Abstract
Introduction
Bladder reconstruction is a huge challenge in the field of urology. In recent years, perfusion methods have brought promising results in the field of tissue engineering. We prepared bladder decellularized scaffolds by improved perfusion, which may be suitable for bladder reconstruction.
Methods
We prepared decellularized scaffolds of rat bladder by perfusion of SDS (0.5% sodium dodecyl sulfate), SDS-SDC (0.5% sodium dodecyl sulfate +0.5% sodium deoxycholate). Histological characteristics of bladder decellularized scaffolds were assessed by Hematoxylin and eosin, Masson, and DAPI staining. Moreover, we also prepared a murine bladder transplantation model to evaluate the regenerative potential of scaffolds.
Results
Hematoxylin and eosin, Masson, and DAPI staining indicated almost no cellular component residues in the SDS-SDC group. Histological analysis (hematoxylin and eosin staining, Masson staining), CD31 and F4/80 staining analysis, one month after implantation, revealed that the decellularized scaffolds had regenerative characteristics, and the SDS-SDC scaffold had better regenerative properties than the SDS scaffold.
Conclusions
We successfully prepared the decellularized scaffold for the rat bladder by perfusion. Our results showed that the SDS-SDC scaffold had better decellularization efficiency and reconstruction ability than the SDS scaffold, which provides a new perspective on bladder reconstruction materials.
Keywords: Urinary bladder, Bladder acellular matrix, Bladder regeneration, Tissue engineering
1. Introduction
Various conditions including bladder exstrophy and chronic inflammation due to injury may lead to impaired bladder function and eventually, bladder reconstruction [1]. Currently, bladder reconstruction remains one of the significant challenges in the field of urology. Although enterocystoplasty has been applied for bladder reconstruction for decades [2], it is associated with complications such as urinary tract infections and urinary incontinence [3], which seriously affect patients' quality of life. Therefore, some new methods are also needed to circumvent these adverse effects.
The bladder has been reported to be reconstructed using the stomach [4] and intestinal tissues [5]. However, bladder reconstruction using gastric and intestinal tissue is associated with unsatisfactory complications such as stones, metabolic imbalances, and absorption defects [6,7]. Synthetic materials such as polycaprolactone [8,9], polylactides [10], and poly-lactic-co-glycolic acid co-polymers [11] have been used for bladder reconstruction. Similarly, synthetic materials have been reported to cause stones, graft contracture, urinary tract infections, and rejection reactions [12]. A previous study found that the extracellular matrix (ECM) is highly conserved across species [13] and can influence cell adhesion, proliferation, and migration [14]. Meanwhile, it is also biocompatible and degrades over time when implanted [15]. Consequently, the extracellular matrix may be a promising material for bladder reconstruction.
Currently, there are limited studies on bladder decellularization via perfusion. Decellularization by perfusion can shorten the distance of decellularization reagents from deep tissue, reduce the processing time of reagents, and better preserve the three-dimensional structure of the whole organ [16,17]. Previous reports demonstrated that perfusion methods can prepare decellularized scaffolds with in vivo reconstruction abilities [18,19], suggesting that perfusion methods have broad applicability and may also be used for bladders. Moreover, the in vivo reconstruction effect of scaffolds is also an important indicator. Although previous studies [[20], [21], [22]] attempted to construct bladder scaffolds, the in vivo reconstructive effects of scaffolds have not been evaluated. SDS (Sodium dodecyl sulfate) is a widely used decellularization reagent. Nevertheless, excessive usage of SDS can cause collagen to become denaturized, whereas SDC (Sodium deoxycholate) can preserve the extracellular matrix structure. Meanwhile, it has been shown that combining different detergents for decellularization can achieve effective decellularization [23]. Therefore, we prepared the bladder scaffold for the first time using SDS-SDC perfusion and evaluated its effectiveness in vivo. Our results showed that bladder scaffolds prepared by perfusion of SDS-SDC had less residual cellular components, caused less immune rejection in mice, and might be used for bladder reconstruction.
2. Methods
2.1. Specimen procurement
Twenty male Kunming mice and thirty-five male SD rats that were 8 weeks old were purchased from the Laboratory Animal Center at Chongqing Medical University. All procedures were performed according to the National Institute of Health (NIH) Guide for the Care and Use of Laboratory Animals.
