Abstract
Adoptive transfer of natural killer (NK) cells has been proposed as a novel immunotherapy for malignant tumours resistant to current therapeutic modalities. Several clinical studies have demonstrated that the NK cell-infusion is well tolerated without severe side effects and shows promising results in haematological malignancies. However, patients with malignant solid tumours do not show significant responses to this therapy. Such disappointing results largely arise from the inefficient delivery of infused NK cells and the impairment of their functions in the tumour microenvironment (TME). Tumour-associated macrophages (TAMs) are the most abundant stromal cells in the TME of most solid tumours, and a high TAM density correlates with poor prognosis of cancer patients. Although our knowledge of the interactions between TAMs and NK cells is limited, many studies have indicated that TAMs suppress NK cell cytotoxicity against cancer cells. Therefore, blockade of TAM functions can be an attractive strategy to improve NK cell-based immunotherapies. On the other hand, macrophages are reported to activate NK cells under certain circumstances.
This essay presents our current knowledge about mechanisms by which macrophages regulate NK cell functions and discusses possible therapeutic approaches to block macrophage-mediated NK cell suppression.
Keywords: cancer, immunotherapy, macrophage, natural killer cell, tumor microenvironments
Introduction
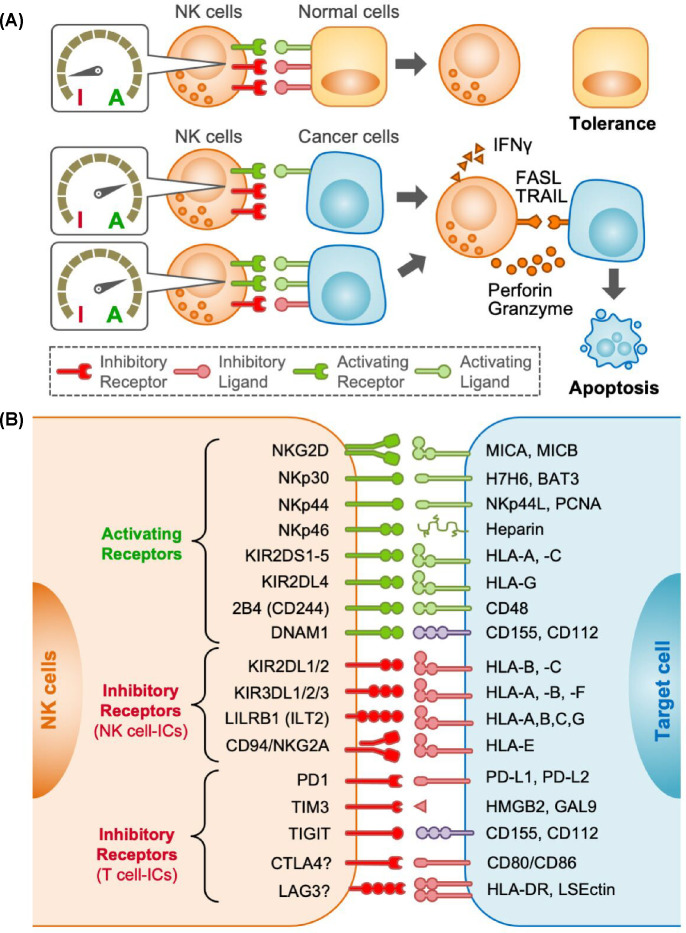
Immunotherapies such as immune checkpoint inhibitors (ICIs) aim at boosting cytotoxic CD8+ T cell activation, which requires antigen presentation from targets via MHC class I (MHC-I) molecules. In many cases, however, malignant tumour cells reduce or lack MHC-I and/or tumour-specific antigens and avoid T cell attack. NK cells, however, do not require antigen presentation to kill targets. Instead, NK cell activation is regulated by a net balance of activating and inhibitory signals (Figure 1A). When inhibitory ligands are expressed in target cells whilst no or low activating ligands are expressed, inhibitory signals overwhelm activating signals, and thus NK cells do not attack them (tolerance). However, when inhibitory ligands are down-regulated (‘missing-self’) or activating ligands are up-regulated (induced-self) in target cells, NK cells are engaged to kill the targets via the secretion of cytotoxic vesicles containing perforin and granzymes, expression of death ligands such as Fas ligand (FASL) and tumour necrosis factor-related apoptosis inducing ligand (TRAIL), and production of interferon-γ (IFNγ) [1].
Figure 1. Regulation of NK cell responses against target cells.
(A) Natural killer (NK) cells express various receptors that transmit activation (A) or inhibitory (I) signals. NK cell response to target cells (being tolerant or triggering apoptosis) is determined by the overall levels of each signalling input. When target cancer cells reduce inhibitory ligands or increase activating ligands, activation signals overweigh inhibitory signals in NK cells. Activated NK cells induce apoptosis by secreting cytotoxic vesicles containing perforin and granzyme-B or presenting death ligands such as FASL and TRAIL. Activated NK cells also secrete interferon-γ (IFNγ) that induces apoptosis in certain cancer cells and regulates anti-immune reactions. (B) Canonical NK cell activating receptors (green) and inhibitory receptors (red) expressed by human NK cells and their ligands in target cells are shown. Potential inhibitory receptors known as T-cell immune checkpoints (ICs) are also shown. CD155 and CD112 (purple) can transmit both activation and inhibitory signals depending on the receptors. CTLA4 and LAG3 involvement in NK cell regulation is unclear and shown with ”?”.
Therefore, the adoptive transfer of NK cells has been proposed as an alternative immunotherapy for cancer cells that evade T cell-mediated elimination [2]. Clinical trials have shown that NK cell infusion therapy is safe and effective for haematological cancers (e.g., leukaemia). However, no clear effects have been observed on solid tumours [3], which is believed to be primarily due to the presence of an immunosuppressive tumour micro-environment (TME) along with the inefficient delivery of therapeutic NK cells [4,5].
Solid tumours consist of a range of immune cells such as regulatory T cells, myeloid-derived suppressor cells, neutrophils, and macrophages that can suppress anti-tumour immune reactions [6,7]. Among these cells, macrophages are most abundant in most solid tumours, and high macrophage infiltration correlates with poor patient prognosis [8]. Moreover, numerous animal studies have identified that these tumour-associated macrophages (TAMs) are differentiated from monocytes recruited to the TME and promote tumour progression [9]. Since these recruited macrophages possess immunosuppressive phenotypes, they are considered a potential target for cancer immunotherapies [10]. Solid tumours, however, include macrophages derived from embryonic precursors (i.e., tissue-resident macrophages) that can contribute to antitumour immunity [11,12]. Furthermore, macrophages change their pro-inflammatory phenotype to anti-inflammatory or vice versa in response to environmental factors [13]. Such diversity and plasticity allow TAMs to regulate immune responses positively or negatively depending on the context. Consistent with this, contradictory effects of TAMs on NK cell functions are reported. For example, TAMs isolated from mouse mammary tumours impair the cytotoxicity of NK cells [14] and the higher TAM density correlates with a lower ratio of activated NK cells in human gastric cancer [15], which suggests NK cell suppressive roles for TAMs. In human liver cancer, however, NK cells in the peri-tumoral stroma where they co-localize with TAMs express an activation marker CD69 more frequently than NK cells in the TAM-sparse intra-tumoral region [16], which implies that TAMs promote NK cell function under certain circumstances.
