Abstract
Background
Malakoplakia is a rare disorder 75% of the reported cases affect mainly the genitourinary tract, its occurrence in the adrenal gland is extremely rare.
Case presentation
A 65-year-old female patient presented to the emergency department for chronic abdominal pain. Radiographic and biochemical studies revealed a left adrenal incidentaloma and left adrenalectomy was performed. Histological examination showed the presence of Michaelis-Gutmann bodies, compatible with a malakoplakia of the adrenal gland.
Conclusions
Malakoplakia is a rare disorder, with non-standardized treatment, medical and surgical therapies appear to be effective in treating the condition.
Keywords: Malakoplakia, Adrenal incidentaloma, Adrenal malakoplakia, Incidental finding
Highlights
-
•
Patients with an adrenal incidentaloma should undergo clinical, biochemical and imaging tests to determine the presence/absence of symptoms and signs caused by an excess of adrenal hormones.
-
•
When the tumor is < 4 cm, the risk of adrenal cancer is less than 2%, but when the size is ≥ 6 cm, the risk increases to 25%.
-
•
Malakoplakia appears to be related to other conditions, such as Urinary Tract Infections, immunosuppression, and is thought to be the result of abnormal macrophage function.
-
•
Treatment of malakoplakia is not standardized, but both medical and surgical therapies appear to be effective in curing the condition.
1. Introduction
Malakoplakia is a rare disorder that was first described in 1902 by Michaelis and Gutmann and has been most frequently associated with immunosuppression, renal transplantation, diabetes, chronic use of systemic corticosteroids and previous E. coli infection.1 Currently less than 500 cases of this disease have been reported in the world literature in almost 75% malakoplakia has mainly affected the genitourinary tract with the bladder being the most commonly affected organ (40%), the renal parenchyma (16%), the prostate and rarely the ureter (11%),2,3 occurrence in the adrenal gland is extremely rare with less than 5 documented cases of adrenal malakoplakia in more than a century of knowledge of this disease.4
The exact etiology is unknown, but it seems to be caused by a defect in the phagocytic functions of histiocytes in response to infection with the most frequent etiological agents being E. coli or Proteus, resulting in a chronic inflammatory process.5 The lesions are characterized by the presence of large macrophages, foamy histiocytes (known as Von Hansemann cells) containing Michaelis-Gutmann bodies.6
In the literature there is no standard for diagnosis and treatment and it is variable depending on the affected area, in most cases there are only reports of cases of bladder malakoplakia,3 so due to the rarity of the presentation the treatment is focused on the different affected organs, in the case of adrenal involvement and that in this case it was presented as an incidental lesion simulating a neoplastic lesion, the initial approach is performed as an adrenal incidentaloma, the treatment and follow-up performed can pose real difficulties in its management.
2. Case presentation
A 65-year-old female patient presented to the emergency department with a history of chronic abdominal pain, vague location, mild intensity and multiple failed therapeutic interventions, accompanied by low urinary symptoms, with a history of poorly controlled Type 2 diabetes and recurrent urinary tract infections.