2.2. Rat bladder decellularization
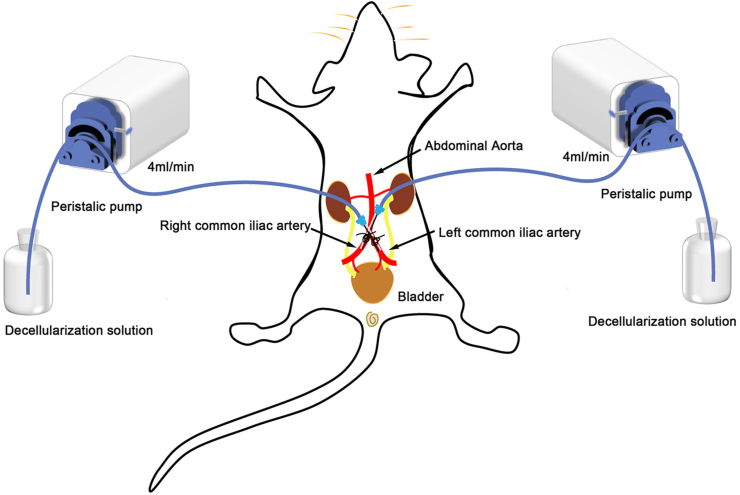
Rats were euthanized by CO2 inhalation followed by cervical dislocation and blood was removed by apical perfusion with 0.01 M PBS. All steps of decellularization were performed on an ultraclean table at room temperature. The rat bladder is decellularized by the following steps (perfusion rate of 4 ml/min for all steps) (Table 1): (a) Two syringe needles (22G, with the tips removed in advance) were inserted into each of the rights and left common iliac arteries and tied with 5.0 surgical thread (Fig. 1). (b) Infusion of deionized water. (c) Infusion of 0.5% SDS (Solarbio, China) or 0.5% SDS+0.5% SDC (Solarbio, China). (d) Infusion of 0.01 M PBS. Finally, the bladder matrix was disinfected with 70% ethanol and washed with 0.01 M PBS for 72 h at 4 °C. The solution was refreshed every day. Lastly, the decellularized bladders were kept at 4 °C in PBS with 100 U/ml of penicillin and 100 U/ml of streptomycin.
Table 1.
Rat bladder decellularization protocol.
| SDS scaffold | SDS-SDC scaffold |
|---|---|
| (a) 0.01 M PBS for 15 min | (a) 0.01 M PBS for 15 min |
| (b) Deionized water for 1 h | (b) Deionized water for 1 h |
| (c) 0.5%SDS for 4 h | (c) 0.5%SDS+0.5%SDC for 4 h |
| (d) 0.01 M PBS for 3 h | (d) 0.01 M PBS for 3 h |
Fig. 1.
Schematic diagram of a rat decellularized bladder prepared by perfusion.
The left and right peristalic pumps each perfuse the same reagent at the same rate.
2.3. Hematoxylin and Eosin(H&E) and Masson staining
After rats and Kunming mice were euthanized by CO2 inhalation followed by cervical dislocation, fresh, decellularized and transplanted tissue samples were removed and fixed in 4% paraformaldehyde in 0.1 M PBS overnight and dehydrated in 30% sucrose-PBS until the tissue sank to the bottom. Bladder tissue was then frozen and sliced (10 μm thickness). H&E staining and Masson staining were performed according to the reagent instructions (Solarbio, China). After Masson staining, we randomly selected five fields (200X) of vision in each section and the muscle of areas were analyzed by Image-pro Plus 6.0.
2.4. DNA analysis
The effectiveness of nuclei removal in the decellularized bladder matrix compared against native samples was evaluated by 4′-6-diamidino-2-phenylindole (DAPI) staining (Solarbio, China). We randomly selected five fields (100X) of vision in each section and the number of nuclei counted by Image-pro Plus 6.0.
The native bladder (n = 5) and decellularized tissues (each group = 5) were dried on filter paper and weighed [24,25]. Residual DNA was also evaluated by tissue DNA extraction kit (Omega, USA). DNA concentration was determined using a spectrophotometer at 260 nm. DNA content was expressed as a ratio between the weight of DNA per tissue wet weight (ng/mg).
2.5. Immunofluorescence staining and analysis
Fresh and transplanted bladder tissue sections were washed with 0.01 M PBS and blocked with 4% horse serum for 1 h. Sections were then incubated overnight at 4 °C with primary antibodies specific for CD31 (AiFang biological, China) or F4/80 (AiFang biological, China). Next, sections were incubated for 1 h at room temperature with fluorescein Cy3-labeled anti-rabbit IgG. Nuclei were labeled with DAPI (1 mg/ml) for 5 min at room temperature. Sections were mounted in glycerol and visualized by a fluorescence microscope (Leica, Germany). After CD31 staining, the number of vessels was counted [26]. After F4/80 staining, we calculated the area of the positive region using ImageJ. Specimens were visualized using a Leica DM3000 fluorescence microscope. Representative images were acquired under 400x imaging by using LAS X software. We randomly selected five fields of view to calculate the number of vessels and F4/80-positive areas in each field of view.
2.6. In vivo implantation of decellularized bladder
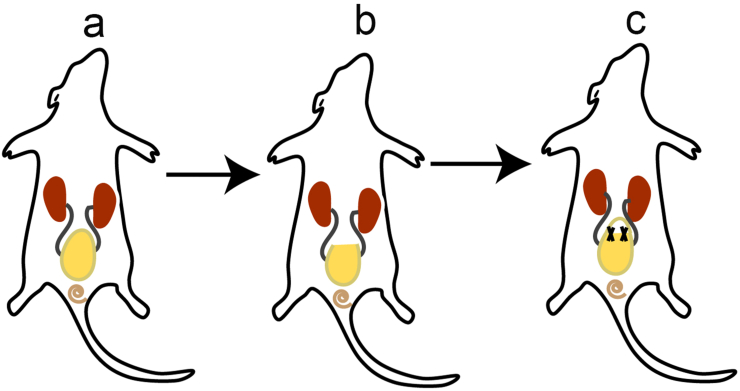
Rat bladder decellularized tissue was transplanted into the bladders of mice and removed one month later. The detailed steps of transplantation are as follows. First, mice were anesthetized with sodium pentobarbital and placed in the supine position on the operation board. Second, the hair was shaved and disinfected with iodophor at a distance of 1 cm above the urethral orifice. Then, the abdomen was opened along the midline with a longitudinal opening of 1 cm to expose the bladder (Fig. 2a). Finally, the bladder tip of the mice was removed (Fig. 2b), and the decellularized bladder tissue was sutured to the remaining bladder with an absorbable suture (6.0) (Fig. 2c). Meanwhile, the control group was sutured immediately after the abdominal incision.
