Abstract
Neuroscientists agree on the value of locating the source of consciousness within the brain. Anaesthesiologists are no exception, and have their own operational definition of consciousness based on phenomenological observations during anaesthesia. The full functional correlates of consciousness are yet to be precisely identified, however rapidly evolving progress in this scientific domain has yielded several theories that attempt to model the generation of consciousness. They have received variable support from experimental observations, including those involving anaesthesia and its ability to reversibly modulate different aspects of consciousness. Aside from the interest in a better understanding of the mechanisms of consciousness, exploring the functional tenets of the phenomenological consciousness states of general anaesthesia has the potential to ultimately improve patient management. It could facilitate the design of specific monitoring devices and approaches, aiming at reliably detecting each of the possible states of consciousness during an anaesthetic procedure, including total absence of mental content (unconsciousness), and internal awareness (sensation of self and internal thoughts) with or without conscious perception of the environment (connected or disconnected consciousness, respectively). Indeed, it must be noted that unresponsiveness is not sufficient to infer absence of connectedness or even absence of consciousness. This narrative review presents the current knowledge in this field from a system-level, underlining the contribution of anaesthesia studies in supporting theories of consciousness, and proposing directions for future research.
Keywords: brain function, consciousness, general anaesthesia, mechanisms, theories
Contrary to common belief, consciousness does not simply disappear during general anaesthesia. The brain of anaesthetised patients goes through a series of different states with variable mental content and perception of the environment.1 As a consequence, the reversible alteration of consciousness by anaesthesia can serve as a basis for designing unique experimental paradigms aimed at exploring the neural correlates of different aspects of consciousness, including perception of the environment, self-awareness, and internal thoughts.2 Understanding these elements paves the way towards a better definition of the nature of human consciousness. Currently, the most prominent ideas about how anaesthesia changes consciousness postulate a disruption of the capacity of the brain to generate information, an unbinding of the integrative information processes, or both.3,4 These ideas emerged from studies exploring the spectrum of anaesthesia-induced changes in brain function, including functional or effective connectivity, topological properties of brain networks, evoked responses and sensory processing, and spatio-temporal dynamics of brain connectivity.2 Most of these studies compared the normal awake state with complete anaesthesia-induced unresponsiveness, thought to correspond to the absence of mental content. Work is underway, which not only explores the functional tenets of anaesthesia-induced unconsciousness, but other additional anaesthesia-induced states of consciousness. Identifying specific and recordable neural signatures of the internal and external awareness systems that underlie these states5 will facilitate the possibility of recognising them in individual patients during anaesthesia.
Parallel to anaesthesia research, and named the ‘hard problem’ of consciousness for neuroscientists,6 several theories have been proposed to model how consciousness is generated by brain activity. Merging knowledge of different origins into a unified framework is important, not only because theories of consciousness can guide anaesthesia research in the quest for identifying the signatures of the brain states of anaesthesia, but also because anaesthesia can help to confirm them. Consequently, this narrative review first aims at describing, from a system perspective, the most prominent theories explaining the generation of consciousness by the brain and how experiments involving anaesthesia, if any, have supported them. Secondly, we describe the current knowledge about the functional correlates of the different anaesthesia-induced brain states and their identified recordable signatures. Finally, we propose some future directions for research in this field.
The neuroscientific conceptualisation of consciousness
The word ‘consciousness’ has various meanings, depending on the perspective from which it is approached, be it philosophy, religion, or neuroscience. Indeed, the study of consciousness has only recently entered the scope of neuroscience. As a vital starting point from which to launch testable scientific investigations, it is agreed that consciousness is generated by brain activity. This means that there must be neuroanatomical and neurophysiological substrates of consciousness, or neural correlates of consciousness (NCCs). NCCs are defined as the minimal set of neuronal mechanisms sufficient for any phenomenological aspect of consciousness to emerge.7 Theoretically, NCCs can be related to a specific mental content (content-specific NCC), or denote the minimal neural mechanisms that are together necessary and sufficient for presence of mental content (full NCC).8 The different phenomenological aspects of consciousness must first be precisely defined before identifying their corresponding NCC. Given the complexity of consciousness and its phenomenology, several teams of scientists have proposed theories to model how the different aspects of consciousness are generated in the brain.9 These theories are based on different features and constructs, but are not necessarily mutually exclusive.10 Before these are described below, we first give a description of several concepts related to consciousness and its contents in addition to outlining the current knowledge of brain functioning underlying consciousness.
One way of dissecting the components of consciousness is to differentiate phenomenal consciousness and access consciousness.11 The necessity of distinguishing these two entities arose from the observation that ‘unconscious’ processing exists, and that one can almost never explain the totality of an experience at any given time. Phenomenal consciousness characterises the experiential features of consciousness as a whole.12 It can be referred to as a global feeling of an experience (‘what it is like’10), of which not all aspects can be verbalised. Access consciousness is the content of consciousness that can be verbalised or manifested in behavioural terms, such as, for example, describing a good meal at the restaurant in one's own terms, or imperfectly mimicking the behaviour of a recently met friend. It occurs through the availability of conscious information from numerous cognitive processes which mediate functions such as working memory, verbal report or motor behaviours.13 In order for conscious access to arise, perceptual contents have to be available for these functions. Accordingly, it is possible to subdivide the NCC into phenomenal-NCC, that is the minimal neural basis of the phenomenal content of an experience that is different across experiences (e.g. between the experience of red or green), and access-NCC, referring to the neural information that can be consciously reported.11 Another matter of concern for scientists is to determine the features of the ‘phenomenal self’, which can be defined as the conscious experience of being someone. This led to the notion of minimal phenomenal selfhood, corresponding in its simplest form to the conscious experience of being oneself, a distinct entity with several capabilities such as global self-control and attention, and spatio-temporal orientation of own body.14,15 If an episode of consciousness occurs during anaesthesia, elements of access consciousness, phenomenal consciousness, and phenomenal selfhood are exhibited. However, there has been more scientific interest in access consciousness and phenomenal selfhood because of their reportability, which allows them to be studied more readily.
Consciousness can also be approached through its main clinical features, namely wakefulness, awareness, and responsiveness. This is a clinically useful and operational perspective for anaesthesiologists. Wakefulness (or the degree of arousal) can be defined as the prerequisite for the ability to open eyes, spontaneously or following a stimulus.16 It is sustained by complex neural systems emerging from the brainstem, projecting to the cortex, and controlling the sleep-wake cycle.17,18 Awareness results from the synergy of all functions mediated through cortical-subcortical interactions,19,20 including cognitive and affective features, external perceptions and, for humans, phenomenal selfhood.21 Awareness can itself be divided into two components, namely internal and external awareness. Internal awareness is composed of internal mental processes (inner speech or mind wandering). External awareness (connectedness) is the conscious perception of the environment through sensory modalities.5 These concepts enable a description of the majority of physiological, pathological or drug-induced consciousness states (Table 1). For example, in disconnected consciousness, there is internal but no external awareness25 (e.g. in dreams experienced during general anaesthesia or sleep). Connected consciousness is a state of connection with the environment, in which both external and internal awareness is present.2,22 Understanding and manipulating connectedness is particularly relevant during general anaesthesia, where we attempt to prevent patients perceiving their environment.
Table 1.
Operational classification of phenomenological consciousness states related to access consciousness and phenomenological self, and the relative contribution of wakefulness, internal awareness, and external awareness to those states. Adapted with permission from Martial and colleagues.22 NREM, non-rapid eye movement sleep; REM, rapid eye moment sleep; MCS−, minimally conscious state −, with non-linguistic signs of conscious awareness; MCS+, minimally conscious state with clear evidence of expressive language functions. a Patients with partially preserved cortical activity compatible with MCS but not showing any behavioural sign of conscious awareness (although cognitive-motor dissociation may exist, and appropriate cortical responses to active paradigms be present as evidenced by functional neuroimaging). b Reportable internal consciousness experience with features that are specific to near-death experiences (out-of-body experience,…) but not associated to a critical event potentially leading to death.