This Essay discusses current understanding of macrophage effects on NK cell functions and potential approaches to prevent the macrophage-mediated NK cell suppression.
NK cell activation by macrophages
The activation signal is transduced to human NK cells through natural killer cell protein group 2D (NKG2D) upon binding of its ligands MICA and MICB (RAE1 and MULT1 in mice), as well as via NKp30, NKp44, NKp46, activating killer cell immunoglobulin-like receptors (KIRs), 2B4, and DNAM1 that bind to different ligands [17,18] (Figure 1B).
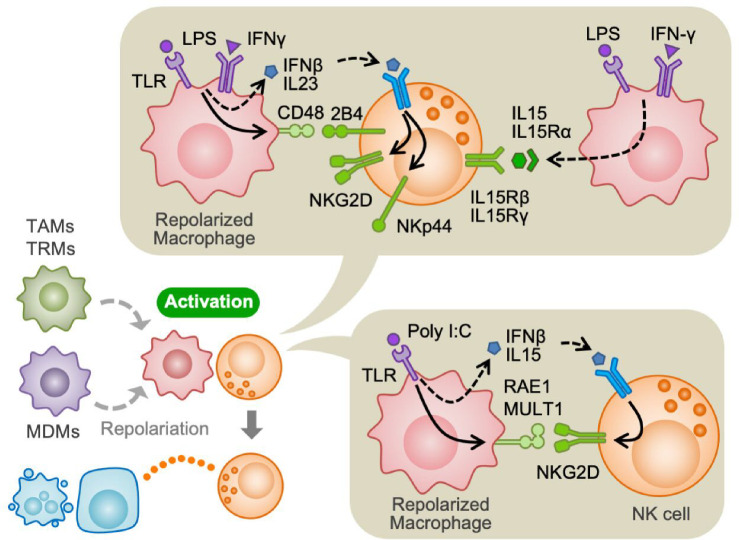
It has been reported that tissue-resident (peritoneal) macrophages treated with poly I:C, a toll-like receptor (TLR) agonist, increase NK cell cytotoxicity against target cancer cells by increasing NKG2D expression on NK cells via secretion of IFNβ and IL15. Poly I:C also increases the expression of NKG2D ligands (RAE1, MULT1) in macrophages, which collectively enhance activating signals in NK cells [19]. Consistent with this result, poly I:C administration suppresses the metastatic growth of melanoma cells in mice by activating NK cells. In this model, alveolar macrophages from tumour-bearing mice treated with poly I:C promoted NK cell cytotoxicity in vitro [20]. Another study demonstrated that macrophages from the ascites of ovarian cancer patients significantly increased the NK cell-mediated cytolysis of ovarian cancer cells upon treatment with lipopolysaccharide (LPS) [21]. Similarly, human monocyte-derived macrophages (MDMs) activated by LPS promote proliferation, IFNγ secretion, and cytotoxicity of NK cells in vitro. Such macrophage-mediated NK cell activation requires CD48 expressed on macrophages and its receptor 2B4 expressed on NK cells [22]. It is also reported that co-culture of NK cells with human MDMs treated with LPS and IFNγ increases expression of activation markers (CD69 and CD107) and IFNγ in NK cells. Mechanistically, LPS and IFNγ increase CD48 expression on MDMs and promote IFNγ secretion from NK cells through 2B4. In addition, LPS/IFNγ stimulated MDMs produce IFNβ and IL23 and thereby enhance the expression of activating receptors NKG2D and NKp44 on NK cells. Moreover, LPS/IFNγ stimulated MDMs express high levels of IL15/IL15Rα membrane-bound complex, which also promotes NK cell activation [23].
Collectively, these data suggest that macrophages in TME can promote NK cell cytotoxicity when they are exposed to inflammatory stimuli such as TLR agonists, e.g., poly I:C, LPS, and/or IFNγ (Figure 2).
Figure 2. Activation of NK cells by macrophages.
Tumour-associated macrophages (TAMs), tissue-resident macrophages (TRMs) and monocyte-derived macrophages (MDMs) repolarize upon stimulation with IFNγ and/or toll-like receptor (TLR) ligands such as poly I:C and lipopolysaccharide (LPS). Repolarized macrophages activate NK cells by expressing activating ligands (RAE1, MULT1, CD48), inducing activating receptors in NK cells (NKG2D, 2B4, NKp44), and secreting IL-15. NK cells activated by macrophages increase cancer cell killing capability (indicated by an orange dot line). RAE1 and MULT1 are NKG2D ligands in mice.
NK cell inhibition by macrophages
Persistent engagement of activating ligands
It is reported that persistent engagement of activating ligands induces tolerance of NK cells [24,25], which could be a mechanism behind TAM-mediated NK cell suppression. In mice injected with melanoma cells, membrane NKG2D expression in tumour-infiltrating NK cells is downregulated whereas such a reduction is not found in Rae1 knockout mice. In this model, TAMs increase RAE1 expression on their surfaces. Interestingly, sustained exposure (longer than 8 hours) to RAE1+ macrophages reduces NK cell activation induced by an agonistic NKp46 antibody, whereas such suppression is blocked by a recombinant NKG2D ligand. These results suggest that persistent engagement with NKG2D ligands on TAMs causes NKG2D down-regulation and subsequent desensitization in NK cells [26]. It is also reported that expression of NK cell activation markers (e.g., CD69, TRAIL, granzyme B) is increased by short-term (2 days) exposure to TAMs from human liver cancer, whereas long-term (8 days) exposure decreases. This macrophage-induced NK cell activation and subsequent dysfunction is attenuated by blocking 2B4 on NK cells [16]. Since prolonged contact with CD48+ cells internalizes membrane 2B4 expression in NK cells [27], it is likely that TAMs expressing activating ligands initially promote NK cell activation but lead to NK cell inactivation by persistent engagement (Figure 3A).
Figure 3. Suppression of NK cells by macrophages.
(A) Short-term interaction with TAMs expressing activating ligands (RAE1, CD48) promotes NK cell activation via activating receptors such as NKG2D and 2B4 (left). However, persistent engagement causes downregulation of activating receptors in NK cells and desensitizes NK cells (right). (B) Macrophages stimulated with poly I:C enhance NK cell cytotoxicity against cancer cells despite they express high levels of the inhibitory ligand, MHC class I (MHC-I). Thus, MHC-I expressed in macrophages may not inactivate NK cells persistently but protect themselves from NK cell killing. Ly49 is a mouse homologue of human inhibitory killer cell immunoglobulin-like receptor (KIR). (C) NK cells from cancer patients express canonical T cell immune checkpoint receptors such as PD1, TIM3, and TIGIT that suppress NK cell activation. Although TAMs can express their ligands (i.e., PD-L1, GAL9, CD155) depending on the tumour context, the extent to which they contribute to NK cell suppression remains unclear. (D) TAMs can produce growth arrest-specific gene 6 (GAS6) that inhibits NK cell activation through its receptors such as AXL. TAMs suppresses NK cell functions also by secreting transforming growth factor-β (TGFβ) or by expressing membrane-bound TGFβ.