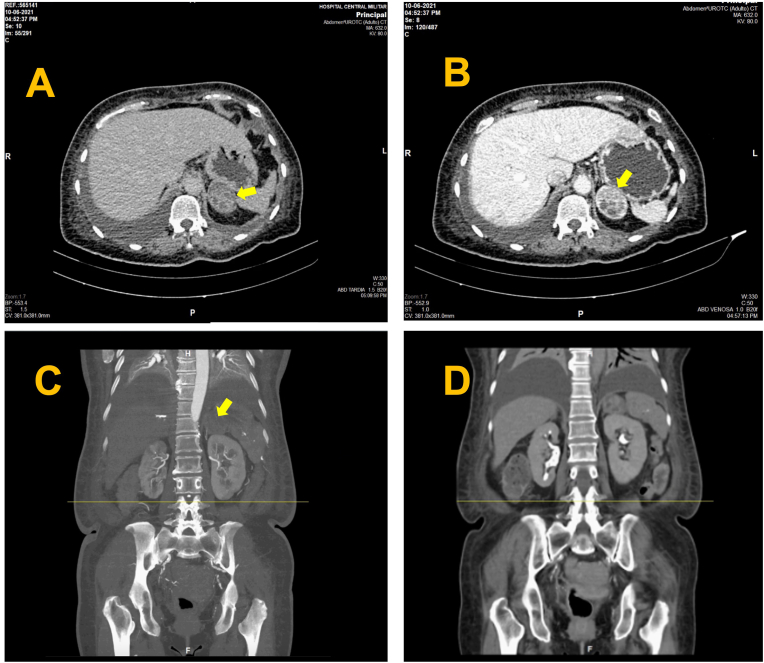
On physical examination, vital signs were within normal limits and no abnormalities were observed, except for mild tenderness to palpation in the epigastric and suprapubic area. The hemogram and serum creatinine were normal, C-Reactive Protein presented an elevation of 15,7 mg/L, the urianalysis was positive for nitrites and leukocytes and the urine culture was positive for multi-sensitive Escherichia coli, it was decided to perform an Abdominal CT Scan with Contrast that revealed enlargement of the left adrenal gland with a well-defined adrenal mass of regular contours that preserved the interface with the adjacent structures, the dimensions in axial section were 4.5x2 cm., in Non-enhanced CT (NECT) phase with heterogeneous density 21 Hounsfield unit (HU), in the Systemic venous phase it was observed with heterogeneous enhancement with a density of up to 112 HU and in the Delayed phase density of 62 HU, an absolute washout index of 55% was calculated (Fig. 1). Hospitalization was decided for analgesic management and antibiotic therapy and approach of the left adrenal Incidentaloma.
Fig. 1.
A) Axial image of late-phase contrast-enhanced computed tomography showing enlargement of the left adrenal gland with an adrenal mass of well-delimited regular contours (arrow) that preserves the interface with adjacent structures with dimensions in an axial section of 4.5 × 2.0 cm, with an average density of 62UH. B) Axial image of computed tomography with contrast in early venous portal phase is observed with heterogeneous enhancement (arrow), with a density of an average of 112UH. C) Coronal image of computed tomography with contrast in early systemic arterial phase: this lesion does not present hypervascular behavior (arrow). D) Coronal image of Delayed phase: an absolute washout rate of 55% is calculated.
Within the management of an incidental mass dexamethasone suppression test (DST) 1 mg overnight was performed with a result of 13,5 nmol/L excluding asymptomatic hypercortisolism, 24-h urine metanephrine test was performed excluding pheochromocytoma, because the patient did not present hypertension or hypokalemia determination of plasma aldosterone/renin activity ratio was not performed to exclude primary aldosteronism, sex hormone levels were determined in our patient with the following findings: estradiol 48,826 pg/mL, testosterone 39,9 ng/dL and Dehydroepiandrosterone 36 mcg/dL.
After the diagnostic approach was completed surgical resection of the adrenal mass was decided due to findings suggestive of malignancy in the CT scan: tumor size (≥4 cm), Hounsfield unit (HU) value in Non-enhanced CT phase ≥10 and an absolute contrast wash out rate <60%, robotic-assisted laparoscopic Suprarenalectomy was performed; a mass of 29 g was identified with measurements of 5 × 4 × 3 cm., spherical shape, smooth surface, brown color with areas of capsular rupture and firm consistency.
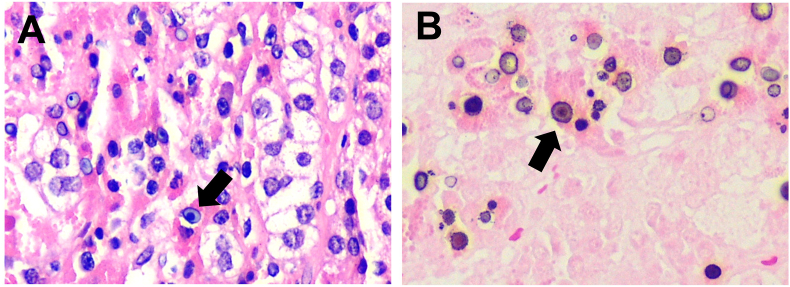
Histological examination showed adrenal gland parenchyma with the presence of multiple granulomas with a dense infiltrate of histiocytes with granular eosinophilic cytoplasm (PAS positive), in addition, abundant circular basophilic structures formed by concentric laminar calcifications (Michaelis-Gutmann bodies) were identified, which are positive to iron (Perls), calcium (Von-Kossa) and PAS, no malignant characteristics or pathogenic microorganisms were identified (Fig. 2).