Fig. 2.
Murine bladder transplantation model.
(a) Expose the mice's bladder. (b) Remove the bladder's top. (c) Integrate the bladder matrix into the bladder wall.
2.7. Statistical analysis
Histograms were created by using GraphPad Prism 8 software (San Diego, CA, USA). Results of DAPI staining, Masson staining, CD31 staining, and F4/80-positive area content (Control, SDS, SDS-SDC) are presented as the mean ± S.E.M. One-way ANOVA followed with Tukey's multiple comparisons test was used to analyze the differences between each group. A P value of <0.05 was considered significant and is indicated in the graphs (∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001).
3. Results
3.1. SDS-SDC treatment had a higher decellularization efficiency than SDS treatment
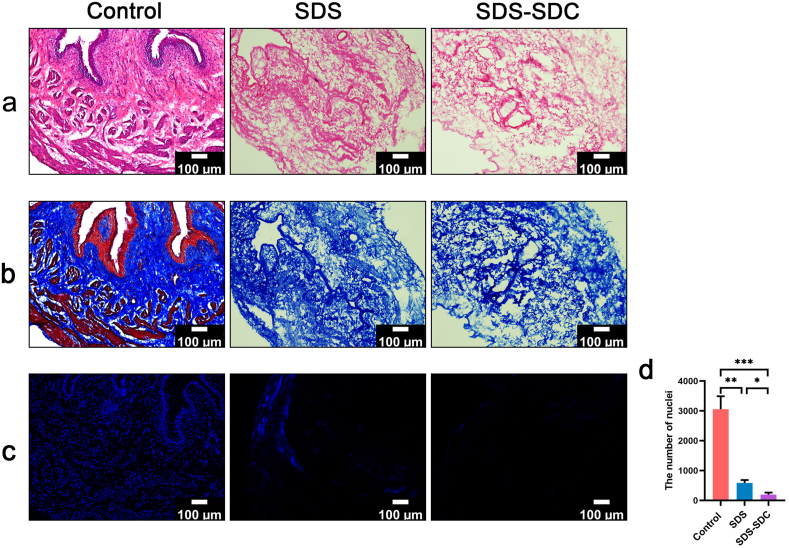
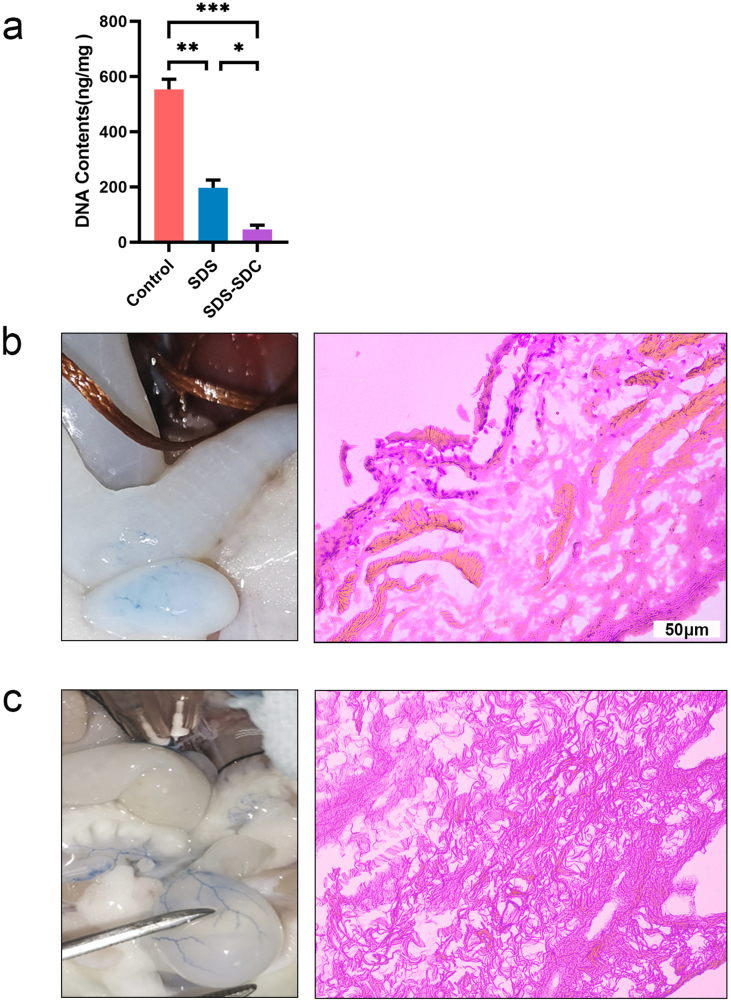
We stained the remaining cellular parts with H&E, Masson, and DAPI to evaluate the decellularization efficiency of SDS and SDS-SDC. H&E staining showed no cellular remnants in decellularized bladder tissue compared to natural bladder tissue (Fig. 3a). Masson staining also showed no muscle fibers in either the SDS group or the SDS-SDC group compared to natural bladder tissue (Fig. 3b). DAPI staining showed that the nuclei were removed mainly from the SDS group and the SDS-SDC group compared with the control group, while more nuclei remained in the SDS group (Fig. 3c and d). At the same time, DNA quantification also demonstrated that the SDS-SDC group had the least amount of residual DNA (Supplementary Fig. 2a).