Inhibition by immune checkpoint (IC) molecules
Canonical NK cell-ICs
The most characterized inhibitory receptors in human NK cells are inhibitory KIRs, leukocyte immunoglobulin-like receptors (LILRs), and NKG2A/CD94 that all bind to MHC-I [17,28]. They are known as canonical NK cell immune checkpoints (ICs) as they involve in NK cell tolerance [18] (Figure 1B).
A few studies have shown that a subset of TAMs express high levels of MHC-I (HLA-A) in human melanoma, liver cancer, and lung cancer [29–31]. However, MHC-I is not essential for suppressing NK cells in some in vitro models. For example, human NK cells co-cultured with MHC-Ihigh autologous monocytes do not impair their ability to lyse target cancer cells [32], and blockades of mouse MHC-I molecules or their receptors (Ly49) do not restore NK cell suppression induced by MHC-Ihigh bone marrow-derived macrophages (BMDMs) [33]. It is also reported that RAW264.7 mouse macrophage cell line expresses high MHC-I (H2-Qa1) upon stimulation with poly I:C, and these MHC-Ihigh macrophages enhance rather than suppress NK cell cytotoxicity. Interestingly, blockade of H2-Qa1 increases the NK cell-mediated macrophage lysis [19]. It is therefore possible that MHC-I on macrophages protect themselves from NK cell killing but may not inactivate NK cells persistently (Figure 3B).
Canonical T cell-ICs
Several ICs such as programmed cell death protein 1 (PD1), cytotoxic T-lymphocyte associated protein 4 (CTLA4), lymphocyte-activation gene 3 (LAG3), T-cell immunoglobulin and mucin domain 3 (TIM3), and T-cell immunoreceptor with immunoglobulin and ITIM domain (TIGIT) are known to regulate CD8+ T cell tolerance. However, their involvements in NK cell regulation are less understood.
PD1 expression is very low in naïve and activated NK cells from healthy donors but increased in NK cells from cancer patients [18]. In the lymph node of lymphoma patients, expressions of PD1 and its ligands PD-L1/PD-L2 are increased in NK cells and TAMs respectively. Interestingly, activation of PD-1hi NK cells is suppressed by human PD-L1hi MDMs, and this suppression is blocked by anti-PD1 antibodies [34]. CTLA4 competitively inhibits the binding of the co-stimulatory receptor CD28 and its ligands CD80/CD86, which is essential for T-cell activation. However, human NK cells do not express CTLA4 and are not co-stimulated by CD80 [35]. LAG3 is a receptor for MHC class II and LSECtin and regulates CD4+ and CD8+ T cell functions. However, blocking LAG3 does not affect human NK cell activity toward MHC-II+ target cells [36]. These data suggest minor or no involvement of CTLA4 or LAG3 in NK cell regulation. TIM3 is expressed by naïve NK cells from healthy donors and is upregulated in patients with melanoma, gastric cancer, and lung cancer [37–40]. It is reported that TIM3 cross-linking by antibodies suppresses human NK cell cytotoxicity [37], and TIM3 blockade reverses the impaired cytotoxicity or exhausted phenotype of patient-derived NK cells [39,40]. Interestingly, expression of HMGB1, a TIM3 ligand, is up-regulated in LPS-treated human MDMs and lactate-treated THP-1 macrophages [41,42]. Moreover, a subset of TAMs in invasive bladder cancer expresses another ligand galectin-9 (GAL9) [43]. However, some studies demonstrated that TIM3 ‘promotes’ rather than inhibits IFNγ secretion and cytotoxicity of human NK cells [44,45]. TIGIT is also expressed by NK cells in healthy donors and suppresses IFNγ production and cytotoxicity of NK cells upon engagement with its ligand CD155 [46–48]. In a mouse colon cancer model, TIGIT blockade prevents NK cell exhaustion and promotes NK cell-dependent tumour immunity [49]. Interestingly, TAMs from human colorectal cancer express high levels of CD155 compared with macrophages from adjacent normal tissues. Moreover, THP-1 macrophages reduce IFNγ and granzyme B expression of CD8+ T cells via a CD155 dependent mechanism [50], although their effects on NK cells are not investigated.
Taken together, current data suggest that TAMs may utilize signalling pathways mediated by PD1 and TIGIT to suppress NK cells in TME. However, further studies are necessary to define the role of these ICs in TAM-mediated NK cell regulation (Figure 3C).
Inhibition by non-IC inhibitory molecules
A few molecules other than ICs are suggested to cause NK cell dysfunction. For example, sialic acid-binding immunoglobulin-like lectins (SIGLECs) and carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) are expressed by activated NK cells and their blockade enhances NK cell cytotoxicity against cancer cells [51,52]. However, it has not been investigated whether TAMs utilize these pathways to suppress NK cells. Receptors for growth arrest-specific gene 6 (GAS6), i.e., AXL, TYRO3, and MER, are also expressed by NK cells and are essential for their functional maturation [53]. Interestingly, GAS6 is reported to be expressed by TAMs in mouse models of breast and colon cancer [54] and inhibit IFNγ production and degranulation of NK cells in vitro by attenuating NKG2D activating receptor [55]. GAS6 is therefore an intriguing factor that may cause NK cell dysfunction within the TME. Another well-known NK cell inhibitory molecule is transforming growth factor β (TGFβ). For example, TAMs from human gastric cancer impair NK cell IFNγ expression, which is rescued by TGFβ blockade [15]. TAMs isolated from mouse mammary tumours and mouse BMDMs stimulated with IL-4 also inhibit NK cell activation by secreting TGFβ [14]. In a mouse model of metastatic breast cancer, TAMs from the tumour-bearing lung express membrane-bound TGFβ, and anti-TGFβ blocking antibody reverses TAM-mediated impairment of NK cell cytotoxicity [33]. As a potential mechanism, TGF reduces NKG2D surface expression on NK cells [56].
Collectively, these data suggest that TAMs constrain NK cell cytotoxicity by expressing GAS6 and/or TGFβ, although it remains unclear how much TAM-derived GAS6 contributes to NK cell suppression (Figure 3D).
TAM targeting strategies that potentially improve NK cell efficacy
Although several TAM-targeting strategies have been investigated in tumour context [10], their effects on the NK cell-infusion therapy remain unknown. Nevertheless, some pre-clinical studies suggest their potential to enhance endogenous NK cell functions (Figure 4).
