces concentrations of digestible, metabolizable, and net energy in diets fed to pigs, but energy values in wheat bran determined by the difference procedure are not different from values estimated from a linear regression procedure. J Anim Sci. 2016;94(7):3012–3021. doi: 10.2527/jas.2016-0352. [DOI] [PubMed] [Google Scholar]
- Jha R., Berrocoso J.D. Review: Dietary fiber utilization and its effects on physiological functions and gut health of swine. Animal. 2015;9(9):1441–1452. doi: 10.1017/S1751731115000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y.J., Li H., Xie P.F., Li Z.H., Li H.W., Yin Y.L., et al. Stages of pregnancy and weaning influence the gut microbiota diversity and function in sows. J Appl Microbiol. 2019;127(3):867–879. doi: 10.1111/jam.14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L.R. Regulation of gastrointestinal mucosal growth. Physiol Rev. 1988;68(2):456–502. doi: 10.1152/physrev.1988.68.2.456. [DOI] [PubMed] [Google Scholar]
- Kim B.G., Lindemann M.D., Cromwell G.L., Balfagon A., Agudelo J.H. The correlation between passage rate of digesta and dry matter digestibility in various stages of swine. Livest Sci. 2007;109(1–3):81–84. [Google Scholar]
- Knudsen K.E.B. Carbohydrate and lignin contents of plant materials used in animal feeding. Anim Feed Sci Technol. 1997;67(4):319–338. [Google Scholar]
- Labussière E., Maxin G., Dubois S., van Milgen J., Bertrand G., Noblet J. Effect of feed intake on heat production and protein and fat deposition in milk-fed veal calves. Animal. 2009;3(4):557–567. doi: 10.1017/S1751731108003777. [DOI] [PubMed] [Google Scholar]
- Labussière E., Dubois S., van Milgen J., Noblet J. Wageningen Acad Publ; Wageningen: 2015. Modelling gas exchanges to partition heat production between fasting, thermic effect of feeding and physical activity in farm animals. [Google Scholar]
- Le Gall M., Warpechowski M., Jaguelin-Peyraud Y., Noblet J. Influence of dietary fibre level and pelleting on the digestibility of energy and nutrients in growing pigs and adult sows. Animal. 2009;3(3):352–359. doi: 10.1017/S1751731108003728. [DOI] [PubMed] [Google Scholar]
- Le Goff G., Noblet J. Comparative total tract digestibility of dietary energy and nutrients in growing pigs and adult sows. J Anim Sci. 2001;79(9):2418–2427. doi: 10.2527/2001.7992418x. [DOI] [PubMed] [Google Scholar]
- Le Goff G., Le Groumellec L., van Milgen J., Dubois S., Noblet J. Digestibility and metabolic utilisation of dietary energy in adult sows: influence of addition and origin of dietary fibre. Br J Nutr. 2002;87(4):325–335. doi: 10.1079/BJNBJN2001528. [DOI] [PubMed] [Google Scholar]
- Li Z., Li Y., Lv Z., Liu H., Zhao J., Noblet J., et al. Net energy of corn, soybean meal and rapeseed meal in growing pigs. J Anim Sci Biotechnol. 2017;8(1):44–54. doi: 10.1186/s40104-017-0169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.A., Lan Y.F., Zhang S., Wang X.L. Comparative analysis of gut microbiota between healthy and diarrheic horses. Front Vet Sci. 2022;9 doi: 10.3389/fvets.2022.882423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu Z., Huang B., Li Z., Wang Z., Chen Y., Zhang S., et al. Net energy of oat bran, wheat bran, and palm kernel expellers fed to growing pigs using indirect calorimetry. Anim Sci J. 2019;90(1):98–107. doi: 10.1111/asj.13124. [DOI] [PubMed] [Google Scholar]
- Lyu Z., Wang L., Wang J., Wang Z., Zhang S., Wang J., et al. Oat bran and wheat bran impact net energy by shaping microbial communities and fermentation products in pigs fed diets with or without xylanase. J Anim Sci Biotechnol. 2020;11(1):99–115. doi: 10.1186/s40104-020-00505-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFabe D.F., Cain D.P., Rodriguez-Capote K., Franklin A.E., Hoffman J.E., Boon F., et al. Neurobiological effects of intraventricular propionic acid in rats: possible role of short chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders. Behav Brain Res. 2007;176(1):149–169. doi: 10.1016/j.bbr.2006.07.025. [DOI] [PubMed] [Google Scholar]
