Abstract
Background
Flow-mediated dilation (FMD) measures vascular endothelial function by evaluating the vasodilatory response of blood vessels to increased blood flow. Nevertheless, the association between FMD and stroke incidence in a general population remains unclear. This study investigated the association between vascular endothelial function and stroke incidence in the general Japanese population.
Methods
Based on cohort data from the Tohoku Medical Megabank Community-based Cohort Study, participants aged ≥18 years were recruited from Iwate Prefecture, with the final sample comprising 2952 subjects.
Results
The FMD level was 0.5%–27.1%, with a median of 5.0% (interquartile, 4.2%–11.3%). The mean follow-up period was 5.5 ± 1.8 years (range, 0.6–6.9 years). After dividing the participants into two subgroups according to the median FMD value, a multivariate Cox regression analysis adjusting for gender, age, smoking, alcohol consumption, systolic blood pressure, low-density lipoprotein cholesterol, estimated glomerular filtration rate, N-terminal pro-brain natriuretic peptide, high-sensitivity cardiac troponin T and hemoglobin A1c revealed that a lower FMD value was strongly associated with incidences of total stroke (hazard ratio[HR] = 2.13, 95% confidence interval[CI] = 1.48–3.07, p < 0.001), ischemic stroke (HR = 3.33, 95%CI = 2.00–5.52, p < 0.001), nonlacunar stroke (HR = 2.77, 95%CI = 1.49–5.16, p = 0.001), and lacunar stroke (HR = 5.12, 95%CI = 1.74–16.05, p = 0.003).
Conclusions
This study showed that a low FMD value might reflect vascular endothelial dysfunction and then was associated with ischemic stroke incidence in the general Japanese population, suggesting that FMD can be used as a tool to identify future stroke risk.
Keywords: Atherosclerosis, Cerebrovascular disease, Flow-mediated dilation, Follow-up study, Ischemic stroke
Highlights
-
•
This study investigated the association between vascular endothelial function and stroke incidence in the general Japanese population.
-
•
The present study found that a lower FMD value (<5.0%) was strongly associated with incidences of total stroke, ischemic stroke, nonlacunar stroke and lacunar stroke.
1. Introduction
Stroke remains a common and life-threatening disease in the general population, despite recent advances in stroke management, such as intravenous thrombolysis with recombinant tissue plasminogen activators [1]. Although preventive treatments are available for stroke risk factors such as hypertension, diabetes, dyslipidemia, smoking, alcohol consumption, and atrial fibrillation, the incidence remains high [1].
Atherosclerosis is one of the risk factors for stroke and is caused by atheroma formation due to the accumulation of low-density lipoprotein (LDL) cholesterol in the arterial wall through an inflammatory mechanism [[2], [3], [4], [5], [6]]. The initial stage of this atherogenic process is believed to be a deterioration of vascular endothelial function, starting with a decrease in the activity of nitric oxide secreted by vascular endothelial cells [7].
Flow-mediated dilation (FMD) uses ultrasound and a cuff to evaluate the dilatation rate of blood vessels at rest and after de-energization and can determine whether an endothelial function is deteriorating [8,9]. A recent study reported that a low FMD value was associated with the occurrence of cardiovascular events in patients with a history of coronary artery disease [10]. Another study also reported that FMD values were a significant predictor of cardiovascular events in adults [11]. Nonetheless, it has been uncertain whether vascular endothelial dysfunction is associated with stroke incidence in the general population.
Therefore, this study was conducted to evaluate whether vascular endothelial function measuring FMD is associated with stroke incidence in the general Japanese population.
2. Methods
2.1. Study population
Cohort data from the Tohoku Medical Megabank (TMM) Community-based Cohort Study, a prospective cohort study, were used in this investigation [12,13]. Participants were recruited from Iwate Prefecture between May 2013 and March 2016, with the study population comprising individuals aged ≥18 years. Participants were excluded from the study if they had pacemaker implantation, continuous dialyzes, and self-reported cardiovascular diseases (CVD), including myocardial infarction, angina pectoris, aortic aneurysm, aortic dissection, heart failure, atrial fibrillation, ventricular fibrillation and stroke, and intake of vasodilatory drugs including calcium antagonist and nitric acid medicine. Participants lacking details regarding baseline characteristics and laboratory or follow-up data were also excluded. This study was approved by the Ethics Committee of Iwate Medical University (HGH25–2, MH2022-147), and informed consent was obtained from all participants. Also, this study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution's human research committee.
2.2. Collection of clinical data
Data on participants’ lifestyles and medical history were collected using self-administered questionnaires, and height, weight, and blood pressure measurements were obtained through physical examinations. Participants consuming more than 34 g of ethanol daily were classified as drinkers [14]. A participant was categorized as a current or former smoker if they are currently engaging in smoking or have a history of smoking. Hypertension was defined as a systolic blood pressure of ≥140 mmHg or a diastolic blood pressure of ≥90 mmHg, a previous diagnosis of hypertension or the current use of antihypertensive drugs [15]. Diabetes mellitus was characterized by a glycated hemoglobin (HbA1c) level of ≥6.5%, a non-fasting blood glucose level of ≥200 mg/dL, a previous diagnosis of diabetes mellitus or the current use of anti-diabetic medicationsn [16]. Dyslipidemia was identified by a low-density lipoprotein (LDL) cholesterol level of ≥140 mg/dL, a previous diagnosis of dyslipidemia, or the current use of antidyslipidemic agents [17].