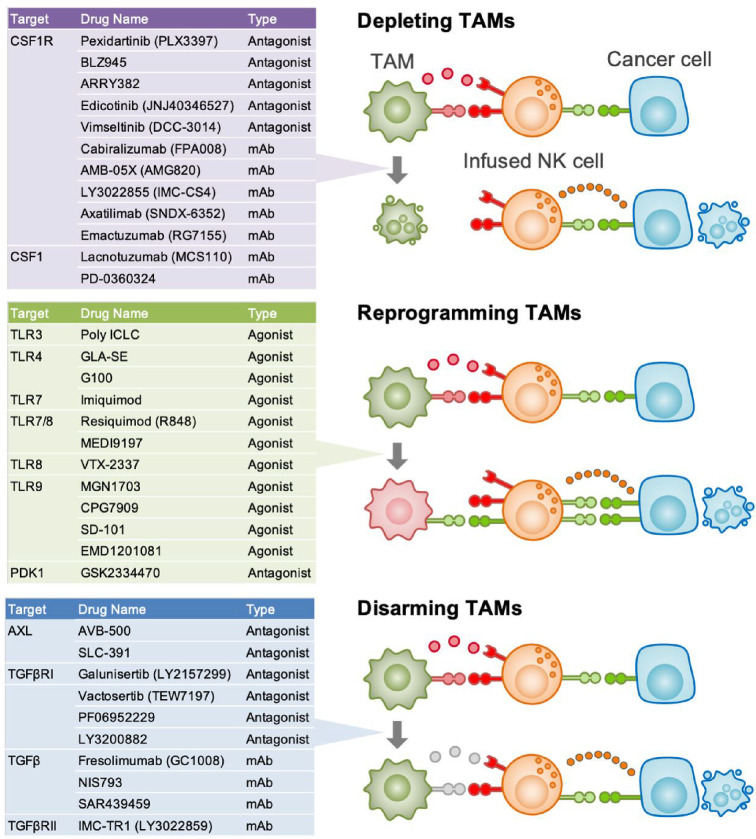
Figure 4. Current TAM-targeting strategies that potentially enhance NK cell function.
Anti-tumour efficacy of therapeutically infused NK cells could be enhanced by combining with TAM targeting, such as depletion, reprogramming, and disarming (i.e., blocking inhibitory ligands in TAMs). Tables show selective examples of compounds for each strategy that are currently tested in clinical trials as monotherapies or in combination with chemotherapeutics or immune checkpoint inhibitors. Note that effects of these compounds on the NK cell-based immunotherapies have not been investigated yet. CSF1R and TGFβR are the receptor for CSF11 and TGFβ, respectively.
Depleting TAMs
Colony-stimulating factor 1 (CSF1) signalling is essential for the survival of most macrophage populations including TAMs, and thus CSF1 receptor (CSF1R) blockade reduces the number of TAMs in solid tumours. In a mouse breast cancer model, Csf1r knockout suppresses TAM accumulation and activates NK cells in metastatic tumours. Importantly, adoptive transfer of NK cells significantly reduces early metastatic tumour expansion only in TAM-depleted mice, suggesting that TAM depletion not only enhances intrinsic NK cell activity but also improves the efficacy of infused NK cells in suppressing metastatic tumour development [33].
Given pro-tumour functions of TAMs, several CSF1R antagonists have been tested as monotherapy or in combination with ICIs in some malignant tumours [10]. However, it is unknown whether these inhibitors improve NK cells’ anti-tumour functions in the TME. Importantly, a lung cancer mouse model demonstrated that a CSF1R inhibitor reduces the number of NK cells in the TME and enhances tumour metastasis [57]. This study suggests that CSF1R blockade may compromise NK cell functions as CSF1 signalling is essential not only for TAMs but also tissue-resident macrophages that can potentially support NK cells [58].
Another potential target is extracellular signal-regulated kinase 5 (ERK5) signalling since myeloid cell-selective knockout of ERK5 blocks TAM proliferation and metastatic tumour loads in a metastatic melanoma mouse model [59]. However, further studies are needed to determine whether NK cell functions are enhanced by TAM deletion via ERK5 blockade.
Reprogramming TAMs
Macrophages alter their effects on NK cells depending on signals they receive. As described above (Figure 2), TLR signals prompt macrophages to activate NK cells. In a mouse model of squamous cell carcinoma, intra-tumoral injection of a TLR agonist induces repolarization of TAMs and suppresses tumour growth when combined with ICI treatment. NK cells contribute to the suppressive effect of this combination therapy whereas CD8+ T cells are also responsible for it [60]. Similar results were found in a mouse melanoma model, although NK cells are not involved in tumour suppression in this model [61].
Another potential target to reprogramme TAM is macrophage receptor with collagenous structure (MARCO), a scavenger receptor predominantly expressed by TAMs. Administration of anti-MARCO antibody induces repolarization of TAMs and suppresses tumour growth in mouse models of solid tumours [62]. In a mouse melanoma model, TAMs reprogrammed by anti-MARCO antibody activate NK cells to kill tumour cells via TRAIL [63].
3-Phosphoinositide-dependent kinase 1 (PDK1) is also a potential target since myeloid-selective knockout of PDK1 alters the phenotype of TAMs, enhances IFNγ expression in NK cells, and suppresses tumour growth in a mouse breast cancer model [64].
Although several TLR agonists and PDK1 inhibitors/antibodies are being tested in clinical trials, their therapeutic effects have not yet been reported [65,66]. Moreover, further pre-clinical studies are needed to determine whether these strategies can enhance NK cell infusion therapy efficacy.
Disarming TAMs
Blocking NK cell suppressive molecules expressed by TAMs is another potential strategy. In mouse models of metastatic breast cancer and melanoma, treatment with a GAS6 receptor inhibitor markedly reduces tumour cell metastases through NK cell activation [67]. The administration of anti-Gas6 neutralising antibodies also promotes NK cell activation and suppresses metastatic tumour development in a mouse pancreatic cancer model [68]. However, their effects on TAM-mediated suppression of infused NK cells have not yet been clarified.
Since TGFβ signalling involves not only immune suppression but also malignant characteristics of tumour cells, several antibodies and inhibitors of TGFβ receptor have been developed and tested in clinical trials [69]. In a mouse breast cancer model, an anti-TGFβ antibody inhibits TAM-mediated NK cell suppression in vitro, and a selective TGFβ receptor antagonist partially but significantly suppresses early metastatic tumour growth when combined with NK cell infusion in vivo [33]. The combination therapy, however, failed to inhibit the late metastatic tumour expansion. It is thus possible that blockade of other NK suppressive molecules in addition to TGFβ might be required for TAM-targeting/NK cell-infusion combination therapy to eliminate established tumours.
Future research directions
Current preclinical data suggest that combining TAM targeting with NK cell infusion could be a novel form of immunotherapy, although it has not been applied clinically yet. To facilitate the development of effective therapeutic approaches targeting TAM–NK cell interactions, it is crucial to address the following challenges.
Most in vitro and pre-clinical studies on TAM–NK cell interactions utilize endogenous NK cells or freshly isolated NK cells that may not necessarily represent NK cells being infused in clinical trials. Recently, a technique for producing NK cells from human-induced pluripotent stem cells (iPSCs) has been established, which is considered a next-generation tool for NK cell infusion therapy. It will be imperative to investigate whether and how TAMs affect the efficacy of these therapeutic NK cells.