| Phenomenological state |
Wakefulness |
Internal awareness |
External awareness (connectedness) |
Responsiveness to verbal command |
|
|---|---|---|---|---|---|
| Normal awake consciousness | ++++ | ++++ | ++++ | ++++ | |
| General anaesthesia | Deep | 0 | 0 | 0 | 0 |
| Disconnected consciousness (with dreams; e.g. ketamine anaesthesia) | 0 | ++ | 0 | 0 | |
| Connected consciousness episodes | ++ | ++ | ++ | ++ | |
| Sleep | NREM sleep without dream | 0 | 0 | 0 | 0 |
| NREM sleep with dreams | 0 | ++ | 0 | 0 | |
| REM sleep with dreams | 0 | +++ | 0 | 0 | |
| REM sleep with lucid dream23 | 0 | +++ | Unknown | Possible | |
| Severe brain injury | Coma without dreams/near-death experiences | 0 | 0 | 0 | 0 |
| Coma with dreams/near-death experiences | 0 | 0 | Unknown | 0 | |
| Unresponsive wakefulness syndrome | + (fluctuating) | 0 | 0 | 0 | |
| Minimally conscious state | + (fluctuating) | + | + | 0 (MCS−) or + (MCS+) | |
| Minimally conscious state or non-behavioural minimally conscious statea,24 | + (fluctuating) | Possible | Possible | 0 (behaviourally, but appropriate cortical response may be present) | |
| Emergence from minimally conscious state | ++++ (fluctuating) | ++ | ++ | + | |
| Locked-in syndrome | ++++ | ++++ | ++++ | ++++ | |
| Others | Drug-induced and psychotic hallucinations | ++++ | ++++ | Variable | Variable |
| Near-death experience | 0 | +++ | Unknown | Unknown | |
| Near-death-like experiencesb | Variable | +++ | Variable | Variable | |
Connected consciousness must not be mistaken with responsiveness, the possibility to deliver a behavioural response. Volitional responsiveness, when present, is crucial to infer connected consciousness, however, even the absence of which does not equate to the absence of internal or external awareness.1,22,26 Additionally, it must not be confounded with reflex, automatic motor responses such as arm withdrawal in response to noxious stimulation. In fact, connected consciousness during anaesthesia is not necessarily associated with movements or responsiveness1,27, 28, 29 and it is therefore difficult to detect. Its incidence has been estimated to be close to 5% after laryngoscopy, with higher incidences in young patients (11%) and women (13%),30 using the isolated forearm technique. This technique preserves one arm from muscle paralysis via a tourniquet, which enables response to command in such responders through squeezing of the hand. Although rarely followed by explicit recall but frequently ignored, the real consequences for patients of this frequent event demand attention.31
The regulation of wakefulness and the generation of a mental content
Wakefulness
The degree of wakefulness determines the level of cortical arousal, where the synergy of cortical functions generates awareness. Wakefulness is modulated by the interplay between brainstem nuclei and cortical regions.32 In the neurotypical individual, two factors drive wakefulness: sleep pressure, or the biological circadian rhythm indicating when to sleep according to clock time and environmental cues, and sleep homeostasis, or the sleep debt increasing with length of wakefulness and decreasing with accumulating sleep time.33 The main timekeeper of circadian rhythms is the suprachiasmatic nucleus of the hypothalamus.34 Circadian rhythm manifests at the cellular and network level, involving several pathways and neurotransmitters35 and modulating brain activity,36 cognition,37 and cortical excitability.38 Sleep pressure, or sleep homeostasis, is mostly mediated by the concentration of adenosine diphosphate (ADP). ADP is a waste product of neural activity,39 and high concentrations of ADP are correlated with deeper slow-wave sleep.40 Adenosine inhibits neurons involved in the arousal circuits and excites neurons that promote sleep.32,41
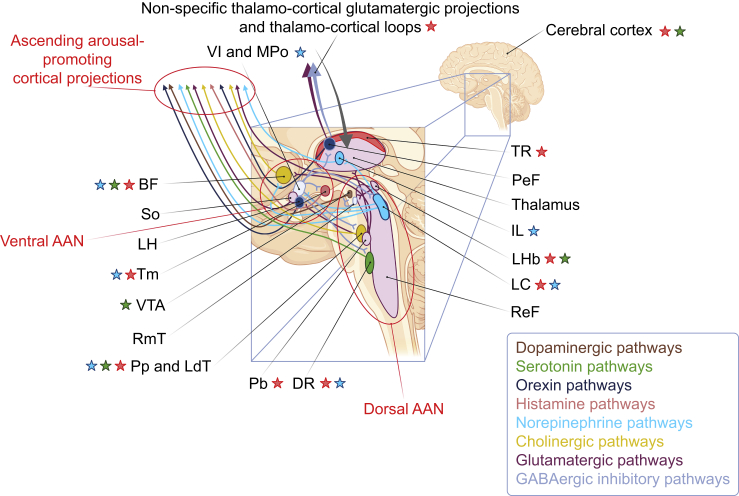
The balance between wakefulness and sleep is a bi-stable flip-flop system,42 mediated by a delicate and complex neuroanatomical organisation with mutually inhibiting arousal-promoting and arousal-inhibiting networks.43 The arousal system is a variegated circuit involving several neurotransmitter pathways including neurons with large and diffuse projections. The most critical are glutamatergic and gamma-aminobutyric acid (GABA)ergic ones, but their activity is regulated by neuromodulators such as norepinephrine, serotonin, histamine, orexin, and acetylcholine.44 The ascending reticular activation system or, as recently referred to, the ascending arousal network (AAN), is a key source of neuromodulators for maintenance of wakefulness.45 AAN includes, among others, the reticular formation and the mesencephalic reticular formation (excitatory glutamatergic input to the cortex), the raphe nuclei (serotonin), the tuberomammillary nucleus (histamine), and the locus coeruleus (norepinephrine).32 The AAN is composed of a dorsal and a ventral pathway. The dorsal pathway innervates the non-specific thalamo-cortical system, a glutamatergic system in which discharge rate differentiates wakefulness and rapid eye movement sleep from non-rapid eye movement sleep and is responsible for a cortical up-state.46 The ventral pathway involves the lateral hypothalamus and the basal forebrain, where neurons produce orexin and acetylcholine, respectively.47,48
Modulation of the AAN by anaesthetics has been mainly studied in animal models.49 It is still unknown whether the alterations of consciousness by anaesthetics are attributable to changes in cortical arousal after a direct effect on the AAN, or whether these are secondary to initial cortical effects. Propofol, sevoflurane, and xenon reproducibly decrease the activity of the thalamus and of the AAN,50 whereas halothane causes locus coeruleus hyperpolarisation.50,51 Histaminergic neurotransmission has also been proposed as a key target,52 and cholinergic neurotransmission.53,54 The activity of the locus coeruleus is inhibited by α2-adrenoceptor agonist sedatives such as dexmedetomidine. This inhibition in turn activates the inhibitory ventro-lateral preoptic nucleus in the basal forebrain, which then exerts its inhibitory activity on a series of cortical arousal-promoting nuclei.55 This is mechanistically similar to non-rapid eye movement sleep.52,56 Through the inhibition of inhibitory interneurons, ketamine promotes cholinergic, dopaminergic, and other aminergic neurotransmission emerging from subcortical nuclei.57 Other subcortical modulations by anaesthetic agents have been described, notably on thalamo-cortical interactions, which appear to be selective. The non-specific thalamo-cortical connectivity is affected first, whereas interactions related to sensory processing are altered at higher doses.58 Notably, anaesthesia-induced unconsciousness can be reversed by deep brain stimulation of the central thalamus in non-human primates,59, 60, 61 indicating the importance of thalamo-cortical communication for consciousness. However these observations of anaesthesia reversal may be linked to an unspecific arousal effect on the cortex, as is observed after indirect boosting of cholinergic neurotransmission.54,62
Aside from these observations, several arguments also support a direct, region-specific, and dose-dependent effect of anaesthetic agents on cortical function.2,63 Agents with a primarily GABAergic effect are known to directly influence the activity of cortical pyramidal neurons.64 Ketamine inhibits GABAergic inhibitory interneurons at the cortical level, and produces a hyperglutamatergic state leading to the activation of specific cortical regions, the limbic system, and the hippocampus.65,66 Additionally, through an effect on other types of interneurons, ketamine causes spontaneously active cortical neurons to become silent, whereas previously silent neurons become active.67
The truth likely resides between these extremities of primarily a cortical or subcortical effect of anaesthetics, and likely depends on the considered agent and dose. However, the net result translates into cortical consequences that lead to the different anaesthetic states of consciousness. A summary of the known effects of anaesthetic agents on the AAN and on the cortex is provided in Figure 1.
Figure 1.
Schematic representation of the complex systems regulating cortical arousal and controlling the sleep-wake cycle. Each assembly of neurons interacts with a series of other nuclei through complex excitatory and inhibitory projections (which are not all drawn for the sake of clarity). The final outputs of this complex network are ascending arousal-promoting or arousal-inhibiting cortical projections. Top-down cortical control of the activity of the subcortical structures also occurs. Stars indicate structures the activity of which is thought to be influenced by the administration of anaesthetic agents. For example, agents with a predominant gamma-aminobutyric acid (GABA)ergic effect (propofol or halogenated vapours; red star) potentiate the inhibition of cortical pyramidal neurons by inhibitory interneurons, boost the inhibitory effect of TR neurons on the cortex, and increase the inhibition of cortical arousal-promoting nuclei by the Vl and MPo, including the histaminergic Tm, the noradrenergic LC, the serotonergic DR, and the cholinergic BF, Pp, and LdT. The effects of ketamine (green star) are complex, and dose dependent. Through N-methyl-D-aspartate (NMDA) glutamate receptor antagonism, ketamine is known to inhibit GABAergic inhibitory interneurons at the cortical level, producing a hyper-glutamatergic state and the activation of specific cortical regions (anterior cingulate, medial prefrontal cortex, insula, and precuneus), the limbic system, and the hippocampus.65 66 This inhibitory interneuron inhibition also promotes cholinergic, dopaminergic, and other aminergic neurotransmission emerging from subcortical nuclei (e.g. TM, BF, Pp, and LdT). Aside from these effects, ketamine also inhibits excitatory-to-excitatory coupling in the cortex, provoking the inhibition of specific cortical regions.68 Alpha2-adrenergic agonists such as dexmedetomidine block the release of norepinephrine by LC. This has a direct inhibitory effect on cortical arousal-promoting nuclei such as BF and IL, or promotes the inhibition of other arousal-promoting nuclei (e.g. Tm, DR, PeF, Pp, and LdT) by Vl and MPo.55 Inspired by Moody and colleagues48 and from Purdon and colleagues.64 Drawn with BioRender. AAN, ascending arousal network; BF, basal forebrain; DR, dorsal raphe; IL, intralaminar nucleus of the thalamus; LC, locus coeruleus; LH, lateral hypothalamus; LHb, lateral habenula; Pb, parabrachial nucleus; PeF, perifornical area; Pp and LdT, pedunculo-pontine and latero-dorsal tegmentum nuclei; ReF, reticular formation; RmT, rostro-medial tegmental nucleus; So, supra-optic nucleus; Tm, tubero-mamillary nucleus; TR, thalamic reticular nucleus; Vl and MPo, ventro-lateral and median preoptic nuclei; VTA, ventral tegmental area.
Awareness
It is now commonly accepted that the emergence of awareness results from the collaborative work of, or communication between brain regions within and between brain networks.69 Adequate functioning of those networks requires an aroused cortex, but also involves subcortical structures.19,20 This exchange of information is thought to correspond to the aforementioned-mentioned NCCs.
Exploring brain communication and exchange of information
Exploring brain communication noninvasively is possible through recording physiological signals from regional brain activity. The signal can be spontaneous or evoked, and recorded through magnetoencephalography (change in the magnetic field produced by neuronal activity), positron emission tomography (regional oxygen consumption or glucose metabolism, or regional cerebral blood flow), functional magnetic resonance imaging (fMRI, blood oxygen level-dependent or BOLD signal, a marker of regional cerebral blood flow), electroencephalography (EEG, electrical signal produced by neuronal activity), coupled or not with transcranial magnetic stimulation (TMS, stimulation of a cortical region to study the nature and spread of the evoked response), or functional near-infrared spectroscopy (fNIRS).2 Various characterisations and properties of the recorded signals are thereby analysed. For example, the activity evoked by a stimulus,70 synchrony in activity (functional connectivity), directional connectivity (effective connectivity),71 cortical excitability,38 complexity of interactions,72,73 amount of integrated information,74 or spatio-temporal interaction dynamics75, 76, 77 between brain regions, and within or between identified brain networks can be studied. Such networks can be further characterised by methods derived from the graph theory, or topological properties.78 In this case, a region is considered as a node that is linked to other nodes by edges. The node degree corresponds to its number of connections with other nodes. Nodes with a high degree (i.e. highly connected) play a crucial role for information transfer.79 These nodes spontaneously and incessantly reconfigure themselves to maintain an optimised state of organisation.80 Rather than being composed of static functional brain networks such as the default mode, salience, dorsal attention, executive control, sensory, or motor networks, the functional organisation of the brain is thought to occupy complex spatio-temporal dynamic of states of connectivity within and between brain networks. The brain activity underlying these networks can be characterised as existing in a state of criticality, thus hovering between several unstable (or metastable) states within a multi-stable attractor landscape.81
As one can see, the measures thought to reflect exchange of information within the brain are numerous (see Supplementary Table S1 for some examples). Each of them has been tentatively proposed to correspond to an NCC, according to changes observed when passing from one consciousness state to the other. However, it is difficult to confirm their effective NCC nature, because some of them can support consciousness but are not mandatory (e.g. those related to the AAN activity), or can display concomitant changes that are indeed independent from changes in a consciousness state.8,82
Theories of consciousness
Several theories framing how brain activity can generate conscious content have emerged. Each of them focuses on a different feature,83 but none encompass the totality of the aforementioned concepts. It is beyond the scope of this paper to review all of them.9 In this review, we primarily focus on those theories addressing the phenomenological aspects of consciousness (access consciousness, phenomenal consciousness, and phenomenal selfhood), that is the subjective experience of an individual, as subjective experience during anaesthesia is the most important aspect for our patients. We also limit our description to the ones that have gained most attention in recent years and may have direct applications to the domain of anaesthesia. According to the classification of Seth and Bayne,9 we detail most prominent examples of global workspace theories (global neuronal workspace theory; GNW), integrated information theories (integrated information theory; IIT), re-entry and predictive processing theories (recurrent processing theory; RPT; predictive processing; PP), and higher-order theories (higher-order theory; HOT).