Peripheral venous blood samples were collected from the upper arm in resting, seated positions for blood examinations. The blood samples were centrifuged, and the supernatant was collected and frozen at −80 °C before analysis. Serum high-sensitivity cardiac troponin T (hs-cTnT) levels were measured using the EcLusys high-sensitivity troponin T assay (Roche Diagnostics K.K., Tokyo, Japan), with a detection limit of 3 ng/L and a 99th percentile upper reference limit of 13.5 ng/L [18]. The estimated glomerular filtration rate (eGFR) was calculated using serum creatinine levels according to the 2002 KDOQI Clinical Practice Guidelines for Chronic Kidney Disease, using the following equation: eGFR = 194 × serum cystatin C−1.094 × age−0.287 [ × 0.739 for women] mL/min/1.73 m [19]. [20].
2.3. FMD measurement
Endothelium-dependent FMD was evaluated through vascular response to reactive hyperemia in the brachial artery. A high-resolution linear artery transducer and computer-assisted analysis software (UNEXEF18G, UNEX Co, Nagoya, Japan) was used to measure brachial artery diameter. FMD measurements were performed by operators in accordance with the guidelines established by the International Brachial Artery Reactivity Task Force [21].
The process begins with placing a blood pressure cuff around the forearm, followed by a longitudinal scan of the brachial artery. A specialized probe holder maintains a stable position for the transducer, promoting consistent imaging. The system automatically tracks the artery diameter and displays the real-time changes in FMD mode. We documented pulsed Doppler flow velocities at baseline and during peak hyperemic flow.
Before initiating the measurement, the cuff was inflated to a pressure of 180 mmHg or at least 50 mmHg above the systolic blood pressure to ensure complete occlusion of the brachial artery. We captured a 30-s baseline artery image before inflating the cuff for 5 min. Following cuff deflation, we recorded artery images for an additional 5 min. We collected pulsed Doppler velocity signals at baseline and immediately following cuff deflation. We quantified changes in the brachial artery diameter as a percentage change relative to the pre-inflation diameter. The system automatically calculated the FMD using the formula: percentage FMD = ([peak diameter − baseline diameter]/baseline diameter), which served as the primary metric for analysis. We calculated the blood flow volume by integrating the angle-corrected Doppler flow velocity with the heart rate and the vessel's cross-sectional area. We defined reactive hyperemia as the maximum percentage increase in flow following cuff deflation compared to baseline flow.
To ensure standardized practices, the core laboratory at Iwate Medical University provided training for all participating sonographers, which covered FMD measurement techniques, scanning procedures, and record analysis. We conducted preliminary tests to evaluate both inter-assay and intra-assay variability. Two sonographers examined fifteen volunteer subjects on separate days, conducting each session twice. A core laboratory later evaluated the collected images. The Supplemental Figure displays the results of the preliminary FMD tests, indicating statistically significant positive correlations, thus affirming the reliability of the measurements (day 1 and day 2 for sonographer 1: r = 0.94, p < 0.05; day 1 and day 2 for sonographer 2: r = 0.96, p < 0.05; comparison between sonographer 1 and 2: r = 0.95, p < 0.05).
2.4. Follow-up study
A follow-up survey evaluating all-cause death, migration, and stroke incidence was performed in a post-baseline study through December 31, 2019. Death and migration were confirmed using official resident registration data provided by local government offices. Stroke incidence was ascertained using the Iwate Stroke Registry data from all regional hospitals [22]. The stroke registration program, coordinated by the Iwate Prefecture government and Iwate Medical Association, ensured comprehensive data recording by verifying medical records from all facilities within the survey area. Upon a stroke patient's discharge, registration forms were mailed to the registration office of the Iwate Medical Association. The research team, comprising physicians and trained research nurses, visited the hospitals to review medical charts.
The stroke diagnostic criteria for this study were based on the International Statistical Classification of Diseases and Related Health Problems criteria established by the World Health Organization [23,24]. Patients with transient ischemic attack and traumatic hemorrhagic stroke were excluded from registration. Stroke was defined as a sudden onset of focal neurological deficit persisting for ≥24 h, confirmed via brain computed tomography or magnetic resonance imaging. Brain computed tomography was performed to diagnose cases involving implanted medical devices, claustrophobia, tattoos, or surgically placed magnetic dentures. Classification of stroke into ischemic stroke, including lacunar, nonlacunar and hemorrhagic stroke, was performed using neuroimaging and medical records.