Macrophages with different origins and activation status play distinct roles in immune regulation. Although RNA sequencing data have defined various TAM subsets with distinct transcriptomic profiles [70], their spatial localization in the TME or functions in the NK cell regulation are largely unknown. Emerging technologies such as spatial transcriptomics and multiplex immunostaining will facilitate answer these questions and thereby develop selective TAM targeting approaches.
Macrophages stimulated with IFNγ and/or TLR ligands promote NK cell activation (Figure 2). These pro-inflammatory ligands, however, can also direct macrophages to express PD-L1 that inhibits anti-tumour immunity [71–73]. This highlights the need to investigate which factors in TME change macrophage functions in regulating NK cells by what mechanisms.
Antibodies against PD1, PD-L1 or CTLA4 have achieved substantial success in the treatment of some cancers, but they are not effective in a large cohort of cancer patients. This prompted the investigation of other inhibitory pathways and led to the identification of novel immune checkpoint receptors such as TIM3 and TIGIT. Similarly, the simultaneous targeting of different immunosuppressive pathways will be required to improve the efficacy of NK cell-infusion therapy, and thus, the identification of novel inhibitory ligands expressed by NK cell-suppressive macrophages is critical.
NK cell-derived cytokines impact dendritic and T-cell functions, promoting a conducive environment for tumour elimination [74,75]. While NK cells are also known to alter the macrophage phenotype in infectious diseases, little is known in tumour context [76,77]. Exploring the influence of endogenous and adoptively transferred NK cells on TAM function is crucial for developing therapeutics selectively targeting NK cell suppressive macrophage subsets.
Cancer patients typically undergo chemotherapy or radiotherapy before trying immunotherapy. These conventional therapies have a negative impact on the survival of ‘intrinsic’ NK cells. However, they can increase tumour cell susceptibility to NK cells and create a favourable environment for “transferred” NK cells, which may benefit NK cell-infusion therapy [78,79]. Moreover, chemotherapy and radiotherapy can change the phenotype of TAMs to be immunostimulatory [80,81], although their effects on NK cell functions are unknown. Moreover, the response of the TME to chemotherapy and radiation could vary in individual cases. Therefore, it is important to define the effects of conventional treatments on TAM–NK cell interactions under different conditions (e.g., type of therapeutic, treatment schedule, tumour types) to design effective clinical trials. Advanced humanized mouse models supporting the engraftment of human macrophages and NK cells will greatly help answer this pertinent question.
Summary
TAMs constrain NK cells’ anti-tumour functions by downregulating activating receptors (NKG2D, 2B4) in NK cells or by expressing distinct immunosuppressive ligands such as PD-L1, GAL9, CD155, GAS6, and TGFβ.
The TAMs’ NK cell-suppressive functions can be altered by targeting a distinct receptor or kinase such as TLR, MARCO, and PDK1.
Blockade of TAM-derived immunosuppressive ligands such as GAS6 and TGFβ could inhibit NK cell dysfunction in the TME.
TAM targeting is a prospective strategy for improving NK cell-based immunotherapies, although further research is required to find efficient targets for blocking TAM-mediated NK cell suppression.
Abbreviations
- BMDM
bone marrow-derived macrophage
- CSF1
colony-stimulating factor 1
- CSF1R
CSF1 receptor
- CTLA4
cytotoxic T-lymphocyte associated protein 4
- GAL9
galectin-9
- GAS6
growth arrest-specific gene 6
- IC
immune checkpoint
- ICI
immune checkpoint inhibitor
- IFNγ
interferon-γ
- IL
interleukin
- KIR
killer cell immunoglobulin-like receptor
- LAG3
lymphocyte-activation gene 3
- LILR
leukocyte immunoglobulin-like receptor
- LPS
lipopolysaccharide
- MARCO
macrophage receptor with collagenous structure
- MDM
monocyte-derived macrophage
- MHC-I
major histocompatibility complex class I
- NK
natural killer
- NKG2D
natural killer cell protein group 2D
- PD1
programmed cell death protein 1
- PDK1
3-phosphoinositide-dependent kinase 1
- TAM
tumour-associated macrophage
- TGFβ
transforming growth factor-β
- TIGIT
T-cell immunoreceptor with immunoglobulin and ITIM domain
- TIM3
T-cell immunoglobulin and mucin domain 3
- TLR
Toll-like receptor
- TME
tumour microenvironment
- TRAIL
tumour necrosis factor-related apoptosis inducing ligand
Competing Interests
The author declares that there are no competing interests associated with the manuscript.
Funding
This work is supported by the UK Medical Research Council [grant number MR/S006982/1 and MR/N022556/1].
Open Access
Open access for this article was enabled by the participation of The University of Edinburgh in an all-inclusive Read & Publish agreement with Portland Press and the Biochemical Society under a transformative agreement with JISC.
References
- 1.Myers J.A. and Miller J.S. (2021) Exploring the NK cell platform for cancer immunotherapy. Nat. Rev. Clin. Oncol. 18, 85–100 10.1038/s41571-020-0426-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shimasaki N., Jain A. and Campana D. (2020) NK cells for cancer immunotherapy. Nat. Rev. Drug Discov. 19, 200–218 10.1038/s41573-019-0052-1 [DOI] [PubMed] [Google Scholar]
- 3.Suen W.C.W., Lee W.Y.W., Leung K.T., Pan X.H. and Li G. (2018) Natural killer cell-based cancer immunotherapy: a review on 10 years completed clinical trials. Cancer Invest. 36, 431–457 10.1080/07357907.2018.1515315 [DOI] [PubMed] [Google Scholar]
- 4.Carlsten M. and Childs R.W. (2015) Genetic manipulation of NK cells for cancer immunotherapy: Techniques and clinical implications. Front. Immunol. 6, 266 10.3389/fimmu.2015.00266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vitale M., Cantoni C., Pietra G., Mingari M.C. and Moretta L. (2014) Effect of tumor cells and tumor microenvironment on NK-cell function. Eur. J. Immunol. 44, 1582–1592 10.1002/eji.201344272 [DOI] [PubMed] [Google Scholar]
- 6.Sharma P., Hu-Lieskovan S., Wargo J.A. and Ribas A. (2017) Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 168, 707–723 10.1016/j.cell.2017.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smyth M.J., Ngiow S.F., Ribas A. and Teng M.W.L. (2016) Combination cancer immunotherapies tailored to the tumour microenvironment. Nat. Rev. Clin. Oncol. 13, 143–158 10.1038/nrclinonc.2015.209 [DOI] [PubMed] [Google Scholar]