The global neuronal workspace hypothesis
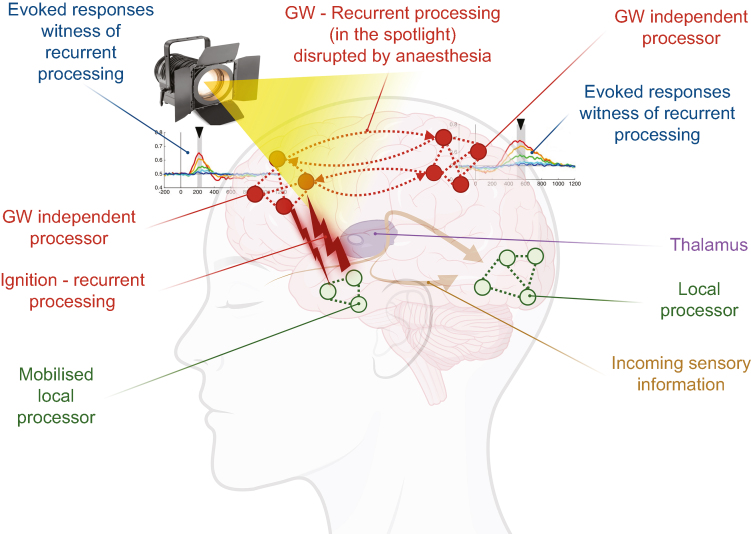
GNW is a framework that explains consciousness by a widespread broadcasting of information through the brain. GNW considers that information only becomes conscious once it is sent towards the independent processors of a global workspace. Therefore, any information that is not broadcasted remains unconscious (Fig 2). In brief, local cortical processors are linked by a core set of highly interconnected areas, which can select pieces of information generated by local processors, amplify them, and broadcast the resulting set of information to all other processors of the global workspace in a top-down manner, rendering it conscious and reportable verbally. Processes of information selection, amplification, and sustainability are critical to trigger ignition and occur through recurrent processing phenomena (feedforward and feedback transfer of information or connectivity). The core set of highly interconnected brain regions is mainly composed of prefrontal and parietal areas. If the feedforward signal generated by local processors (sensory information or information of another nature) is strong enough, it reaches the prefrontal cortex, which in turn triggers ignition into the reverberant network, involving not only the posterior parietal cortex, but also other cortical and subcortical regions, including the thalamus and cerebellum.13
Figure 2.
The global neuronal workspace hypothesis. In this theory, information (sensory or of another nature) generated within local processors of the brain becomes conscious if the feedforward signal from those processors is strong enough to generate ignition. Ignition allows the reverberation of the information within a global workspace, which is composed of highly interconnected independent processors. The independent processors (and particularly the frontal ones) can select relevant information, amplify it, and sustain it, and therefore control the process of ignition through recurrent processing (feedforward and feedback communication). Once in the global workspace, the information is broadcasted to all other processors of the workspace, again through recurrent processing, and becomes conscious (like being in the spotlight of a theatre). The global workspace is mainly composed of prefrontal and parietal areas, but also of other cortical and subcortical regions, including the thalamus and cerebellum (not shown in the figure, for the sake of clarity). Evoked responses to sensory stimuli and their timing are thought to be witnesses of recurrent processing within the global workspace, and of information reaching the field of consciousness. Anaesthesia is known to disrupt recurrent processing. Inspired by Seth and Bayne9 and Sergent and colleagues.70 Drawn with BioRender. GW, global workspace.
In this model, not all perceptual content enters conscious awareness. This is because of the all-or-none ignition of workspace activity, which allows the availability of a limited number of representations at a time.84 One could consider a theatre where only the small portion of the stage shown by the spotlight is perceived, while the remaining parts are ignored until they, in turn, enter the spotlight. A global workspace framework also explains why something can be partially reported (access consciousness), even if the entire experience itself (phenomenal consciousness) is richer than the one expressed. There is a ‘winner-takes-all’ competition among representations to be broadcasted within the global workspace.11 This has led to a long history of research looking for a ‘global signature’ of such broadcasting activity.85
Neural signatures of ignition have been previously identified in human and non-human primates,13 which are suppressed by anaesthesia.86 The most recent finding in this area is that, when a stimulus is consciously perceived, independently from being reported or not, a late electroencephalographic evoked activity is observed 250–300 ms after the stimulus (P3). This late activity is first seen in frontal areas, and then transferred to parietal regions, where it remains sustained up to 800–900 ms after the stimulus. This type of activity could be a signature of ignition and reverberant activity within the global workspace.70 Its maintenance or disappearance during anaesthesia and its associated possible different phenomenological brain states (unconsciousness, disconnected consciousness, and connected consciousness) are beginning to be studied.
It has been suggested that general anaesthesia primarily occurs via disruption of the reverberant global workspace functioning, and therefore of conscious access to information. Supporting this, EEG studies of directional connectivity in anaesthesia have shown that the functional connections from the frontal cortex towards more posterior areas (reflecting a frontal broadcasting of information) are pertinent,82,87,88,89 and the large-scale network top-down organisation,90,91 feedback (from higher- to lower-order cortical networks),91, 92, 93, 94, 95, 96, 97 and feedforward (the opposite pathway) connectivity,98, 99, 100, 101, 102 underlining the importance of recurrent processing. These effects do not preclude from basic sensory processing by lower-order sensory networks, as attested by the preservation of their connectivity when subjects are unresponsive.56,87,88,89
However, recent findings contradict the P3 as being a signature of ignition.103 P3 is observed during an awake connected state in response to oddball tones, but a similar response occurs in disconnected individuals in response to standard tones, as if all incoming sensory stimuli were interpreted as equally surprising despite not reaching the field of consciousness. This is associated with an increase in feedback connectivity within the auditory network thought to generate the P3 response.103
Others advocate that the dynamic state of the brain is differently affected by anaesthetic agents, without ever disappearing completely.104,105 Suppression of consciousness by anaesthesia would constrain the brain state towards more stable fluctuations and less temporal complexity,104,106 remaining further from critical dynamics,80,111 particularly regarding the dynamics of the main nodes of GNW.107 However, this view hardly explains how anaesthetised patients can be disconnected from their environment (i.e. sensory information does not reach the global workspace), while a mental content is still present in the form of a dream, indicating adequate functioning of the reverberant network. In this case, the feedforward sensory signal must be sufficiently and selectively attenuated as not to reach the prefrontal cortex, hence precluding it from ignition, while other internal inputs can still undergo ignition and reach the global workspace, thus enabling consciousness.
The integrated information theory
IIT bases its assumption on the phenomenological individual experience: conscious experience exists intrinsically (it has cause-effect power, meaning that it is possible to change its state), is structured (i.e. composed of different phenomenal units), is specific (it corresponds to a precise assembly of phenomenal units), is unitary (the conscious experience cannot be subdivided into a subset of other independent experiences), and is definite (regarding its composing elements and in time).108 Symmetrically, the physical substrate of consciousness must have an irreducible intrinsic cause-effect power that has the same properties.108 Hence, conscious experience corresponds to an irreducible maximum of integrated information generated by its physical substrate.9 The amount of integrated information is symbolised by the information theoretic quantity Φ, a measure of complexity. Complexity is a discrete measure that can be used to differentiate conscious states (e.g. normal wakefulness and awareness, dreaming, and hallucinogenic states) from the unconscious ones (e.g. deep sleep without dreams, general anaesthesia),109 but its value is not easy to determine with precision.109 This has led to a major criticism of the theory, some arguing that it is untestable. The solution to the problem could lie in trying to link some elements of consciousness with measures of information dynamics.110 Hence, some proxies for Φ have been proposed, based on the EEG power, frequency, functional connectivity, and modularity,74 based on the information content of the brain's response to TMS (perturbational complexity index or PCI72,111, 112, 113, 114, 115, 116, 117, 118), or obtained through interpretable deep learning analysis of the EEG [explainable consciousness indicator or ECI, able to distinguish the level of arousal (ECIaro) and awareness (ECIawa)].73 PCI or ECI critical values are suggested to clinically differentiate patients as a function of their level of arousal and awareness, such as minimally conscious state patients (fluctuating level of arousal, partially preserved awareness), unresponsive wakefulness syndrome patients (fluctuating level of arousal, no awareness),73,112 patients anesthetised with propofol or xenon (low level of arousal, no awareness), or patients anaesthetised with ketamine (low level of arousal, distorted internal awareness).73
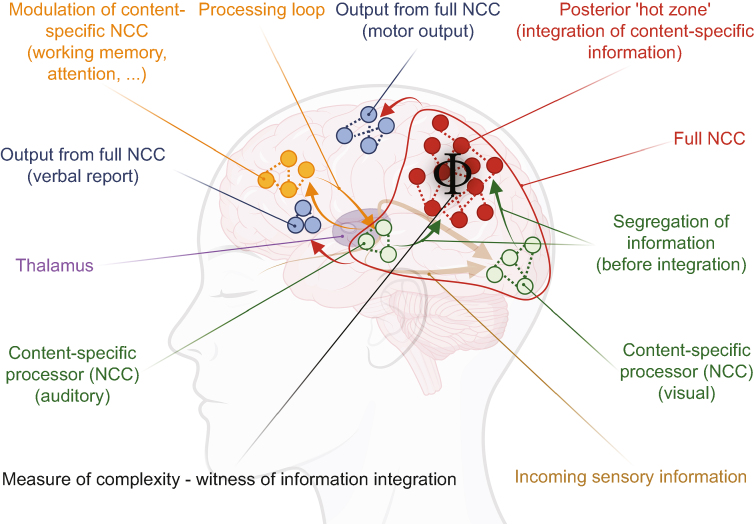
As opposed to GNW, IIT proposes that a circumscribed posterior zone of the brain plays a key regulating role in the integration of information, the ‘posterior hot zone’ (Fig 3). To consciously perceive the external world, a condition is first to be sensitive to incoming stimuli.113 The information related to incoming stimuli is separately processed in segregated modules before being integrated. Those processes are thought to be reflected in surrogate measures of brain activity such as the BOLD signal of fMRI. Indeed, BOLD signal frequency is found to be narrower, lower, and in a dynamically distinct range during segregation, while being wider, and dynamically coherent during integration.114 The lower-order processing of information would occur in content-specific, segregated NCCs (e.g. face recognition in the occipital cortex). The integration of all information emerging from the content-specific NCCs would occur in the full NCC complex, namely the ‘posterior hot zone’ composed of temporal, parietal, and occipital cortical regions. This functioning would be influenced by enabling factors (e.g. the AAN determining the level of arousal), and factors modulating the activity of content-specific NCCs (e.g. attention and working memory, and sensory input to the cortex). The full NCC would provide output for motor responses and verbal report.8
Figure 3.