2.5. Statistical analysis
Baseline characteristics were expressed as mean ± standard deviation for continuous variables and as numbers and percentages for categorical variables. Continuous variables were compared across quartiles using analysis of variance for normally distributed variables, whereas the Kruskal–Wallis test was applied for skewed variables. The chi-squared test was used to analyze categorical variables across quartiles. Event-free curves were estimated using the Kaplan–Meier method and compared via the log-rank test. The association between FMD and incident stroke was examined using Cox hazard models in an unadjusted crude model and a multivariate adjustment model, adjusted for sex, age, smoking, drinking, systolic blood pressure, LDL cholesterol, eGFR, N-terminal pro-brain natriuretic peptide (NT-proBNP), hs-cTnT, and hemoglobin A1c. All data were analyzed using IBM SPSS Statistics, version 25, for Windows (IBM Corp., Armonk, NY, USA), with p values < 0.05 deemed statistically significant.
2.6. Data availability
The datasets analyzed in the current study are not publicly available due to ethical reasons but are available upon request after approval from the Ethical Committee of Iwate Medical University, the Ethical Committee of Tohoku University, and the Materials and Information Distribution Review Committee of the TMM Project.
3. Results
3.1. Baseline characteristics of the study population
Table 1 shows the baseline characteristics of the study participants. Among 3865 individuals who participated in the Tohoku Medical Megabank CommCohort Study, this study was intended for 2952 individuals, except for the exclusion criteria (self-reported CVD: n = 221, participants taking vasodilatory drugs: n = 603, lacking baseline characteristics and laboratory and follow-up data: n = 89). The FMD level was 0.5%–27.1%, with a median value of 5.0% (interquartile, 4.2%–11.3%). After dividing the participants into two subgroups according to the median FMD value, significant differences were observed in age, gender, body mass index, prevalence of smoking, diabetes mellitus, hypertension, hs-cTnT, uric acid, eGFR, hemoglobin A1c, high-density lipoprotein cholesterol, and systolic blood pressure (Table 1).
Table 1.
Baseline characteristics of participants.
| Parameter | All | FMDa ≥ 5.0% | FMD <5.0% | p value |
|---|---|---|---|---|
| Number | 2952 | 1486 | 1466 | |
| Age | 63.0 ± 8.3 | 61.2 ± 8.7 | 64.3 ± 7.6 | <0.001 |
| Women, % | 1859 (63.0) | 1045 (70.3) | 814 (55.5) | <0.001 |
| Body mass index, kg/m2 | 23.2 ± 3.3 | 22.9 ± 3.2 | 23.6 ± 3.3 | 0.001 |
| Current or former smoker, % | 897 (36.2) | 399 (26.9) | 498 (34.0) | <0.001 |
| Drinker, % | 1095 (37.1) | 540 (36.4) | 555 (37.9) | 0.12 |
| Diabetes, % | 270 (9.1) | 123 (8.3) | 147 (10.0) | 0.031 |
| Hypertension, % | 839 (28.4) | 326 (21.9) | 513 (35.0) | <0.001 |
| Dyslipidemia, % | 472 (16.0) | 238 (16.0) | 234 (16.0) | 0.952 |
| Median NT-proBNPb, pg/mL | 48.0 (29.0–81.0) | 47.0 (28–78) | 50.0 (29–83) | 0.188 |
| Median hs-cTnTc, ng/L | 5 (3–7) | 5 (3–7) | 6 (4–8) | <0.001 |
| Uric acid, mg/dL | 5.0 ± 1.2 | 4.9 ± 1.2 | 5.1 ± 1.3 | <0.001 |
| eGFRd, mL/min/1.73 m2 | 79.2 ± 15.5 | 80.1 ± 15.7 | 78.2 ± 15.4 | 0.002 |
| Hemoglobin A1c, % | 5.6 ± 0.6 | 5.6 ± 0.5 | 5.6 ± 0.6 | 0.001 |
| HDLe-cholesterol, U/L | 63.7 ± 16.8 | 64.7 ± 16.7 | 62.7 ± 16.9 | 0.001 |
| LDLf-cholesterol, U/L | 122.2 ± 30.4 | 122.1 ± 31.0 | 122.2 ± 29.8 | 0.635 |
| Systolic blood pressure, mmHg | 131.7 ± 17.8 | 131.1 ± 19.6 | 133.5 ± 18.7 | <0.001 |
| FMD, % | 5.0 (4.2–11.3) | 7.8 (6.4–9.2) | 4.2 (3.6–4.7) | not applicable |
Values are mean ± SD, or number of subjects (percentage).
Flow-mediated dilation.
N-terminal pro-brain natriuretic peptide.
High-sensitivity cardiac troponin T.
Estimated glomerular filtration rate.
High-density lipoprotein.
Low-density lipoprotein.
3.2. Follow-up study
The mean follow-up period was 5.5 ± 1.8 years (range, 0.6–6.9 years), during which all-cause death, hemorrhagic stroke, and ischemic stroke were identified in 76, 44, and 100 participants, respectively. Among the 100 participants with ischemic stroke, nonlacunar stroke and lacunar stroke were identified in 72 and 28 participants, respectively.