- Montagne L., Pluske J.R., Hampson D.J. A review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals. Anim Feed Sci Technol. 2003;108(1–4):95–117. [Google Scholar]
- Moter V., Stein H.H. Effect of feed intake on endogenous losses and amino acid and energy digestibility by growing pigs. J Anim Sci. 2004;82(12):3518–3525. doi: 10.2527/2004.82123518x. [DOI] [PubMed] [Google Scholar]
- Niu Q., Li P., Hao S., Kim S., Du T., Hua J., et al. Characteristics of gut microbiota in sows and their relationship with apparent nutrient digestibility. Int J Mol Sci. 2019;20(4):870–882. doi: 10.3390/ijms20040870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noblet J., Shi X.S., Dubois S. Metabolic utilization of dietary energy and nutrients for maintenance energy-requirements in sows - basis for a net energy system. Br J Nutr. 1993;70(2):407–419. doi: 10.1079/bjn19930135. [DOI] [PubMed] [Google Scholar]
- Noblet J., Fortune H., Shi X.S., Dubois S. Prediction of net energy value of feeds for growing pigs. J Anim Sci. 1994;72(2):344–354. doi: 10.2527/1994.722344x. [DOI] [PubMed] [Google Scholar]
- NRC . 11th rev. National Academic Press; Washington, DC: 2012. Nutrient requirements of swine. [Google Scholar]
- Okeke B.C., Lu J. Characterization of a defined cellulolytic and xylanolytic bacterial consortium for bioprocessing of cellulose and hemicelluloses. Appl Biochem Biotechnol. 2011;163(7):869–881. doi: 10.1007/s12010-010-9091-0. [DOI] [PubMed] [Google Scholar]
- Prosky L., Asp N.G., Schweizer T.F., Devries J.W., Furda I. Determination of insoluble and soluble in foods and food-products-collaborative study. J AOAC Int. 1992;75(2):360–367. [PubMed] [Google Scholar]
- Ramonet Y., Meunier-Salaun M.C., Dourmad J.Y. High-fiber diets in pregnant sows: digestive utilization and effects on the behavior of the animals. J Anim Sci. 1999;77(3):591–599. doi: 10.2527/1999.773591x. [DOI] [PubMed] [Google Scholar]
- Ramonet Y., Robert S., Aumaitre A., Dourmad J.Y., Meunier-Salaun M.C. Influence of the nature of dietary fibre on digestive utilization, some metabolite and hormone profiles and the behaviour of pregnant sows. Anim Sci. 2000;70(11):275–286. [Google Scholar]
- Rijnen M., Verstegen M.W.A., Heetkamp M.J.W., Haaksma J., Schrama J.W. Effects of dietary fermentable carbohydrates on behavior and heat production in group-housed sows. J Anim Sci. 2003;81(1):182–190. doi: 10.2527/2003.811182x. [DOI] [PubMed] [Google Scholar]
- Serena A., Jorgensen H., Knudsen K.E.B. Digestion of carbohydrates and utilization of energy in sows fed diets with contrasting levels and physicochemical properties of dietary fiber. J Anim Sci. 2008;86(9):2208–2216. doi: 10.2527/jas.2006-060. [DOI] [PubMed] [Google Scholar]
- Shao Y., Zhou J., Xiong X., Zou L., Kong X., Tan B., et al. Differences in gut microbial and serum biochemical indices between sows with different productive capacities during perinatal period. Front Microbiol. 2020;10(11):3047–3058. doi: 10.3389/fmicb.2019.03047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi B., He W., Su G., Xu X., Shan A. The effect of increasing neutral detergent fiber level through different fiber feed ingredients throughout the gestation of sows. Animals (Basel) 2021;11(2):415–430. doi: 10.3390/ani11020415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenburg E.D., Zheng H.J., Joglekar P., Higginbottom S.K., Firbank S.J., Bolam D.N., et al. Specificity of polysaccharide use in intestinal bacteroides species determines diet-induced microbiota alterations. Cell. 2010;141(7):1241–1252. doi: 10.1016/j.cell.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H.Q., Tan C.Q., Wei H.K., Zou Y., Long G., Ao J.T., et al. Effects of different amounts of konjac flour inclusion in gestation diets on physio-chemical properties of diets, postprandial satiety in pregnant sows, lactation feed intake of sows and piglet performance. Anim Reprod Sci. 2015;152(9):55–64. doi: 10.1016/j.anireprosci.2014.11.003. [DOI] [PubMed] [Google Scholar]