The information integration theory (IIT). IIT postulates that the physical substrates of consciousness (neural correlates of consciousness, NCC) are of different natures. It distinguishes content-specific NCCs, which process specific aspects of information (auditory, visual, …), and full NCCs, which integrate all pertinent information into a mental content. Before reaching the full NCC, information from content-specific NCCs is modulated by specific brain functions such as working memory and attention, and segregated. The ‘posterior hot zone’, a hub involving parietal, occipital, and temporal cortical regions, plays a key role in controlling information integration by the full NCC. Integration of information can be quantified by the value Φ, a measure of brain activity complexity. Proxies of Φ have been proposed (see main text for details). The full NCC generates behavioural outputs, including motor and verbal report ones. Inspired by Seth and Bayne9 and Boly and colleagues.8 Drawn with BioRender. NCC, neural correlate of consciousness.
Using high-density EEG data acquired during propofol-induced unresponsiveness, dynamic causal modelling showed a selective breakdown of posterior parietal and medial feedforward fronto-parietal connectivity, and of parietal inter-network connectivity. These factors were found to be associated with loss of consciousness.115 This constitutes an argument in favour of the ‘posterior hot zone’ functional reality, in addition to the fact that posterior regions appear most relevant for quantifying arousal and awareness using ECI.73 It also supports the importance of communication between this zone and more anterior regions such as the anterior cingulate cortex for the integration of information and conscious content generation. This is in line with other anaesthesia and sleep studies investigating disconnected consciousness.116, 117, 118
The recurrent processing theory
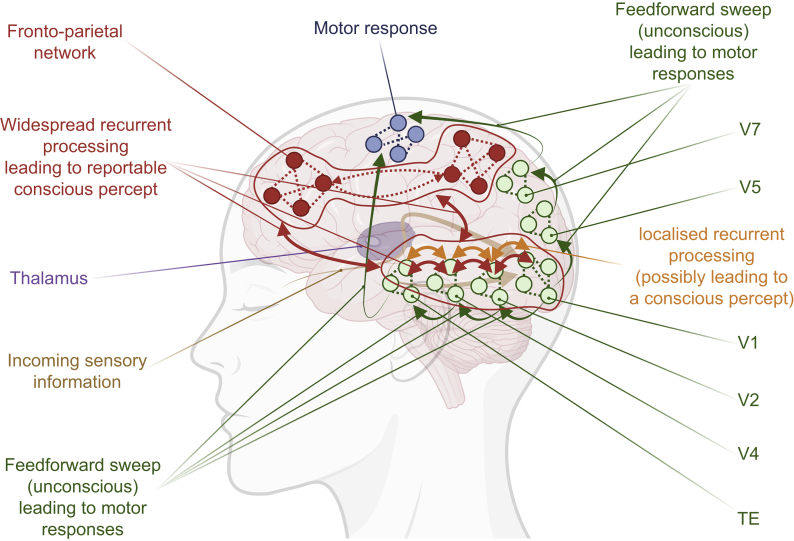
RPT assumes that a percept becomes conscious after several steps only, which are the ‘feedforward sweep’ and local ‘recurrent processing’119 (Fig 4). In other words, it assumes that consciousness necessitates reverberation of the activity in the sensory areas, and therefore that consciousness is sustained by the continuous interplay between low- and high-level areas. The originality here is the assumption that information reverberating locally is still available and experienced, even if not broadcasted. The theory still makes a difference between what can be reported and what is really experienced, in the same line as phenomenal and access consciousness. Partial report paradigms have supported RPT, through the exploration of the dissociation between the amount of remembered information, and its estimation.121,122 This theory has received little but significant support from anaesthesia studies in humans.123,124
Figure 4.
The recurrent processing theory (RPT). RPT has emerged following the study of visual perception. After sensory information incoming to the cortex (through the optic pathways, for example), feedforward sweep (green arrows) of information between visual areas (V1, V2, V4, V5, V7, and TE) occurs very rapidly for the interpretation of all the elements of the information (shape, localisation, …). It can lead to motor responses without any conscious perception of visual information. Localised recurrent processing (orange arrows) between visual areas allows fine tuning of information content, and may lead to a conscious percept. The occurrence of a reportable conscious percept would necessitate widespread recurrent processing (red arrows), not only between visual areas, but also with and within the fronto-parietal network. Inspired by Lamme.119 Drawn with BioRender. TE, temporal visual area; V1, V2, V4, V5, and V7, visual areas 1, 2, 4, 5, and 7, respectively.
Predictive processing
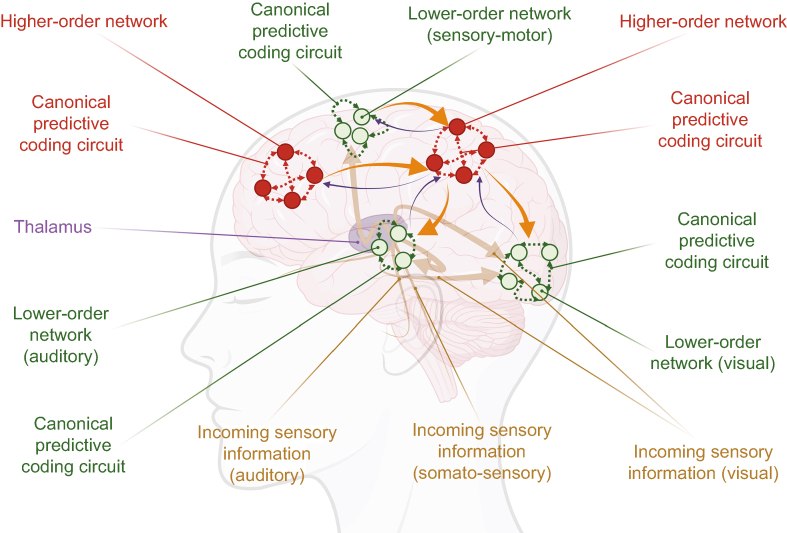
PP is currently grasping the attention of neuroscientists. PP is not a pure theory of consciousness, but rather a framework for understanding how the mind is organised. According to such view, the brain is a Bayesian machine, the role of which is to minimise surprise or free energy.125 As such, perception can be thought of as a ‘controlled hallucination’, a model that the brain constructs to navigate its surrounding.126 Lately, PP has served as a starting point to investigate modified states of consciousness, such as psychedelic experiences.127 According to PP,120 higher-order functional units constantly generate hypotheses of the sensory world and compare them against incoming sensory information. A feedback of the prediction is returned to sensory lower-order networks, and, in case of mismatch, a feedforward error message is sent to higher-order units with estimation of the precision of this information. Connected consciousness would therefore require balanced feedforward and feedback communication between higher-order and lower-order functional units. Alterations in the prediction, feedback, or both to lower-order sensory systems would result in unconsciousness, and alterations of feedforward messages, precision estimates, or both would characterise disconnected consciousness (Fig 5).
Figure 5.
Predictive processing. Higher-order functional units generate hypotheses of the sensory world within canonical predictive coding circuits, and confront them to incoming sensory information (also processed in canonical predictive coding circuits of lower-order networks). A feedback of the prediction is returned to sensory lower-order networks (orange arrows), and, in case of mismatch, a feedforward error message is sent to higher-order units with estimation of the precision of this information (blue thin arrows). Alteration of feedback to lower-order sensory systems would result in unconsciousness, and alteration of feedforward in disconnected consciousness. Inspired by Seth and Bayne9 and Sanders and colleagues.120 Drawn with BioRender.
The higher-order theory
In contrast to RPT, HOT128 is based on the assumption that the mere activation of sensory regions is not sufficient for the emergence of phenomenal consciousness, but that a hierarchically higher-order region is instead necessary (Supplementary Fig. S1). Lower-order representations, in the sensory areas, for example, would become conscious when targeted by the right type of meta-representation, which would come from higher-order functional regions. The prefrontal cortex would play a key role in this metacognition.9 Interestingly, HOT implies the presence of an inner awareness, which makes it different from other theories such as GNW. Additionally, part of the added value of HOT is an accounting of emotive feeling and mental states,129 which have strong influence on conscious experience.128 Like RPT, HOT has not been investigated in studies involving anaesthesia, but several studies exploring consciousness networks during anaesthesia make the distinction between lower-order (or sensory) networks, and higher-order ones.