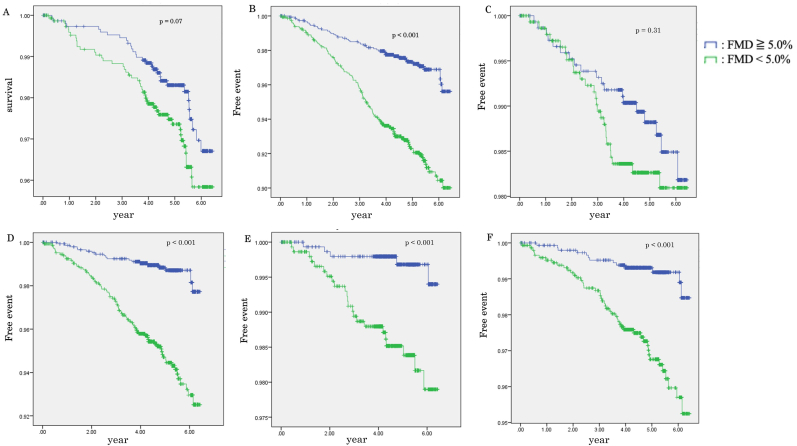
A figure shows the Kaplan–Meier curves for all-cause death and stroke-free rates based on the medium FMD value in all participants. Total stroke-, ischemic stroke-, nonlacunar stroke-, and lacunar stroke-free rates were lower in the lower FMD subgroup (<5.0%) than in the higher FMD subgroup (≥5.0%) (all p values < 0.05, log-rank test) (Figure). No significant differences were observed in the incidences of all-cause death or hemorrhagic stroke between the higher and lower FMD subgroups (Figure).
Fig. 1.
Kaplan–Meier survival and event-time curve according to flow-mediated dilation. (A) All-cause death, (B) total stroke-free, (C) hemorrhagic stroke-free, (D) ischemic stroke-free, (E) lacunar stroke-free, (F) nonlacunar stroke-free probability based on the medium according to flow-mediated dilation values (5.0%).
The results of the Cox hazard model analysis are shown in Table 2. The unadjusted model demonstrated that the lower FMD subgroup had a significantly higher risk for total stroke (hazard ratio [HR] = 2.74, 95% confidence interval [CI] = 1.98–3.88, p < 0.001), ischemic stroke (HR = 4.15, 95% CI = 2.54–6.78, p < 0.001), nonlacunar stroke (HR = 3.75, 95% CI = 2.03–6.93, p < 0.001), and lacunar stroke (HR = 4.77, 95% CI = 1.81–12.56, p = 0.002) than the higher FMD subgroup (Table 2). No significant association was observed between all-cause mortality, hemorrhagic stroke, and the lower FMD subgroup (Table 2).
Table 2.
Hazard ratios for incident stroke according to FMDa levels.
| Model | FMD ≥5.0% |
FMD <5.0% |
|---|---|---|
| HRb | HR (95% CIc), p value | |
| Number of participants | 1486 | 1466 |
| All-cause death | ||
| Person-years | 7514.6 | 7370.3 |
| Number of deaths | 10 | 66 |
| Model 1 | 1 (reference) | 1.55 (0.98–2.46), p = 0.06 |
| Model 2 | 1 (reference) | 1.37 (0.84–2.23), p = 0.21 |
| Total stroke | ||
| Person-years | 7420.4 | 7140.4 |
| Number of cases | 39 | 105 |
| Model 1 | 1 (reference) | 2.74 (1.94–3.88), p < 0.001 |
| Model 2 | 1 (reference) | 2.13 (1.48–3.07), p < 0.001 |
| Hemorrhagic stroke | ||
| Number of cases | 19 | 25 |
| Model 1 | 1 (reference) | 1.37 (0.75–2.49), p = 0.30 |
| Model 2 | 1 (reference) | 1.09 (0.58–2.07), p = 0.77 |
| Ischemic stroke | ||
| Number of cases | 20 | 80 |
| Model 1 | 1 (reference) | 4.15 (2.54–6.78), p < 0.001 |
| Model 2 | 1 (reference) | 3.33 (2.00–5.52), p < 0.001 |
| Nonlacunar stroke | ||
| Number of cases | 15 | 57 |
| Model 1 | 1 (reference) | 3.75 (2.03–6.93), p < 0.001 |
| Model 2 | 1 (reference) | 2.77 (1.49–5.16), p = 0.001 |
| Lacunar stroke | ||
| Number of cases | 5 | 23 |
| Model 1 | 1 (reference) | 4.77 (1.81–12.56), p = 0.002 |
| Model 2 | 1 (reference) | 5.12 (1.74–15.05), p = 0.003 |
Model 1 was univariate Cox regression analysis using FMD values.