- 8.Cassetta L. and Pollard J.W. (2018) Targeting macrophages: Therapeutic approaches in cancer. Nat. Rev. Drug Discov. 17, 887–904 10.1038/nrd.2018.169 [DOI] [PubMed] [Google Scholar]
- 9.Kitamura T., Qian B.Z. and Pollard J.W. (2015) Immune cell promotion of metastasis. Nat. Rev. Immunol. 15, 73–86 10.1038/nri3789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cassetta L. and Kitamura T. (2018) Macrophage targeting: opening new possibilities for cancer immunotherapy. Immunology 155, 285–293 10.1111/imm.12976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cotechini T., Atallah A. and Grossman A. (2021) Tissue‐resident and recruited macrophages in primary tumor and metastatic microenvironments: Potential targets in cancer therapy. Cells 10, 960 10.3390/cells10040960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramos R.N., Missolo-Koussou Y., Gerber-Ferder Y., Bromley C.P., Bugatti M., Núñez N.G.et al. (2022) Tissue-resident FOLR2+ macrophages associate with CD8+ T cell infiltration in human breast cancer. Cell 185, 1189–1207 10.1016/j.cell.2022.02.021 [DOI] [PubMed] [Google Scholar]
- 13.Gao J., Liang Y. and Wang L. (2022) Shaping polarization of tumor-associated macrophages in cancer immunotherapy. Front. Immunol. 13, 888713 10.3389/fimmu.2022.888713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krneta T., Gillgrass A., Poznanski S., Chew M., Lee A.J., Kolb M.et al. (2017) M2-polarized and tumor-associated macrophages alter NK cell phenotype and function in a contact-dependent manner. J. Leukoc. Biol. 101, 285–295 10.1189/jlb.3A1215-552R [DOI] [PubMed] [Google Scholar]
- 15.Peng L.S., Zhang J.Y., Teng Y.S., Zhao Y.L., Wang T.T., Mao F.Y.et al. (2017) Tumor-associated monocytes/macrophages impair NK-cell function via TGFβ1 in human gastric cancer. Cancer Immunol. Res. 5, 248–256 10.1158/2326-6066.CIR-16-0152 [DOI] [PubMed] [Google Scholar]
- 16.Wu Y., Kuang D.M., Pan W.D., Wan Y.L., Lao X.M., Wang D.et al. (2013) Monocyte/macrophage-elicited natural killer cell dysfunction in hepatocellular carcinoma is mediated by CD48/2B4 interactions. Hepatology 57, 1107–1116 10.1002/hep.26192 [DOI] [PubMed] [Google Scholar]
- 17.Paul S. and Lal G. (2017) The molecular mechanism of natural killer cells function and its importance in cancer immunotherapy. Front. Immunol. 8, 1124 10.3389/fimmu.2017.01124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lanuza P.M., Pesini C., Arias M.A., Calvo C., Ramirez-Labrada A. and Pardo J. (2020) Recalling the Biological Significance of Immune Checkpoints on NK Cells: A chance to overcome LAG3, PD1, and CTLA4 inhibitory pathways by adoptive NK cell transfer? Front. Immunol. 10, 3010 10.3389/fimmu.2019.03010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Z., Zhang C., Zhang J. and Tian Z. (2012) Macrophages help NK cells to attack tumor cells by stimulatory NKG2D ligand but protect themselves from NK killing by inhibitory ligand Qa-1. PLoS ONE 7, e36928 10.1371/journal.pone.0036928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sommariva M., Le Noci V., Storti C., Bianchi F., Tagliabue E., Balsari A.et al. (2017) Activation of NK cell cytotoxicity by aerosolized CpG-ODN/poly(I:C) against lung melanoma metastases is mediated by alveolar macrophages. Cell. Immunol. 313, 52–58 10.1016/j.cellimm.2017.01.004 [DOI] [PubMed] [Google Scholar]
- 21.Bellora F., Castriconi R., Dondero A., Pessino A., Nencioni A., Liggieri G.et al. (2014) TLR activation of tumor-associated macrophages from ovarian cancer patients triggers cytolytic activity of NK cells. Eur. J. Immunol. 44, 1814–1822 10.1002/eji.201344130 [DOI] [PubMed] [Google Scholar]
- 22.Nedvetzki S., Sowinski S., Eagle R.A., Harris J., Ric Vély F., Pende D.et al. (2007) Reciprocal regulation of human natural killer cells and macrophages associated with distinct immune synapses. Blood 109, 3776–3785 10.1182/blood-2006-10-052977 [DOI] [PubMed] [Google Scholar]
- 23.Mattiola I., Pesant M., Tentorio P.F., Molgora M., Marcenaro E., Lugli E.et al. (2015) Priming of human resting nk cells by autologous M1 macrophages via the engagement of IL-1β, IFN-β, and IL-15 pathways. J. Immunol. 195, 2818–2828 10.4049/jimmunol.1500325 [DOI] [PubMed] [Google Scholar]
- 24.Oppenheim D.E., Roberts S.J., Clarke S.L., Filler R., Lewis J.M., Tigelaar R.E.et al. (2005) Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nat. Immunol. 6, 928–937 10.1038/ni1239 [DOI] [PubMed] [Google Scholar]
- 25.Coudert J.D., Scarpellino L., Gros F., Vivier E. and Held W. (2008) Sustained NKG2D engagement induces cross-tolerance of multiple distinct NK cell activation pathways. Blood 111, 3571–3578 10.1182/blood-2007-07-100057 [DOI] [PubMed] [Google Scholar]
- 26.Deng W., Gowen B.G., Zhang L., Wang L., Lau S., Iannello A.et al. (2015) A shed NKG2D ligand that promotes natural killer cell activation and tumor rejection. Science 348, 136–139 10.1126/science.1258867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sandusky M.M., Messmer B. and Watzl C. (2006) Regulation of 2B4 (CD244)-mediated NK cell activation by ligand-induced receptor modulation. Eur. J. Immunol. 36, 3268–3276 10.1002/eji.200636146 [DOI] [PubMed] [Google Scholar]
- 28.Pende D., Falco M., Vitale M., Cantoni C., Vitale C., Munari E.et al. (2019) Killer Ig-like receptors (KIRs): Their role in NK cell modulation and developments leading to their clinical exploitation. Front. Immunol. 10, 1179 10.3389/fimmu.2019.01179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Modak M., Mattes A.K., Reiss D., Skronska-Wasek W., Langlois R., Sabarth N.et al. (2022) CD206+ tumor-associated macrophages cross-present tumor antigen and drive antitumor immunity. JCI Insight 7, e155022 10.1172/jci.insight.155022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang T., Dai L., Shen S., Yang Y., Yang M., Yang X.et al. (2022) Comprehensive molecular analyses of a macrophage-related gene signature with regard to prognosis, immune features, and biomarkers for immunotherapy in hepatocellular carcinoma based on WGCNA and the LASSO algorithm. Front. Immunol. 13, 843408 10.3389/fimmu.2022.843408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mulder K., Patel A.A., Kong W.T., Piot C., Halitzki E., Dunsmore G.et al. (2021) Cross-tissue single-cell landscape of human monocytes and macrophages in health and disease. Immunity 54, 1883–1900 10.1016/j.immuni.2021.07.007 [DOI] [PubMed] [Google Scholar]
- 32.Kloss M., Decker P., Baltz K.M., Baessler T., Jung G., Rammensee H.-G.et al. (2008) Interaction of Monocytes with NK cells upon Toll-like receptor-induced expression of the NKG2D ligand MICA 1. J. Immunol. 181, 6711–6719 10.4049/jimmunol.181.10.6711 [DOI] [PubMed] [Google Scholar]