Fig. 3.
Histological characteristics of native (Control) and decellularized rat bladders with SDS and SDS-SDC.
Sections were stained with H&E (a), Masson (b), and DAPI (c). (d) Statistical results of the number of nuclei in different group. 0.5% sodium dodecyl sulfate group (SDS), 0.5% SDS+0.5% sodium deoxycholate group (SDS-SDC). (n = 5) (∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001).
3.2. SDS and SDS-SDC scaffolds could promote bladder regeneration
Muscle tissue plays a vital role in the expansion and contraction of the bladder. Masson staining can distinguish collagen from muscle tissue, where blue regions show collagen extracellular matrix and red regions show muscle tissue. We used Masson staining to assess muscle regeneration in decellularized bladder tissue after transplantation.
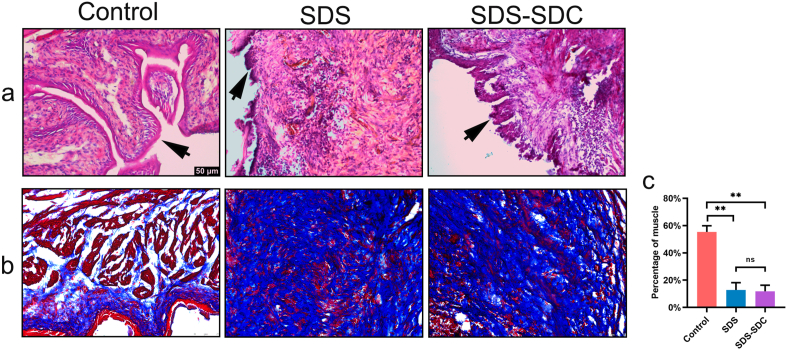
We transplanted decellularized bladder tissue into the bladders of mice and removed them for examination after one month. By H&E staining, we found regenerated uroepithelium in the SDS group and SDS-SDC group (Fig. 4a). Furthermore, we also found regenerative muscle fibers in the SDS group and the SDS-SDC group (Fig. 4b and c).
Fig. 4.
Variation of the SDS group, the SDS-SDC group, and the control group one month after in vivo.
Sections were used for H&E (a) and Masson staining (b). (c) Statistical results of the proportion of muscle in different group. The black arrow denotes uroepithelium. (n = 5) (∗∗p < 0.01; ns, no significant).
3.3. The SDS-SDC scaffold could better promote vascular formation
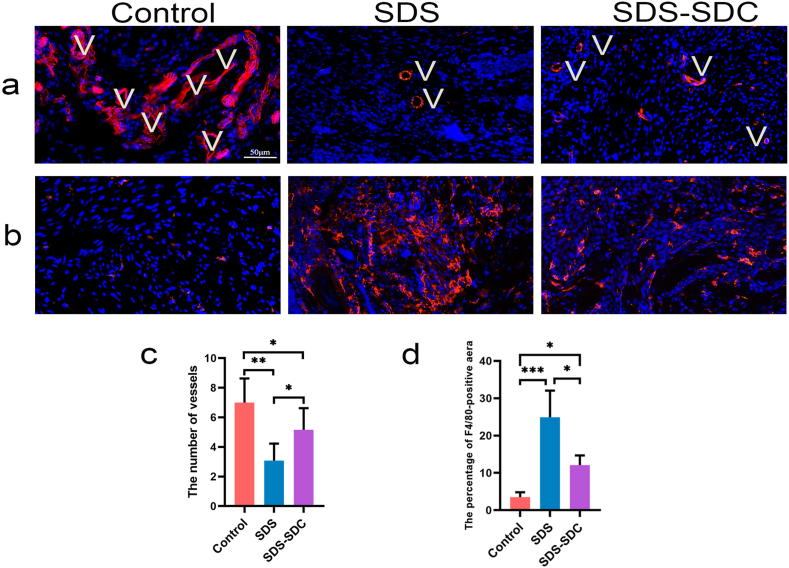
Vessels transport nutrients and excrete waste, and the number of vessels is essential for regenerating decellularized tissues. We marked the blood vessels with CD31 so we could find the blood vessels in the bladder tissue that was healing.