Model 2 was multivariate Cox regression analysis using FMD values adjusted for sex, age, smoking, drinker, systolic blood pressure, low-density lipoprotein cholesterol, high-sensitivity cardiac troponin T, estimated glomerular filtration rate, N-terminal pro-brain natriuretic peptide, and hemoglobin A1c.
Flow-mediated dilation.
Hazard ratio.
Confidence interval.
A multivariate Cox analysis also revealed that the lower FMD subgroup was significantly associated with a higher risk for total stroke (HR = 2.13, 95% CI = 1.48–3.07, p < 0.001), ischemic stroke (HR = 3.33, 95% CI = 2.00–5.52, p < 0.001), nonlacunar stroke (HR = 2.77, 95% CI = 1.49–5.16, p = 0.001), and lacunar stroke (HR = 5.12, 95% CI = 1.74–16.05, p = 0.003) than the higher FMD subgroup (Table 2). However, the lower FMD subgroup showed no remarkable association with all-cause mortality (HR = 1.37, 95% CI = 0.84–2.23, p = 0.212) and hemorrhagic stroke (HR = 1.09, 95% CI = 0.58–2.07, p = 0.77) (Table 2).
The cox regression analysis demonstrated that the lower FMD subgroup had the strongest association with stroke incidence in the stroke risk factors (FMD <5.0%: HR = 2.13, 95% CI = 1.48–3.07; hs-cTnT >14 ng/dL: HR = 1.51, 95% CI = 1.23–1.85; hypertension: HR = 1.35, 95% CI = 1.26–1.52; age: HR = 1.10, 95% CI = 1.02–1.23; dyslipidemia: OR = 1.39, 95% CI = 1.18–1.50, Table 3). In addition, FMD as a continuous variable was association with stroke incidence (HR = 1.27, 95% CI = 1.09–1.45, Table 3)
Table 3.
Association between stroke incidence and risk factor, including FMDa levels, using cox regression analysis.
| Variables | HRb (95%CIc) | P value |
|---|---|---|
| Age | 1.10 (1.02–1.23) | <0.001 |
| Women | 0.91 (0.82–0.94) | 0.003 |
| Current or former smoker | 1.41 (1.27–1.53) | <0.001 |
| Drinker | 1.15 (1.10–1.22) | <0.001 |
| Diabetes | 1.24 (1.15–1.44) | <0.001 |
| Hypertension | 1.35 (1.26–1.52) | <0.001 |
| Dyslipidemia | 1.39 (1.18–1.50) | <0.001 |
| FMD <5.0% | 2.13 (1.48–3.07) | <0.001 |
| FMD as a continuous variable | 1.27 (1.09–1.45) | <0.001 |
| NT-proBNPd > 48 pg/mL | 1.06 (1.02–1.11) | 0.01 |
| hs-cTnTe > 0.014 ng/L | 1.51 (1.23–1.85) | <0.001 |
| eGFRf < 60 mL/min/1.73 m2 | 1.08 (1.03–1.14) | 0.004 |
Flow-mediated dilation.
Hazard ratio.
Confidence interval.
N-terminal pro-brain natriuretic peptide.
High-sensitivity cardiac troponin T.
Estimated glomerular filtration rate.
4. Discussion
According to the Kaplan–Meier curves, the total stroke- and ischemic stroke-free rates were lower in the lower FMD subgroup than in the higher FMD subgroup. The multivariate Cox analysis revealed an association between ischemic stroke and lower FMD value. The logistic analysis also suggested that a low FMD value was strongly associated with stroke incidence compared with classical stroke risk factors.
This study elucidates the association between FMD values and stroke risk factors, clearly indicating that subjects in the lower FMD subgroup had a greater susceptibility to hypertension, dyslipidemia, diabetes mellitus, increased hs-cTnT levels, increased BMI and escalated systolic blood pressure. Previous studies have similarly reported a link between decreased FMD values and stroke risk factors such as elevated systolic blood pressure, increased BMI, dyslipidemia, and diabetes [[25], [26], [27], [28]]. The consistent association between low FMD values and stroke risk factors across the present study and previous studies reinforces the premise that vascular endothelial dysfunction is associated with stroke risk factors [28].
The present study also establishes an association between total stroke and ischemic stroke incidence and the group characterized by low FMD values. Furthermore, it associates both lacunar and nonlacunar infarction incidences with this low FMD group. A previous study compared the FMD values of healthy individuals with those of patients with ischemic stroke, revealing that patients with all ischemic stroke subtypes had decreased FMD values compared with those of their healthy counterparts [29]. Nevertheless, there has been no previous report evaluating FMD values to determine stroke incidence in the general Japanese population. A cohort study involving patients with a history of coronary artery disease echoed the findings of our study, reporting higher stroke incidence at low FMD values [10]. Moreover, research conducted in Europe and the United States indicated a higher incidence of CVD within the low FMD group but found no significant discrepancy regarding stroke alone [30]. Thus, to our knowledge, the present investigation is the first global study demonstrating a correlation between FMD levels and stroke incidence within a general population cohort. One explanatory factor for the significant difference observed is the incidence of stroke and the baseline characteristics. Data on stroke incidence reported in 2016 revealed a prevalence of 1.6% in the United States and 1.9% in Japan, yet the present investigation observed a markedly higher incidence rate of 4.6% [31,32]. This difference can be attributed to factors such as the elevated smoking rate (36% in this study compared with the significantly lower average of 16% in Japan) and systolic blood pressure exceeding the Japanese average by 10 mmHg [33,34]. Such conditions may contribute to the higher incidence of stroke observed in this study.