Fig. 2.
A) Adrenal gland with necrosis and Michaelis-Gutmann bodies (arrow). Micrograph at 40×. Hematoxylin-Eosin. B) Abundant cytoplasmic laminated mineralized concretions Michaelis-Gutmann bodies (arrow). Photomicrograph at 40×.
Due to the extreme rarity of the lesion and the reports, it was decided to follow up with CT scan, currently with one year of follow-up after surgery the patient has a good renal function without any complication finding.
3. Discussion
Incidentally adrenal gland lesions usually do not present any symptoms when there is no abnormality in hormone secretion, in some cases there may be flank pain, abdominal discomfort, abdominal distention caused by pressure from nearby tissues, but it is impossible to differentiate malignant neoplasms based on clinical features alone.7 All patients found to have adrenal Incidentaloma should undergo clinical, biochemical, and imaging tests to determine the presence/absence of symptoms and signs caused by adrenal hormone excess and to determine suspected malignancy, hormone hypersecretion can be found in 12%–23% of patients with adrenal Incidentaloma,8 the screening test for asymptomatic hypercortisolism may be the nocturnal dexamethasone suppression test, 24-hour urine free cortisol measurement, or a midnight cortisol measurement,9 in the case of Pheochromocytoma plasma free metanephrine is the most sensitive screening test.10
The main objective of imaging studies in adrenal Incidentaloma is to identify malignant tumors and to define their treatment by surgical resection, the attenuation coefficient of non-contrast CT expressed in Hounsfield Units (HU) is the best way to distinguish between benign and malignant tumors.11 Attenuation coefficient less than or equal to 10 HU on noncontrast CT as a diagnostic criteria for benign adenoma has a specificity of 71%–79% with a sensitivity of 96%–98%.12 When the tumor is < 4 cm, the risk of adrenal cancer is less than 2%, but when the size is ≥ 6 cm, the risk increases to 25%. Therefore, surgical removal is recommended when an adrenal tumor is ≥ 4 cm,13 other findings compatible with malignancy are irregular or heterogeneous margins, as well as an attenuation coefficient of 10 HU or higher on noncontrast CT, washout of the contrast agent after 10–15 minutes is another diagnostic support as malignant lesions usually show rapid contrast enhancement but slow contrast washout, therefore a washout of less than 60% indicates likelihood of malignant lesions.14,15 Adrenalectomy should also be considered in cases of functional adrenal tumors accompanied by clinical symptoms.16
With regard to the pathologic finding in this case, malakoplakia is a rare inflammatory and granulomatous disease, first identified by Michaelis and Gutmann in 1902 and its histologic features were described by Von Hansemann in 1903,1 affecting mainly the urinary tract, especially the bladder, followed by the kidney, prostate and, rarely the ureter.5 However it can also affect other parts of the body: the tonsils, spleen, pancreas, adrenal glands, lymph nodes, lung and skin, and potentially any organ.17, 18, 19 The exact etiology is still unclear however a close relationship has been observed with recurrent urinary tract infections (especially E. coli) and immunosuppression (diabetes, kidney transplantation), malakoplakia is thought to be the result of deficient phagocytosis resulting in inadequate clearance of bacteria, which generates a granulomatous reaction caused by the accumulation of bacterial degradation products partially digested bacteria calcify and accumulate in macrophages, forming the pathognomonic Michaelis-Gutmann bodies, the sine qua non condition of malakoplakia remains the demonstration of Michaelis-Gutmann bodies on microscopic section. These inclusion bodies are invariably located within macrophages have a diameter of 5–10 μm. and a dense matrix core, these nucleus appear to be composed mainly of calcium hydroxyapatite with variable amounts of iron; they stain positively with periodic acid Schiff, von Kossa and Prussian blue staining.6
Immunodeficiency states have been reported in approximately 40% of all cases of malakoplakia however a clear causal relationship has not yet been demonstrated, this condition is a diagnostic challenge since according to the site of presentation it can be a mimic of neoplastic lesions such as prostatic malakoplakia that can be misdiagnosed as prostatic adenocarcinoma, renal malakoplakia mistaken for xanthogranulomatous pyelonephritis and carcinoma,20 skin malakoplakia that can be mistaken for granular cell myoblastoma, foreign body granuloma or any cutaneous xanthogranulomatous lesion,17 and the one shown in our case report which mimicked a neoplasm of the adrenal gland.