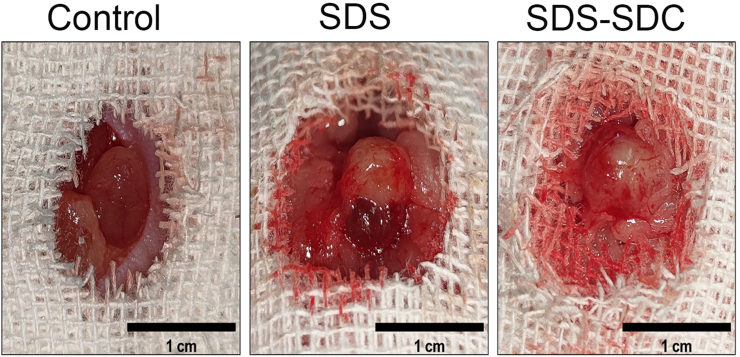
One month after surgery, we found that vessels appeared in the control, SDS and SDS-SDC groups (Fig. 5). CD31 staining also proved that vessels appeared in all groups (Fig. 6a). Moreover, the number of vessels regenerated in the SDS-SDC group was significantly greater than in the SDS group (∗p < 0.05) (Fig. 6c).
Fig. 5.
Morphology of each group one month after surgery.
Fig. 6.
One month after surgery, staining with CD31 and F4/80 of regrown bladder domes supported by different decellularized bladder matrices and the control group.
(a) CD31 staining. (b) F4/80 staining. (c) Analysis of CD31+ vessels present in the control and different scaffold groups. (d) Analysis of the F4/80-positive area in different groups. V denotes vessels. (n = 5) (∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001).
3.4. The SDS-SDC scaffold caused a reduced inflammatory response
After biomaterial implantation, the host inflammatory response is a normal response to injury and the presence of foreign objects. Macrophages are one of the main types of cells that control the immune and inflammatory processes of the host that are caused by biomaterials.
By F4/80 staining, we found a large number of macrophages in the SDS group compared to the control group and relatively fewer in the SDS-SDC group than in the SDS group (∗p < 0.05) (Fig. 6b and d).
4. Discussion
In this article, we successfully prepared for the first time a decellularized scaffold of the rat bladder by vascular perfusion of SDS-SDC, transplanted it into mice and promoted bladder tissue regeneration. The preparation of a bladder acellular matrix by perfusion decellularization offers a promising alternate approach for bladder tissue engineering and functional organ replacement. The biomaterial used for bladder reconstruction should have similar structure and properties to the natural bladder. It should also be non-toxic and have good biocompatibility. Previous studies have shown that the composition and expression patterns of the ECM are specific to a particular anatomical location to direct or support the attachment and function of site-appropriate cell [27,28]. The natural scaffold, extracellular matrix, which we used to remove cellular components and nuclei, fits exactly the above requirements.
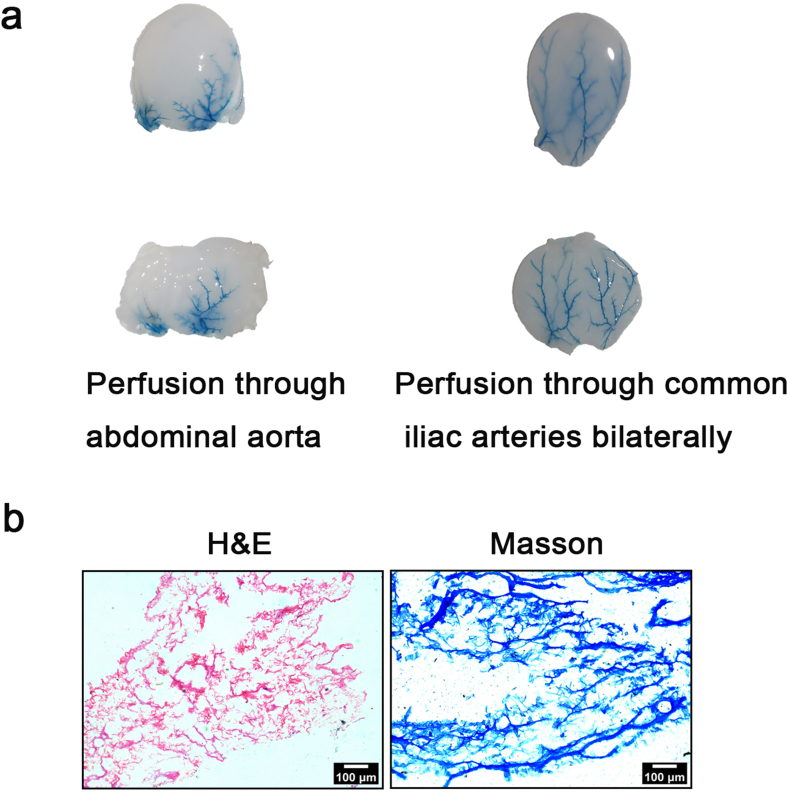
The choice of perfusion rate, time, and concentration of reagents is critical for perfusion preparation of decellularized scaffolds. In this article, we refer to previous studies to determine the concentration of SDS [29], SDC [22]. Our perfusion rate is referenced and adapted from previous study [20]. In our preliminary experiment, we found that perfusion was performed through the abdominal aorta, where one side could be inaccessible to the perfusion reagents (Supplementary Fig. 1a). Meanwhile, more nuclear components of cells remained by 4 h SDS-SDC perfusion through the abdominal aorta (Supplementary Fig. 2b). The vascular distribution is not uniform on both sides of the bladder, which may lead to uneven perfusion pressure, which in turn leads to poor entry of decellularized reagents from one side. In contrast, reagents perfused through the common iliac artery closest to the bladder side were more likely to enter the bladder (Supplementary Fig. 1a). Therefore, we chose to perform simultaneous perfusion through the common iliac arteries bilaterally. Moreover, we also found that 0.5% SDS destroyed most of the bladder structures after 6 h of perfusion (Supplementary Fig. 1b). Consequently, we use 4 h as the treatment time. After perfusion of 4 h SDS, the structure of the bladder was preserved, but a lot of nuclei remained. Conversely, our results showed that after 4 h of SDS-SDC perfusion, the structure of the bladder was preserved and almost no nuclei remained (Supplementary Fig. 2c), demonstrating that the 4 h treatment time was appropriate.