The present study detected a higher stroke risk than classical CVD risk factors among the low FMD subgroup through logistic analysis. Although an association exists between the low FMD values and CVD risk factors, the former is recognized for decreased endothelial nitric oxide synthase (eNOS) activity, independent of CVD risk factors [35]. eNOS is an enzyme that generates nitric oxide. This signaling molecule plays a crucial role in regulating and maintaining vascular tone, as well as cell migration, proliferation, maturation, leukocyte adhesion, and platelet aggregation. Decreased eNOS activity is correlated with endothelial dysfunction, a state characterized by improper endothelium functioning, leading to impaired vasodilation, inflammation, and thrombosis [36]. Reduced eNOS expression is reported to contribute to stroke development [37], possibly underlying the observed higher stroke contribution by the low FMD values.
There was no significant discrepancy between total mortality and cerebral hemorrhage, which could be attributed to the lack of a definitive cause of death determination in this study. Moreover, cerebral hemorrhage may be induced by cerebral amyloid angiopathy and hypertension [38,39]. Furthermore, β-amyloid protein accumulation within the cerebral cortex and leptomeningeal vessels has been identified as a risk determinant for intracerebral hemorrhage, particularly in elderly populations [38]. These insights suggest a distinction in the pathogenesis of ischemic stroke and cerebral hemorrhage.
Our study has several limitations. It depended on self-reported history, which may have included patients with previous strokes. Although this investigation excluded patients diagnosed with atrial fibrillation, no evaluation was performed for new-onset cases during the follow-up phase.
5. Conclusions
In conclusion, our study revealed that a low FMD value might reflect vascular endothelial dysfunction and then was associated with ischemic stroke incidence in the general Japanese population, suggesting that FMD can be used as a tool to identify future stroke risk.
Author statement
Harutomo Numazaki: Methodology, Formal analysis, Writing. Takahito Nasu: Conceptualization, Methodology, Formal analysis, Writing – review & editing, Supervision. Mamoru Satoh: Writing – review & editing. Yuka Kotozaki: Data curation. Kozo Tannno: Data curation. Koichi Asahi: Data curation. Hideki Ohmomo: Data curation. Atsushi Shimizu: Data curation. Shinichi Omama: Data curation. Yoshihiro Morino: Writing – review & editing, Project administration. Kenji Sobue: Data curation, Project administration. Makoto Sasaki: Data curation, Project administration.
Funding support
This work was supported by the Tohoku Medical Megabank Project (Special Account for Reconstruction from the Great East Japan Earthquake) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) and the Japan Agency for Medical Research and Development (AMED). This research was supported by AMED under Grant Numbers JP17km0105003 and P187km0105004.
Declarations of interest
None.
Acknowledgments
The authors thank the members of the Iwate Tohoku Medical Megabank Organization of Iwate Medical University (IMM) and the Tohoku Medical Megabank Organization of Tohoku University (ToMMo) for their encouragement and support. We are grateful to the Tohoku Medical Megabank Project participants.
Handling editor: D Levy
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcrp.2023.200216.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Tsao C.W., Aday A.W., Almarzooq Z.I., Alonso A., Beaton A.Z., Bittencourt M.S., Boehme A.K., Buxton A.E., Carson A.P., Commodore-Mensah Y., et al. Heart disease and stroke statistics-2022 update: a report from the American Heart Association. Circulation. 2022;145:e153–e639. doi: 10.1161/cir.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 2.Adams H.P., Jr., Bendixen B.H., Kappelle L.J., Biller J., Love B.B., Gordon D.L., Marsh E.E., 3rd Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 3.Ross R. Atherosclerosis–an inflammatory disease. N. Engl. J. Med. 1999;340:115–126. doi: 10.1056/nejm199901143400207. [DOI] [PubMed] [Google Scholar]
- 4.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 5.Hansson G.K. Inflammation and immune response in atherosclerosis. Curr. Atherosclerosis Rep. 1999;1:150–155. doi: 10.1007/s11883-999-0011-0. [DOI] [PubMed] [Google Scholar]