As for the natural history of malakoplakia progression depends in part on the organ system involved; for example, gastrointestinal malakoplakia has been found to be much more aggressive than genitourinary malakoplakia.2 There are currently no validated treatment guidelines, but both medical and surgical therapies appear to be effective in treating the condition and preventing recurrences.2,3,5
4. Conclusions
Patients with an adrenal incidentaloma should undergo clinical, biochemical and imaging examinations to determine the presence/absence of symptoms and signs caused by an excess of adrenal hormones and to determine the suspicion of malignancy, malakoplakia is a rare disorder with non-standardized treatment, both medical and surgical therapies seem to be effective in curing the condition and avoiding recurrences.
5. Informed consent
Written informed consent was obtained from the patient.
Section headings
Inflammation and Infection.
Formatting of funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Contributor Information
Orión Erenhú Rodríguez González, Email: orion.rodriguez.g@gmail.com.
Jesus Eduardo Osorio, Email: jeduardosorio@gmail.com.
Edgar Iván Bravo Castro, Email: briv_edca@hotmail.com.
References
- 1.Stamatiou K., Chelioti E., Tsavari A., et al. Renal failure caused by malakoplakia lesions of the urinary bladder. Nephro-Urol Mon. 2014;6(4) doi: 10.5812/numonthly.18522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stanton M.J., Maxted W. Malacoplakia: a study of the literature and current concepts of pathogenesis, diagnosis and treatment. J Urol. 1981;125(2):139–146. doi: 10.1016/s0022-5347(17)54940-x. [DOI] [PubMed] [Google Scholar]
- 3.Long J.P., Jr., Althausen A.F. Malacoplakia: a 25-year experience with a review of the literature. J Urol. 1989;141(6):1328–1331. doi: 10.1016/s0022-5347(17)41297-3. [DOI] [PubMed] [Google Scholar]
- 4.McClure J., Hadden D.R., Mudd D.G., Parks T.G. Adrenocortical hyperactivity with disseminated malacoplakia. J Clin Pathol. 1977;30(3):206–211. doi: 10.1136/jcp.30.3.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hina S., Hasan A., Iqbal N., Shabbir M.U., Sheikh A. Malakoplakia of the urinary bladder and unilateral ureter. Journal of the College of Physicians and Surgeons--Pakistan : JCPSP. 2019;29(6):582–584. doi: 10.29271/jcpsp.2019.06.582. [DOI] [PubMed] [Google Scholar]
- 6.Rabani S., Rabani S.H. Bladder malakoplakia simulating neoplasm in a young girl: report of a case and review of literature. Urol J. 2019;16(6):614–615. doi: 10.22037/uj.v0i0.4428. [DOI] [PubMed] [Google Scholar]
- 7.Lenert J.T., Barnett C.C., Jr., Kudelka A.P., et al. Evaluation and surgical resection of adrenal masses in patients with a history of extra-adrenal malignancy. Surgery. 2001;130(6):1060–1067. doi: 10.1067/msy.2001.118369. [DOI] [PubMed] [Google Scholar]
- 8.Caplan R.H., Strutt P.J., Wickus G.G. Subclinical hormone secretion by incidentally discovered adrenal masses. Arch Surg. 1994;129(3):291–296. doi: 10.1001/archsurg.1994.01420270067016. [DOI] [PubMed] [Google Scholar]
- 9.Zeiger M.A., Siegelman S.S., Hamrahian A.H. Medical and surgical evaluation and treatment of adrenal incidentalomas. J Clin Endocrinol Metabol. 2011;96(7):2004–2015. doi: 10.1210/jc.2011-0085. [DOI] [PubMed] [Google Scholar]