We found that rat bladder decellularized scaffolds were better prepared by using SDS-SDC. SDS-SDC scaffold have better decellularization efficiency, milder levels of inflammation and superior regenerative capacity. SDS was previously used to prepare decellularized scaffolds for the bladder [30]. However, excessive use of SDS leads to denaturation of collagen [31]. SDC can preserve the natural collagen fibril structure [32], but its decellularization efficiency is insufficient [22]. Therefore, we used SDS combined with SDC to obtain bladder decellularized scaffolds, reducing the processing time of SDS and obtaining better results.
Currently, synthetic materials used for bladder reconstruction suffer from graft contracture, stone formation, and other problems. To evaluate the reconstruction effect of the two decellularized scaffolds, we prepared a bladder reconstruction (or enlargement model) by removing the mice's apical part of the bladder. In multiple studies using different materials for bladder reconstruction, the recovery of the muscular layer was difficult [33,34]. In our experimental results, there was some muscle and uroepithelial regeneration in both scaffolds. However, the muscle tissue content regenerated by both scaffolds was much less than that of the control group. These results demonstrate that both SDS and SDS-SDC scaffolds can promote bladder tissue regeneration. This offers a new hope for bladder reconstruction. However, further efforts are needed to obtain better regeneration results.
Adequate blood supply is a crucial factor in tissue regeneration. It has been reported that tissues thicker than 0.8 mm require blood vessels to provide oxygen and nutrients to all cells [35]. Our results found the presence of neovascularization in both the SDS-SDC and SDS groups, but the number of vessels in the SDS-SDC was more significant. Additionally, we discovered fewer macrophages in the SDS-SDC group than in the SDS group. This may be because the residual cellular component within the SDS-SDC group was lower than the SDS group, causing less immune rejection and more favorable tissue regeneration. However, the specific mechanisms of tissue regeneration are complex, involving the action of many cells and cytokines and a series of steps that require more research and investigation.
Our study also has some limitations. We found that SDS-SDC was superior to SDS for the same time of decellularization, there was still a difference in the revascularization capacity between the SDS-SDC group and the control group, and further improvement is needed to improve the revascularization capacity.
5. Conclusion
We successfully prepared decellularized scaffolds of rat bladder by the perfusion method. Compared with the natural bladder, both scaffolds retained the structure of the bladder. The in vivo transplantation results also showed that both scaffolds could promote bladder tissue regeneration, but the SDS-SDC group showed better results. This study provides a new idea for bladder reconstruction materials.
Disclosure
Ethics approval
The present studies have been performed according to the Declaration of Helsinki, and the procedures have been approved by the ethics committee of The First Affiliated Hospital of Chongqing Medical University.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China to Fei Gao [NO. 82370691] and Mei Yang [NO. 81971230, 81671312]. Furthermore, it is supported by Chongqing Science and health joint project [NO. 2020GDRC007], and supported by Senior Medical Talents Program of Chongqing for Yong and Middle-aged [NO. 204216qn] and Reserve Talents Program for academic Leaders of the First Affiliated Hospital of Chongqing Medical University [NO. XKTS070] to Fei Gao. Finally, this work was also sponsored by Natural Science Foundation of Chongqing, China to Fei Gao (No. CSTB2023NSCQ-MSX0072), to Mei Yang (No. CSTB2023NSCQ- MSX0565) and to John Ogooluwa Aremu (No. CSTB2023NSCQ-BHX0192.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.reth.2023.09.005.
Contributor Information
Mei Yang, Email: yangmei503@cqmu.edu.cn.
Fei Gao, Email: gaofei@hospital.cqmu.edu.cn, gaofei667@yahoo.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
Supplementary Fig. 1.
Determination of perfusion mode and histological characteristics of prolonged perfusion of SDS.
(a) Macroscopic view of Evan's blue perfusion through the abdominal aorta or the common iliac arteries bilaterally. (b) H&E and Masson staining after perfusion of 0.5% SDS for 6 h.
Supplementary Fig. 2.
DNA quantification and two perfusion modes.