- 6.Celermajer D.S., Sorensen K.E., Gooch V.M., Spiegelhalter D.J., Miller O.I., Sullivan I.D., Lloyd J.K., Deanfield J.E. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 7.Cooke J.P., Dzau V.J. Nitric oxide synthase: role in the genesis of vascular disease. Annu. Rev. Med. 1997;48:489–509. doi: 10.1146/annurev.med.48.1.489. [DOI] [PubMed] [Google Scholar]
- 8.Moens A.L., Goovaerts I., Claeys M.J., Vrints C.J. Flow-mediated vasodilation: a diagnostic instrument, or an experimental tool? Chest. 2005;127:2254–2263. doi: 10.1378/chest.127.6.2254. [DOI] [PubMed] [Google Scholar]
- 9.Green D.J., Dawson E.A., Groenewoud H.M., Jones H., Thijssen D.H. Is flow-mediated dilation nitric oxide mediated?: a meta-analysis. Hypertension. 2014;63:376–382. doi: 10.1161/hypertensionaha.113.02044. [DOI] [PubMed] [Google Scholar]
- 10.Maruhashi T., Soga J., Fujimura N., Idei N., Mikami S., Iwamoto Y., Iwamoto A., Kajikawa M., Matsumoto T., Oda N., et al. Endothelial dysfunction, increased arterial stiffness, and cardiovascular risk prediction in patients with coronary artery disease: FMD-J (flow-mediated dilation Japan) Study A. J. Am. Heart Assoc. 2018;7 doi: 10.1161/jaha.118.008588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeboah J., Crouse J.R., Hsu F.C., Burke G.L., Herrington D.M. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the cardiovascular health study. Circulation. 2007;115:2390–2397. doi: 10.1161/circulationaha.106.678276. [DOI] [PubMed] [Google Scholar]
- 12.Kuriyama S., Yaegashi N., Nagami F., Arai T., Kawaguchi Y., Osumi N., Sakaida M., Suzuki Y., Nakayama K., Hashizume H., et al. The Tohoku medical megabank project: design and mission. J. Epidemiol. 2016;26:493–511. doi: 10.2188/jea.JE20150268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hozawa A., Tanno K., Nakaya N., Nakamura T., Tsuchiya N., Hirata T., Narita A., Kogure M., Nochioka K., Sasaki R., et al. Study profile of the Tohoku medical megabank CommunitybBased cohort study. J. Epidemiol. 2021;31:65–76. doi: 10.2188/jea.JE20190271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiyohara Y., Kato I., Iwamoto H., Nakayama K., Fujishima M. The impact of alcohol and hypertension on stroke incidence in a general Japanese population. The Hisayama study. Stroke. 1995;26:368–372. doi: 10.1161/01.str.26.3.368. [DOI] [PubMed] [Google Scholar]
- 15.Shimamoto K., Ando K., Fujita T., et al. Japanese society of hypertension committee for guidelines for the management of hypertension. The Japanese society of hypertension guidelines for the management of hypertension (JSH 2014) Hypertens. Res. 2014;37:253–390. doi: 10.1038/hr.2014.20. [DOI] [PubMed] [Google Scholar]
- 16.Haneda M., Noda M., Origasa H., et al. Japanese clinical practice guide- line for diabetes 2016. Diabetol Int. 2018;9:1–45. doi: 10.1111/jdi.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kinoshita M., Yokote K., Arai H., et al. Committee for Epidemiology and Clinical Management of Atherosclerosis. Japan atherosclerosis society (JAS) guidelines for prevention of atherosclerotic cardiovascular diseases 2017. J. Atherosclerosis Thromb. 2018;25:846–984. doi: 10.5551/jat.GL2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giannitsis E., Kurz K., Hallermayer K., Jarausch J., Jaffe A.S., Katus H.A. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin. Chem. 2010;56:254–261. doi: 10.1373/clinchem.2009.132654. [DOI] [PubMed] [Google Scholar]
- 19.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am. J. Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 20.Matsuo S., Imai E., Horio M., Yasuda Y., Tomita K., Nitta K., Yamagata K., Tomino Y., Yokoyama H., Hishida A. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 21.Corretti M.C., Anderson T.J., Benjamin E.J., Celermajer D., Charbonneau F., Creager M.A., Deanfield J., Drexler H., Gerhard-Herman M., Herrington D., et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J. Am. Coll. Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 22.Omama S., Yoshida Y., Ogawa A., Onoda T., Okayama A. Differences in circadian variation of cerebral infarction, intracerebral haemorrhage and subarachnoid haemorrhage by situation at onset. J. Neurol. Neurosurg. Psychiatry. 2006;77:1345–1349. doi: 10.1136/jnnp.2006.090373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. International Statistical Classification of Diseases and Related Health Problems, 10th rev. Geneva: WHO. http://www.who.int/classifications/icd/ICD10Volume2_en_2010. pdf (accessed November 27, 2017)..