- 33.Brownlie D., Doughty-Shenton D., Soong D.Y.H., Nixon C., Carragher N.O., Carlin L.M.et al. (2021) Metastasis-associated macrophages constrain antitumor capability of natural killer cells in the metastatic site at least partially by membrane bound transforming growth factor β. J. Immunother. Cancer 9, e001740 10.1136/jitc-2020-001740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vari F., Arpon D., Keane C., Hertzberg M.S., Talaulikar D., Jain S.et al. (2018) Immune evasion via PD-1/PD-L1 on NK cells and monocyte/macrophages is more prominent in Hodgkin lymphoma than DLBCL. Blood 131, 1809–1819 10.1182/blood-2017-07-796342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lang S., Vujanovic N.L., Wollenberg B. and Whiteside T.L. (1998) Absence of B7.1-CD28/CTLA-4-mediated co-stimulation in human NK cells. Eur. J. Immunol. 28, 780–786 [DOI] [PubMed] [Google Scholar]
- 36.Huard B., Tournier M., Triebel F. and Johnson R.W. (1998) LAG-3 does not define a specific mode of natural killing in human. Immunol. Lett. 61, 109–112 10.1016/S0165-2478(97)00170-3 [DOI] [PubMed] [Google Scholar]
- 37.Ndhlovu L.C., Lopez-Vergè S., Barbour J.D., Jones R.B., Jha A.R., Long B.R.et al. (2012) Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood. 119, 3734–3743 10.1182/blood-2011-11-392951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Z., Zhu J., Gu H., Yuan Y., Zhang B., Zhu D.et al. (2015) The clinical significance of abnormal Tim-3 expression on nk cells from patients with gastric cancer. Immunol. Invest. 44, 578–589 10.3109/08820139.2015.1052145 [DOI] [PubMed] [Google Scholar]
- 39.Xu L., Huang Y., Tan L., Yu W., Chen D., Lu C.et al. (2015) Increased Tim-3 expression in peripheral NK cells predicts a poorer prognosis and Tim-3 blockade improves NK cell-mediated cytotoxicity in human lung adenocarcinoma. Int. Immunopharmacol. 29, 635–641 10.1016/j.intimp.2015.09.017 [DOI] [PubMed] [Google Scholar]
- 40.Da Silva I.P., Gallois A., Jimenez-Baranda S., Khan S., Anderson A.C., Kuchroo V.K.et al. (2014) Reversal of NK-cell exhaustion in advanced melanoma by Tim-3 blockade. Cancer Immunol. Res. 2, 410–422 10.1158/2326-6066.CIR-13-0171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lolmede K., Campana L., Vezzoli M., Bosurgi L., Tonlorenzi R., Clementi E.et al. (2009) Inflammatory and alternatively activated human macrophages attract vessel-associated stem cells, relying on separate HMGB1- and MMP-9-dependent pathways. J. Leukoc. Biol. 85, 779–787 10.1189/jlb.0908579 [DOI] [PubMed] [Google Scholar]
- 42.Gao X., Zhou S., Qin Z., Li D., Zhu Y. and Ma D. (2023) Upregulation of HMGB1 in tumor-associated macrophages induced by tumor cell-derived lactate further promotes colorectal cancer progression. J. Transl. Med. 21, 53 10.1186/s12967-023-03918-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qi Y., Chang Y., Wang Z., Chen L., Kong Y., Zhang P.et al. (2019) Tumor-associated macrophages expressing galectin-9 identify immunoevasive subtype muscle-invasive bladder cancer with poor prognosis but favorable adjuvant chemotherapeutic response. Cancer Immunol. Immunother. 68, 2067–2080 10.1007/s00262-019-02429-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gleason M.K., Lenvik T.R., McCullar V., Felices M., O'Brien M.S., Cooley S.A.et al. (2012) Tim-3 is an inducible human natural killer cell receptor that enhances interferon gamma production in response to galectin-9. Blood 119, 3064–3072 10.1182/blood-2011-06-360321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Audenaerde J.R.M., De Waele J., Marcq E., Van Loenhout J., Lion E., Van den Bergh J.M.J.et al. (2017) Interleukin-15 stimulates natural killer cell-mediated killing of both human pancreatic cancer and stellate cells. Oncotarget 8, 56968–56979 10.18632/oncotarget.18185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li M., Xia P., Du Y., Liu S., Huang G., Chen J.et al. (2014) T-cell immunoglobulin and ITIM domain (TIGIT) receptor/poliovirus receptor (PVR) ligand engagement suppresses interferon-γ production of natural killer cells via β-arrestin 2-mediated negative signaling. J. Biol. Chem. 289, 17647–17657 10.1074/jbc.M114.572420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stanietsky N., Simic H., Arapovic J., Toporik A., Levy O., Novik A.et al. (2009) The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc. Natl. Acad. Sci. U.S.A. 106, 17858–17863 10.1073/pnas.0903474106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu S., Zhang H., Li M., Hu D., Li C., Ge B.et al. (2013) Recruitment of Grb2 and SHIP1 by the ITT-like motif of TIGIT suppresses granule polarization and cytotoxicity of NK cells. Cell Death. Different. 20, 456–464 10.1038/cdd.2012.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Q., Bi J., Zheng X., Chen Y., Wang H., Wu W.et al. (2018) Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat. Immunol. 19, 723–732 10.1038/s41590-018-0132-0 [DOI] [PubMed] [Google Scholar]
- 50.Zhu X., Liang R., Lan T., Ding D., Huang S., Shao J.et al. (2022) Tumor-associated macrophage-specific CD155 contributes to M2-phenotype transition, immunosuppression, and tumor progression in colorectal cancer. J. Immunother. Cancer 10, e004219 10.1136/jitc-2021-004219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khan M., Arooj S. and Wang H. (2020) NK cell-based immune checkpoint inhibition. Front. Immunol. 11, 167 10.3389/fimmu.2020.00167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dankner M., Gray-Owen S.D., Huang Y.H., Blumberg R.S. and Beauchemin N. (2017) CEACAM1 as a multi-purpose target for cancer immunotherapy. Onco. Immunol. 7, e1328336 10.1080/2162402X.2017.1328336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Caraux A., Lu Q., Fernandez N., Riou S., Di Santo J.P., Raulet D.H.et al. (2006) Natural killer cell differentiation driven by Tyro3 receptor tyrosine kinases. Nat. Immunol. 7, 747–754 10.1038/ni1353 [DOI] [PubMed] [Google Scholar]
- 54.Loges S., Schmidt T., Tjwa M., Van Geyte K., Lievens D., Lutgens E.et al. (2010) Malignant cells fuel tumor growth by educating infiltrating leukocytes to produce the mitogen Gas6. Blood 115, 2264–2273 10.1182/blood-2009-06-228684 [DOI] [PubMed] [Google Scholar]