(a) DNA quantification results for different tissues. (b) Evan's blue staining and H&E staining after 4 h of SDS-SDC perfusion through the abdominal aorta. (c) Results of Evan's blue staining and H&E staining after 4 h perfusion of SDS-SDC in bilateral common iliac arteries. (n = 5) (∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001)
References
- 1.Yoo J.J., Olson J., Atala A., Kim B. Regenerative medicine strategies for treating neurogenic bladder. Int Neurourol J. 2011;15:109–119. doi: 10.5213/inj.2011.15.3.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwon T.G., Yoo J.J., Atala A. Local and systemic effects of a tissue engineered neobladder in a canine cystoplasty model. J Urol. 2008;179:2035–2041. doi: 10.1016/j.juro.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Krueger H., Noonan V.K., Trenaman L.M., Joshi P., Rivers C.S. The economic burden of traumatic spinal cord injury in Canada. Chronic Dis Inj Can. 2013;33:113–122. [PubMed] [Google Scholar]
- 4.Singla A.K. Surgery: outcomes of gastric-segment bladder reconstruction. Nat Rev Urol. 2012;9:362–363. doi: 10.1038/nrurol.2012.87. [DOI] [PubMed] [Google Scholar]
- 5.Lin H.-K., Godiwalla S.Y., Palmer B., Frimberger D., Yang Q., Madihally S.V., Fung K.-M., Kropp B.P. Understanding roles of porcine small intestinal submucosa in urinary bladder regeneration: identification of variable regenerative characteristics of small intestinal submucosa. Tissue Eng Part B. 2014;20:73–83. doi: 10.1089/ten.teb.2013.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis N.F., Mulvihill J.J.E., Mulay S., Cunnane E.M., Bolton D.M., Walsh M.T. Urinary bladder vs gastrointestinal tissue: a comparative study of their biomechanical properties for urinary tract reconstruction. Urology. 2018;113:235–240. doi: 10.1016/j.urology.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 7.Martini A., Villari D., Nicita G. Editorial comment to: ileal versus sigmoid neobladder as bladder substitute after radical cystectomy for bladder cancer: a meta-analysis. Int J Surg. 2017;37:13–14. doi: 10.1016/j.ijsu.2016.11.137. [DOI] [PubMed] [Google Scholar]
- 8.Yu D.-S., Lee C.-F., Chen H.-I., Chang S.-Y. Bladder wall grafting in rats using salt-modified and collagen-coated polycaprolactone scaffolds: preliminary report. Int J Urol. 2007;14:939–944. doi: 10.1111/j.1442-2042.2007.01871.x. [DOI] [PubMed] [Google Scholar]
- 9.Shakhssalim N., Rasouli J., Moghadasali R., Aghdas F.S., Naji M., Soleimani M. Bladder smooth muscle cells interaction and proliferation on PCL/PLLA electrospun nanofibrous scaffold. Int J Artif Organs. 2013;36:113–120. doi: 10.5301/ijao.5000175. [DOI] [PubMed] [Google Scholar]
- 10.Kajbafzadeh A.-M., Esfahani S.A., Sadeghi Z., Elmi A., Monajemzadeh M. Application of different scaffolds for bladder wall regeneration: the bladder as a natural bioreactor. Tissue Eng. 2012;18:882–887. doi: 10.1089/ten.TEA.2011.0202. [DOI] [PubMed] [Google Scholar]
- 11.Jayo M.J., Jain D., Wagner B.J., Bertram T.A. Early cellular and stromal responses in regeneration versus repair of a mammalian bladder using autologous cell and biodegradable scaffold technologies. J Urol. 2008;180:392–397. doi: 10.1016/j.juro.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 12.Elbahnasy A.M., Shalhav A., Hoenig D.M., Figenshau R., Clayman R.V. Bladder wall substitution with synthetic and non-intestinal organic materials. J Urol. 1998;159:628–637. [PubMed] [Google Scholar]
- 13.van der Rest M., Garrone R. Collagen family of proteins. FASEB J. 1991;5:2814–2823. [PubMed] [Google Scholar]
- 14.Theocharis A.D., Skandalis S.S., Gialeli C., Karamanos N.K. Extracellular matrix structure. Adv Drug Deliv Rev. 2016;97 doi: 10.1016/j.addr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Fu Q., Deng C.-l., Liu W., Cao Y.-l. Urethral replacement using epidermal cell-seeded tubular acellular bladder collagen matrix. BJU Int. 2007;99:1162–1165. doi: 10.1111/j.1464-410X.2006.06691.x. [DOI] [PubMed] [Google Scholar]
- 16.Badylak S.F., Taylor D., Uygun K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu Rev Biomed Eng. 2011;13:27–53. doi: 10.1146/annurev-bioeng-071910-124743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crapo P.M., Gilbert T.W., Badylak S.F. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233–3243. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song J.J., Guyette J.P., Gilpin S.E., Gonzalez G., Vacanti J.P., Ott H.C. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat Med. 2013;19:646–651. doi: 10.1038/nm.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sabetkish S., Kajbafzadeh A.-M., Sabetkish N., Khorramirouz R., Akbarzadeh A., Seyedian S.L., Pasalar P., Orangian S., Beigi R.S.H., Aryan Z., Akbari H., Tavangar S.M. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix liver scaffolds. J Biomed Mater Res. 2015;103:1498–1508. doi: 10.1002/jbm.a.35291. [DOI] [PubMed] [Google Scholar]