- 24.World Health Organization . MONICA Manual, Part IV, Event Registration. November 1990. MONICA Project. Section 2: stroke event registration data component.http://www.thl.fi/publications/monica/manual/part4/iv-2.htm [Google Scholar]
- 25.Pedralli M.L., Marschner R.A., Kollet D.P., Neto S.G., Eibel B., Tanaka H., Lehnen A.M. Different exercise training modalities produce similar endothelial function improvements in individuals with prehypertension or hypertension: a randomized clinical trial Exercise, endothelium and blood pressure. Sci. Rep. 2020;10:7628. doi: 10.1038/s41598-020-64365-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esposito K., Ciotola M., Schisano B., Gualdiero R., Sardelli L., Misso L., Giannetti G., Giugliano D. Endothelial microparticles correlate with endothelial dysfunction in obese women. J. Clin. Endocrinol. Metab. 2006;91:3676–3679. doi: 10.1210/jc.2006-0851. [DOI] [PubMed] [Google Scholar]
- 27.Tushuizen M.E., Nieuwland R., Rustemeijer C., Hensgens B.E., Sturk A., Heine R.J., Diamant M. Elevated endothelial microparticles following consecutive meals are associated with vascular endothelial dysfunction in type 2 diabetes. Diabetes Care. 2007;30:728–730. doi: 10.2337/dc06-1473. [DOI] [PubMed] [Google Scholar]
- 28.Benincasa G., Coscioni E., Napoli C. Cardiovascular risk factors and molecular routes underlying endothelial dysfunction: novel opportunities for primary prevention. Biochem. Pharmacol. 2022;202 doi: 10.1016/j.bcp.2022.115108. [DOI] [PubMed] [Google Scholar]
- 29.Santos-García D., Blanco M., Serena J., Arias S., Millán M., Rodríguez-Yáñez M., Leira R., Dávalos A., Castillo J. Brachial arterial flow mediated dilation in acute ischemic stroke. Eur. J. Neurol. 2009;16:684–690. doi: 10.1111/j.1468-1331.2009.02564.x. [DOI] [PubMed] [Google Scholar]
- 30.Yeboah J., Folsom A.R., Burke G.L., Johnson C., Polak J.F., Post W., Lima J.A., Crouse J.R., Herrington D.M. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation. 2009;120:502–509. doi: 10.1161/circulationaha.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Global, Regional, and National Burden of Stroke, 1990-2016: a Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;vol. 18:439-458. doi: 10.1016/s1474-4422(19)30034-1. [DOI] [PMC free article] [PubMed]
- 32.Saito I., Yamagishi K., Kokubo Y., Yatsuya H., Iso H., Sawada N., Inoue M., Tsugane S. Association between mortality and incidence rates of coronary heart disease and stroke: the Japan public health center-based prospective (JPHC) study. Int. J. Cardiol. 2016;222:281–286. doi: 10.1016/j.ijcard.2016.07.222. [DOI] [PubMed] [Google Scholar]
- 33.Katoh K. Health advocacy for reducing smoking rates in Hamamatsu, Japan. Hypertens. Res. 2020;43:634–647. doi: 10.1038/s41440-020-0418-0. [DOI] [PubMed] [Google Scholar]
- 34.Sugiura T., Takase H., Machii M., Hayashi K., Nakano S., Takayama S., Seo Y., Dohi Y. Blood pressure variability and the development of hypertensive organ damage in the general population. J. Clin. Hypertens. 2022;24:1405–1414. doi: 10.1111/jch.14526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nezu T., Hosomi N., Aoki S., Kubo S., Araki M., Mukai T., Takahashi T., Maruyama H., Higashi Y., Matsumoto M. Endothelial dysfunction is associated with the severity of cerebral small vessel disease. Hypertens. Res. 2015;38:291–297. doi: 10.1038/hr.2015.4. [DOI] [PubMed] [Google Scholar]
- 36.Tran N., Garcia T., Aniqa M., Ali S., Ally A., Nauli S.M. Endothelial nitric oxide synthase (eNOS) and the cardiovascular system: in physiology and in disease states. Am. J. Biomed. Sci. Res. 2022;15:153–177. [PMC free article] [PubMed] [Google Scholar]
- 37.Tan X.L., Xue Y.Q., Ma T., Wang X., Li J.J., Lan L., Malik K.U., McDonald M.P., Dopico A.M., Liao F.F. Partial eNOS deficiency causes spontaneous thrombotic cerebral infarction, amyloid angiopathy and cognitive impairment. Mol. Neurodegener. 2015;10:24. doi: 10.1186/s13024-015-0020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garg P.K., Bartz T.M., Burke G., Gottdiener J.S., Herrington D., Heckbert S.R., Kizer J.R., Sotoodehnia N., Mukamal K.J. Brachial flow-mediated dilation and risk of atrial fibrillation in older adults: the cardiovascular health study. Vasc. Health Risk Manag. 2021;17:95–102. doi: 10.2147/vhrm.S297720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Donnell H.C., Rosand J., Knudsen K.A., Furie K.L., Segal A.Z., Chiu R.I., Ikeda D., Greenberg S.M. Apolipoprotein E genotype and the risk of recurrent lobar intracerebral hemorrhage. N. Engl. J. Med. 2000;342:240–245. doi: 10.1056/nejm200001273420403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed in the current study are not publicly available due to ethical reasons but are available upon request after approval from the Ethical Committee of Iwate Medical University, the Ethical Committee of Tohoku University, and the Materials and Information Distribution Review Committee of the TMM Project.