Caveats of consciousness theories
Each proposed theory has been challenged. For example, in opposition to assertions of GNW and HOT, anterior regions of the brain (frontal and prefrontal) have been involved in behavioural report, subjective report, and executive control rather than consciousness per se.9 In addition, the identification of their neural correlates is impeded by the ability to validate the proposed models of brain communication, and to distinguish the real NCCs of mental content from those that are only prerequisites for consciousness, or simply independent correlates.8 Stakeholders of these theories have different conceptions about consciousness, and communication between them can be uneasy. This has been a limiting factor for the reproducibility of results. The development of collaborative and adversarial projects will likely progress the situation thereby allowing the direct testing of opposing hypotheses130 (see, e.g., https://www.templetonworldcharity.org/accelerating-research-consciousness-our-structured-adversarial-collaboration-projects).130,131 Such collaborations have already led to studies confirming some prediction of IIT and GNW, but also challenging them at the same time (questioning the reality of the posterior hot zone for IIT, and ignition and the role of the prefrontal cortex for GNW).132
Future directions
Recent progress is incrementally elucidating the complex nature of the effects of anaesthetic agents on the functional properties of consciousness. The current scientific literature contains a significant number of publications detailing changes in physiological brain signals when comparing a consciousness state to another, including studies using anaesthesia to modify consciousness. Those findings do not necessarily confirm that the considered physiological signal property really corresponds to an NCC, for the aforementioned reasons, and are not necessarily linked to a specific theory of consciousness. Through its ability to precisely and reversibly modulate different aspects of consciousness, anaesthesia offers unique possibilities to further progress in this domain, provided that researchers correctly assess consciousness states, namely connectedness and the presence of a mental content in an otherwise unresponsive subject. Progresses in these domains could help to provide evidence for theories of consciousness, and lead to the development of monitors facilitating, in patients with altered consciousness and unresponsive (either after brain damage or during anaesthesia), the detection of awareness of the environment, or the detection of disconnected consciousness. Such innovations might be closer than initially expected.
Current knowledge about anaesthesia-induced brain functional changes
Most studies investigating the brain functional changes induced by general anaesthesia have focused on single-drug sedation and have compared the full waking state with a state of unresponsiveness, supposed to be reflective of an absence of mental content. Functional changes that are common to all anaesthetic agents include a breakdown of higher-order networks (those involved in higher-order cognitive functions), and particularly of fronto-parietal connectivity, a relative preservation of lower-order networks (mainly sensory-motor networks, although between-network communication with higher-order networks might be compromised), an alteration of long distance cortico-cortical communication, and an alteration of the spatio-temporal dynamics in network interactions. Changes in communication dynamics involve a limitation of the repertoire of possible configurations, reduced complexity, reconfiguration of network structure, reduced efficiency, increased clustering and segregation, and a breakdown of the posterior ‘hot zone’.78 Authors disagree on the involved changes in communication directionality, both feedback91, 92, 93, 94 and feedforward,98, 99, 100, 101 suggesting that specific changes in feedforward or feedback connectivity throughout the cortical hierarchy might be involved in the mechanisms leading to the different anaesthesia-related brain states. As most of these studies were focused on the differences between wakefulness and drug-induced unresponsiveness thought to reflect unconsciousness, they did not specifically attempt to identify correlates of residual mental content and covert connectedness during anaesthesia. Therefore, we must consider the possibility that observed unresponsiveness-related functional changes may not correspond to a state of unconsciousness. This consideration renders the identification of the functional elements of anaesthesia-related brain states even more challenging.
Functional correlates of the states of consciousness during anaesthesia as a basis to identify their recordable signatures
The phenomenological state of disconnection from the environment supposes that incoming sensory stimuli are not consciously perceived. Knowing that functional connectivity within lower-order sensory-motor networks is usually preserved during deep sedation with a series of anaesthetic agents,89,133 indicating possible residual information processing at that level, disconnected consciousness could involve a disruption of communication between low-order sensory systems and higher-order cognitive systems, while the latter keep on maintaining internal information processing for the generation of mental content. Arguments that support this hypothesis are still scarce. Interestingly, propofol titration to loss of overt responsiveness during the performance of an auditory verbal memory task increases functional connectivity between the precuneus and other cortical regions, particularly the dorsal prefrontal and visual cortices.134 This could be in relation to a disconnected endogenous mentation. Strong evidence of a disruption of communication between lower-order and higher-order networks during disconnected consciousness, for example through a topological reconfiguration, is not yet available. Another possibility could be that disconnected consciousness occurs when information processing in lower-order networks is no longer generating information content (as assessed by the entropy of the principal components of the regional BOLD signal), while information content within higher-order networks remains of a high enough quality to generate internal mentation.135 Contrarily to this, others have shown that connected—compared with disconnected consciousness—necessitates preserved regional activity in midline structures of the brain including the thalamus, cingulate cortices, and angular gyri, which are components of higher-order consciousness networks.136 However, regional brain activity does not necessarily account for connectivity, and generation and exchange of information, rendering the interpretation of this finding difficult. It must also be kept in mind that the functional integrity of the dorsal anterior insular cortex appears to be necessary for a behavioural response to sensory stimuli,97 but this does not necessarily mean that such integrity is related to the conscious perception of external information.
Along with functional brain imaging studies trying to identify the functional correlates of anaesthesia-related brain states, the quest for easily recordable physiological markers of those states has now started. Slow-wave activity saturation in the EEG has long been advocated as a signature of perception loss during anaesthesia,137 but is probably not specific enough to distinguish between unconsciousness and disconnected consciousness. A frontal α-δ EEG pattern is almost constantly observed during surgical anaesthesia, and was first seen as indicative of unconsciousness.64 However, patients with this pattern might still be responsive to command, whereas patients without it can be unresponsive.138 It has been hypothesised that evoked occipital TMS-EEG α power is a marker of sensory information processing and hence of connectedness, whereas evoked low-γ power is necessary for consciousness, either connected or disconnected.139 In another study, disconnected consciousness was associated with broad spatial and spectral EEG changes, whereas unconsciousness was associated with focal decreases in activity in anterior and posterior cingulate cortices.116 Other examples are the perturbational distance metric, which assesses the ‘dynamical stability’ of a conscious state against perturbation, and hence its reversibility,140 or the above-mentioned PCI and ECI, whose value above a threshold is thought to correspond to the presence of awareness and to the level of arousal.72,73 The above-mentioned evoked cortical EEG responses to an auditory oddball paradigm may serve to screen for disconnection from the environment. During normal connected consciousness, oddball tones produce a P3 scalp EEG response. This P3 is absent during standard tone delivery. When disconnection occurs during sedation (while internal consciousness is still present), a similar oddball-evoked P3 response is observed during standard tones delivery. This could be as a result of increased feedback signalling in the auditory network, provoking a disruption of normal predictive coding processes, in that all incoming auditory stimuli become similarly surprising.103 The importance of attention processes in regulating access of sensory information to consciousness is considered in some of the above-described theories of consciousness (mainly in GNW, where it is associated with ignition, and in IIT). Attention is thought to be regulated by some of the higher-order consciousness networks (executive control network, dorsal attention network, and their relationship with the default-mode network), which can be differently and selectively affected by anaesthetic agents.87 Attention has recently been proposed as a core element of consciousness,141 and plays a role in patients' ability to give a purposeful response to a stimulus during anaesthesia.142 It also determines the ability to recall events after the procedure, as shown by a newly developed EEG-based index of attention (the cognitive effort index; CEI), which displays higher values in sedated patients who will have recall after the procedure.143
Despite these initial explorations into the discovery of specific markers of anaesthetic brain states, further research using optimal models to reliably discriminate the consciousness states of anaesthesia is needed. For instance, the isolated forearm technique and iterative awakening to ask for the presence of dreams, associated or not to sensory stimulation paradigms will aid progression in this respect.
Authors’ contributions
Literature search, writing and revising paper: all authors.
Accountable for all aspects of the work: all authors.
Declaration of interest
VB has had financial relationships with the following companies: Orion Pharma, Medtronic, Edwards, and Elsevier. PC and NA are research fellows and OG is research associate at F.R.S-FNRS. The other authors declare that they have no conflicts of interest.
Funding
The Department of Anesthesia and Intensive Care Medicine (Liege University Hospital, Liege, Belgium), the Belgian National Fund for Scientific Research (FNRS), the European Union's Horizon 2020 Framework Programme for Research and Innovation (945539) (Human Brain Project SGA3), the BIAL Foundation, the Mind Science Foundation, the Leon Fredericq Foundation, and the GIGA Doctoral School for Health Sciences.