- 20.Consolo F., Brizzola S., Tremolada G., Grieco V., Riva F., Acocella F., Fiore G.B., Soncini M. A dynamic distention protocol for whole-organ bladder decellularization: histological and biomechanical characterization of the acellular matrix. J Tissue Eng Regen Med. 2016;10:E101–E112. doi: 10.1002/term.1767. [DOI] [PubMed] [Google Scholar]
- 21.Vishwakarma S.K., Sarwar S., Adil M.A.M., Khan A.A. Biofabrication of cell-laden allografts of goat urinary bladder scaffold for organ reconstruction/regeneration. Tissue Cell. 2020;67 doi: 10.1016/j.tice.2020.101443. [DOI] [PubMed] [Google Scholar]
- 22.Mayorca-Guiliani A.E., Willacy O., Madsen C.D., Rafaeva M., Elisabeth Heumüller S., Bock F., Sengle G., Koch M., Imhof T., Zaucke F., Wagener R., Sasaki T., Erler J.T., Reuten R. Decellularization and antibody staining of mouse tissues to map native extracellular matrix structures in 3D. Nat Protoc. 2019;14:3395–3425. doi: 10.1038/s41596-019-0225-8. [DOI] [PubMed] [Google Scholar]
- 23.Alshaikh A.B., Padma A.M., Dehlin M., Akouri R., Song M.J., Brännström M., Hellström M. Decellularization of the mouse ovary: comparison of different scaffold generation protocols for future ovarian bioengineering. J Ovarian Res. 2019;12:58. doi: 10.1186/s13048-019-0531-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu S., Liu Y., Bharadwaj S., Atala A., Zhang Y. Human urine-derived stem cells seeded in a modified 3D porous small intestinal submucosa scaffold for urethral tissue engineering. Biomaterials. 2011;32:1317–1326. doi: 10.1016/j.biomaterials.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Xiao S., Wang P., Zhao J., Ling Z., An Z., Fu Z., Fu W., Zhou J., Zhang X. Bladder acellular matrix prepared by a self-designed perfusion system and adipose-derived stem cells to promote bladder tissue regeneration. Front Bioeng Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.794603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao Y., He Y., Guo J.-H., Wu J.-S., Zhou Z., Zhang M., Li W., Zhou J., Xiao D.-D., Wang Z., Sun K., Zhu Y.-J., Lu M.-J. Time-dependent bladder tissue regeneration using bilayer bladder acellular matrix graft-silk fibroin scaffolds in a rat bladder augmentation model. Acta Biomater. 2015;23 doi: 10.1016/j.actbio.2015.05.032. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y., Cui C.-B., Yamauchi M., Miguez P., Roach M., Malavarca R., Costello M.J., Cardinale V., Wauthier E., Barbier C., Gerber D.A., Alvaro D., Reid L.M. Lineage restriction of human hepatic stem cells to mature fates is made efficient by tissue-specific biomatrix scaffolds. Hepatology. 2011;53:293–305. doi: 10.1002/hep.24012. [DOI] [PubMed] [Google Scholar]
- 28.Petersen T.H., Calle E.A., Zhao L., Lee E.J., Gui L., Raredon M.B., Gavrilov K., Yi T., Zhuang Z.W., Breuer C., Herzog E., Niklason L.E. Tissue-engineered lungs for in vivo implantation. Science. 2010;329:538–541. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sullivan D.C., Mirmalek-Sani S.-H., Deegan D.B., Baptista P.M., Aboushwareb T., Atala A., Yoo J.J. Decellularization methods of porcine kidneys for whole organ engineering using a high-throughput system. Biomaterials. 2012;33:7756–7764. doi: 10.1016/j.biomaterials.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 30.Bolland F., Korossis S., Wilshaw S.-P., Ingham E., Fisher J., Kearney J.N., Southgate J. Development and characterisation of a full-thickness acellular porcine bladder matrix for tissue engineering. Biomaterials. 2007;28:1061–1070. doi: 10.1016/j.biomaterials.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Hwang J., San B.H., Turner N.J., White L.J., Faulk D.M., Badylak S.F., Li Y., Yu S.M. Molecular assessment of collagen denaturation in decellularized tissues using a collagen hybridizing peptide. Acta Biomater. 2017;53:268–278. doi: 10.1016/j.actbio.2017.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou J., Fritze O., Schleicher M., Wendel H.-P., Schenke-Layland K., Harasztosi C., Hu S., Stock U.A. Impact of heart valve decellularization on 3-D ultrastructure, immunogenicity and thrombogenicity. Biomaterials. 2010;31:2549–2554. doi: 10.1016/j.biomaterials.2009.11.088. [DOI] [PubMed] [Google Scholar]
- 33.Brown A.L., Farhat W., Merguerian P.A., Wilson G.J., Khoury A.E., Woodhouse K.A. 22 week assessment of bladder acellular matrix as a bladder augmentation material in a porcine model. Biomaterials. 2002;23:2179–2190. doi: 10.1016/s0142-9612(01)00350-7. [DOI] [PubMed] [Google Scholar]
- 34.Shi C., Chen W., Chen B., Shan T., Jia W., Hou X., Li L., Ye G., Dai J. Bladder regeneration in a canine model using a bladder acellular matrix loaded with a collagen-binding bFGF. Biomater Sci. 2017;5:2427–2436. doi: 10.1039/c7bm00806f. [DOI] [PubMed] [Google Scholar]
- 35.Nomi M., Atala A., Coppi P.D., Soker S. Principals of neovascularization for tissue engineering. Mol Aspects Med. 2002;23:463–483. doi: 10.1016/s0098-2997(02)00008-0. [DOI] [PubMed] [Google Scholar]