- Thiex N.J., Anderson S., Gildemeister B. Crude fat, diethyl ether extraction, in feed, cereal grain, and forage (Randall/Soxtec/submersion method): collaborative study. J AOAC Int. 2003;86(5):888–898. [PubMed] [Google Scholar]
- van Milgen J., Noblet J., Dubois S., Bernier J.F. Dynamic aspects of oxygen consumption and carbon dioxide production in swine. Br J Nutr. 1997;78(3):397–410. doi: 10.1079/bjn19970159. [DOI] [PubMed] [Google Scholar]
- van Soest P.J., Robertson J.B., Lewis B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. 1991;74(10):3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- Villamide M.J. Methods of energy evaluation of feed ingredients for rabbits and their accuracy. Anim Feed Sci Technol. 1996;57(3):211–223. [Google Scholar]
- Wang Z.Y., Chen Y.F., Ding J., Liu H., Lyu Z.Q., Dong W.X., et al. Net energy content of five fiber-rich ingredients fed to pregnant sows. Anim Sci J. 2019;90(8):939–947. doi: 10.1111/asj.13211. [DOI] [PubMed] [Google Scholar]
- Wang Z.J., Wang W.H., Xu S., Ding J., Zeng X.F., Liu H., et al. Diets enriched with finely ground wheat bran alter digesta passage rate and composition of the gut microbiome in sows. Anim Nutr. 2023;12(9):32–41. doi: 10.1016/j.aninu.2022.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters J.L., Ley R.E. The human gut bacteria Christensenellaceae are widespread, heritable, and associated with health. BMC Biol. 2019;17:83. doi: 10.1186/s12915-019-0699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilfart A., Montagne L., Simmins H., Noblet J., van Milgen J. Digesta transit in different segments of the gastrointestinal tract of pigs as affected by insoluble fibre supplied by wheat bran. Br J Nutr. 2007;98(1):54–62. doi: 10.1017/S0007114507682981. [DOI] [PubMed] [Google Scholar]
- Woodworth J.C., Goodband R.D., Dritz S.S., Tokach M.D., DeRouchey J.M., Swanson A.J., et al. Effects of increased lysine and energy feeding duration prior to parturition on sow and litter performance, piglet survival, and colostrum quality. J Anim Sci. 2020;98(5):1–10. doi: 10.1093/jas/skaa105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M.G., Tokach M.D., Noblet J., Aherne F.X., Dritz S.S., Goodband R.D., et al. Influence of Carnichrome on the energy balance of gestating sows. J Anim Sci. 2004;82(7):2013–2022. doi: 10.2527/2004.8272013x. [DOI] [PubMed] [Google Scholar]
- Yu M., Gao T., Liu Z., Diao X. Effects of dietary supplementation with high fiber (Stevia Residue) on the fecal flora of pregnant sows. Animals (Basel) 2020;10(12):2247–2266. doi: 10.3390/ani10122247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebrowska T., Low A.G. The influence of diets based on whole wheat, wheat flour and wheat bran on exocrine pancreatic secretion in pigs. J Nutr. 1987;117(7):1212–1216. doi: 10.1093/jn/117.7.1212. [DOI] [PubMed] [Google Scholar]
- Zhang G.F., Liu D.W., Wang F.L., Li D.F. Estimation of the net energy requirements for maintenance in growing and finishing pigs. J Anim Sci. 2014;92(7):2987–2995. doi: 10.2527/jas.2013-7002. [DOI] [PubMed] [Google Scholar]
- Zhuo Y., Feng B., Xuan Y., Che L., Fang Z., Lin Y., et al. Inclusion of purified dietary fiber during gestation improved the reproductive performance of sows. J Anim Sci Biotechnol. 2020;11(1):47–64. doi: 10.1186/s40104-020-00450-5. [DOI] [PMC free article] [PubMed] [Google Scholar]