Handling Editor: Phil Hopkins
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bjao.2023.100224.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Sanders R.D., Tononi G., Laureys S., Sleigh J.W. Unresponsiveness ≠ unconsciousness. Anesthesiology. 2012;116:946–959. doi: 10.1097/ALN.0b013e318249d0a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonhomme V., Staquet C., Montupil J., et al. General anesthesia: a probe to explore consciousness. Front Syst Neurosci. 2019;13:36. doi: 10.3389/fnsys.2019.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hudetz A.G. General anesthesia and human brain connectivity. Brain Connect. 2012;2:291–302. doi: 10.1089/brain.2012.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mashour G.A. Cognitive unbinding: a neuroscientific paradigm of general anesthesia and related states of unconsciousness. Neurosci Biobehav Rev. 2013;37:2751–2759. doi: 10.1016/j.neubiorev.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vanhaudenhuyse A., Demertzi A., Schabus M., et al. Two distinct neuronal networks mediate the awareness of environment and of self. J Cogn Neurosci. 2011;23:570–578. doi: 10.1162/jocn.2010.21488. [DOI] [PubMed] [Google Scholar]
- 6.Klein C., Barron A.B. How experimental neuroscientists can fix the hard problem of consciousness. Neurosci Conscious. 2020;2020:niaa009. doi: 10.1093/nc/niaa009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crick F., Koch C. Towards a neurobiological theory of consciousness. Semin Neurosci. 1990;2:263–275. [Google Scholar]
- 8.Boly M., Massimini M., Tsuchiya N., Postle B.R., Koch C., Tononi G. Are the neural correlates of consciousness in the front or in the back of the cerebral cortex? clinical and neuroimaging evidence. J Neurosci. 2017;37:9603–9613. doi: 10.1523/JNEUROSCI.3218-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seth A.K., Bayne T. Theories of consciousness. Nat Rev Neurosci. 2022;23:439–452. doi: 10.1038/s41583-022-00587-4. [DOI] [PubMed] [Google Scholar]
- 10.Northoff G., Lamme V. Neural signs and mechanisms of consciousness: is there a potential convergence of theories of consciousness in sight? Neurosci Biobehav Rev. 2020;118:568–587. doi: 10.1016/j.neubiorev.2020.07.019. [DOI] [PubMed] [Google Scholar]
- 11.Block N. Two neural correlates of consciousness. Trends Cogn Sci. 2005;9:46–52. doi: 10.1016/j.tics.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Fazekas P., Overgaard M. Perceptual consciousness and cognitive access: an introduction. Philos Trans R Soc Lond B Biol Sci. 2018;373 doi: 10.1098/rstb.2017.0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mashour G.A., Roelfsema P., Changeux J.-P., Dehaene S. Conscious processing and the global neuronal workspace hypothesis. Neuron. 2020;105:776–798. doi: 10.1016/j.neuron.2020.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blanke O., Metzinger T. Full-body illusions and minimal phenomenal selfhood. Trends Cogn Sci. 2009;13:7–13. doi: 10.1016/j.tics.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Seth A.K., Tsakiris M. Being a beast machine: the somatic basis of selfhood. Trends Cogn Sci. 2018;22:969–981. doi: 10.1016/j.tics.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Bernat J.L. Chronic consciousness disorders. Annu Rev Med. 2009;60:381–392. doi: 10.1146/annurev.med.60.060107.091250. [DOI] [PubMed] [Google Scholar]
- 17.Schiff N.D. Central thalamic contributions to arousal regulation and neurological disorders of consciousness. Ann N Y Acad Sci. 2008;1129:105–118. doi: 10.1196/annals.1417.029. [DOI] [PubMed] [Google Scholar]
- 18.Satpute A.B., Kragel P.A., Barrett L.F., Wager T.D., Bianciardi M. Deconstructing arousal into wakeful, autonomic and affective varieties. Neurosci Lett. 2019;693:19–28. doi: 10.1016/j.neulet.2018.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lutkenhoff E.S., Johnson M.A., Casarotto S., Massimini M., Monti M.M. Subcortical atrophy correlates with the perturbational complexity index in patients with disorders of consciousness. Brain Stimul. 2020;13:1426–1435. doi: 10.1016/j.brs.2020.07.012. [DOI] [PubMed] [Google Scholar]
- 20.Luppi A.I., Cain J., Spindler L.R.B., et al. Mechanisms underlying disorders of consciousness: bridging gaps to move toward an integrated translational science. Neurocrit Care. 2021;35:37–54. doi: 10.1007/s12028-021-01281-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naccache L. Visual consciousness explained by its impairments. Curr Opin Neurol. 2015;28:45–50. doi: 10.1097/WCO.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 22.Martial C., Cassol H., Laureys S., Gosseries O. Near-death experience as a probe to explore (disconnected) consciousness. Trends Cogn Sci. 2020;24:173–183. doi: 10.1016/j.tics.2019.12.010. [DOI] [PubMed] [Google Scholar]
- 23.Konkoly K.R., Appel K., Chabani E., et al. Real-time dialogue between experimenters and dreamers during REM sleep. Curr Biol. 2021;31:1417–1427.e6. doi: 10.1016/j.cub.2021.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thibaut A., Panda R., Annen J., et al. Preservation of brain activity in unresponsive patients identifies MCS Star. Ann Neurol. 2021;90:89–100. doi: 10.1002/ana.26095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown E.N., Lydic R., Schiff N.D. General anesthesia, sleep, and coma. N Engl J Med. 2010;363:2638–2650. doi: 10.1056/NEJMra0808281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boly M., Sanders R.D., Mashour G.A., Laureys S. Consciousness and responsiveness: Lessons from anaesthesia and the vegetative state. Curr Opin Anaesthesiol. 2013;26:444–449. doi: 10.1097/ACO.0b013e3283628b5d. [DOI] [PubMed] [Google Scholar]
- 27.Linassi F., Zanatta P., Tellaroli P., Ori C., Carron M. Isolated forearm technique: a meta-analysis of connected consciousness during different general anaesthesia regimens. Br J Anaesth. 2018;121:198–209. doi: 10.1016/j.bja.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 28.Radek L., Kallionpää R.E., Karvonen M., et al. Dreaming and awareness during dexmedetomidine- and propofol-induced unresponsiveness. Br J Anaesth. 2018;121:260–269. doi: 10.1016/j.bja.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 29.Sanders R.D., Gaskell A., Raz A., et al. Incidence of connected consciousness after tracheal intubation: a prospective, international, multicenter cohort study of the isolated forearm technique. Anesthesiology. 2017;126:214–222. doi: 10.1097/ALN.0000000000001479. [DOI] [PubMed] [Google Scholar]
- 30.Lennertz R., Pryor K.O., Raz A., et al. Connected consciousness after tracheal intubation in young adults: an international multicentre cohort study. Br J Anaesth. 2023;130:e217–e224. doi: 10.1016/j.bja.2022.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hudson A.E. Presumption of insensibility during general anaesthesia. Br J Anaesth. 2023;130:e209–e212. doi: 10.1016/j.bja.2022.09.010. [DOI] [PubMed] [Google Scholar]
- 32.Brown R.E., Basheer R., McKenna J.T., Strecker R.E., McCarley R.W. Control of sleep and wakefulness. Physiol Rev. 2012;92:1087–1187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borbély A.A., Daan S., Wirz-Justice A., Deboer T. The two-process model of sleep regulation: a reappraisal. J Sleep Res. 2016;25:131–143. doi: 10.1111/jsr.12371. [DOI] [PubMed] [Google Scholar]
- 34.Mohawk J.A., Green C.B., Takahashi J.S. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenwasser A.M., Turek F.W. Neurobiology of circadian rhythm regulation. Sleep Med Clin. 2015;10:403–412. doi: 10.1016/j.jsmc.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 36.Muto V., Jaspar M., Meyer C., et al. Local modulation of human brain responses by circadian rhythmicity and sleep debt. Science. 2016;353:687–690. doi: 10.1126/science.aad2993. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt C., Collette F., Cajochen C., Peigneux P. A time to think: circadian rhythms in human cognition. Cogn Neuropsychol. 2007;24:755–789. doi: 10.1080/02643290701754158. [DOI] [PubMed] [Google Scholar]
- 38.Ly J.Q.M., Gaggioni G., Chellappa S.L., et al. Circadian regulation of human cortical excitability. Nat Commun. 2016;7 doi: 10.1038/ncomms11828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greene R.W., Bjorness T.E., Suzuki A. The adenosine-mediated, neuronal-glial, homeostatic sleep response. Curr Opin Neurobiol. 2017;44:236–242. doi: 10.1016/j.conb.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Radulovacki M., Virus R.M., Djuricic Nedelson M., Green R.D. Adenosine analogs and sleep in rats. J Pharmacol Exp Ther. 1984;228:268–274. [PubMed] [Google Scholar]
- 41.Korkutata M., Saitoh T., Cherasse Y., et al. Enhancing endogenous adenosine A2A receptor signaling induces slow-wave sleep without affecting body temperature and cardiovascular function. Neuropharmacology. 2019;144:122–132. doi: 10.1016/j.neuropharm.2018.10.022. [DOI] [PubMed] [Google Scholar]
- 42.McGinty D., Szymusiak R. The sleep-wake switch: a neuronal alarm clock. Nat Med. 2000;6:510–511. doi: 10.1038/74988. [DOI] [PubMed] [Google Scholar]
- 43.Sulaman B.A., Wang S., Tyan J., Eban-Rothschild A. Neuro-orchestration of sleep and wakefulness. Nat Neurosci. 2023;26:196–212. doi: 10.1038/s41593-022-01236-w. [DOI] [PubMed] [Google Scholar]
- 44.Jones B.E. Arousal and sleep circuits. Neuropsychopharmacology. 2020;45:6–20. doi: 10.1038/s41386-019-0444-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edlow B.L., Takahashi E., Wu O., et al. Neuroanatomic connectivity of the human ascending arousal system critical to consciousness and its disorders. J Neuropathol Exp Neurol. 2012;71:531–546. doi: 10.1097/NEN.0b013e3182588293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gent T.C., Bandarabadi M., Herrera C.G., Adamantidis A.R. Thalamic dual control of sleep and wakefulness. Nat Neurosci. 2018;21:974–984. doi: 10.1038/s41593-018-0164-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jones B.E. Principal cell types of sleep–wake regulatory circuits. Curr Opin Neurobiol. 2017;44:101–109. doi: 10.1016/j.conb.2017.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moody O.A., Zhang E.R., Vincent K.F., et al. The neural circuits underlying general anesthesia and sleep. Anesth Analg. 2021;132:1254–1264. doi: 10.1213/ANE.0000000000005361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bao W.-W., Jiang S., Qu W.-M., Li W.-X., Miao C.-H., Huang Z.-L. Understanding the neural mechanisms of general anesthesia from interaction with sleep-wake state: a decade of discovery. Pharmacol Rev. 2023;75:532–553. doi: 10.1124/pharmrev.122.000717. [DOI] [PubMed] [Google Scholar]
- 50.Franks N.P. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9:370–386. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- 51.Sirois J.E., Lei Q., Talley E.M., Lynch C., Bayliss D.A. The TASK-1 two-pore domain K+ channel is a molecular substrate for neuronal effects of inhalation anesthetics. J Neurosci. 2000;20:6347–6354. doi: 10.1523/JNEUROSCI.20-17-06347.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu X., Franks N.P., Wisden W. Sleep and sedative states induced by targeting the histamine and noradrenergic systems. Front Neural Circuits. 2018;12:4. doi: 10.3389/fncir.2018.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meuret P., Backman S.B., Bonhomme V., Plourde G., Fiset P. Physostigmine reverses propofol-induced unconsciousness and attenuation of the auditory steady state response and bispectral index in human volunteers. Anesthesiology. 2000;93:708–717. doi: 10.1097/00000542-200009000-00020. [DOI] [PubMed] [Google Scholar]