- 55.Chirino L.M., Kumar S., Okumura M., Sterner D.E., Mattern M., Butt T.R.et al. (2020) TAM receptors attenuate murine NK-cell responses via E3 ubiquitin ligase Cbl-b. Eur. J. Immunol. 50, 48–55 10.1002/eji.201948204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lazarova M. and Steinle A. (2019) Impairment of NKG2D-mediated tumor immunity by TGF-β. Front. Immunol. 10, 2689 10.3389/fimmu.2019.02689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beffinger M., de Lara P.T., Tugues S., Vermeer M., Montagnolo Y., Ohs I.et al. (2018) CSF1R-dependent myeloid cells are required for NK mediated control of metastasis. JCI Insight 3, e97792 10.1172/jci.insight.97792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Michel T., Poli A., Domingues O., Mauffray M., Thérésine M., Brons N.H.C.et al. (2012) Mouse lung and spleen natural killer cells have phenotypic and functional differences, in part influenced by macrophages. PloS ONE 7, e51230 10.1371/journal.pone.0051230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Giurisato E., Lonardi S., Telfer B., Lussoso S., Risa-Ebrí B., Zhang J.et al. (2020) Extracellular-regulated protein kinase 5-mediated control of p21 expression promotes macrophage proliferation associated with tumor growth and metastasis. Cancer Res. 80, 3319–3330 10.1158/0008-5472.CAN-19-2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sato-Kaneko F., Yao S., Ahmadi A., Zhang S.S., Hosoya T., Kaneda M.M.et al. (2017) Combination immunotherapy with TLR agonists and checkpoint inhibitors suppresses head and neck cancer. JCI Insight 2, e93397 10.1172/jci.insight.93397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh M., Khong H., Dai Z., Huang X.F., Wargo J.A., Cooper Z.A.et al. (2014) Effective Innate and Adaptive Antimelanoma Immunity through Localized TLR7/8 Activation. J. Immunol. 193, 4722–4731 10.4049/jimmunol.1401160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Georgoudaki A.M., Prokopec K.E., Boura V.F., Hellqvist E., Sohn S., Östling J.et al. (2016) Reprogramming tumor-associated macrophages by antibody targeting inhibits cancer progression and metastasis. Cell Reports 15, 2000–2011 10.1016/j.celrep.2016.04.084 [DOI] [PubMed] [Google Scholar]
- 63.Eisinger S., Sarhan D., Boura V.F., Ibarlucea-Benitez I., Tyystjärvi S., Oliynyk G.et al. (2020) Targeting a scavenger receptor on tumor-associated macrophages activates tumor cell killing by natural killer cells. Proc. Natl. Acad. Sci. U.S.A. 117, 32005–32016 10.1073/pnas.2015343117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.He Y., Du J. and Dong Z. (2020) Myeloid deletion of phosphoinositide-dependent kinase-1 enhances NK cell-mediated antitumor immunity by mediating macrophage polarization. Onco. Immunol. 9, 1–14 10.1080/2162402X.2020.1774281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cen X., Liu S. and Cheng K. (2018) The role of toll-like receptor in inflammation and tumor immunity. Front. Pharmacol. 9, 878 10.3389/fphar.2018.00878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang N., Fu J., Li Z., Jiang N., Chen Y. and Peng J. (2022) The landscape of PDK1 in breast cancer. Cancers 14, 811 10.3390/cancers14030811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Paolino M., Choidas A., Wallner S., Pranjic B., Uribesalgo I., Loeser S.et al. (2014) The E3 ligase Cbl-b and TAM receptors regulate cancer metastasis via natural killer cells. Nature 507, 508–512 10.1038/nature12998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ireland L., Luckett T., Schmid M.C. and Mielgo A. (2020) Blockade of stromal Gas6 alters cancer cell plasticity, activates NK cells, and inhibits pancreatic cancer metastasis. Front. Immunol. 11, 297 10.3389/fimmu.2020.00297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu S., Ren J. and ten Dijke P. (2021) Targeting TGFβ signal transduction for cancer therapy. Signal Transduct. Target. Ther. 6, 8 10.1038/s41392-020-00436-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ma R.Y., Black A. and Qian B.Z. (2022) Macrophage diversity in cancer revisited in the era of single-cell omics. Trends Immunol. 43, 546–563 10.1016/j.it.2022.04.008 [DOI] [PubMed] [Google Scholar]
- 71.Yamazaki T., Akiba H., Iwai H., Matsuda H., Aoki M., Tanno Y.et al. (2002) Expression of programmed death 1 ligands by murine T cells and APC. J. Immunol. 169, 5538–5545 10.4049/jimmunol.169.10.5538 [DOI] [PubMed] [Google Scholar]
- 72.Loke P. and Allison J.P. (2003) PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells. Proc. Natl. Acad. Sci. U.S.A. 100, 5336–5341 10.1073/pnas.0931259100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cai H., Zhang Y., Wang J. and Gu J. (2021) Defects in macrophage reprogramming in cancer therapy: the negative impact of PD-L1/PD-1. Front. Immunol. 12, 690869 10.3389/fimmu.2021.690869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barry K.C., Hsu J., Broz M.L., Cueto F.J., Binnewies M., Combes A.J.et al. (2018) A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nat. Med. 24, 1178–1191 10.1038/s41591-018-0085-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bonavita E., Bromley C.P., Jonsson G., Pelly V.S., Sahoo S., Walwyn-Brown K.et al. (2020) Antagonistic inflammatory phenotypes dictate tumor fate and response to immune checkpoint blockade. Immunity 53, 1215.e8–1229.e8 10.1016/j.immuni.2020.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vivier E., Tomasello E., Baratin M., Walzer T. and Ugolini S. (2008) Functions of natural killer cells. Nat. Immunol. 9, 503–510 10.1038/ni1582 [DOI] [PubMed] [Google Scholar]
- 77.Russo E., Laffranchi M., Tomaipitinca L., Del Prete A., Santoni A., Sozzani S.et al. (2021) NK cell anti-tumor surveillance in a myeloid cell-shaped environment. Front. Immunol. 12, 787116 10.3389/fimmu.2021.787116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Toffoli E.C., Sheikhi A., Höppner Y.D., de Kok P., Yazdanpanah-Samani M., Spanholtz J.et al. (2021) Natural killer cells and anti-cancer therapies: reciprocal effects on immune function and therapeutic response. Cancers 13, 711 10.3390/cancers13040711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Suen W.C., Lee W.Y., Leung K.T., Pan X.H. and Li G. (2018) Natural killer cell-based cancer immunotherapy: a review on 10 years completed clinical trials. Cancer Invest. 36, 431–457 10.1080/07357907.2018.1515315 [DOI] [PubMed] [Google Scholar]
- 80.Larionova I., Cherdyntseva N., Liu T., Patysheva M., Rakina M. and Kzhyshkowska J. (2019) Interaction of tumor-associated macrophages and cancer chemotherapy. Oncoimmunology 8, 1596004 10.1080/2162402X.2019.1596004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Beach C., MacLean D., Majorova D., Arnold J.N. and Olcina M.M. (2022) The effects of radiation therapy on the macrophage response in cancer. Front. Oncol. 12, 1020606 10.3389/fonc.2022.1020606 [DOI] [PMC free article] [PubMed] [Google Scholar]