- 10.Funder J.W., Carey R.M., Mantero F., et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metabol. 2016;101(5):1889–1916. doi: 10.1210/jc.2015-4061. [DOI] [PubMed] [Google Scholar]
- 11.Vikram R., Yeung H.D., Macapinlac H.A., Iyer R.B. Utility of PET/CT in differentiating benign from malignant adrenal nodules in patients with cancer. AJR. American journal of roentgenology. 2008;191(5):1545–1551. doi: 10.2214/AJR.07.3447. [DOI] [PubMed] [Google Scholar]
- 12.Terzolo M., Stigliano A., Chiodini I., Loli P., Furlani L., Arnaldi G., Reimondo G., Pia A., Toscano V., Zini M., Borretta G., Papini E., Garofalo P., Allolio B., Dupas B., Mantero F., Tabarin A., Italian Association of Clinical Endocrinologists AME position statement on adrenal incidentaloma. Eur J Endocrinol. 2011;164(6):851–870. doi: 10.1530/EJE-10-1147. [DOI] [PubMed] [Google Scholar]
- 13.Fassnacht M., Tsagarakis S., Terzolo M., et al. European Society of Endocrinology clinical practice guidelines on the management of adrenal incidentalomas, in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2023;189(1):G1–G42. doi: 10.1093/ejendo/lvad066. [DOI] [PubMed] [Google Scholar]
- 14.Ilias I., Sahdev A., Reznek R.H., Grossman A.B., Pacak K. The optimal imaging of adrenal tumours: a comparison of different methods. Endocr Relat Cancer. 2007;14(3):587–599. doi: 10.1677/ERC-07-0045. [DOI] [PubMed] [Google Scholar]
- 15.Ansquer C., Scigliano S., Mirallié E., et al. 18F-FDG PET/CT in the characterization and surgical decision concerning adrenal masses: a prospective multicentre evaluation. Eur J Nucl Med Mol Imag. 2010;37(9):1669–1678. doi: 10.1007/s00259-010-1471-8. [DOI] [PubMed] [Google Scholar]
- 16.Lee J.M., Kim M.K., Ko S.H., Koh J.M., Kim B.Y., Kim S.W., Kim S.K., Kim H.J., Ryu O.H., Park J., Lim J.S., Kim S.Y., Shong Y.K., Yoo S.J., Korean Endocrine Society, Committee for Clinical Practice Guidelines Clinical guidelines for the management of adrenal incidentaloma. Endocrinology and metabolism (Seoul, Korea) 2017;32(2):200–218. doi: 10.3803/EnM.2017.32.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kyriakou G., Gialeli E., Vryzaki E., Koumoundourou D., Glentis A., Georgiou S. Malacoplakia of the skin: overview of a rare clinical entity. Dermatol Online J. 2019;25(6) 13030/qt3dc495vk. PMID: 31329385. [PubMed] [Google Scholar]
- 18.Meredith T., Dharan N., Killen L., et al. Colonic malakoplakia in a dual stem cell and cardiac transplant recipient: a case report and literature review. Transpl Infect Dis : an official journal of the Transplantation Society. 2021;23(2) doi: 10.1111/tid.13488. [DOI] [PubMed] [Google Scholar]
- 19.Samian C., Ghaffar S., Nandapalan V., Santosh S. Malakoplakia of the parotid gland: a case report and review of localised malakoplakia of the head and neck. Ann R Coll Surg Engl. 2019;101(5):309–312. doi: 10.1308/rcsann.2019.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller O.S., Finck F.M. Malacoplakia of the kidney: the great impersonator. J Urol. 1970;103(6):712–717. doi: 10.1016/s0022-5347(17)62032-9. [DOI] [PubMed] [Google Scholar]