- 54.Plourde G., Chartrand D., Fiset P., Font S., Backman S.B. Antagonism of sevoflurane anaesthesia by physostigmine: effects on the auditory steady-state response and bispectral index. Br J Anaesth. 2003;91:583–586. doi: 10.1093/bja/aeg209. [DOI] [PubMed] [Google Scholar]
- 55.Nelson L.E., Lu J., Guo T., Saper C.B., Franks N.P., Maze M. The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology. 2003;98:428–436. doi: 10.1097/00000542-200302000-00024. [DOI] [PubMed] [Google Scholar]
- 56.Guldenmund P., Vanhaudenhuyse A., Sanders R.D., et al. Brain functional connectivity differentiates dexmedetomidine from propofol and natural sleep. Br J Anaesth. 2017;119:674–684. doi: 10.1093/bja/aex257. [DOI] [PubMed] [Google Scholar]
- 57.Kokkinou M., Ashok A.H., Howes O.D. The effects of ketamine on dopaminergic function: meta-analysis and review of the implications for neuropsychiatric disorders. Mol Psychiatry. 2018;23:59–69. doi: 10.1038/mp.2017.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu X., Lauer K.K., Ward B.D., Li S.J., Hudetz A.G. Differential effects of deep sedation with propofol on the specific and nonspecific thalamocortical systems: a functional magnetic resonance imaging study. Anesthesiology. 2013;118:59–69. doi: 10.1097/ALN.0b013e318277a801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tasserie J., Uhrig L., Sitt J.D., et al. Deep brain stimulation of the thalamus restores signatures of consciousness in a nonhuman primate model. Sci Adv. 2022;8:eabl5547. doi: 10.1126/sciadv.abl5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bastos A.M., Donoghue J.A., Brincat S.L., et al. Neural effects of propofol-induced unconsciousness and its reversal using thalamic stimulation. eLife. 2021;10 doi: 10.7554/eLife.60824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Afrasiabi M., Redinbaugh M.J., Phillips J.M., et al. Consciousness depends on integration between parietal cortex, striatum, and thalamus. Cell Syst. 2021;12:363–373.e11. doi: 10.1016/j.cels.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meuret P., Backman S.B.S.B., Bonhomme V., Plourde G., Fiset P. Physostigmine reverses propofol-induced unconsciousness and attenuation of the auditory steady state response and bispectral index in human volunteers. Anesthesiology. 2000;93:708–717. doi: 10.1097/00000542-200009000-00020. [DOI] [PubMed] [Google Scholar]
- 63.Luppi A.I., Craig M.M., Pappas I., et al. Consciousness-specific dynamic interactions of brain integration and functional diversity. Nat Commun. 2019;10:4616. doi: 10.1038/s41467-019-12658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Purdon P.L., Sampson A., Pavone K.J., Brown E.N. Clinical electroencephalography for anesthesiologists. part i: background and basic signatures. Anesthesiology. 2015;123:937–960. doi: 10.1097/ALN.0000000000000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kraguljac N.V., Frölich M.A., Tran S., et al. Ketamine modulates hippocampal neurochemistry and functional connectivity: a combined magnetic resonance spectroscopy and resting-state fMRI study in healthy volunteers. Mol Psychiatry. 2017;22:562–569. doi: 10.1038/mp.2016.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Höflich A., Hahn A., Küblböck M., et al. Ketamine-dependent neuronal activation in healthy volunteers. Brain Struct Funct. 2017;222:1533–1542. doi: 10.1007/s00429-016-1291-0. [DOI] [PubMed] [Google Scholar]
- 67.Cichon J., Wasilczuk A.Z., Looger L.L., Contreras D., Kelz M.B., Proekt A. Ketamine triggers a switch in excitatory neuronal activity across neocortex. Nat Neurosci. 2023;26:39–52. doi: 10.1038/s41593-022-01203-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sleigh J., Harvey M., Voss L., Denny B. Ketamine - more mechanisms of action than just NMDA blockade. Trends Anaesth Crit Care. 2014;4:76–81. [Google Scholar]
- 69.Smith S.M., Fox P.T., Miller K.L., et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sergent C., Corazzol M., Labouret G., et al. Bifurcation in brain dynamics reveals a signature of conscious processing independent of report. Nat Commun. 2021;12:1149. doi: 10.1038/s41467-021-21393-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Crone J.S., Lutkenhoff E.S., Bio B.J., Laureys S., Monti M.M. Testing proposed neuronal models of effective connectivity within the cortico-basal ganglia-thalamo-cortical loop during loss of consciousness. Cereb Cortex. 2017;27:2727–2738. doi: 10.1093/cercor/bhw112. [DOI] [PubMed] [Google Scholar]
- 72.Casali A.G., Gosseries O., Rosanova M., et al. A theoretically based index of consciousness independent of sensory processing and behavior. Sci Transl Med. 2013;5:198ra105. doi: 10.1126/scitranslmed.3006294. [DOI] [PubMed] [Google Scholar]
- 73.Lee M., Sanz L.R.D., Barra A., et al. Quantifying arousal and awareness in altered states of consciousness using interpretable deep learning. Nat Commun. 2022;13:1064. doi: 10.1038/s41467-022-28451-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim H., Hudetz A.G., Lee J., et al. Estimating the integrated information measure phi from high-density electroencephalography during states of consciousness in humans. Front Hum Neurosci. 2018;12:42. doi: 10.3389/fnhum.2018.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Del Pozo S.M., Laufs H., Bonhomme V., Laureys S., Balenzuela P., Tagliazucchi E. Unconsciousness reconfigures modular brain network dynamics. Chaos. 2021;31 doi: 10.1063/5.0046047. [DOI] [PubMed] [Google Scholar]
- 76.Golkowski D., Larroque S.K., Vanhaudenhuyse A., et al. Changes in whole brain dynamics and connectivity patterns imaging. Anesthesiology. 2019;130:898–911. doi: 10.1097/ALN.0000000000002704. [DOI] [PubMed] [Google Scholar]
- 77.Luppi A.I., Cabral J., Cofre R., Destexhe A., Deco G., Kringelbach M.L. Dynamical models to evaluate structure-function relationships in network neuroscience. Nat Rev Neurosci. 2022;23:767–768. doi: 10.1038/s41583-022-00646-w. [DOI] [PubMed] [Google Scholar]
- 78.Lee U., Mashour G.A. Role of network science in the study of anesthetic state transitions. Anesthesiology. 2018;129:1029–1044. doi: 10.1097/ALN.0000000000002228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Blain-Moraes S., Tarnal V., Vanini G., et al. Network efficiency and posterior alpha patterns are markers of recovery from general anesthesia: a high-density electroencephalography study in healthy volunteers. Front Hum Neurosci. 2017;11:328. doi: 10.3389/fnhum.2017.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee H., Golkowski D., Jordan D., et al. Relationship of critical dynamics, functional connectivity, and states of consciousness in large-scale human brain networks. Neuroimage. 2019;188:228–238. doi: 10.1016/j.neuroimage.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 81.Demertzi A., Tagliazucchi E., Dehaene S., et al. Human consciousness is supported by dynamic complex patterns of brain signal coordination. Sci Adv. 2019;5:eaat7603. doi: 10.1126/sciadv.aat7603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mashour G.A., Hudetz A.G. Neural correlates of unconsciousness in large-scale brain networks. Trends Neurosci. 2018;41:150–160. doi: 10.1016/j.tins.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hanson J.R., Walker S.I. Formalizing falsification for theories of consciousness across computational hierarchies. Neurosci Conscious. 2021;2021 doi: 10.1093/nc/niab014. niab014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dehaene S., Sergent C., Changeux J.P. A neuronal network model linking subjective reports and objective physiological data during conscious perception. Proc Natl Acad Sci U S A. 2003;100:8520–8525. doi: 10.1073/pnas.1332574100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bekinschtein T.A., Dehaene S., Rohaut B., Tadel F., Cohen L., Naccache L. Neural signature of the conscious processing of auditory regularities. Proc Natl Acad Sci U S A. 2009;106:1672–1677. doi: 10.1073/pnas.0809667106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Noel J.-P., Ishizawa Y., Patel S.R., Eskandar E.N., Wallace M.T. Leveraging nonhuman primate multisensory neurons and circuits in assessing consciousness theory. J Neurosci. 2019;39:7485–7500. doi: 10.1523/JNEUROSCI.0934-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bonhomme V., Vanhaudenhuyse A., Demertzi A., et al. Resting-state network-specific breakdown of functional connectivity during ketamine alteration of consciousness in volunteers. Anesthesiology. 2016;125:873–888. doi: 10.1097/ALN.0000000000001275. [DOI] [PubMed] [Google Scholar]
- 88.Palanca B.J.A., Mitra A., Larson-Prior L., Snyder A.Z., Avidan M.S., Raichle M.E. Resting state functional magnetic resonance imaging correlates of sevoflurane-induced unconsciousness. Anesthesiology. 2015;123:346–356. doi: 10.1097/ALN.0000000000000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Boveroux P., Vanhaudenhuyse A., Bruno M.-A., et al. Breakdown of within- and between-network resting state functional magnetic resonance imaging connectivity during propofol-induced loss of consciousness. Anesthesiology. 2010;113:1038–1053. doi: 10.1097/ALN.0b013e3181f697f5. [DOI] [PubMed] [Google Scholar]
- 90.Ferrarelli F., Massimini M., Sarasso S., et al. Breakdown in cortical effective connectivity during midazolam-induced loss of consciousness. Proc Natl Acad Sci U S A. 2010;107:2681–2686. doi: 10.1073/pnas.0913008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Boly M., Moran R., Murphy M., et al. Connectivity changes underlying spectral EEG changes during propofol-induced loss of consciousness. J Neurosci. 2012;32:7082–7090. doi: 10.1523/JNEUROSCI.3769-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Peltier S.J., Kerssens C.A.C., Hamann S.B., Sebel P.S., Byas-Smith M., Hu X.P. Functional connectivity changes with concentration of sevoflurane anesthesia. Neuroreport. 2005;16:285–288. doi: 10.1097/00001756-200502280-00017. [DOI] [PubMed] [Google Scholar]
- 93.Lee U., Kim S., Noh G.J., Choi B.M., Hwang E., Mashour G.A. The directionality and functional organization of frontoparietal connectivity during consciousness and anesthesia in humans. Conscious Cogn. 2009;18:1069–1078. doi: 10.1016/j.concog.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 94.Ku S.-W.W., Lee U., Noh G.-J.J., Jun I.-G.G., Mashour G.A. Preferential inhibition of frontal-to-parietal feedback connectivity is a neurophysiologic correlate of general anesthesia in surgical patients. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jordan D., Ilg R., Riedl V., et al. Simultaneous electroencephalographic and functional magnetic resonance imaging indicate impaired cortical top-down processing in association with anesthetic-induced unconsciousness. Anesthesiology. 2013;119:1031–1042. doi: 10.1097/ALN.0b013e3182a7ca92. [DOI] [PubMed] [Google Scholar]
- 96.Ranft A., Golkowski D., Kiel T., et al. Neural correlates of sevoflurane-induced unconsciousness identified by simultaneous functional magnetic resonance imaging and electroencephalography. Anesthesiology. 2016;125:861–872. doi: 10.1097/ALN.0000000000001322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Warnaby C.E., Seretny M., Mhuircheartaigh R.N., et al. Anesthesia-induced suppression of human dorsal anterior insula responsivity at loss of volitional behavioral response. Anesthesiology. 2016;124:766–778. doi: 10.1097/ALN.0000000000001027. [DOI] [PubMed] [Google Scholar]
- 98.Barrett A.B., Murphy M., Bruno M.A., et al. Granger causality analysis of steady-state electroencephalographic signals during propofol-induced anaesthesia. PLoS One. 2012;7 doi: 10.1371/journal.pone.0029072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Maksimow A., Silfverhuth M., Långsjö J., et al. Directional connectivity between frontal and posterior brain regions is altered with increasing concentrations of propofol. PLoS One. 2014;9 doi: 10.1371/journal.pone.0113616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nicolaou N., Hourris S., Alexandrou P., Georgiou J. EEG-based automatic classification of ‘awake’ versus ‘anesthetized’ state in general anesthesia using granger causality. PLoS One. 2012;7 doi: 10.1371/journal.pone.0033869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nicolaou N., Georgiou J. Neural network-based classification of anesthesia/awareness using granger causality features. Clin EEG Neurosci. 2014;45:77–88. doi: 10.1177/1550059413486271. [DOI] [PubMed] [Google Scholar]
- 102.Sanders R.D., Banks M.I., Darracq M., et al. Propofol-induced unresponsiveness is associated with impaired feedforward connectivity in cortical hierarchy. Br J Anaesth. 2018;121:1084–1096. doi: 10.1016/j.bja.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Casey C.P., Tanabe S., Farahbakhsh Z., et al. Dynamic causal modelling of auditory surprise during disconnected consciousness: the role of feedback connectivity. Neuroimage. 2022;263 doi: 10.1016/j.neuroimage.2022.119657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li D., Vlisides P.E., Kelz M.B., et al. Dynamic cortical connectivity during general anesthesia in healthy volunteers. Anesthesiology. 2019;130:870–884. doi: 10.1097/ALN.0000000000002656. [DOI] [PubMed] [Google Scholar]
- 105.Vlisides P.E., Li D., Zierau M., et al. Dynamic cortical connectivity during general anesthesia in surgical patients. Anesthesiology. 2019;130:885–897. doi: 10.1097/ALN.0000000000002677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tagliazucchi E., Chialvo D.R.D.R., Siniatchkin M., et al. Large-scale signatures of unconsciousness are consistent with a departure from critical dynamics. J R Soc Interface. 2016;13 doi: 10.1098/rsif.2015.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Barttfeld P., Uhrig L., Sitt J.D., Sigman M., Jarraya B., Dehaene S. Signature of consciousness in the dynamics of resting-state brain activity. Proc Natl Acad Sci U S A. 2015;112:887–892. doi: 10.1073/pnas.1418031112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tononi G., Boly M., Massimini M., Koch C. Integrated information theory: from consciousness to its physical substrate. Nat Rev Neurosci. 2016;17:450–461. doi: 10.1038/nrn.2016.44. [DOI] [PubMed] [Google Scholar]
- 109.Sarasso S., Casali A.G., Casarotto S., Rosanova M., Sinigaglia C., Massimini M. Consciousness and complexity: a consilience of evidence. Neurosci Conscious. 2021;7:1–24. doi: 10.1093/nc/niab023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mediano P.A.M., Rosas F.E., Bor D., Seth A.K., Barrett A.B. The strength of weak integrated information theory. Trends Cogn Sci. 2022;26:646–655. doi: 10.1016/j.tics.2022.04.008. [DOI] [PubMed] [Google Scholar]
- 111.Comolatti R., Pigorini A., Casarotto S., et al. A fast and general method to empirically estimate the complexity of brain responses to transcranial and intracranial stimulations. Brain Stimul. 2019;12:1280–1289. doi: 10.1016/j.brs.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 112.Casarotto S., Comanducci A., Rosanova M., et al. Stratification of unresponsive patients by an independently validated index of brain complexity. Ann Neurol. 2016;80:718–729. doi: 10.1002/ana.24779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee H., Noh G.J., Joo P., et al. Diversity of functional connectivity patterns is reduced in propofol-induced unconsciousness. Hum Brain Mapp. 2017;38:4980–4995. doi: 10.1002/hbm.23708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bharath R.D., Panda R., Saini J., Sriganesh K., Rao G.S.U. Dynamic local connectivity uncovers altered brain synchrony during propofol sedation. Sci Rep. 2017;7:8501. doi: 10.1038/s41598-017-08135-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ihalainen R., Gosseries O., de Steen F Van, et al. How hot is the hot zone? Computational modelling clarifies the role of parietal and frontoparietal connectivity during anaesthetic-induced loss of consciousness. Neuroimage. 2021;231 doi: 10.1016/j.neuroimage.2021.117841. [DOI] [PubMed] [Google Scholar]
- 116.Casey C.P., Tanabe S., Farahbakhsh Z., et al. Distinct EEG signatures differentiate unconsciousness and disconnection during anaesthesia and sleep. Br J Anaesth. 2022;128:1006–1018. doi: 10.1016/j.bja.2022.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Siclari F., Baird B., Perogamvros L., et al. The neural correlates of dreaming. Nat Neurosci. 2017;20:872–878. doi: 10.1038/nn.4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Siclari F., Bernardi G., Cataldi J., Tononi G. Dreaming in NREM sleep: a high-density EEG study of slow waves and spindles. J Neurosci. 2018;38:9175–9185. doi: 10.1523/JNEUROSCI.0855-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lamme V.A.F. Towards a true neural stance on consciousness. Trends Cogn Sci. 2006;10:494–501. doi: 10.1016/j.tics.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 120.Sanders R.D., Casey C., Saalmann Y.B. Predictive coding as a model of sensory disconnection: relevance to anaesthetic mechanisms. Br J Anaesth. 2021;126:37–40. doi: 10.1016/j.bja.2020.08.017. [DOI] [PubMed] [Google Scholar]
- 121.Pinto Y., Vandenbroucke A.R., Otten M., Sligte I.G., Seth A.K., Lamme V.A.F. Conscious visual memory with minimal attention. J Exp Psychol Gen. 2017;146:214–226. doi: 10.1037/xge0000255. [DOI] [PubMed] [Google Scholar]
- 122.van Gaal S., Lamme V.A.F. Unconscious high-level information processing: implication for neurobiological theories of consciousness. Neuroscientist. 2012;18:287–301. doi: 10.1177/1073858411404079. [DOI] [PubMed] [Google Scholar]
- 123.Alkire M.T., Hudetz A.G., Tononi G. Consciusness and anesthesia. Science. 2008;7:876–880. doi: 10.1126/science.1149213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lamme V.A., Zipser K., Spekreijse H. Figure-ground activity in primary visual cortex is suppressed by anesthesia. Proc Natl Acad Sci U S A. 1998;95:3263–3268. doi: 10.1073/pnas.95.6.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kilner J.M., Friston K.J., Frith C.D. Predictive coding: an account of the mirror neuron system. Cogn Process. 2007;8:159–166. doi: 10.1007/s10339-007-0170-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Seth A.K. Interoceptive inference, emotion, and the embodied self. Trends Cogn Sci. 2013;17:565–573. doi: 10.1016/j.tics.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 127.Carhart-Harris R.L., Friston K.J. REBUS and the anarchic brain: toward a unified model of the brain action of psychedelics. Pharmacol Rev. 2019;71:316–344. doi: 10.1124/pr.118.017160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Brown R., Lau H., LeDoux J.E. Understanding the higher-order approach to consciousness. Trends Cogn Sci. 2019;23:754–768. doi: 10.1016/j.tics.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 129.LeDoux J.E., Brown R. A higher-order theory of emotional consciousness. Proc Natl Acad Sci U S A. 2017;114:E2016–E2025. doi: 10.1073/pnas.1619316114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Melloni L., Mudrik L., Pitts M., et al. An adversarial collaboration protocol for testing contrasting predictions of global neuronal workspace and integrated information theory. PLoS One. 2023;18 doi: 10.1371/journal.pone.0268577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Melloni L., Mudrik L., Pitts M., Koch C. Making the hard problem of consciousness easier. Science. 2021;372:911–912. doi: 10.1126/science.abj3259. [DOI] [PubMed] [Google Scholar]
- 132.Consortium C., Ferrante O., Gorska-Klimowska U., et al. An adversarial collaboration to critically evaluate theories of consciousness. bioRxiv [Internet] Cold Spring Harbor Laboratory; 2023. https://www.biorxiv.org/content/early/2023/06/26/2023.06.23.546249 Available from: [Google Scholar]
- 133.Amico E., Marinazzo D., Di Perri C., et al. Mapping the functional connectome traits of levels of consciousness. Neuroimage. 2017;148:201–211. doi: 10.1016/j.neuroimage.2017.01.020. [DOI] [PubMed] [Google Scholar]
- 134.Liu X., Li S.-J.J., Hudetz A.G. Increased precuneus connectivity during propofol sedation. Neurosci Lett. 2014;561:18–23. doi: 10.1016/j.neulet.2013.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu X., Lauer K.K.K., Ward B.D., et al. Regional entropy of functional imaging signals varies differently in sensory and cognitive systems during propofol-modulated loss and return of behavioral responsiveness. Brain Imaging Behav. 2019;13:514–525. doi: 10.1007/s11682-018-9886-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Scheinin A., Kantonen O., Alkire M., et al. Foundations of human consciousness: imaging the twilight zone. J Neurosci. 2021;41:1769–1778. doi: 10.1523/JNEUROSCI.0775-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Warnaby C.E., Sleigh J.W., Hight D., Jbabdi S., Tracey I. Investigation of slow-wave activity saturation during surgical anesthesia reveals a signature of neural inertia in humans. Anesthesiology. 2017;127:645–657. doi: 10.1097/ALN.0000000000001759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gaskell A.L.L., Hight D.F.F., Winders J., et al. Frontal alpha-delta EEG does not preclude volitional response during anaesthesia: prospective cohort study of the isolated forearm technique. Br J Anaesth. 2017;119:664–673. doi: 10.1093/bja/aex170. [DOI] [PubMed] [Google Scholar]
- 139.Darracq M., Funk C., Polyakov D., et al. Evoked alpha power is reduced in disconnected consciousness during sleep and anesthesia. Sci Rep. 2018;8:16664. doi: 10.1038/s41598-018-34957-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sanz Perl Y., Pallavicini C., Pérez Ipiña I., et al. Perturbations in dynamical models of whole-brain activity dissociate between the level and stability of consciousness. PLoS Comput Biol. 2021;17 doi: 10.1371/journal.pcbi.1009139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Graziano M.S.A. A conceptual framework for consciousness. Proc Natl Acad Sci U S A. 2022;119 doi: 10.1073/pnas.2116933119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Deng F., Taylor N., Owen A.M., Cusack R., Naci L. Responsiveness variability during anaesthesia relates to inherent differences in brain structure and function of the frontoparietal networks. Hum Brain Mapp. 2023;44:2142–2157. doi: 10.1002/hbm.26199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Baron Shahaf D., Weissman A., Priven L., Shahaf G. Identifying recall under sedation by a novel EEG based index of attention-a pilot study. Front Med. 2022;9 doi: 10.3389/fmed.2022.880384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.